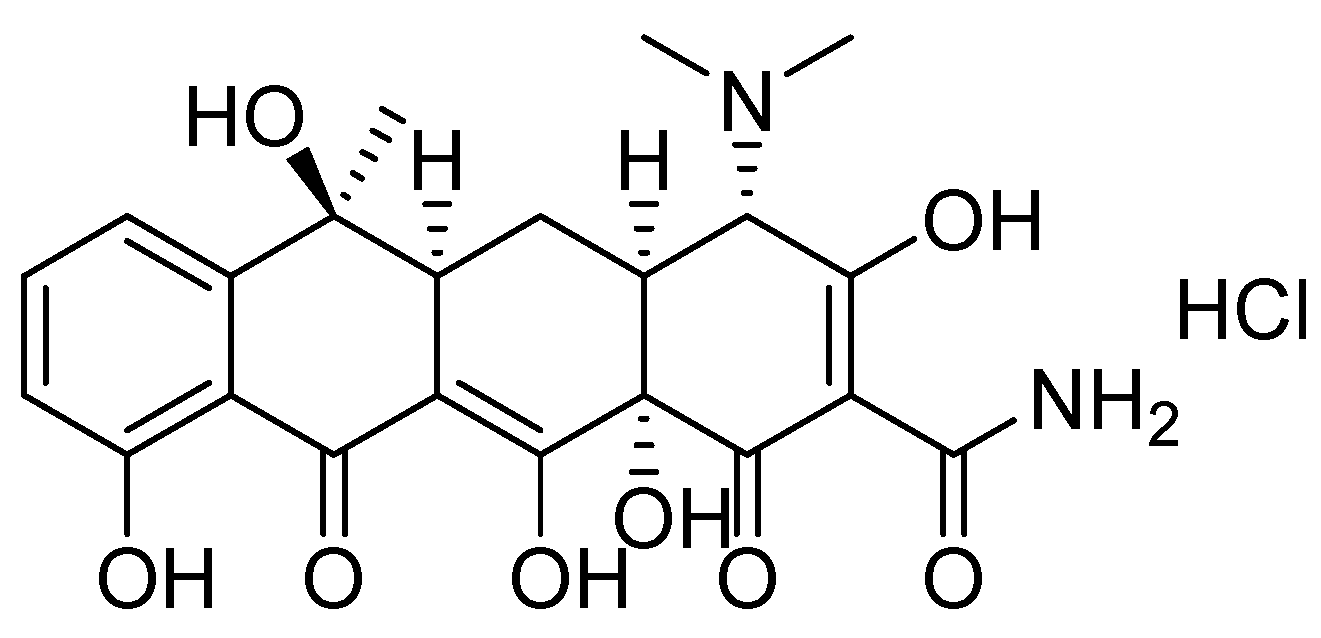
Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
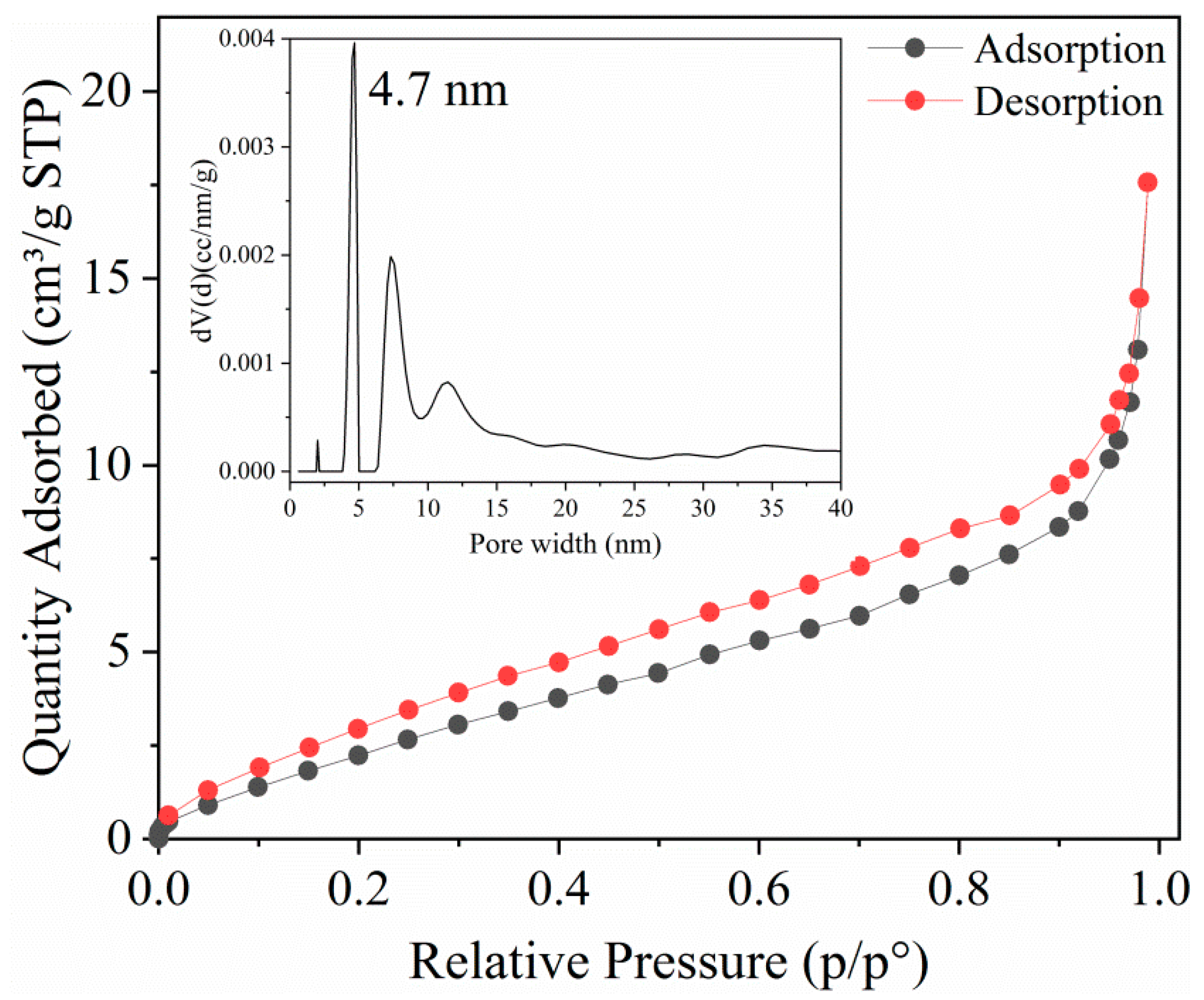
N2 Adsorption-Desorption Isotherms Analysis
2.2. Tetracycline Adsorption Studies
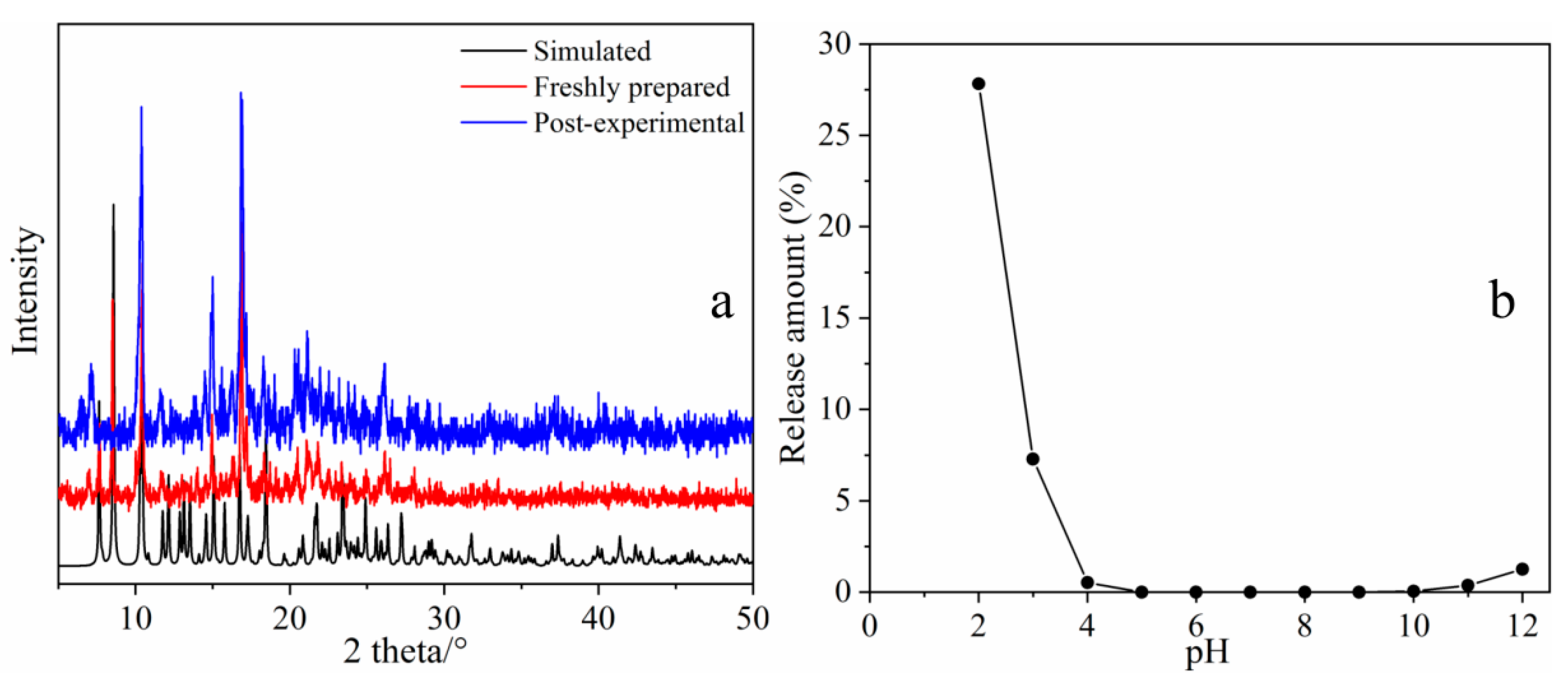
2.2.1. Effect of pH on the Tetracycline Adsorption
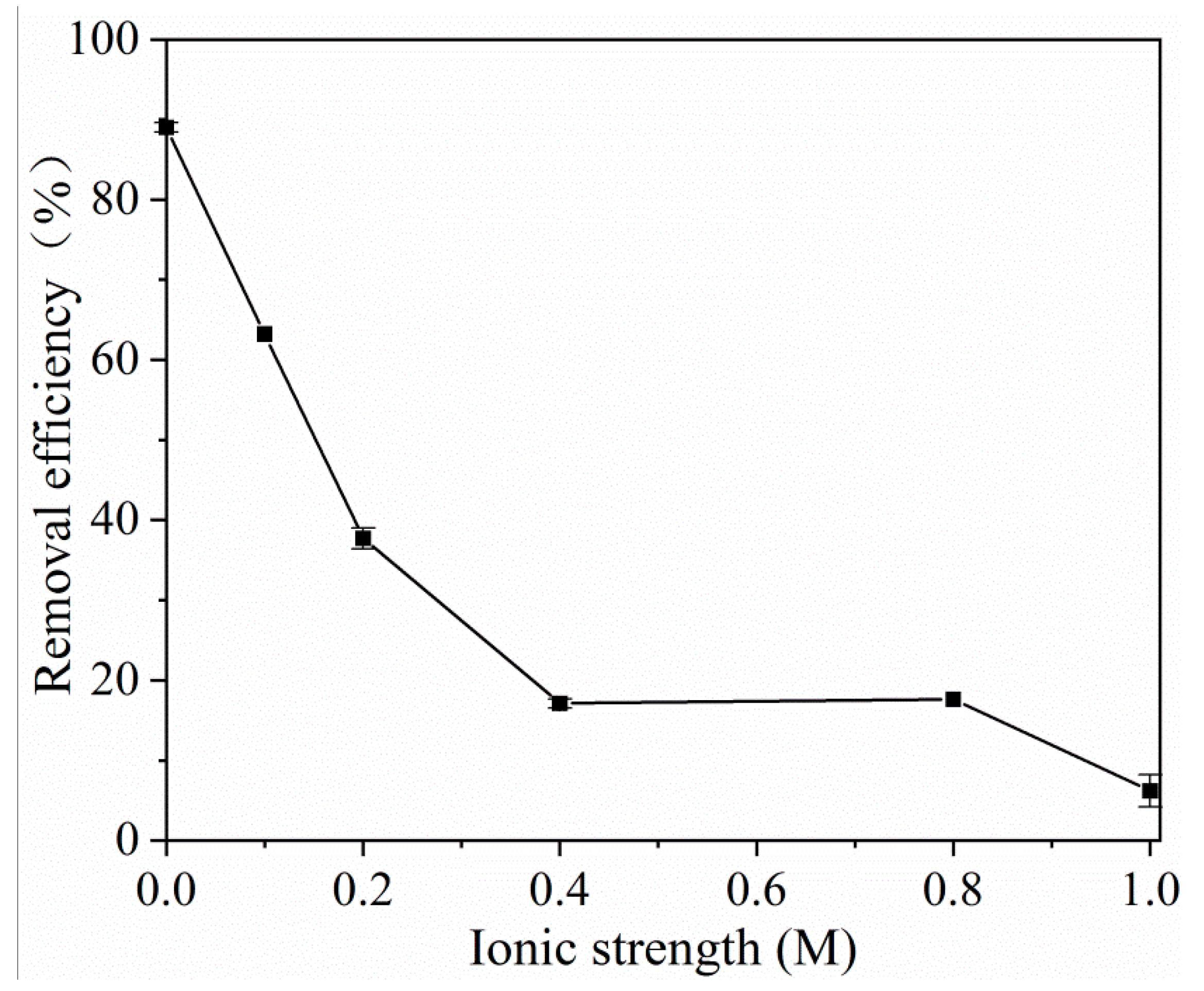
2.2.2. Effect of Ionic Strength on the Tetracycline Adsorption
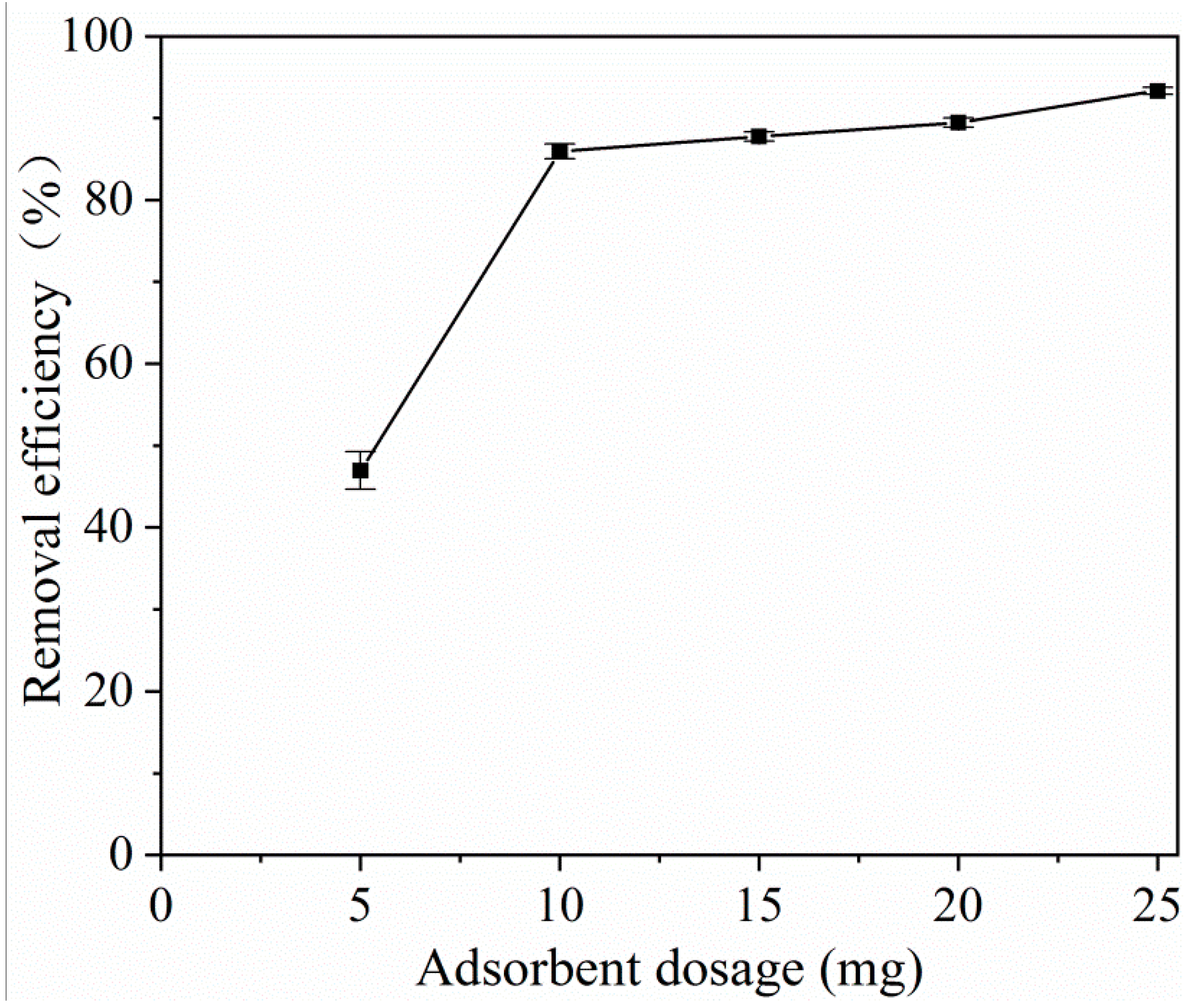
2.2.3. Effect of the Adsorption Dose on the Tetracycline Adsorption
2.2.4. Adsorption Kinetics for Tetracycline on MOF 1
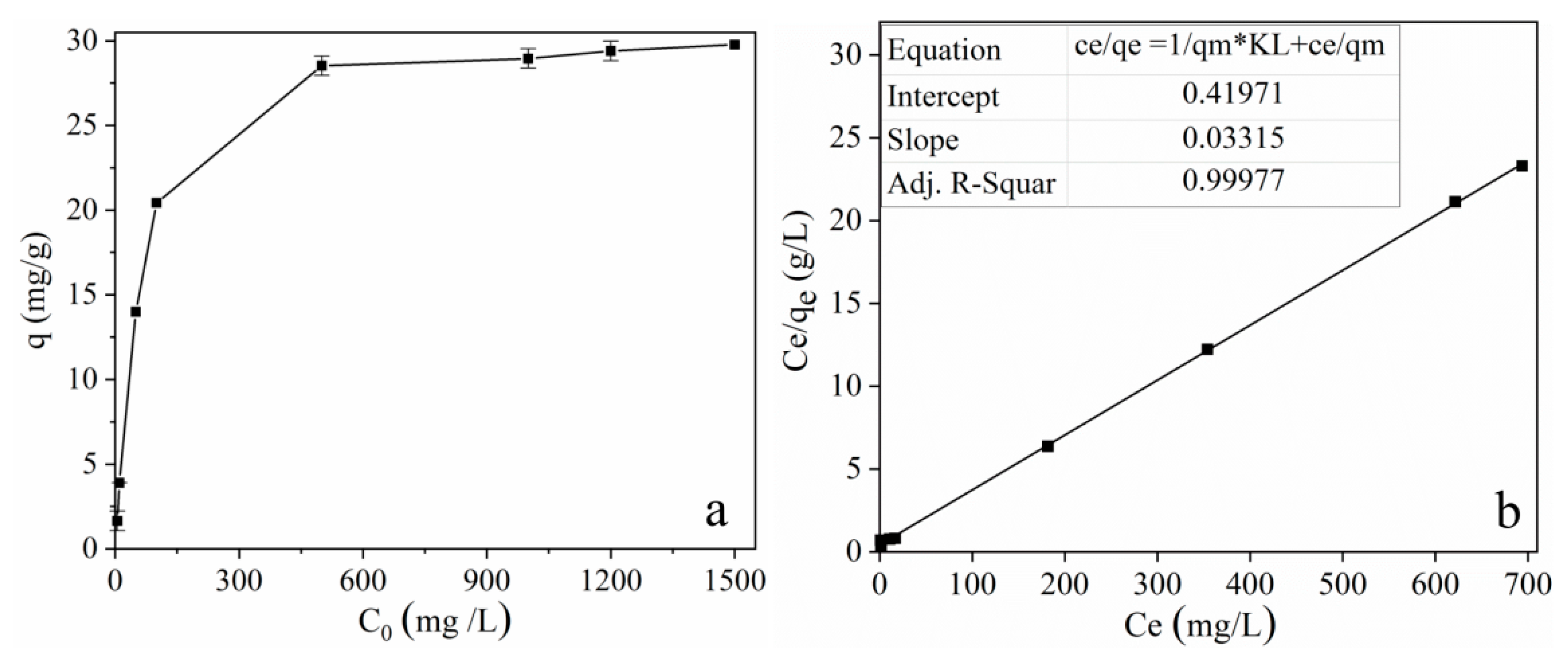
2.2.5. Adsorption isotherms for tetracycline on MOF 1
2.2.6. Potential Mechanism of Tetracycline Adsorption
2.3. Study on the Stability of MOF 1
2.4. Treatment of Tetracycline in Sewage and Dairy Products
3. Experimental Section
3.1. Chemicals and Methods
3.2. Synthesis of Ligand (L) and Adsorbent (MOF 1)
3.3. Sample Preparation
3.4. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Zhong, D.; Miao, H.; Yang, X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta 2015, 140, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, R.; Wang, Y.; Sun, L.; Chen, L.; Dai, X.; Pan, J.; Pan, G.; Yan, Y. Surface-imprinted fluorescence microspheres as ultrasensitive sensor for rapid and effective detection of tetracycline in real biological samples. Sens. Actuators B Chem. 2018, 263, 533–542. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, M.; Zeng, Z. Risks of neonicotinoid pesticides. Science 2013, 340, 1403. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Cao, J.; Chen, Y.-P.; Li, X.; Xiong, W.-P.; Zhou, Y.-Y.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. Mn-doped zirconium metal-organic framework as an effective adsorbent for removal of tetracycline and Cr (VI) from aqueous solution. Microporous Mesoporous Mater. 2019, 277, 277–285. [Google Scholar] [CrossRef]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin–A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef]
- Donoghue, D.J. Antibiotic residues in poultry tissues and eggs: Human health concerns? Poult. Sci. 2003, 82, 618–621. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Sandulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework for tetracycline detection in honey. Food Control 2016, 59, 424–429. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Larsen, J.L. Incidence, distribution, and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl. Environ. Microbiol. 2001, 67, 5675–5682. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.; Zhu, S.; Feng, Y.; Zhuo, Y.; Dou, Y. One-pot synthesis of high fluorescent carbon nanoparticles and their applications as probes for detection of tetracyclines. Biosens. Bioelectron. 2014, 56, 6–11. [Google Scholar] [CrossRef]
- Ramezani, M.; Danesh, N.M.; Lavaee, P.; Abnous, K.; Taghdisi, S.M. A novel colorimetric triple-helix molecular switch aptasensor for ultrasensitive detection of tetracycline. Biosens. Bioelectron. 2015, 70, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, X.; Jia, L.; Zhou, T.; Ma, T.; Xu, Z.; Cao, J.; Ge, Z.; Bi, N.; Zhu, T. A novel visual ratiometric fluorescent sensing platform for highly-sensitive visual detection of tetracyclines by a lanthanide-functionalized palygorskite nanomaterial. J. Hazard. Mater. 2018, 342, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.; Fierro, V.; De Yuso, A.M.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-D.; Wang, Y.-J.; Sun, R.-J.; Zhou, D.-M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-F.; Zhu, M.-P.; Chen, J.P.; Yuan, Z.-H.; Zhong, L.-B.; Zheng, Y.-M. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane–Implications for antibiotics recovery. Sep. Purif. Technol. 2015, 153, 76–83. [Google Scholar] [CrossRef]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Fan, X.; Quan, X.; Tan, F.; Zhang, Y.; Gao, J. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: In comparison with powder activated carbon. J. Colloid Interface Sci. 2015, 447, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, W.; Chen, B.; Zhao, Y.; Liu, D.; Sun, Y.; Gong, B. Removal of tetracycline from aqueous solution by MCM-41-zeolite A loaded nano zero valent iron: Synthesis, characteristic, adsorption performance and mechanism. J. Hazard. Mater. 2017, 339, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef]
- Jin, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Xu, R.; Zhang, Y.; Cao, J.; Li, X.; Zhou, C. Cu and Co nanoparticles co-doped MIL-101 as a novel adsorbent for efficient removal of tetracycline from aqueous solutions. Sci. Total Environ. 2019, 650, 408–418. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P. Stable metal–organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–organic framework nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Li, K.; Li, J.-J.; Zhao, N.; Xie, T.-T.; Di, B.; Xu, L.-L. Thioether-based recyclable metal–organic frameworks for selective and efficient removal of Hg2+ from water. Dalton Trans. 2019, 48, 17800–17809. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal–organic framework UiO-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]
- Rathod, M.; Haldar, S.; Basha, S. Nanocrystalline cellulose for removal of tetracycline hydrochloride from water via biosorption: Equilibrium, kinetic and thermodynamic studies. Ecol. Eng. 2015, 84, 240–249. [Google Scholar] [CrossRef]
- Guler, U.A.; Sarioglu, M. Removal of tetracycline from wastewater using pumice stone: Equilibrium, kinetic and thermodynamic studies. J. Environ. Health Sci. Eng. 2014, 12, 79. [Google Scholar] [CrossRef]
- Kang, J.; Liu, H.; Zheng, Y.-M.; Qu, J.; Chen, J.P. Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan. J. Colloid Interface Sci. 2010, 344, 117–125. [Google Scholar] [CrossRef]
- Pouretedal, H.; Sadegh, N. Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, W.; Li, X.; Yang, Z.; Cao, J.; Jia, M.; Xu, R.; Zhang, Y. Functionalized MIL-53 (Fe) as efficient adsorbents for removal of tetracycline antibiotics from aqueous solution. Microporous Mesoporous Mater. 2019, 290, 109642. [Google Scholar] [CrossRef]
- Yu, L.-L.; Cao, W.; Wu, S.-C.; Yang, C.; Cheng, J.-H. Removal of tetracycline from aqueous solution by MOF/graphite oxide pellets: Preparation, characteristic, adsorption performance and mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 289–296. [Google Scholar] [CrossRef]
- Tu, C.; Guo, Y.; Dai, Y.; Wei, W.; Wang, W.; Wu, L.; Wang, A. Determination of Chloramphenicol in Honey and Milk by HPLC Coupled with Aptamer—Functionalized Fe3O4/Graphene Oxide Magnetic Solid—Phase Extraction. J. Food Sci. 2019, 84, 3624–3633. [Google Scholar] [CrossRef]
Sample Availability: Samples of the ligands and adsorbents are available from the authors. |

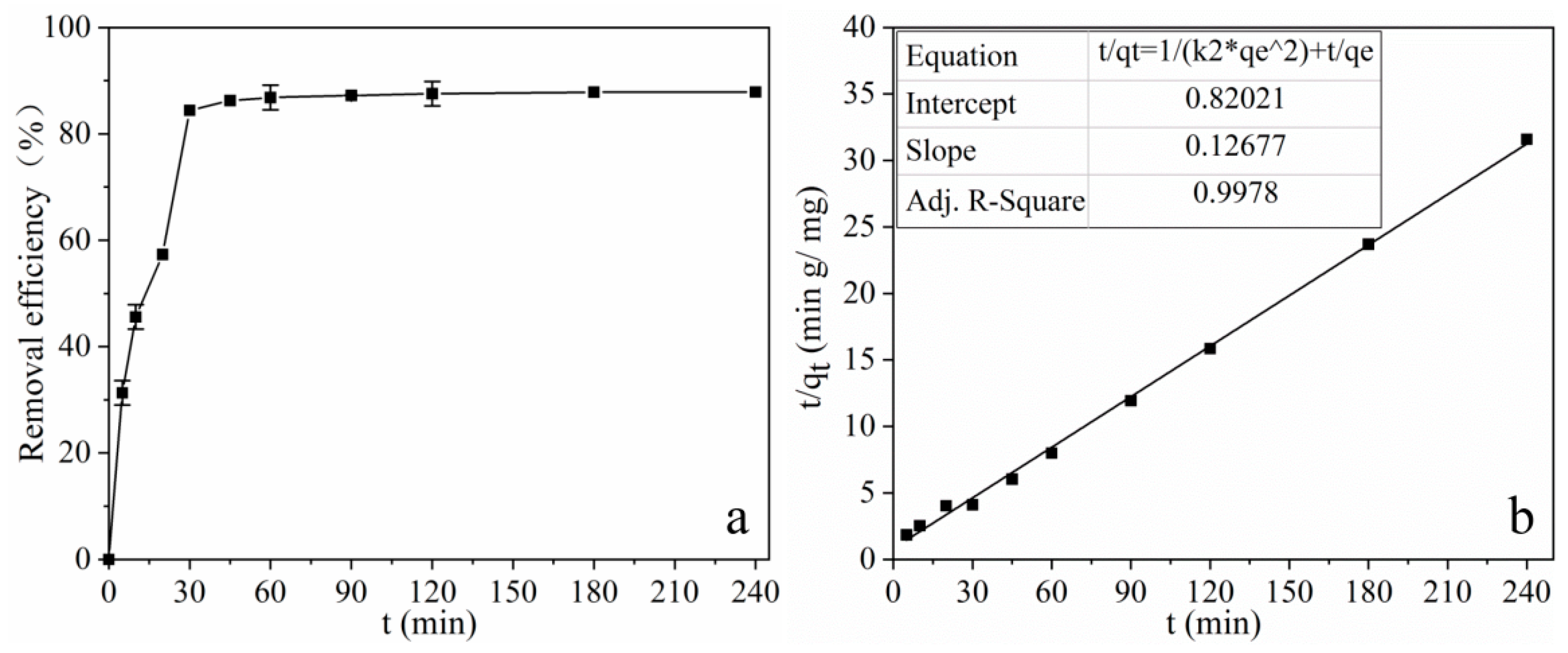
| Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | |||||||
|---|---|---|---|---|---|---|---|---|
| qe,exp | k1 | qe,cal | R2 | Δqe | k2 | qe,cal | R2 | Δqe |
| 7.60 | 0.0403 | 2.59 | 0.8919 | −67.13% | 0.01959 | 7.89 | 0.9978 | −3.65% |
| Langmuir Adsorption Isotherm | Freundlich Adsorption Isotherm | |||||
|---|---|---|---|---|---|---|
| qm,exp | qm,cal | KF | R2 | KF | n | R2 |
| 29.78 | 30.17 | 0.07898 | 0.9998 | 1.219 | 2.6483 | 0.8111 |
| Adsorbent | Adsorption Kinetics Model | Adsorption Isotherm Model | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| UiO-66 | pseudo-second-order | Langmuir model | 23.10 | 33 |
| Nanocellulose | pseudo-second-order | Redlich–Peterson | 7.73 | 34 |
| Pumice stone | pseudo-second-order | Langmuir and Freundlich model | 20.02 | 35 |
| Chitosan | - | Langmuir model | 23.92 | 36 |
| Activated carbon | pseudo-second-order | Langmuir model | 1.98 | 37 |
| MOF 1 | pseudo-second-order | Langmuir model | 29.78 | This study |
| Samples | Sewage | Dairy Products | ||||||
|---|---|---|---|---|---|---|---|---|
| Name | NJ | HZ | Pure milk | Yogurt | ||||
| Concentrations (mg L−1) | 5.0 | 50.0 | 5.0 | 50.0 | 5.0 | 50.0 | 5.0 | 50.0 |
| Removal efficiency (%) | 96.3 | 79.7 | 90.6 | 76.0 | 97.3 | 63.5 | 99.2 | 71. 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, J.-j.; Zhao, N.; Ma, Y.; Di, B. Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF. Molecules 2020, 25, 1312. https://doi.org/10.3390/molecules25061312
Li K, Li J-j, Zhao N, Ma Y, Di B. Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF. Molecules. 2020; 25(6):1312. https://doi.org/10.3390/molecules25061312
Chicago/Turabian StyleLi, Kan, Jing-jing Li, Ni Zhao, Ying Ma, and Bin Di. 2020. "Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF" Molecules 25, no. 6: 1312. https://doi.org/10.3390/molecules25061312
APA StyleLi, K., Li, J.-j., Zhao, N., Ma, Y., & Di, B. (2020). Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF. Molecules, 25(6), 1312. https://doi.org/10.3390/molecules25061312





