Abstract
The increasing resistance of rice sheath blight caused by Rhizoctonia solani highlights the need for highly effective and environmentally benign agents. Natural β-carboline alkaloids were simplified to obtain a series of indole derivatives, and their fungicidal activity and preliminary mode of action against R. solani were also evaluated. The initial hit indole (7) displayed significant fungicidal activity with an EC50 value of 25.56 μg/mL, and was selected for further optimization. Importantly, compound 55, the most active compound, had an EC50 value of 0.62 μg/mL, and approximately 300-fold more potent than validamycin A (EC50 = 183.00 μg/mL). In vivo bioassay also demonstrated that compound 55 showed better fungicidal activities than validamycin A. Moreover, the mechanism studies revealed that compound 55 not only caused remarkable morphological and structural alterations of R. solani hyphae, but also induced the loss of mitochondrial membrane potential and interfered with DNA synthesis. Therefore, compound 55 showed superior fungicidal activity against R. solani, and the elucidated mode of action supported the potential application of compound 55 against rice sheath blight.
1. Introduction
Rice sheath blight, caused by Rhizoctonia solani Kühn, is one of the most serious rice diseases worldwide [1], and not only has led to devastating loss in rice production—up to 50% under favorable conditions—but also causes quality issues [2,3]. For example, in China, the sheath blight infects almost 18 million hectares of rice in 2016 [4]. Although fungicides such as validamycin [5] are currently the most effective and reliable method to manage this disease [6], pathogen resistance, residual toxicity and environmental problems may arise with constant and indiscriminate fungicide usage, which highlights the need for highly effective and environmentally benign agents to control rice sheath blight [7].
In the quest for novel fungicidal agents, natural bioactive products have been and remain an excellent source of novel structure and serve as templates and inspiration for fungicides [8,9]. However, natural bioactive products are often victims of several deficiencies such as paucity or difficulty of obtainment from natural sources, as some natural product leads possess rather large, complex, or labile chemical structures which are difficult to obtain quantitatively by chemical synthesis, and most are not optimally ideal for direct use because of these weak activities [10]. Therefore, structural modification or simplification of active lead compounds from natural products has proven to be a successful way to discover fungicides with new modes of action, such as strobilurins fungicides.
Among natural product-based fungicides, β-carboline alkaloids and their saturated analogues, dihydro-β-carboline and tetrahydro-β-carboline alkaloids, isolated from medicinal genus Peganum [11], have possessed potent fungicidal activity with a broad spectrum [12,13,14]. For instant, the representative harmala alkaloids harmine (Figure 1, 1), harmaline (2) and tetrahydroharmine (3) displayed marginal and similar fungicidal activity against R. solani [13]. In our previous work, we had introduced urea [15] and oxadiazole [16] groups into the 3 position of β-carboline scaffold and obtained two potent compounds 5 and 6 with respective EC50 values of 69.52 and 4.24 μg/mL against R. solani. Continued exploration of the β-carboline through optimization of the ring C of β-carboline led to 1,2,4,9-tetrahydro-3-thia-9-aza-fluorene derivatives with greater fungicidal efficacy giving rise to a highly effective agent 4 (EC50 = 2.35 μg/mL) [17], which indicated that the ring C of β-carboline was attractive for further simplifications. Therefore, in this study, we further simplified the β-carboline scaffold with an opening of ring C, and obtained a potent fungicidal template indole scaffold [18]. The structure-activity relationship (SAR) of indole compounds was then systematically evaluated and the preliminary mechanism of action was also elucidated.
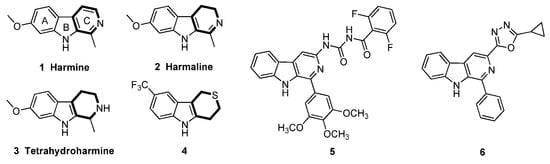
Figure 1.
The structure of the β-carboline alkaloids and their derivatives.
2. Results and Discussion
2.1. Chemistry
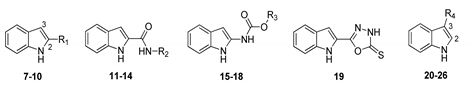
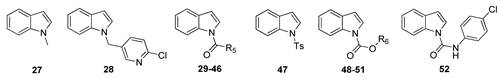
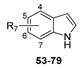
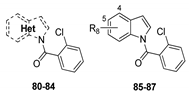
The synthesis of intermediates and target compounds were performed as shown in the Supporting Information. N-substituted indole-2-carboxamides 11–14 were achieved in a good yield through coupling of indole-2-carboxylic acid and various amines with HCTU as a coupling reagent [19]. Indole-2-carboxylic acid was treated with diphenylphosphoryl azide (DPPA) to give the intermediate indole-2-carboxazide. Subsequently, rearrangment of this intermediate in various alcohols yielded the substituted indole-2-carbamates 15–18 [20]. Compound 19 was prepared according to our previously reported procedure [21]. N-benzylation of indole was accomplished using 2-chloro-5-chloromethylpyridine in the presence of sodium hydride, furnishing 1-(6-chloro-3-pyridylmethyl)-1H-indole 28. Compounds 29–46 were prepared through acylation with various acyl chlorides under tetrabutylammonium hydrogen sulfate and potassium hydroxide [22]. Indole could be readily converted into indole-1-carboxylates 48–51 on treatment with various chloroformates, and into 1-(1H-indol-1-yl)-3-(4-chlorophenyl)urea 52 on treatment with 4-chlorophenyl isocyanate in the presence of sodium hydride [23]. Substituted indoles 55, 56, 58, 60 and 74 on 4- or 5-position were synthesized from substituted 2-nitrotoluene with DMF-DMA through Leimgruber–Batcho procedure [24]. Likewise, N-benzoylation of aza-indoles and substituted indoles with 2-chlorobenzoyl chloride to obtain the corresponding derivatives 80–87. All target compounds were purified by column chromatography and confirmed on the basis of 1H-NMR data.
2.2. Biological Activity
2.2.1. In Vitro Fungicidal Activity against R. Solani
Firstly, a series of 2- or 3- position substituted indole derivatives were obtained through simplification of the β-carboline scaffold with an opening of ring C, and these ring C modified seco-structures 7–26 were screened for the in vitro fungicidal activity against R. solani and the results were shown in Table 1. Of these compounds, indole (7, EC50 = 25.56 μg/mL) displayed superior fungicidal activity, and was 7-fold as potent as validamycin A (EC50 = 183.00 μg/mL). Additionally, it can be seen quite clearly that the structural changes in general were detrimental to activity and no favorable substitution could be found for the 2- or 3-position, even after extensive probing with a variety of diverse groups (8–26). These findings implied that indole was a potent fungicidal template for further modification and derivatization.

Table 1.
In vitro fungicidal activity of compounds 7–26. a
The biological data for compounds 27–52 with a focus on varying substituents in the 1-position of the indole moiety were shown in Table 2. Here it can be seen that methyl (27) or very bulky 6-chloro-3-pyridylmethyl compound (28) displayed similar activity. Compounds 29–34 bearing linear alkyl groups showed similar potency with similar EC50 values. Interestingly, as the length of alkyl group increased, the activity of 29–34 correspondingly decreased at the concentration of 50 μg/mL, while the opposite results were obtained at the low concentration (10 μg/mL). In the 1-position, a cyclopropionyl (35) was favorable for activity, bulky cycloalkyl acyl groups (36–38) less so, but in contrast, good activity accompanied substitution with a range of benzoyl groups (39–43), particularly the decoration of the electron-withdrawing substituents on the ortho- position of benzoyl ring was recommended for the enhancement of activity than the meta- and para-counterparts (2-CF3 > 3-CF3 > 4-CF3). The fungicidal activity of ortho-substituted compounds (40–43) increased following 2-Cl ≈ 2-Br > 2-F > 2-CF3. For compounds 48–52, the carboxylate substituents (48–51) was beneficial for the improvement of the fungicidal activity, while urea group (52) was detrimental to activity.

Table 2.
In vitro fungicidal activity of compounds 27–52. a
The SAR for the ring A of indole scaffold was probed next, and the results were depicted in Table 3. In the 4-position, compounds containing electron donating groups such as methyl (53) or methoxy group (54) retained fungicidal activity. For example, compound 53 (EC50 = 4.99 μg/mL) was over 5-fold more potent than indole (7, EC50 = 25.56 μg/mL). On the other hand, the introduction of a small electron withdrawing group (55–60) yielded highly active compounds, whereas a more polar derivative (61, 4-COOH) and a bulkier substituent (62, 4-COOCH3) showed inferior activity. Of particular note, compound 55 displayed the greatest inhibitory activity with the EC50 value of 0.62 μg/mL, and an over 40-fold higher activity than indole. In the 5-position, methyl (63), chloro (66), and bromo (67) were favorable for activity, a nitro (68) was tolerated, while the more polar (69–71) or bulky substituents (74 and 75) led to a loss of activity, which indicated that electronegativity and size of substituents were crucial for activity. A similar trend in activity was observed for compounds 76–79 bearing methyl or methoxy substitution at the 6- or 7-position of the indole ring.

Table 3.
In vitro fungicidal activity of compounds 53–79. a
The replacement of a CH group with a N atom in aromatic and heteroaromatic rings can have many important effects, such as molecular and physicochemical properties and intra- and inter-molecular interactions, which can translate to improved pharmacological profiles [25]. To evaluate whether a similar effect existed in indole ring, compounds 80–84 were synthesized and investigated (Table 4). Although these compounds displayed significant fungicidal activity against R. solani, with EC50 values ranging from 9.21–48.63 μg/mL, they were less potent than their corresponding counterpart 41 (EC50 = 2.15 μg/mL). According to the Table 1, Table 2, Table 3 and Table 4, we found that the compounds harboring 1-O-Cl-C6H5 (41), 4-F (55), 4-Cl (56), or 5-Cl (66), with respective EC50 values of 2.15, 0.62, 1.25 and 7.25 μg/mL, which were better than that of indole (25.56 μg/mL). Combined with these favorable substituents, compounds 85–87 resulted. Unfortunately, it can be seen that for compounds 85 and 86 this combination was detrimental to activity with respective EC50 values of 2.36 and 9.01 μg/mL as compared to compounds 55 and 56, while a neutral effect was observed for compound 87. The detailed structure-activity relationships of indole compounds were summarized in Figure 2.

Table 4.
In vitro fungicidal activity of compounds 80–87. a
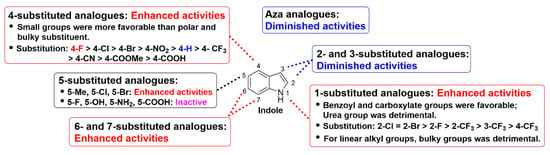
Figure 2.
Detailed structure-activity relationships of indole compounds against R. solani.
2.2.2. In Vivo Protective and Curative Effects against Rice Sheath Blight
Among the target compounds, compounds 55, 56, 66 and 85–87 displayed potent in vitro fungicidal activity against R. solani and, therefore, were selected to assess the in vivo protective effect through detached leaf assay. As shown in Table 5 and Figure 3, most of the tested compounds displayed excellent in vivo protective activity. To our delight, compound 55 possessed the highest fungicidal activity and the inhibitory effect at 50 μg/mL was up to 100%, which was comparable in activity to the commercial fungicide validamycin A (100% at 50 μg/mL).

Table 5.
In vivo protective activity against R. solani using detached leaf assay.

Figure 3.
In vivo protective effect of selected compounds against R. solani using detached leaf assay.
The most potent compounds 55, 56, 66 and 85 were further evaluated for their in vivo protective and curative activities against rice sheath blight in greenhouse experiment. As illustrated in Table 6 and Figure 4, most of these compounds displayed significant in vivo protective and curative activities against R. solani, of which compound 55 (79.23% and 89.08% at 200 μg/mL; 74.09% and 77.09% at 100 μg/mL) exhibited the highest fungicidal activity and was more potent than validamycin A (78.80% and 75.80% at 200 μg/mL; 65.74% and 67.45% at 100 μg/mL).

Table 6.
In vivo protective and curative effects against R. solani using greenhouse experiment.

Figure 4.
In vivo protective and curative activities of selected compounds against R. solani.
2.2.3. Effect of 55 on the Hyphae Morphology of R. Solani
The inhibitory activity of compound 55 on the sclerotia germination and formation of R. solani was tested, and the results showed that compound 55 displayed strong inhibitory effects of sclerotia formation and germination in a dose-dependent manner and both the inhibitory rates reached 100% at the concentration of 10 μg/mL, which indicated that compound 55 could significantly inhibit sclerotia formation and germination of R. solani, thereby reducing the primary infection sources and controlling the disease (Figure S1).
2.3. Preliminary Mechanism of Compound 55 against R. Solani
2.3.1. Effect of 55 on the Hyphae Morphology of R. Solani
The mycelia of R. solani grew smoothly along the surface of 55-free medium with a low density and a regular colony. However, the mycelia was dense as compared to the control mycelia, and the edge of the colony was irregular in the presence of 55, which implied that this compound could seriously depress the growth of mycelia.
The morphological variations of R. solani hyphae treated with 1 μg/mL 55 were revealed under SEM and the results were presented in Figure 5. The control mycelia were loose with a smooth surface and an intact structure (Figure 5a,b). However, the 55-treated mycelia were dense with a coarse surface and locally folding and entangling, even appeared severely shrunken and distorted (Figure 5c,d).
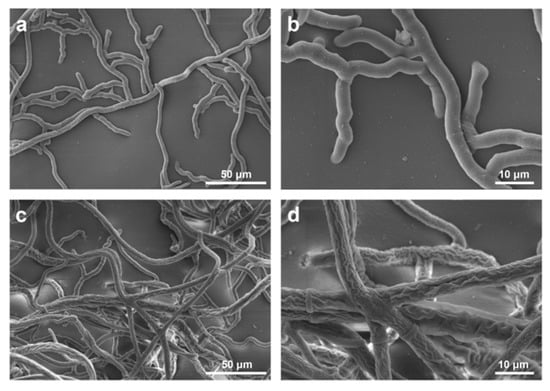
Figure 5.
SEM of R. solani hyphae treated with 0 (a,b) or 1 μg/mL 55 (c,d).
TEM was used to observe the definitive ultrastructural features of R. solani hyphae in response to compound 55. As shown in Figure 6a–e, the control hyphal cell displayed the characteristic cellular features with obvious cell walls (CW), cell membranes (CM), septa (S) and septal pore caps (SP), and abundant organelles in cytoplasm, such as vacuole (V) and mitochondria (M). In contrast, the cell walls of the 55-treated hyphae became abnormally thickened (Figure 6f,g). Moreover, the organelles were disorganized and caused abnormal changes. For instance, obvious cavitations and a slight swelling of mitochondrial matrix were observed after exposure to compound 55 (Figure 6h,j,k). It was notable that the septal pore caps of 55-treated hyphae disappeared (Figure 6i).
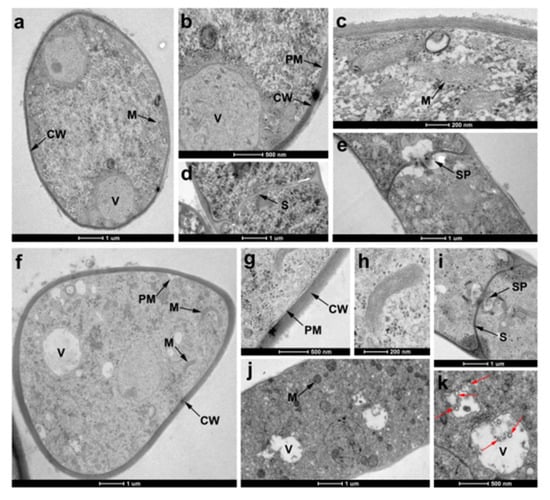
Figure 6.
TEM of R. solani hyphae treated without (a–e) or with 1 μg/mL 55 (f–k). (a) transverse of untreated hyphae, and many organelles were observed such as mitochondria (M) and vacuole (V); (b) cell wall (CW) of untreated hyphae; (c) mitochondria of untreated hyphae; (d) longitudinal of untreated hyphae, and spectra (S) and was uniform; (e) septal pore caps (SP) of untreated hyphae was visible; (f,g) transverse of 55-treated hyphae, and cell wall was thickening; (h) mitochondria of 55-treated hyphae was swollen; (i) longitudinal of 55-treated hyphae, and the septal pore caps disappeared; (j,k) longitudinal of 55-treated hyphae (loss of matrix in vacuoles and obvious vacuolization), and red arrow represented vacuolization.
2.3.2. Effect of 55 on the Endogenous ROS Production and Cell Membrane Permeability
Reactive oxygen species (ROS), byproducts of cellular metabolism, are primarily generated in the mitochondria. ROS accumulation could lead to cell damage and is considered as a fungicidal mechanism [26]. The effect of compound 55 on the endogenous ROS induction was determined by ROS assay using DCFH-DA staining. Surprisingly, the fluorescence intensity in the 55-treated mycelia reduced obviously as compared to that in the control mycelia (Figure 7a,b), indicating that this compound inhibited the intracellular ROS generation. This result was opposed to our previous findings for β-carboline [16] and 1,2,4,9-tetrahydro-3-thia-9-aza-fluorene derivatives [17]. Next, we examined the effect of 55 on the cell membrane permeability through evaluating the conductivity alterations of the R. solani hyphae in response to 55, and the results showed that compound 55 had no effect on cell membrane permeability (Figure S2a).
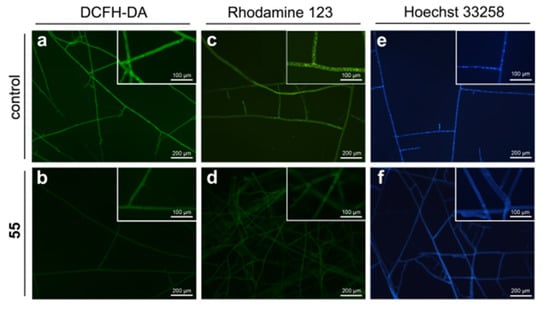
Figure 7.
The mode of action of 55 against R. solani. (a,b) The intracellular ROS generation of the hyphae was detected by fluorescent micrographs using DCFH-DA staining; (c,d) The mitochondrial membrane potential of the hyphae was evaluated by fluorescent micrographs with Rhodamine 123 staining; (e,f) nuclear morphology of R. solani hyphae stained by Hoechst 33258.
2.3.3. Effect of 55 on the Mitochondrial Membrane Potential (MMP)
The effect of compound 55 on mitochondrial membrane potential (MMP) was detected using the potential-dependent distributional probe Rhodamine 123 and the results were presented in Figure 7c,d. The fluorescence intensity of 55-treated hyphae dramatically decreased as compared to that of the control hyphae, which demonstrated that compound 55 could affect the MMP, disrupt the normal function of mitochondria, ultimately resulting in cell death.
2.3.4. Effect of 55 on the Nuclear Morphology
Hoechst 33258 staining was utilized to observe the nuclear morphology of R. solani hyphae exposed to 55. The results showed that the cell nucleus of the control hyphal showed uniform blue color and were observed as intense, discrete signals. In contrast, the nucleus of 55-treated hyphae displayed faint signals, and most of which did not show discrete nuclear signals (Figure 7e,f). Moreover, the number of nuclei in per hyphal cell treated with 0 or 1 μg/mL 55 was determined by a quantitative analysis (Figure S2b), and the results showed that the number of nuclei in per 55-treated hyphal cell (average 3.67 nuclei) was fewer than that of per untreated hyphae cell (average 5.94 nuclei). Taken together, these findings suggested that compound 55 could reduce DNA contents and the number of cell nuclei of R. solani hyphae, thereby interfering with DNA synthesis.
3. Materials and Methods
3.1. Chemistry
All commercial reagents and solvents were of reagent grade and used without purification. The 1H-NMR spectral data were obtained using a Bruker Avance-600 superconducting nuclear magnetic resonance instrument (Bruker Company, Hamburg, Gemany). The synthesis and structural characterization of compounds 11–87 were showed in the Supporting Information.
3.2. Biological Assay
3.2.1. In Vitro and in Vivo Fungicidal Activity against R. Solani
The fungi R. solani GD-118 was provided by our laboratory, and the rice cultivar (Xiangyazhan) was planted according to our previously reported method [15]. The in vitro and in vivo fungicidal activity against R. solani were tested following our previously reported method [17].
3.2.2. Inhibition of Compound 55 on the Sclerotia Formation and Germination of R. Solani
The inhibitory activity of compound 55 on the sclerotia formation and germination of R. solani was evaluated using our previous method [17].
3.3. Morphology Observation of R. Solani Hyphae
The morphology changes of R. solani hyphae treated with 1 μg/mL compound 55 were detected under scanning electron microscopy (SEM) and transmission electron microscopy (TEM) using our previously reported method [16].
3.4. Reactive Oxygen Species (ROS) Generation
The R. solani hyphae were treated with 0 and 1 μg/mL compound 55 for 48 h, and then were cut and placed on a sterile slide before continued incubation at 25 °C for 24 h. The hyphae were stained with 10 μM DCFH-DA solution (Beyotime, Shanghai, China). After incubation at 37 °C for 30 min in the darkness, the samples were observed and photographed using a fluorescence microscopy (Nikon ECLIPES 80 i, Tokyo, Japan).
3.5. Mitochondrial Membrane Potential (MMP)
Rhodamine 123 was used as an indicator to detect the effect of 55 on the MMP of R. solani using our previously reported method [16].
3.6. Karyological Analysis
The mycelial specimens were fixed with stain-fixative at 4 °C overnight. After washing twice with 0.1 M PBS, the fixed hyphae were stained with 1 mL Hoechst 33258 solution (Beyotime) at 25 °C for 10 min and the samples were observed using a fluorescence microscopy.
3.7. Detection of Cell Membrane Permeability
The effect of compound 55 on the cell membrane permeability was evaluated through the conductivity changes of R. solani mycelial using our previously reported method [16].
4. Conclusions
Simplification of β-carboline alkaloids through an opening of ring C obtained a potent fungicidal template indole scaffold. The structure-activity relationship (SAR) of indole compounds was then systematically evaluated and the preliminary mode of action of compound 55 was also elucidated. Among these compounds, compound 55 showed the most active against R. solani with an EC50 value of 0.62 μg/mL, and was approximately 300-fold more potent than validamycin A. In vivo bioassay also demonstrated that compound 55 displayed superior protective and curative activities as compared to validamycin A. Moreover, the preliminary mechanism studies revealed that compound 55 caused remarkable morphological and structural alterations of R. solani hyphae, induced the loss of mitochondrial membrane potential and reduced the DNA contents and number of cell nuclei. Due to these positive results, further development of 55-related compounds as potential fungicidal candidates is definitely warranted.
Supplementary Materials
Supplementary Materials are available online.
Author Contributions
Writing—original draft preparation, Z.Z.; methodology, J.Z., Z.Z., Q.Z. and Z.J.; writing—review and editing, Z.Z. and G.Z.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31572335), the National Key Research and Development Program of China (Grant No. 2018YFD0200300), and the China Guangdong Province Science and Technology Innovation Strategy Special Fund (Grant No. 2018B020217003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Groth, D.E. Azoxystrobin rate and timing effects on rice sheath blight incidence and severity and rice grain and milling yields. Plant Dis. 2005, 89, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.N.; Rush, M.C. Rice sheath blight: A major rice disease. Plant Dis. 1983, 67, 829–832. [Google Scholar] [CrossRef]
- Marchetti, M.A. Potential impact of sheath blight on yield and milling quality of short-statured rice lines in the Southern United States. Plant Dis. 1983, 67, 162–165. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, Y.; Wang, C.; Liu, S.; Liao, X. Effects and inhibition mechanism of phenazine-1-carboxamide on the mycelial morphology and ultrastructure of Rhizoctonia solani. Pestic. Biochem. Phys. 2018, 147, 32–39. [Google Scholar] [CrossRef]
- Boukaew, S.; Klinmanee, C.; Prasertsan, P. Potential for the integration of biological and chemical control of sheath blight disease caused by Rhizoctonia solani on rice. World J. Microb. Biot. 2013, 29, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Mew, T.W. Applying rice seed-associated antagonistic bacteria to manage rice sheath blight in developing countries. Plant Dis. 2004, 88, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Liu, Y.H.; Ma, X.Y.; Feng, Z.; Ma, Z.H. Characterization of sensitivity of Rhizoctonia solani, causing rice sheath blight, to mepronil and boscalid. Crop Prot. 2009, 28, 381–386. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Ichikawa, S. Function-Oriented Synthesis: How to design simplified analogues of antibacterial nucleoside natural products? Chem. Rec. 2016, 16, 1106–1115. [Google Scholar] [CrossRef]
- Kartal, M.; Altun, M.L.; Kurucu, S. HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. J. Pharmaceut. Biomed. 2003, 31, 263–269. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Liu, Y.; Wang, L.; Wang, Q. Synthesis and antiviral and fungicidal activity evaluation of β-carboline, dihydro-β-carboline, tetrahydro-β-carboline alkaloids, and their derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.B.; Chen, S.H.; Zhu, S.W.; Luo, J.J.; Zhang, Y.M.; Weng, Q.F. Synthesis and fungicidal activity of β-carboline alkaloids and their derivatives. Molecules 2015, 20, 13941–13957. [Google Scholar] [CrossRef]
- Khan, H.; Mubarak, M.S.; Amin, S. Antifungal potential of alkaloids as an emerging therapeutic target. Curr. Drug Targets 2017, 18, 1825–1835. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zeng, Y.; Jiang, Z.Y.; Shu, B.S.; Sethuraman, V.; Zhong, G.H. Design, synthesis, fungicidal property and QSAR studies of novel β-carbolines containing urea, benzoylthiourea and benzoylurea for the control of rice sheath blight. Pest Manag. Sci. 2018, 74, 1736–1746. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Jiang, Z.Y.; Zhu, Q.; Zhong, G.H. Discovery of β-carboline oxadiazole derivatives as fungicidal agents against rice sheath blight. J. Agric. Food Chem. 2018, 66, 9598–9607. [Google Scholar] [CrossRef]
- Xi, J.M.; Zhang, Z.J.; Zhu, Q.; Zhong, G.H. Evolution from natural β-carboline alkaloids to obtain 1,2,4,9-tetrahydro-3-thia-9-aza-fluorene derivatives as potent fungicidal agents against Rhizoctonia solani. Int. J. Mol. Sci. 2018, 19, 4044. [Google Scholar] [CrossRef]
- Singh, T.P.; Singh, O.M. Recent progress in biological activities of indole and indole alkaloids. Mini-Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar] [CrossRef]
- Mistry, S.N.; Shonberg, J.; Draperjoyce, C.J.; Klein, H.C.; Michino, M.; Shi, L.; Christopoulos, A.; Capuano, B.; Scammells, P.J.; Lane, J.R. Discovery of a novel class of negative allosteric modulator of the dopamine D2 receptor through fragmentation of a bitopic ligand. J. Med. Chem. 2015, 58, 6819–6843. [Google Scholar] [CrossRef]
- Jiang, H.; Zhuang, D.M.; Huang, Y.; Cao, X.; Yao, J.; Li, J.; Wang, J.Y.; Zhang, C.; Jiang, B. Design, synthesis, and biological evaluation of novel trifluoromethyl indole derivatives as potent HIV-1 NNRTIs with an improved drug resistance profile. Org. Biomol. Chem. 2014, 12, 3446–3458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zhang, J.J.; Jiang, Z.Y.; Zhong, G.H. Design, synthesis and bioactivity evaluation of novel β-carboline 1,3,4-oxadiazole derivatives. Molecules 2017, 22, 1811. [Google Scholar] [CrossRef]
- Tomakinian, T.; Guillot, R.; Kouklovsky, C.; Vincent, G. Direct oxidative coupling of N-acetyl indoles and phenols for the synthesis of benzofuroindolines related to phalarine. Angew Chem. Int. Edit. 2014, 53, 11881–11885. [Google Scholar] [CrossRef] [PubMed]
- Kirchberg, S.; Fröhlich, R.; Studer, A. Stereoselective palladium-catalyzed carboaminoxylations of indoles with arylboronic acids and TEMPO. Angew Chem. Int. Edit. 2009, 48, 4235–4238. [Google Scholar] [CrossRef] [PubMed]
- Batcho, A.D.; Leimgruber, W. Indoles from 2-methylnitrobenzenes by condensation with formamide acetals followed by reduction: 4-benzyloxyindole. Org. Synth. 1985, 63, 214. [Google Scholar] [CrossRef]
- Pennington, L.D.; Moustakas, D.T. The necessary nitrogen atom: A versatile high-impact design element for multiparameter optimization. J. Med. Chem. 2017, 60, 3552–3579. [Google Scholar] [CrossRef]
- Hwang, J.H.; Hwang, I.S.; Liu, Q.H.; Woo, E.R.; Lee, D.G. (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie 2012, 94, 1784–1793. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 11–87 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).