Abstract
A novel approach for the synthesis of unsymmetrically substituted dibenzo[b,f][1,5]diazocine-6,12(5H,11H)diones has been developed. This facile three-step method uses variously substituted 1H-benzo[d][1,3]oxazine-2,4-diones (isatoic anhydrides) and 2-aminobenzoic acids as a starting materials. The obtained products were further transformed into N-alkyl-, N-acetyl- and dithio analogues. Developed procedures allowed the synthesis of unsymmetrical dibenzo[b,f][1,5]diazocine-6,12(5H,11H)diones and three novel heterocyclic scaffolds: benzo[b]naphtho[2,3-f][1,5]diazocine-6,14(5H,13H)dione, pyrido[3,2-c][1,5]benzodiazocine-5,11(6H,12H)-dione and pyrazino[3,2-c][1,5]benzodiazocine-6,12(5H,11H)dione. For 11 of the compounds crystal structures were obtained. The preliminary cytotoxic effect against two cancer (HeLa, U87) and two normal lines (HEK293, EUFA30) as well as antibacterial activity were determined. The obtained dibenzo[b,f][1,5]diazocine(5H,11H)6,12-dione framework could serve as a privileged structure for the drug design and development.
1. Introduction
Tricyclic dibenzodiazepines and their structural analogues (dibenzoazepines, dibenzothiazepines and others) having two non-polar, aromatic benzene or heterocyclic rings separated by a seven-membered ring containing heteroatoms such as sulfur, nitrogen or oxygen (1, Figure 1), are very popular, privileged structures, useful for the development of drugs, as well as compounds with various biological activities and applications (Figure 1). Modulation of biological properties is possible by skillful chemical modifications, and variation of substituents and functional groups located in the six-membered benzene rings and the seven-membered heterocyclic ring. This group of compounds includes such important drugs as imipramine (2; a dibenzoazepine with antidepressant activity and a serotonin and norepinephrine reuptake inhibitor) [1], clobenzepam (3; dibenzodiazepine; antihistaminic and anticholinergic) [2], quetiapine (4; dibenzothiazepine; antipsychotic activity; dopamine, serotonin, and adrenergic receptors antagonist) [3], oxcarbazepine (5; dibenzoazepine; anticonvulsant activity; voltage-sensitive sodium channels blocker) [4,5], and nevirapine (6; dipyridodiazepine; anti-HIV; non-nucleoside reverse transcriptase inhibitor) [6]. Additionally, we recently reported [7] the synthesis of structurally related, tricyclic pyrazinebenzodiazepines type 7. Surprisingly, these compounds exhibited a promising cytotoxic effect against two cancer cell lines: LoVo (human colon cancer) and MV-4-11 (biphenotypic B myelomonocytic leukemia) [8].
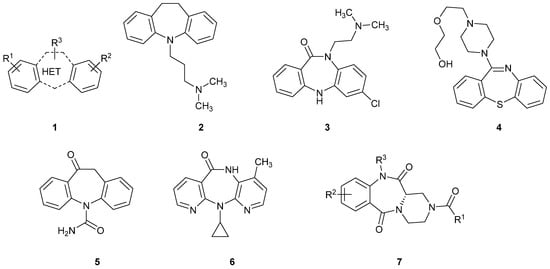
Figure 1.
Tricyclic 7-th membered dibenzoheterocycles and their analogues.
Tricyclic dibenzoheterocycles 2–5, and their tricyclic analogues possessing pyridine 6 or piperidine 7 rings are widely used in the design and search for new compounds of biological importance. On the other hand, analogous tricyclic systems containing two non-polar, aromatic benzene rings separated by a larger, eight-membered heterocyclic ring—tricyclic dibenzodiazocines and their structural analogs (8, Figure 2) are not practically used in the design of biologically active compounds despite their high potential. This situation is caused by the lack of appropriate synthetic routes leading to this type of compounds. A rare example of the application of such heterocyclic scaffolds in the design of compounds exhibiting biological activity involves the use of compound 9 (Figure 2) as a chemosensitizer, abolishing the activity of a glycoprotein (GP 170) located in the cell membrane, and increasing the penetration of the drug into the cell [9,10].
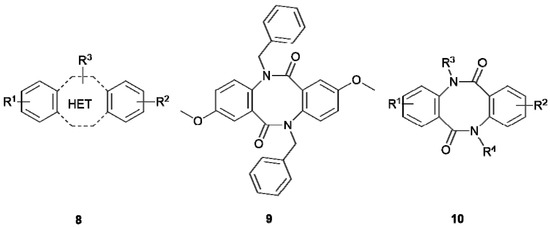
Figure 2.
Tricyclic, 8-membered dibenzoheterocycles.
In our continuous research on the development of medicinally relevant compounds possessing the dilactam structure [7,8], we recently focused on the dibenzo[b,f][1,5]diazocine scaffold 10 as a possible framework for the design of biologically active substances. Our literature survey revealed that symmetrical compounds type 10 (R1 = R2, R3 = R4) could be obtained in the dimerization reaction of substituted 2-aminobenzoic acids (anthranilic acids) in the presence of phosphorus oxychloride [9] or in the dimerization reaction of alkyl 2-aminobenzoates after treatment with sodium hydride [11]. These methods, based on the dimerization of two moieties of 2-aminobenzoic acids or their esters, allow for high-yield syntheses of symmetrical dibenzo[b,f][1,5]diazocines but are completely unsuitable for synthesis of more complex and unsymmetrical compounds type 10, possessing various substituents in the aromatic and dilactam rings (R1 ≠ R2, R3 ≠ R4). It is obvious that by limiting the ability to modify the structure of a compound, one reduces its potential use in the design of biologically active agents. This limits the modulation of biological activity, as well as physicochemical properties, where the key to success is the skilful introduction and modification of substituents and side chains attached to the heterocyclic scaffold. Compounds possessing the dibenzo[b,f][1,5]diazocine structure 10, could be also treated as small cyclic dipeptides, consisting of two aromatic β-amino acid units. Consequently, synthetic efforts have been directed toward cyclisation of 2-(2-aminobenzamido)benzoic acids 11 (Scheme 1) in the presence of classical peptide coupling reagents [11,12]. Unfortunately, as reported [12], and confirmed in our laboratory, this approach led exclusively to the formation of yellow-coloured, bicyclic products possessing the 2-(2-aminophenyl)-4H-benzo[d][1,3]oxazin-4-one structure 12. We also observed, and proved by the single-crystal X-ray diffraction analysis, that crystallization of compound 12 (R1 = R2 = H) from water-methanol mixture, in the presence of p-toluenesulphonic acid (1 equivalent), led to its hydrolysis to 2-(2-aminobenzamido)benzoic acid 11 (R1 = R2 = H), crystallized as a 4-toluenesulphonate salt 11*TsOH (for the crystallographic details see the Supporting Information file). The application of some other cyclisation agents such as polyphosphate ester (PPE) [12] or thionyl chloride [13] for cyclisation of primary amides also led to the product 12, while treatment of the N-methyl amide with PPE resulted in the formation of the dibenzo[b,f][1,5]diazocine scaffold [12]. These results suggest that the use of cyclisation agents such as carbodiimides, PPE or thionyl chloride requires substitution or protection of amide group is necessary to avoid formation of 12.
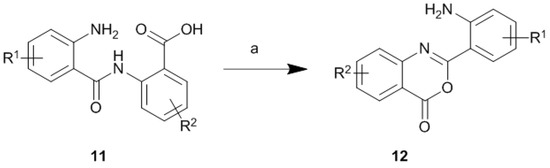
Scheme 1.
DCC-mediated cyclisation of 2-(2-aminobenzamido)benzoic acid 11: Reagents and Conditions: (a) DCC, DMF, rt, 18 h.
The only attempt toward the synthesis of unsymmetrical compounds type 10 (R1 ≠ R2, R3 ≠ R4) was reported in 2004 [13], where unprotected 2-aminobenzoic acids were initially treated with thionyl chloride, and the obtained intermediates were coupled with a second molecule of unprotected, differentially C-substituted 2-aminobenzoic acids. That short letter was only limited to the general procedure of synthesis and physicochemical data for just one representative product containing the dibenzo[b,f][1,5]diazocine structure. In contrast to the high yields of syntheses reported in the article, in our hands this method led to the complex mixture of products, including benzo[d][1,3]oxazin-4-ones 12 and uncyclized 11. For this reason, we focused on the search for the alternative methods for the synthesis of unsymmetrical dibenzo[b,f][1,5]diazocines 10 (R1 ≠ R2, R3 ≠ R4). In this paper, we present a high-yielded, three-step procedure based on the synthesis of unsymmetrical and variously substituted 2-(2-aminobenzamido)benzoic acids esters, followed by basic cyclisation in the presence of sodium hydride.
2. Results and Discussion
2.1. Chemistry
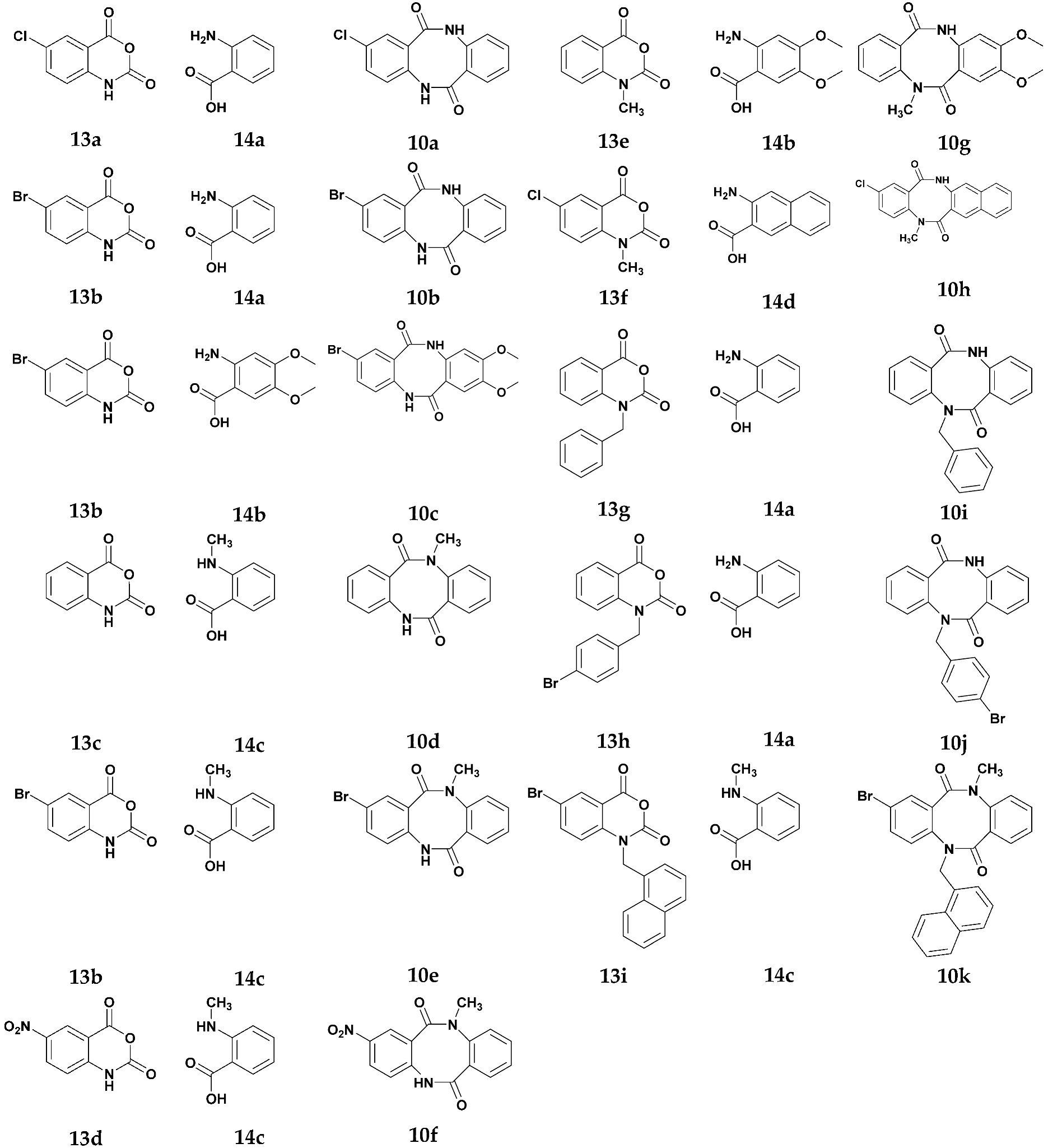
In the present study, we developed two different synthetic approaches to the preparation of asymmetrically substituted dibenzo[b,f][1,5]diazocine scaffold 10. The first method is based on the application of variously N- and C- and N,C-disubstituted 1H-benzo[d][1,3]oxazine-2,4-diones (isatoic anhydrides) 13a–i as coupling partners with variously N- and C-substituted, unprotected 2-aminobenzoic acids 14a–d (Scheme 2).
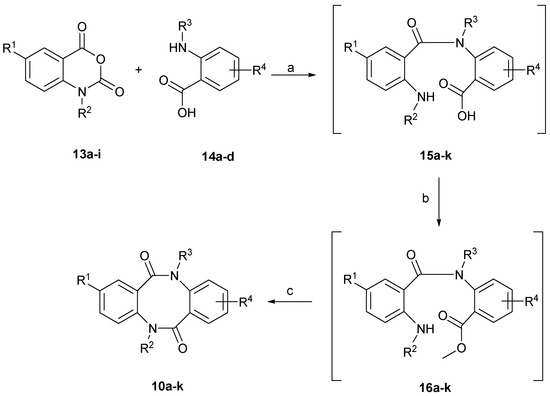
Scheme 2.
First synthetic strategy to the dibenzo[b,f][1,5]diazocine scaffold. Reagents and Conditions: (a) NaOH, H2O, 80 °C, 30 min; (b) H2SO4, MeOH, reflux, 72 h; (c) THFanh., 60% NaH, reflux, 18 h.
The previous literature reported one example of the application of 1H-benzo[d]- [1,3]oxazine-2,4-diones for the synthesis of the dibenzo[b,f][1,5]diazocine scaffold 10, which was limited to symmetrically substituted products [14]. We observed that unsubstituted 1H-benzo[d][1,3]oxazine-2,4-dione (13c), C-substituted: 6-chloro (13a), 6-bromo- (13b) and 6-nitro-1H-benzo[d][1,3]oxazine-2,4-dione (13d), N-substituted: 1-methyl- (13e), 1-benzyl- (13g) and 1-(4-bromobenzyl)-1H-benzo[d][1,3]oxazine-2,4-dione (13h), as well as C,N-disubstituted: 6-chloro-1-methyl- (13f) and 6-bromo-1-(naphthalen-1-ylmethyl)-1H-benzo[d][1,3]oxazine-2,4-dione (13i) could be used as coupling partners with unsubstituted 2-aminobenzoic acid (14a), C-substituted: 4,5-dimethoxy-2-aminobenzoic acid (14b) and 3-amino-2-naphthoic acids (14d) and N-substituted 2-(methylamino)benzoic acid (14c) (Table 1). The coupling step requires the presence of base, one equivalent of sodium hydroxide, and is performed in an aqueous solution at 80 °C [11]. The obtained non-symmetrically substituted 2-(2-aminobenzamido)benzoic acids 15a–k were not isolated but directly transformed into the appropriate methyl esters 16a–k, by refluxing 15a–k in methanol, in the presence of concentrated sulfuric acid [11]. The final cyclisation step of crude methyl 2-(2-aminobenzamido)benzoates 16a–k was performed with sodium hydride in refluxing anhydrous THF, resulting in dibenzo[b,f][1,5]diazocines 10a–k. In the case of compounds 10a–e, 10h and 10j the yield of this three-step synthesis was in the range of 30–35%, giving quite high efficiency for a single step synthesis (average yield for one synthesis step). In the case of compounds 10f, 10g and 10i, we observed slightly lower efficiency, in the 25–29% range, while compound 10k was obtained with 18% yield.

Table 1.
Synthesized compounds.
Unfortunately, the unsubstituted 2-aminonicotinic acid (18a) and 3-amino-2-pyrazinecarboxylic acid (18b) failed to react with 1H-benzo[d][1,3]oxazine-2,4-diones, which forced us to investigate an alternative method for coupling of 18a-b with 2-aminobenzoic acids. In the second method, 2-aminonicotinic acid (18a) and 3-amino-2-pyrazinecarboxylic acid (18b) were transformed in the first synthesis step into 2-amino-N-sulfinylnicotinoyl chloride (19a) and 3-amino-N-sulfinylpyrazine-2-carbonyl chloride (19b) after treatment with SOCl2 in boiling toluene (Scheme 3) [13].
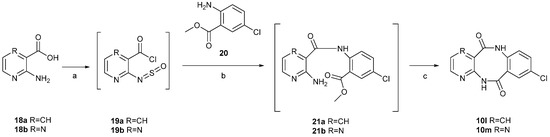
Scheme 3.
Second synthetic strategy to obtain the tricyclic diazocine-5,11(6H,12H)-dione scaffold. Reagents and Conditions: (a) SOCl2, toluene, reflux, 3 h; (b) toluene, rt, 48 h; (c) THFanh., 60% NaH, reflux, 18 h.
The resulting crude intermediates 19a,b underwent reaction with 2-amino-5-chlorobenzoate (20) to form methyl 2-(2-aminonicotinamido)-5-chlorobenzoate (21a) and methyl 2-(2-aminopyrazine-3-carboxamido)-5-chlorobenzoate (21b). Intermediates 21a,b were not isolated, but rather directly treated with sodium hydride in refluxing anhydrous THF, which led to the formation of 8-chloropyrido[3,2-c][1,5]benzodiazocine-5,11(6H,12H)-dione (10l), in 17% yield, and 8-chloropyrazino[3,2-c][1,5]benzodiazocine-6,12(5H,11H)-dione (10m), in 19% yield (total yield for the three-step synthesis).
We also performed post-cyclisation modifications of the reported dibenzo[b,f][1,5]diazocines, including alkylation, acylation and thiolation of 8-membered dilactam diazocine-6,12-dione ring (Scheme 4). The treatment of 5-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10d) with ethyl bromoacetate, in the presence of 60% sodium hydride in mineral oil, led to the formation of ethyl 2-(11-methyl-6,12-dioxo-11,12-dihydrodibenzo[b,f][1,5]diazocin-5(6H)-yl)acetate (10n), in 78% yield. Heating of 5-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10d) in boiling acetic anhydride for 3 h, resulted in the N-acyl derivative, 5-acetyl-11-methyldibenzo[b,f][1,5]diazocine- 6,12(5H,11H)-dione (10o), in 89% yield. Finally, heating of 8-bromo-2,3-dimethoxy- dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10c) with p-tolyl Davy-reagent in boiling anhydrous THF for 18 h, led to the formation of the corresponding dithiolactam derivative, 8-bromo-2,3-dimethoxydibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dithione (10p), in 58% yield.
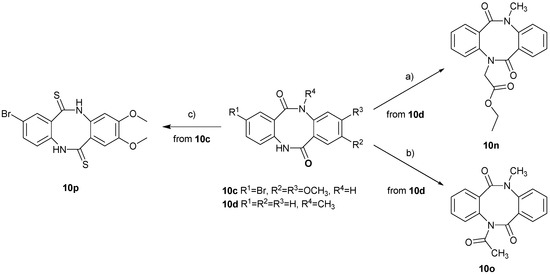
Scheme 4.
Reagents and Conditions: (a) ethyl bromoacetate, 60% NaH, DMSO, rt, 18 h; (b) Ac2O, reflux, 3 h; (c) p-tolyl Davy-reagent, THFanh., reflux, 18 h.
2.2. Crystallographic Analysis
Attempts to obtain single-crystals suitable for X-ray diffraction measurements of all synthesized dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-diones 10a–o were undertaken. However, they were successful in only eight cases (10b, 10g, 10h, 10i, 10j, 10l, 10m and 10o) (Figure 3). The crystal structures of three intermediates (11*TsOH, 13i and 14c) were also determined (Figure 1, Figure 2 and Figure 3 (ESI)). Crystals appropriate for diffractometric analysis were grown by slow evaporation from ethanol (10h, 10j, 10m), acetone (10b, 10l), ethyl acetate (10g, 13i), hexane:ethyl acetate (14c), cyclohexane:ethyl acetate (10o), and water:methanol (11*TsOH) at room temperature.
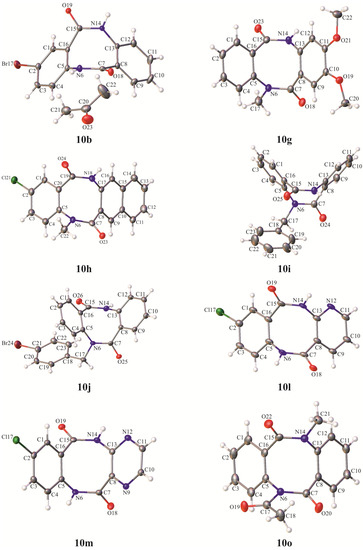
Figure 3.
Asymmetric unit of the crystal lattice of final products 10b, 10g–j, 10l, 10m, 10o with crystallographic atom numbering. Displacement ellipsoids are drawn at the 50% probability level. The H-atoms are shown as small spheres of arbitrary radius.
The investigated compounds crystallized in the monoclinic P21/c (11*TsOH, 13i, 14c, 10b, 10g, 10l, and 10m) or C2/c (10i), orthorhombic P212121 (10h) or Pbca (10o) and triclinic P-1 (10j) space groups, with one molecule of a compound in the asymmetric unit of the crystal lattice (Figure 4, Figures S1 and S2). The asymmetric unit of 10b contains one molecule of solvent (acetone). The conformation of 10a-o resembles a butterfly. The refinement parameters and details of the crystallographic data are presented in Table S1 (ESI). The values of valence and torsion angles, together with bond lengths are presented in Tables S2−S4 (ESI).
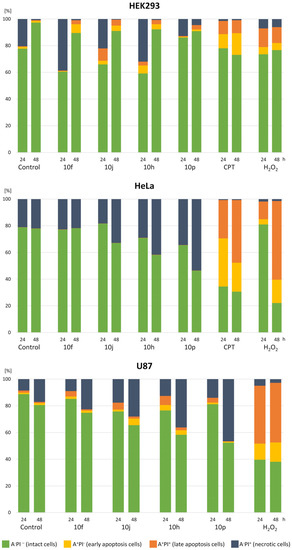
Figure 4.
Flow cytometry analysis of HEK293, HeLa and U87 cells stained with Annexin-FITC (A) and propidium iodide (PI). Cells were treated with 200 µM compounds for 24 or 48 h. Camptothecin (10 μM) and H2O2 (100 μM) were used as experimental controls.
2.3. Cytotoxic and Antibacterial Effect of dibenzo[b,f][1,5]diazocine-6,12-diones 10a–p
We evaluated the cytotoxic efficacy of 16 synthesized dibenzo[b,f][1,5]diazocine-6,12-diones 10a–p on two normal (HEK293, EUFA30) and two cancerous (HeLa, U87) cell lines (concentrations tested: 1–200 µM; Table 2). Among the tested compounds, five showed a cytotoxic effect while maintaining the selectivity of action—10b, 10f, 10h, 10j and 10p—their IC50 ranged from several dozen (lowest—97.3 µM) to several hundred (higher—205.7 µM). Nevertheless, the IC50 for normal cell lines was higher than for cancerous ones. For the majority of the remaining compounds, the IC50 values were above 1 mM or could not be calculated.

Table 2.
IC50 (μM) of dibenzo[b,f][1,5]diazocine-6,12-diones 10a–p based on the survival of non-cancerous (HEK293, EUFA30) and cancerous (U87, HeLa) cells after 24 and 48 h of treatment. “*”-Proliferation inhibition at 200 µM, the highest concentration used.
Although the evaluated compounds showed a rather weak cytotoxic effect, we were able to establish some relationships between the structure of the compounds and their activity. 2-Bromodibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10b) showed a very weak cytotoxic effect with some selectivity for the HeLa tumor cell line (IC50 = 97.3 μM). Its N-methyl derivative, 2-bromo-11-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10e), showed a weaker cytotoxic effect than the parent compound (IC50 ˃ 200 μM for HeLa). The introduction of an additional naphthalen-1-ylmethyl group resulted in the formation of 2-bromo-11-methyl- 5-(naphthalen-1-ylmethyl)dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10k) showing noticeable cytotoxicity on all cell lines tested (IC50 = 87.6 μM—EUFA, 119.0 μM—HEK293, 75.3 μM—HeLa, 75.4 μM—U87). We observed that the presence of an additional, large and hydrophobic substituent attached to the dilactam ring was beneficial for enhancement of the cytoxic effect. 2-Chlorodibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10a) and 8-bromo-2,3-dimethoxydibenzo- [b,f][1,5]diazocine-6,12(5H,11H)-dione (10c) showed no cytotoxic effect in the concentration range tested. Structural modifications of 10a: introduction of another benzene ring into the heterocyclic scaffold and a methyl group into the dilactam ring led to 2-chloro-5-methyl- benzo[b]naphtho[2,3-f][1,5]diazocine-6,14(5H,13H)-dione (10h). These structural modifications resulted in the enhancement of the cytotoxicity of 10h which exhibited cytotoxic effect for all tested cell lines (IC50 = 170.5 μM—EUFA, ˃ 200 μM—HEK293, 107.4 μM—HeLa, 148.6 μM—U87). Finally, the conversion of 10c into its dithiolactam analogue: 8-bromo-2,3-dimethoxy- dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dithione (10p) caused the appearance of a cytotoxic effect on cancer cell lines (IC50 ˃ 200 μM—EUFA, ˃ 200 μM—HEK293, 115.2 μM—HeLa, 170.2 μM—U87) suggesting that such structural modification is beneficial for increasing the cytotoxic effect of these compounds.
To measure the induction of apoptosis, cells were treated with 200 µM of 10f, 10h, 10j, 10k and 10p compound, for 24 and 48 h (Figure 4). The experiment was performed on one normal (HEK293) and two cancer (HeLa, U87) cell lines. After 24 h of treatment, the HEK293 cells showed significant increase in necrosis and late apoptosis, as follows: 20% and 0.4% in control cells, 39% and 0.5% for 10f, 32% and 3% for 10h, 22% and 10% for 10j. After 48 h of treatment, a slight increase in early and late apoptosis was observed, namely, 6.6% and 3% for 10f, 3.8% and 3.2% for 10h, 4% and 4.4% for 10j, in comparison to 1.3% and 0.5% in control cells.
The HeLa cells were the most sensitive to compound 10p, with a significant increase only in necrotic phase. After 24 h, the percentage of necrotic cells was 34.5% in comparison to 21.1% in the non-treated control, and 53.4% in comparison to 22.1% in control cells after 48 h treatment. The U87 cells were sensitive to three out of five compounds tested. In the control, after 24 h, there were 1.2% of cells in early apoptosis, 1.6% in late apoptosis, and 8.6% in necrosis. For 10h treatment, cells were at 4.1% in early apoptosis and 6.9% in late apoptosis; for 10j at 5.2% in late apoptosis and 17.8% in necrosis; and for 10p at 14% in necrosis. After 48 h, we observed a significant increase in necrotic phase only, as follows: in control to 17%, for 10h to 36.2%, for 10j to 28.1%, and for 10p to 46.6%.
Although compound 10k showed cytotoxicity to all tested human cell lines, we did not observe any changes in the apoptosis/necrosis phases in relation to control. It is possible that the compound shows not cytotoxic but cytostatic activity.
Many different natural products possessing a lactam ring (such as β-lactam antibiotics [15]) or dilactam ring (diketopiperazines [16]) exhibit a strong antimicrobial effect. For this reason, we evaluated compounds 10a–o for their antibacterial activity. Antibacterial studies were carried out against two Gram-positive (Bacillus megaterium, Staphylococcus aureus RN4220) and six Gram-negative bacterial strains (Escherichia coli AB1157, Pseudomonas putida KT2440, Shewanella oneidensis MR-1, Salmonella typhimurium TA98, Salmonella typhimurium TA100, Pseudomonas aeruginosa PAO1). The results showed none of the synthesized compounds exhibited antibacterial properties at the concentrations of 12.5, 33.3, 50 and 100 µM. Even though the obtained products exhibited rather weak cytotoxic and no antibacterial activity in the tested range of concentrations, further research with the use of dibenzo[b,f][1,5]diazocine-6,12(5H,11H)diones as privileged structures useful in the design of bioactive compounds is underway in our laboratory and will be published in due course.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
Commercially available chemicals were of reagent grade and used as received. Purification of the compounds was performed by column chromatography on silica gel 60 M (0.040–0.063 mm, E. Merck, Darmstadt, Germany). Thin layer chromatography (TLC), using silica gel plates (Kieselgel 60F254, E. Merck), was used to monitor reaction progress. A B-540 Melting Point apparatus (Büchi, New Castle, DE, USA) was used to measure melting points. The 1H-NMR and 13C-NMR spectra, in DMSO-d6 and CDCl3, were recorded at the Department of Chemistry, University of Warsaw, using an AVANCE III HD 300 MHz spectrometer (Bruker, Billerica, MA, USA); shift values in parts per million are relative to the SiMe4 internal reference. The resonance assignments were based on peak multiplicity and peak integration of recorded spectra, Multiplets were assigned as bs (broad singlet), s (singlet), d (doublet), t (triplet) dd (doublet of doublet), ddd (doublet of doublet of doublet) and m (multiplet). An LTQ Orbitrap Velos instrument (Thermo Scientific, Waltham, MA, USA) located at the Mass Spectrometry Laboratory of the Institute of Biochemistry and Biophysics PAS (Warsaw, Poland) was used to record high resolution mass spectra. A 6200 FT/IR spectrometer (Jasco, Easton, MD, USA) at the Laboratory of Optical Spectroscopy (Institute of Organic Chemistry PAS, Warsaw, Poland) was used to record IR spectra.
3.1.2. General Procedure for the Synthesis of 2-(2-aminobenzamido)benzoic acids 15a–k
A suspension of 1H-benzo[d][1,3]oxazine-2,4-dione 13a–i (1 equiv.), 2-aminobenzoic acid 14a–d (1 equiv.) and sodium hydroxide (1 equiv.) in water (10 mL/mmol) was heated at 80 °C for 30 min until the evolution of carbon dioxide had ceased and a clear solution had formed. After cooling the reaction mixture, the obtained solution was diluted with water, and the crude product was precipitated by addition of glacial acetic acid, filtered and dried under vacuum. In cases of N-alkyl 1H-benzo[d][1,3]oxazine-2,4-diones where a gummy-like residue was formed, the crude product was extracted with ethyl acetate, the organic phase was washed with brine and dried over anhydrous magnesium sulfate. Evaporation of the solvent left a glass-like residue which was used in next step without further purification.
3.1.3. General Procedure for the Synthesis of 2-(2-aminobenzamido)benzoic acids methyl esters 16a–k
2-(2-Aminobenzamido)benzoic acids 15a–k were dissolved in methanol (ca. 10 mL/mmol) then, concentrated sulfuric acid was added (0.5 mL/mmol) (caution: exothermic). The resulted solution was refluxed for 72 h. The excess of methanol was evaporated and the resulting residue was added to water. The pH was adjusted to 8 by addition of NaOH and the crude products were extracted with ethyl acetate. The combined organic layers were washed with 1 N sodium hydroxide, water and brine, and then dried over anhydrous magnesium sulfate. Evaporation of solvent yielded a dark residue of crude methyl esters 16a–k, which were used in the next step without further purification.
3.1.4. General Procedure for the Synthesis of dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-diones 10a–k from 2-(2-aminobenzamido)benzoic acids methyl esters 16a–k
2-(2-Aminobenzamido)benzoic acids methyl esters 16a–k were dissolved in the anhydrous THF (20 mL/mmol) then 60% sodium hydride in mineral oil (2 equiv.) was added and the resulted solution was refluxed for 18 h. The excess of THF was evaporated, the obtained residue was poured into 1 N HCl and the crude product was extracted with ethyl acetate. The combined organic layers were washed with 1 N HCl, water and brine, then dried over anhydrous magnesium sulfate. Crude products were purified by column chromatography using hexane/EtOAc 1:1 then 2:8 v/v as eluent.
2-Chlorodibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10a): Yield 33%, colourless crystals, m.p. 275–276 °C, R.f. = 0.40 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 10.33 (s, 1H, NH), 10.25 (s, 1H, NH), 7.45–7.21 (m, 5H, HAr), 7.11 (d, 1H, J = 2.4 Hz, HAr), 7.09 (d, 1H, J = 1.8 Hz, HAr); 13C-NMR (DMSO-d6) δ 169.1, 167.6, 135.3, 134.4, 133.8, 133.3, 131.4, 130.7, 130.4, 128.2, 127.7, 127.6, 127.5, 125.9; IR (KBr): cm−1 3183, 3055, 2900, 1658, 1601, 1578, 1484, 1415, 1366, 1261, 1229, 1145, 1111; HRMS (ESI): m/z [M+H]+ calcd for C14H10ClN2O2: 273.04253, 275.03958, found: 273.04202, 275.03904;
2-Bromodibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10b): Yield 35%, colourless crystals, m.p. 283–284 °C, R.f. = 0.43 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 10.33 (s, 1H, NH), 10.24 (s, 1H, NH), 7.53 (dd, 1H, J = 2.4, 8.4 Hz, HAr), 7.49 (d, 1H, J = 2.4 Hz, HAr), 7.42–7.21 (m, 3H, HAr), 7.10 (d, 1H, J = 7.8 Hz, HAr), 7.03 (d, 1H, J = 8.4 Hz, HAr); 13C-NMR (DMSO-d6) δ 169.0, 167.5, 135.5, 134.4, 134.2, 133.3, 133.2, 130.7, 130.6, 128.2, 127.8, 127.5, 125.9, 119.6; IR (KBr): cm−1 3182, 3056, 2900, 1657, 1602, 1480, 1411, 1362, 1260, 1229, 1142, 1100; HRMS (ESI): m/z [M+H]+ calcd for C14H10BrN2O2: 316.99202, 318.98997, found: 316.99140, 318.98930;
8-Bromo-2,3-dimethoxydibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10c): Yield 33%, colourless crystals, m.p. 195–196 °C, R.f. = 0.28 (ethyl acetate). 1H-NMR (DMSO-d6) δ 10.099 (s, 1H, NH), 10.057 (s, 1H, NH), 7.54 (dd, 1H, J = 2.4, 8.4 Hz, HAr), 7.46 (d, 1H, J = 2.4 Hz, HAr), 7.01 (d, 1H, J = 8.4 Hz, HAr), 6.81 (s, 1H, HAr), 6.65 (s, 1H, HAr), 3.72 (s, 3H, OCH3), 3.70 (s, 3H, OCH3); 13C-NMR (DMSO-d6) δ 169.0, 167.6, 150.0, 147.7, 135.6, 134.6, 133.2, 130.6, 127.85, 127.77, 124.9, 119.4, 110.4, 109.1, 55.70, 55.66; IR (KBr): cm−1 3499, 3185, 3064, 2934, 2848, 1678, 1646, 1607, 1517, 1470, 1401, 1342, 1264, 1224, 1176, 1108, 1078, 1027; HRMS (ESI): m/z [M+H]+ calcd for C16H14BrN2O4: 377.01315, 379.01110, found: 377.01246, 379.01037;
5-Methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10d): Yield 31%, colourless crystals, m.p. 258–259 °C, R.f. = 0.36 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 10.25 (s, 1H, NH), 7.45–7.15 (m, 7H, HAr), 7.07–6.98 (m, 1H, HAr), 3.33 (s, 1H, CH3, partially overlapped with H2O signal); 13C-NMR (DMSO-d6) δ 168.9, 167.4, 140.1, 134.7, 134.4, 133.3, 130.9, 130.2, 128.2, 127.9, 127.5, 127.1, 125.7, 125.4, 36.5; IR (KBr): cm−1 3257, 3067, 2979, 2934, 1991, 1938, 1836, 1673, 1623, 1599, 1575, 1470, 1415, 1386, 1351, 1302, 1260, 1226, 1185, 1161, 1141, 1082, 1034; HRMS (ESI): m/z [M+H]+ calcd for C15H13N2O2: 253.09715, found: 253.09682;
2-Bromo-11-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10e): Yield 30%, colourless crystals, m.p. 247–248 °C, R.f. = 0.46 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 10.30 (s, 1H, NH), 7.53–7.25 (m, 6H, HAr), 7.00 (d, 1H, J = 8.4 Hz, HAr), 3.32 (s, 1H, CH3, partially overlapped with H2O signal); 13C-NMR (DMSO-d6) δ 186.7, 165.8, 139.7, 136.3, 134.2, 133.1, 132.9, 131.2, 130.3, 128.5, 127.6. 127.5, 125.8, 119.4, 36.5; IR (KBr): cm−1 3172, 3087, 2881, 1681, 1635, 1597, 1473, 1427, 1399, 1370, 1345, 1300, 1274, 1240, 1180, 1135, 1079, 1038, 1014; HRMS (ESI): m/z [M+H]+ calcd for C15H12BrN2O2: 331.00767, 333.00562, found: 331.00718, 333.00503;
11-Methyl-2-nitrodibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10f): Yield 25%, colourless crystals, m.p. 225–226 °C, R.f. = 0.46 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 10.74 (s, 1H, NH), 8.18-8.09 (m, 2H, HAr), 7.50–7.41 (m, 2H, HAr), 7.36–7.26 (m, 3H, HAr), 3.37 (s, 1H, CH3, overlapped with H2O signal); 13C-NMR (DMSO-d6) δ 168.6, 165.3, 145.5, 140.5, 139.6, 134.9, 132.4, 131.4, 128.7, 127.8, 126.4, 125.9, 125.3, 123.6, 36.6; IR (KBr): cm−1 3177, 3056, 2907, 1946, 1712, 1676, 1628, 1528, 1485, 1335, 1260, 1239, 1178, 1137, 1089; HRMS (ESI): m/z [M+H]+ calcd for C15H12N3O4: 298.08223, found: 298.08160;
2,3-Dimethoxy-11-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10g): Yield 29%, colourless crystals, m.p. 271–272 °C, R.f. = 0.23 (ethyl acetate). 1H-NMR (, DMSO-d6) δ 9.97 (s, 1H, NH), 7.46–7.37 (m, 2H, HAr), 7.37–7.27 (m, 3H, HAr), 6.77 (s, 1H, HAr), 6.59 (s, 1H, HAr), 3.68 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 3.30 (s, 1H, CH3); 13C-NMR (DMSO-d6) δ 168.9, 167.3, 149.6, 147.4, 140.5, 133.3, 130.9, 128.1, 129.0, 127.6, 126.1, 125.6, 110.2, 108.7, 55.65, 55.62, 36.6; IR (KBr): cm−1 3193, 3000, 2936, 2841, 1674, 1626, 1513, 1478, 1454, 1431, 1398, 1364, 1302, 1258, 1223, 1156, 1133, 1105, 1082, 1036, 1021; HRMS (ESI): m/z [M+H]+ calcd for C17H17N2O4: 313.11828, found: 313.11760;
2-Chloro-5-methylbenzo[b]naphtho[2,3-f][1,5]diazocine-6,14(5H,13H)-dione (10h): Yield 34%, beige crystals, m.p. 270–271 °C, R.f. = 0.56 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 10.59 (s, 1H, NH), 7.98–7.82 (m, 3H, HAr), 7.62 (s, 1H, HAr), 7.58–7.36 (m, 5H, HAr), 3.37 (s, 1H, CH3); 13C-NMR (DMSO-d6) δ 167.6, 167.3, 138.6, 135.6, 133.4, 133.0, 132.6, 131.8, 131.1, 131.0, 128.1, 128.0, 127.78, 127.76, 127.5, 127.1, 126.9, 123.6, 36.5; IR (KBr): cm−1 3157, 3117, 3052, 2965, 2924, 2887, 1939, 1913, 1839, 1768, 1670, 1630, 1594, 1475, 1420, 1351, 1302, 1274, 1228, 1164, 1129, 1106; HRMS (ESI): m/z [M+H]+ calcd for C19H14ClN2O2: 337.07383, 339.07088, found: 337.07313, 339.07024;
5-Benzyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10i): Yield 29%, beige crystals, m.p. 212–213 °C, R.f. = 0.60 (hexane:ethyl acetate). 1H-NMR (DMSO-d6) δ 10.26 (s, 1H, NH), 7.37–7.18 (m, 12H, HAr), 7.05–7.00 (m, 1H, HAr), 5.32 (d, 1H, J = 15.0 Hz, CH2), 4.81 (d, 1H, J = 15.0 Hz, CH2); 13C-NMR (DMSO-d6) δ 168.9, 167.8, 138.9, 136.7, 134.8, 134.3, 133.9, 130.8, 130.3, 128.3, 128.2, 127.9, 127.84, 127.76, 127.3, 127.1, 125.9, 125.4, 52.4; IR (KBr): cm−1 3166, 3037, 2952, 2898, 1969, 1829, 1735, 1664, 1638, 1598, 1486, 1454, 1402, 1379, 1301, 1240, 1153, 1104, 1076, 1042; HRMS (ESI): m/z [M+H]+ calcd for C21H17N2O2: 329.12845, found: 329.12792;
5-(4-Bromobenzyl)dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10j): Yield 30%, colourless crystals, m.p. 222–223 °C, R.f. = 0.66 (hexane:ethyl acetate). 1H-NMR (DMSO-d6) δ 10.24 (s, 1H, NH), 7.51–7.15 (m, 12H, HAr), 7.06–6.98 (m, 1H, HAr), 5.35 (d, 1H, J = 15.0 Hz, CH2), 4.72 (d, 1H, J = 15.0 Hz, CH2); 13C- NMR (DMSO-d6) δ 168.6, 167.7, 138.6, 136.1, 134.8, 134.2, 133.9, 131.2, 130.9, 130.35, 130.32, 128.4, 127.8, 127.7, 127.1, 126.0, 125.4, 120.5, 51.7; IR (KBr): cm−1 3219, 3059, 2939, 1901, 1672, 1633, 1598, 1488, 1467, 1395, 1353, 1307, 1285, 1258, 1217, 1158, 1103, 1068, 1024, 1009; HRMS (ESI): m/z [M+H]+ calcd for C21H16BrN2O2: 407.03897, 409.03692, found: 407.03841, 409.03626;
2-Bromo-11-methyl-5-(naphthalen-1-ylmethyl)dibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10k): Yield 18%, colourless crystals, m.p. 255–256 °C, R.f. = 0.40 (hexane:ethyl acetate 7:3 v/v). 1H-NMR (DMSO-d6) δ 8.18–8.06 (m, 1H, HAr), 7.98–7.88 (m, 1H, HAr), 7.82 (d, 1H, J = 8.1 Hz, HAr), 7.60–7.22 (m, 9H, HAr), 7.16–7.07 (m, 2H, HAr), 6.16 (d, 1H, J = 15.0 Hz, CH2), 4.98 (d, 1H, J = 15.0 Hz, CH2), 3.05 (s, 1H, CH3); 13C-NMR (DMSO-d6) δ 166.8, 164.5, 139.8, 137.3, 136.6, 133.7, 133.4, 133.3, 131.4, 131.3, 131.0, 130.0, 128.6, 128.5, 128.4, 128.3, 127.6, 127.4, 126.3, 125.8, 125.7, 125.0, 123.3, 120.5, 48.8, 35.8; IR (KBr): cm−1 3039, 3037, 3006, 2978, 2930, 2904, 2875, 1731, 1663, 1641, 1597, 1510, 1469, 1452, 1422, 1409, 1355, 1285, 1259, 1209, 1175, 1155, 1123, 1082, 1042, 1016; HRMS (ESI): m/z [M+H]+ calcd for C26H20BrN2O2: 471.07027, 473.06819, found: 471.07027, 473.06822;
3.1.5. General Procedure for the Synthesis of 8-chloropyrido[3,2-c][1,5] benzodiazocine-5,11- (6H,12H)-dione (10l) and 8-chloropyrazino[3,2-c][1,5]benzodiazocine- 6,12(5H,11H)-dione (10m)
Synthesis of 2-amino-N-sulfinylnicotinoyl chloride (19a) and 3-amino-N-sulfinyl-pyrazine-2-carbonyl chloride (19b).
2-Aminonicotinic acid (18a) or 3-amino-2-pyrazinecarboxylic acid (18b) was suspended in dry toluene (10 mL/mmol), then thionyl chloride (5 equiv.) was added dropwise. The obtained slurry was refluxed for 3 h, until clear solution was formed. The excess of solvent was evaporated, and the residue was co-evaporated with toluene to remove traces of thionyl chloride. Crude products 19a,b were used in the next step without further purification.
Synthesis of methyl 2-(2-aminonicotinamido)-5-chlorobenzoate (21a) and methyl 2-(2-aminopyrazine-3-carboxamido)-5-chlorobenzoate (21b).
2-Amino-N-sulfinylnicotinoyl chloride (19a) or 3-amino-N-sulfinylpyrazine-2-carbonyl chloride (19b) (1 equiv.) was dissolved in dry toluene (10 mL/mmol), then the solution of methyl 2-amino-5-chlorobenzoate (20) (1 equiv.) in toluene (10 mL/mmol) was added dropwise. The mixture was stirred at ambient temperature for 48 h, then the solvent was evaporated and the obtained residue was dissolved in ethyl acetate. The organic phase was washed two times with 1 N NaOH, water and brine, then dried over anhydrous magnesium sulfate. Evaporation of the solvent resulted in crude methyl esters 21a-b which were used in next step without further purification.
Cyclisation of methyl 2-(2-aminonicotinamido)-5-chlorobenzoate (21a) and methyl 2-(2-aminopyrazine-3-carboxamido)-5-chlorobenzoate (21b).
Methyl 2-(2-aminonicotinamido)-5-chlorobenzoate (21a, 1 equiv.) and methyl 2-(2-aminopyrazine-3-carboxamido)-5-chlorobenzoate (21b, 1 equiv.) were dissolved in anhydrous THF (20 mL/mmol) then 60% sodium hydride in mineral oil (2 equiv.) was added. The resulting solution was refluxed for 18 h. The excess of THF was evaporated, residue was poured into 1 N HCl and the crude product was extracted with ethyl acetate. The combined organic layers were washed with 1 N HCl, water and brine, then dried with anhydrous magnesium sulfate. Crude products 10l,m were purified by column chromatography using hexane/EtOAc 1:1 then 2:8 v/v as eluent.
8-Chloropyrido[3,2-c][1,5]benzodiazocine-5,11(6H,12H)-dione (10l): Yield 17%, colourless crystals, m.p. 308-309 °C, R.f. = 0.26 (hexane:ethyl acetate). 1H-NMR (DMSO-d6) δ 10.84 (s, 1H, NH), 10.46 (s, 1H, NH), 8.46 (dd, 1H, J = 1.8, 4.8 Hz, HAr), 7.84 (dd, 1H, J = 1.8, 7.5 Hz, HAr), 7.49–7.40 (m, 2H, HAr), 7.48 (dd, 1H, J = 4.8, 7.8 Hz, HAr), 7.18–7.11 (m, 1H, HAr); 13C-NMR (DMSO-d6) δ 167.7, 167.3, 150.6, 147.0, 138.1, 134.6, 133.6, 131.7, 130.8, 128.0, 127.90, 127.64, 123.1; IR (KBr): cm−1 3182, 3066, 2937, 2902, 1924, 1675, 1596, 1486, 1459, 1433, 1410, 1328, 1281, 1254, 1226, 1150, 1110; HRMS (ESI): m/z [M+H]+ calcd for C13H9ClN3O2: 274.03778, 276.03483, found: 274.0372, 276.03436;
8-Chloropyrazino[3,2-c][1,5]benzodiazocine-6,12(5H,11H)-dione (10m): Yield 19%, colourless crystals, m.p. 346–347 °C, R.f. = 0.35 (hexane:ethyl acetate). 1H-NMR (DMSO-d6) δ 11.24 (s, 1H, NH), 10.77 (s, 1H, NH), 8.65–8.50 (m, 2H, HAr), 7.55–7.40 (m, 2H, HAr), 7.30–7.18 (m, 2H, HAr); 13C-NMR (DMSO-d6) δ 167.1, 165.5, 144.9, 144.5, 144.1, 143.7, 134.1, 132.8, 132.2, 131.1, 128.2, 127.8; IR (KBr): cm−1 3249, 3164, 3057, 2961, 2891, 1928, 1685, 1571, 1537, 1484, 1458, 1406, 1343, 1276, 1236, 1211, 1150, 1133, 1108, 1064; HRMS (ESI): m/z [M+H]+ calcd for C12H8ClN4O2: 275.03303, 277.03008, found: 275.03247, 277.02957;
3.1.6. Synthesis of ethyl 2-(11-methyl-6,12-dioxo-11,12-dihydrodibenzo[b,f][1,5]diazocin- 5(6H)-yl)acetate (10n)
5-Methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10d, 252 mg, 1 mmol, 1 equiv.) was dissolved in anhydrous DMSO (5 mL), then 60% NaH dispersed in mineral oil (48 mg, 1.2 mmol, 1.2 equiv.) was added. The resulted suspension was stirred at room temperature for 30 min. until evolution of gas ceased. Then, ethyl bromoacetate (133 μL, 1.2 mmol, 1.2 equiv.) was added dropwise, and the resulting solution was stirred for 18 h at room temperature. The next day, the reaction mixture was poured into water (20 mL) and extracted with ethyl acetate (3 × 20 mL). Combined organic layers were washed with brine (1 × 20 mL) and dried over anhydrous magnesium sulfate, followed by evaporation under reduced pressure. Crude product was purified by column chromatography using hexane/EtOAc 1:1 then 2:8 v/v as eluent. Yield 78% (264 mg), colourless crystals, m.p. 125–126 °C, R.f. = 0.66 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 7.45-7.15 (m, 8H, HAr), 4.62 (d, 1H, J = 17.1 Hz, CH2), 4.47 (d, 1H, J = 17.1 Hz, CH2), 4.23–4.10 (m, 2H, CH2), 1.22 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (DMSO-d6) δ 168.3, 167.6, 166.9, 140.1, 139.0, 134.3, 133.2, 131.0, 130.8, 128.2, 128.1, 127.6, 127.2, 125.6, 125.2, 60.9, 51.1, 36.1, 14.0; IR (KBr): cm−1 3458, 3300, 3058, 2981, 2936, 1953, 1840, 1739, 1656, 1599, 1456, 1411, 1411, 1375, 1324, 1308, 1281, 1260, 1217, 1120, 1088, 1056, 1023; HRMS (ESI): m/z [M+H]+ calcd for C19H19N2O4: 339.13393, found: 339.13393;
3.1.7. Synthesis of 5-acetyl-11-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10o)
The dispersion of 5-methyldibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10d) (504 mg, 2 mmol, 1 equiv.) in acetic anhydride (10 mL) was refluxed for 3 h. The excess anhydride was evaporated under reduced pressure and the residue was crystallized from a mixture of cyclohexane and ethyl acetate (9:1) to give pure product. Yield 89% (524 mg), colourless crystals, m.p. 193–194 °C, R.f. = 0.74 (hexane:ethyl acetate 2:8 v/v). 1H-NMR (DMSO-d6) δ 7.46-7.19 (m, 8H, HAr), 3.34 (s, 1H, CH3), 2.61 (s, 1H, CH3); 13C-NMR (DMSO-d6) δ 171.5, 168.6, 166.7, 139.7, 135.4, 134.3, 133.8, 132.1, 130.3, 129.4, 129.0, 128.3, 127.8, 127.0, 126.2, 36.0, 26.7; IR (KBr): cm−1 3412, 3379, 3296, 3061, 2938, 1975, 1941, 1718, 1703, 1658, 1599, 1485, 1453, 1420, 1371, 1304, 1251, 1206, 1142, 1084, 1038, 1013; HRMS (ESI): m/z [M+H]+ calcd for C17H15N2O3: 295.10772, found: 295.10735;
3.1.8. Synthesis of 8-bromo-2,3-dimethoxydibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dithione (10p)
Bromo-2,3-dimethoxydibenzo[b,f][1,5]diazocine-6,12(5H,11H)-dione (10c, 376 mg, 1 mmol, 1 equiv.) was dissolved in dry THF and then p-tolyl Davy-reagent (873 mg, 2 mmol, 2 equiv.) was added. The resulting suspension was refluxed for 18 h, then the reaction mixture was cooled to room temperature. The obtained clear, yellow solution was evaporated with small amount of chromatographic silica gel. The crude product was purified by column chromatography using hexane/EtOAc 9:1 then 7:3 v/v as eluent. Yield 58% (237 mg), yellow solid, m.p. 186–187 °C (decomposition), R.f. = 0.27 (hexane:ethyl acetate 7:3 v/v). 1H-NMR (DMSO-d6) δ 12.30 (s, 1H, NH), 12.28 (s, 1H, NH), 7.56–7.49 (m, 2H, HAr), 6.99 (d, 1H, J = 5.1 Hz, HAr), 6.88 (s, 1H, HAr), 6.64 (s, 1H, HAr), 3.73 (s, 3H, OMe), 3.70 (s, 3H, OMe); 13C-NMR (DMSO-d6) δ 200.2, 197.5, 149.8, 148.0, 141.2, 133.1, 132.9, 131.2, 131.1, 126.9, 126.6, 120.4, 111.4, 107.7, 55.9, 55.7; IR (KBr): cm−1 3439, 3248, 2925, 2644, 1602, 1506, 1403, 1345, 1265, 1227,1200, 1141, 1079, 1030; HRMS (ESI): m/z [M+H]+ calcd for C16H14BrN2O2S2: 408.96746, 410.96541, found: 408.96725, 410.96512;
3.1.9. General Procedure for the Synthesis of 1-Substituted 1H-benzo[d][1,3]oxazine-2,4-diones 13f–i
To a stirred solution of 6-chloro-1H-benzo[d][1,3]oxazine-2,4-dione (13a), 6-bromo-1H- benzo[d][1,3]oxazine-2,4-dione (13b) or 1H-benzo[d][1,3]oxazine-2,4-dione (13c) (1 equiv.) in DMSO (10 mL/mmol), 60% sodium hydride (1.5 equiv.) was added and the resulting suspension was stirred for 15 min. at room temperature, until the evolution of gas ceased. Then, the appropriate halide (1.5 equiv.): methyl iodide for 13a, 1-(bromomethyl)naphthalene for 13b or benzyl bromide and 4-bromobenzyl bromide for 13c, was added and the resulting mixture was stirred at room temperature for 18 h. Next day, the reaction mixture was poured into water (100 mL/10 mL DMSO) and extracted with ethyl acetate (3 × 100 mL/100 mL H2O). Combined organic layers were washed with brine (100 mL/300 mL EtOAc) and dried over anhydrous magnesium sulfate. Crude products were crystallized from mixture of ethyl acetate and hexane.
6-Chloro-1-methyl-1H-benzo[d][1,3]oxazine-2,4-dione (13f): Yield 62%, yellow crystals, m.p. 201–202 °C, R.f. = 0.33 (hexane:ethyl acetate 7:3). 1H-NMR (DMSO-d6) δ 7.94 (d, 1H, J = 2.4 Hz, HAr), 7.89 (dd, 1H, J = 2.4, 9.0 Hz, HAr), 7.48 (d, 1H, J = 9.0 Hz, HAr), 3.46 (s, 1H, Me); 13C-NMR (DMSO-d6) δ 158.0, 147.4, 141.1, 136.6, 127.9, 127.6, 117.1, 113.3, 31.9; HRMS (ESI): m/z [M+H]+ calcd for C9H7ClNO3: 212.01090, 214.00795, found: 212.01049, 214.00754;
1-Benzyl-1H-benzo[d][1,3]oxazine-2,4-dione (13g): Yield 57%, beige crystals, m.p. 141–142 °C, R.f. = 0.54 (hexane:ethyl acetate 7:3). 1H-NMR (DMSO-d6) δ 8.04 (dd, 1H, J = 1.5, 7.8 Hz, HAr), 7.73 (ddd, 1H, J = 1.5, 7.2, 8.7 Hz, HAr), 7.46–7.20 (m, 7H, HAr), 5.30 (s, 2H, CH2); 13C-NMR (DMSO-d6) δ 158.9, 148.3, 141.3, 137.0, 135.3, 129.5, 128.6, 127.4, 126.6, 123.7, 115.1, 112.1, 47.6; HRMS (ESI): m/z [M+H]+ calcd for C15H12NO3: 254.08116, found: 254.08104;
1-(4-Bromobenzyl)-1H-benzo[d][1,3]oxazine-2,4-dione (13h): Yield 62%, beige crystals, m.p. 182–183 °C, R.f. = 0.50 (hexane:ethyl acetate 7:3). 1H-NMR (DMSO-d6) δ 8.04 (dd, 1H, J = 1.5, 7.8 Hz, HAr), 7.74 (ddd, 1H, J = 1.5, 7.2, 8.7 Hz, HAr), 7.85–7.49 (m, 2H, HAr), 7.44–7.36 (m, 2H, HAr), 7.35–7.26 (m, 1H, HAr), 7.22 (dd, 1H, J = 8.4 Hz, HAr), 5.27 (s, 2H, CH2); 13C-NMR (DMSO-d6) δ 158.8, 148.3, 141.2, 137.0, 134.8, 131.5, 129.5, 129.0, 123.8, 120.5, 115.0, 112.2, 47.0; HRMS (ESI): m/z [M+H]+ calcd for C15H11BrNO3: 331.99168, 333.98964, found: 331.99138, 333.98930;
6-Bromo-1-(naphthalen-1-ylmethyl)-1H-benzo[d][1,3]oxazine-2,4-dione (13i): Yield 51%, white solid, m.p. 230–231 °C, R.f. = 0.74 (hexane:ethyl acetate 7:3). 1H-NMR (DMSO-d6) δ 8.26–8.16 (m, 1H, HAr), 8.15 (d, 1H, J = 2.4 Hz, HAr), 8.04–7.97 (m, 1H, HAr), 7.92–7.78 (m, 2H, HAr), 7.72–7.56 (m, 2H, HAr), 7.42–7.33 (m, 2H, HAr), 7.02 (d, 1H, J = 9.0 Hz, HAr), 5.73 (s, 2H, CH2); 13C-NMR (DMSO-d6) δ 157.9, 147.8, 140.8, 139.2, 133.3, 131.0, 129.9, 129.5, 128.7, 127.7, 126.5, 126.2, 125.4, 123.0, 122.2, 117.7, 115.3, 114.52, 114.48, 46.4; HRMS (ESI): m/z [M+H]+ calcd for C19H13BrNO3: 382.0073, 384.00529 found: 382.0645, 384.00443;
3.1.10. DCC-mediated Synthesis of 2-(2-aminophenyl)-4H-benzo[d][1,3]oxazin-4-one (12, R1 = R2 = H)
A suspension of 1H-benzo[d][1,3]oxazine-2,4-dione (13c, 489 mg, 3 mmol, 1 equiv.), 2-aminobenzoic acid (14a, 411 mg, 3 mmol, 1 equiv.), sodium hydroxide (120 mg, 3 mmol, 1 equiv.) in water (30 mL) was heated at 80 °C for 30 min. until the evolution of carbon dioxide ceased and clear solution was formed. After cooling, the obtained solution was diluted with water, crude product was precipitated by addition of glacial acetic acid and the resulting precipitate was dried under reduced pressure. The crude 2-(2-aminobenzamido)benzoic acid (11) was dissolved in 50 mL of DMF, then N,N’-dicyclohexylcarbodiimide (DCC) (681 mg, 3.3 mmol, 1.1 equiv.) was added and the reaction mixture was stirred at room temperature for 18 h. The next day, the obtained solution was poured into water (100 mL) and the product was extracted with ethyl acetate (3 × 100 mL). Combined organic layers were washed with brine (1 × 50 mL), dried over anhydrous magnesium sulfate followed by evaporation of volatiles under reduced pressure. Crude product was purified by column chromatography using hexane: ethyl acetate 9:1 v/v as eluent. yield 84% (600 mg), yellow solid, m.p. 171–172 °C, R.f. = 0.69 (hexane:ethyl acetate 7:3). 1H-NMR (CDCl3) δ 8.19 (d, 1H, J = 4.8 Hz, HAr), 8.08 (d, 1H, J = 4.8 Hz, HAr), 7.76 (t, 1H, J = 4.7 Hz, HAr), 7.56 (d, 1H, J = 5.1 Hz, HAr), 7.44 (t, 1H, J = 4.5 Hz, HAr), 7.26 (t, 1H, J = 4.7 Hz, HAr), 6.78–6.68 (m, 2H, HAr), 6.47 (bs, 2H, NH2); 13C-NMR (CDCl3) δ 159.4, 158.0, 149.8, 146.7, 136.5, 133.7, 129.7, 128.7, 127.8, 126.4, 116.9, 116.8, 116.6, 110.1; IR (KBr): cm−1 3448, 3311, 1743, 1625, 1592, 1550, 1489, 1471, 1447, 1356, 1331, 1310, 1235, 1220, 1167, 1054, 1013; HRMS (ESI): m/z [M+H]+ calcd for C14H11N2O2: 239.08150, found: 239.08128;
3.1.11. The Synthesis of methyl 2-amino-5-chlorobenzoate (20)
To stirred slurry of 6-chloro-1H-benzo[d][1,3]oxazine-2,4-dione (13a, 591 mg, 3 mmol, 1 equiv.) in methanol (30 mL), sodium methoxide (486 mg, 9 mmol, 3 equiv.) was added, and the reaction mixture was refluxed for 3 h. After cooling down, the excess of solvent was evaporated under reduced pressure; the obtained residue was treated with water (100 mL) and extracted with ethyl acetate (3 × 50 mL). Combined organic layers were washed with water (1 × 100 mL), brine (1 × 50 mL) and dried over anhydrous magnesium sulfate. Evaporation of solvent gave pure product as slight yellow oil which solidified upon storage. Yield 97% (538 mg), m.p. 70–71 °C, R.f. = 0.80 (hexane:ethyl acetate 7:3). 1H-NMR (CDCl3) δ 7.81 (d, 1H, J = 2.4 Hz, HAr), 7.19 (dd, 1H, J = 2.4, 9.0 Hz, HAr), 6.59 (d, 1H, J = 9.0 Hz, HAr), 5.73 (bs, 2H, NH2), 3.86 (s, 3H, Me); 13C-NMR (CDCl3) δ 167.7, 149.1, 134.2, 130.5, 120.7, 118.1, 111.6, 51.9; HRMS (ESI): m/z [M+H]+ calcd for C8H9ClNO2: 186.03163, 188.02868 found: 186.03171, 188.02870.
3.2. X-ray Data Collection and Data Refinement
Good quality single-crystals of 10b, 10g, 10h, 10i, 10j, 10l, 10m, 10o 11*TsOH, 13i and 14c, were selected for the X-ray diffraction experiments at T = 100(2) K. Diffraction data were collected on a SuperNova Dual Source diffractometer (Agilent Technologies, Yarnton, Oxfordshire, UK) with CuKα radiation (λ = 1.54184 Å) (10g, 10h, 10i, 10j, 10l, 10m, 10o 11*TsOH, 13i and 14c) or an Agilent Technologies SuperNova Single Source diffractometer with MoKα radiation (λ = 0.71073 Å) (10b), using CrysAlis RED software [17]. The analytical numeric absorption correction using a multifaceted crystal model based on expressions derived by Clark and Reid [18] (10b, 10l, 10j 10h, 11*TsOH and 13i) and multi-scan empirical absorption correction using spherical harmonics (10i, 10g, 10m, 10o and 14c), implemented in SCALE3 ABSPACK scaling algorithm, were applied [17]. The structural determination procedure was carried out using the SHELX package [19]. The structures were solved with direct methods and then successive least-square refinement was carried out based on the full-matrix least-squares method on F2 using the SHELXL programme [19]. All H-atoms linked to the N and O-atoms were located on a Fourier difference map and refined as riding with Uiso(H) = xUeq(N,O), where x = 1.2 for the amine and 1.5 for the hydroxyl H-atoms, respectively. In all the cases, the N−H bonds were subject to the DFIX 0.87 restraint. In the case of 14c, length of the O−H bond was restrained to 0.82 Å. Other H-atoms were positioned geometrically, with C–H equal to 0.93, 0.97 and 0.98 Å for the aromatic, methylene and methine H-atoms, respectively, and constrained to ride on their parent atoms with Uiso(H) = xUeq(C), where x = 1.2 for the aromatic, methylene and methine H-atoms. In the case of 10i, a few distinct peaks on the difference Fourier map indicated the presence of a disordered solvent molecule. However, all of the attempts to model disordered solvents used for crystallization failed. Therefore, the solvent contribution was removed by applying the appropriate MASK procedure in the Olex2 programme [20]. Calculated total solvent accessible volume was 982.1 Å3 occupied by 286.6 electrons per unit cell. The figures for this publication were prepared using Olex2 programme [20].
3.3. Cell Culturing
Following cell lines: HeLa, U87, HEK293, EUFA30 were cultured in DMEM medium (Life Technology, Carlsbad, CA, USA) with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and 0.1% antibiotics (penicillin, streptomycin, Life Technology, Carlsbad, CA, USA). Cells were grown in atmosphere of 5 and 95% CO2 and air, respectively, at 37 °C.
3.4. Cytotoxicity Assay
Exponentially growing cells at the density of 2 × 103 cells/well were seeded onto a 96-well plate, cultured for 18 h, and treated with newly synthesized compounds at concentrations 1–200 µM, or with DMSO as a control, for 24 or 48 h. Alamar Blue (Thermo Fisher Scientific, Waltham, MA, USA) was added accordingly to manufacturer protocol. After 4 h, light emission at 590 nm was measured with excitation at 560 nm using a scanning multiwell spectrophotometer (DTX 880, Beckman Coulter, Brea, CA, USA). The experiments were repeated at least three times with three replicates for each inhibitor concentration. After background subtraction, inhibition rates, IC50, were calculated as the component concentration inhibiting cell growth by 50%. All the calculations were performed using Origin 9.0 software.
3.5. Flow Cytometry
The apoptosis detection kit (Annexin V-FITC BD Biosciences, Franklin Lakes, NJ, USA) was used to detect apoptosis by flow cytometry. Cells were seeded in 6-well plates at concentration of 5 × 105 cells/well, cultured for 18 h, and tested compound was applied for indicated time. Afterwards, cells were washed with PBS, resuspended in binding buffer at concentration of 2 × 106 cells/mL. The anti-Annexin V FITC-conjugated antibody and propidium iodide were added to 100 µl aliquots. Then, mixtures were incubated for 15 min at room temperature, supplemented with binding buffer to 500 µl and processed by BD FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed in Flowing Software version 2.5.1 (Flowing Software, http://www.uskonaskel.fi/flowingsoftware).
3.6. Disk Diffusion Test
Bacterial inoculum was spread on LB plates solidified with 1.5% agar. Standard 6 mm paper discs were placed on the surface of the plates and 4 µL of tested compound at the desired concentration plotted on the discs.
4. Conclusions
In this article we have presented that asymmetrically substituted dibenzo[b,f][1,5]- diazocine-6,12(5H,11H)diones, which may be treated as convenient privileged structures useful in the design of biologically active compounds, can be obtained according to two, general methods based on reactions of: (a) 1H-benzo[d][1,3]oxazine-2,4-diones and unprotected 2-aminobenzoic acids, and (b) unprotected 2-aminobenzoic acid analogues, activated with thionyl chloride, and 2-aminobenzoic acid esters. The procedures described allow a wide range of modifications of all structural elements of the dibenzo[b,f][1,5]diazocine-6,12(5H,11H)dione framework: the introduction of various substituents and functional groups into benzene rings as well as diverse substituents and side chains attached to the eight-membered diazocine ring. We have shown that these modifications can be introduced both, through the selection of appropriately modified building blocks as well as further chemical modifications of the obtained dibenzo[b,f][1,5]diazocine-6,12(5H,11H)dione scaffold. The wide range of synthetic methods has also allowed to obtain three new heterocyclic frameworks: benzo[b]naphtho[2,3-f][1,5]diazocine-6,14(5H,13H)-dione 10h, pyrido[3,2-c][1,5]benzo- diazocine-5,11(6H,12H)-dione 10l, and pyrazino[3,2-c][1,5]benzodiazocine-6,12(5H,11H)-dione 10m. The usefulness of the synthetic methods has been confirmed by obtaining a representative library of 16 compounds, evaluated as possible cytotoxic and antimicrobial agents.
Supplementary Materials
1H-NMR, 13C-NMR, IR, HRMS (ESI) and X-Ray analysis data are available online. CCDC 1956772−19567781 and 1976509 contain the supplementary crystallographic data for this paper. These data can be obtained freely via http://www.ccdc.cam.ac.uk/data_request/cif, by e-mailing data_request@ccdc.cam.ac.uk, or by contacting directly the Cambridge Crystallographic Data Centre (12 Union Road, Cambridge CB2 1EZ, UK. Fax: +44-1223-336033).
Author Contributions
Design, conception and writing were performed by A.M., B.B., D.T., D.G. Biological data analysis was performed by D.G., E.G., D.M. Synthesis and structure elucidation were performed by B.B., A.M. Crystallographic analysis was performed by D.T., K.W. All authors reviewed and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the MNiSW grant (Diamentowy Grant 0072/DIA/2016/45, B.B.). This study was also supported by the National Science Centre Poland MAESTRO grant-DEC-2012/04/A/ST5/00609 (D.T. and K.W.). This study was carried out at the Biological and Chemical Research Centre, University of Warsaw, established within the project co-financed by European Union from the European Regional Development Fund under the Operational Programme Innovative Economy, 2007–2013. The X-ray diffraction data collection was accomplished at the Core Facility for Crystallographic and Biophysical Research to support the development of medicinal products. The „Core facility for crystallographic and biophysical research to support the development of medicinal products” project is carried out within the TEAM-TECH Core Facility programme of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund. The equipment used was sponsored in part by the Centre for Preclinical Research and Technology (CePT), a project co-sponsored by European Regional Development Fund and Innovative Economy, The National Cohesion Strategy of Poland.
Acknowledgments
We are greatly indebted to Małgorzata Łobodzka for bacterial strains. We thank Jacek Olędzki for recording the ES-MS spectra.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gilman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Saxena, M. Developments in antihistamines (H1). Prog. Drug Res. 1992, 39, 35–125. [Google Scholar] [PubMed]
- Kasper, S.; Muller-Spahn, F. Review of quetiapine and its clinical applications in schizophrenia. Expert Opin. Pharmacother. 2000, 1, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Goa, K.L. Oxcarbazepine an update of its efficacy in the management of epilepsy. CNS Drugs 2001, 15, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Kallis, M.M.; Huff, N.A. Oxcarbazepine, an antiepileptic agent. Clin. Ther. 2001, 23, 680–700. [Google Scholar] [CrossRef]
- Murphy, R.L.; Montaner, J. Drug Evaluations anti-infectives: Nevirapine: A review of its development, pharmacological profile and potential for clinical use. Expert Opin. Investig. Drugs 1996, 5, 1183–1199. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Trzybiński, D.; Wilczek, M.; Psurski, M.; Bagiński, M.; Bieszczad, B.; Mroczkowska, M.; Woźniak, K. (S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12 (11H,12aH)-dione—Synthesis and crystallographic studies. Molbank 2017, 2017, M964. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Psurski, M.; Bagiński, M.; Bieszczad, B.; Mroczkowska, M.; Wilczek, M.; Czajkowska, J.; Trzybiński, D.; Woźniak, K.; Wietrzyk, J. Novel (S)-1,3,4,12a-tetrahydropyrazino[2,1-c] [1,4]benzodiazepine-6,12(2H,11H)-dione derivatives: Selective inhibition of MV-4-11 biphenotypic B myelomonocytic leukemia cells’ growth is accompanied by reactive oxygen species overproduction and apoptosis. Bioorg. Med. Chem. Lett. 2018, 28, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Nonnenmacher, E.; Hevertt, A.; Mahamoudt, A.; Aubert, C.; Molnartt, J.; Barbe, J. A novel route to new dibenzo[b,f][1,5]diazocine derivatives as chemosensitizers. Org. Prep. Proced. Int. 1997, 29, 711–715. [Google Scholar] [CrossRef]
- Nonnenmacher, E.; Brouant, P.; Mrozek, A.; Karolak-Wojciechowska, J.; Barbe, J. Structure and conformational analysis of 5,11-dibenzyldibenzo[b,f][1,5]diazocine-6,12-dione. A novel approach for new chemosensitizers. J. Mol. Struct. 2000, 522, 263–269. [Google Scholar] [CrossRef]
- Hoorfar, A.; Ollis, W.D.; Price, J.A.; Stephanidou Stephanatou, J.; Stoddart, J.F. Conformational behaviour of medium-sized rings. Part 11. Dianthranilides and trianthranilides. J. Chem. Soc. Perkin Trans. 1982, 1649–1699. [Google Scholar] [CrossRef]
- Nadkarni, S.S.; Hosangadi, B.D. Studies in large ring compounds: Part IX—Observations on cyclodehydration of anthraniloylanthranilic acids. Ind. J. Chem. 1988, 27B, 225–228. [Google Scholar]
- Hassner, A.; Sun, B.; Gellermann, G.; Meir, S. Dilactams. Synthesis of nonsymmetrical dibenzodiazocinediones. Tetrahedron Lett. 2004, 45, 1377–1379. [Google Scholar] [CrossRef]
- Yokoyama, A.; Karasawa, M.; Taniguchi, M.; Yokozawa, T. Successive formation of two amide linkages between two benzene rings. Chem. Lett. 2013, 42, 641–642. [Google Scholar] [CrossRef]
- Aoki, H.; Okuhara, M. Natural β-lactam antibiotics. Ann. Rev. Microbial. 1980, 34, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Yarnton, UK, 2008.
- Clarck, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. A 1995, A51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 10a–p are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).