Abstract
Ring cleavage of cyclic ether substituents attached to a boron cage via an oxonium oxygen atom are amongst the most versatile methods for conjoining boron closo-cages with organic functional groups. Here we focus on much less tackled chemistry of the 11-vertex zwitterionic compound [10-(O-(CH2-CH2)2O)-nido-7,8-C2B9H11] (1), which is the only known representative of cyclic ether substitution at nido-cages, and explore the scope for the use of this zwitterion 1 in reactions with various types of nucleophiles including bifunctional ones. Most of the nitrogen, oxygen, halogen, and sulphur nucleophiles studied react via nucleophilic substitution at the C1 atom of the dioxane ring, followed by its cleavage that produces six atom chain between the cage and the respective organic moiety. We also report the differences in reactivity of this nido-cage system with the simplest oxygen nucleophile, i.e., OH−. With compound 1, reaction proceeds in two possible directions, either via typical ring cleavage, or by replacement of the whole dioxane ring with -OH at higher temperatures. Furthermore, an easy deprotonation of the hydrogen bridge in 1 was observed that proceeds even in diluted aqueous KOH. We believe this knowledge can be further applied in the design of functional molecules, materials, and drugs.
1. Introduction
Eleven vertex 7,8-Dicarba-nido-dodecahydroundecaborate(1−) ion [1] belongs to the most studied boron cluster anions due to its easy availability from ortho-carborane [2], open pentagonal C2B3 plane with three stereochemically distinctive sites for substitution and an extra hydrogen atom sitting on it [3,4]. It is well recognized that the coupling of the [nido-7,8-C2B9H12]− ion with tetrahydrofuran and dioxane promoted by various metal halides, such as FeCl3 [5] or HgCl2 [6,7], yields the neutral zwitterionic compounds [10-(CH2)4O)-nido-7,8-C2B9H11] or [10-(O-(CH2-CH2)2O)- nido-7,8-C2B9H11] (1). More recently, we reported that the dioxane derivative 1 can be produced in high yield by reaction of the neutral carborane nido-C2B9H13 with dioxane used as solvent [8]. This is a metal free, straightforward, and clean reaction in which the dioxane ring is attached to the nido-cluster by an oxonium atom to the open-face boron site B(10) that is position located opposite the cluster carbon atoms.
This compound belongs to a large and rapidly growing family of species that contain an ether ring attached to the boron cage via an oxonium atom [9,10,11,12]. All other members are derived from closo-borate ions such as cobalt bis(dicarbollide) [13,14], decaborate [15,16], dodecaborate [17,18,19], or 1-carba-undecahydroundecaborate [20]; most of which have been used in numerous emerging applications, among others, advanced materials [21,22], organic conducting polymers [23,24], radionuclide partitioning [25,26], biology, and medicine [8,27,28,29,30,31,32,33,34,35,36,37]. Surprisingly enough, the nido- system from this family of compounds, namely compound 1, has largely remained outside the stream of main interest, despite its potential for the introduction of open-cage boron polyhedra to functional molecules and materials. This is even more surprising in the light of recent advances in the use of [7,8-C2B9H12]– as a pharmacophore in drug design [30,38,39,40]. In this regard, the ring cleavage methodology brings a level of high versatility to a broad scope of applications. Furthermore, the resulting substituents are located on the cage in a symmetrically located position and hence the products are free from stereochemical complications such as the presence of diastereoisomeric pairs or chirality [7]. The compound, and its derivatives arising from ring cleavage, can potentially serve as new charge-compensated [41,42] or common type of ligands in the synthesis of metallacarboranes.
With regards to ring cleavage of the zwitterion 1 using nitrogen nucleophiles, only reactions with the azide ion [7] and butyl amine have been reported, the later within the synthesis of model inhibitors of HIV-Protease that bear two dicarbollide ions units interconnected with long aliphatic chains to a central n-alkylammonium group [8,36]. Compounds of this type are sometimes referred to as “dumbbells” [43]. Similar double cluster compounds resulted also from reactions of 1 with dihydroxy benzenes as oxygen nucleophile [44]. These compounds were shown to adopt crown-ether like arrangements. The reactions with other nucleophiles have been reported only for isomers of hydroxybenzoic acid [7], which lead to anionic products with one boron cage per molecule and thiourea [45].
We have been involved in the design and exploration of building blocks based on boron polyhedra, which enable the facile merging of boron cages with various organic molecules or biomolecules, particularly those applicable in drug design [40,46,47,48]. Herein we present an overview of the reactions of 1 with a variety of possible nucleophiles. Some of these reactions resulted in unexpected findings such as the easy deprotonation of the starting zwitterion even in aqueous solutions, or the detachment and substitution of the whole dioxane ring at higher temperatures. The conditions for ring cleavage have thus to be carefully considered and optimized in order for this method be used successfully for functionalization, and it is these considerations that are described herein.
2. Results and Discussion
2.1. Reaction Pathways of 1 with Nucleophiles
According to the literature considerations [49], three different reaction pathways (A, B, and C below) can apply when ether rings attached to boron anions via oxonium oxygen atom, such as dioxane in compound 1 (or other tetrahydrofurane, tetrahydropyrane, etc. derivatives) are attacked with various nucleophiles, as schematically shown for the current system in Scheme 1.
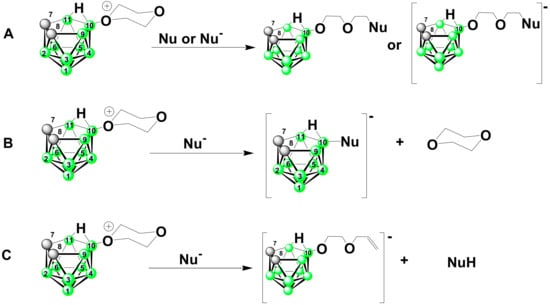
Scheme 1.
Possible reaction pathways that may apply to the reactions of the compounds containing cyclic ethers with oxonium atoms with nucleophiles, according to Ref. [49]. (A) Nucleophilic substitution on the ether ring at the α-carbon adjacent to the oxonium atom. This leads to the cleavage of the ring and an attachment of the nucleophile at terminal carbon atom of the diethyleneglycol chain. (B) Substitution of the cyclic ether by the anionic nucleophile can lead to Nu- substituted boron cluster and to the elimination of the cyclic ether. (C) Hofmann-type dealkylation at the oxonium atom, when the anionic nucleophile Nu– acts as a base abstracting the α-proton of the ring. This results in formation of a protonated nucleophile HNu and formation of a terminal double bond in the aliphatic chain.
To our knowledge, only the first pathway A was reported, taking into account all cyclic ether derivatives of boron clusters [9,11]. Even with anionic nucleophiles such as O−, Cl−, Br−, I−, N3−, and R-O−, all reactions reported in the literature led to nucleophilic substitution producing opened ether ring with nucleophile moiety attached at the carbon originally present in α-position to oxonium oxygen.
The ring cleavage of dioxane ring in 10-(O-(CH2-CH2)2O)-nido-7,8-C2B9H11 with nitrogen nucleophiles proceeds (Scheme 2) in a straightforward manner according to pathway A, i.e., via nucleophilic substitution that produce compounds with ammonio functions attached to the cage via a dietlyleneglycol chain [7,8]. We illustrate here reactions with amines only in three examples that highlight affinity trends observed between various types of nucleophiles (see Scheme 2). The synthesis of the simplest derivative that contains terminal –NH3 group is usually less straightforward than for primary and secondary amines. Thus, for other cages, protection-deprotection schemes have been sometimes reported, typically using potassium phtalimide (see e.g., Ref. [50]). Nevertheless, the amine derivative [10-(H3N-(CH2-CH2O)2-nido-7,8-C2B9H11] (2) can be easily prepared by reaction of 1 with NH3 in THF as is shown here and its molecular structure is depicted in Figure 1. (see the crystallographic discussion below; crystallographic data are given in the Materials Section and Supplementary Information).
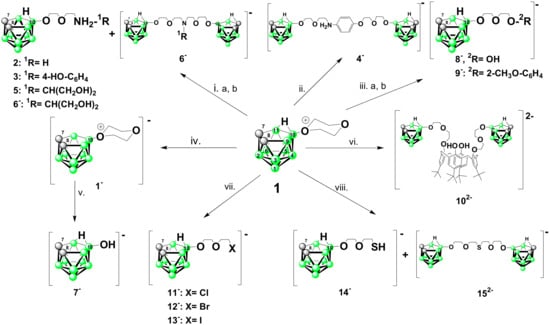
Scheme 2.
Reaction pathways observed in this study for cleavage of the ring in 1 by various nucleophiles; i. Amine in an excess, (a) THF for 2, ACE® pressure flask, (b) for 3, THF r.t.; ii. 1-HO-C6H4-4-NH2, K2CO3 in CH3CN, 80 °C; iii. (a) for 8−: KOH water-ether, r.t. (b) for 9−: K2CO3, 1-HO-2-CH3O-C6H4 reflux; iv. 2.5 M KOH r.t.; v. 2.5 M KOH, reflux, vi. K2CO3, CH3CN, t-Bu-calix[4]arene 0.5 equiv.; vii. Bu4NX, 2 equiv. ether, r.t.; viii. NaSH.H2O in excess, THF, r.t.

Figure 1.
The molecular structure of 1, ORTEP diagram, 30% probability level. Selected interatomic distances [Å] and angles [°]: O1 C12 1.484(2), O1 C15 1.490(2), O1 B10 1.518(3), B10 B6 1.771(3), B10 B5 1.771(3), B10 B9 1.778(3), B10 B11 1.855(4), O2 C13 1.419(3), O2 C14 1.420(3), B11 C7 1.623(4), B11 B6 1.798(4), B11 B2 1.811(4), C7 C8 1.552(3), C7 B2 1.705(4), C7 B3 1.718(4), B9 C8 1.606(3), B9 B5 1.767(4), B9 B4 1.784(4), C8 B4 1.725(4), C8 B3 1.731(3), B6 B2 1.763(4), B6 B1 1.794(4), B6 B5 1.811(4), B3 B2 1.760(4), B3 B4 1.763(4), B3 B1 1.774(4), B2 B1 1.753(4), C12 O1 C15 110.86(15), C12 O1 B10 115.64(15), C15 O1 B10 118.60(15), O1 B10 B6 116.41(16), O1 B10 B5 115.16(17), B6 B10 B5 61.48(15), O1 B10 B9 124.11(18), B6 B10 B9 107.75(17), B5 B10 B9 59.72(14), O1 B10 B11 125.94(18), B6 B10 B11 59.40(14), B5 B10 B11 107.75(17), B9 B10 B11 105.10(17).
To test further the differences between reactivity of different types of nucleophiles in this system, a single molecule, 4-aminophenol was used, which contains a weakly basic and nucleophilic amino group (pKa 5.48) and an acidic phenolic function (pKa 10.30) [51] that are separated by a phenyl ring. As expected, in the absence of a base, the ring cleavage proceeds at room temperature exclusively and quantitatively with the nitrogen end of the molecule binding via an ammonio group to the cluster to give [10-HO-C6H4-H2N-(CH2-CH2O)2-nido-7,8-C2B9H11] (3). However, when K2CO3 is present in dry acetonitrile, the reaction at 80 °C proceeds apparently at both nitrogen and oxygen ends of the 4-aminophenol to give as a main product the double-cluster anionic species [nido-7,8-C2B9H11-10-(CH2-CH2O)2-H2N-C6H4-O-10′-(CH2-CH2O)2-nido-7,8-C2B9H11]− (4−). The mode of bonding could be distinguished from salt isolated directly from the reaction in the form with 4-ammonium phenol cation. 1H and 13C-NMR spectra of 4- then show inequivalent signals of all eight CH2 groups attached to different, oxygen and nitrogen sites of aminophenol. Also aromatic signals of the central phenol unit in 13C-NMR are split into two sets. After metathesis to potassium salt these signals shrunk together and only 3 overlapping signals could be observed in the range of CH2 protons in 1H-NMR. Unfortunately, no crystal could be grown for XRD analysis that precluded unequivocal confirmation of the structure. Only about 15% of 3 (by NMR and HPLC) could be found in the reaction mixture stirred in CH3CN with K2CO3 at room temperature for 72 h. Then, another dicluster compound forms in addition to 4−, which structure probably corresponds to N,N-substitution at aminophenol (according to 1H and 13C-NMR spectra of a crude product), however the analytically pure compound could not be isolated from complex mixture.
Similarly, the reaction of 1 with two equivalents of 2-amino-1,3-propanediol (Serinol), a carbohydrate mimetic that contains amino function and two hydroxyl groups on an aliphatic hydrocarbon chain, proceeds without the necessity for another base, and preferentially at amino group, to give a main product that contains a terminal serinol moiety N-attached to the diethyleneglycol chain in [10-(HOCH2)2CHNH2-(CH2-CH2O)2-nido-7,8-C2B9H11] (5). The second product corresponds to an anion containing a secondary amine as the central moiety to which two cluster units are bound [(HOCH2)2CHNH-(10,10′-(CH2-CH2O)2-nido-7,8-C2B9H11)2]− (6−).
A fundamental difference between compound 1, which is of an open-cage nido-geometry, with that of the more-studied closo-cages, is the presence in 1 of a bridging hydrogen atom. With the aim to investigate the relevance of this hydrogen bridge to the reactivity of zwitterion 1, the basic protonation/ deprotonation of 1 was studied with KOH as base. The sparingly water soluble starting compound dissolves only slightly upon addition of aqueous KOH at room temperature up to 2.5 M concentration. Poor solubility was responsible for weak signals observed in NMR spectrum that precluded carry out NMR titration in deuterium oxide. However, when the compound was dissolved in 100 μL of CD3CN and treated with 500 μL of 2.5 M KOH in D2O, 11B-NMR spectrum of freshly prepared sample showed appreciable shift of signals of boron atoms B(1 and 10), with upfield shift 11.3 ppm (see Figure S1 in Supplementary Information). Such a high shift of boron position B(1) to lower frequency is well known from the literature and could be ascribed to the absence of the extra hydrogen atom [52,53]. Also, another diagnostic peak for hydrogen bridge in 1H [11B]-NMR spectrum at approx. −0.6 ppm completely disappeared. However, this could be also ascribed to an exchange of the hydrogen atom for deuterium. Indeed, additions of 0.5 equivalents of KOH to solution of 1 in D2O, to which 50% of THF-d8 was added for complete dissolution, caused mainly by the exchange of the bridge proton for deuterium with partial disappearing of the signals for B(1) at −40.9 ppm originally present in the spectrum, when new signal at −41.2 ppm appeared. After addition of 1.5 equivalents of KOH both signals were no longer present and moved by incremental shift ca. 10 ppm to higher field to −51.7 ppm (see Figure S2 in Supplementary Information), apparently due to complete removal of the extra hydrogen atom. However, it should be noted a partial degradation and formation of side products also occurred in THF-d8 solution. Therefore, this could partly affect actual concentrations of the species 1 and 1− and KOH in solution.
Such an easy and complete abstraction of the hydrogen bridge from 11-vertex cages by diluted solution of potassium hydroxide was never reported, furthermore carried out in water at room temperature. In contrast, the hydrogen bridge of the parent ion [C2B9H12]− can be abstracted only by a strong base in anhydrous solvents and the ratio of deprotonated species in 12 M KOH has been estimated around 1% [54]. Even in the case of the similarly univalent tricarbollide ion [7,8,9-C3B8H12]–, a strong base (e.g., NaH, RLi, or Tl+ and/or heating) and anhydrous solvents are necessary for deprotonation [55,56,57], though the neutral ammonium derivative exhibits equilibrium between two tautomeric forms, depending on polarity of the solvent, with a proton sitting either on the cage or at tBuNH2- group or in both positions [58,59].
Herein we also report x-ray structure of protonated form of the zwitterionic compound 1 (see Figure 2, the crystallographic discussion below, Materials Section and Supplementary Information), unfortunately the structure of the corresponding potassium salt could not be obtained.
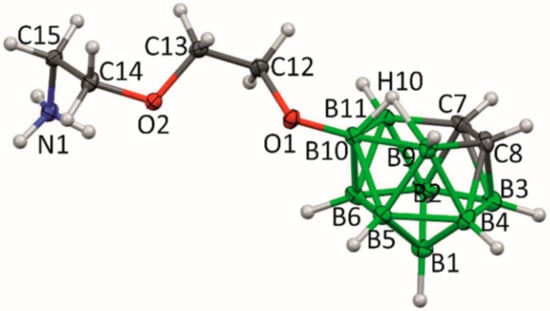
Figure 2.
The molecular structure of 2, ORTEP diagram, 40% probability level. Selected interatomic distances [Å] and angles [°]: O1 C12 1.4400(18), O1 B10 1.459(2), N1 C15 1.490(2), B1 B4 1.762(3), B1 B2 1.771(3), B1 B3 1.772(3), B1 B6 1.791(3), B1 B5 1.799(3), O2 C14 1.4304(18), O2 C13 1.4351(19), B2 C7 1.721(2), B2 B6 1.746(3), B2 B3 1.764(3), B2 B11 1.788(3), B3 C8 1.713(2), B3 C7 1.727(3), B3 B4 1.757(3), B4 C8 1.711(3), B9 B10 1.850(2), B10 B11 1.836(2), C12 O1 B10 113.49(12), B4 B1 B2 107.94(13), B4 B1 B3 59.62(11), B2 B1 B3 59.74(11), B4 B1 B6 108.05(13), B2 B1 B6 58.70(11), B3 B1 B6 106.19(13), B4 B1 B5 59.23(10), B2 B1 B5 107.59(13), B3 B1 B5 106.31(13), B6 B1 B5 61.06(10).
Further, the ring cleavage reaction by KOH was studied under several experimental conditions. Heating with 2.5 M aqueous KOH led to almost quantitative substitution of the ether by -OH to give the [10-HO-7,8-C2B9H11]− anion (7−). This pathway corresponds to the reaction type B (Scheme 1), and is anomalous among all borate ion derivatives bearing a cyclic ether moiety. However, this mechanism we observed only for the OH- ion as nucleophile and at higher temperatures. In this case, the deprotonation probably proceeds in the first step and the whole ring in [10-(O(CH2-CH2)2O)-nido-7,8-C2B9H11]− anion is then replaced by OH−, according to pathway B in Scheme 1. This compound is known to form upon hydride abstraction from [C2B9H12]− ion by AlCl3 in acetone [60]. Conditions described herein thus provide an alternative synthetic procedure to this symmetrically substituted 10-hydroxyderivative.
Under mild conditions, when the compound 1 is heated in a biphasic system of ether and 2.5 M KOH for 48 h, the nucleophilic addition proceeds via pathway A instead and the expected product, with a hydroxyl group attached via a diethylene glycol chain [10-HO-(CH2CH2O)2-7,8-C2B9H11]− (8−) (see Scheme 2), is obtained in a good yield. However, use of KOH in other solvents such as dry dioxane, THF or acetonitrile led to quite complicated product mixtures that contained both, 7− and 8− as well as some other species that presumably originate from reactions with solvent and from degradation of the cage. Details are not reported here.
The ring cleavage with other oxygen nucleophiles follows the usual pathway A corresponding to SN substitution. The previously reported reactions with phenolate ions led solely to pathway A and the corresponding phenols attached to the cage by a six atom linker [7,44]. Also reactions with 2-methoxy-phenol (guaiacol) as an example of naturally occurring compound and t-Bu-calix[4]arene, as an archetype for organic platform with four phenolic -OH sites, provided the expected products (9− and 102−) according to the pathway A, when the respective compounds were reacted with K2CO3 and 1 in dry acetonitrile. A compound with a terminal guaiacol moiety [10-(2-CH3O-C6H4O)-(CH2-CH2O)2-nido-7,8-C2B9H11]− (9−) was formed in high yield. This product was synthesized also with regard to easy crystallization observed previously with metal bis(dicarbollide) analogues [61,62], apparently due to five coordination sites (four oxygens and B-H site of the cage) available for alkali metal complexation. However, in this particular case these expectations proved groundless, apparently also due to different nido-system.
The reaction with two equivalents of 1 with t-Bu-calix[4]arene in presence of K2CO3 resulted in a doubly substituted product 102− that has two boron cages in symmetric positions 1,3- at the narrow rim of the platform that are located in positions separated by unsubstituted calix[4]arene phenol rings. The dicesium salt of the main isolated species adopts a cone-conformation at room temperature in solution as evidenced from NMR data that shows two signals for phenol ring, two AX doublets (Δδ = 1.21 ppm) for bridging -CH2- groups and two tBu signals in a typical range. This could be interpreted via the analogy of a similar known derivative, in which a structure containing two cobalt bis(dicarbollide) and diethyleneglycol chains are responsible for complexation of one caesium cation above the platform. The second Cs+ was found incorporated inside the tBu-calix[4]arene cavity [63]. Therefore, the presence of two caesium cations in 102− apparently also helps to fix t-Bu-calix[4]arene in cone-conformation even in hot water or acetone solutions used for crystallization and NMR measurements, respectively.
Considering other nucleophiles, halogen anions X− (X = Cl−, Br−, I−) react with 1 readily and under mild conditions following the typical reaction pathway A, i.e., the cleavage of the ring producing the SN substitutions with halogen. Thus, stirring of the zwitterion 1 with two equivalents of Bu4NX (X = Cl, Br, I) in diethyl ether or THF at room temperature provides after 12 h quantitative conversion to the [10-X-(CH2-CH2O)2-nido-7,8-C2B9H11]Bu4N salts of the respective anions (11– to 13–) (X = Cl, Br, I). The compounds with terminal halogens can readily serve as valuable synthons in various alkylation reactions. Despite low nucleophilic action of halide ions observed in the reactions of tetrahydropyrane derivative of [B12H12]− ion [49], where the ring could not be cleaved, the reactions of 1 proceed smoothly like in the case of cobalt bis(dicarbollide) ion [64]. On the other hand, this can be a source of problems when trying to accomplish reactions with other nucleophiles and a source of halide ions, e.g., Cl−, is also present in a reaction mixture. Thus, according to our experience, all amines have to be carefully released from respective hydrochlorides and adequately purified before use to avoid side reactions with the chloride anion.
Another known type of strong nucleophile is represented by thiolate ion. Since thiol derivatives, may provide interactions with thiol groups in protein structure and are suitable functional group for further labelling, e.g., by fluorescent groups we focused here also on their synthesis. The ring cleavage with sodium hydrogen sulphide leads to nucleophilic substitution of type A resulting in a compound with a terminal –SH group [10-HS-(CH2-CH2O)2-nido-7,8-C2B9H11]− (14−). This compound is identical with product that was prepared previously by two step procedure that comprised ring opening of 1 with thiourea followed by alkaline hydrolysis [45]. This derivative may be a good building block for bio-conjugation and has also potential for use in BNCT as easily available alternative of doubly charged [B12H11SH]2− (BSH) [65]. Also, a double cage compound (152−) that contains central thioether moiety [S-(10,10′-(CH2-CH2O)2-nido-7,8-C2B9H11)2]2− forms as a side product of this reaction, even if an excess of sodium sulphide and a larger dilution were applied. Both compounds were isolated by chromatography and fully characterized.
2.2. Crystallography
The molecular structures of two compounds from the series, the starting zwitterion 1 and ammonium derivative 2 were unambiguously confirmed by sc-XRD determination. Both compounds have the eleven-vertex nido-type structures with the oxygen substitution at the B10 atom. Compound 1 crystallizes in the chiral orthorhombic space group, while 2 in the monoclinic P21/n. All the structural parameters, e.g., interatomic distances and angles in both molecules are close to the appropriate values found for related 10-(2-dimethylsulfonioethoxy)-7,8-dicarba-nido-undecaborate [66]. Namely, the C7–C8 distances (see in captions of Figure 1 and Figure 2) are the shortest within the cluster but still a bit longer than the typical Csp3-Csp3 bond distance. C-B separations are around the mean value 1.6 Å and the B-B distances of ~1.85 Å for atoms bound with the bridging H atoms are the longest in all boron clusters. The placement of the bridging hydrogen atom between B10 and B11 atoms in 1 is unequivocal as seen mainly from the distinct distances 1.778(3) Å for B10–B9 and 1.855(4) Å for B10–B11, resp. On the other hand, in 2 with similar separations of 1.850(2) Å for B9−B10 and 1.836(2) Å for B10–B11, the position is selected according the appearance of a stronger maximum on the Fourier electron density map. The major differences are also seen in the O1 bonding patterns, where in 1 the connections of this atom by three ‘covalent’ bonds are taking place, while ~ 0.05 Å shorter connections to B10 and C12 are observed in 2. Similar C-O and B-O distances, as found for 1, were already reported for 1,4-dioxane derivatives of cobalt bis(dicarbollide) [14] and closo-dodecaborate [10], with the exception of the B-O distance in the latter compound, which is elongated by 0.03 Å. The direct comparison of 1 with recently published 10-Et2O-7,8-C2B9H11 derivative [67] shows essentially the same interatomic distances and angles with only negligible differences in the structural parameters. However a closer look revealed the fact that the crystals of compounds 1 and 10-Et2O-7,8-C2B9H11 virtually correspond to two different enantiomers (considering the asymmetric position of extra hydrogen atoms).
In addition, the supramolecular architecture differs a lot. The molecules in the crystals of 1 are connected only by non-covalent C-H…H-B and C-H…O interactions, in the crystals of 2, also classical H-bridging between N-H and three oxygen atoms of the OCH2CH2O groups are detected.
3. Materials and Methods
3.1. General
The starting trimethylammonium salt of dicarbollide anion was purchased from Katchem Ltd. Prague, Czech Republic. The starting compound 1 was prepared according to described procedure [8]. 1,4-dioxane, tetrahydrofuran (THF) and ethylene glycol dimethyl ether (DME) were dried with sodium diphenyl ketyl and distilled prior to use. Acetonitrile was dried over molecular sieves 4A (Fluka, Buchs, Switzerland). Other chemicals and solvents were obtained from Aldrich, Merck Lachema a.s., Neratovice and Penta Ltd., Prague, Czech Republic and used without purification. Analytical TLC was carried out on Silufol (Lachema, Votice, Czech Republic), silica gel TLC plates in CH2Cl2-CH3CN mixture. Unless otherwise specified, column chromatography was performed on high purity silica gel (Merck Grade, Type 7754, 70–230 mesh, 60 Å). All reactions were performed using standard Schlenk type vacuum or inert-atmosphere techniques. Some operations, such as column chromatography and crystallization, were carried out in the air.
The identities of all the reported compounds were unambiguously verified by a combination of 11B, 1H and 13C-NMR spectral data (complete assignment of the resonances) Mass Spectrometry (two decimal place resolution); elemental analysis; TLC; and other methods. The purity was also assayed by a previously developed analytical HPLC method with DAD detection, under chromatographic conditions specified in the paragraph below and was ≥95% for all compounds. For chemical analyses the Me4N+ or Bu4N+salts were used.
3.2. Instrumental Techniques
1H, 1H[11B], 1H[11Bselective], 13C, and 11B-NMR spectra were measured on a Varian Mercury 400Plus and a on Jeol 600 MHz spectrometers. The spectra of all compounds were measured immediately after dissolution in particular deuterated solvent, usually acetonitrile-d3 unless otherwise stated. 11B-NMR (192 MHz) chemical shifts are given in ppm to high-frequency (low field) to F3B OEt2 as the external reference. Residual solvent 1H resonances were used as internal secondary standards. The NMR data are presented in the text as follows: 11B-NMR: 11B chemical shifts δ (ppm), multiplicity. Signal assignments are based on [11B-11B] COSY NMR spectroscopy. 1H-NMR (600 MHz) and 13C (150 MHz): chemical shifts δ are given in ppm relative to Me4Si (0 ppm) as the external standard, coupling constants J(H,H) are in Hz.
Mass spectrometry measurements were performed on Thermo-Finnigan LCQ-Fleet Ion Trap instruments (San Jose, CA, USA) using electrospray ionization (ESI) for ionic species with detection of negative or positive ions. For ESI, samples dissolved in acetonitrile (concentrations approx. 100 ng mL−1) were introduced to the ion source by infusion. Molecular ions [M]− were detected for all univalent anions as base peaks in the spectra. Full agreement between the experimental and calculated isotopic distribution pattern was observed for all isolated compounds. The isotopic distribution in the boron plot of all peaks was in complete agreement with the calculated spectral pattern. The data are presented for the most abundant mass in the boron distribution plot (100%) and for the peak on the right side of the boron plot corresponding to the m/z value.
HPLC: A Merck-Hitachi LaChrom Series 7000 HPLC system equipped with a DAD 7450 detector and a L7250 Programmable Autosampler was used. Anion separation was carried out according to a previously reported Ion-Pair RP method [68] for separation of hydrophobic borate anions on a RP Separon SGX C8 column, 7 μm (250 × 4 mm I. D.), Tessek Prague. The mobile phase was 4.5 mmol hexylamine acetate in 45% aqueous CH3CN (pH 6.0). Samples with concentration of approximately 1 mg∙mL−1 in the mobile phase were injected. DAD detection was carried out at fixed wavelengths 220, 225, 235, 260 nm; samples of concentration approximately 1 mg∙mL−1 in the mobile phase were injected.
Elemental analyses were performed on a Thermo Fisher Scientific FlashSmart Organic Elemental Analyzer (Austin, TX, USA) using a V2O5 catalyst weighted with the sample for combustion of the samples in oxygen. Most of compounds for EA were measured as K+ or Na+ or were converted to respective Me4N+ or Bu4N+ salts after careful drying in vacuum. The alkali metal and Me4N+ salts of compounds 6− and 8− tend to strongly bind water and sodium from the chromatographic support, thus for elemental analysis were converted to respective PPh4+ salts by precipitation with PPh4Cl. All compounds were dried for 12 h in vacuum at 80 °C before analyses.
X-ray crystallography: The X-ray data for the compound 1 and 2 (colorless crystals by slow evaporation of a methanol/water solution) was collected at 150(2)K with a Bruker D8-Venture diffractometer equipped with Mo (Mo/Kα radiation; λ = 0.71073 Å) microfocus X-ray (IµS) source, Photon CMOS detector (Karlsruhe, Germany) and Oxford Cryosystems cooling device (Oxford, United Kingdom) was used for data collection. The frames for both 1 and 2 were integrated with the Bruker SAINT software package (Karlsruhe, Germany) using a narrow-frame algorithm. Data were corrected for absorption effects using the Multi-Scan method (SADABS). The ratios of minimum to maximum apparent transmission were 0.892 and 0.921. The structures were solved and refined using XT-version 2014/5 and SHELXL-2017/1 software implemented in APEX3 v2016.9-0 (Bruker AXS, Karlsruhe, Germany) system [69]. Rint = ∑|Fo2 − Fo,mean2|/∑Fo2, S = [∑(w(Fo2 − Fc2)2)/(Ndiffrs − Nparams)]½ for all data, R(F) = ∑||Fo| − |Fc||/∑|Fo| for observed data, wR(F2) = [∑(w(Fo2 − Fc2)2)/(∑w(Fo2)2)]½ for all data. Hydrogen atoms were mostly localized on a difference Fourier map; however, to ensure uniformity of treatment of crystal, all hydrogen were recalculated into idealized positions (riding model) and assigned temperature factors Hiso(H) = 1.2 Ueq (pivot atom) or of 1.5 Ueq (methyl). H atoms in methylene moieties were placed with C-H distances of 0.96 Å, 0.82Å for N-H and 1.1 Å for B-H and C-H bonds in the carborane cages. For crystallographic data and structure refinement see ESI. Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC nos. 1973450-1973451. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EY, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
3.3. Synthetic Procedures
Deprotonation of the [10-(O-(CH2-CH2)2O)-nido-7,8-C2B9H11] (1)
400 μL of 2.5 M KOH in D2O was added to a 100 μL of 1.25 M solution of 1 in CD3CN in a 5 mm NMR tube. The insoluble zwitterion dissolved during 10 min. under shaking and the respective anion formed, from which the bridge was fully abstracted as evidenced by significant changes in 11B and 1H-NMR. The sample was measured immediately after dissolution. After several hours the salt starts to crystallize out of the solution. After acidification with few drops of 3 M HCl this ion reverts back to neutral zwitterion.
NMR data for the starting zwitterion [10-(O-(CH2-CH2)2O)-nido-7,8-C2B9H11] (1) 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.6 (s, 1B, B10), −13.3 (d, 2B, J = 146 Hz, B9,11), −18.0 (d, 2B, J = 137 Hz, B5,6), −23.2 (d, 3B, J = 151 Hz, B2,4,3), −40.8 (d, 1B, J = 146 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 4.48 (t, 4 H, J = 4.2 Hz, CH2O), 3.83 (t, 4 H, J = 4.8 Hz, CH2O), 1.99 (br. s, 2 H, CHcarborane); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 82.3 (CH2O), 64.8 (CH2O), 41.3 (CHcarborane).
NMR data for the deprotonated [10-(O-(CH2-CH2)2O)-nido-7,8-C2B9H10]− (1−) 11B-NMR (192.6 MHz, D2O) δ (ppm) = −22.5 (d, 2B, J = 120 Hz, B9,11), −24.8 (d, 2B, J = 111 Hz, B5,6), −30.0 (2d + s, 4B, J = 129 Hz, B2,4,3,10), −52.1 (d, 1B, J = 128 Hz, B1); 1H-NMR (600 MHz, D2O: δ (ppm) = 4.12 (t, 4 H, J = 4.2 Hz, CH2O), 3.73 (t, 4 H, J = 4.8 Hz, CH2O), 0.65 (br. s, 2H, CHcarborane); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 79.6 (CH2O), 65.6 (CH2O), 24.0 (CHcarborane).
[10-H3N-(CH2-CH2O)2-nido-7,8-C2B9H11] (2)
The zwitterion 1 (205 mg, 0.927 mmol) was dissolved in THF (15 mL) in a glass ACE® pressure tube, which was cooled down to −78 °C under nitrogen and then ammonia (3.0 g, 0.176 mol) was condensed into this solution. The tube was closed by a stopper and heated up. The reaction mixture was stirred at room temperature for 1 h 30 min, and then the solvent was removed on a rotary evaporator. MS of the reaction mixture showed the mass of the expected product and, in addition also a peak of the respective double-cluster compound. This however was not isolated in analytical purity. The pure compound 2 was isolated by chromatography on silica gel column 15 × 1.5 cm I.D. elution with CH2Cl2-CH3CN mixture from 5:1 to 1:1 b.v. The respective fractions were evaporated and the product was dried in vacuum for 3 h; white solid, yield 0.103 g, 47%. 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −11.4 (s, 1B, B10), −13.5 (d, 2B, J = 138 Hz, B9,11), −18.3 (d, 2B, J = 133 Hz, B5,6), −24.6 (d, 2B, J = 151 Hz, B2,4), −25.8 (d, 1B, B3, J = 137 Hz), −41.5 (d, 1B, J = 137 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 6.82 (br. s, 2H, H2N), 3.64 (m, 4H), 3.55 (m, 2H), 3.05 (t, 2H), 1.63 (br. s, 2 H, CHcarborane); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 6.82 (br. s, 3H, H3N), 3.64 (m, 4H), 3.55 (m, 2H), 3.05 (t, 2H, J = 5 Hz), 2.06 (br. s, 2 H, BH), 1.94 (br. s, 1 H, BH), 1.63 (br. s, 2 H, CHcarborane), 1.38 (br. s, 2 H, BH), 1.24 (br. s, 1 H, BH), 1.09 (br. s, 1 H, BH), −0.60 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 71.0 (CH2N), 70.0 (CH2O), 66.1 (CH2O), 39.7 (CH2O), 39.4 (CHcarborane); MS (ESI−) m/z (%) found: 237.40 (100), 238.36 (50) [M − H]−, calcd. 237.25 (100), 238.24 (48); Analysis found (%): C 30.12, H 9.04, N 5.86; calcd. for C6B9H21O2N C 30.47, H 8.95, N 5.92.
[10-HO-C6H4-H2N-(CH2-CH2O)2-nido-7,8-C2B9H11] (3)
The zwitterion 1 (300 mg, 1.36 mmol) was dissolved in THF (25 mL) and then solid 4-aminophenol (190 mg, 1.74 mmol) was added under stirring. The reaction mixture was stirred at room temperature for 24 h until the spot of starting compound disappeared on the TLC, and then the solvent was removed on a rotary evaporator. The product was crystallized from CH2Cl2-hexane, filtered and dried in vacuum; yellowish solid, yield 416 mg, 93%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −11.3 (s, 1B, B10), −13.5 (d, 2B, J = 133 Hz, B9,11), −18.3 (d, 2B, J = 131 Hz, B5,6), −24.6 (d, 2B, J = 153 Hz, B2,4), −25.7 (d, 1B, B3), −41.5 (d, 1B, J = 142 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 7.16 (dd, 2 H, J = 9.0 Hz, ArH), 6.85 (dd, 2 H, J = 8.4 Hz, ArH), 4.26 (br. s, 2 H, H2N), 3.72 (m, 2 H, CH2N), 3.61 (m, 4 H, CH2O), 3.35 (br t, 2 H, J = 4.8 Hz, CH2O), 1.61 (br. s, 2 H, CHcarborane); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 7.16 (dd, 2 H, J = 9.0 Hz, ArH), 6.85 (dd, 2 H, J = 8.4 Hz, ArH), 4.26 (br. s, 2 H, H2N), 3.72 (m, 2 H, CH2N), 3.61 (m, 4 H, CH2O), 3.35 (br t, 2 H, J = 4.8 Hz, CH2O), 2.06 (br. s, 2 H, BH), 1.78 (br. s, 1 H, BH), 1.61 (br. s, 2 H, CHcarborane), 1.59 (br. s, 2 H, BH), 1.24 (br. s, 1 H, BH), 1.06 (br. s, 1 H, BH), −0.58 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 156.9 (ArC), 127.9 (ArC), 123.9 (ArC), 116.3 (ArC), 70.3 (CH2N), 70.1 (CH2O), 64.1 (CH2O), 49.8 (CH2O), 39.6 (CHcarborane); MS (ESI−) m/z (%) found: 329.40 (100), 330.36 (54) [M − H]−, calcd. 329.27 (100), 330.27 (53); Analysis found (%): C 43.79, H 7.57, N 4.40; calcd. for C12B9H25O3N 43.86, H 7.67, N 4.26.
[Nido-7,8-C2B9H11-10-(CH2-CH2O)2-H2N-C6H4-O-10′-(CH2-CH2O)2-nido-7,8-C2B9H11]K (K.4)
The zwitterion 1 (330 mg, 1.50 mmol) was dissolved in dry CH3CN (15 mL) and then solid K2CO3 (1.0 g, 7.2 mmol) was added followed by 1-HO-C6H4-4-NH2 (390 mg, 3.38 mmol). The reaction mixture was stirred at 80 °C for 24 h. After cooling down, the excess of K2CO3 was filtered off under nitrogen and the solvent was removed in vacuum. The crude product, which contained ca. 10% of compounds 3 was dissolved in CH2Cl2 and poured on a top of silica gel column. The pure compound 4- was then isolated by chromatography on silica gel column 25 × 1.5 cm I.D. elution with CH2Cl2-CH3CN mixture from 5:1 to 2:1 b.v. The respective fractions were evaporated and the product was dried in vacuum for 3 h; white solid, yield of 4− in the form of salt with 1-HO-C6H4-4-NH3+ cation after isolation was 181 mg, 37%. This salt was then dissolved in ethyl acetate (25 mL) shaken with diluted HCl (4 × 20 mL), then with 5% KOH (4 × 20 mL) and aqueous KCl (10%, 5 × 20 mL) and water. The organic layer was then separated, water (5 mL) was added and the volatiles were removed in vacuum. The K.4 was then dried at 40 °C in vacuum for 6 h, yield 135 mg, 31%. [HOC6H4NH3]4: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.7 (s, 1B, B10), −13.5 (d, 2B, J = 135 Hz, B9,11), −18.5 (d, 2B, J = 133 Hz, B5,6), −24.8 (d, 2B, J = 151 Hz, B2,4), −26.2 (d, 1B, J = 164 Hz, B3), −41.6 (d, 1B, J = 140 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 6.92 (d, 2 H, J = 8.4 Hz, ArH), 6.73 (d, 2 H, J = 9.0 Hz, ArH), 6.60 (d, 2 H, J = 9.0 Hz, ArH), 6.54 (d, 1 H, J = 9.0 Hz, ArH), 6.52 (d, 1 H, J = 9.0 Hz, ArH), 6.48 (d, 2 H, J = 9.0 Hz, ArH), 3.70 (br. s, 2 H, H2N), 3.57 (t, 4 H, J = 4.2 Hz CH2O), 3.5 (br s, 2 H, CH2N), 3.47 (t, 2 H, J = 4.8 Hz, CH2O), 3.43 (t, 2 H, J = 4.8 Hz, CH2O), 3.38 (t, 2 H, J = 4.8 Hz, CH2O), 3.11 (t, 2 H, J = 5.4 Hz, CH2O), 3.09 (t, 2 H, J = 5.4 Hz, CH2O), 1.58 (br. s, 4 H, CHcarborane); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 6.92 (d, 2 H, J = 8.4 Hz, ArH), 6.73 (d, 2 H, J = 9.0 Hz, ArH), 6.60 (d, 2 H, J = 9.0 Hz, ArH), 6.54 (d, 1 H, J = 9.0 Hz, ArH), 6.52 (d, 1 H, J = 9.0 Hz, ArH), 6.48 (d, 2 H, J = 9.0 Hz, ArH), 3.70 (br. s, 2 H, H2N), 3.57 (t, 4 H, J = 4.2 Hz CH2O), 3.5 (br s, 2 H, CH2N), 3.47 (t, 2 H, J = 4.8 Hz, CH2O), 3.43 (t, 2 H, J = 4.8 Hz, CH2O), 3.38 (t, 2 H, J = 4.8 Hz, CH2O), 3.11 (t, 2 H, J = 5.4 Hz, CH2O), 3.09 (t, 2 H, J = 5.4 Hz, CH2O), 2.17 (br. s, BH), 2.07 (br. s, BH), 1.78 (br. s, 1 H, BH), 1.60 (br. s, 2 H, CHcarborane), 1.25 (br. s, 1 H, BH), 1.07 (br. s, BH), 0.69 (br. s, 1 H, BH), 0.32, −0.53 (br. s, 1 H, μ-H), −0.61 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 152.4 (ArC), 148.9 (ArC), 148.8 (ArC), 142.4 (ArC), 142.3 (ArC), 140.7 (ArC), 122.9 (ArC), 115.9 (ArC), 115.9 (ArC), 115.8 (ArC), 115.8 (ArC), 114.5 (ArC), 71.6 (CH2), 71.4 (CH2), 69.4 (CH2), 68.7 (CH2O), 67.7 (CH2O), 55.0 (CH2O), 44.6 (CH2O), 39.2 (CHcarborane), 39.2 (CHcarborane), 38.9 (CHcarborane);) K.4: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.4 (s, 1B, B10), −13.5 (d, 2B, J = 135 Hz, B9,11), −18.5 (d, 2B, J = 133 Hz, B5,6), −24.9 (d, 2B, J = 151 Hz, B2,4), −26.2 (d, 1B, J = 164 Hz, B3), −41.6 (d, 1B, J = 140.18 Hz, B1); 1H-NMR (600 MHz, CD3CN) δ (ppm) = 6.92 (d, 2 H, J = 8.4 Hz, ArH), 6.76 (d, 2 H, J = 9.0 Hz, ArH), 3.54 (br. t, 4 H, CH2O, CH2N), 3.51 (m, 4 H, CH2O), 3.45 (t, 4 H, J = 4.8 Hz, CH2O), 1.55 (br. s, 2 H, CHcarborane); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) −0.61 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 116.9 (ArC), 115.9 (ArC), 71.6 (CH2), 71.5 (CH2), 69.5 (CH2), 55.4 (CH2O), 55.2 (CH2O), 38.9 (CHcarborane); MS (ESI−) m/z (%) found: 549.56 (100), 551.52 (15) [M]−, calcd. 549.50 (100), 552.49 (14); Analysis found (%): C 36.48, H 7.33, N 2.38, calcd. for C12B9H25O3NK: C 36.82, H 7.38, N 2.39.
[10-(HOCH2)2CHNH2-(CH2-CH2O)2-nido-7,8-C2B9H11] (5) and [(HOCH2)2CHNH-N,N-(10,10′-(CH2-CH2O)2-nido-7,8-C2B9H11)2]− (6−)
The reaction of 1 (300 mg, 1.36 mmol) in THF (25 mL) 2-Amino-1,3-propanediol (Serinol) (0.250 g, 2.75 mmol) was carried out in analogous way to the synthesis of 3. The reaction mixture was stirred at room temperature for 16 h until the spot of starting compound disappeared on the TLC, and then the solvent was removed on a rotary evaporator. The crude product was dissolved in aqueous methanol and the disubstituted compound 6− was precipitated by Me4NCl and the solid contained also a small amount of Me4N5. This mixture was separated by column chromatography on silica gel using CH2Cl2-CH3CN from 5:1 to 3:1 b.v. isolating Me4N6. The solution was made slightly alkaline by few drops of 5% KOH and the anionic form of compound 5− was precipitated by aqueous Et4NCl and left to stand for 1h. The semi-solid material that settled down to glass was separated by decantation, washed with water (4 × 5 mL) and dried in vacuum. The solid material was dissolved in ethyl acetate (30 mL) and treated by diluted HCl (3 M, 30 mL). The organic layer was separated and the aqueous phase extracted by ethyl acetate (2 × 15 mL). Combined organic extracts were evaporated to dryness and the zwitterionic product 5 was then dried in vacuum at 50 °C for 8 h.
Data for 5: Semi-solid material, yield 320 mg, 73%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −11.8 (s, 1B, B10), −13.4 (d, 2B, J = 135 Hz, B9,11), −18.4 (d, 2B, J = 130 Hz, B5,6), −24.4 (d, 2B, J = 149 Hz, B2,4), −25.5 (d, 1B, B3), −41.4 (d, 1B, J = 144 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 7.56 (q, 2H, J = 7.8 Hz, NH), 4.77 (br s, 2 H, OH), 4.29 (dd, 2 H, J = 4.2 Hz, CH2O), 4,22 (d, 1H, J = 5.4 Hz, CH), 3.91 (t, 4 H, J = 5.4 Hz, CH2O), 3.76 (t, 2 H, J = 5.4 Hz, CH2O), 3.75 (m, 2 H, CH), 3.64 (t, 2 H, J = 4.8 Hz, CH2O), 3.28 (br. t, 2 H, J = 4.2 Hz, CH2O), 1.66 (br. s, 2 H, CHcarborane); 1H[11B]-NMR (400 MHz, Acetone-d6): δ (ppm) = 4.37 (br s, 2 H, OH), 3.80 (br s, 2 H, CH2N), 3.71 (br. s, 1 H, CHN), 3.77 (br. t, 2 H, J = 7.2 Hz, CH2O), 3.59 (br t, 2 H, J = 7.2 Hz, CH2O), 3.54 (2 H, J = 4.2 Hz, CH2O), 3.41 (s, 2 H, J = 4.2 Hz, CH2O), 3.22 (br. t, 4 H, CH2O), 2.15 (br. s, 2 H, BH), 1.53 (br. s, 1 H, BH), 1.52 (br. s, 2 H, CHcarborane), 1.41 (br. s, 2 H, BH), 1.17 (br. s, 2 H, BH), 0.45 (br. s, 1 H, BH), −0.51 (br. s, 2 H, μ-H); 13C-NMR (100.6 MHz, Acetone-d6) δ (ppm) = 70.5 (CH2N), 64.8 (CH2O), 60.0 (CH2O), 55.1 (CHN), 45.2 (CH2O), 40.0 (CHcarborane); MS (ESI−) m/z (%) found: 311.40 (100), 312.40 (52) [M − H]−, calcd. 311.28 (100), 312.28 (51); Analysis found (%): C 35.73, H 8.87, N 4.40 calcd. for C9B9H27N1O4 35.59 H 8.72, N 4.49.
Data for 6−: Viscous semi-solid material, yield 55 mg, 6%: 11B-NMR (192.6 MHz, Acetone-d6) δ (ppm) = −11.0 (s, 2B, B10), −13.4 (d, 4B, J = 137 Hz, B9,11), −18.2 (d, 4B, J = 129 Hz, B5,6), −24.6 (d, 4B, J = 151 Hz, B2,4), −25.8 (d, 2B, B3), −41.3 (d, 2B, J = 136 Hz, B1); 1H-NMR (600 MHz, Acetone-d6): δ (ppm) = 7.98 (br s, 1 H, NH), 4.70 (br s, 2 H, OH), 3.96 (dd, 4 H, J = 7.8 and 4.2 Hz, CHCH2N), 3.88 (t, 4 H, J = 7.2 Hz, CH2O), 3.85 (q, 4 H, J = 6.0 Hz, CH2N), 3.61 (s, Me4N), 3.58 (t, 4 H, J = 5.4 Hz, CH2O), 3.48 (m, 1 H, CH), 3.60 (4 H, CH2O), 1.53 (br. s, 4 H, CHcarborane); 1H[11B]-NMR (400 MHz, Acetone-d6): δ (ppm) = 7.98 (br s, 1 H, NH), 3.80 (br s, 2 H, CH2N), 3.71 (br. s, 1 H, CHN), 3.77 (br. t, 2 H, J = 7.2 Hz, CH2O), 3.59 (br t, 2 H, J = 7.2 Hz, CH2O), 3.54 (2 H, J = 4.2 Hz, CH2O), 3.41 (s, 2 H, J = 4.2 Hz, CH2O), 3.21 (br. t, 4 H, CH2O), 2.151 (br. s, 4 H, BH), 1.57 (br. s, 2 H, BH), 1.48 (br. s, 2 H, CHcarborane), 1.37 (br. s, 4 H, BH), 1.25 (br. s, 4 H, BH), 0.48 (br. s, 2 H, BH), −0.50 (br. s, 2 H, μ-H); 13C-NMR (100.6 MHz, Acetone-d6) δ (ppm) = 71.3, 70.0, 65.1, 65.0, 58.1 (Me4N), 45.1, 38.4 (CHcarborane); MS (ESI−) m/z (%) found: 531.56 (100), 534.50 (10) [M]−, calcd. 531.51 (100), 534.50 (13); Analysis for Ph4P6 found (%): C 53.5, H 7.61, N 1.65 calcd. for C39B18H64O6N1P1 C 53.94, H 7.43, N 1.61.
[10-HO-7,8-nido-C2B9H11]K (K.7)
The zwitterion 1 (300 mg, 1.36 mmol) was poured into 2.5 M KOH (25 mL) and the reaction mixture was stirred and heated to reflux temperature for 3 h. After cooling down the crude product was extracted from the aqueous reaction mixture into ethylacetate (3 × 20 mL). The combined organic extracts were washed with water (20 mL), water (3 mL) was added and the solvent was removed in a rotary evaporator and then the crude product was dried in vacuum for 2 h. The crude product was purified by column chromatography on a silica gel column 25 × 2.5 cm I.D. using CH2Cl2-CH3CN solvent mixture 7:3 b.v.; white solid, yield 195 mg, 76%: 11B-NMR (128.32 MHz, CD3CN) δ (ppm) = −12.1, −12.5 (s + d, 3B, B10, 9,11), −17.5 (d, 2B, B5,6), −25.1 (d, 2B, B2,4), −27.0 (d, 1B, B3), −41.6 (d, 1B, B1); 1H[11B]-NMR (600 MHz, CD3CN): δ (ppm) = 2.18 (br. s, 2 H, BH), 1.50 (br. s, 1 H, CHcarborane), 1.45 (br. s, 2 H, BH), 1.37 (br. s, 2 H, BH), 1.14 (br. s, 1 H, BH), 1.434 (br. s, 1 H, BH), −0.38 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 38.3 (CHcarborane); MS (ESI−) m/z (%) found: 150.32 (100), 151.28 (50), (M-H), calcd. 149.17 (100), 151.17 (48); Analysis found (%): C 12.89, H 6.01, calcd. for C2B9H12OK: C 12.74, H 6.42. The data are in agreement with those reported for synthesis of 7– by another method [60].
[10-HO-(CH2CH2O)2-nido-7,8-C2B9H11]K (K8)
The zwitterion 1 (300 mg, 1.36 mmol) was dissolved in Et2O (30 mL) and then degassed 2.5 M KOH (25 mL) was injected through septum. The reaction mixture was stirred and heated to reflux temperature of ether for 48 h. After cooling down the ether layer was separated, the aqueous phase was extracted twice with ether (10 mL), water (3 mL) was added to combined ether extracts and the organic solvent was evaporated in a rotary evaporator and then dried in vacuum for 2 h. The crude product was purified by column chromatography on a silica gel column 25 × 2.5 cm I.D. using CH2Cl2-CH3CN solvent mixture 9:1 to 2:1 b.v.; white solid, yield 228 mg, 61%; 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −11.3 (s, 1B, B10), −13.5 (d, 2B, B9,11), −18.4 (d, 2B, B5,6), −24.6 (d, 2B, B2,4), −25.9 (d, 1B, B3), −41.5 (d, 1B, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 3.62 (t, 4 H, CH2O), 3.492–3.435 (m, 4 H, CH2O), 1.604 (br. s, 2 H, CHcarborane); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 71.4 (CH2O), 71.1 (CH2O), 69.51 (CH2O), 60.9 (CH2O), 39.2 (CHcarborane); MS (ESI−) m/z (%) found: 238.33 (100), 239.25 (45), calcd. 238.23 (100), 239.22 (48); Analysis found for PPh48 (%): C 62.26, H 6.64, calcd. for C30B9H40O3P1 C 62.46, H 6.99.
[10-(2-CH3O-C6H4O)-(CH2-CH2O)2-nido-7,8-C2B9H11]K (K9)
The zwitterion 1 (300 mg, 1.36 mmol) was dissolved in dry CH3CN (15 mL) and then solid K2CO3 (1.0 g, 7.25 mmol) was added and 1-HO-2-CH3O-C6H4 (0.3 mL, 2.69 mmol) was injected through septum. The reaction mixture was stirred at room temperature for 24 h, then the excess of K2CO3 was filtered off under nitrogen and the solvent was removed in vacuum. The residue was dissolved in CH2Cl2 and poured on top of a silica gel column 25 × 2.2 cm I.D. Elution with CH2Cl2 led to recovery of a small amount of the starting material. The product was then isolated by continuous elution with CH2Cl2-CH3CN mixture from 9:1 to 1:1 b.v. The respective fractions were evaporated and the product was dried in vacuum for 2.5 h; white solid, yield 185 mg, 40%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.8 (s, 1B, B10), −13.5 (d, 2B, J = 132 Hz, B9,11), −18.4 (d, 2B, J = 131 Hz, B5,6), −24.7 (d, 2B, J = 151 Hz, B2,4), −26.1 (d, 1B, J = 164 Hz, B3), −41.6 (d, 1B, J = 140 Hz, B1) 1H-NMR (600 MHz, CD3CN): δ (ppm) = 6.99–6.86 (m, 4 H, ArH), 4.09 (t, 2 H, J = 4.2 Hz, CH2O), 3.82 (s, 3 H, CH3O), 3.73 (t, 2 H, J = 4.2 Hz, CH2O), 3.61 (br. t, 2 H, J = 3.6 Hz, CH2O), 3.54 (t, 2 H, J = 4.2 Hz, CH2O), 1.59 (br. s, 1 H, CHcarborane); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 6.99–6.86 (m, 4 H, ArH), 4.09 (t, 2 H, J = 4.2 Hz, CH2O), 3.82 (s, 1 H, CH3O), 3.73 (t, 2 H, J = 4.2 Hz, CH2O), 3.61 (br. t, 2 H, J = 3.6 Hz, CH2O), 3.53 (t, 2 H, J = 4.2 Hz, CH2O), 2.16 (br. s, 2 H, BH), 2.07 (br. s, 1 H, BH), 1.93 (br. s, 2 H, BH), 1.60 (br. s, 2 H, CHcarborane), 1.25 (br. s, 1 H, BH), 1.09 (br. s, 1 H, BH), −0.57 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 148.7 (ArC), 147.3 (ArC), 121.7 (ArC), 121.2 (ArC), 113.0 (ArC), 111.7 (ArC), 71.4 (CH3O), 68.8 (CH2O), 68.3 (CH2O), 67.6 (CH2O), 55.5 (CH2O), 39.0 (CHcarborane); MS (ESI−) m/z (%) found: 344.40 (100), 345.40 (55) [M − H]−, calcd. 344.27 (100), 345.27 (53); Analysis found (%): C 40.62, H 7.04, calcd. for C13B9H26O4K C 40.80, H 6.85.
[tBu-calix[4]arene-1,3-(10-(CH2-CH2O)2-nido-7,8-C2B9H11)2]− (102−)
Dry acetonitrile (20 mL) was injected into flask containing carefully dried (8 h at 95 °C) tBu-calix[4]arene (430 mg, 66 mmol) and solid K2CO3 (1.0 g, mmol) and the slurry was stirred for 30 min. Then, a solution of compound 1 (300 mg, 1.36 mmol in CH3CN, 5 mL) was injected through rubber septum. The reaction mixture was stirred at a temperature of 50 °C for 36 h, then the excess of K2CO3 was filtered off under nitrogen, washed with CH3CN (2 × 5 mL) and the combined organic fractions were evaporated on a vacuum rotary evaporator. The residue was dissolved in ether (30 mL), the solution was filtered through a paper filter from oily brownish impurities and then shaken with diluted HCl (3 M, 3 × 30 mL). Water (25 mL) was added and ether was evaporated with a part of water reducing volume to 20 mL. Methanol was added until dissolution of a turbid solid and the product was precipitated by excess of aqueous CsCl. The voluminous white precipitate was collected and crystallized twice from hot water, to which MeOH was added dropwise until dissolution; the bath was then left slowly cool down. The product was collected by filtration after two days of crystallization, washed with water, and dried in a vacuum for 3 h at room temperature and then at 50 °C for 6 h; white solid, yield 375 mg, 42%: 11B-NMR (192.6 MHz, acetone-d6) δ (ppm) = −10.4 (s, 2B, B10), −14.0 (d, 4B, J = 124 Hz, B9,11), −18.2 (d, 4B, J = 128 Hz, B5,6), −24.5 (d, 4B, J = 146 Hz, B2,4), −26.1 (d, 2B, J = 146 Hz, B3), −41.4 (d, 2B, J = 137 Hz, B1); 1H-NMR (600 MHz, Acetone-d6): δ (ppm) = 7.18 (s, 4 H, ArH), 6.90 (s, 4 H, ArH), 4.38 (d, 4 H, J = 20.4 Hz, CH2), 4.20 (m, 4 H, CH2O), 3.95 (br. t, 4 H, J = 3.6 Hz, CH2O), 3.70 (br. t, 8 H, CH2O), 3.42 (d, 4 H, J = 18.0 Hz, CH2), 1.42 (br. s, 2 H, CHcarborane), 1.27 (s, 18 H, tBu), 0.91 (s, 18 H, tBu); 1H[11B]-NMR (400 MHz, Acetone-d6): δ (ppm) = 7.18 (s, 4 H, ArH), 6.90 (s, 4 H, ArH), 4.38 (d, 4 H, J = 20.4 Hz, CH2), 4.20 (m, 4 H, CH2O), 3.95 (br. t, 4 H, J = 3.6 Hz, CH2O), 3.70 (br. t, 8 H, CH2O), 3.42 (d, 4 H, J = 18.0 Hz, CH2), 2.33 (br. s, 4 H, BH), 1.54 (br. s, 4 H, BH), 1.51 (br. s, 4 H, BH), 1.42 (br. s, 2 H, CHcarborane), 1.18 (br. s, 2 H, BH), 1.27 (s, 18 H, tBu), 0.91 (s, 18 H, tBu); 0.51 (br. s, 2 H, BH), −0.40 (br. s, 2 H, μ-H); 13C-NMR (100.6 MHz, Acetone-d6) δ (ppm) = 150.6 (ArC), 146.9 (ArC), 141.6 (ArC), 132.8 (ArC), 127.9 (ArC), 125.8 (ArC), 125.5 (ArC), 75.8 (CH2O), 72.9 (CH2O), 69.4 (CH2O), 69.0 (CH2O), 39.1 (CHcarborane), 33.7 (CH2), 33.6 (CH2), 31.3 (tBu), 30.6 (tBu); MS (ESI−) m/z (%) found: 544.08 (100), 545.48 (24) [M]2−, calcd. 543.93 (100), 545.43 (24); Analysis found (%): C 49.15, H 6.80, calcd. for C56B18H92O8Cs2 C 49.54, H 6.84.
General Procedure for Synthesis of [10-X-(CH2-CH2O)2-nido-7,8-C2B9H11]Bu4N (X = Cl, Br, I) (11–13)
Dry diethyl ether (20 mL) was injected into flask containing the starting zwitterion 1 (270 mg, 1.22 mmol) and Bu4NX (X=Cl, Br and I) (2.50 mmol). The reaction mixture was stirred at room temperature for 24 h. Ether solutions were the washed with water (4 × 20 mL), separated, evaporated on a rotary evaporator to dryness and then dried for 3 h in vacuum. The products were then crystalized from CH2Cl2-hexane.
Data for [10-Cl-(CH2-CH2O)2-nido-7,8-C2B9H11]Bu4N (11): White solid, yield 395 mg, 65%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.2 (s, 1B, B10), −13.5 (d, 2B, J = 133 Hz, B9,11), −18.5 (d, 2B, J = 133 Hz, B5,6), −25.1 (d, 2B, J = 148 Hz, B2,4), −26.4 (d, 1B, B3), −41.7 (d, 1B, J = 140 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 3.63 (dd, 4 H, J = 9.6 Hz, CH2O), 3.50 (br. t, 2 H, CH2O), 3.46 (t, 2 H, J = 5.4 Hz, CH2Cl), 3.05 (m, 8 H, CH2N, Bu4N+), 1.56 (m, 8 H, CH2, Bu4N+), 1.50 (br. s, 2 H, CHcarborane), 1.32 (m, 8 H, CH2, Bu4N+), 0.94 (t, 12 H, CH3, Bu4N+); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 3.63 (dd, 4 H, J = 9.6 Hz, CH2O), 3.50 (br. t, 2 H, CH2O), 3.46 (t, 2 H, J = 5.4 Hz, CH2), 3.05 (m, 8 H, CH2N, Bu4N+), 1.88 (br. s, 2 H, BH), 1.56 (m, 8 H, CH2, Bu4N+), 1.50 (br. s, 2 H, CHcarborane), 1.32 (m, 8 H, CH2, Bu4N+), 0.94 (t, 12 H, CH3, Bu4N+), 1.41 (br. s, 2 H, BH), 1.17 (br. s, 2 H, BH), 0.45 (br. s, 1 H, BH), −0.50 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 72.3 (CH2), 70.9 (CH2), 69.3 (CH2), 58.39 (CH2N, Bu4N+), 43.6 (CH2), 38.4 (CHcarborane), 23.5 (CH2, Bu4N+), 19.4 (CH2, Bu4N+), 12.9 (CH3, Bu4N+); MS (ESI-) m/z (%) found: 256.32 (100), 257.28 (65) [M]−, calcd. 256.19 (100), 257.19 (67); Analysis found (%): C 52.96, H 10.66, N 2.70 calcd. for C22B9H55O2ClN C 53.01, H 11.12, N 2.80.
Data for [10-Br-(CH2-CH2O)2-nido-7,8-C2B9H11]Bu4N (12): White solid, yield 456 mg, 69%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.2 (s, 1B, B10), −13.5 (d, 2B, J = 135 Hz, B9,11), −18.5 (d, 2B, J = 130 Hz, B5,6), −25.1 (d, 2B, J = 148 Hz, B2,4), −26.4 (d, 1B, J = 161 Hz, B3), −41.7 (d, 1B, J = 137 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 3.71 (t, 2 H, J = 6.0 Hz, CH2O), 3.49 (br t, 2 H, CH2O), 3.47 (m, 4 H, J = 9.6 Hz, CH2O, CH2Br), 3.06 (m, 8 H, CH2N, Bu4N+), 1.57 (m, 8 H, CH2, Bu4N+), 1.50 (br. s, 2 H, CHcarborane), 1.32 (m, 8 H, CH2, Bu4N+), 0.94 (t, 12 H, CH3, Bu4N+); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 3.71 (t, 2 H, J = 6.0 Hz, CH2O), 3.49 (br t, 2 H, CH2O), 3.47 (m, 4 H, J = 9.6 Hz, CH2O, CH2Br), 3.06 (m, 8 H, CH2N, Bu4N+), 2.21 (br. s, 1 H, BH), 1.57 (m, 8 H, CH2, Bu4N+), 1.50 (br. s, 2 H, CHcarborane), 1.32 (m, 8 H, CH2, Bu4N+), 1.03 (br. s, 4 H, BH) 0.94 (t, 12 H, CH3, Bu4N+); 0.26 (br. s, 1 H, BH), −0.64 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 71.9 (CH2), 70.8 (CH2), 69.3 (CH2), 58.4 (CH2N, Bu4N+, CH2), 38.2 (CHcarborane), 23.4 (CH2, Bu4N+), 19.4 (CH2, Bu4N+), 13.0 (CH3, Bu4N+); MS (ESI−) m/z (%) found: 300.32 (100), 302.20 (90) [M]−, calcd. 300.15 (100), 301.14 (92); Analysis found (%): C 48.61, H 10.18, N 2.52 calcd. for C22B9H55O2BrN C 48.67, H 10.21, N 2.58.
Data for [10-I-(CH2-CH2O)2-nido-7,8-C2B9H11]Bu4N (13): White solid, yield 516 mg, 71%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.4 (s, 1B, B10), −13.5 (d, 2B, J = 135 Hz, B9,11), −18.5 (d, 2B, J = 133 Hz, B5,6), −25.0 (d, 2B, J = 149 Hz, B2,4), −26.3 (d, 1B, J = 169 Hz, B3), −41.6 (d, 1B, J = 138 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 3.66 (t, 2 H, J = 6.0 Hz, CH2O), 3.53 (br t, 2 H, CH2O), 3.47 (t, 2 H, J = 5.4 Hz, CH2O), 3.26 (t, 2 H, J = 6.3 Hz, CH2I), 3.05 (m, 8 H, CH2N, Bu4N+), 1.57 (m, 8 H, CH2, Bu4N+), 1.52 (br. s, 2 H, CHcarborane), 1.33 (m, 8 H, CH2, Bu4N+), 0.94 (t, 12 H, CH3, Bu4N+); 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 3.66 (t, 2 H, J = 6.0 Hz, CH2O), 3.53 (br t, 2 H, CH2O), 3.47 (t, 2 H, J = 5.4 Hz, CH2O), 3.26 (t, 2 H, J = 6.3 Hz, CH2I), 3.08 (m, 8 H, CH2N, Bu4N+), 1.88 (br. s, 1 H, BH), 1.56 (m, 8 H, CH2, Bu4N+), 1.50 (br. s, 2 H, CHcarborane), 1.58 (m, 8 H, CH2, Bu4N+), 1.53 (br. s, 4 H, BH), 1.20 (br. s, 2 H, BH), 1.01 (t, 12 H, CH3, Bu4N+), 0.97 (br. s, 1 H, BH), 0.23 (br. s, 1 H, BH), −0.64 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 71.5 (CH2O), 71.4 (CH2), 69.4 (CH2), 58.4 (CH2N, Bu4N+, CH2), 38.9 (CHcarborane), 23.4 (CH2, Bu4N+), 19.4 (CH2, Bu4N+), 13.0 (CH3, Bu4N+); MS (ESI-) m/z (%) found: 348.16 (100), 349.16 (45) [M]−, calcd. 348.13 (100), 349.13 (48); Analysis found (%): C 44.62, H 9.38, N 2.34 calcd. for C22B9H55O2IN C 44.80, H 9.40, N 2.37.
[10-HS-(CH2-CH2O)2-nido-7,8-C2B9H11]− (14−) and [S-(10,10’-(CH2-CH2O)2-nido-7,8-C2B9H11)2]2−
Distilled THF (60 mL) was injected into flask containing the starting zwitterion 1 (423 mg, 1.92 mmol). 724 mg of NaHS.H2O was added the reaction mixture was stirred at room temperature for 24 h. The mixture was extracted by ether, evaporated on a rotary evaporator to dryness and then dried for 3 h in vacuum. This mixture was separated by column chromatography on silica gel using CH2Cl2-CH3CN from 5:1 to 1:1 b.v. isolating 14− and 152−. 14− and 152− were dried under vacuum for 8 h at 80 °C.
Data for Na14: White solid, yield 137 mg, 26%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.5 (s, 1B, B10), −13.4 (d, 2B, J = 137 Hz, B9,11), −18.5 (d, 2B, J = 133 Hz, B5,6), −24.9 (d, 2B, J = 150 Hz, B2,4), −26.2 (d, 1B, J = 168 Hz, B3), −41.6 (d, 1B, J = 142 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 3.51 (t, 4 H, J = 6.0 Hz, CH2O), 3.44 (t, 2 H, J = 5.0 Hz, CH2O), 2.62 (q, 2 H, J = 7.0 Hz, CH2S), 1.70 (t, 1H, J = 8.0 Hz, SH), 1.54 (br. s, 2 H, CHcarborane). 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 3.51 (t, 4 H, J = 6.0 Hz, CH2O), 3.44 (t, 2 H, J = 5.0 Hz, CH2O), 2.62 (q, 2 H, J = 7.0 HzHz, CH2S), 1.70 (t, 1H, J = 8.0 Hz, SH), 2.03 (br. s, BH), 1.54 (br. s, 2 H, CHcarborane), 1.33 (br. s, BH), 1.21 (br. s, BH), 1.02 (br. s, BH), −0.61 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 72.4, 71.4, 69.1, 38.4 (CHcarborane), 23.69. MS (ESI−) m/z (%) found: 254.32 (100), 255.32 (45) [M]−, calcd. 254.21 (100), 255.20 (48); Analysis found (%): C 25.97, H 7.02 calcd. for C6B9H20O2SNa C 26.06, H 7.29.
Data for Na215: White solid, yield 27 mg, 5%: 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = 11B-NMR (192.6 MHz, CD3CN) δ (ppm) = −10.8 (s, 2B, B10), −13.4 (d, 4B, J = 137 Hz, B9,11), −18.5 (d, 4B, J = 133 Hz, B5,6), −24.9 (d, 4B, J = 150 Hz, B2,4), −26.2 (d, 2B, J = 168 Hz, B3), -41.6 (d, 2B, J = 161 Hz, B1); 1H-NMR (600 MHz, CD3CN): δ (ppm) = 3.59 (t, 2 H, J = 7.0 Hz, CH2O), 3.56 (br. t, 2 H, CH2O), 3.48 (t, 2 H, J = 5.0 Hz), 2.71 (t, 2H, J = 6.0 Hz, CH2S), 1.57 (br. s, 2 H, CHcarborane). 1H[11B]-NMR (400 MHz, CD3CN): δ (ppm) = 3.59 (t, 2 H, J = 7.0 Hz, CH2O), 3.56 (br. t, 2 H, CH2O), 3.48 (t, 2 H, J = 5.0 Hz), 2.71 (t, 2H, J = 6.0 Hz, CH2S), 2.06 (br. s, BH), 1.35 (br. s, BH), 1.57 (br. s, 2 H, CHcarborane), 1.23 (br. s, BH), 1.04 (br. s, BH), −0.55 (br. s, 1 H, μ-H); 13C-NMR (100.6 MHz, CD3CN) δ (ppm) = 71.3, 69.7, 68.9, 38.9 (CHcarborane), 31.2. MS (ESI−) m/z (%) found: 496.56 (100), 497.60 (72) [M]−, calcd. 496. 42 (100), 497.41 (82); Analysis found (%): C 27.46, H 7.41 calcd. for C12B18H38O4S1Na2 C 27.77, H 7.38.
4. Conclusions
In this article we have been focussing on the basic chemistry of the dioxane derivative 1 of the [nido-C2B9H12]− ion. Ring cleavage reactions with various nucleophilic reagents have been explored, revealing various patterns in reactivity that we have documented in this manuscript. Unlike in previously reported reactions of compounds that contain a cyclic ether attached via an oxonium atom to other (closo-) boron cages, the zwitterion 1 exhibits some different features that apparently are a consequence of its nido-structure. These differences are connected with the presence of the extra hydrogen atom, its unexpectedly easy abstraction from the molecule and dual reaction pathways observed for the resulting anion, which exchanges dioxane ring with OH− as the nucleophile. On the other hand, attacks of nucleophiles such as NH2R, Cl−, Br−, I−, S−, SH−, and RO− proceed at the carbon atom in the α-position relative to the oxonium atom of the ether ring and produce compounds in which the terminal nucleophile moiety is bound to the polyhedral cage via a six-atom diethyleneglycol pendant group. Therefore, the neutral compound 1 and products of its reactions described herein can serve as valuable building blocks for a variety of purposes in the construction of advanced functional molecules, materials, compounds for BNCT, drug design, etc. They also offer the possibility to serve as ligands in synthesis of a broad range of new metal dicarbollide complexes. The advantage of all the compounds described herein is the symmetric position of the substituent on the cage, which may be of high relevance for the synthesis of biologically active compounds.
Supplementary Materials
The following are available online: CIF file for crystallographic structures of compounds 1 and 2, Crystallographic collection data, Table S1: NMR and MS spectral data for compounds 2 to 15, Figures S1–S16 and Figures S17–S33, resp.
Author Contributions
Investigation, M.B. and S.E.A.; methodology and writing—review and editing, S.E.A.; methodology, formal analysis and data curation D.B. and Z.R.; X-ray data curation, Z.R.; conceptualization, writing—original draft preparation, review and supervision, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by from the Czech Science Foundation Grant Number. 18-27648S.
Acknowledgments
Support from Institutional Support No. 61388980 and Program PPLZ number L200321851 for Suzan El Anwar, Ph.D. from Czech Academy of Sciences is highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hawthorne, M.F.; Young, D.C.; Garrett, P.M.; Owen, D.A.; Schwerin, S.G.; Tebbe, F.N.; Wegner, P.A. Preparation and characterization of (3)-1,2- and (3)-1,7-dicarbadodecahydroundecaborate(-1) Ions. J. Am. Chem. Soc. 1968, 90, 862–865. [Google Scholar] [CrossRef]
- Plešek, J.; Heřmánek, S.; Štíbr, B. Potassium Dodecahydro-7,8-Dicarba-Nido-Undecaborate(1-), K[7,8-C2B9H12], Intermediates, Stock Solution, and Anhydrous Salt. Inorg. Synth. 1983, 22, 231–234. [Google Scholar]
- Grimes, R.N. Eleven-Vertex Carboranes. In Carboranes, 3rd ed.; Academic Press Ltd-Elsevier Science Ltd.: London, UK, 2016; pp. 179–247. [Google Scholar]
- Frank, R.; Adhikari, A.K.; Auer, H.; Hey-Hawkins, E. Electrophile-Induced Nucleophilic Substitution of the nido-Dicarbaundecaborate Anion nido-7,8-C2B9H12-by Conjugated Heterodienes. Chem. Eur. J. 2014, 20, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Young, D.C.; Howe, D.V.; Hawthorne, M.F. Ligand derivatives of (3)-1, 2-dicarbadodecahydroundecaborate (-1). J. Am Chem. Soc. 1969, 91, 859–862. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Kalinin, V.N.; Zhigareva, G.G. Oxidation of dicarbadodecahydroundecaborate anions by mercuric-chloride in tetrahydrofuran and pyridine. Bull. Acad. Sci. USSR 1979, 28, 2198–2199. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Abramova, E.N.; Lobanova, I.A.; Sivaev, I.B.; Bragin, V.I.; Petrovskii, P.V.; Tsupreva, V.N.; Sorokina, O.V.; Bregadze, V.I. Synthesis of functional derivatives of 7,8-dicarba-nido-undecaborate anion by ring-opening of its cyclic oxonium derivatives. Collect. Czech. Chem. Commun. 2007, 72, 1676–1688. [Google Scholar] [CrossRef]
- Řezáčová, P.; Řezáčová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Šašková, K.G.; et al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. J. Med. Chem. 2009, 52, 7132–7141. [Google Scholar]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008, 977–992. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Kulikova, N.Y.; Nizhnik, E.A.; Vichuzhanin, M.V.; Starikova, Z.A.; Semioshkin, A.A.; Bregadze, V.I. Practical synthesis of 1,4-dioxane derivative of the closo-dodecaborate anion and its ring opening with acetylenic alkoxides. J. Organomet. Chem. 2008, 693, 519–525. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Cyclic Oxonium Derivatives as an Efficient Synthetic Tool for the Modification of Polyhedral Boron Hydrides. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 623–637. [Google Scholar]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press, Elsevier: London, UK, 2016; pp. 1–1041. [Google Scholar]
- Dash, B.P.; Satapathy, R.; Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Hosmane, N.S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849, 170–194. [Google Scholar] [CrossRef]
- Plešek, J.; Heřmánek, S.; Franken, A.; Císařová, I.; Nachtigal, C. Dimethyl sulfate induced nucleophilic substitution of the bis(1,2-dicarbollido)-3-cobalt(1-) ate ion. Syntheses, properties and structures of its 8,8′-mu-sulfato, 8-phenyl and 8-dioxane derivatives. Collect. Czech. Chem. Commun. 1997, 62, 47–56. [Google Scholar] [CrossRef]
- Matveev, E.Y.; Akimov, S.S.; Kubasov, A.S.; Nichugovskii, A.I.; Nartov, A.S.; Retivov, V.M.; Zhizhina, K.Y.; Kuznetsov, N.T. Reaction of the B10H9O2C4H8 (-) anion with C-nucleophiles. Russ. J. Inorg. Chem. 2017, 62, 808–813. [Google Scholar] [CrossRef]
- El Anwar, S.; Laila, Z.; Ramsubhag, R.; Tlais, S.; Safa, A.; Dudley, G.; Naoufal, D. Synthesis and characterization of click-decahydrodecaborate derivatives by the copper(I) catalyzed 3+2 azide-alkyne cycloaddition reaction. J. Organomet. Chem. 2018, 865, 89–94. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Semioshkin, A.A.; Brellochs, B.; Sjoberg, S.; Bregadze, V.I. Synthesis of oxonium derivatives of the dodecahydro-closo-dodecaborate anion B12H12 (2-). Tetramethylene oxonium derivative of B12H12 (2-) as a convenient precursor for the synthesis of functional compounds for boron neutron capture therapy. Polyhedron 2000, 19, 627–632. [Google Scholar] [CrossRef]
- Bernard, R.; Cornu, D.; Gruner, B.; Dozol, J.F.; Miele, P.; Bonnetot, B. Synthesis of B12H12 (2-) based extractants and their application for the treatment of nuclear wastes. J. Organomet. Chem. 2002, 657, 83–90. [Google Scholar] [CrossRef]
- Bernard, R.; Cornu, D.; Perrin, M.; Scharff, J.P.; Miele, P. Synthesis and X-ray structural characterisation of the tetramethylene oxonium derivative of the hydrodecaborate anion. A versatile route for derivative chemistry of B10H10 (2-). J. Organomet. Chem. 2004, 689, 2581–2585. [Google Scholar] [CrossRef]
- Grűner, B.; Císařová, I.; Čáslavský, J.; Bonnetot, B.; Cornu, D. Synthesis of 12-hydroxy and 12-dioxane derivatives of the closo-1-carbadodecaborate(1-) ion. Variations on the Plesek’s cobalt bis(dicarbollide) pattern. Collect. Czech. Chem. Commun. 2002, 67, 953–964. [Google Scholar] [CrossRef]
- Oleshkevich, E.; Teixidor, F.; Rosell, A.; Vinas, C. Merging Icosahedral Boron Clusters and Magnetic Nanoparticles: Aiming toward Multifunctional Nanohybrid Materials. Inorg. Chem. 2018, 57, 462–470. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Polyhedral boron clusters in materials science. N. J. Chem. 2011, 35, 1955–1972. [Google Scholar] [CrossRef]
- Llop, J.; Masalles, C.; Viñas, C.; Teixidor, F.; Sillanpää, R.; Kivekäs, R. The 3,3′-Co(1,2-C2B9H11)(2) (-) anion as a platform for new materials: Synthesis of its functionalized monosubstituted derivatives incorporating synthons for conducting organic polymers. Dalton Trans. 2003, 556–561. [Google Scholar] [CrossRef]
- Nuñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef] [PubMed]
- Rais, J.; Grűner, B. Extraction with Metal bis(Dicarbollide) Anions; Metal bis(Dicarbollide) Extractants and their Applications in Separation Chemistry. In Ion Exchange, Solvent Extraction, 1st ed.; Marcus, Y., SenGupta, A.K., Eds.; Marcel Dekker: New York, NY, USA, 2004; Volume 17, pp. 243–334. [Google Scholar]
- Grűner, B.; Rais, J.; Selucký, P.; Lučaníkova, M. Recent Progress in Extraction Agents Based on Cobalt Bis(Dicarbollides) for Partitioning of Radionuclides from High-Level Nuclear Waste. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; Crc Press-Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 463–490. [Google Scholar]
- Hao, E.; Vicente, M.G.H. Expeditious synthesis of porphyrin-cobaltacarborane conjugates. Chem. Commun. 2005, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.H.; Jensen, T.J.; Courtney, B.H.; Vicente, M.G.H. Synthesis and cellular studies of porphyrin-cobaltacarborane conjugates. Bioconjugate Chem. 2005, 16, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, A.B.; Plešek, J.; Kříž, O.; Lesnikowski, Z.J. A nucleoside conjugate containing a metallacarborane group and its incorporation into a DNA oligonucleotide. Angew. Chem. Int. Edit. 2003, 42, 5740–5743. [Google Scholar] [CrossRef] [PubMed]
- Lesnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef] [PubMed]
- Gabel, D. Boron clusters in medicinal chemistry: Perspectives and problems. Pure Appl. Chem. 2015, 87, 173–179. [Google Scholar] [CrossRef]
- Semioshkin, A.; Bregadze, V.I.; Godovikov, I.; Ilinova, A.; Laskova, Z.J.; Starikova, Z.A. Synthesis and structure of 1-iodo-7-dioxonium-decahydro-closo-dodecaborate. J. Organomet. Chem. 2011, 696, 2760–3765. [Google Scholar] [CrossRef]
- Schaffran, T.; Lissel, F.; Samatanga, B.; Karlsson, G.; Burghardt, A.; Edwards, K.; Winterhalter, M.; Peschka-Suss, R.; Schubert, R.; Gabel, D. Dodecaborate cluster lipids with variable headgroups for boron neutron capture therapy: Synthesis, physical-chemical properties and toxicity. J. Organomet. Chem. 2009, 694, 1708–1712. [Google Scholar] [CrossRef]
- Cígler, P.; Kožíšek, M.; Rezáčova, P.; Brynda, J.; Otwinowski, Z.; Pokorná, J.; Plešek, J.; Grűner, B.; Dolečková-Marešová, L.; Máša, M.; et al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc. Natl. Acad. Sci. USA 2005, 102, 15394–15399. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.V. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur. J. Inorg. Chem. 2009, 1433–1450. [Google Scholar] [CrossRef]
- Řezáčová, P.; Cigler, P.; Matějíček, P.; Lepšík, M.; Pokorná, J.; Grűner, B.; Konvalinka, J. Medicinal Application of Carboranes Inhibition of HIV Protease. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 41–70. [Google Scholar]
- Prikaznov, A.V.; Las’kova, Y.N.; Semioshkin, A.A.; Sivaev, I.B.; Kisin, A.V.; Bregadze, V.I. Synthesis of boron-containing tyrosine derivatives based on the closo-decaborate and closo-dodecaborate anions. Russ. Chem. Bull. 2011, 60, 2550–2554. [Google Scholar] [CrossRef]
- Neumann, W.; Xu, S.; Sarosi, M.B.; Scholz, M.S.; Crews, B.C.; Ghebreselasie, K.; Banerjee, S.; Marnett, L.J.; Hey-Hawkins, E. nido-Dicarbaborate Induces Potent and Selective Inhibition of Cyclooxygenase-2. ChemMedChem 2016, 11, 175–178. [Google Scholar] [CrossRef]
- Dabrowska, A.; Matuszewski, M.; Zwolinski, K.; Ignaczak, A.; Olejniczak, A.B. Insight into lipophilicity of deoxyribonucleoside-boron cluster conjugates. Eur. J. Pharm. Sci. 2018, 111, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Brynda, J.; Mader, P.; Šícha, V.; Fábry, M.; Poncová, K.; Bakardiev, M.; Grűner, B.; Cígler, P.; Řezáčová, P. Carborane-Based Carbonic Anhydrase Inhibitors. Angew. Chem.-Int. Edit. 2013, 52, 13760–13763. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.C.; Lee, S.S.; Knobler, C.B.; Hawthorne, M.F. Synthesis of Charge-compensated Dicarbollide Ligand Precursors and their Use in the Preparation of Novel Metallacarboranes. Inorg. Chem. 1991, 30, 2024–2031. [Google Scholar] [CrossRef]
- Timofeev, S.V.; Sivaev, I.B.; Prikaznova, E.A.; Bregadze, V.I. Transition metal complexes with charge-compensated dicarbollide ligands. J. Organomet. Chem. 2014, 751, 221–250. [Google Scholar] [CrossRef]
- Tarres, M.; Viñas, C.; Gonzalez-Cardoso, P.; Hanninen, M.M.; Sillanpää, R.; Dorďovic, V.; Uchman, M.; Teixidor, F.; Matějíček, P. Aqueous Self-Assembly and Cation Selectivity of Cobaltabisdicarbollide Dianionic Dumbbells. Chem. Eur. J. 2014, 20, 6786–6794. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Kazakov, G.S.; Sivaev, I.B.; Bregadze, V.I. Synthesis of podands with nido-carboranyl groups as a basis for construction of crown ethers with an incorporated metallacarborane moiety. Russ. Chem. Bull. 2013, 62, 699–704. [Google Scholar] [CrossRef]
- Kazakov, G.S.; Stogniy, M.Y.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Kirilin, A.D.; Bregadze, V.I. Synthesis of crown ethers with the incorporated cobalt bis(dicarbollide) fragment. J. Organomet. Chem. 2015, 798, 196–203. [Google Scholar] [CrossRef]
- Nekvinda, J.; Rozycka, D.; Rykowski, S.; Wyszko, E.; Fedoruk-Wyszomirska, A.; Gurda, D.; Orlicka-Plocka, M.; Giel-Pietraszuk, M.; Kiliszek, A.; Rypniewski, W.; et al. Synthesis of naphthalimide-carborane and metallacarborane conjugates: Anticancer activity, DNA binding ability. Bioorgan. Chem. 2020, 94, 16. [Google Scholar] [CrossRef]
- Grűner, B.; Brynda, J.; Das, V.; Šícha, V.; Štěpánková, J.; Nekvinda, J.; Holub, J.; Pospíšilová, K.; Fabry, M.; Pachl, P.; et al. Metallacarborane Sulfamides: Unconventional, Specific, and Highly Selective Inhibitors of Carbonic Anhydrase IX. J. Med. Chem. 2019, 62, 9560–9575. [Google Scholar] [CrossRef] [PubMed]
- El Anwar, S.; Assaf, K.I.; Begaj, B.; Samsonov, M.A.; Růžičková, Z.; Holub, J.; Bavol, D.; Nau, W.M.; Gabel, D.; Grűner, B. Versatile, one-pot introduction of nonahalogenated 2-ammonio-decaborate ions as boron cluster scaffolds into organic molecules; host-guest complexation with gamma-cyclodextrin. Chem. Commun. 2019, 55, 13669–13672. [Google Scholar] [CrossRef] [PubMed]
- Peymann, T.; Kuck, K.; Gabel, D. Ring opening of tetrahydropyran attached to undecahydro-closo-dodecaborate(1-) by nucleophiles. Inorg. Chem. 1997, 36, 5138–5139. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Starikova, Z.A.; Sjoberg, S.; Bregadze, V.I. Synthesis of functional derivatives of the 3,3′-Co(1,2-C2B2H11)(2) (-) anion. J. Organomet. Chem. 2002, 649, 1–8. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 5–89. [Google Scholar]
- Heřmánek, S. NMR as a tool for elucidation of structures and estimation of electron distribution in boranes and their derivatives. Inorg. Chim. Acta 1999, 289, 20–44. [Google Scholar] [CrossRef]
- Heřmánek, S. B-11 NMR-spectra of boranes, main-group heteroboranes, and substituted derivatives–Factors influencing chemical-shifts of skeletal atoms. Chem. Rev. 1992, 92, 325–362. [Google Scholar]
- Grimes, R.N. Metallacarboranes of the Transition and Lanthanide Elements. In Carboranes, 3rd ed.; Academic Press, Elsevier: London, UK, 2016; pp. 711–903. [Google Scholar]
- Holub, J.; Grűner, B.; Perekalin, D.S.; Golovanov, D.G.; Lyssenko, K.A.; Petrovskii, P.V.; Kudinov, A.R.; Štíbr, B. Synthesis and rearrangements of aminosubstituted ferra- and ruthenatricarbaboranes. Inorg. Chem. 2005, 44, 1655–1659. [Google Scholar] [CrossRef]
- Grűner, B.; Bačkovský, J.; Sillanpää, R.; Kivekäs, R.; Císařová, I.; Teixidor, F.; Viñas, C.; Štíbr, B. Amino-substituted ferra-bis(tricarbollides)—Metallatricarbaboranes designed for linear molecular constructions. Eur. J. Inorg. Chem. 2004, 1402–1410. [Google Scholar] [CrossRef]
- Grűner, B.; Lehtonen, A.; Kivekäs, R.; Sillanpää, R.; Holub, J.; Teixidor, F.; Viñas, C.; Štíbr, B. Unusual 9 -> 10 rearrangement of the substituted cage carbon in the ferratricarbollide series. Synthesis of the isomeric complexes 2-eta(5)-(C5H5)-10-X-closo-2,1,7,10-FeC3B8H10 (where X = H2N, MeHN, Me2N, and Bu(t)HN). Inorg. Chem. 2000, 39, 2577–2580. [Google Scholar] [CrossRef]
- Bakardjiev, M.; Holub, J.; Hnyk, D.; Císařová, I.; Londesborough, M.G.S.; Perekalin, D.S.; Štíbr, B. Structural dualism in the zwitterionic 7-RR′ NH-nido-7,8,9-C3B8H10 tricarbollide series: An example of absolute tautomerism. Angew. Chem. Int. Edit. 2005, 44, 6222–6226. [Google Scholar] [CrossRef]
- Štíbr, B. On Tautomerism. The Story of Absolute Tautomerism, an Unique Phenomenon in Chemistry. Chem. Listy 2008, 102, 902–905. [Google Scholar]
- Frank, R.; Auer, H.; Hey-Hawkins, E. Functionalisation of the nido-dicarbaborate anion nido-7,8-C2B9H12- by hydride abstraction. J. Organomet. Chem. 2013, 747, 217–224. [Google Scholar] [CrossRef]
- Plešek, J.; Grüner, B.; Heřmánek, S.; Báča, J.; Mareček, V.; Janchenová, J.; Lhotský, A.; Holub, K.; Selucký, P.; Rais, J.; et al. Synthesis of functionalized cobaltacarboranes based on the closo-(1,2-C2B9H11)(2)-3,3′-Co (-) ion bearing polydentate ligands for separation of M3+ cations from nuclear waste solutions. Electrochemical and liquid-liquid extraction study of selective transfer of M3+ metal cations to an organic phase. Molecular structure of the closo- (8-(2-CH3O-C5H4-O)- (CH2CH2O)(2)-1,2-C2B9H10)-(1’,2’-C2B9H11)-3,3′-Co Na determined by X-ray diffraction analysis. Polyhedron 2002, 21, 975–986. [Google Scholar]
- Plešek, J.; Grűner, B.; Macháček, J.; Císařová, I.; Čáslavský, J. 8-Dioxane ferra(III) bis(dicarbollide): A paramagnetic functional molecule as versatile building block for introduction of a Fe(III) centre into organic molecules. J. Organomet. Chem. 2007, 692, 4801–4804. [Google Scholar] [CrossRef]
- Grűner, B.; Mikulášek, L.; Báča, J.; Císařová, I.; Böhmer, V.; Danila, C.; Reinoso-Garcia, M.M.; Verboom, W.; Reinhoudt, D.N.; Casnati, A.; et al. Cobalt bis(dicarbollides)(1-) covalently attached to the calix 4 arene platform: The first combination of organic bowl-shaped matrices and inorganic metallaborane cluster anions. Eur. J. Org. Chem. 2005, 2022–2039. [Google Scholar] [CrossRef]
- Farras, P.; Teixidor, F.; Sillanpää, R.; Viñas, C. A convenient synthetic route to useful monobranched polyethoxylated halogen terminated 3,3-Co(1,2-C2B9H11)(2) (-) synthons. Dalton Trans. 2010, 39, 1716–1718. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Maguire, J.A.; Zhu, Y.; Takagaki, M. Boron and Gadolinium Neutron Capture Therapy for Cancer Treatment, 1st ed.; World Scientific Co., Pte, Ltd.: Hackensack, NJ, USA, 2012. [Google Scholar]
- Šubrtová, V.; Petříček, V.; Hummel, L. Structure of the zwitterionic 10- 2-(2-Dimethylsulfonioethoxy undecahydro-7,8-dicarba-nido-uncecaborate)(1-). Acta Crystallogr. C 1989, 45, 1964–1966. [Google Scholar] [CrossRef]
- Shmal’ko, A.V.; Anufriev, S.A.; Anisimov, A.A.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of cobalt and nickel 6,6-diphenylbis(dicarbollides). Russ. Chem. Bull. 2019, 68, 1239–1247. [Google Scholar] [CrossRef]
- Grűner, B.; Plzák, Z. High-performance liquid chromatographic separations of boron- cluster compounds. J. Chromatogr. A 1997, 789, 497–517. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).