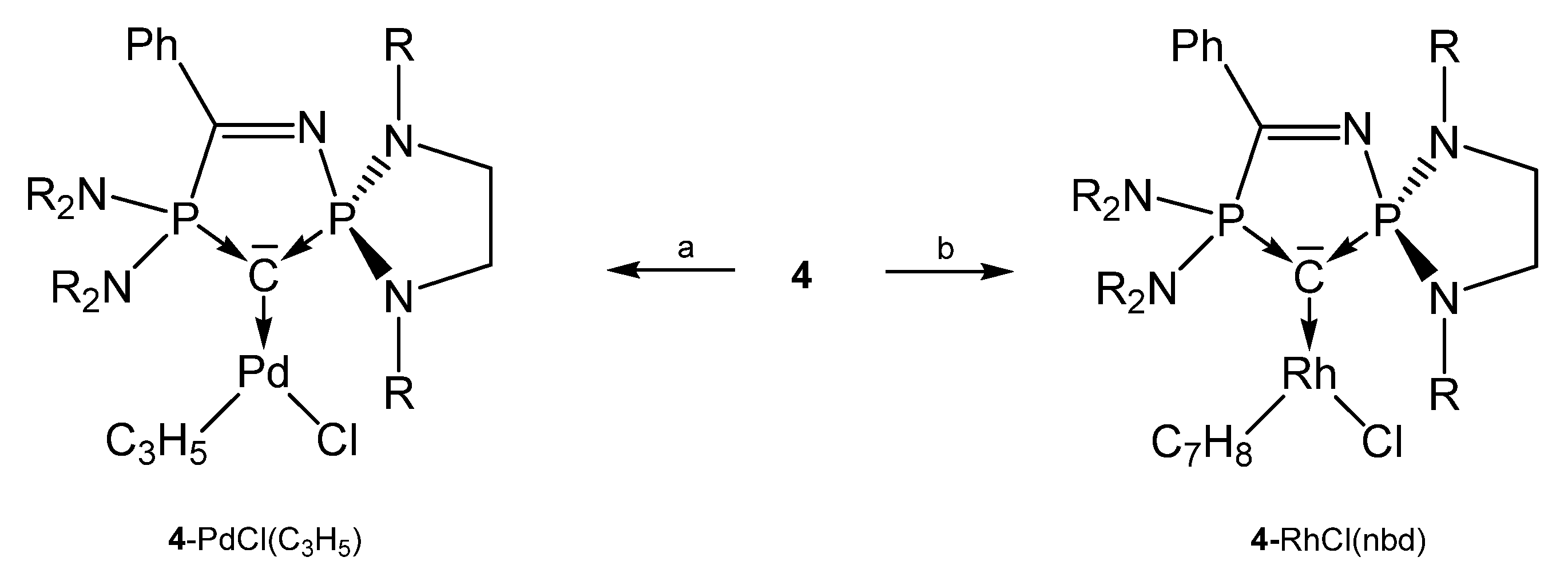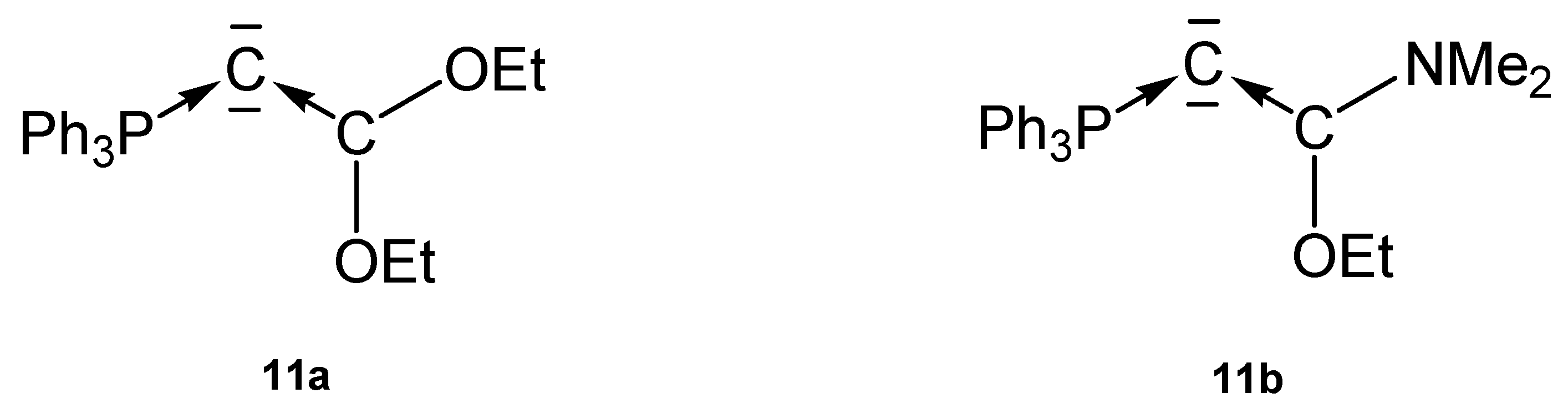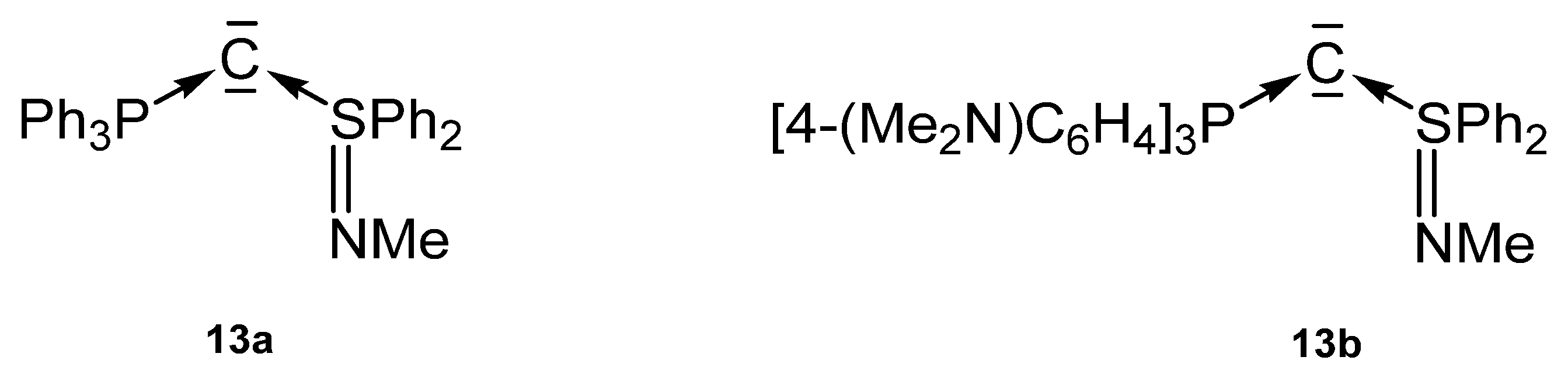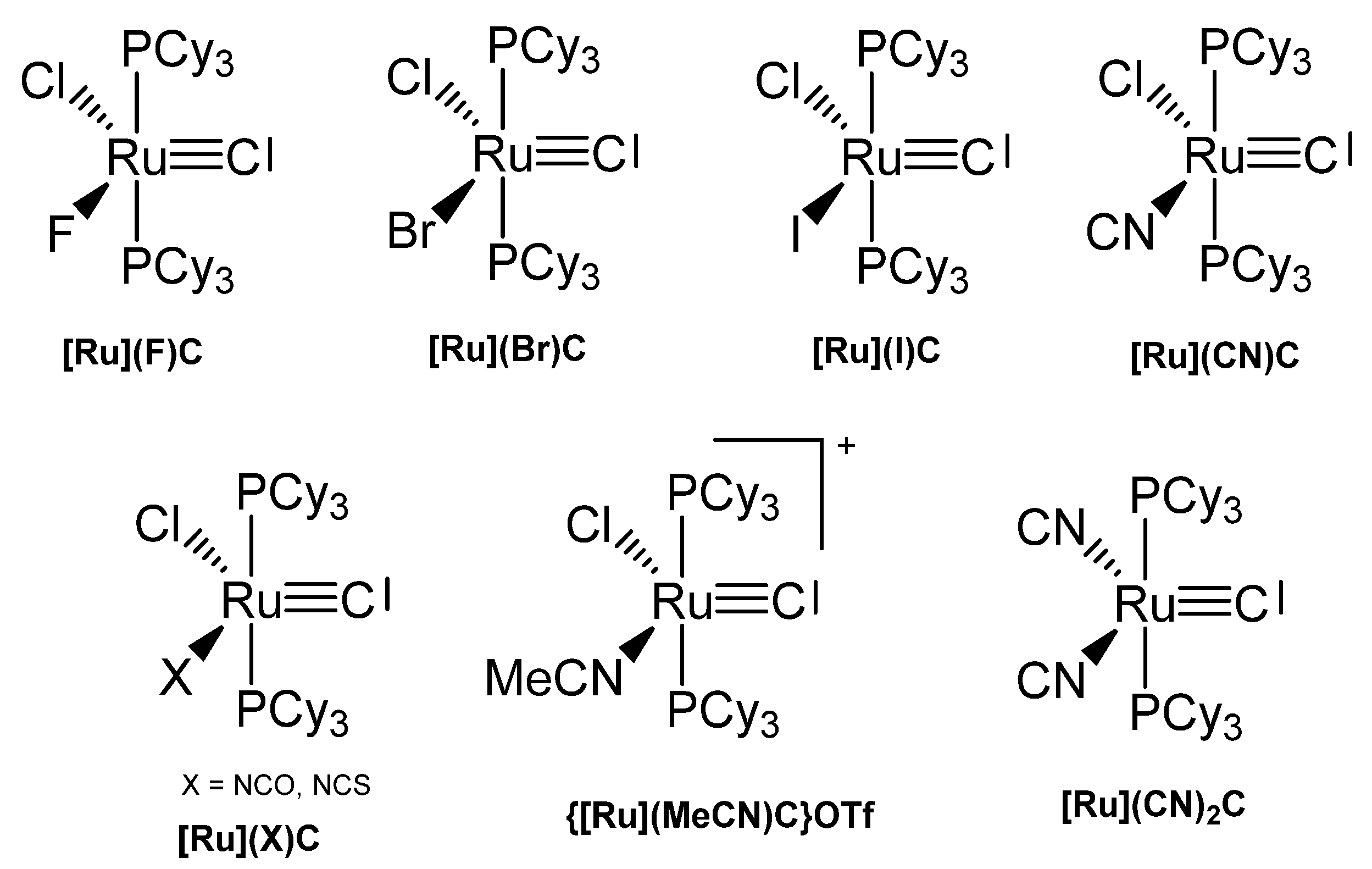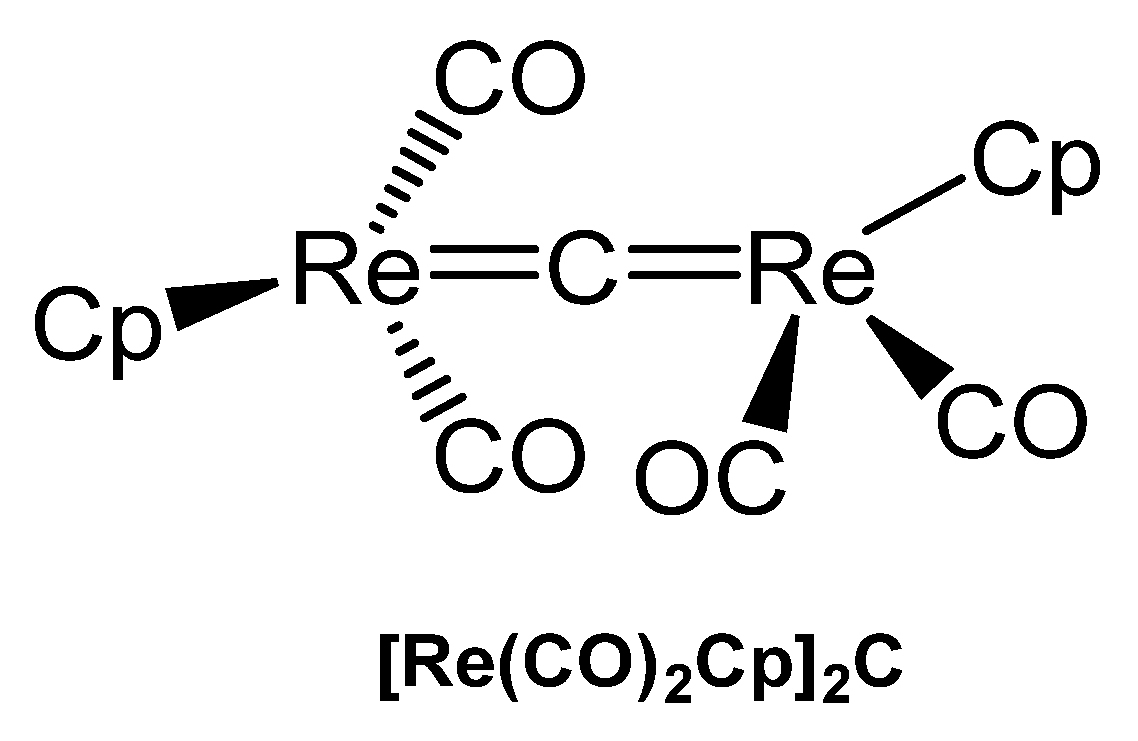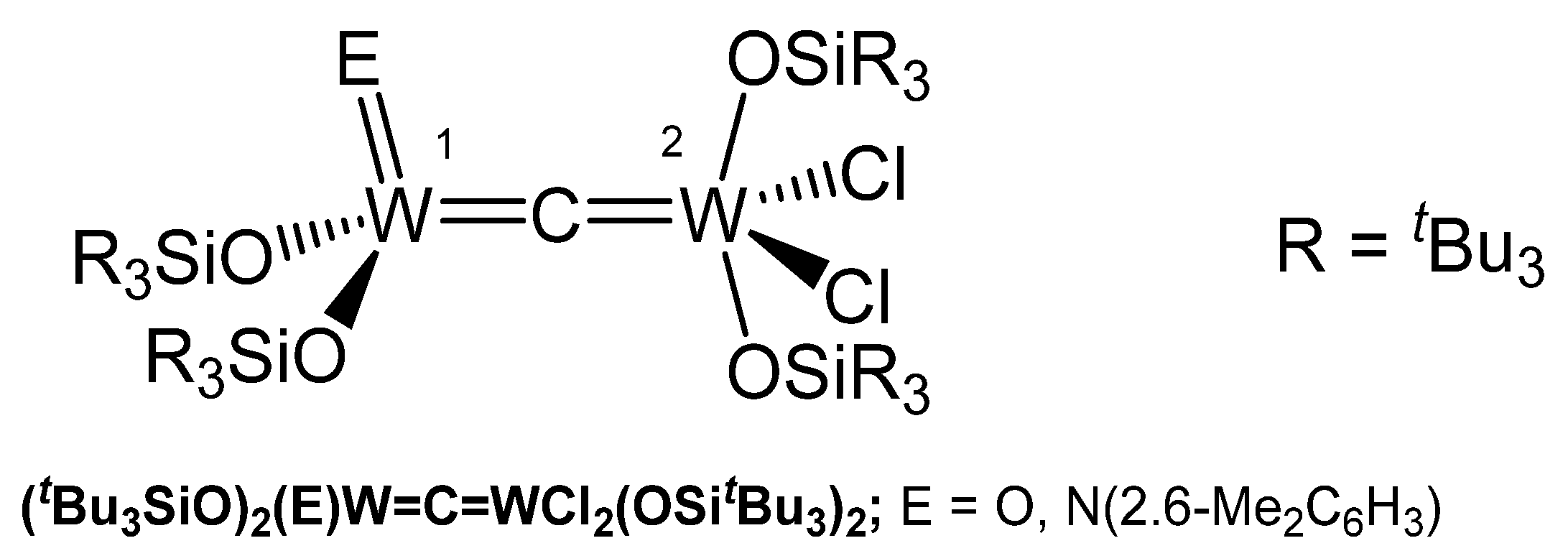Abstract
This review summarizes experimental and theoretical studies of transition metal complexes with two types of novel metal-carbon bonds. One type features complexes with carbones CL2 as ligands, where the carbon(0) atom has two electron lone pairs which engage in double (σ and π) donation to the metal atom [M]⇇CL2. The second part of this review reports complexes which have a neutral carbon atom C as ligand. Carbido complexes with naked carbon atoms may be considered as endpoint of the series [M]-CR3 → [M]-CR2 → [M]-CR → [M]-C. This review includes some work on uranium and cerium complexes, but it does not present a complete coverage of actinide and lanthanide complexes with carbone or carbide ligands.
1. Introduction
Transition metal compounds with metal-carbon bonds are the backbone of organometallic chemistry. Molecules with M-C single bonds are already known since 1849 when Frankland reported the accidental synthesis of diethyl zinc while attempting to prepare free ethyl radicals [1,2]. Molecules with a [M]=CR2 double bond (carbene complexes) or a [M]≡CR triple bond (carbyne complexes) were synthesized much later [3,4,5,6]. Two types of compounds with metal-carbon double or triple bonds having different types of bonds are generally distinguished, which are named after the people who isolated them first. Fischer-type carbene and carbyne complexes are best described in terms of dative bonds following the Dewar–Chatt–Duncan (DCD) model [7,8] [M]⇄CR2 and [M(─)]  CR(+), whereas Schrock-type alkylidenes and alkylidynes are assumed to have electron-sharing double and triple bonds [M]=CR2 and [M]≡CR [9,10,11].
CR(+), whereas Schrock-type alkylidenes and alkylidynes are assumed to have electron-sharing double and triple bonds [M]=CR2 and [M]≡CR [9,10,11].
 CR(+), whereas Schrock-type alkylidenes and alkylidynes are assumed to have electron-sharing double and triple bonds [M]=CR2 and [M]≡CR [9,10,11].
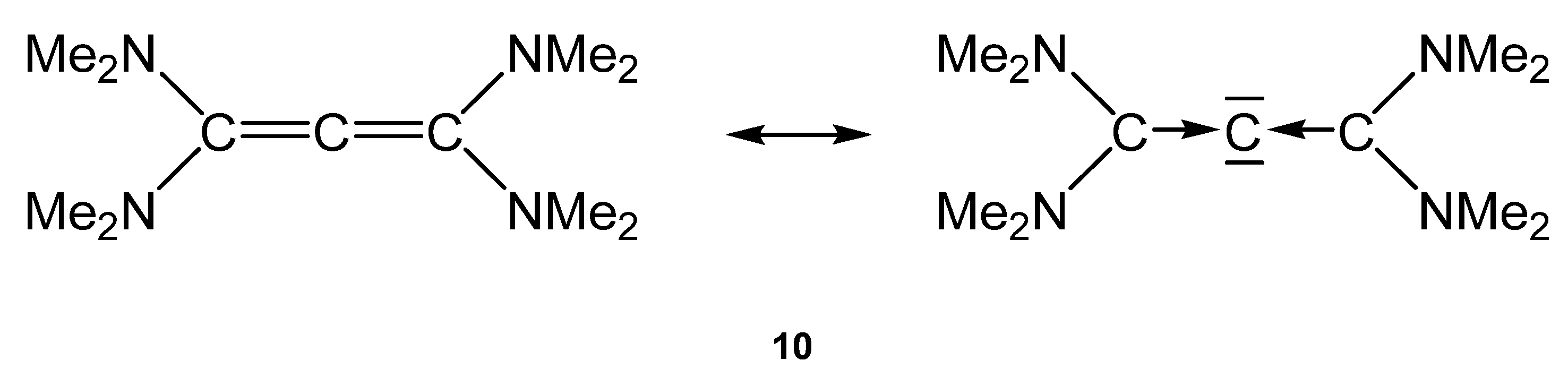
CR(+), whereas Schrock-type alkylidenes and alkylidynes are assumed to have electron-sharing double and triple bonds [M]=CR2 and [M]≡CR [9,10,11].This review deals with transition metal complexes with metal-carbon bonds to two types of ligands, which have only recently been isolated and theoretically studied. One type of ligand are carbones CL2 [12], which are carbon(0) compounds with two dative bonds to a carbon atom in the excited 1D state L→←L where the carbon atom retains its four valence electrons as two lone pairs that can serve as four-electron donors [13,14]. Thus, carbones CL2 are four-electron donor ligands whereas carbenes CR2 are two-electron donors. Carbenes have a formally [15] vacant p(π) orbital that can accept electrons in donor-acceptor complexes M⇄CR2 whereas carbones are double (σ and π) donors in complexes [M]⇇CL2. A good Lewis acid acceptor fragment A for a carbene complex has a vacant σ orbital and an occupied π orbital whereas a suitable acceptor for a carbone is a double Lewis acid with vacant σ and π orbitals as shown in Figure 1a,b. If the Lewis acid A has an occupied π orbital, it would lead to π repulsion with the π lone pair of the carbone CL2, whereby the repulsive interaction is reduced if L is a good π acceptor (Figure 1c). The two electron lone pairs of a carbone may bind to one or two monodentate Lewis acids A or protons or to a single bidentate Lewis acid as shown in Figure 1. The large second proton affinity is a characteristic feature of carbones, which distinguishes them from carbenes [16]. Examples of all cases are known and are described below.
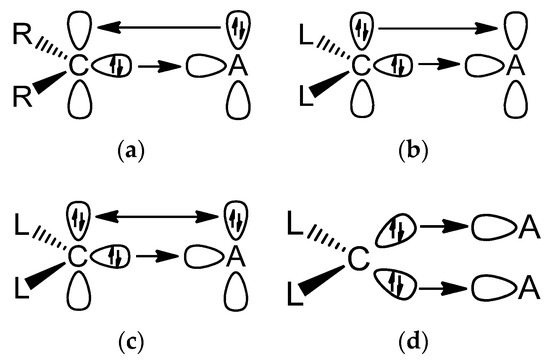
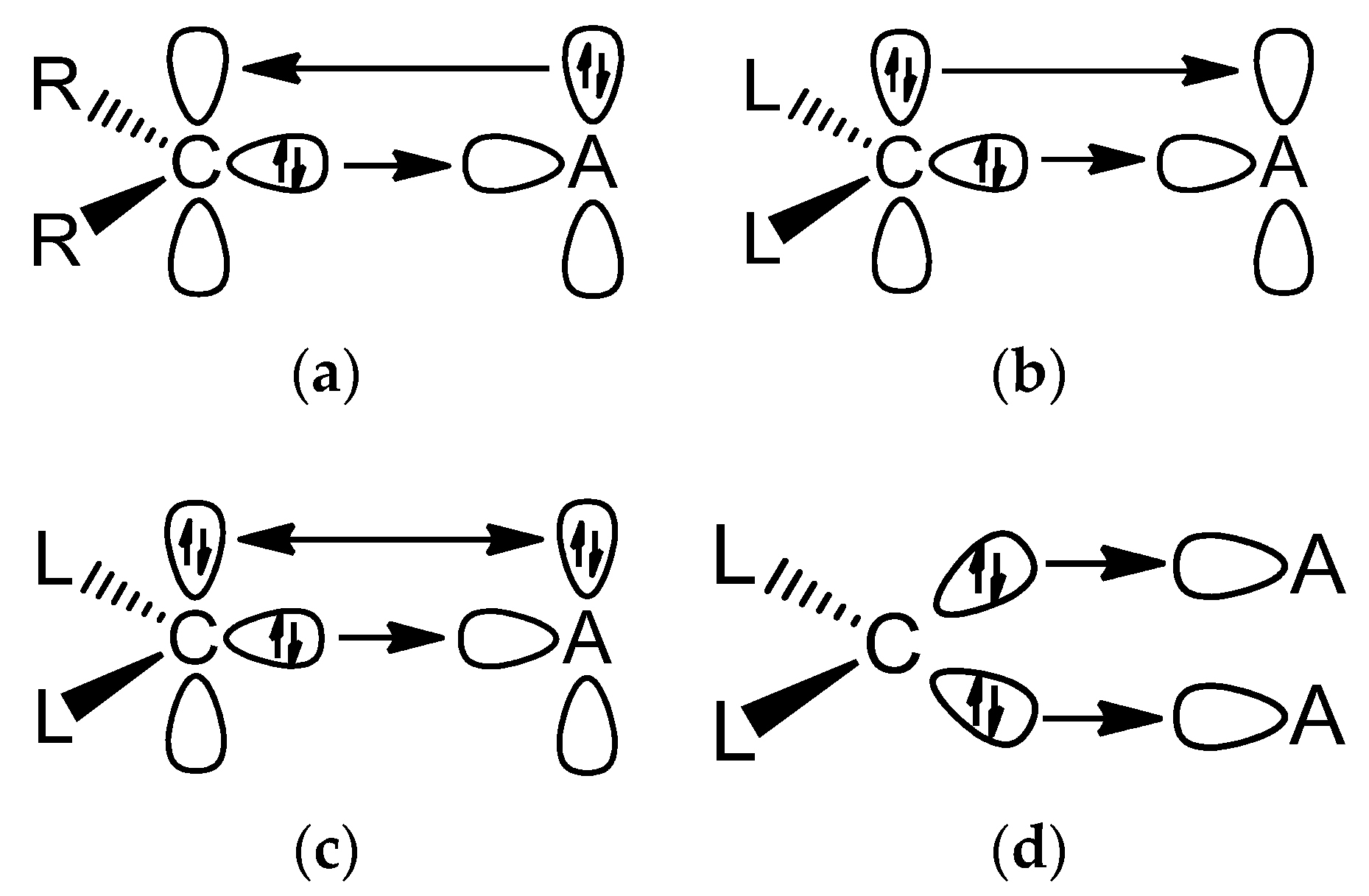
Figure 1.
Schematic representation of the most important orbital interactions between carbene ligands CR2 and carbones CL2 with Lewis acids A.(a) Carbene complex with a monodentate Lewis acid; (b) Carbone with a bidentate Lewis acid; (c) Carbone with a monodentate Lewis acid; (d) Carbone with two monodentate Lewis acids.
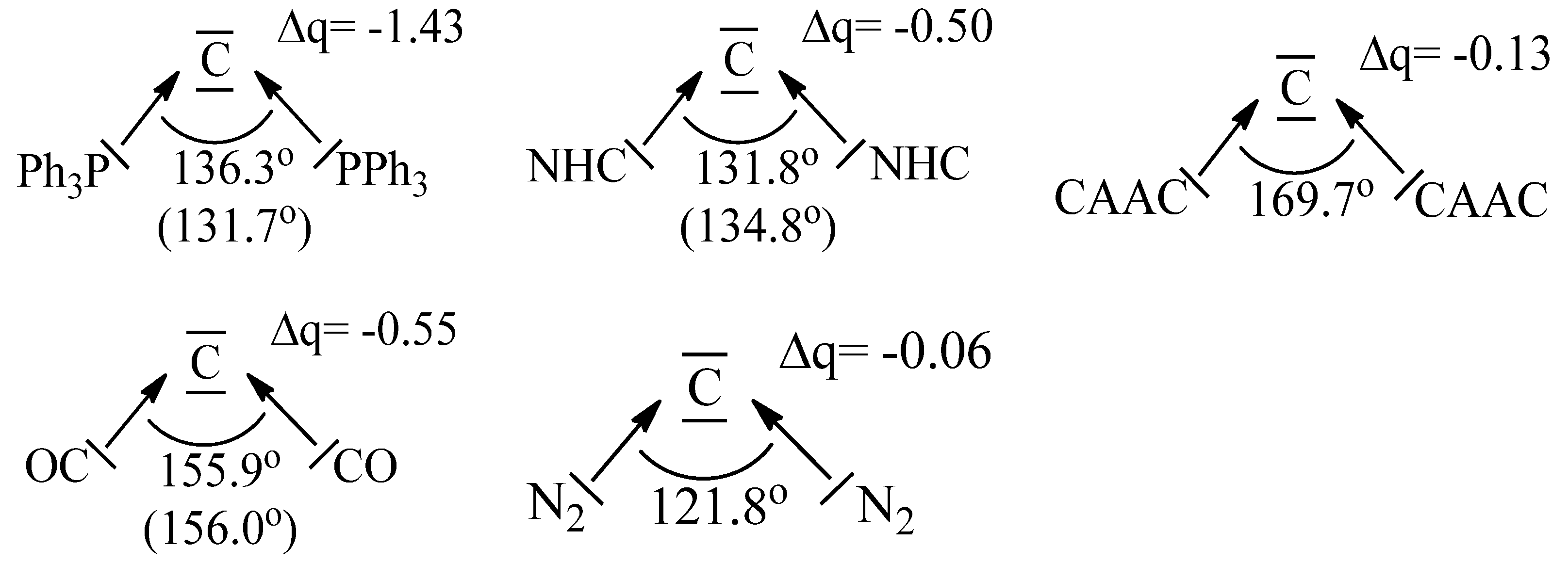
It is important to realize that the two electron lone-pairs of a carbone CL2 may additionally engage in π-backdonation to the ligands L whose strength depends on the availability of vacant π orbitals of the ligands L. Stronger π acceptor ligands L enhance the π-backdonation L←→L which leads to wider bending angles at the carbon atom (Figure 2). The significant bending of free C(CO)2 [17,18] can straightforwardly be explained in terms of dative bonding in carbon suboxide C3O2 [19,20]. The π-acceptor strength of ligands L thus modulates the donor interaction of the carbone CL2.
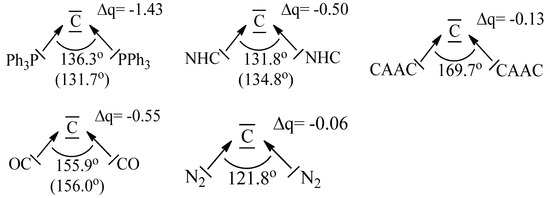
Figure 2.
Calculated and (in parentheses) experimental bond angles of carbones CL2 with different ligands L and partial charges Δq of the divalent carbon atom. The data are taken from [19].
The following list gives some essential features of carbones and their differences to carbenes. At the same time we want to stress that the distinction between carbenes and carbones are just a useful classification of compounds, which are a helpful model to explain the structures and reactivity of molecules. Nature does not exhibit a strict distinction line and there are complexes with electronic structures that have intermediate features between both classes of compounds. Carbenes and carbones are two ordering principles like ionic and covalent bonding. Intermediate cases are common and yet, the two concepts are essential ingredients of chemistry. The first part of this review summarizes experimental and theoretical work about transition metal complexes with carbone ligands [M]-CL2.
- Carbones are neutral carbon(0) compounds of the general formula CL2, which possess two electron lone pairs of electrons of σ and π symmetry, respectively.
- Carbones CL2 have dative σ bonds L→ ←L and weaker π backdonation L← →L which resemble donor-acceptor bonds in transition metal complexes.
- The carbon atom of carbones has very large electron densities and thus, unusually large negative partial charges.
- In contrast to carbenes, carbones exhibit high first and second proton affinities (PAs) in the region of about 290 and 150–190 kcal/mol, respectively. The second PA is a sensitive probe for the divalent C(0) character of a CL2 molecule. Carbones can take up one and two protons with formation of [HCL2]+ cations or [H2CL2]2+ dications, respectively.
- Carbones have a bent equilibrium geometry where the bending angle becomes wider when the ligand L is a better π acceptor.
- Carbones can take up one or two monodentate Lewis acids A building the complexes A←C(L2) and A←C(L2)→A or one bidentate Lewis acid A⇇C(L2).
To the thematic of carbones several review articles were reported previously; A general overview on species that bear two lone pairs of electrons at the same C-center are summarized in [21], transition metal adducts of carbones are described in [22], and those of main group fragments in [23]. Two contributions, [24] and [25], in the series Structure and Bonding (Springer Edition) also deal with carbone transition metal addition compounds.
The second type of transition metal complexes with a carbon ligand features species with a naked neutral carbon atom as a ligand [M]-C, which can be considered as endpoint of the series [M]-CR3 → [M]-CR2 → [M]-CR → [M]-C. Complexes with negatively charged carbon ligands [M]-C─, which are isoelectronic to nitride complexes [M]-N and are termed as carbides, were synthesized in 1997 by Cummins [26]. The first neutral carbon complex [M]-C, which was prepared and structurally characterized was reported in 2002 by by Heppert and co-workers [27]. They isolated the diamagnetic 16 valence electron ruthenium complexes [(PCy3)LCl2Ru(C)] (L = PCy and 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene; Cy = Cyclohexyl) by a metathesis facilitated reaction. Quantum chemical calculations of model compounds suggested that the Ru-C bond in the complexes is best described by an electron-sharing double bond like in Schrock carbenes, which is reinforced by a donor bond [Ru]C| [28]. The field of neutral carbon complexes was systematically explored in recent years by Bendix [29]. This review summarizes in its second part the research in transition metal complexes with a naked carbon atom as ligand [M]-C that has been accomplished since 2002. The review includes some work on uranium and cerium complexes, but it does not present a complete coverage of actinide and lanthanide complexes with carbone or carbide ligands.
2. Transition Metal Complexes with Carbone Ligands [M]-CL2
2.1. Transition Metal Addition Compounds of Symmetrical Carbones C(PR3)2
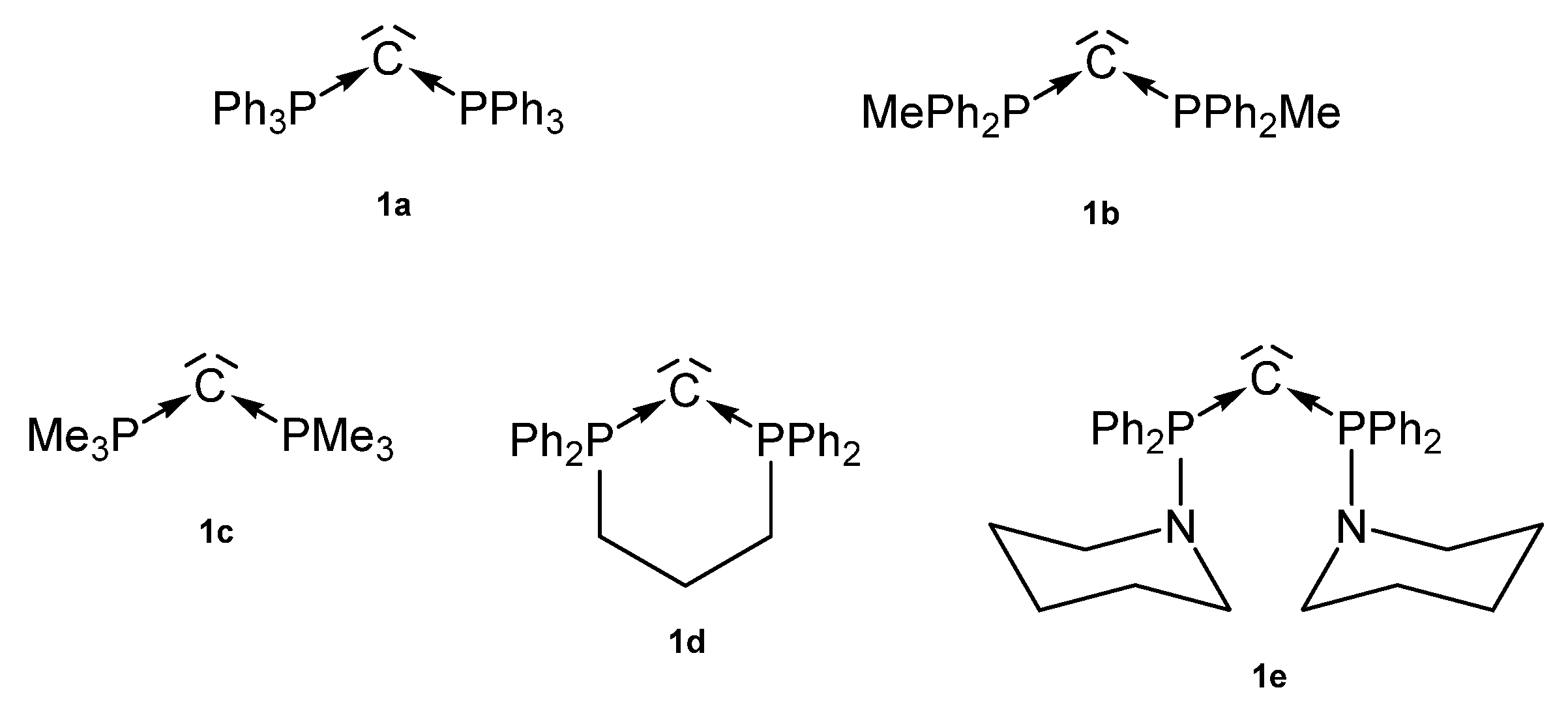
Among the existing carbones with a symmetric P-C-P skeleton, five species (1a–1e) are known today as donor ligands to various transition metal fragments as outlined in Figure 3. From other linear or bent carbones with this skeleton, no transition metal complexes are described so far.
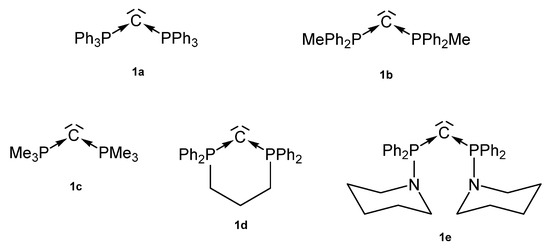
Figure 3.
Symmetric carbones 1a–1e as ligands for transition metal complexes.
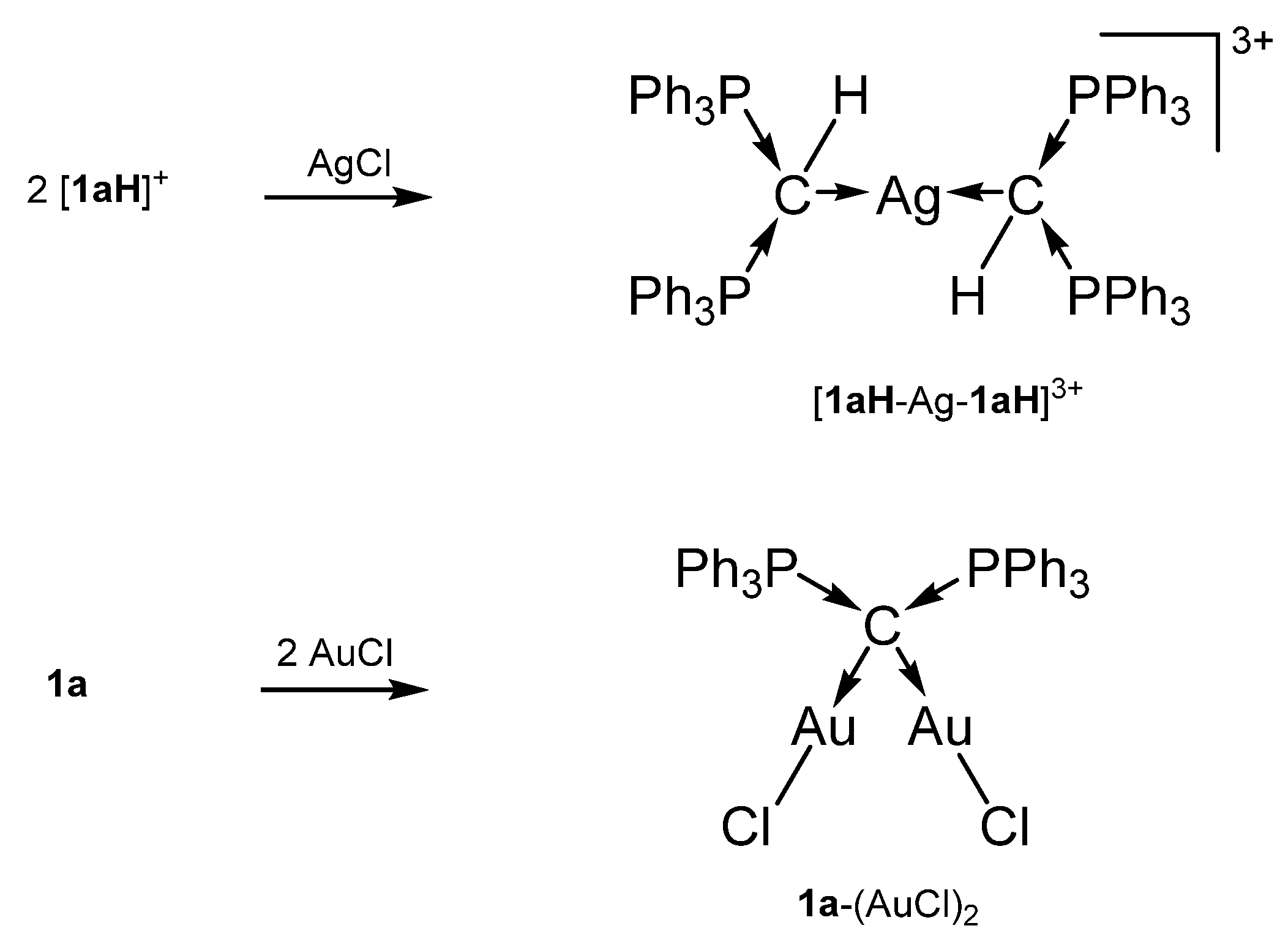
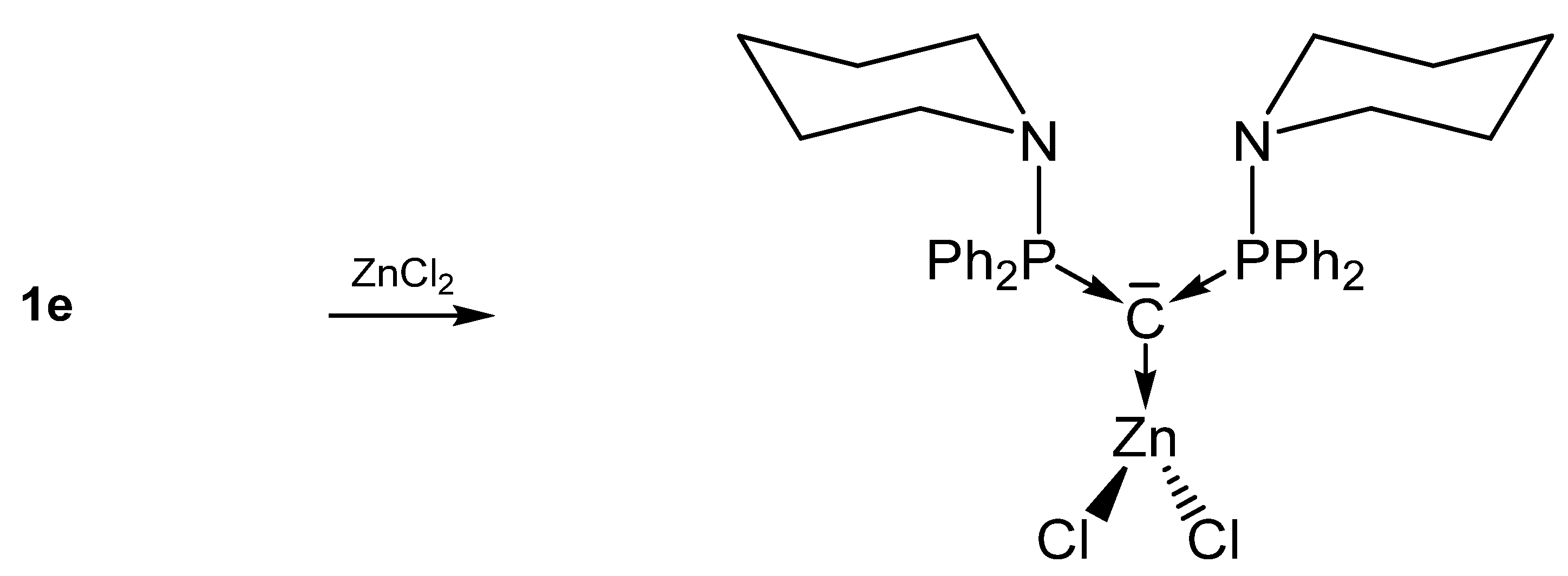
In 1961, 1a was detected by Ramirez [30], and 1b–1d stem from the laboratory of Schmidbaurs group [31]. Later on, a series of related carbones were synthesized, but for which transition metal complexes are unknown so far. Quite recently the new amino substituted carbone 1e was published together with Zn and Rh addition compounds (See Scheme 1) [32]. In the 31P NMR spectra singlets at about −4.50 (1a), −6.70 (1b), −29.6 (1c), −22.45 (1d), and 12.5 ppm (1e) confirm the symmetric array of the compounds. All carbones have a bent structure but a linear form of 1a is realized if crystallized from benzene [33,34]. 1a has a short P-C distance of 1.633(4) Å and the P-C-P angle amounts to 130.1(6)° [35]. The carbone 1b exhibits a slightly longer P-C distance of 1.648(4) Å and the introduction of two less bulky methyl groups allows a more acute P-C-P angle of 121.8(3)° [36]. 1d has similar P-C bond distances of 1.645(12) Å 1.653(14) Å and the acutest P-C-P angle in this series of 116.7(7)° [37,38]. For 1c, gas phase electron diffraction studies result in a P-C distance of 1.594(3) Å and a P-C-P angle of 147.6(5)° assuming an apparent non-linearity but linearity in the average structure [37]. All structural parameters of 1e are close to those of 1a (P-C = 1.632(2) Å, P-C-P angle = 136.5(3)° [32].
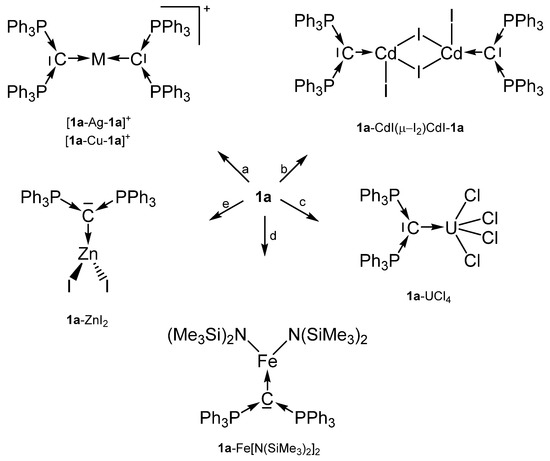
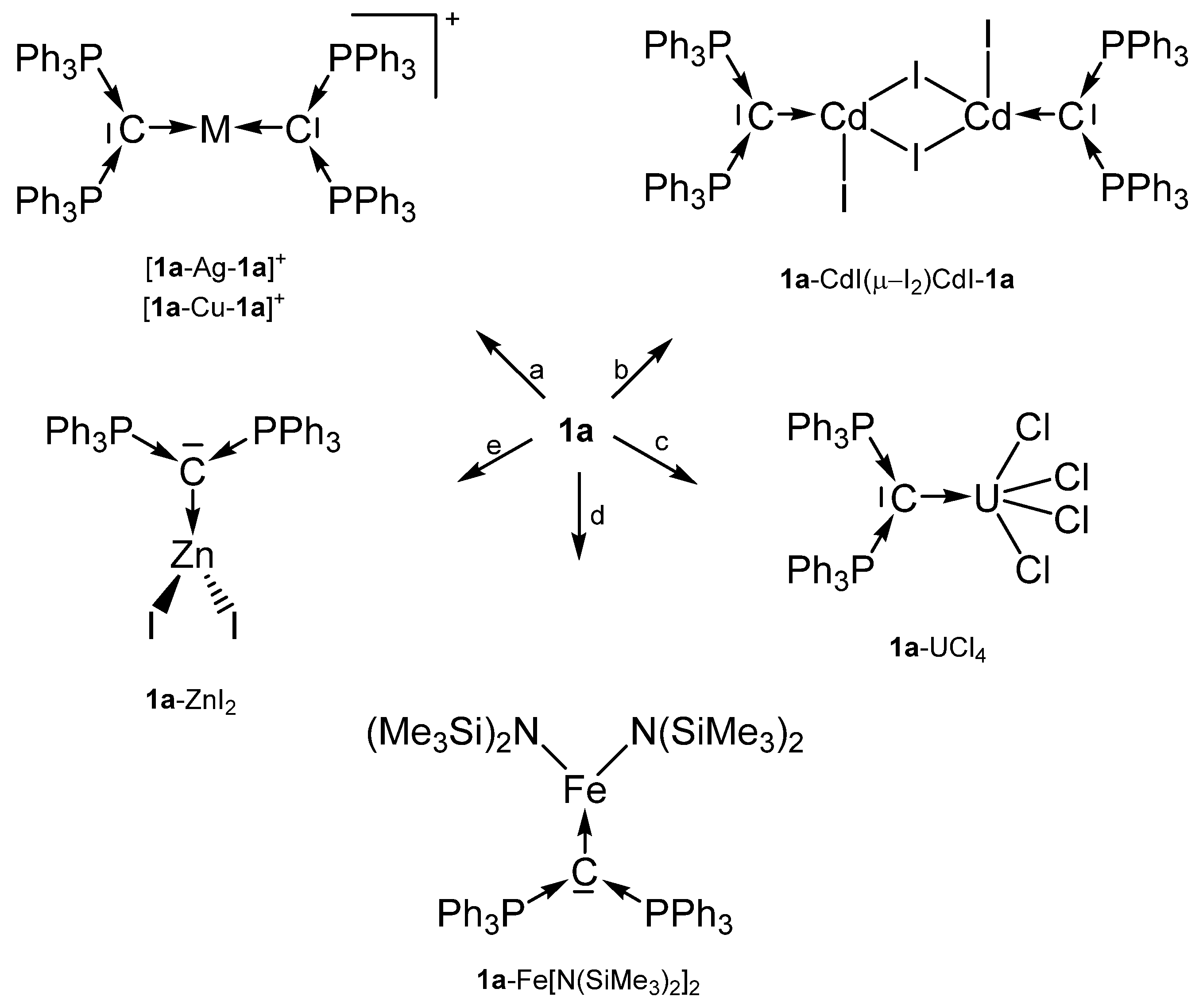
Scheme 1.
Selected transition metal compounds with the carbone 1a as two electron donor ligand; (a) MI, (b) CdI2, (c) UCl4, (d) Fe(N{SiMe3}2)2, (e) ZnI2.
In Table 1, transition metal addition compounds between carbones with the P-C-P core are collected. All compounds show longer P-C bonds than the basic carbones as consequence of the competition of the occupied p orbital at C(0) between the two P-σ* orbitals and those of A.

Table 1.
Transition metal complexes with the carbones 1a to 1e including C-M and P-C bond lengths and P-C-P angles and 31PNMR shifts in ppm.
Occupied d orbitals of Ni in the 1a-Ni(CO)3 complex elongate the C-Ni bond to a carbone (2.110 Å) [39] but this leads to a relative short bond length to a NHC (1.971 Å) moiety [57]. In contrast, UCl4 leads to a short bond to a carbone (2.411 Å) [51] indicating an appreciable U-C double bond character and a long one to a NHC base (2.612 Å) [58,59].
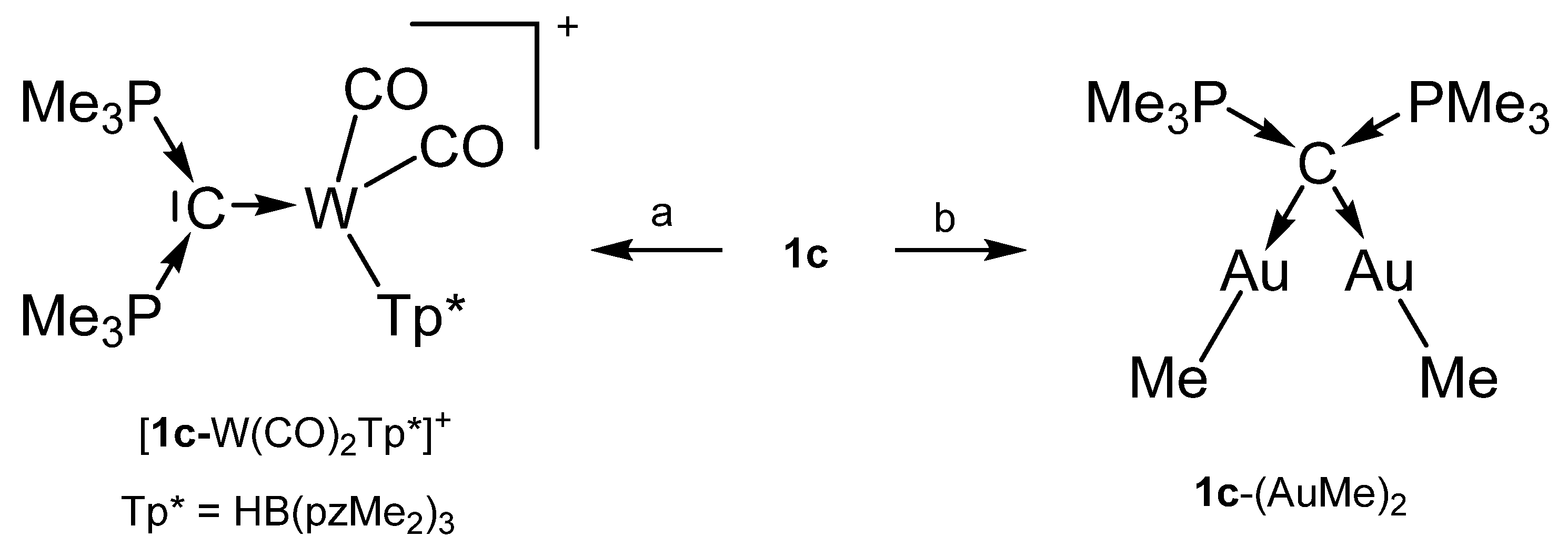
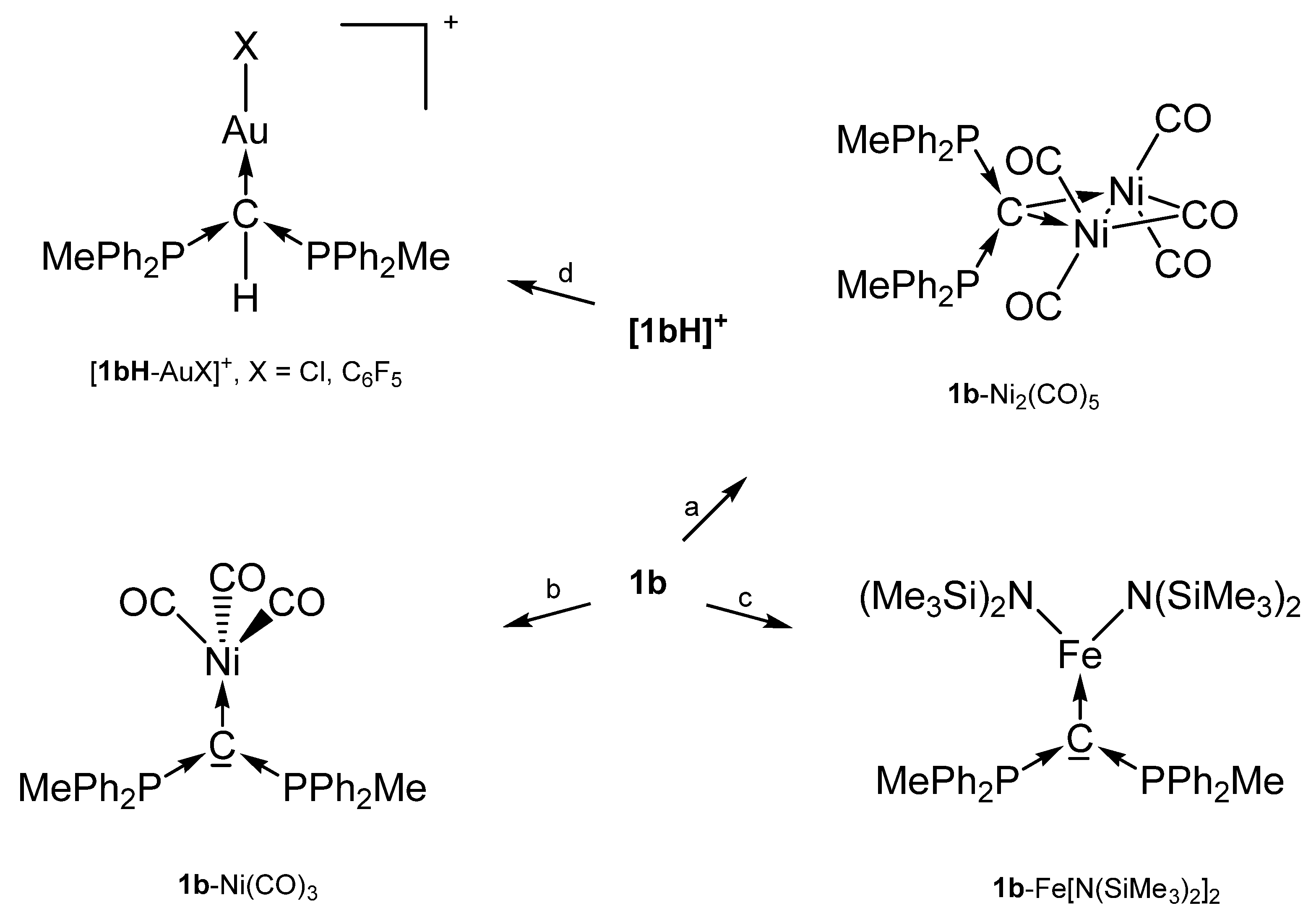
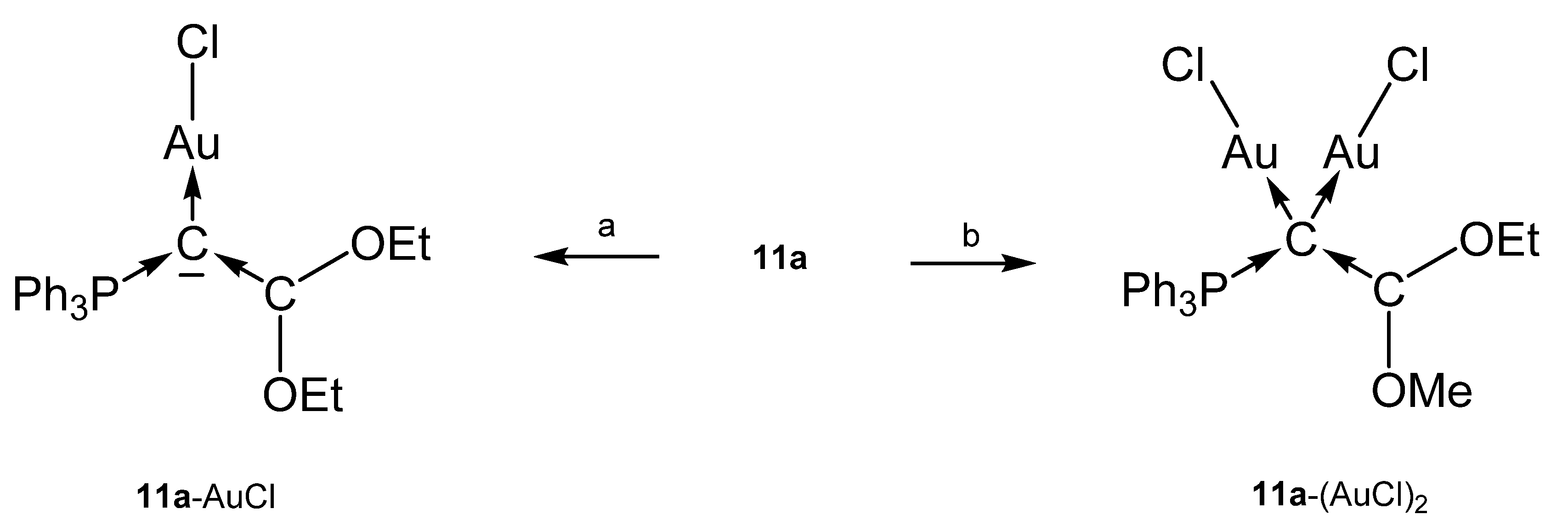
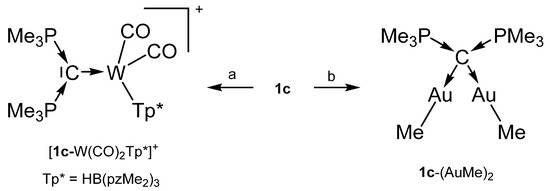
The cation [1a-ReO3]+ holds the longest one with 1.771(8) Å indicating an appreciable C=Re double bond character. This feature applies also in part to 1a-UCl4 and 1c-W(CO)2N3 with elongated P-C bonds(See Scheme 2); a partial C-U double bond is confirmed by theoretical calculations. Similar long P-C bonds are found in the trication [1aH-Ag-1aH]3+, in 1a-(AuCl)2(See Scheme 3), and in 1b-Ni2(CO)5(See Scheme 4), where the carbone provides each two electrons to two accepting Lewis acids as depicted in Figure 1d.
Scheme 2.
Transition metal complex with the carbone 1c as two and four electron donor ligand. (a) [Tp*(CO)2W≡CPMe3]+/PMe3, (b) 1c/2 MeAuPMe3.
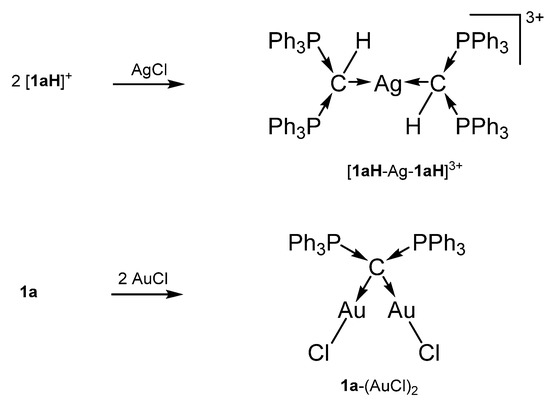
Scheme 3.
Selected transition metal compounds with the carbone 1a as four electron donor ligand.
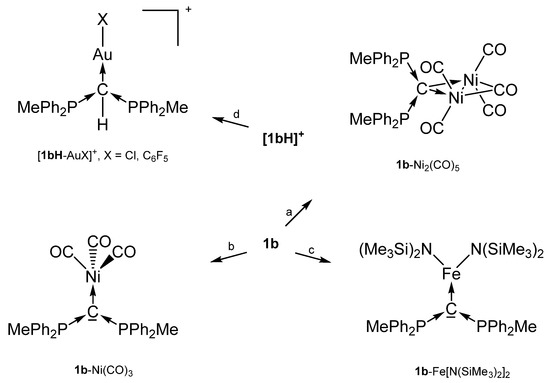
Scheme 4.
Selected transition metal complexes with the carbone 1b as two and four electron donor ligand. (a) Ni(CO)4, (b) Ni(CO)4 under CO atm, (c) Fe(N{SiMe3}2)2, (d) AuX(tht).
The P-C-P angles are in the range between 115° and 132° reflecting the required space of the appropriate Lewis acid. The 31P NMR shift of the carbone 1a amounts to about −5 ppm and those of the related addition compounds are shifted to lower fields and range between 4 ppm and 30 ppm. All iron(II) complexes of 1a and 1b are paramagnetic and 31P NMR spectra could not be obtained.
For the 31P NMR spectrum of the carbone 1b, a shift of −6.70 ppm was recorded [31]. With exception of 1b-Ni(CO)3 which resonate at 2.6 ppm, low field shifts between 12 and 22 ppm were found when 1b act as a four electron donor [40].
Further, 1e-ZnCl2 (See Scheme 5) [32] and 1a-ZnI2 [53] have closely related structural parameters but exhibit shorter C-Zn bond lengths than to related NHC-addition compounds (Δ = 0.051 Å) [60]. In both compounds a nearly perpendicular array of the ZnX2 and the PCP plane are found. No tendency for an additional N-coordination to the amino ligand of 1e is recorded for the ZnCl2 addition compound. In contrast the Rh-C distances in 1e-Rh(CO)2(acac) are longer (Δ = 0.117 Å) than in the corresponding NHC compound [61] and a partial π interaction was found by DFT calculation. Rh also shows no tendency for coordination of the adjacent amino groups [32].
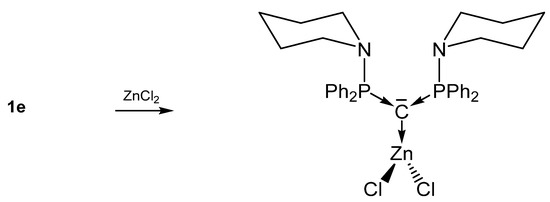
Scheme 5.
Selected transition metal complex with the carbone 1e as two electron donor ligand.
2.2. Transition Metal Addition Compounds of Carbones C(PR3)2 with an Additional Pincer Function
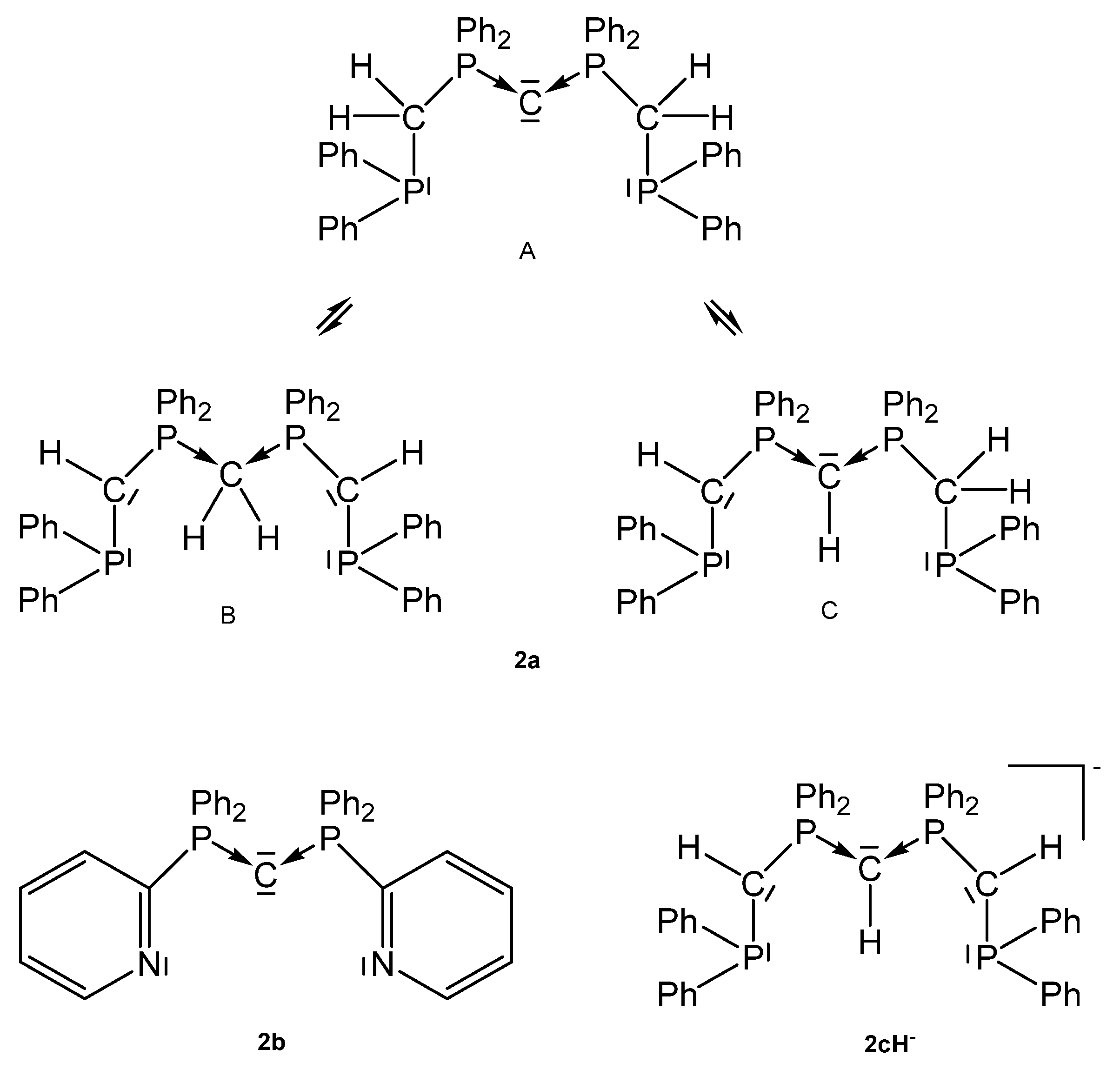
Starting material for 2a is not the free carbone Ph2P-CH2-PPh2-C-PPh2-CH2-PPh2, which could not be prepared so far, but the dication [Ph2P-CH2-PPh2-CH2-PPh2-CH2-PPh2]2+ as reported by Peringer [62]. Later on, Sundermeyer studied the deprotonation of the cation [Ph2P-CH2-PPh2-CH-PPh2-CH2-PPh2]+ by quantum chemical methods giving more or less stable tautomers of 2a, see Figure 4. Deprotonation of the tautomer C of 2a generates the anionic pincer ligand [Ph2P-CH-PPh2-CH-PPh2-CH-PPh2]− [2c]− [63]. The same working group also published the X-ray structure of the pincer ligand 2b with the P-C-P angle of 133.76(13)° and P-C distances of 1.633(2) and 1.642(2) Å; the 31P NMR shift δ = −5.6 ppm [64].
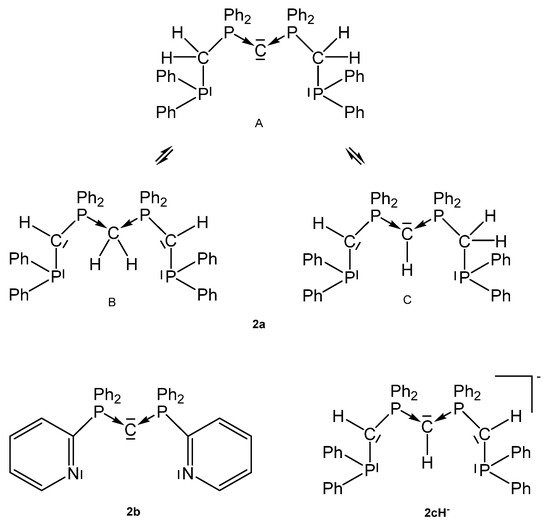
Figure 4.
Tripodal basic pincer ligand 2a with its tautomers, the anionic pincer ligand 2cH− and the pyridyl pincer ligand 2b.
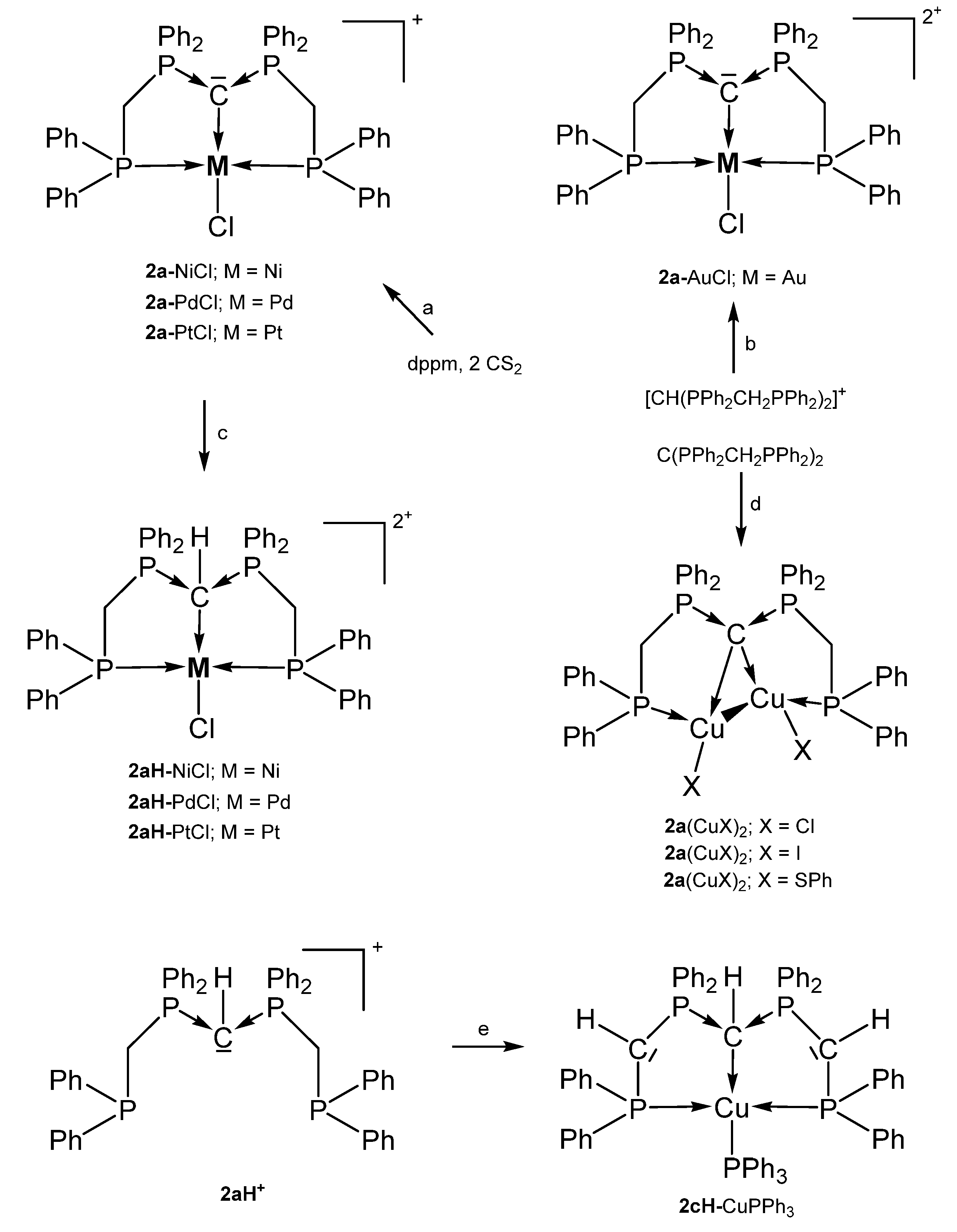
Various cationic complexes where reported with the pincer ligand 2a (See Figure 4) and group 10 metal halides and one dication with the group 11 metal Au. The 31P NMR shifts range between 32 and 41 ppm(See Table 2). As with 1a the carbone carbon atom of 2a is basic enough to accept a proton to generate complexes of the type 2aH-MCl dications with all group 10 elements (See Scheme 6).

Table 2.
Transition metal complexes with the phosphine based pincer ligands 2a and the pyridyl based pincer ligand 2b; C-M and P-C distances are included and 31P NMR shifts in ppm.
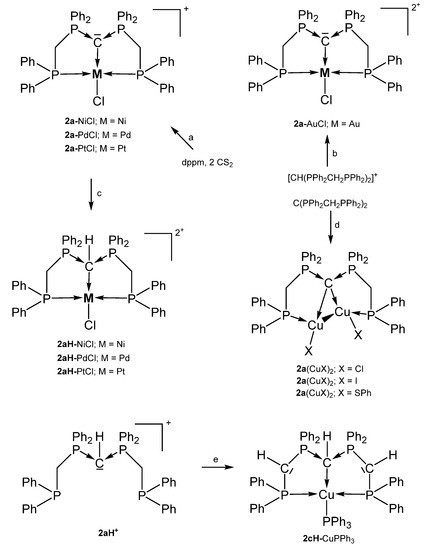
Scheme 6.
Selected compounds with the pincer ligands 2a and 2aH. (a) MCl2 with a mixture of dppm and 2 eq. of CS2, (b) AuCl(tht)/HNO3, (c) HCl. (d) two eq. of CuX. (e) 2 nBuLi, [Cu(NCMe)4]PF6/PPh3.
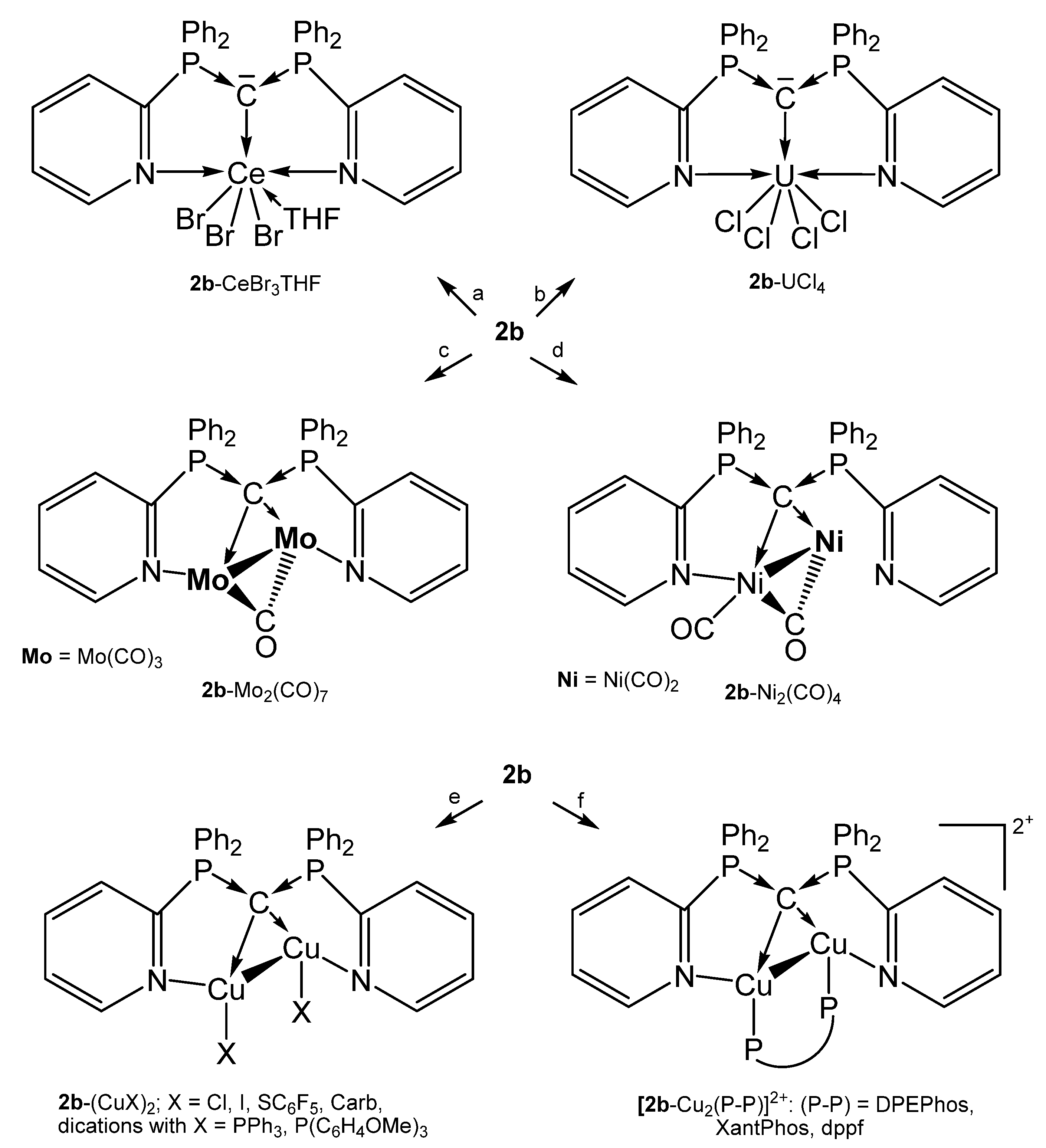
A series of complexes with the N,C,N pincer ligand sym-bis(2-pyridyl)tetraphenylcarbodiphosphorane (2b) were reported recently by the group of Sundermeyer. Remarkable is the molybdenum complex 2b-[Mo2(CO)7] in which 2b provides four pairs of electrons for donation to a Mo2 unit with an Mo-Mo separation of 3.0456(5) Å [64]. This coordination mode is continued in a series of dicopper complexes presented by the same working group and prepared as depicted in Scheme 7. The addition of [Cu]PF6 to 2b followed by treatment with two eq. of PR3 generated the cationic complexes [2b-(CuPPh3)](PF6)2 and [2b-(CuP{C6H4OMe}3](PF6)2, respectively; 2b-(CuCarb)2 was obtained from 2b-(CuCl)2 and two eq. of CarbH/NaOtBu (CarbH = carbazol) [63].
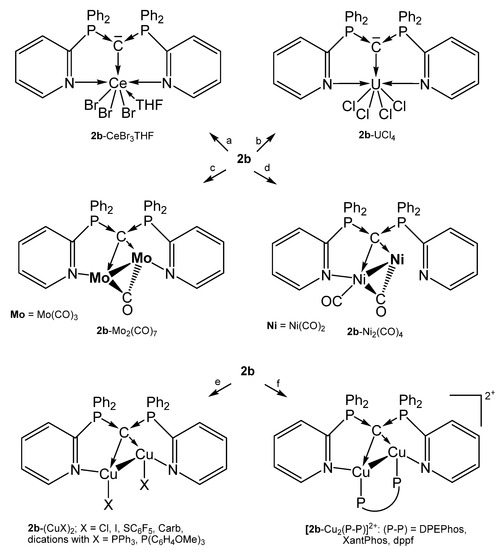
Scheme 7.
Selected compounds with the pincer ligand 2b as two and four electron donor. (a) CeBr3 in THF, (b) UCl4, (c) 2 eq. of Mo(CO)3(NCMe)3, (d) 2 eq. of Ni(CO)4, (e) 2 eq. of CuX, (f) 2 eq. of [Cu]PF6/1 eq. of P-P.
For the cationic complexes [2b-Cu2(P-P)]2+ the chelating ligands are: DPEPhos = bis[(2-diphenylphosphino)phenyl] ether, XantPhos = 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, dppf = 1,10-bis(diphenyl-phosphino)ferrocene. The germinal nature of both Cu(I) centers leads to Cu-Cu distances in the range of 2.55–2.67 Å. Most of the Cu(I) complexes show photoluminescence upon irradiation with UV light at room temperature [63].
Further, 2cH-CuPPh3 is an example of a complex with a deprotonated form of 2a and longer P-C distances are observed due to the protonation of the central carbon atom [63].
2.3. Transition Metal Addition Compounds of Carbones C(PR3)2 with an Additional Ortho Metallated Pincer Function
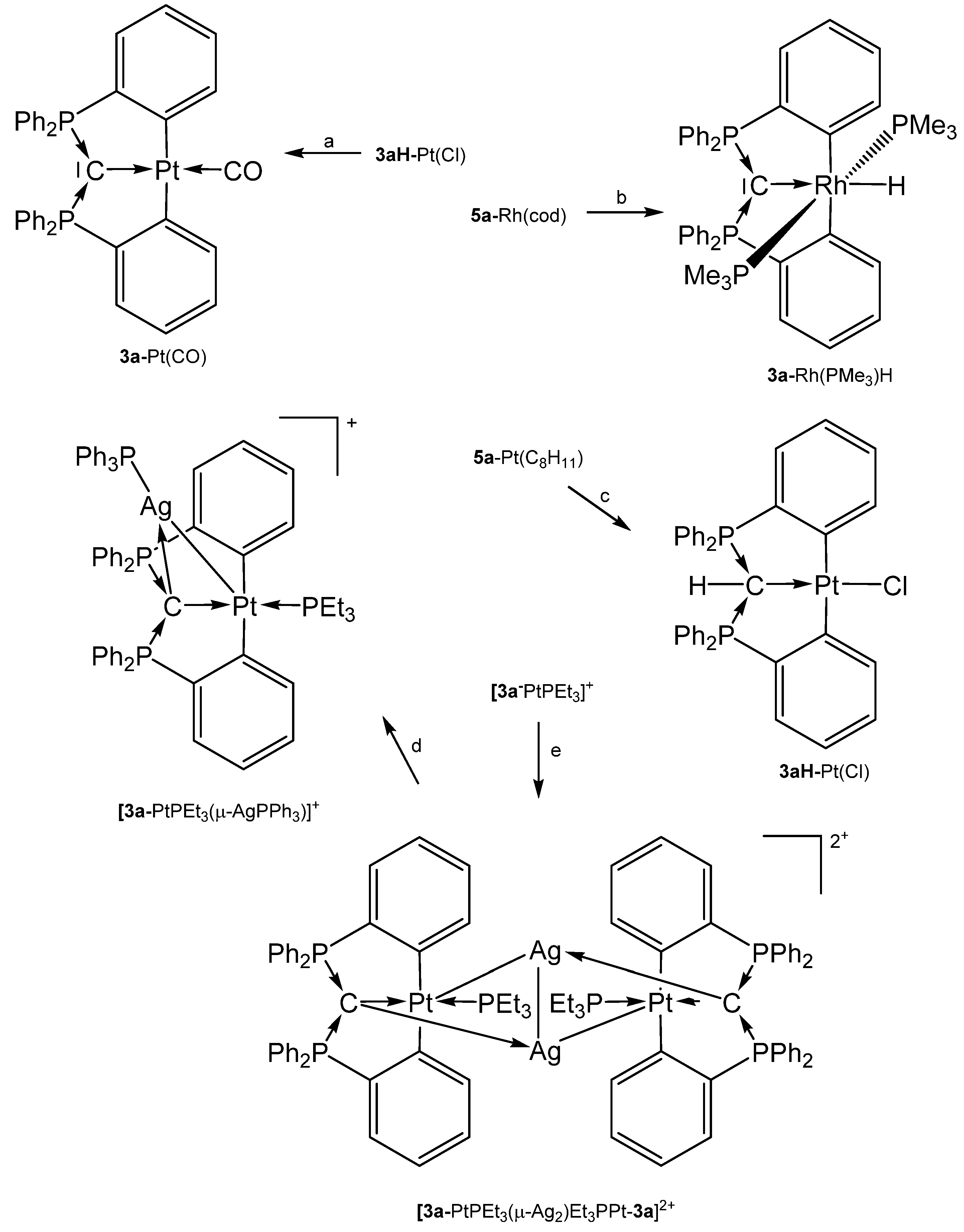
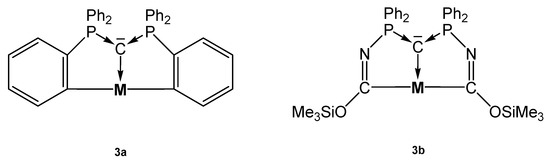
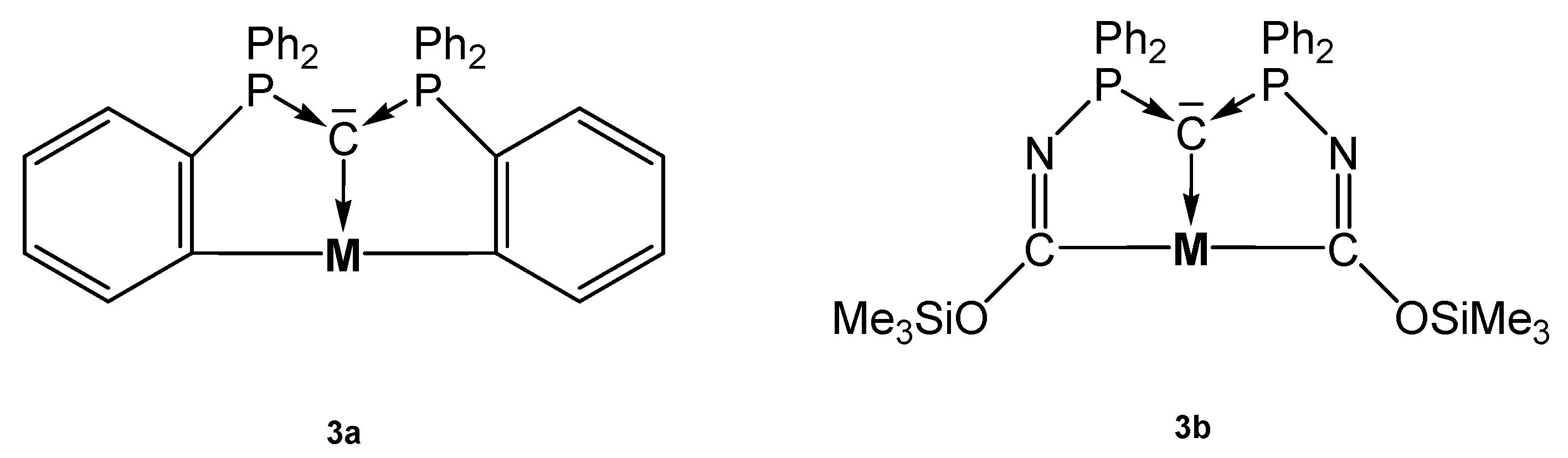
The source for the Rh complex 3a-Rh(PMe3)2H was the half pincer compound 5a-Rh(C6H8) (vide infra) upon reacting with PMe3 under loss of cod (see Scheme 8). 3a-Pt(SMe2) forms upon reacting 1a with [Me2Pt(SMe2)]2 and loss of 4 molecules of CH4 [69]. PEt3 replaces the labile bonded SMe2 group of 3a-Pt(SMe2) to produce 3a-PtEt3, which is transformed with P(OPh)3 into 3a-Pt(OPh)3. The dication [3a-PtPEt3(μ-Ag2)Et3PPt-3a]2+ was obtained upon addition of AgOTf to 3a-PtPEt3. According to the carbone C atom as four electron donor the Pt complexes with μ-Ag functions show long Pt-C distances between 1.737 and 1.749 Å (mean values) and the 31PNMR shifts are in the narrow range of 33 and 36 ppm (See Table 3) [70]. More complicated is the formation of 3a-Pt(CO), which stems from the hydrolysis of the related 3a-Pt(CCl2) complex (not isolated) [71].
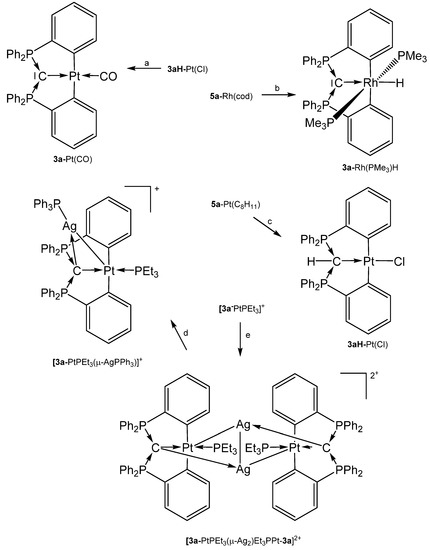
Scheme 8.
Selected addition compounds with the pincer ligand 3a and 3aH and those with the Ag-bridged cations or dication, respectively. (a) from 3aH-PtCl via 3a-Pt(CCl2) and H2O, (b) PMe3, (c) from 5a-Pt(C8H11) (see Scheme 11) and CHCl3, (d) PPh3, (e) 2 AgOTf.

Table 3.
Transition metal complexes with ortho metallated tripodal pincer ligand 3a derived from 1a and the related pincer ligand 3b and 31P NMR shifts.
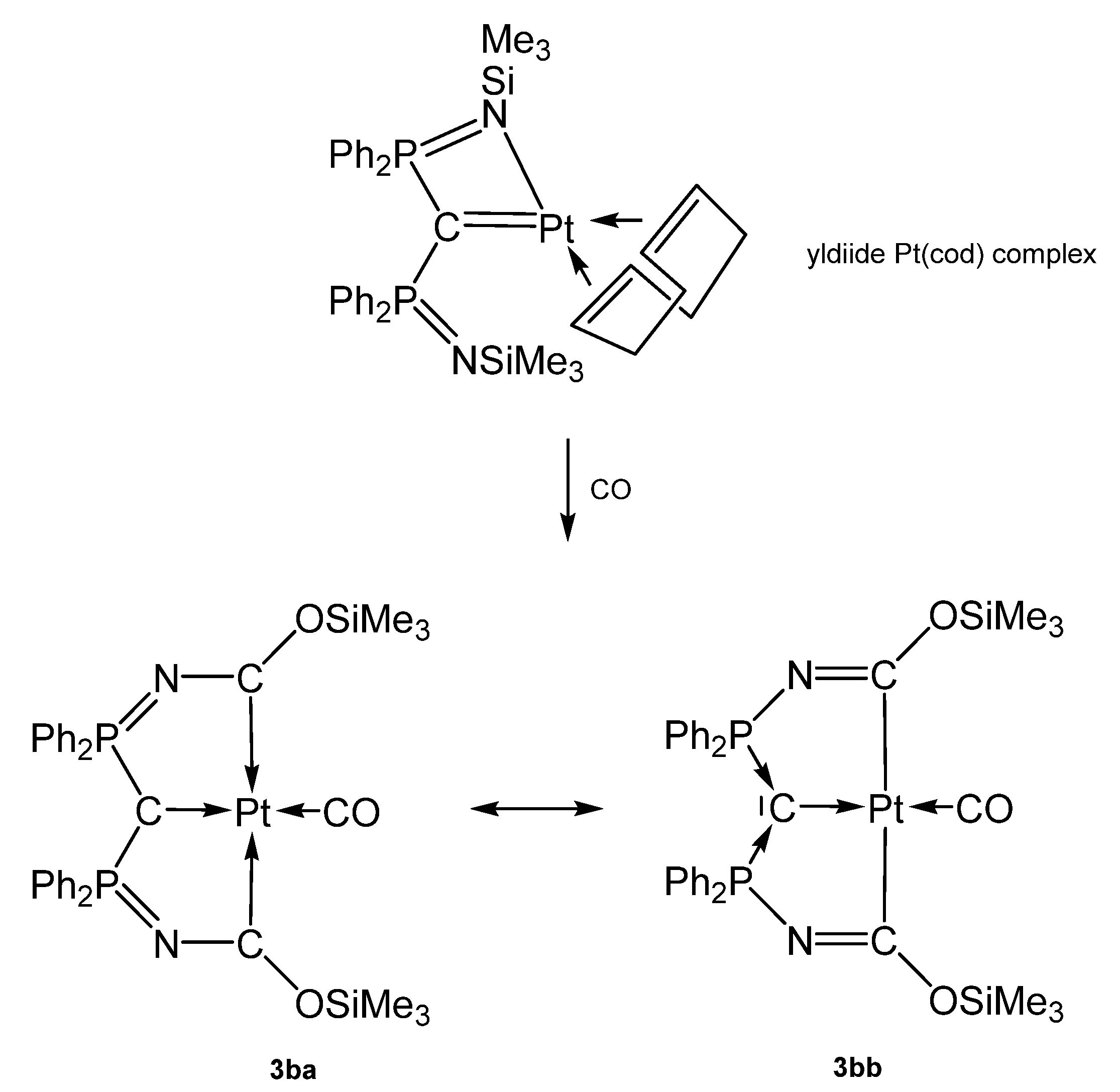
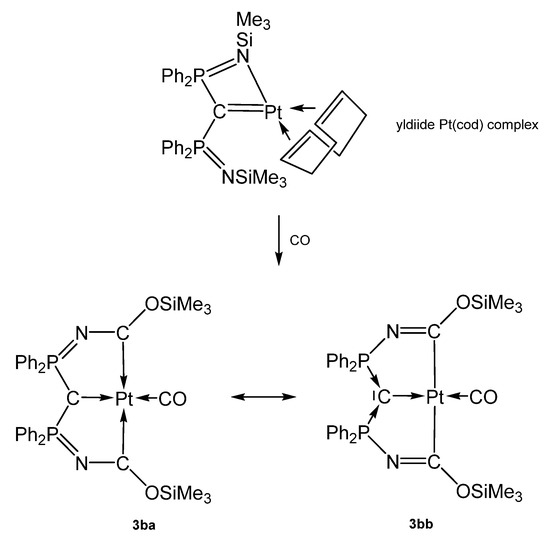
The carbone complex 3b-Pt(CO) was obtained from reacting the yldiide platinum complex (see Scheme 9) with 1 atm CO that inserts into the N-Si bond of the yldiide.
Scheme 9.
Two mesomeric forms of 3b-Pt(CO); 3ba favors a tricarbene coordination at Pt(0) whereas 3bb is consistent Pt(II) forming two C-Pt s-bonds similar to 3a-Pt(CO). The short central C-Pt bond length of 2.002 Å indicates a partial doubly donation of the carbone C atom as shown in Figure 5. The planar environment at Pt is typical for Pt(II) and supports this view [72].
2.4. Transition Metal Complexes with P-C-P Five Membered Ring
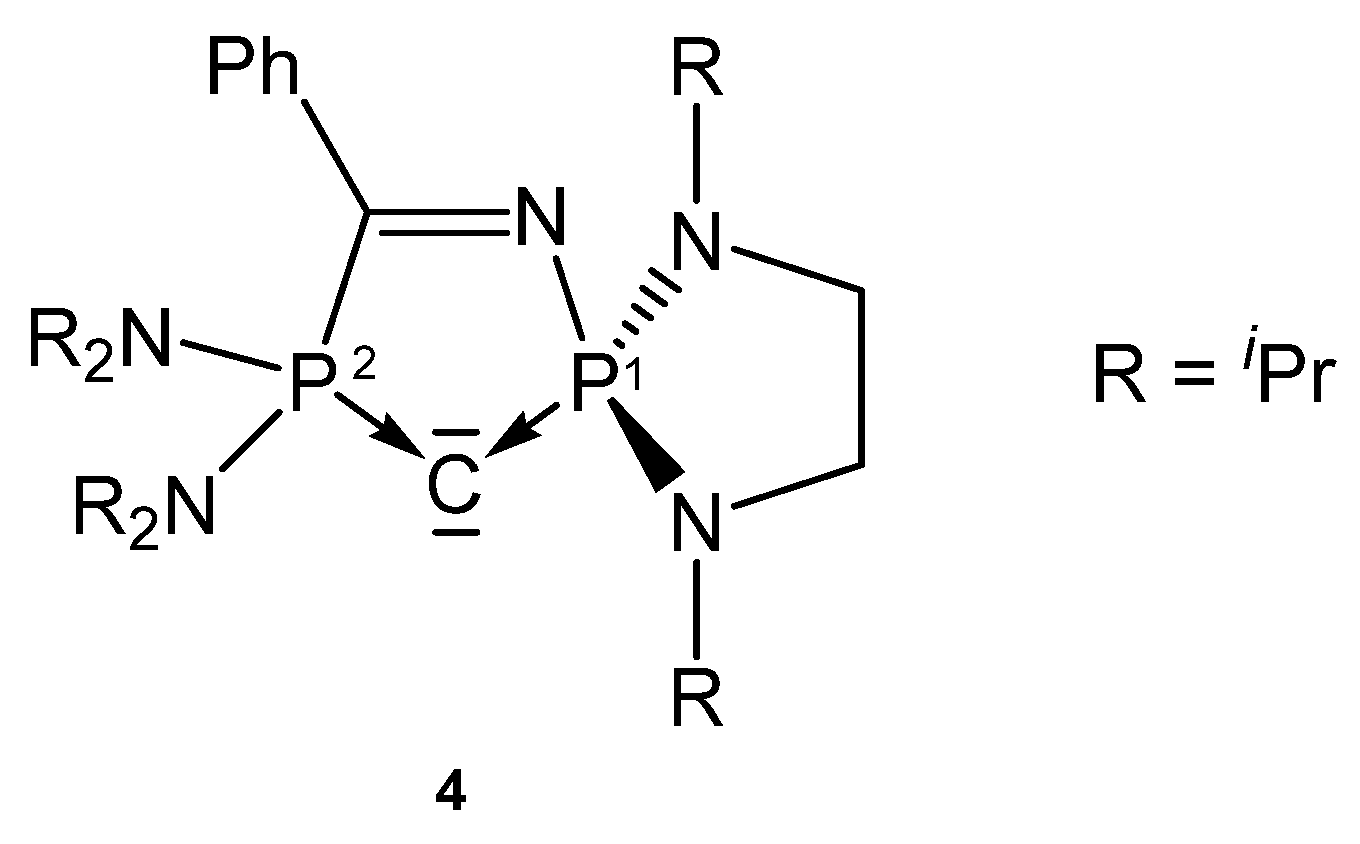
The carbone 4 (see Figure 6) was obtained by deprotonation of the cation [4H]+. According to two P atoms in different chemical environments two doublets in the 31P NMR spectrum were recorded at δ = 60.0 and 71.5 ppm; 2JPP = 153 Hz. From X-ray determination stem the P-C(1) and P-C(2) distances of 1.644(19) and 1.657(17) Å, respectively, and the P-C-P angle amounts to 104.82(10)° [73]. The bond lengths (see Table 4) are close to that reported for the carbone 1a.
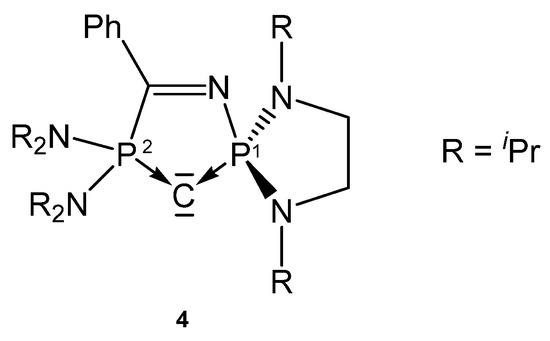
Figure 6.
Structure of compound 4.

Table 4.
Transition metal complexes with the cyclic carbone 4, containing 31P NMR shifts and relevant structural parameters.
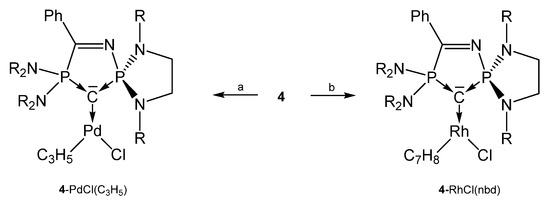
From the cyclic and asymmetric carbone 4 six transition metal complexes (see Scheme 10) are known in which the ligand acts as two electron donor via the C atom. As in the starting compound 4 the P2-C bond distances are slightly longer than P1-C bond. Addition of CuCl and AuCl(SMe2) to 4H+/tBuOK generates the compounds 4-CuOtBu and 4-AuOtBu, respectively. In CH3Cl2 or CHCl3 4-CuOtBu is converted into 4-CuCl [74]. 4-Rh(CO)2Cl stems from the reaction of 4 with [{RhCl(CO)2}2] [73]. 4-CuOtBu and 4-AuOtBu catalyze the hydroamination or hydroalkoxylation of acrylonitrile [74].
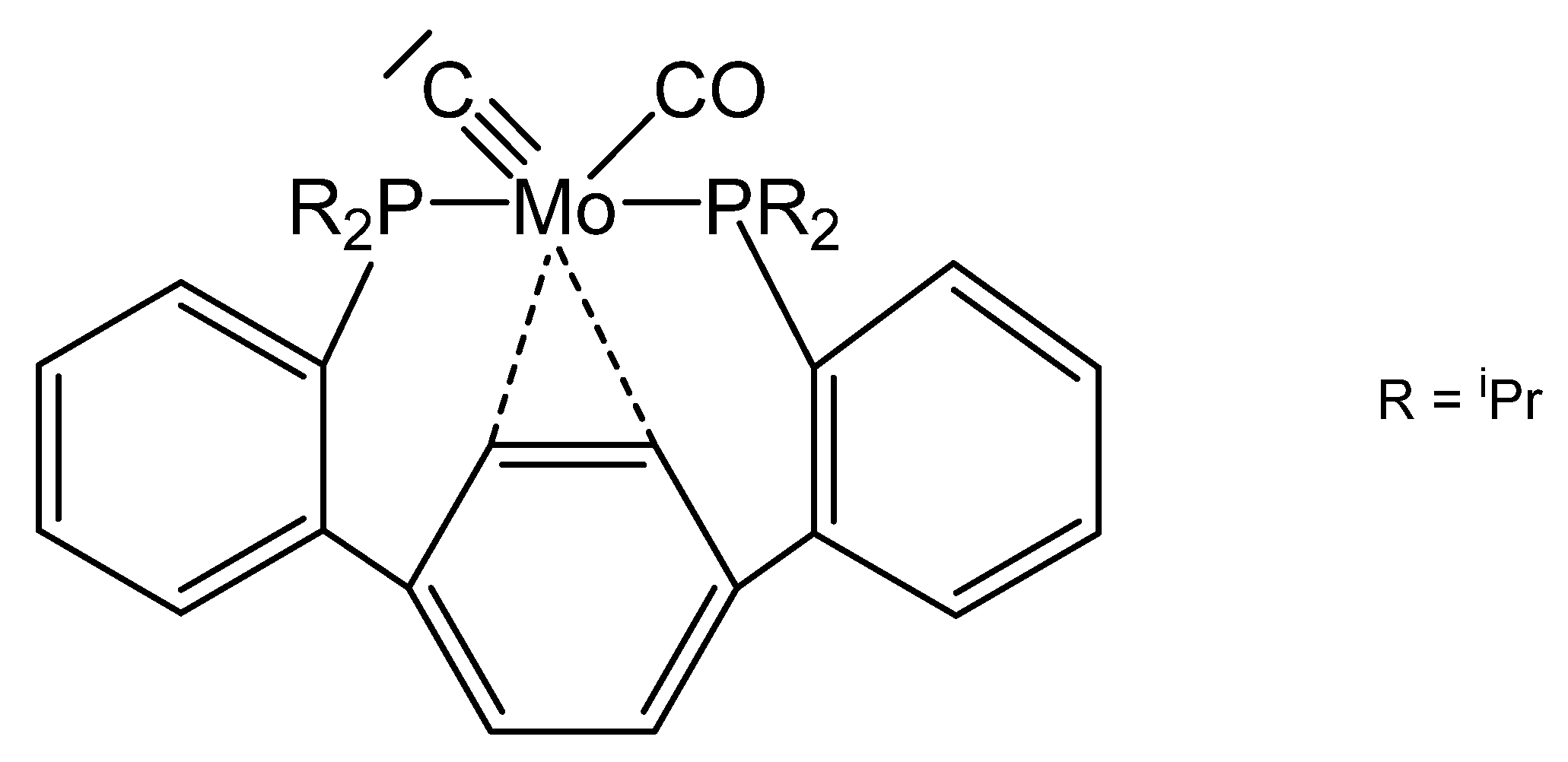
Scheme 10.
Selected complexes with the cyclic carbone 4. R = iPr. a) [{PdCl(allyl)}2], b) [{RhCl(nbd)}2].
2.5. Transition Metal Complexes with Asymmetric P-C-P Ligands
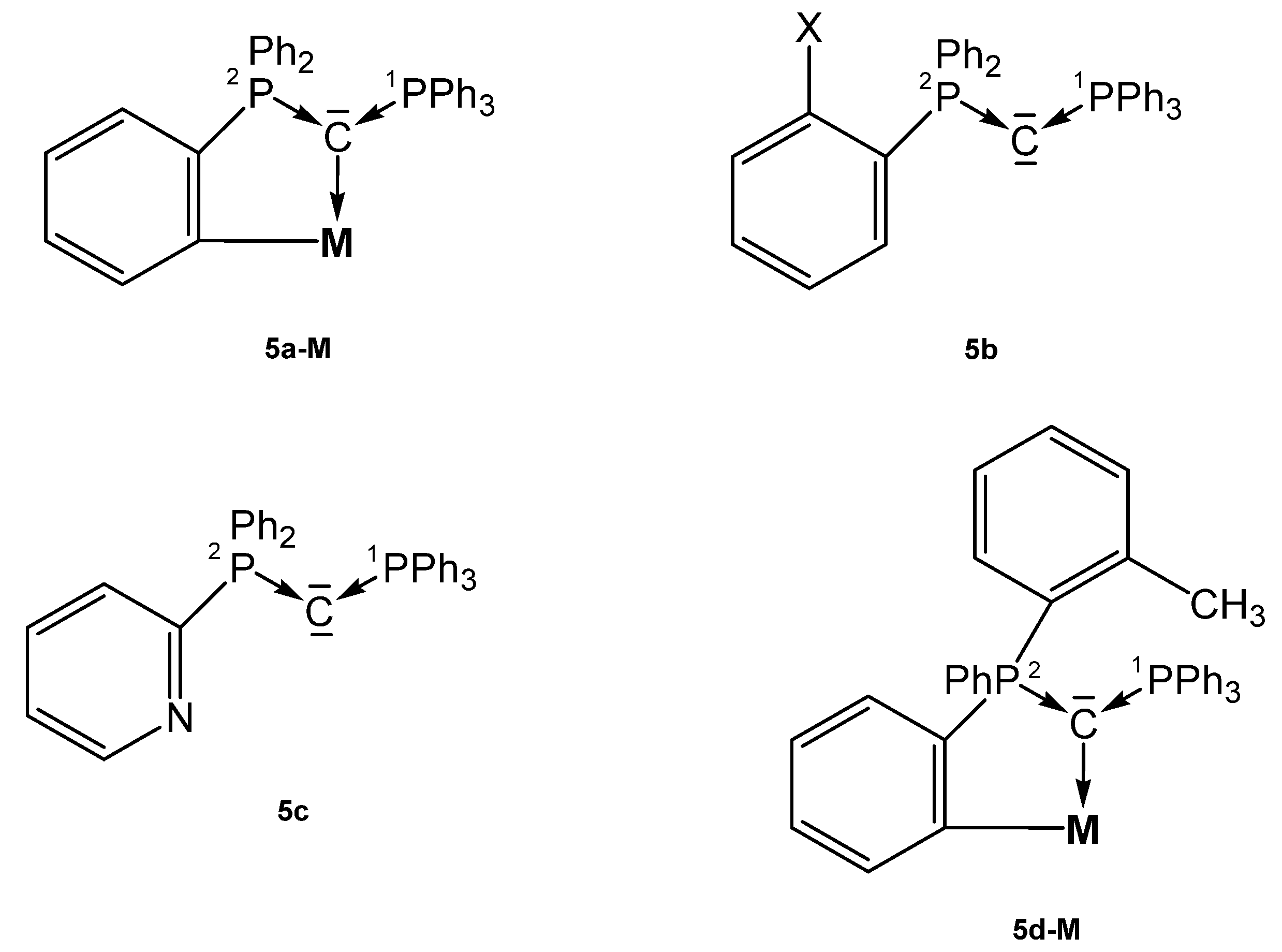
Several asymmetric carbones with orthometallation (5a-M, 5d-M), with an additional donor function (5c), or with a functionalized phenyl ring (5b) were reported that form TM complexes (see Figure 7).
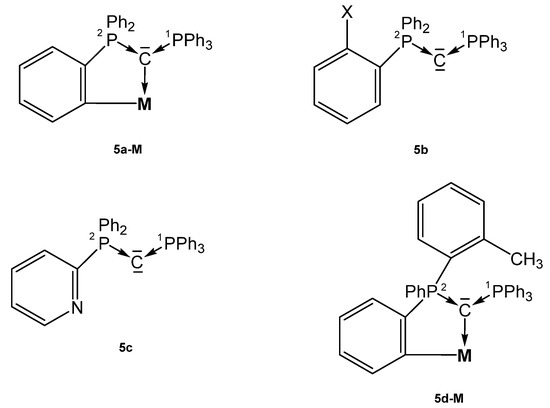
Figure 7.
Structures of compounds 5a-M, 5b, 5c and 5d-M.
The neutral asymmetric carbone 5b (X = PPh2) has the structural parameters P1-C = 1.642(2), P2-C = 1.636(1) Å, and a P-C-P angle of 140.74(8)° (see Table 5); the P atoms resonate at δ = −6.9 and −3.4 ppm (2JPP = 93 Hz) [75]. Those of 5c are P1-C = 1.6416(16) Å, P2-C = 1.6398(17) Å, and P-C-P = 133.25(10)° [76]. Three complexes in which the carbone 1a is half-side orthometallated forming 5a-M complexes are described [69,73,77].

Table 5.
Transition metal complexes with the unsymmetrical carbones 5a–5d; 31P NMR shifts in ppm.
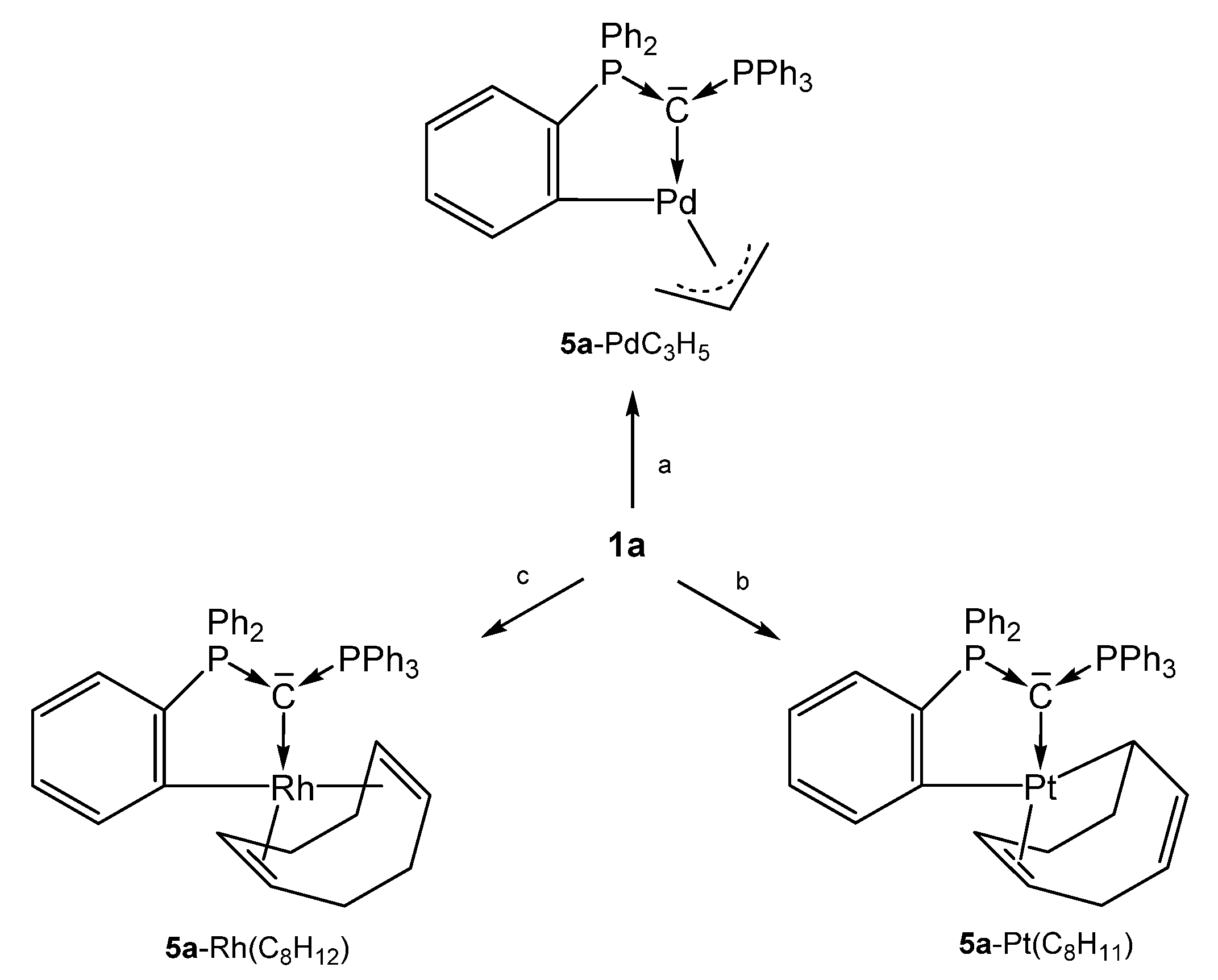
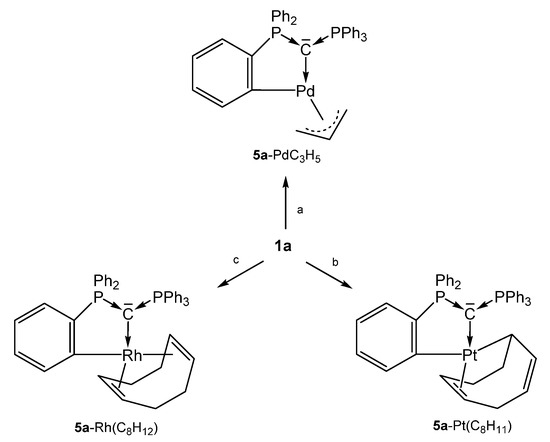
As depicted in Scheme 11, three neutral complexes of 1a are known in which one of its phenyl group is orthometallated to produce the 5a-M core. The 31P NMR shift of the unchanged PPh3 group range between about 6 and 13 ppm whereas for the orthometallated side shifts between 15 and 40 ppm where recorded. Both P-C distances do not differ markedly and amount to about 1.700 Å.
Scheme 11.
Selected structures of transition metal complexes with the carbone 5a; (a) ½ [PdCl(allyl); (b) 1/3 [PtI2(cod)]; (c) ¼ [RhCl(cod)]. All complexes are formed upon release of the cation [1aH]+.
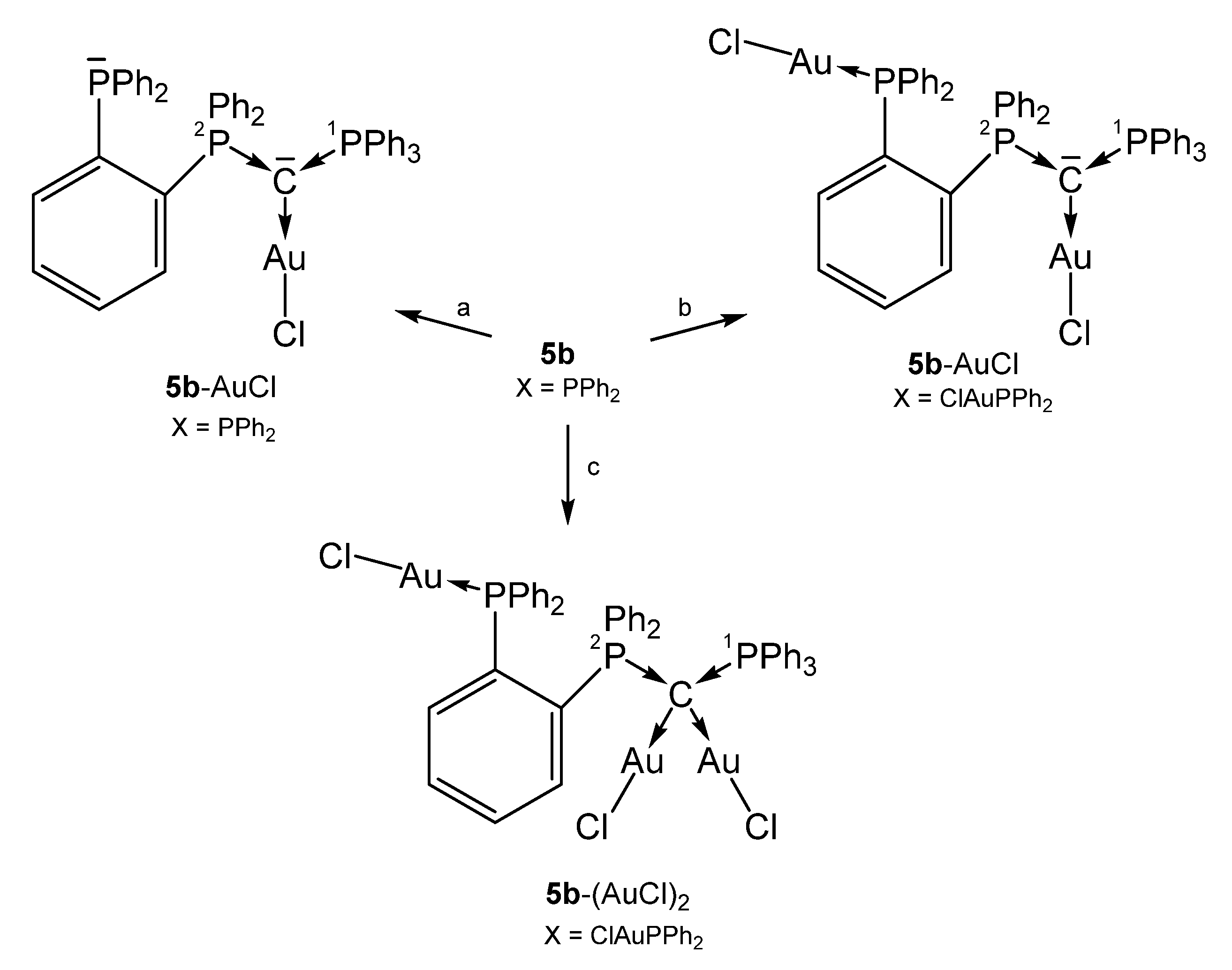
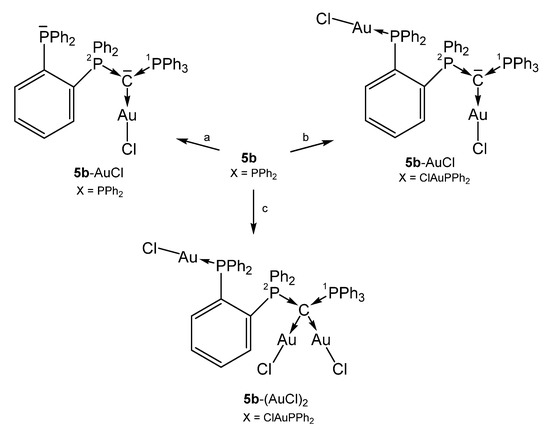
All complexes shown in Scheme 12 have a further PPh2 function at the ortho position of one phenyl group of 1a. In the complex 5b-(AuCl)2 the carbone provides four electrons for donation with typical long P-C distances of about 1.770 Å [75].
Scheme 12.
Selected structures of transition metal complexes with the carbone 5b. (a) [AuCl(tht)], (b) 2 [AuCl(tht), 3 [AuCl(tht)].
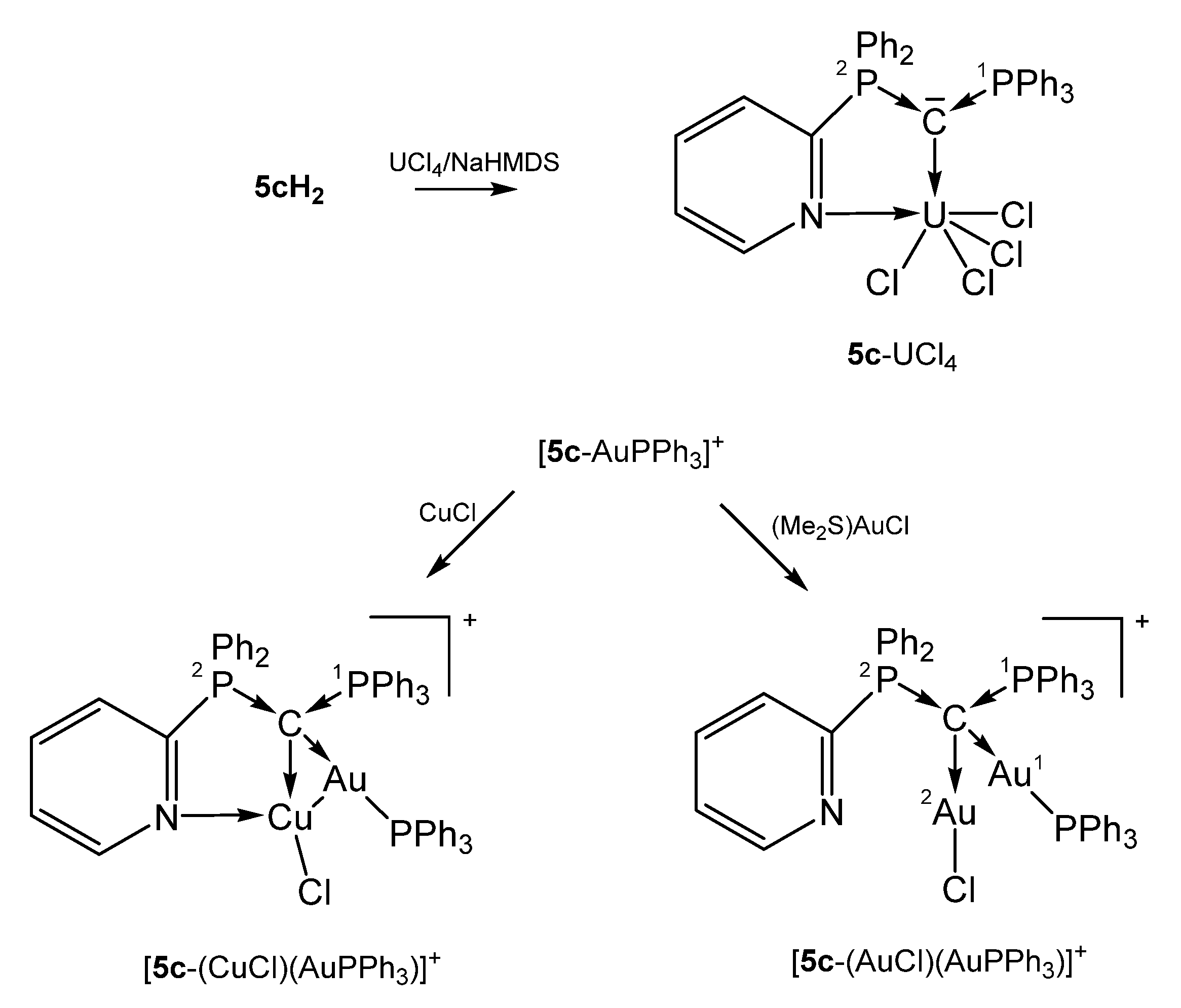
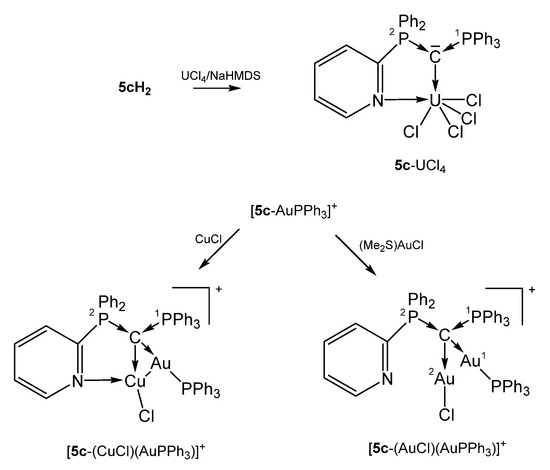
The paramagnetic 5c-UCl4 exhibits a short C-U distance indicative for a double dative bond of the carbone C atom as in 2b-UCl4 and was obtained by reacting UCl4 with the dication 5c-H2/NaHMDS. Upon further coordination of the pyridyl group (U-N = 2.537(4) Å) the U atom attains the coordination number 6 [41].
[5c-AuPPh3]+ was obtained from reacting the carbone 5c with [PPh3AuCl]/Na[SbCl6] (see Scheme 13). In the cationic complex [5c-(CuCl)((AuPPh3)]SbF6, the carbone 5c acts as a six-electron donor with a Cu-N distance of 2.267(6) Å and Cu-Au separation of 2.8483(10) Å. The Cu and Cl atoms are each disordered over two positions with occupancy of about 0.8 to 0.2. If CuCl is replaced by AuCl as in [5c-(AuCl)(AuPPh3)]SbF6 the C-AuPPh3 distance is slightly elongated and no coordination of the pyridyl N atom is observed. The Au-Au separation is with 3.1274(6) Å too long for a metallophilic interaction. In both compounds, the carbone C atom constitutes a chiral center according to four chemical different substituents and acts as a four-electron donor. The PPh3 group resonates between 15 and 27 ppm [76]. In the related symmetric pyridyl-free complex 1a-(AuCl)2, slightly shorter C-Au (2.076(3) Å) were recorded accompanied by longer P-C (1.776(3) Å) bond lengths [51].
Scheme 13.
Selected structures of transition metal complexes with the mono pyridyl substituted carbone 5c.
2.6. Transition Metal Complexes of Carbones with Cyclobutadiene
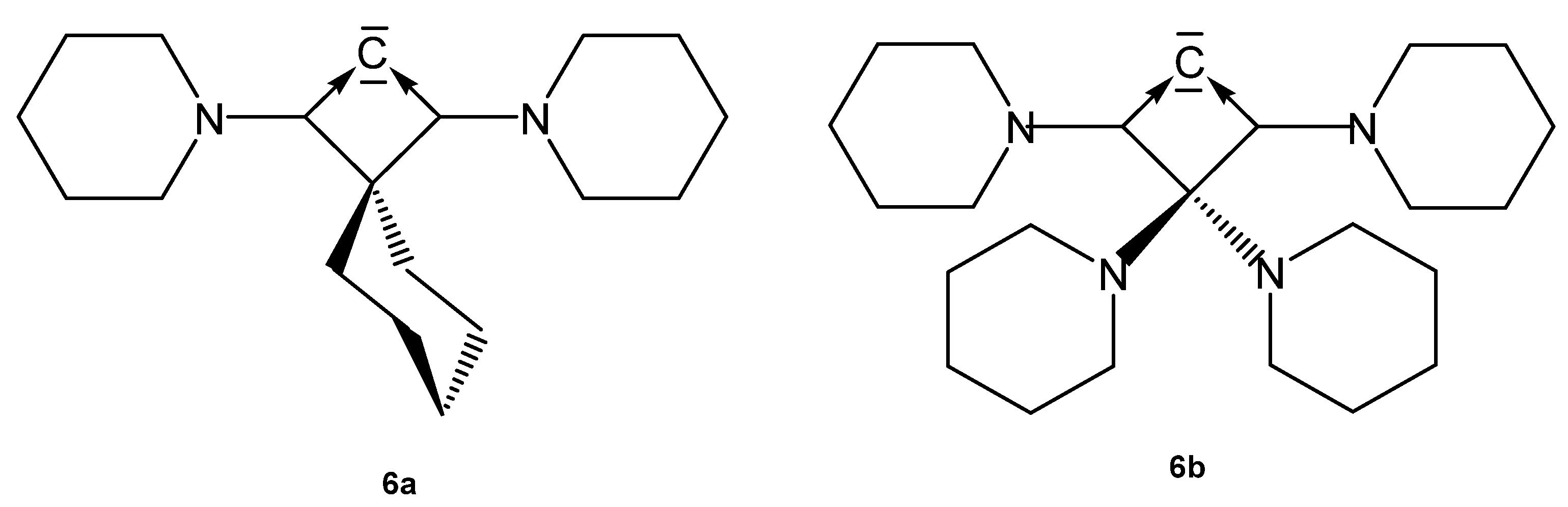
The carbones 6a and 6b (see Figure 8) can also be seen as an all-carbon four-membered ring bent allene (CBA); 6a is stable for several hours at −20° but decomposes when warmed up to −5°. The optimized geometry reveals a very acute allene bond angle of 85.0° and coplanarity of the ring carbon atoms including the two nitrogen atoms. The C=C bonds of the allene fragment amount to 1.423 Å and are significantly longer than in typical linear allenes (1.31 Å). Short CN bonds of 1.36 Å indicate some double bond character. The CCC carbon atom resonates in the 13C NMR spectrum at 151 ppm. The first and second proton affinities (PAs) are very high amounting to 307 and 152 kcal/mol [79].
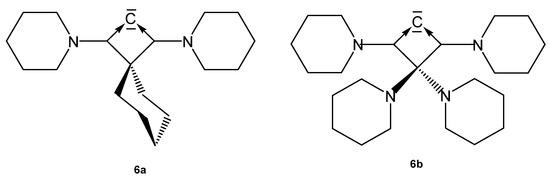
Figure 8.
Structures of compounds 6a and 6b.
The molecular orbitals show that the HOMO and HOMO-1 have clearly the largest coefficients at the central carbon atom and exhibit the typical shape of lone-pair molecular orbitals with σ (HOMO) and π (HOMO-1) symmetry; however, with reversed order with respect to CDPs and CDCs. To emphasize the proximity of 6 to CDP carbones, we use the same symbolism mimicking a metal.
The free CBA 6b could not be obtained, but only the cationic 6bH+ and 6bH22+ are known and used as starting compounds for the syntheses of the related transition metal complexes [80].
The 13C NMR shifts of the central carbon atom are shifted to higher fields relative to the starting free carbone ranging between 124 and 139 ppm (see Table 6).

Table 6.
Transition metal complexes with the all carbon ligand 6; 13C NMR shifts (in ppm) of the donating carbon atom. Distances in Å, angles in deg.
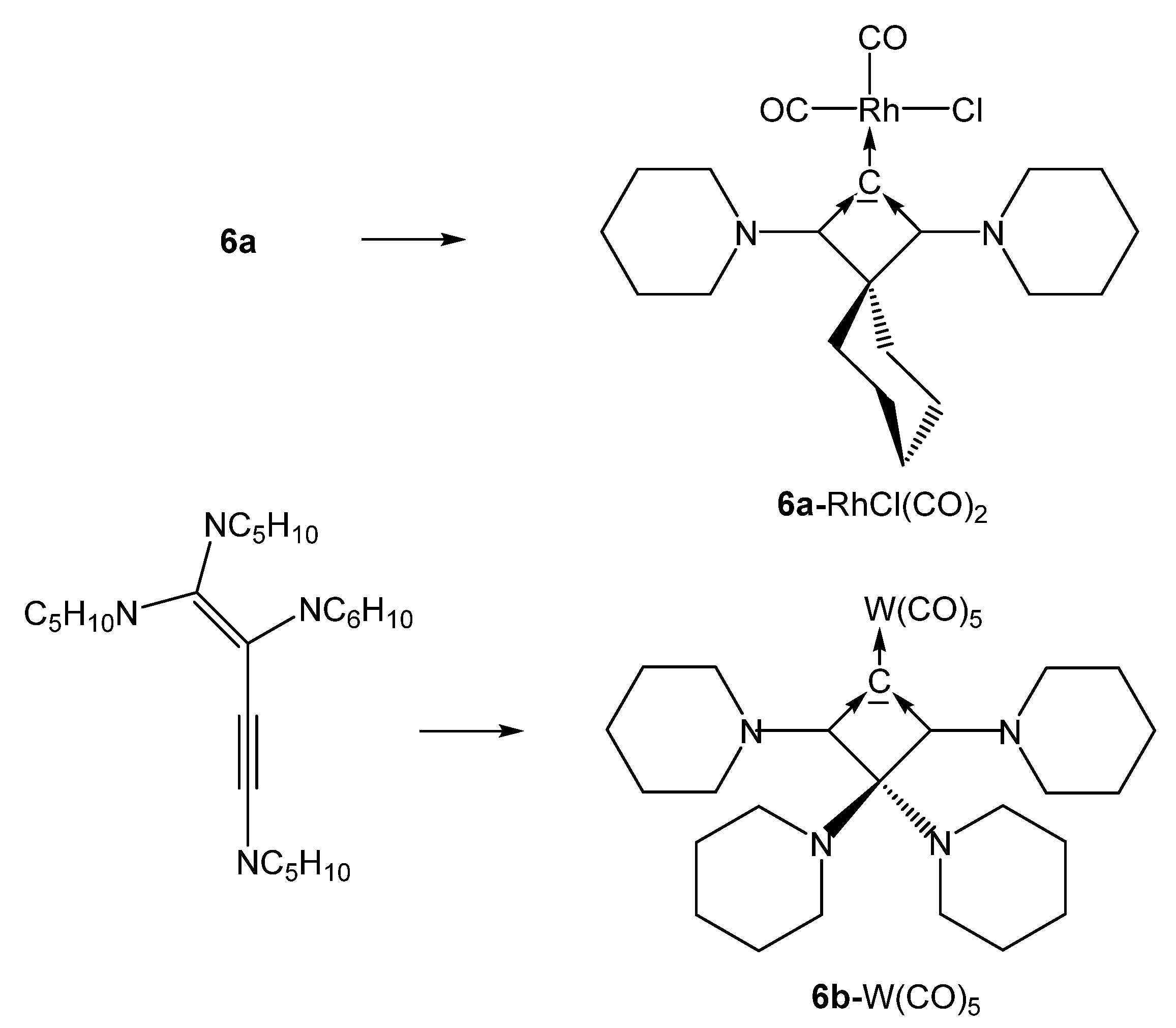
All complexes of the CBA 6a where obtained by reacting the freshly prepared free carbone 6a at −20° with [{MCl(cod)}2] complexes (M = Rh, Ir). The cod ligand can be replaced by bubbling CO through solutions of 6a-MCl(cod) to produce the related 6a-MCl(CO)2 compounds (see Scheme 14) [79].
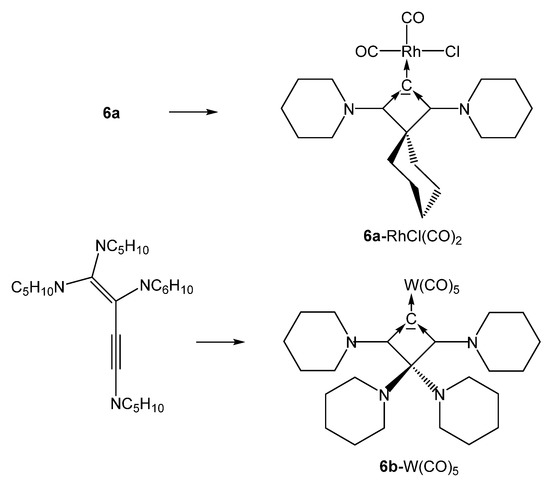
Scheme 14.
Selected structures of complexes with the cyclic carbones 6a and 6b. Preparation see text.
Transition metal complexes with 6b as ligand were obtained by reacting 1,1,2,4-tetrapiperidino-1-buten-3-yne with (a) [(tht)AuCl], (b) [RhCl(CO)2]2, and (c) [(NMe3)W(CO)5] during the reaction rearrangement of the starting buten-3-yne to 6b has occurred [80].
2.7. Carbodicyclopropenylidene
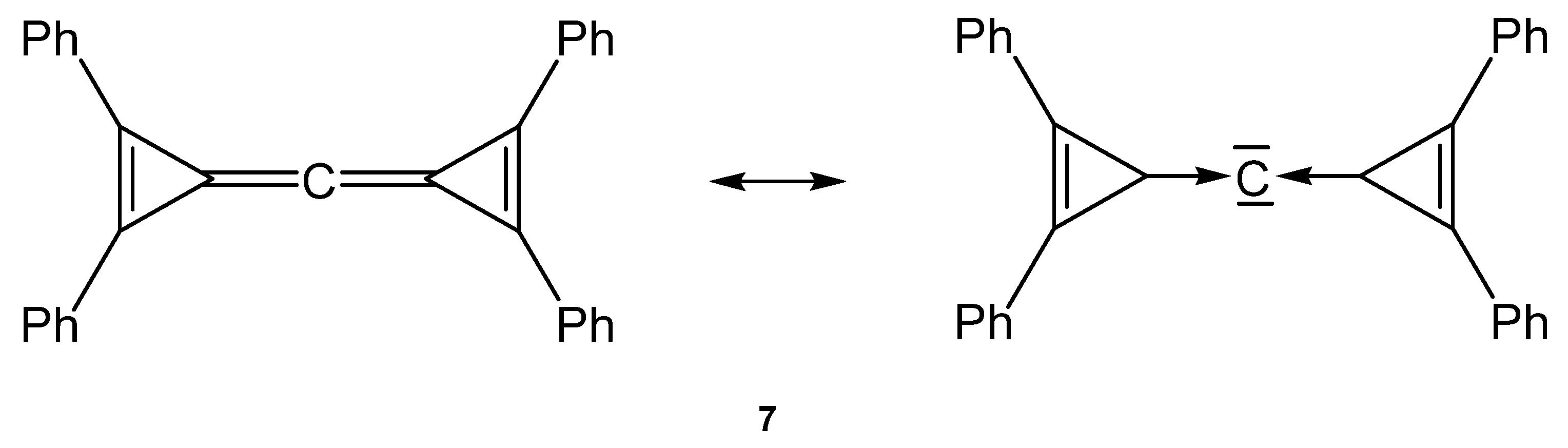
Stephan described the first carbodicarbene stabilized by flanking cyclopropylidenes, named carbodicyclopropylidene 7 (see Figure 9) [81].
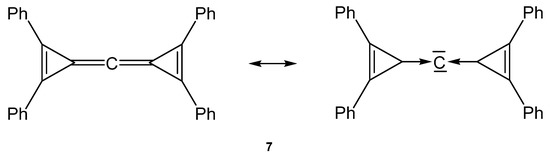
Figure 9.
Possible description of the bonding in the carbone 7.
Neither the neutral singlet 1,2-diphenylcyclopropenylidene as carbene ligand L in 7 nor the carbone tetraphenylcarbodicyclopropenyliden (CDC) 7 itself are stable compounds at room temperature. The free carbene L has only been observed in an argon matrix isolated at 10 K and 7 could be characterized in solution by low temperature NMR spectroscopy; for the central carbon atom a 13C NMR shift at δ = 133 ppm was recorded at −60 °C.
The first and second proton affinities of 7 were determined to be 283 and 153 kcal/mol, respectively. The molecular structure of 7 was determined by computational methods. Calculations reveal that the central carbon atom is in a linear environment the C-C distances were calculated at 1.308 Å and the C-C-C angle to 180°. The energy difference between the linear allenic structure and the bent arrangement is shallow amounting to 6.6 kcal/mol for a bending angle of 140° and 10 kcal/mol for 130°. The highest occupied molecular orbital (HOMO) and HOMO-1 of 7 are degenerate and incorporate the p(π) orbitals of the C2-C1-C2a fragment.
The central C atom is more negatively charged (−0.19 a.u.) than the adjacent C atoms, suggesting nucleophilic character [81].
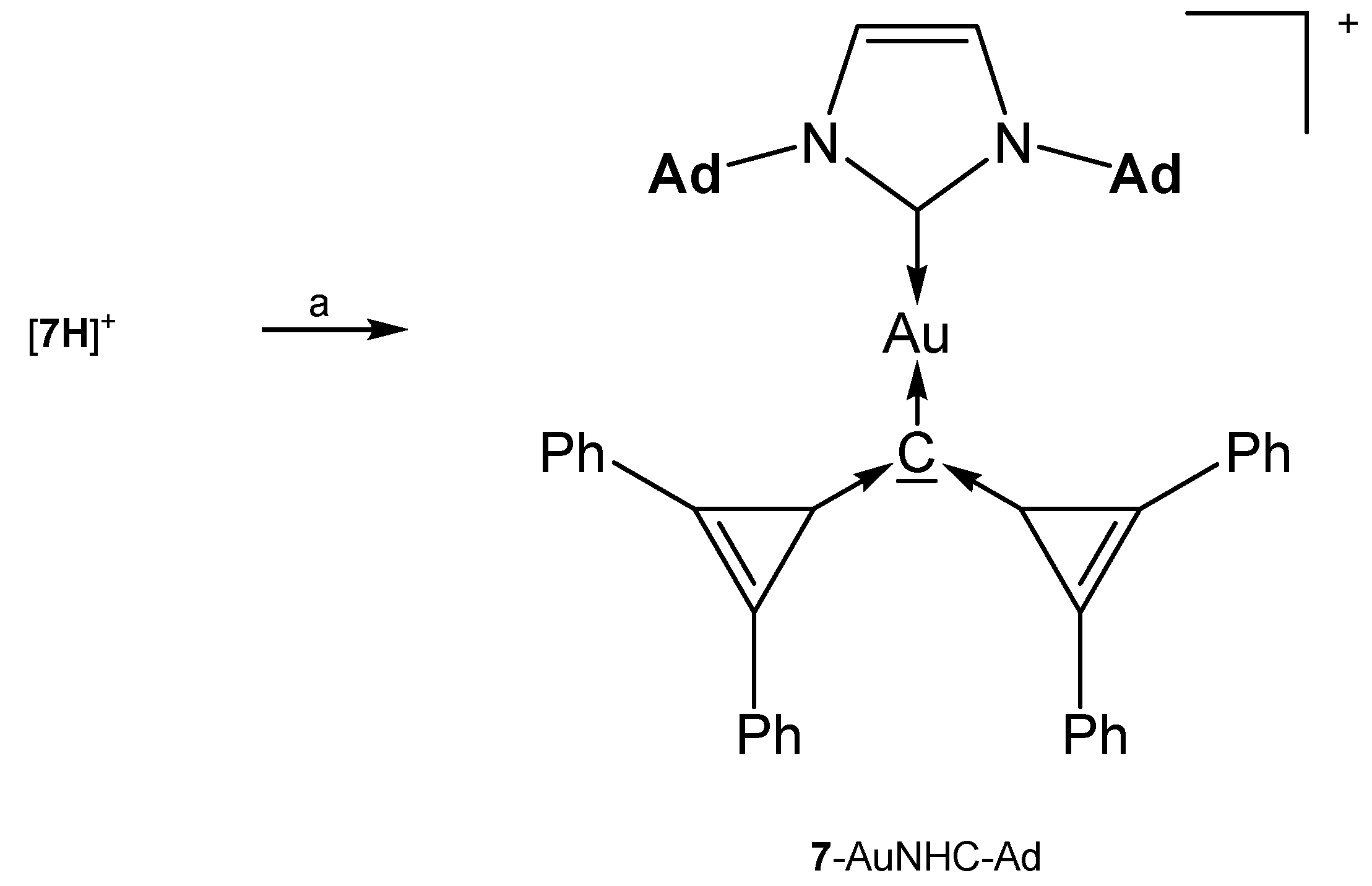
The addition compounds [7-AuNHC-Ad](OTf) and [7-AuNHC-Dipp](OTf) (see Table 7) were prepared from reacting [7H]+ with KHMDS and the related (NHC)AuOTf at −45°(see Scheme 15) [81].

Table 7.
Complexes with the carbone 7. 13C NMR shifts (in ppm) of the donating carbon atom.
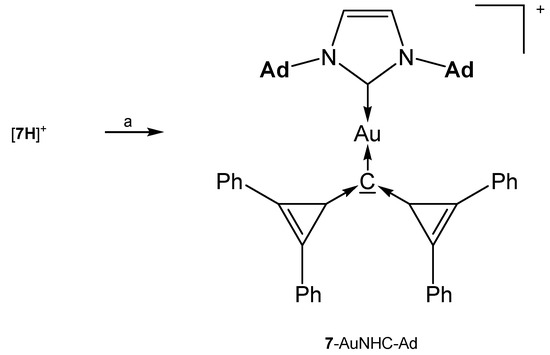
Scheme 15.
Selected structures of complexes with the cyclo propylidene stabilized carbone 7. (a) KHMDS/(NHC)AuOTf.
2.8. Carbodicarbenes
Carbodicarbenes, CDCs, are neutral compounds where a bare carbon atom with its four electrons is stabilized by two NHC ligands which plays the role of a phosphine group as in carbodiphosphoranes, CDPs. Theoretical studies have demonstrated that this class of compounds could be stable and their existence was predicted by Frenking [82] and short times later realized by the group of Bertrand [83].
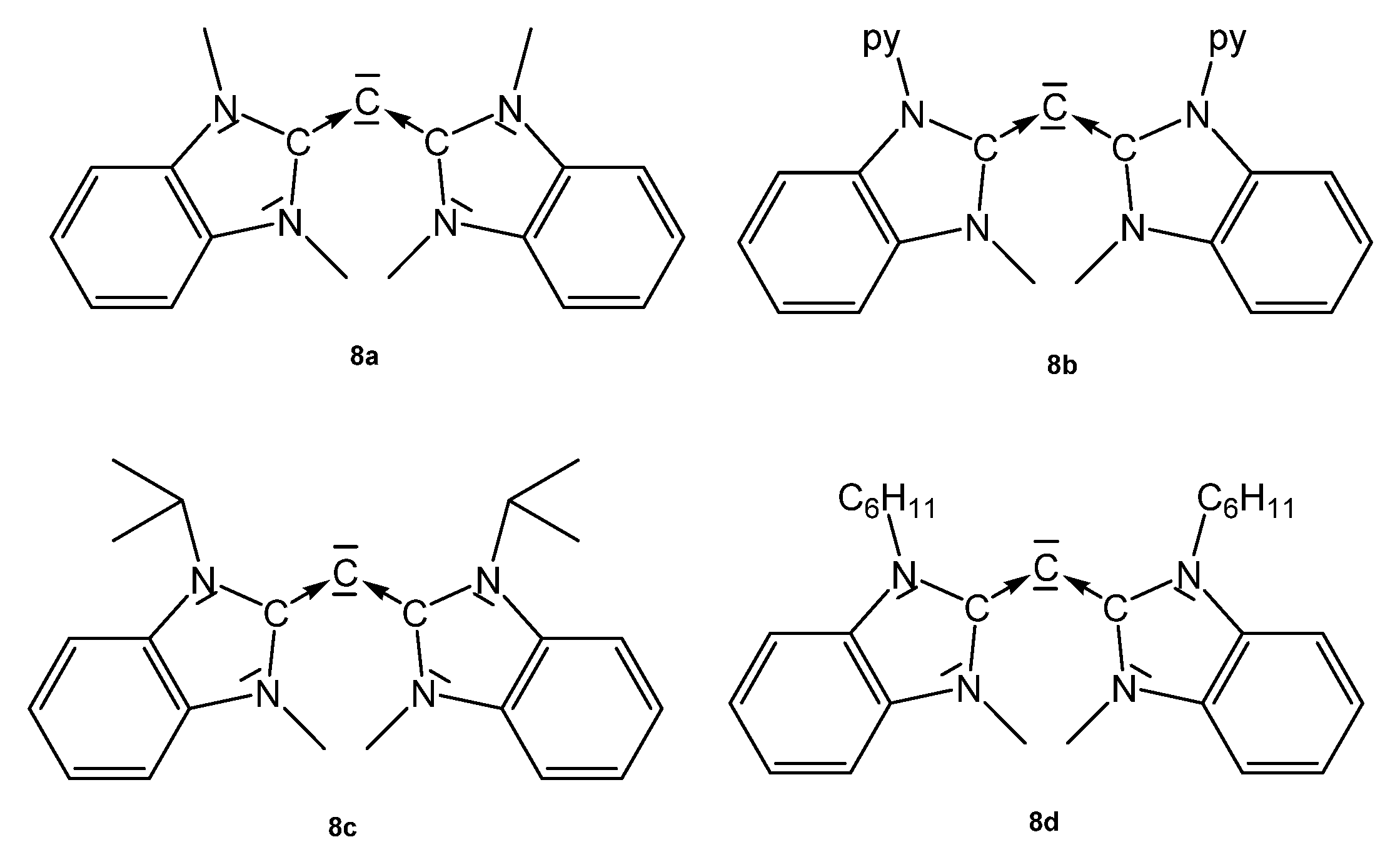
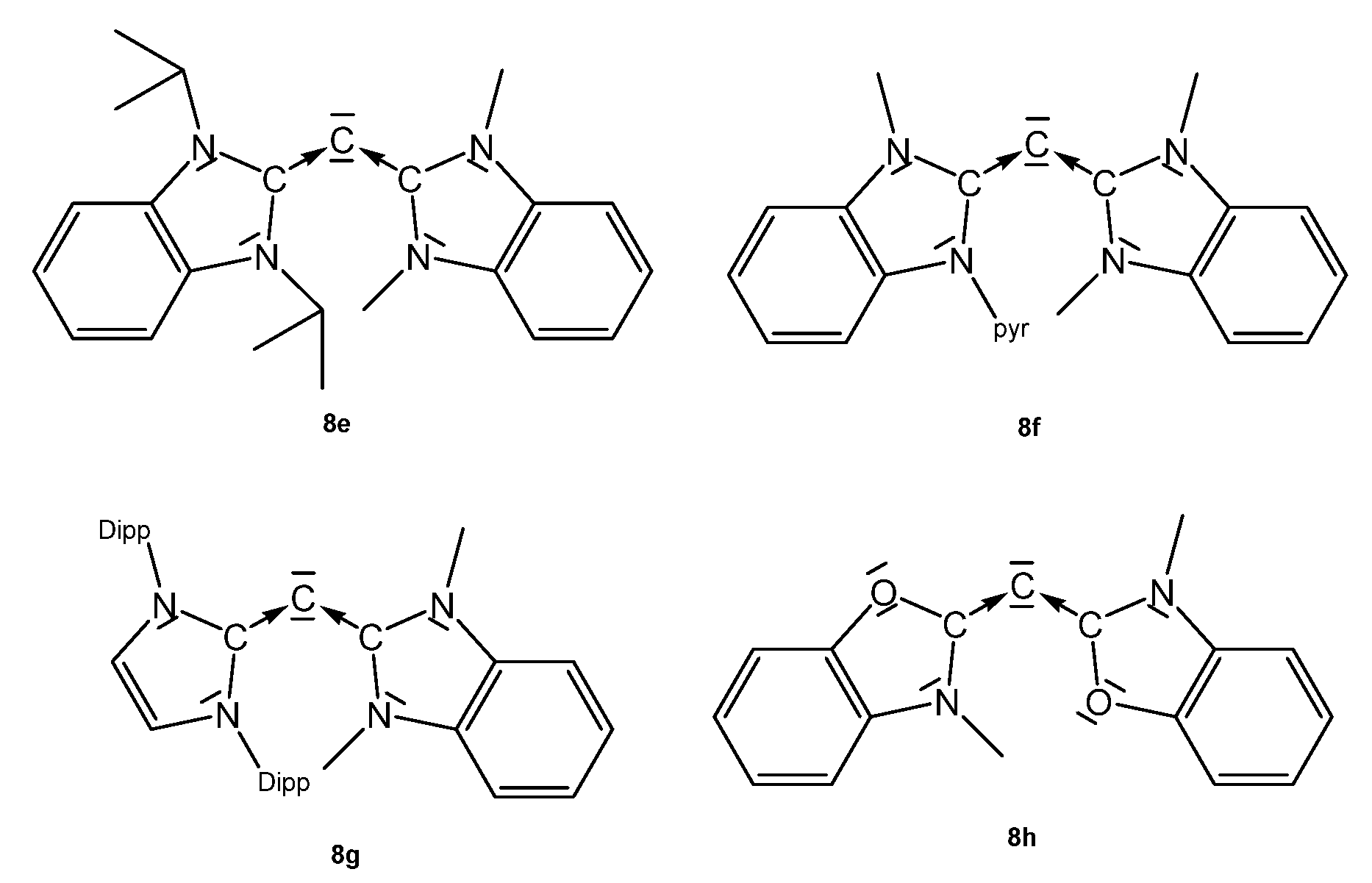
Structural and spectroscopic parameters of the following symmetric CDCs (see Figure 10) are available: 8a, C-C = 1.343(2) Å, C-C-C = 134.8(2)°, 13C NMR 110.2 ppm [83]; 8b, C-C = 1.333(2) Å and 1.324(2) Å, C-C-C = 143.61(15)° [84]; 8c, C-C = 1.335(5) Å, C-C-C = 136.6(5)° (see Table 8) [85].
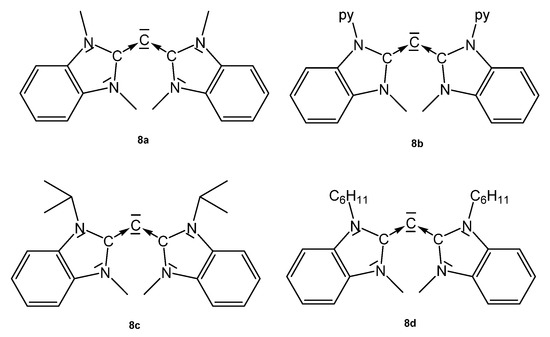
Figure 10.
Symmetrical CDCs from which transition metal complexes are known.

Table 8.
Collection of transition metal complexes with the CDCs 8a–8h. 13C NMR shifts of the central carbon atom (in ppm).
Structural parameters of the unsymmetrical CDCs (see Figure 11) are: 8e, C-C = 1.3401(16) Å and 1.3455(16), C-C-C 137.55(12)°. For 8f, no data are available [90]. 8g: C-C = 1.344(3) Å and, 1.318(3) Å, C-C-C = 146.11(19)° [90]. 8h was obtained at −60° by reacting 8hH+ with KMDS, and characterized spectroscopically. On warming to room temperature, it dimerizes. 13C NMR: δ = 105.5 ppm (see Table 8) [91].
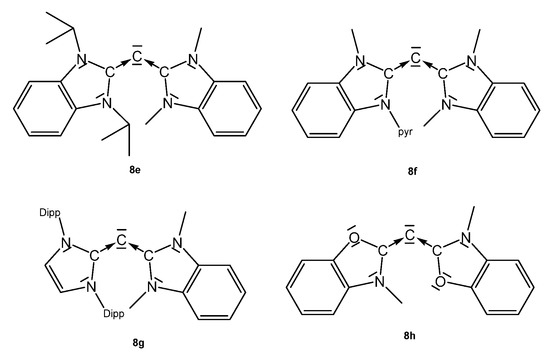
Figure 11.
Unsymmetrical CDCs from which transition metal complexes are reported.
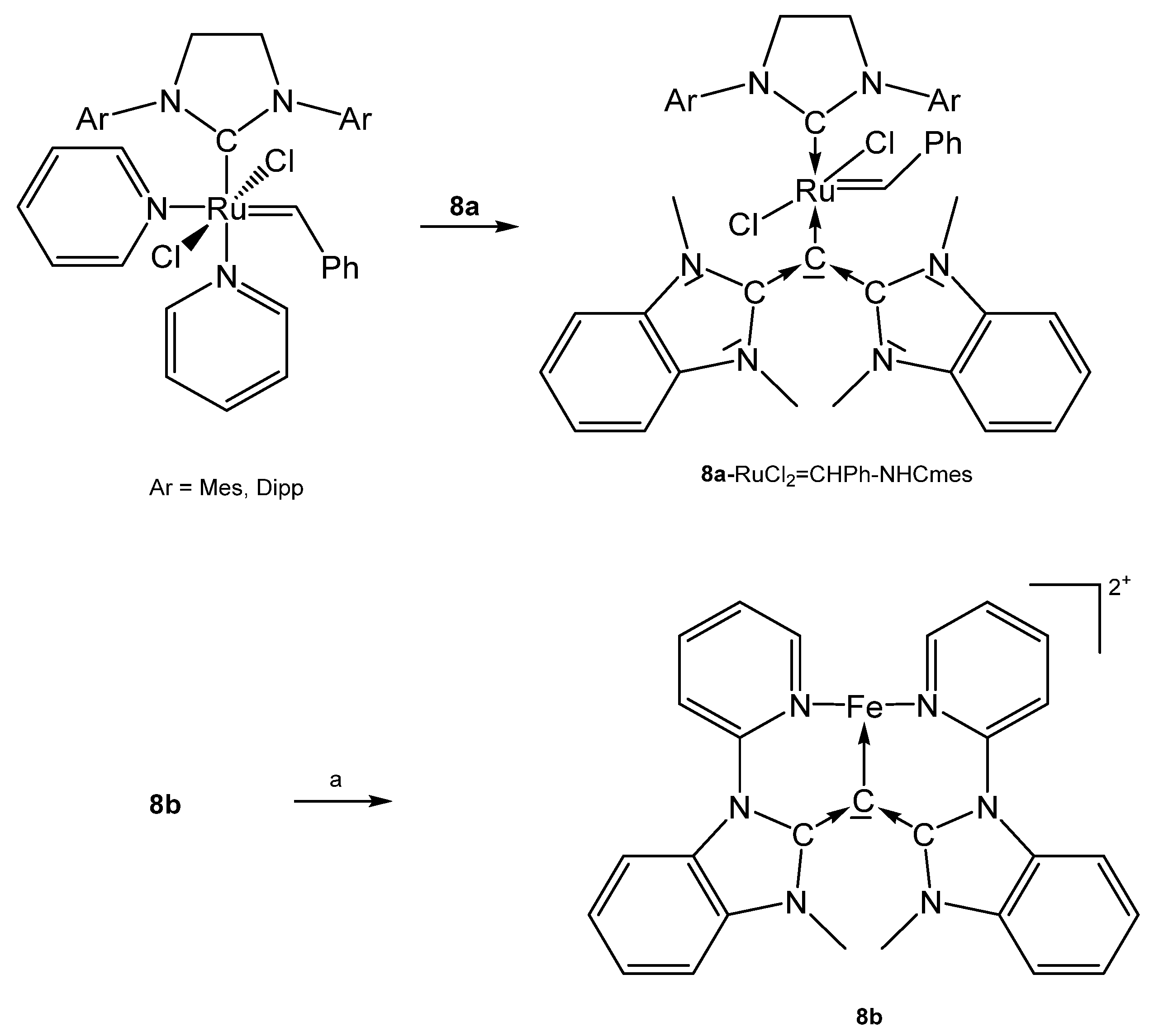
Further, 8a-RhCl(CO)2 was prepared by addition of a suspension of 8a (see Scheme 16) in benzene to a solution of [RhCl(CO)2]2 [83]. [8b-Fe0.5]2+ contains Fe2+ in octahedral environment coordinated by two molecules of 8b. Fe(II) can be successively oxidized to the corresponding tri-, tetra-, and pentacationic species [87].
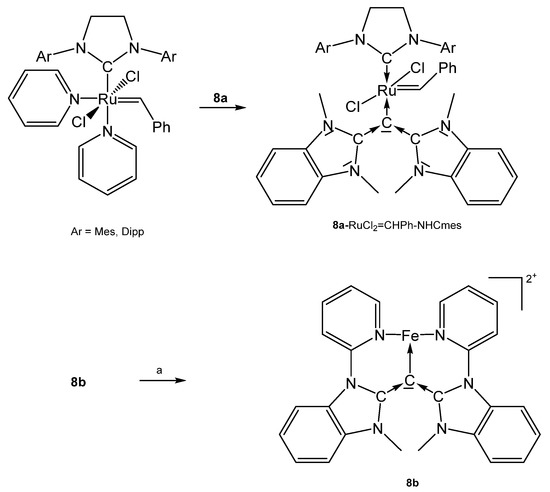
Scheme 16.
Selected structures of transition metal complexes with symmetric CDCs 8a and 8b; (a) Fe(OTf)2(MeCN)2.
The addition compounds 8c-RhCl(CO)2 and 8d-RhCl(CO)2 where obtained upon reacting the appropriate carbone 8c or 8d with [RhCl(CO)2]2. Similarly, the addition of [Pd(allyl)Cl]2 to 8c leads to the allyl complex 8c-PdCl(C3H5) [85].
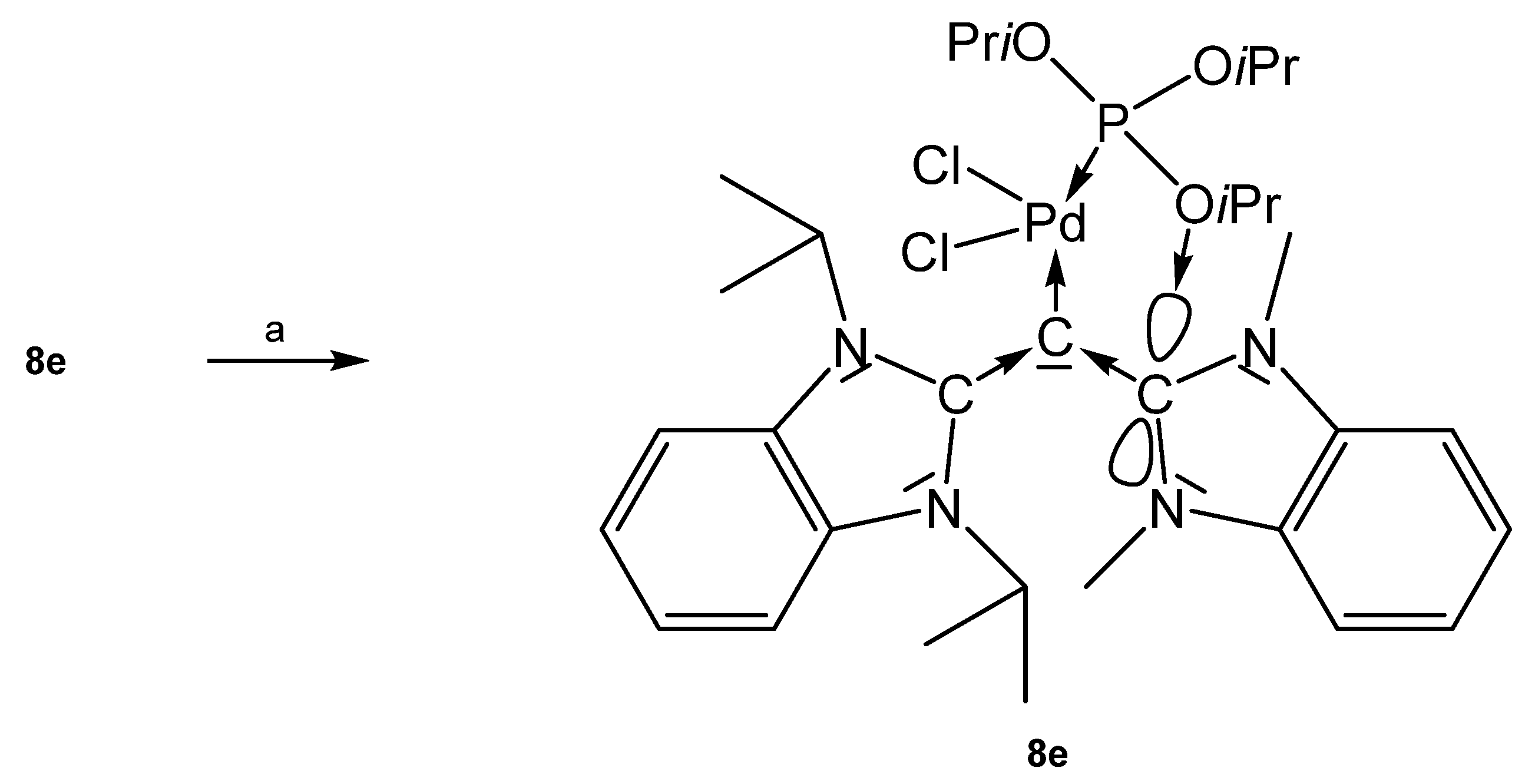
As depicted in Scheme 17, introduction of PdCl2P(OiPr)3 to 8e afforded the complex 8e-PdCl2P(OiPr)3; it features a square planar Pd center with a short interatomic distance of one phosphite oxygen atom and the carbon atom of the NHC molecule of 2.890 Å that is smaller than the sum of van der Waals radii. This indicates strong attractive interaction between the atoms [88]. The three Pd complexes 8e-PdCl2PPh3, 8e-PdCl2PTol3, and 8e-PdCl2PCy3 were obtained by reacting the carbone 8e with the appropriate PdCl2PR3; between the NHC and the aromatic phosphine substituents (Ph or Tol) an unexpected π-π interaction was detected. One Ph and one Tol group are nearly parallel to the imidazole rings with centroid-centroid distances of 3.25 Å (Ph) and 3.30 Å (Tol), respectively [89].
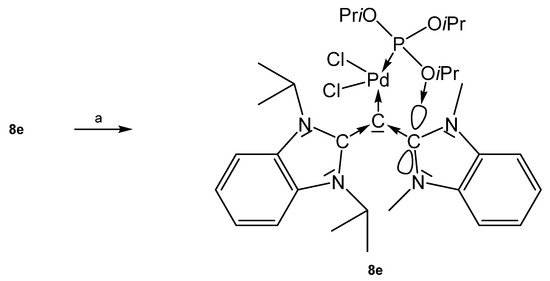
Scheme 17.
Selected structural representation of 8e-PdCl2P(OiPr)3 (a) PdCl2P(OiPr)3.
8f-RhCl(CO)2 and 8g-RhCl(CO)2 stem from reacting the appropriate carbone with [RhCl(CO)2]2 [90]. The cod ligand of [Ir(cod)Cl]2 was replaced by bubbling CO through a mixture with 8h to generate the complex 8h-IrCl(CO)2 [91].
Some experimental findings indicate that carbodicarbenes also have catalytic properties for a wide range of transformations, which are currently being actively studied by several groups. Examples have been reported such as hydrogenation of inert olefins [92], C-C cross-coupling reactions [84], intermolecular hydroamination [93] and hydroheteroarylation [94]. It seems that this area is still in an infant stadium and it can be expected that CDCs may be found useful as catalyst for other reactions.
2.9. Tridentate Cyclic Diphosphino CDCs
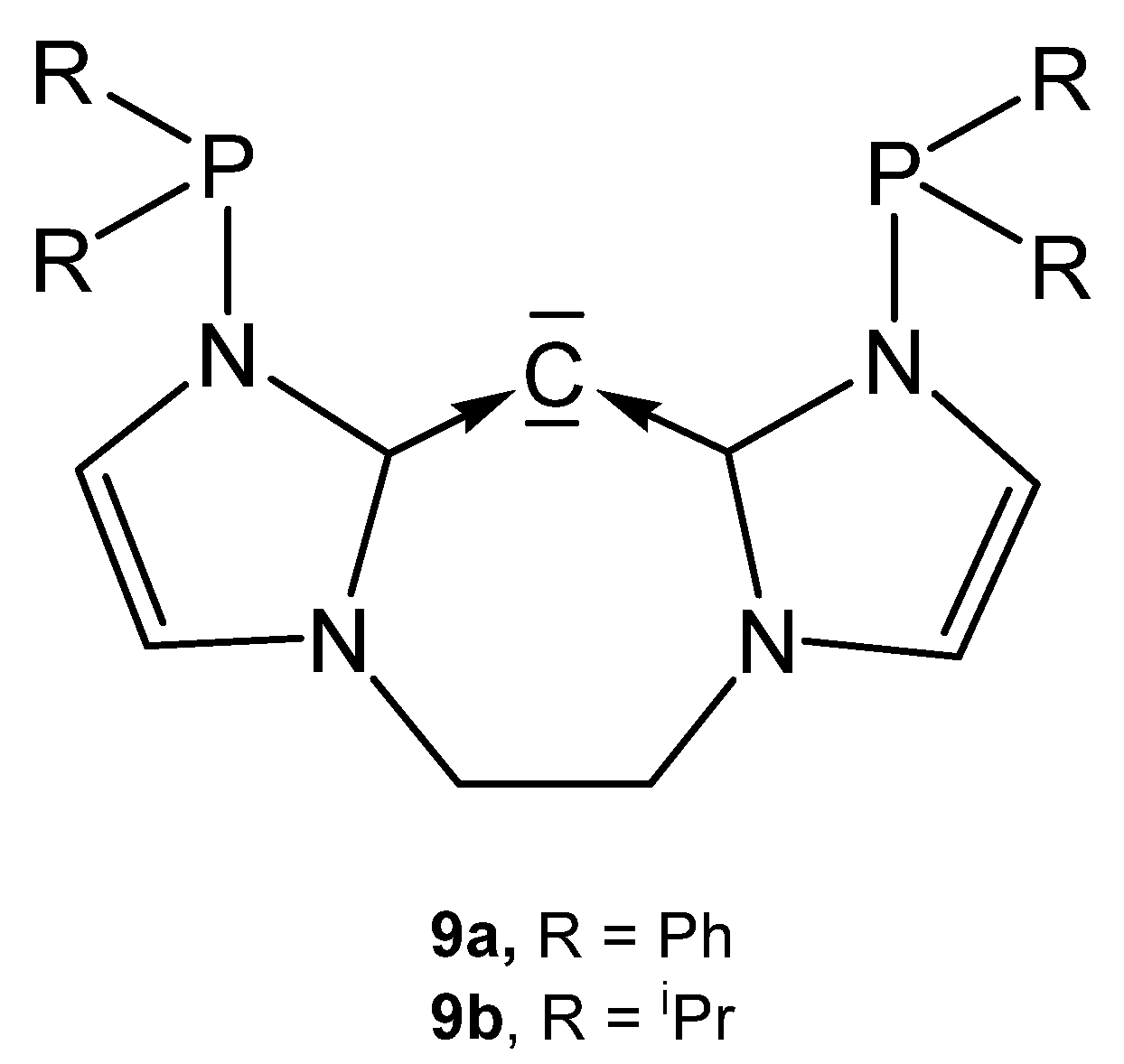
The carbones 9a and 9b in Figure 12 are functionalized carbodicarbene in which the donating carbon atom is part of a seven membered ring.
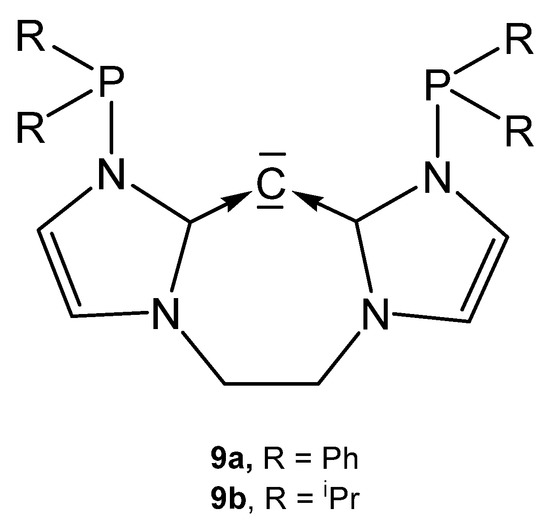
Figure 12.
Hypothetical free carbones 9a and 9b.
The neutral 9a and 9b could not be isolated, source for transition metal complexes are the related cations 9aH+ and 9bH+ (see Table 9) [93].

Table 9.
Transition metal complexes with the carbones 9a and 9b; 13C NMR signal of the central donating carbon atom.
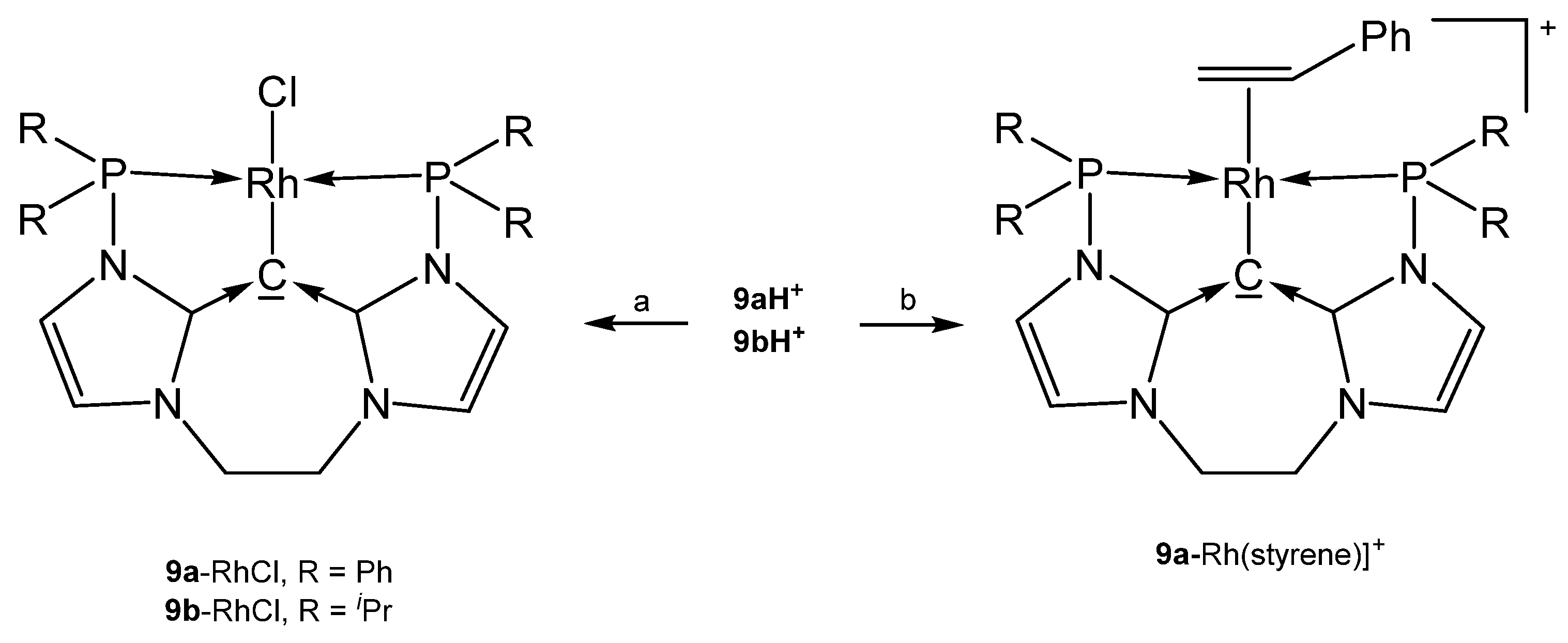
The neutral complexes 9a-RhCl and 9b-RhCl (see Scheme 18) where prepared upon reacting the cations 9aH+ or 9bH+, respectively with [Rh(cod)Cl]2/NaOMe; if treated with AgBF4/MeCN the cationic spezies [9a-Rh(MeCN)]BF4 and [9b-Rh(MeCN)]BF4, respectively, were isolated. The related carbonyl complexes [9a-Rh(CO)]BF4 and [9b-Rh(CO)]BF4 formed similarly upon reaction with [Rh(CO)2Cl]2/NaOMe [93]. The styrene complex [9a-Rh(styrene)]+ was obtained upon treating the related chloro complex with styrene/NaBAr4; the styrene complex catalyzes the hydroarylation of dienes. Protonation of [9a-Rh(CO)]+ with HBF4·OEt2 generates [9aH-Rh(CO)]2+ in which the carbone acts as four-electron donor [94].
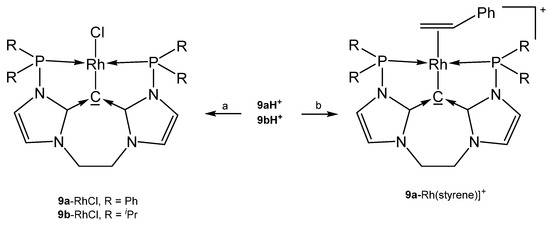
Scheme 18.
Selected structures of transition metal complexes with the carbones 9a and 9b. (a) [Rh(cod)Cl]2/NaOMe, (b) 9a-RhCl/styrene/NaBF4.
2.10. Tetraaminoallene (TAA) Transition Metal Complexes
The 13C NMR shift of the central carbon atom amounts to 142.8 ppm. The first and second PAs of 10 are 282.5 and 151.6 kcal/mol, respectively [16,82].
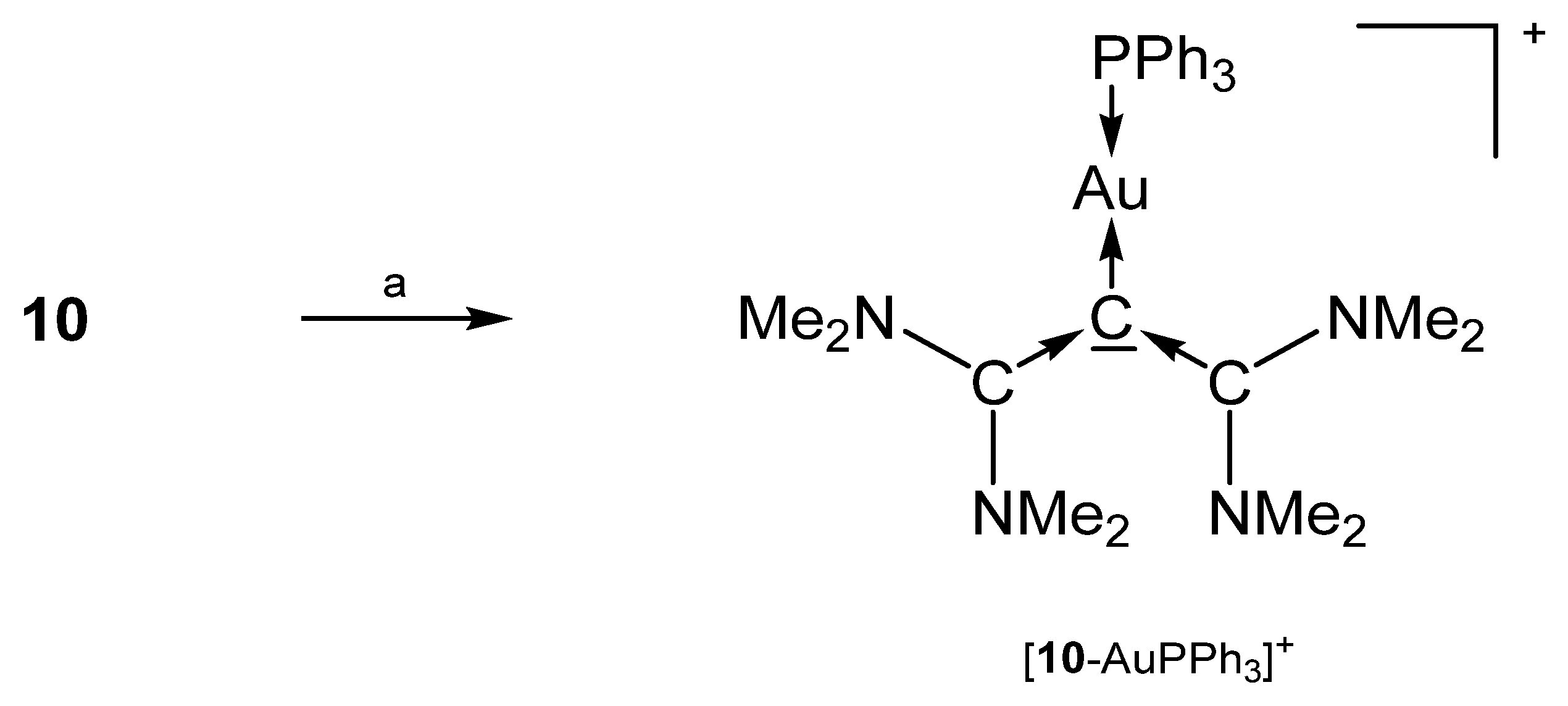
The salt [10-AuPPh3]SbF6 in Scheme 19 is the only transition metal complex of TAA (see Figure 13), which has been reported so far. Both carbene moieties are planar, but are tilted relative to each other, to relieve allylic strain. The Au-C bond lengths amounts to 2.072(3) Å and the slightly different C-C dative bonds has interatomic distances of 1.406(5) and 1.424(5) Å. The central C-C-C bond angle is reported with 118.5(3)° [95].
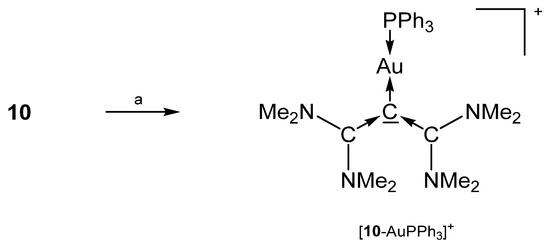
Scheme 19.
Preparation of [10-AuPPh3]SbF6; a) AuClPPh3/NaSbF6.
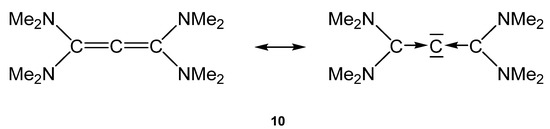
Figure 13.
Bonding description of tetraaminoallene (TAA) (10). TAA’s may have a bent geometry with hidden or masked pairs of electrons, which are delocalized but serve as double donor orbitals in complexes with CO2 and CS2 [96].
2.11. Transition Metal Complexes of Carbones with the P-C-C Skeleton
Mixed carbene-phosphine stabilized carbones from the working group of Bestmann (1974) and Alkarazo (2009).
The crystal structure of 11a in Figure 14 reveals a planar configuration of the carbene ligand C(OEt)2. Short P-C and C-C distances indicate some p back donation; P-C = 1.682(4)Å, C-C = 1.316(10) Å, C-C-C 125.6° (see Table 10) [97].

Figure 14.
In compounds 11 the C(0) atoms are stabilized by a phophine or a carbene ligand.

Table 10.
Transition metal complexes with the mixed carbones 11a and 11b. 31P NMR shifts in ppm.
The neutral Rh complex 11a-RhCl(CO)2 was obtained from reacting the carbone 11a with [Rh(CO)2Cl]2. Similarly, the complex 11b-AuCl results from reaction of 11b with AuCl(SMe2) (Scheme 20) [98].
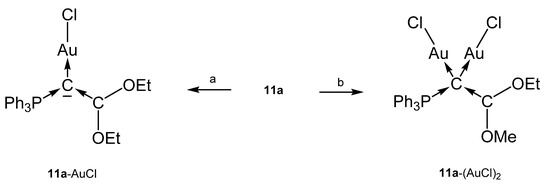
Scheme 20.
Selected structural representation of transition metal complexes of 11a. (a) one equiv. of AuCl(SMe2), (b) two equiv. of AuCl(SMe2).
2.12. Transition Metal Complexes of Carbones with the P-C-Si Skeleton
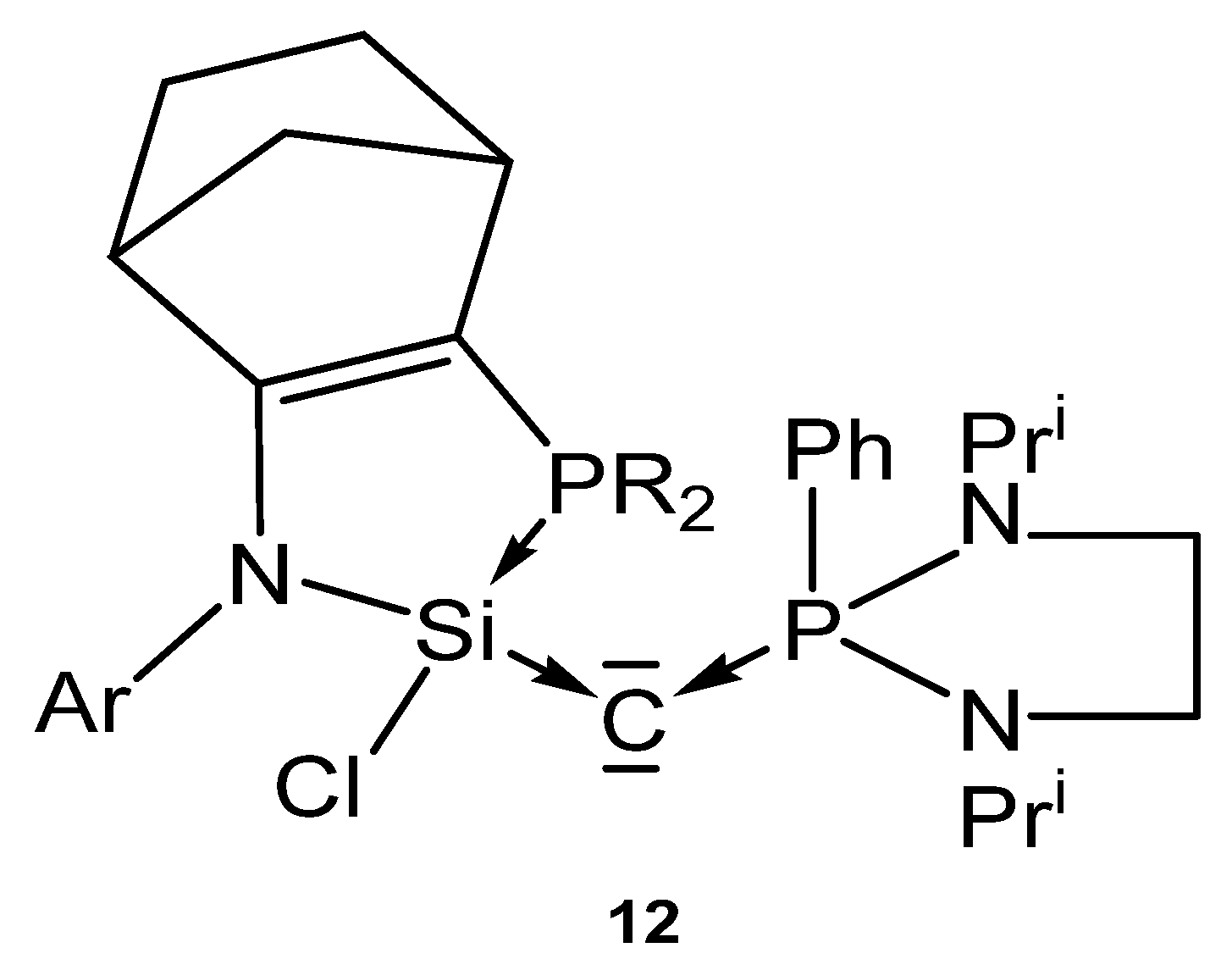
The neutral compound 12 in Figure 15 is a carbone in which the C(0) atom is stabilized by a donor stabilized silylene and a phosphine ligand.
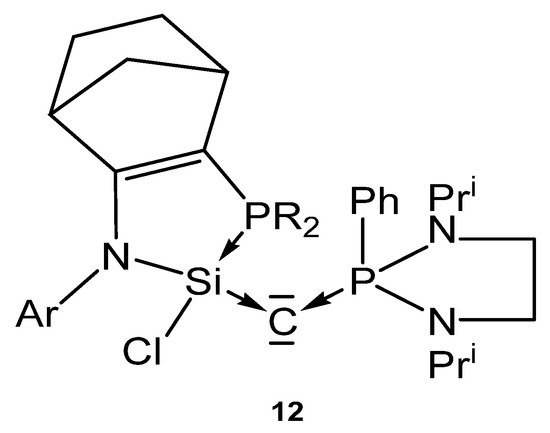
Figure 15.
Carbone complex reported by Kato et al. [99].
The crystal structure of a related compound to 12 (a cyclopentene instead of a cyclohexene ring) shows a P-C distance of 1.6226(4) Å and Si-C distance of 1.6844(4) Å; the Si-C-P angle amounts to 140.03(3)°.
Addition of CuCl generates the complex 12-CuCl. No spectroscopic or structural details are available [99].
2.13. Transition Metal Complexes of Carbones with the P-C-S Skeleton
A series of carbones (13a, 13b) in Figure 16 based on a P-C-S core containing the neutral S(IV) ligands SPh2=NMe (Figure 16) were reported by Fujii [100].
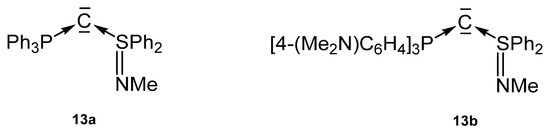
Figure 16.
Carbone complexes reported by Fujii et al. [100].
Crystal structures and 31P NMR shifts of the following basic carbones are available (see Table 11): 13a, δ = −2.64 ppm; 13b, δ = −1.39 ppm, P-C = 1.663(2) Å, S-C = 1.602(2) Å, P-C-S = 125.59(15)°. The authors revealed a high electron density at the central carbon atom.

Table 11.
Collection of transition metal complexes with the carbones 13a and 13b. 31P NMR signals (in ppm) are given.
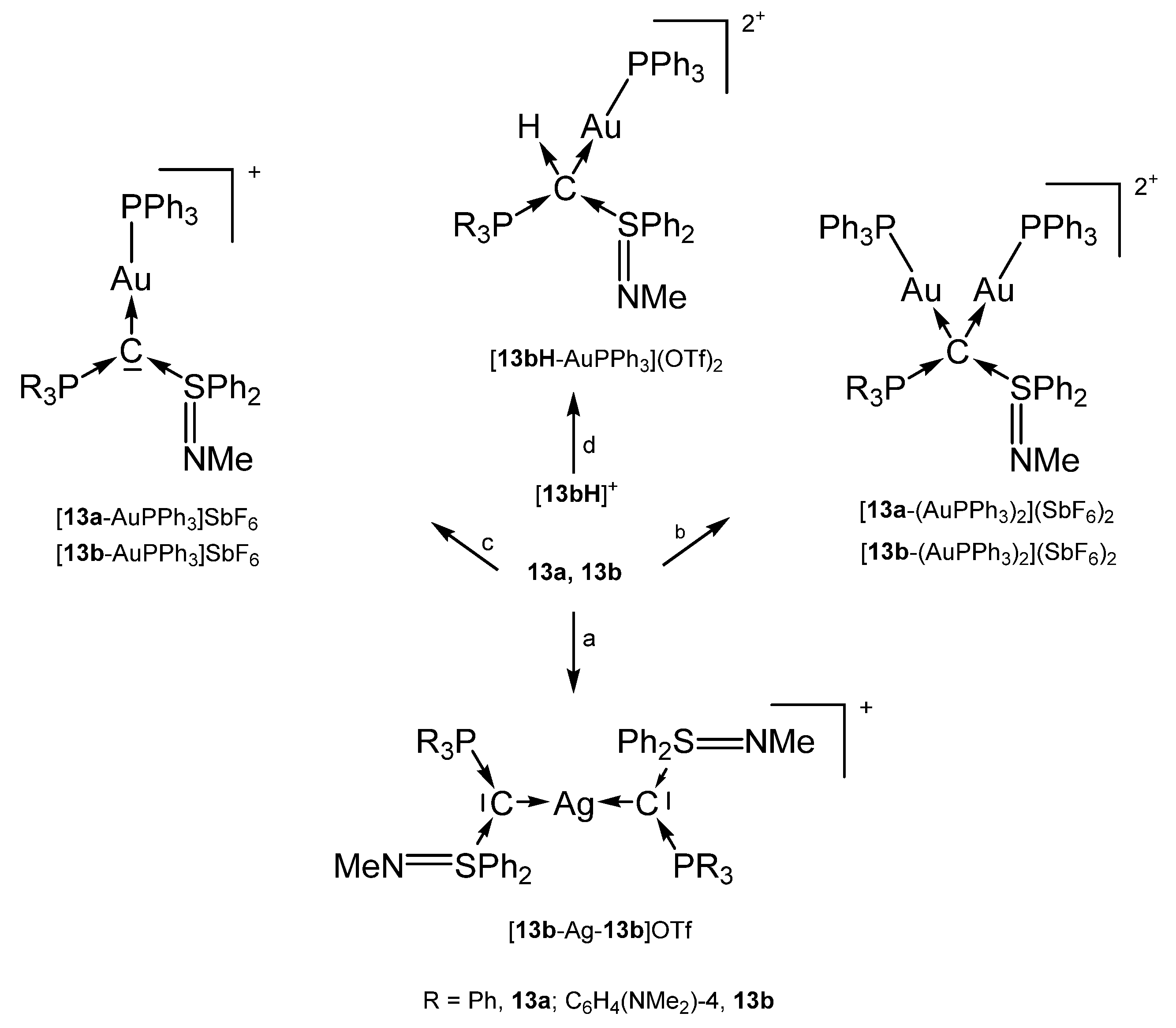
The addition products 13a-AgCl and 13b-AgCl were obtained from reacting [13aH]+ or [13bH]+, respectively with ion exchange resin (Cl− form) and Ag2O/CH2Cl2. For the other products see Scheme 21 [100].
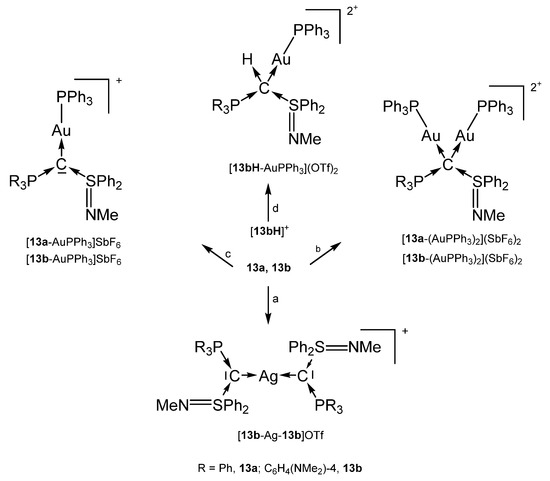
Scheme 21.
Selected structures with the carbones 13a und 13b: (a) 0.5 eq. of AgOTf, (b) 2 eq. of AuCl(PPh3)/2 eq. of AgSbF6, (c) 1 eq. of AuCl(PPh3)/1 eq. of AgSbF6, (d) ion exchange (OH− form), 1 eq. of AuClPPh3/1 eq. of AgOTf [100].
Addition of TM fragments to 13a or 13b in Scheme 21 elongates P-C and S-C bond length as reported for 1a. That of [13bH-AuPPh3](OTf)2 in which 13b acts as four-electron donor are elongated to normal single bonds [100].
2.14. Transition Metal Complex with a P-C-S Core Possessing a Neutral S(II) Ligand
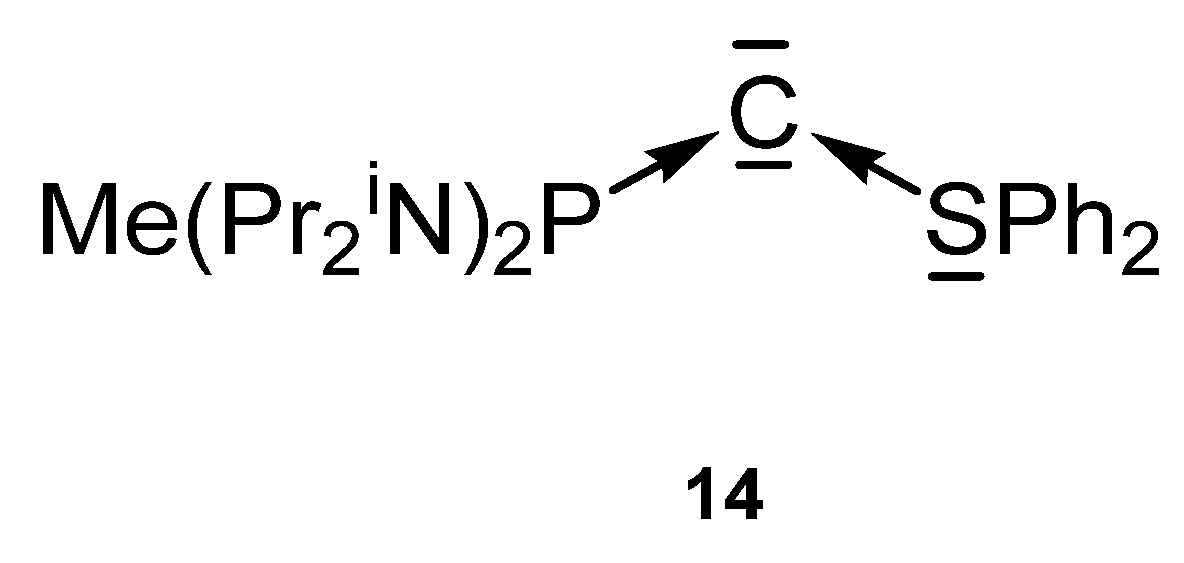
The carbone 14 in Figure 17 contains a phosphine and a S(II) ligand with a free pair of electrons to stabilize the C(0) atom. However, the bare 14 could not be isolated, but only the protonated cation [14H]+ and used as starting material [101].
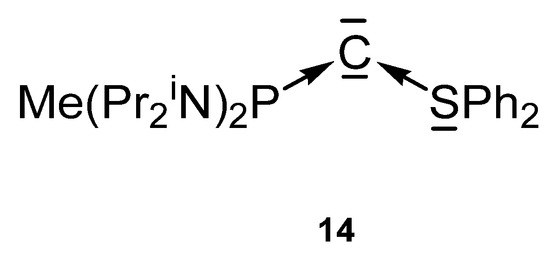
Figure 17.
Mixed P and S stabilized carbone 14.
The transition metal complex [14-CuN(SiMe3)2](OTf) was prepared upon reacting [14H]+ with KHMDS/CuCl. X-ray analysis reveals a Cu-C distance of 1.903(4) Å and the P-C and S-C distances amount to 1.709(5) and 1.677(5) Å, respectively. As found in carbone addition compounds of 13a and 13b the P-C distance is longer than the S-C distance. An acute P-C-S angle of 115.3(2)° was recorded. The 31P NMR signal is shifted to lower fields at 66.5 ppm [101].
2.15. Transition Metal Complexes of Carbones with the S-C-S Skeleton
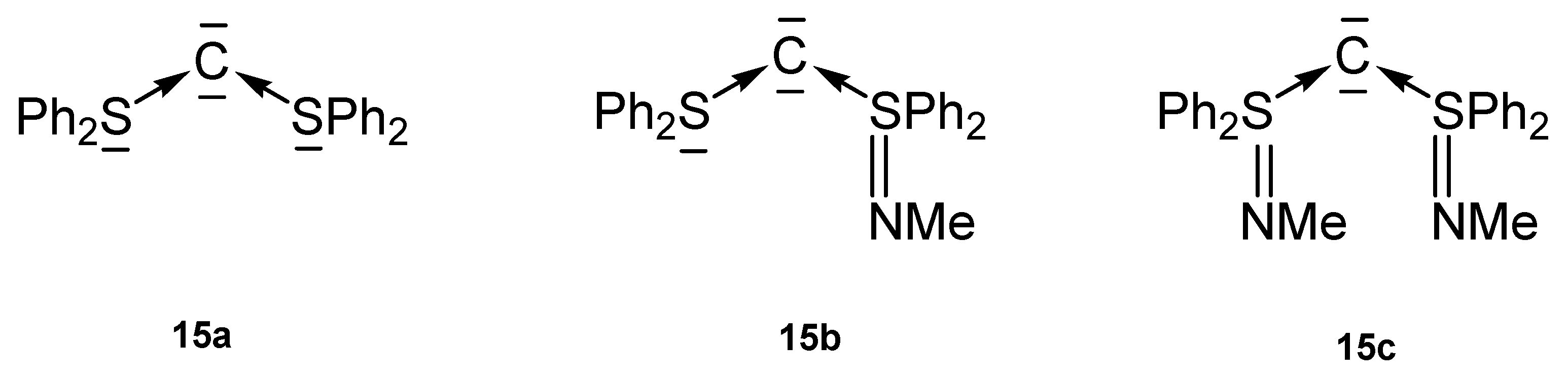
In the carbones 15 (carbodisulfanes, CDS) the central carbon atom is stabilized by two neutral S(II) ligands (15a), or S(II), S(IV) groups (15b), or two S(IV) (15c) ligands (see Figure 18).
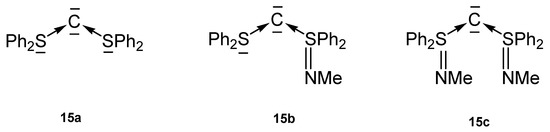
Figure 18.
Sulfur based carbones 15 as ligands for transition metal complexes.
The molecular structure of 15a was investigated computationally (see Table 12) [102]. For the carbones the following parameters were recorded: 15b, C-SII 1.707(2), C-SIV 1.648(2), S-C-S 106.67(14). 13C NMR, δ = 35.4 ppm [103]. 15c, S-C 1.635(4), 1.636(2); S-C-S 116.8(2) [104]. Similar to CDCs the first and second PAs of 15b amount to 288.0 and 184.4 kcal/mol, respectively.

Table 12.
Transition metal complexes with selected bond length (Å) and angles (deg) of the carbone ligands 15a to 15c. 13C NMR signal (in ppm) of the central carbon atom.
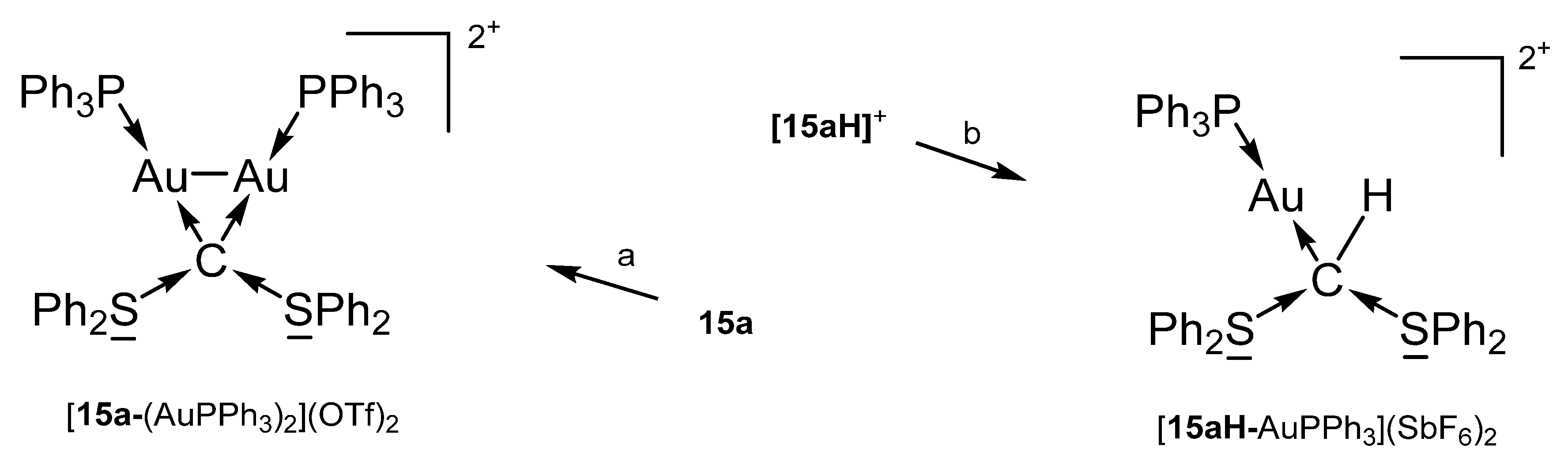
15a-AgCl was obtained from [15aH]+ upon treating with Ag2O/CH2Cl2. The salt [15a-AuPPh3]OTf formed reacting the bare 15a with AuCl(PPh3) followed by addition of NaTfO in THF. [15a-(AuPPh3)2](OTf)2 and [15aH-AuPPh3](SbF6) are sketched in Scheme 22 [102].
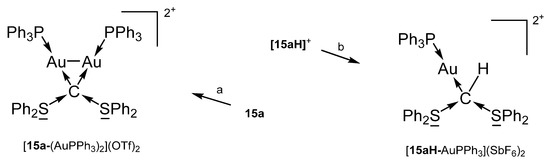
Scheme 22.
Selected of complexes with the carbone 15a. (a) 2 eq AuCl(PPh3), (b) AuCl(PPh3)2.
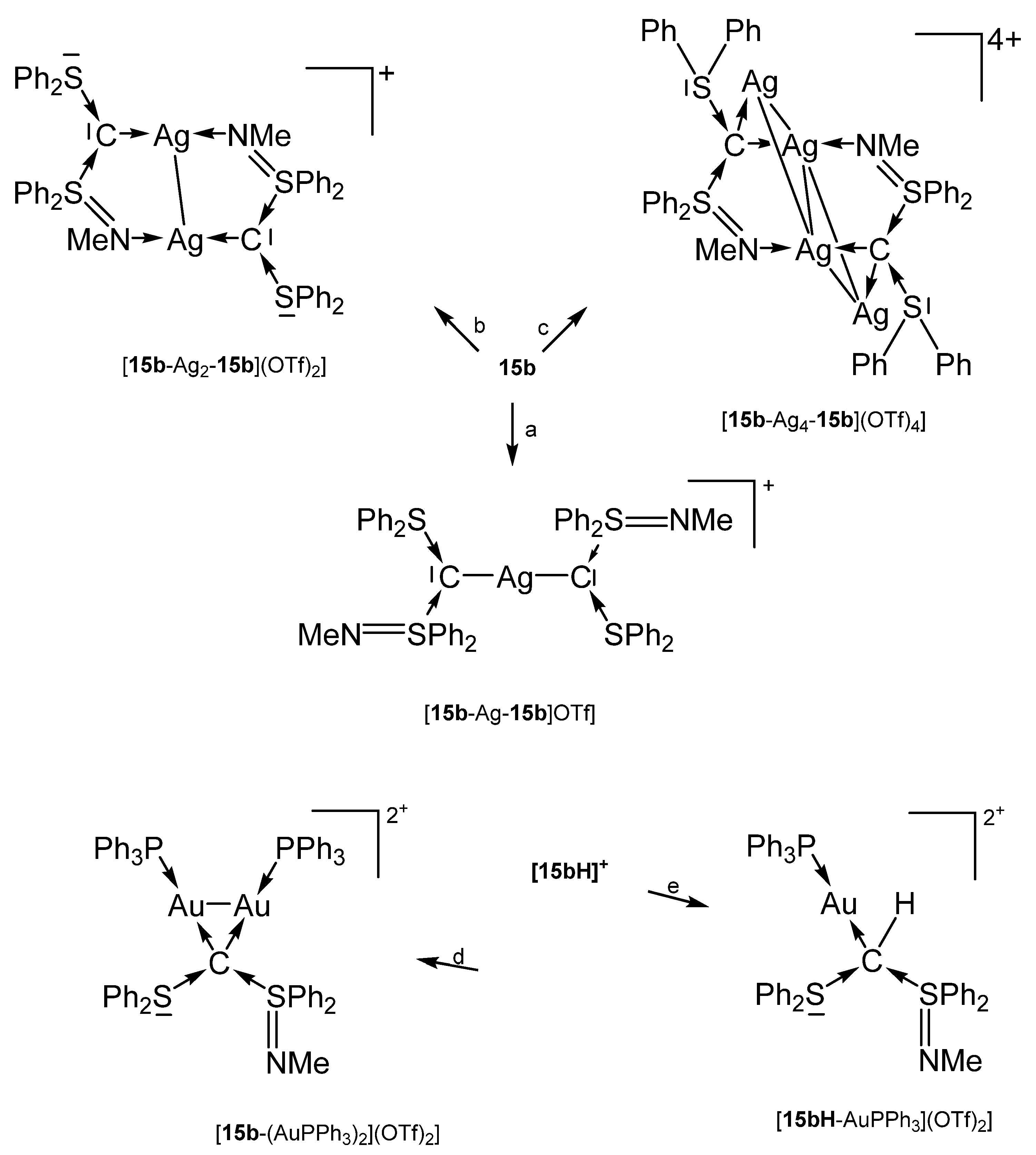
[15b-AuPPh3]OTf was obtained analogously formed from reacting 15b with AuCl(PPh3) followed by addition of NaTfO in THF. For the other compounds, see Scheme 23 [102].
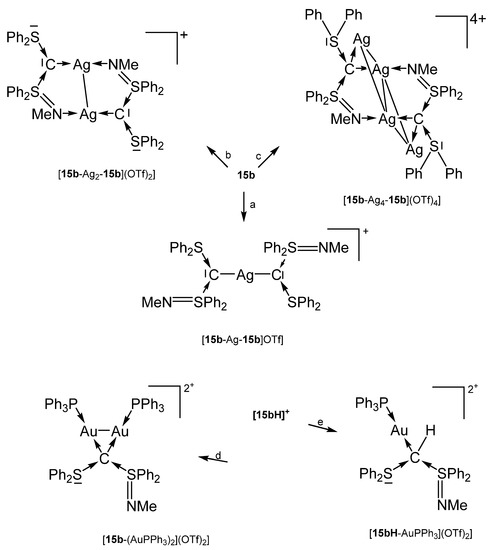
Scheme 23.
Selected of complexes with the carbone 15b. (a) 0.5 eq AgOTf, (b) 1.0 eq AgOTf, (c) 2.0 eq AgOTf, (d) 2 eq AuCl(PPh3), (e) AuCl(PPh3).
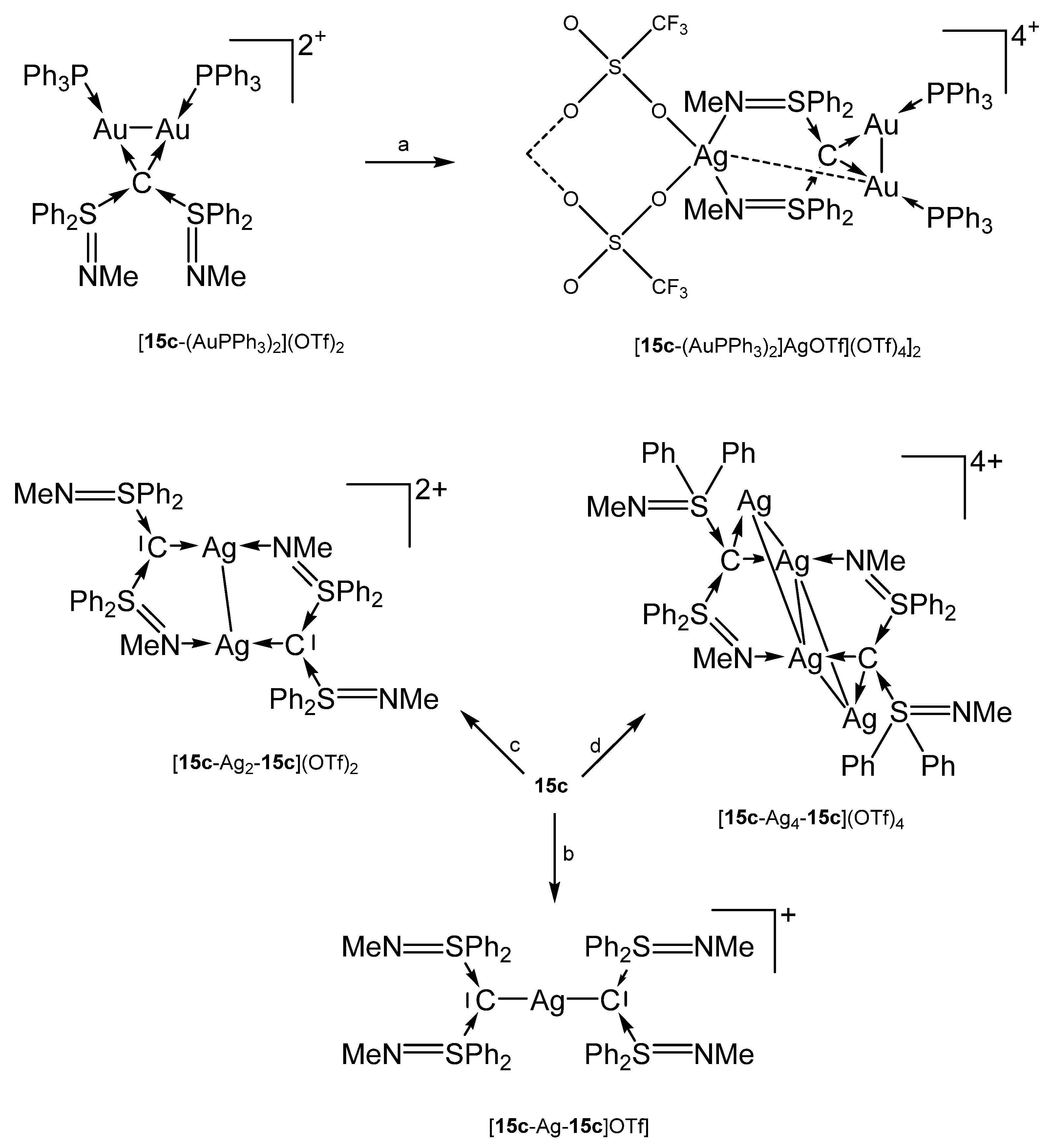
The preparation of [15c-AuPPh3]OTf and 15c-AgCl follows the procedure outlined for the related 15b compounds [102]. For the other compounds, see Scheme 24 [102,105]. The hetero hexametallic cluster {[15c-(AuPPh3)2AgOTf](OTf)4}2 is supported by two carbone ligands that adopt a κ4C,C’,N,N’ coordination mode. The Au-Ag separation amounts to 3.003 Å [102].
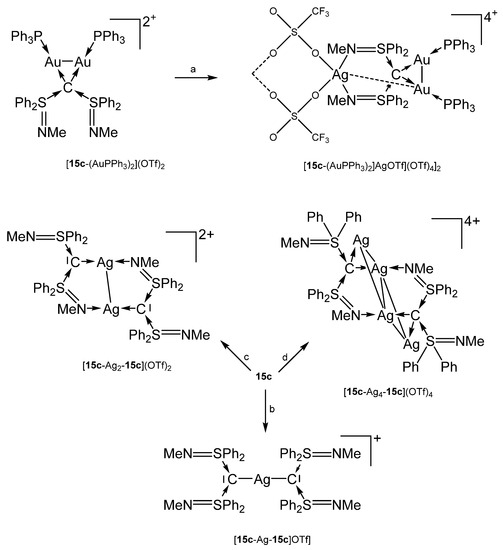
Scheme 24.
Selected complexes with the carbone ligand 15c; (a) AgOTf, (b) 0.5 eq AgOTf, (c) 1.0 eq AgOTf, (d) 2.0 eq AgOTf. {[15c-(AuPPh3)2AgOTf](OTf)4}2 is dimeric linked by two OTf anions.
13C NMR signals of the donating C(0) atoms (if available) of all addition compounds of 15a to 15c are less shielded than that of the basic carbones [102].
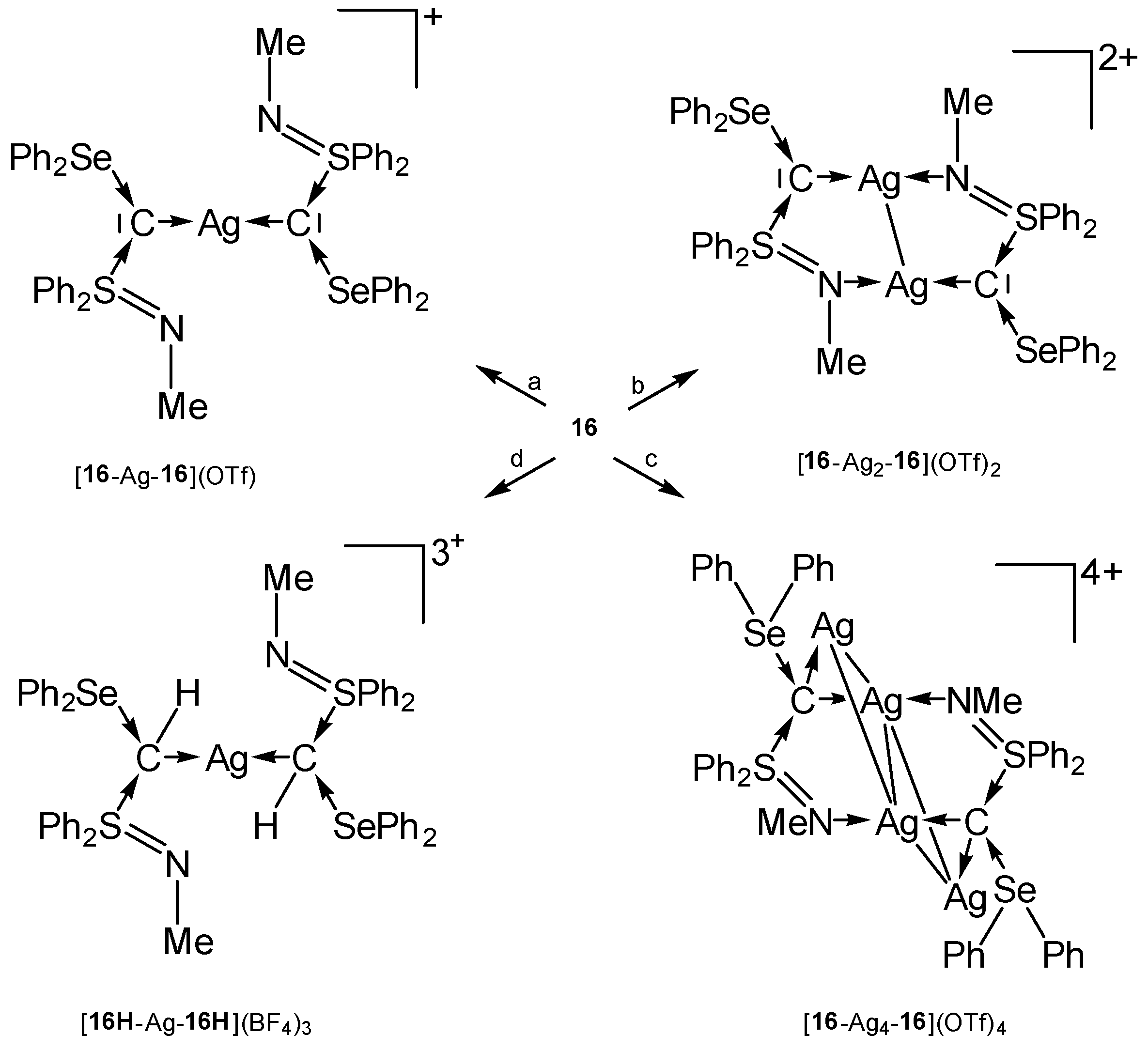
2.16. Transition Metal Complexes of Carbones with the S-C-Se Skeleton (16)
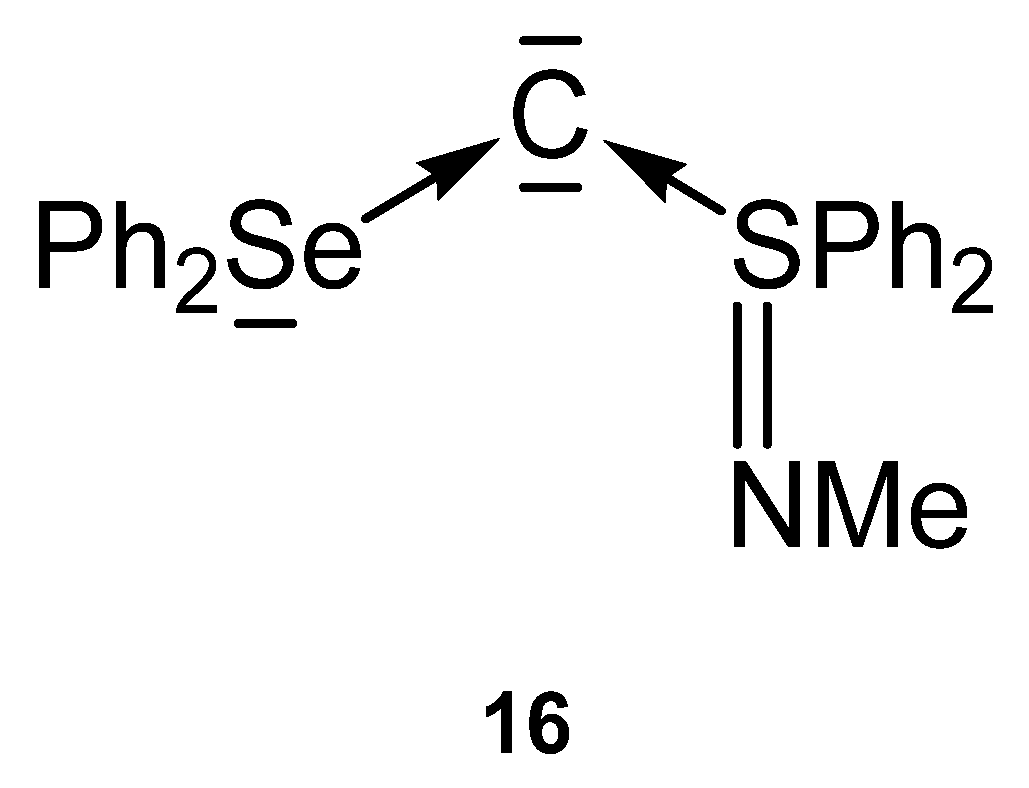
Compound 16 in Figure 19 is the first carbone containing a Se(II) compound together with a S(IV) one as ligand for stabilization of a C(0) atom.
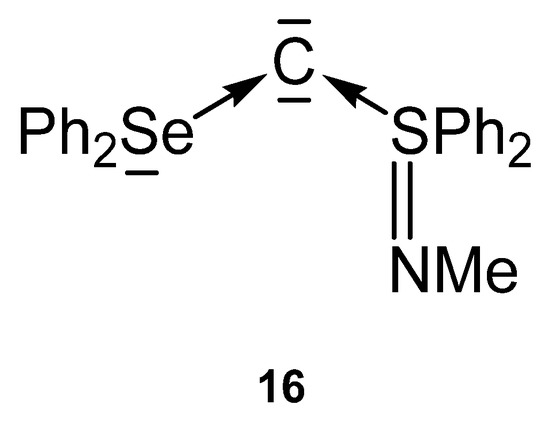
Figure 19.
Carbone with Se and S based ligands L.
The tetranuclear complex [16-Ag4-16]4+ contains a rhomboidal [Ag4]4+ core surrounded by two carbones 16 (see Table 13). In this and in [16H-Ag-16H]3+ the donating C(0) acts as a four-electron donor (see Scheme 25) [105].

Table 13.
Transition metal complexes with selected bond length (Å) and angles (deg) of the carbone 16. 13C NMR signal (in ppm) of the central carbon atom.
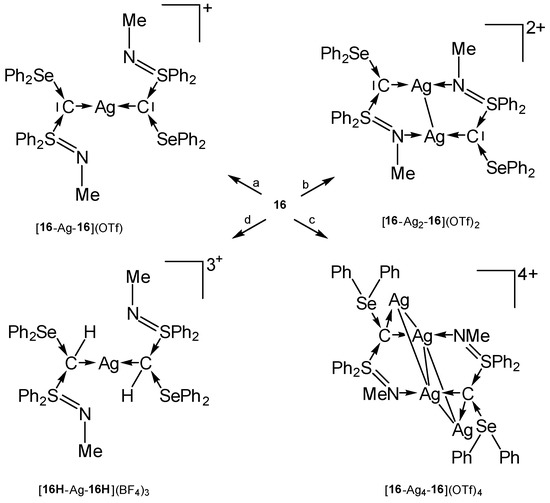
Scheme 25.
Transition metal complexes with the carbone 16 as two and four electron donor. (a) 0.5 eq AgOTf, (b) 1 eq AgOTf, (c) 2.0 eq AgOTf, (d) AgBF4/CH2Cl2.
3. Transition Metal Carbido Complexes [M]-C
The second part of this review summarizes the research of transition metal complexes with a naked carbon atom as ligand [M]-C. They are often termed as carbides, but the bonding situation is clearly different from well-known carbides of the alkaline and alkaline earth elements E, which are salt compounds of acetylene EnC2. The electron configuration of carbon atom in the 1D state (2s22px22py02pz0) is perfectly suited for dative bonding with a transition metal following the DCD model [7] in terms of σ donation and π backdonation [M]  C|. Carbon complexes [M]-C may thus be considered as carbone complexes [M]-CL2 without the ligands L at the carbon atoms. A theoretical study showed in 2000 that the 18 valence electron (VE) complex [(CO)4Fe(C)] is an energy minimum structure with a rather strong Fe-C bond [106]. However, such 18 VE systems could not be synthesized as isolated species but were only found as ligands where the lone-pair electron at the carbon atom serves as donor (see below). It seems that the electron lone-pair at carbon in the 18 VE complexes [M]-C makes the adducts too reactive to become isolated.
C|. Carbon complexes [M]-C may thus be considered as carbone complexes [M]-CL2 without the ligands L at the carbon atoms. A theoretical study showed in 2000 that the 18 valence electron (VE) complex [(CO)4Fe(C)] is an energy minimum structure with a rather strong Fe-C bond [106]. However, such 18 VE systems could not be synthesized as isolated species but were only found as ligands where the lone-pair electron at the carbon atom serves as donor (see below). It seems that the electron lone-pair at carbon in the 18 VE complexes [M]-C makes the adducts too reactive to become isolated.
 C|. Carbon complexes [M]-C may thus be considered as carbone complexes [M]-CL2 without the ligands L at the carbon atoms. A theoretical study showed in 2000 that the 18 valence electron (VE) complex [(CO)4Fe(C)] is an energy minimum structure with a rather strong Fe-C bond [106]. However, such 18 VE systems could not be synthesized as isolated species but were only found as ligands where the lone-pair electron at the carbon atom serves as donor (see below). It seems that the electron lone-pair at carbon in the 18 VE complexes [M]-C makes the adducts too reactive to become isolated.
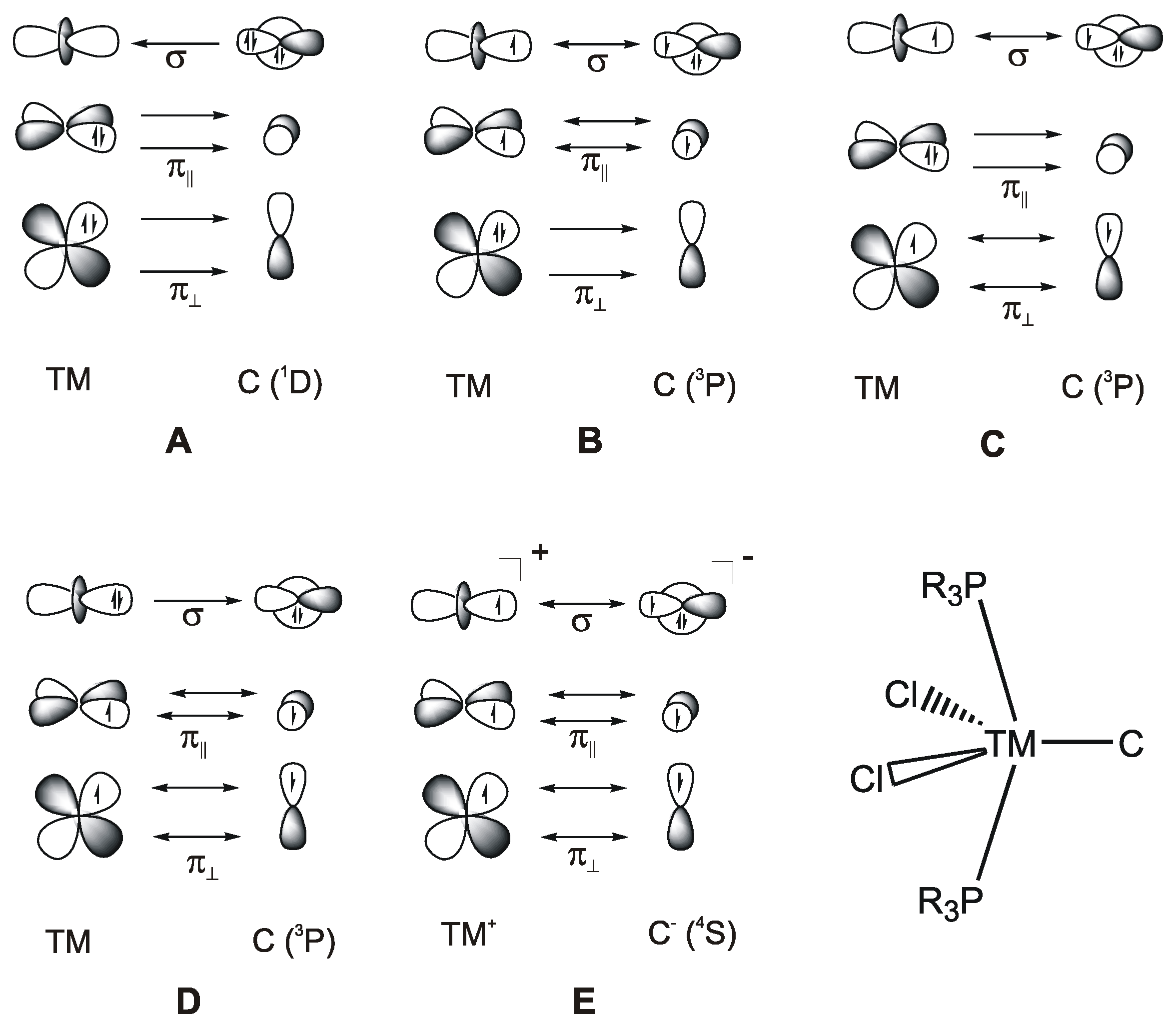
C|. Carbon complexes [M]-C may thus be considered as carbone complexes [M]-CL2 without the ligands L at the carbon atoms. A theoretical study showed in 2000 that the 18 valence electron (VE) complex [(CO)4Fe(C)] is an energy minimum structure with a rather strong Fe-C bond [106]. However, such 18 VE systems could not be synthesized as isolated species but were only found as ligands where the lone-pair electron at the carbon atom serves as donor (see below). It seems that the electron lone-pair at carbon in the 18 VE complexes [M]-C makes the adducts too reactive to become isolated.It came as a surprise when Heppert and co-workers reported in 2002 the first neutral adducts with a naked carbon atom as a ligand, which are the formally 16 VE diamagnetic ruthenium complexes [(PCy3)LCl2Ru(C)] (L= PCy and 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene; Cy = Cyclohexyl) [27]. A subsequent bonding analysis of the model compound [(Me3P)2Cl2Ru-C] considered five different models A–E for the Ru-C bonds that are shown in Figure 20 [28]. It turned out that the best description for the bonding interactions is a combination of electron-sharing and dative bonds. An energy decomposition analysis [107] suggested that the model B provides the most faithful account of the bond, where the σ bond and the π bond in the Cl2M plane come from electron-sharing interactions Cl2M=C whereas the π bond in the P2M plane is due to backdonation (Me3P)2Ru→C. The compounds [(PCy3)LCl2Ru(C)] should therefore be considered as 18 VE Ru(IV) adducts. The following section summarizes the research of transition metal complexes with a naked carbon atom as ligand [M]-C that has been accomplished since 2002.
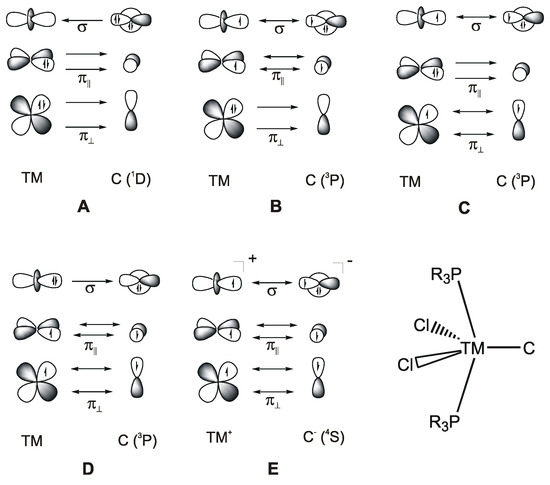
Figure 20.
Bonding models (A–E) for the bonding between a transition metal (TM) and a naked carbon atom in the compound [(R3P)2Cl2Ru-C].
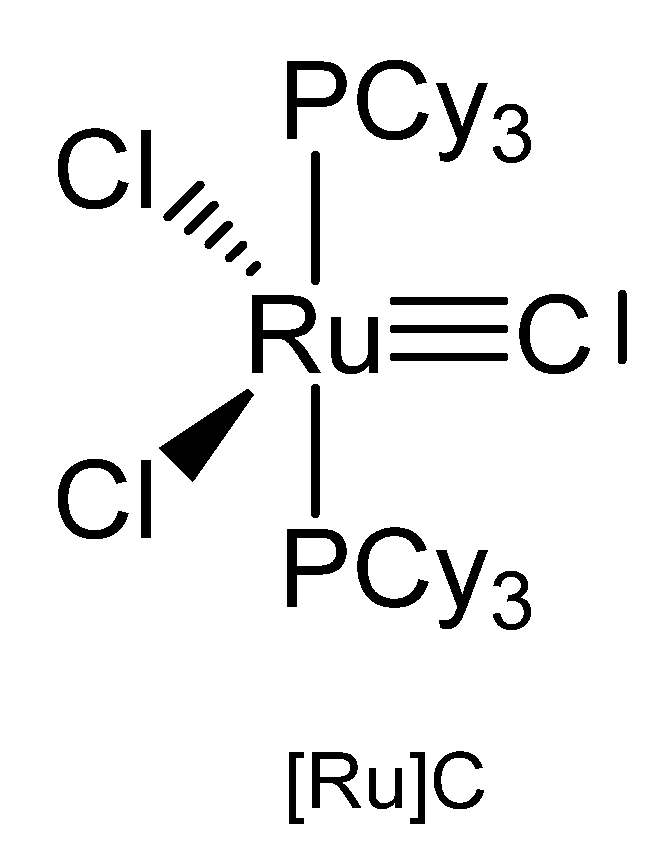
3.1. The System RuCl2(PCy3)2C ([Ru]C)
By far the most known complexes with carbido ligands that have been synthesized and structurally characterized are ruthenium adducts. The progress in the chemistry of ruthenium carbido complexes was reviewed in 2012 by Takemoto and Matsuzaka [108]. In the following, we summarize the present knowledge on ruthenium carbido complexes which has been reported in the literature.
The X-ray analysis of [Ru]C in Figure 21 exhibits a Ru-C distance of 1.632(6) Å. A signal at 471.8 ppm was attributed to the ligand carbon atom [109]. A general route to carbon complexes is described in [110].
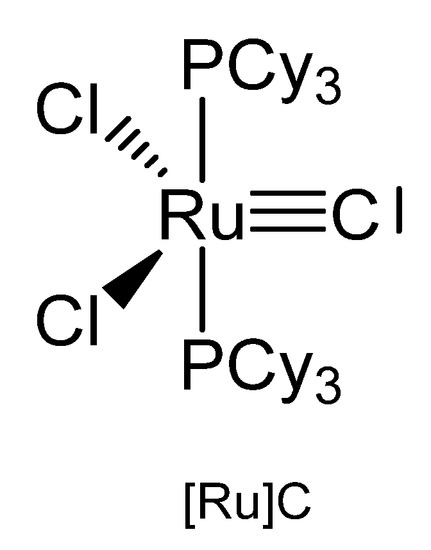
Figure 21.
The [Ru]C core.
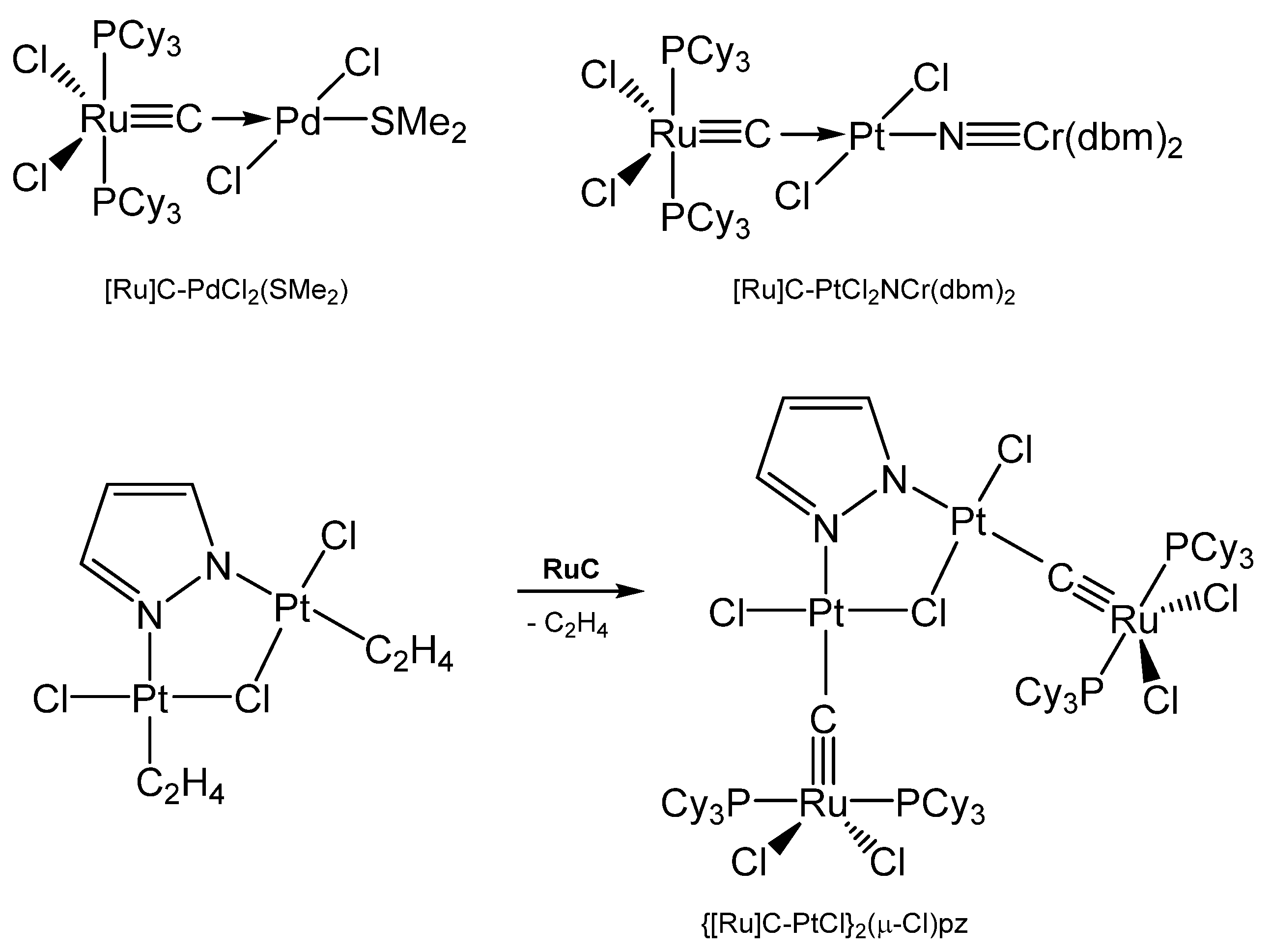
Addition of PdCl2(SMe2)2 gives the complex [Ru]C→PdCl2(SMe2), while with Mo(CO)5(NMe3) the carbonyl complex [Ru]C→Mo(CO)5 is generated (see Table 14) [29,109]. A series of [Ru]C→PtCl2L complexes were obtained by Bendix from reacting the dimeric complex {[Ru]C→PtCl2]2 with various ligands L (L = PPh3, PCy3, P(OPh)3, AsPh3, CNtBu, CNCy). Complexes with bridging ligands L such as {[Ru]C→PtCl2]2bipy, {[Ru]C→PtCl2]2pyz, and {[Ru]C→PtCl2]2pym formed upon displacing ethylene from the related (C2H4)PtCl2-L-PtCl2(C2H4) by [Ru]C. {[Ru]C→PtCl]2(μ-Cl)pz results from an ethylene complex and [Ru]C as depicted in Scheme 26 [111]. A series of Pt, Pd, Rh, Ir, Ag, Ru complexes were presented by Bendix with X-ray data and 13C NMR shifts of the ligand carbon atom ranging between 340 and 412 ppm [112]. Sulfur containing TM complexes with the metals Pd, Pt, Au, and Cu stem from the same laboratory. The sulfur ligands are ttcn = 1,4,7-trithiacyclononane and S4(MCp*)3 (see Figure 22) [113].

Table 14.
Selected structural (in Å and deg) and spectroscopic (13C NMR in ppm) details of [Ru]C addition compounds.
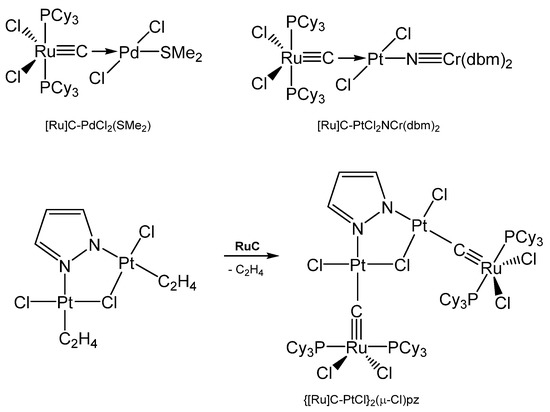
Scheme 26.
Selected [Ru]C→M carbido complexes and synthesis of {[Ru]C→PtCl}2(μ-Cl)pz.
Figure 22.
Spezification of ligands of Table 14.
3.2. The System RuCl2(PCy3)(NHC)C (NHC[Ru]C)
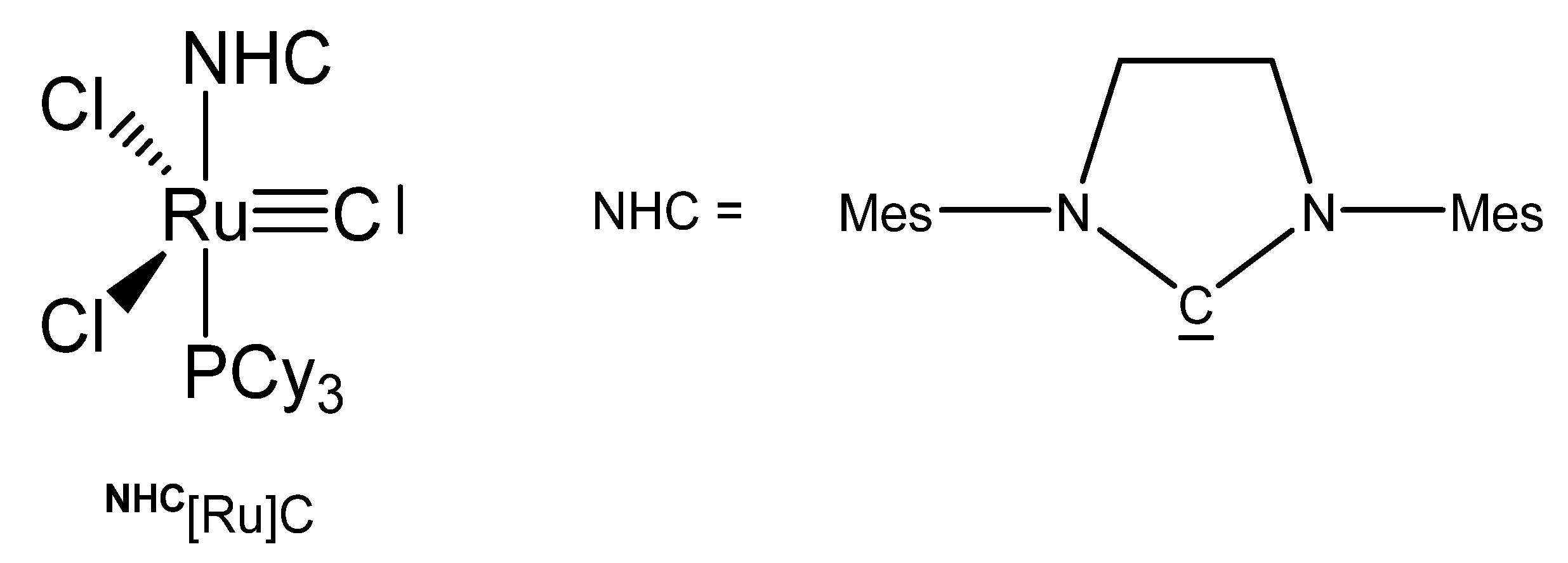
The X-ray analysis of NHC[Ru]C in Figure 23 exhibits a Ru-C distance of 1.605(2) Å. A signal at 471.5 ppm was attributed to the ligand carbon atom. No addition compounds were described so far [27].
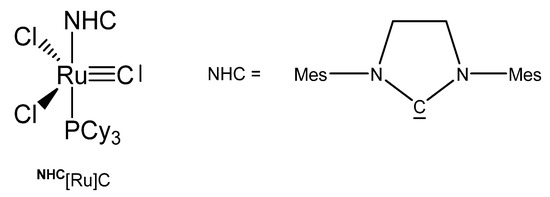
Figure 23.
The NHC[Ru]C core.
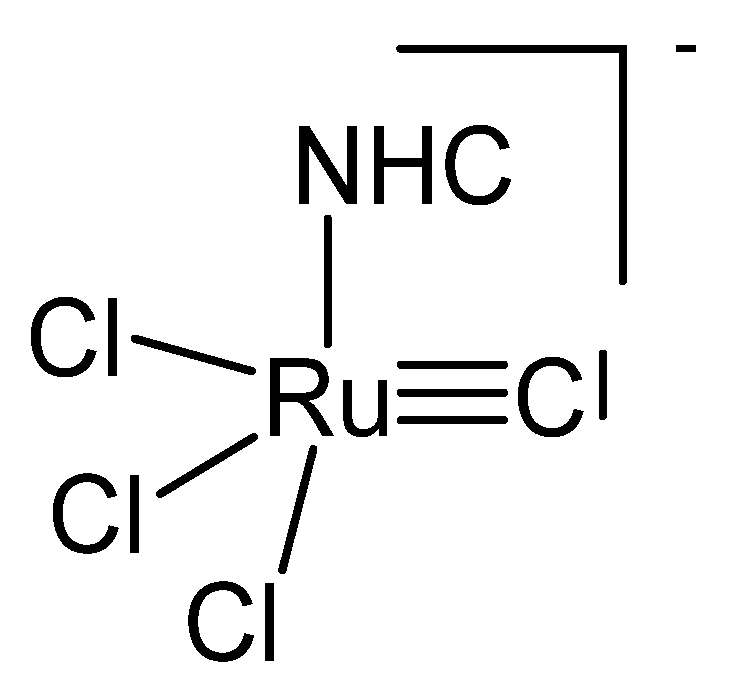
3.3. The System (NHC)Cl3RuC− (NHC[Ru]−C)
Treating the carbene complex (NHC)Cl2(PCy3)Ru=CH2 in Figure 24 at 55° in benzene generated the neutral complex depicted in Figure 25. X-ray analysis revealed a Ru1-C distance of 1.698(4) Å and the Ru2-C distance of 1.875(4) Å with a Ru-C-Ru angle of 160.3(2)°. In the 13C NMR the bridging C atom resonates at the typical value of 414.0 ppm [114].
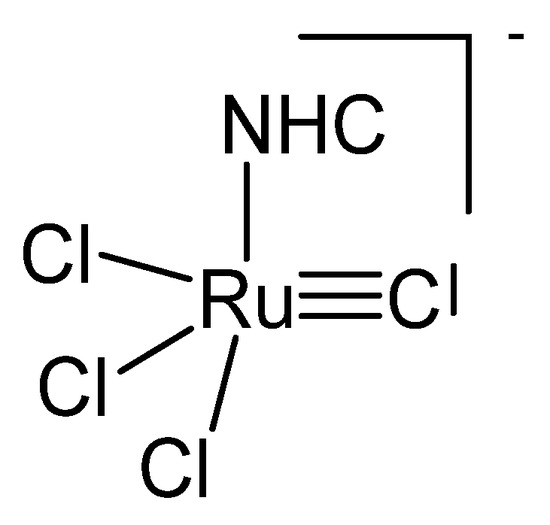
Figure 24.
The NHC[RuCl3]−C core.
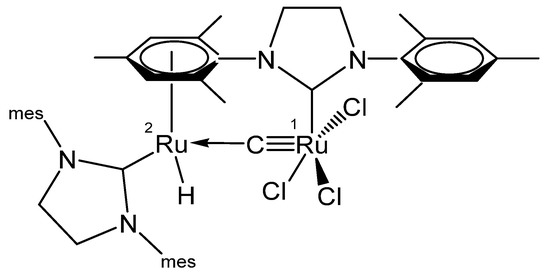
Figure 25.
Structural representation of the Ru carbido complex Ru2(NHC)2(≡C)Cl3H.
3.4. The system RuClX(PCy3)2C ([Ru]XC)
Various carbido complexes were reported in which one or both chloride ions in [Ru]C are replaced by X (X = Br, I, CN, NCO, NCS) (see Figure 26). {[Ru](MeCN)C}OTf is the first cationic carbido complex which is also starting point for most of the substituted carbido complexes. X-ray data for {[Ru](MeCN)C}OTf, [Ru](CN)2C, [Ru](Br)C, and [Ru](NCO)C are available (see Table 15) [115].
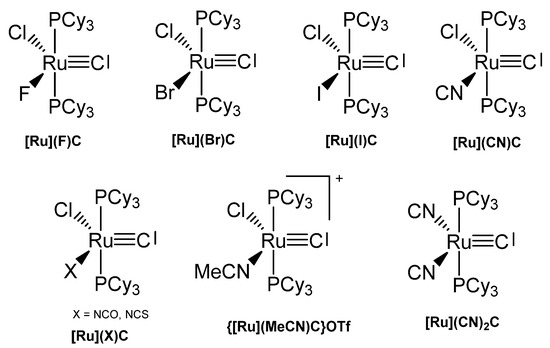
Figure 26.
Carbido compounds of [Ru]XC with various X.

Table 15.
Carbido complexes with the [Ru]XC core.
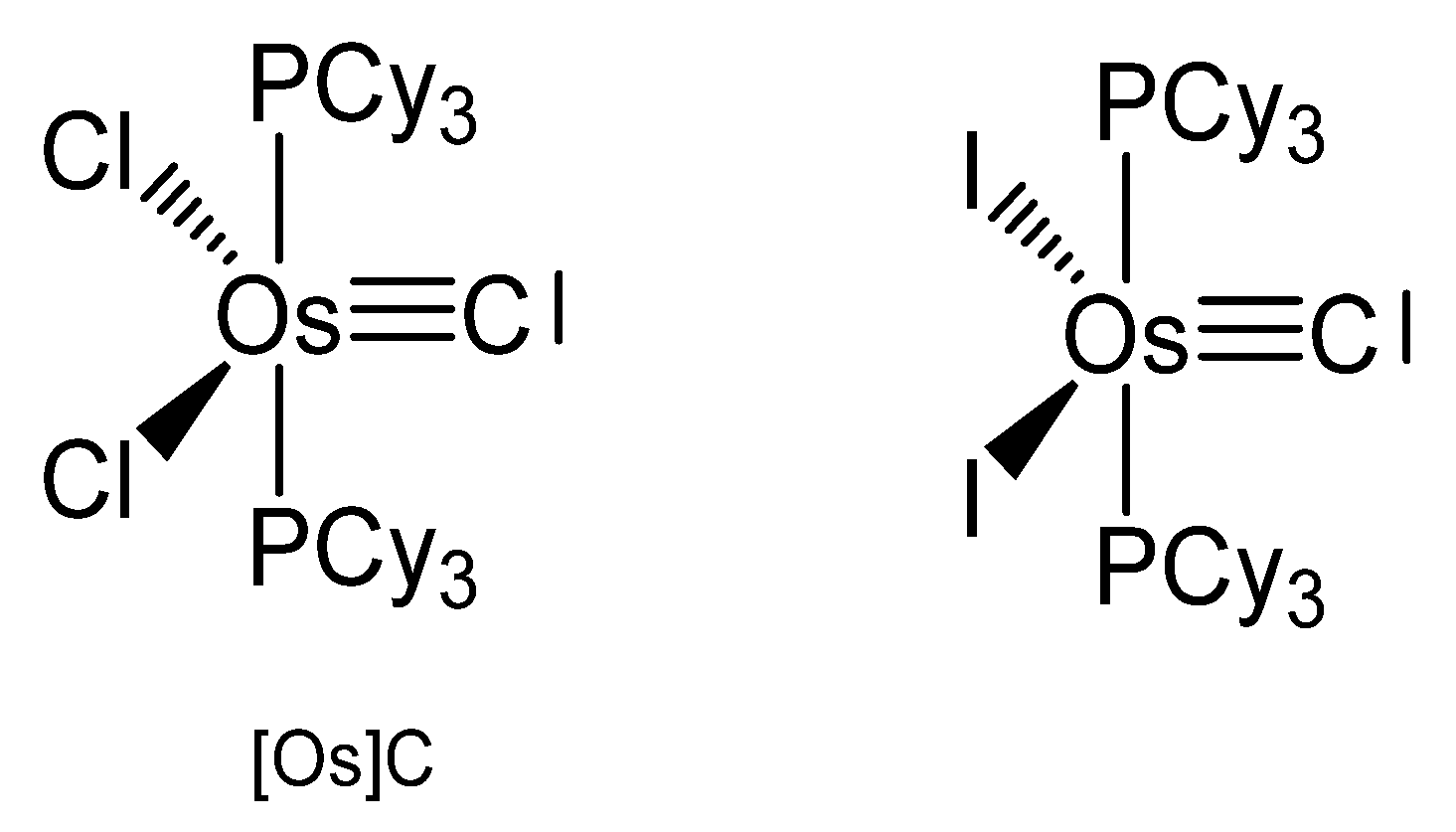
3.5. The Systems OsCl2(PCy3)2C and OsI2(PCy3)2C ([OsX]C)
The carbido complexes [OsX]C in Figure 27 were studied by X-ray analysis. The most important structural parameter is the Os-C separation, which for X = Cl amounts to 1.689(5) Å [116]. Single-crystal X-ray diffraction reveals that molecular [OsX]C adopts an approximately square-pyramidal core geometry, with the carbido ligand occupying the apical position and a short Os-C bond. In the 13C NMR spectrum the signal at 471.8 ppm for X = Cl was attributed to the ligand carbon atom. It was synthesized via S-atom abstraction from the thiocarbonyl complex Os(CS)(PCy3)2Cl2 by Ta(OSi-t-Bu3)3. The diiodo derivative was synthesized from [OsCl]C upon reacting with 10 eq of Me3SiI and exhibits a 13C NMR signal at 446.14 ppm.
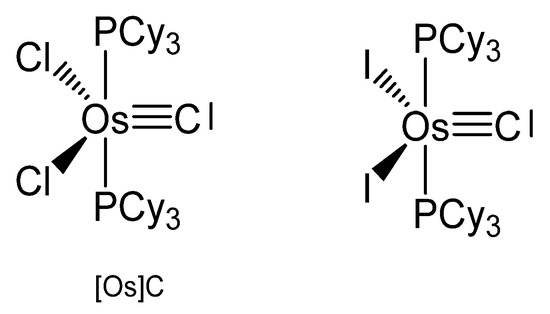
Figure 27.
The [Os]C core.
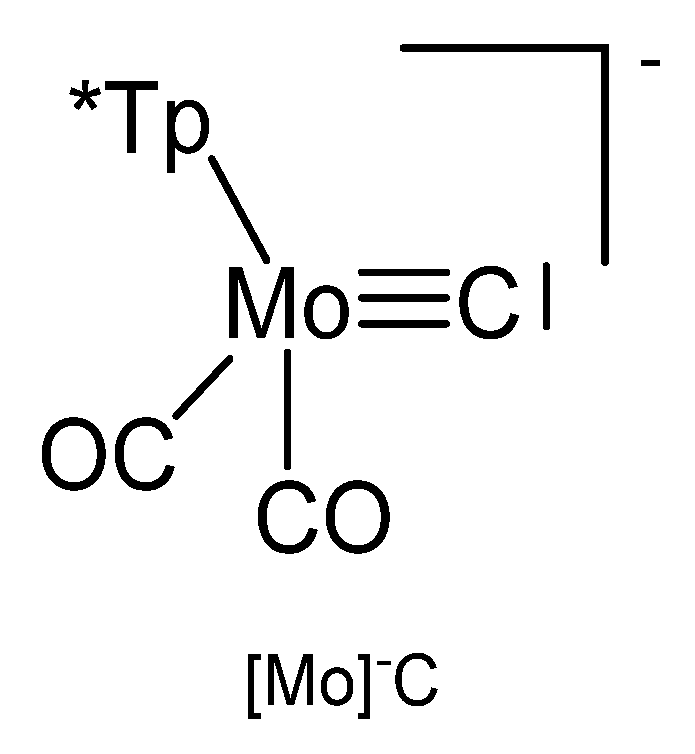
3.6. The System [Tp*Mo(CO)3≡C]− ([Mo]−C)
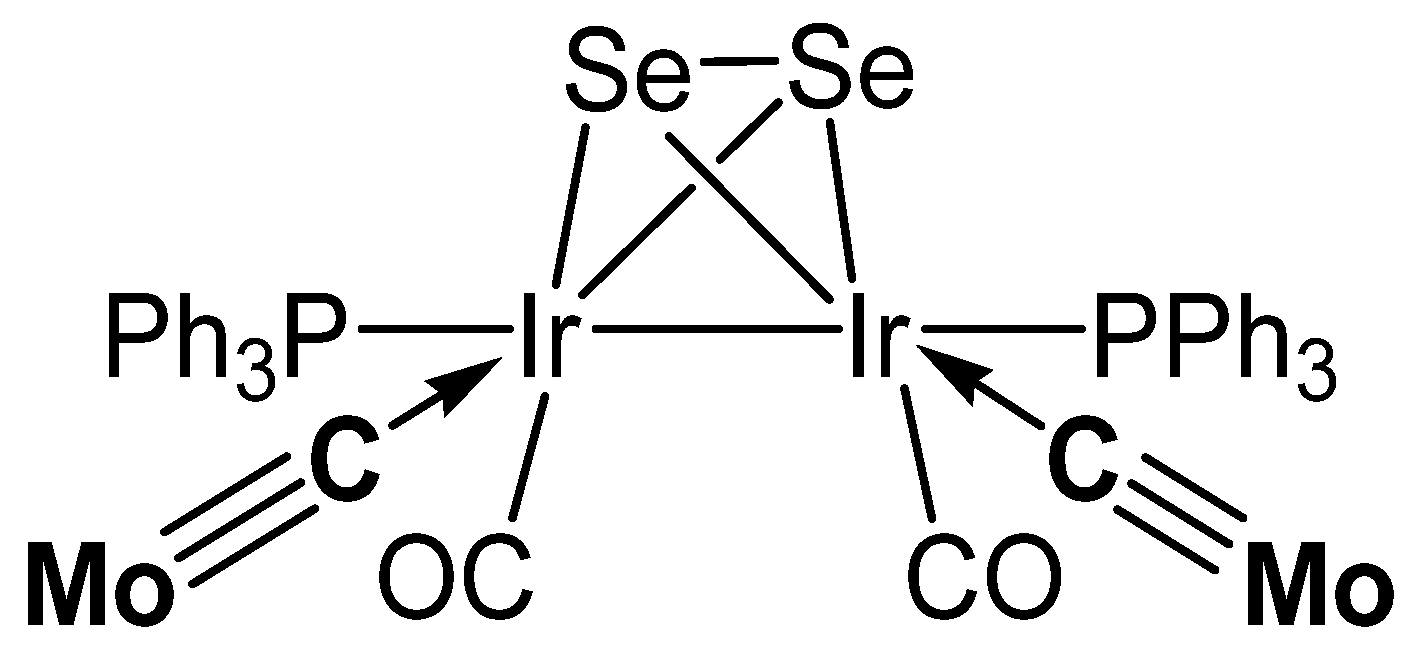
The reaction between Tp*Mo(CO)2CCl (see Figure 28) and KFeCp(CO)2 generates the carbido complex [Mo]C→FeCp(CO)2 (see Table 16) [117]; see alternative synthesis from Tp*Mo(CO)2C-Li and ClFeCp(CO)2 [118]. When Tp*Mo(CO)2CSe was allowed to react with [Ir(NCMe)(CO)(PPh3)2]BF4 the tetranuclear carbido complex (μ-Se2)[Ir2-{[Mo]C}2(CO)2(PPh3)2] was obtained (see Figure 29) [119]. A solution of Tp*Mo(CO)2CBr in THF was treated with BuLi followed by addition of HgCl2 resulted in the formation of the carbido complex [Mo]C→Hg←C[Mo] [120]. The platinum complex [Mo]C→Pt(PPh3)2Br was prepared from reacting [(HB(pz)3]Mo(CO)2CBr with [(PPh3)2Pt(C2H4)] [121].
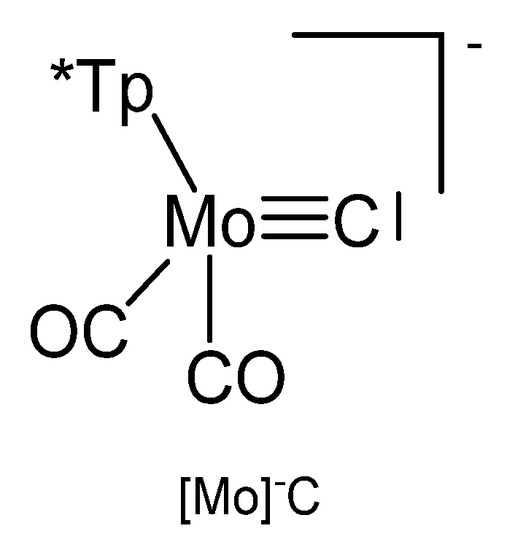
Figure 28.
The [Mo]−C core. Tp* = tris(3,5-dimethylpyrazolyl)borate, [HB(pzMe2)3]− or [HB(pz)3]−.

Table 16.
Compounds with [Mo]−C core with Tp* = [HB(pzMe2)3]− or [HB(pz)3]−.
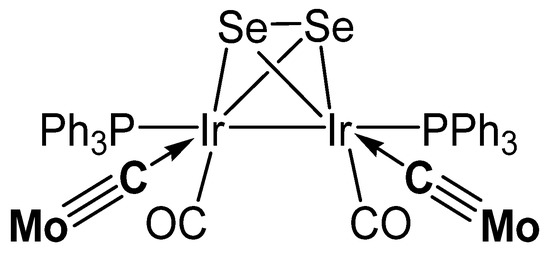
Figure 29.
Selected structure of compounds with the [Mo]−C moiety.
3.7. Unique Mo Carbido Complex
A further unique carbido complex was described recently as shown in Figure 30. A signal at 360.8 ppm in the 13C NMR spectrum was assigned to the ligand carbon atom [123].
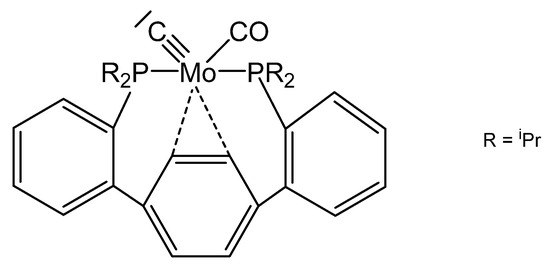
Figure 30.
The carbido complex with the P2(CO)Mo≡C core.
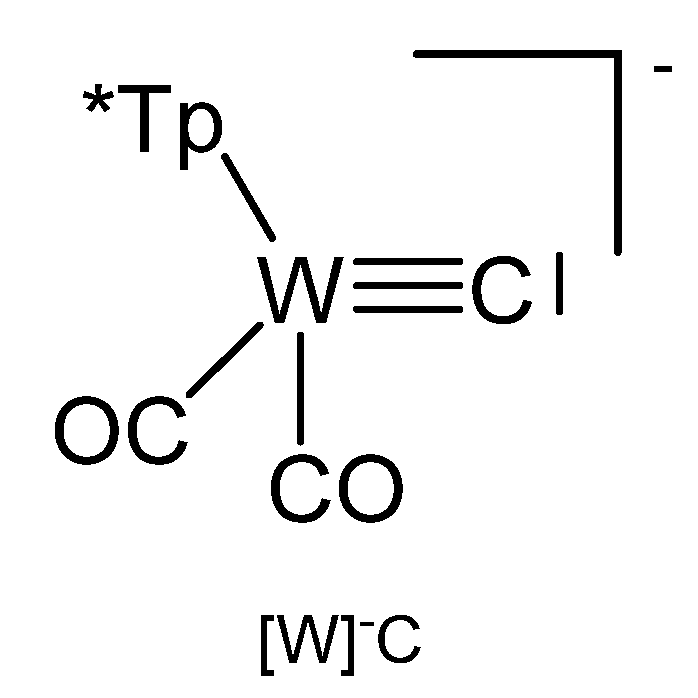
3.8. The System [Tp*W(CO)3≡C]− ([W]−C)
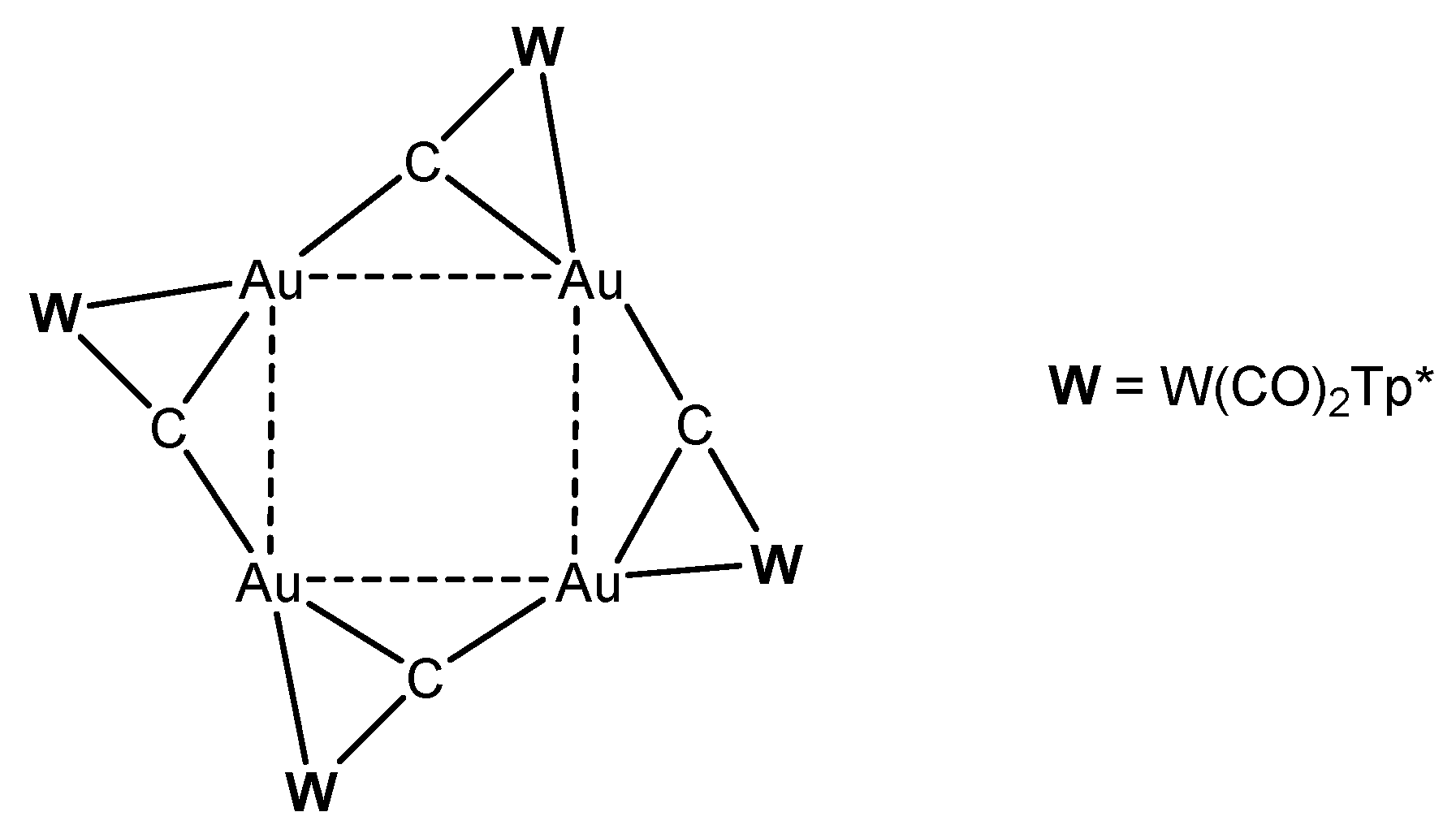
Reaction of [W]C-Li(THF) with NiCl2(PEt3)2 produced the complex [W]C→NiCl(PEt3)2 in Figure 31 [124]. Similarly, with [W]C-Li(THF) and FeCl(CO)2Cp or HgCl2 the compounds [W]C→Fe(CO)2Cp and [W]C→Hg←C[W], respectively, were obtained. [W]C→AuPEt3 was prepared from reacting [W]C→SnMe3 with AuCl(SMe2) followed by addition of PEt3. A similar reaction with AuCl(PPh3) yielded [W]C→AuPPh3. [W]C→AuAsPh3 and [W]C→AuPPh3 form a tetrameric assembly as depicted in Figure 32. The X-ray analysis of the tetrameric unit revealed Au-C distances of 1.995 and 2.078 Å and the W-C distance is 1.877 Å [122].
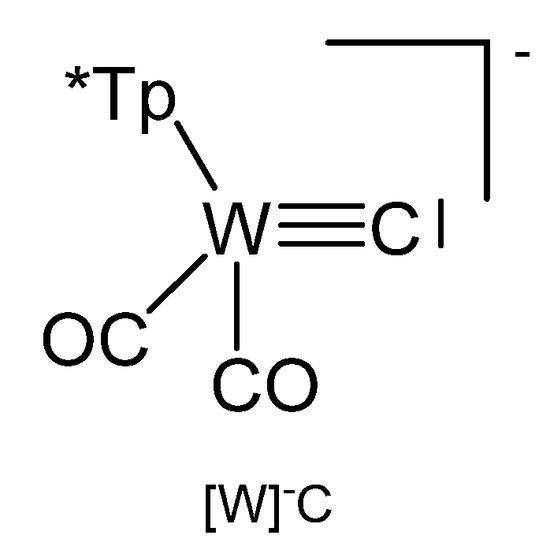
Figure 31.
The [W]−C core. T* = tris(3,5-dimethylpyrazolyl)borate, [HB(pzMe2)3]−.
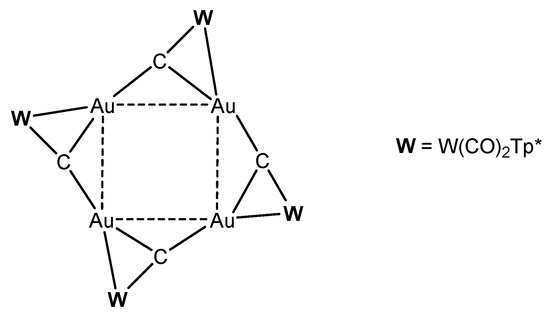
Figure 32.
Tetrameric unit from [W]C→AuAsPh3 and [W]C→AuPPh3 [122].
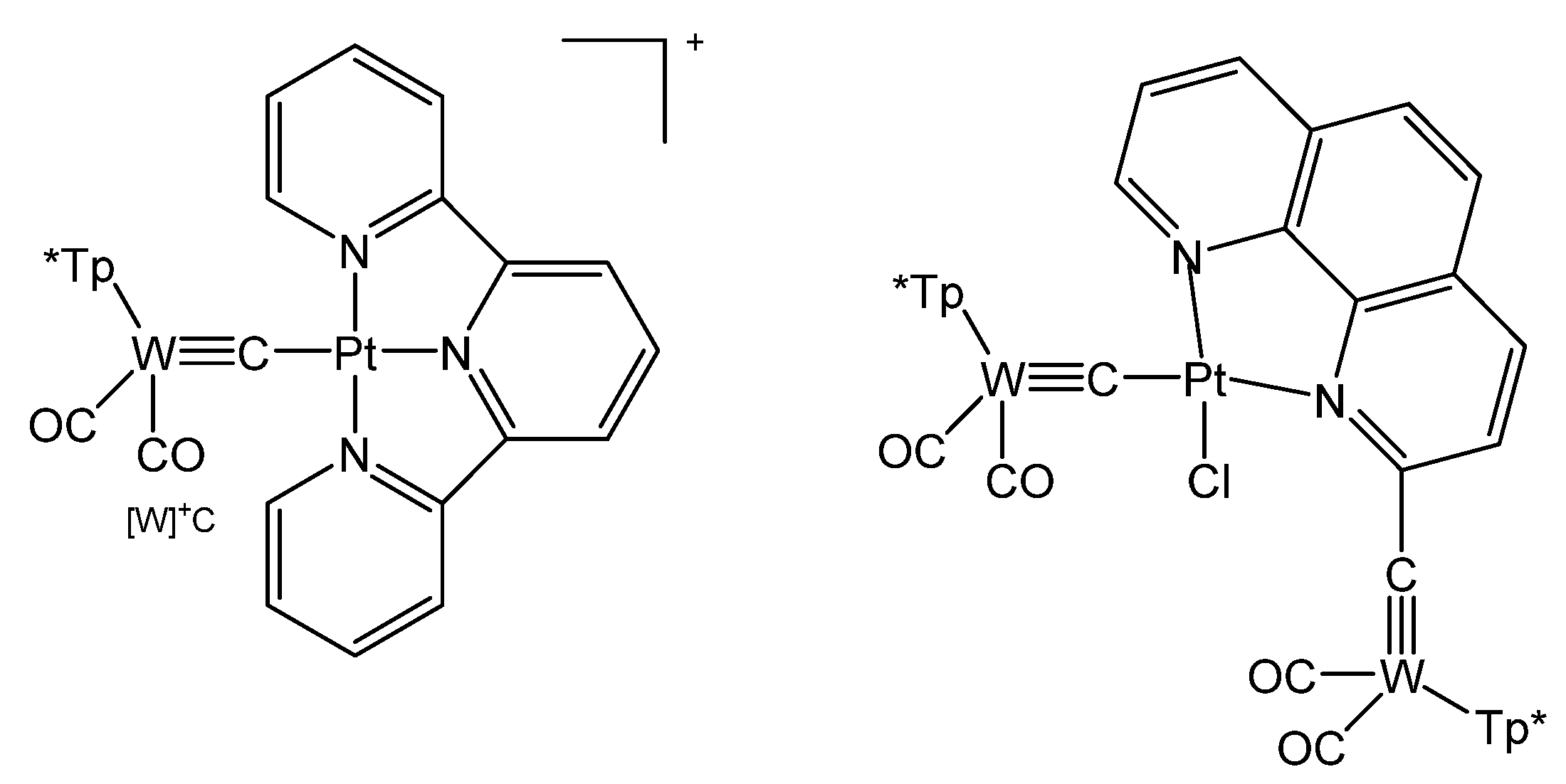
The terpyridine complex salt {[W]C→Pt(terpy)}PF6 was obtained from [W]C-Li and [PtCl(terpy)]PF6; the neutral complex [W]C→PtCl(terpyC[W]) (see Figure 33) was prepared from the same starting material and [PtCl2(phen)] (see Table 17) [125].
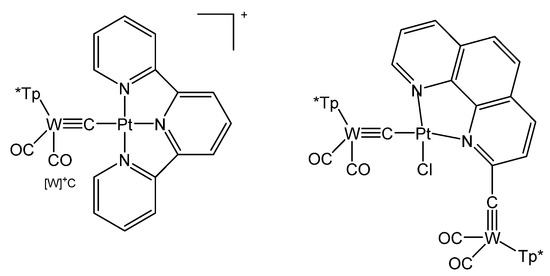
Figure 33.
Structural representation of [W]C→Pt complexes [125].

Table 17.
Compounds with [W]−C core. Tp* = [HB(pzMe2)3]−.
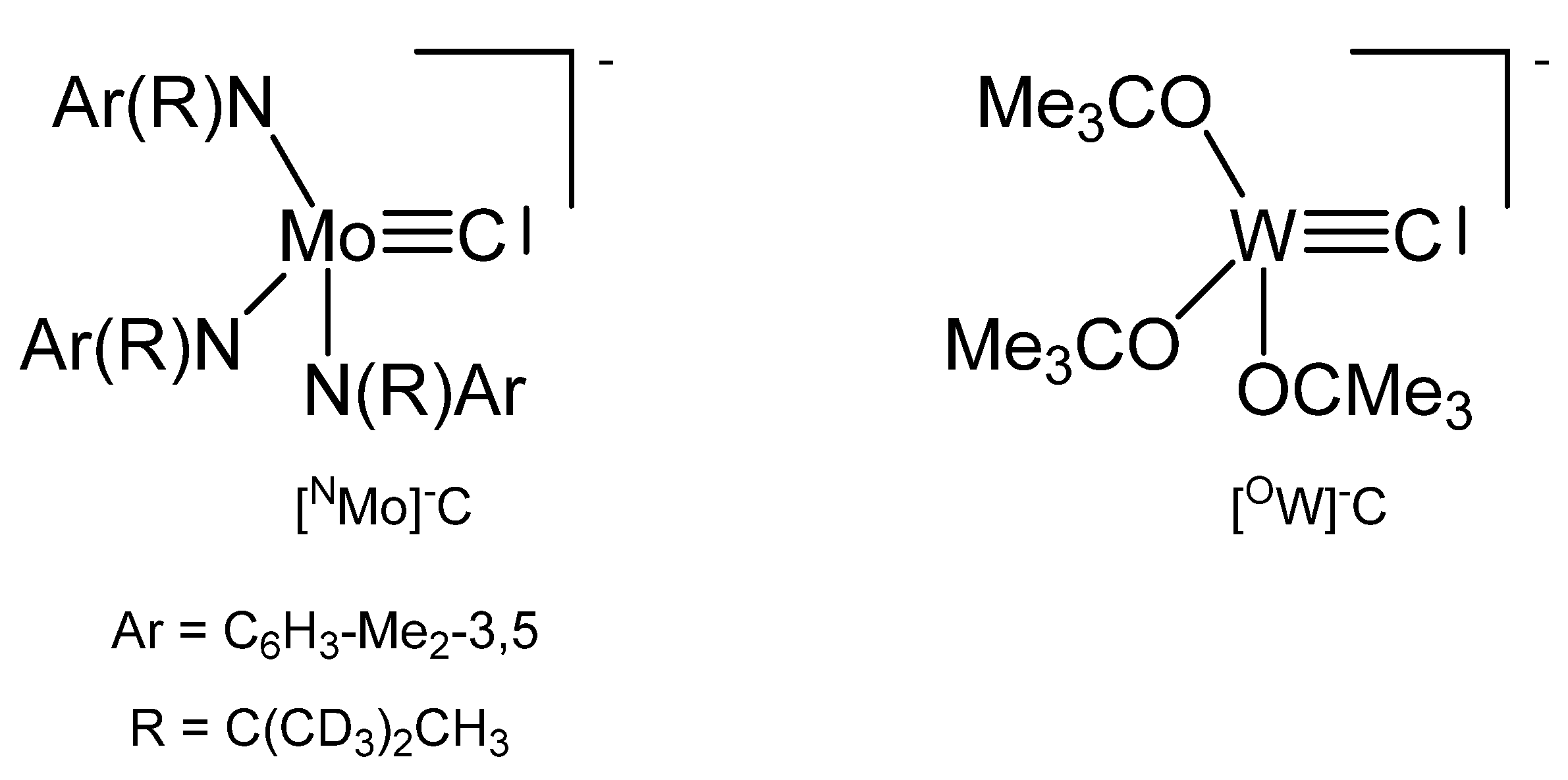
3.9. The Systems N3MoC and O3MoC
The potassium salt of NMOC− in Figure 34 is dimeric with two K+ ions bridging two anions and can be transformed with the crown ethers 2.0-benzo-15-crown-5 and 1.0 2,2,2-crypt into the related ion pairs. X-ray analysis of the crown ether salt revealed a Mo-C distance of 1.713(9) Å [26,126].
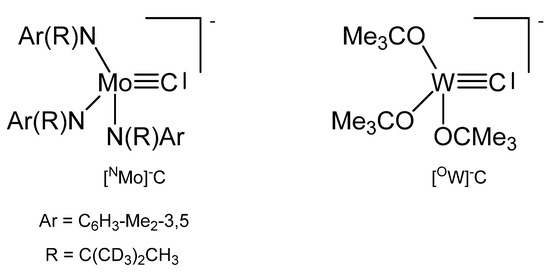
Figure 34.
The [NMo]−C and [OW]−C core.
The complex [OW]C→Ru(CO)2Cp was prepared from reacting [OW]C-Et with Ru(C≡CMe)(CO)2Cp under loss of MeCCEt. The ligand C atom resonates at 237.3 ppm (1JWC = 290.1 Hz). Distances are W-C = 1.75(2) Å, Ru-C = 2.09(2) Å and the W-C-Ru angle amounts to 177(2) ° [127].
3.10. Symmetrically Bridged Carbido Complexes M=C=M
3.10.1. The Fe=C=Fe Core
[Fe(TPP)]2C was obtained from FeIII(TPP)Cl in the presence of iron powder by reacting with CI4 (TPP = 5, 10, 15, 20-tetraphenylporphyrin; according to FeII the complex is diamagnetic [128]. The complex was also obtained upon reacting Fe(TPP) with Me3SiCCl3 [129]; see also [130]. An X-ray analysis was performed in [131] and later in [130]. The Mössbauer spectrum is published in [132]. [Fe(TTP)]2C (TTP = tetratolylporphyrine) was similarly obtained from Fe(TTP) with Me3SiCCl3 [129]. [Fe(oep)]2C (oep = octaethylporphyrine) was prepared from [ClFe(oep)] and HCCl3 and studied by X-ray analysis ans Mössbauer spectroscopy (see Table 18) [132].

Table 18.
Fe-C distances (in Å) and Fe-C-Fe angles (in deg). 13C NMR of the bridging carbon atom in ppm.
The mixed carbido compounds (TPP)Fe=C=Fe(CO)4, and (TCNP)Fe=C=Fe(CO)4 (TCNP = Tetrakis-p-cyanophenylporphyrinate) were synthesized from [(TPP)FeCCl2] or (TCNP)FeCCl2 and [Na2Fe(CO)4]; characterization proceeded via IR spectroscopy [121].
[Fe(pc)]2C was prepared from [ClFe(pc)]− and KOH/HCCl3 [132], or from Fe(pc) and CI4 in the presence of sodium dithionite [95,136], see also [134]. It also forms upon hydrolysis of (Bu4N)2{[(F)Fe(pc)]2C}in acetone [135]. Oxidation with I2 generates {[Fe(pc)]2C}(I3)0.66 which was characterized by IR, Mössbauer spectroscopy and powder X-ray diffraction [95].
A series of six-coordinate N-Base adducts of μ-carbido phthalocyanine complexes were reported. The pyridine adduct [(py)Fe(pc)]2C was obtained y dissolution of [Fe(pc)]2C in warm pyridine [133] and characterized by Mössbauer spectroscopy [136] and X-ray analysis [133]. [Fe(pc)(1-meim)}2C was similarly obtained as the TPP derivate; starting with pcFe and CI4 followed by addition of sodium dithionite gave the μ-carbido bridged dimer; an X-ray diffraction analysis was reportedd (1-meim = 1-methylimidazole, pc = phthalocyanine) [134]. [(4-Mepy)Fe(pc)]2C and [(pip)Fe(pc)]2C were similarly obtained and studied by IR and Mössbauer spectroscopy [136].
[(thf)Fe(pc)]2C forms on dissolving [Fe(pc)] in THF. The asymmetric μ-carbido complex [(thf)(TPP)Fe=C=Fe(pc)(thf)] stems from the reaction of [FeCCl2(TPP)] with [Fe(pc)]-; both compounds were characterized by X-ray analyses [130].
Anionic six-coordinate μ-carbido complexes (Bu4N)2{[(hal)Fe(pc)]2C}were reported (hal = F. Cl. Br) and obtained from reacting [Fe(pc)]2C with (Bu4N)(hal) (F: RT, Cl: 115°, Br: 140°) in solution (F) and in a melt [135].
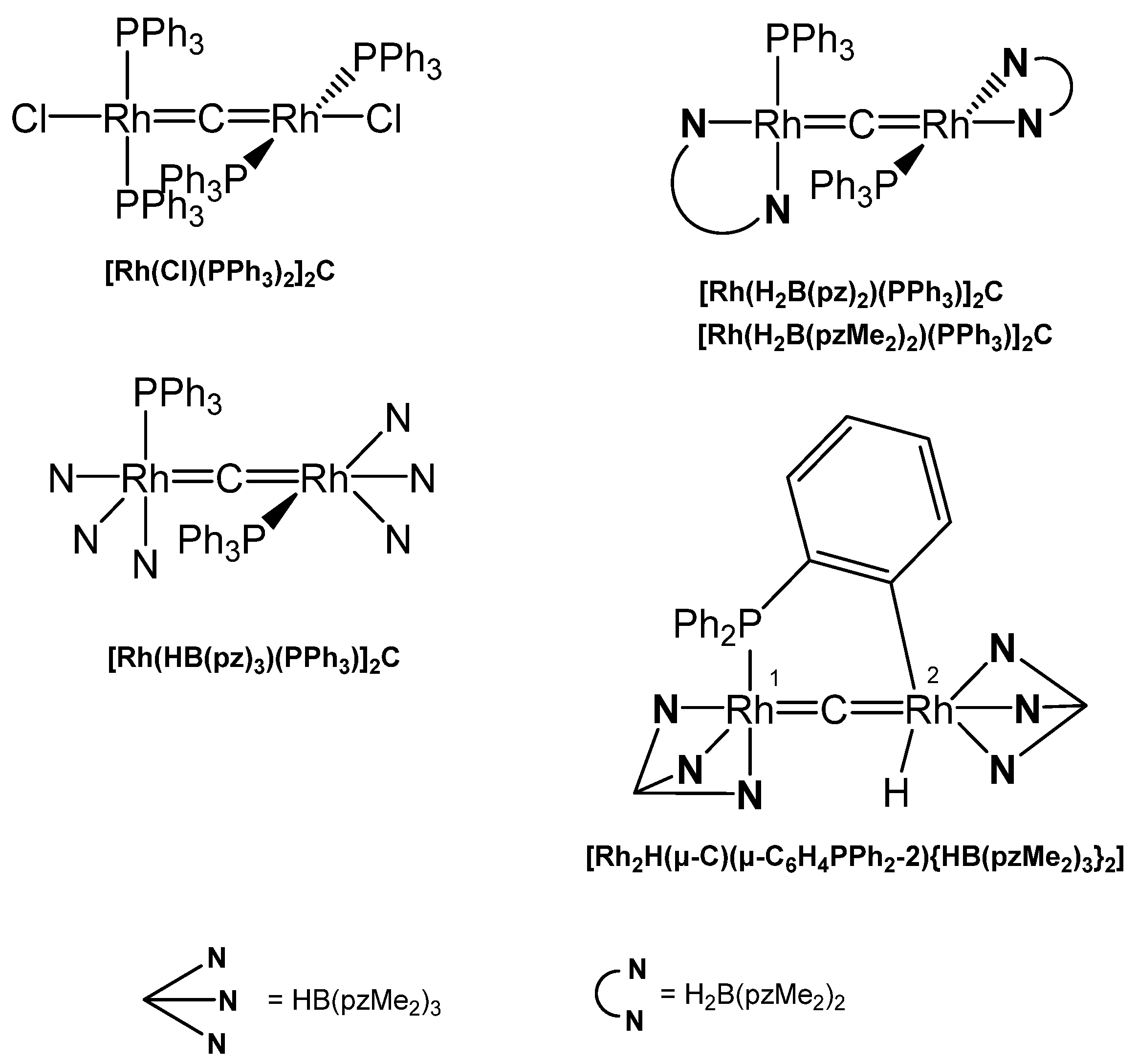
3.10.2. The Rh=C=Rh Core
[Rh(PEt3)2(SGePh3)]2C was obtained upon reacting Rh(PEt3)2(SGePh3)CS with Rh(PEt3)3(Bpin) via the intermediate mixed carbido complex (SGePh3)(PEt3)2Rh=C=Rh(PEt3)2(SBpin) which rearranges to this complex and [Rh(PEt3)2(SBpin)]2. The X-ray analysis was performed (see Table 19) [137] [Rh(PEt3)2(SBpin)]2C was prepared earlier by the same working group from Rh(PEt3)3(Bpin) and 0,5 eq of CS2 (X-ray data (see Table 19). Addition of MeOH generated the carbido complex [Rh(PEt3)2(SH)]2C [138]. [Rh(Cl)(PPh3)2]2C resulted from reacting the thiocarbonyl complex Rh(Cl)(PPh3)2CS with HBCat. The central C atom resonates at 424 ppm (t, 1JRhC = 47 Hz). In the chloro complex the chloride ion can be replaced with K[(H2B(pz)2], K[(H2B(pzMe2)2], or K[(HB(pz)3] to produce the carbido complexes [Rh(H2B(pz)2)(PPh3)]2C, [Rh(H2B(pzMe2)2)(PPh3)]2C, and [Rh(HB(pz)3)(PPh3)]2C, respectively (see Figure 35). The unusual asymmetric carbido complex [Rh2H(μ-C)(μ-C6H4PPh2-2){HB(pzMe2)3}2] contains a RhI atom with a shorter Rh-C distance, while the RhIII –C distance is longer [139].

Table 19.
Rh-C distances (in Å) and Rh-C-Rh angles (in deg). 13C NMR of the bridging carbon atom in ppm.
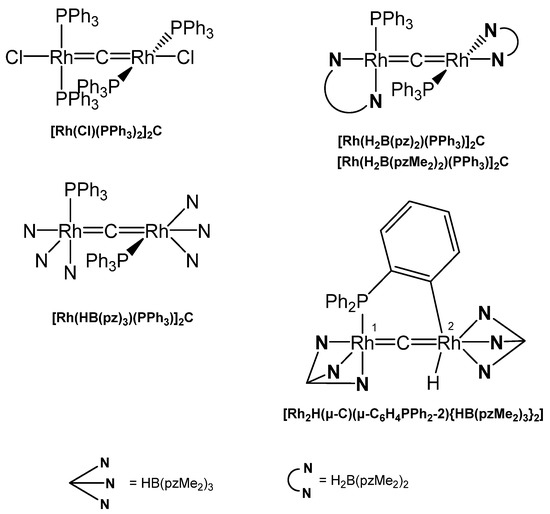
Figure 35.
Selected structures of Rh=C=Rh complexes.
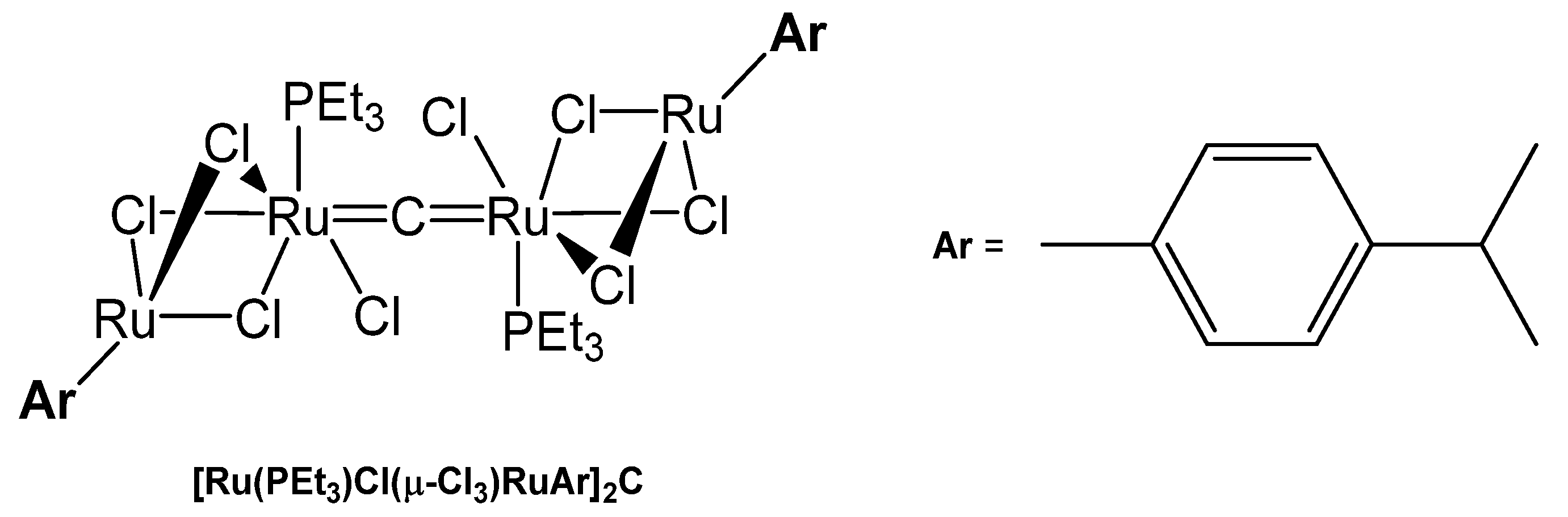
3.10.3. The Ru=C=Ru Core
The tetranuclear carbido complex [Ru(PEt3)Cl(μ-Cl3)RuAr]2C was prepared from the reaction of [(p-cymene)Ru(μ-Cl)3RuCl(C2H4)-(PCy3)] with HCCH in THF. X-ray analysis adopts Ru-C distances of 1.877(9) Å and a Ru-C-Ru angle of 178.8(9)°(see Figure 36) [140].
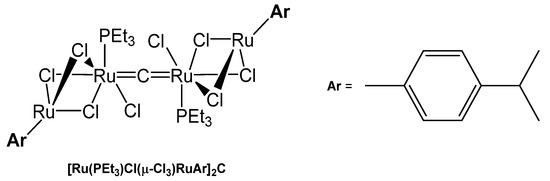
Figure 36.
Structural representation of the Ru carbido complex [Ru(PEt3)Cl(μ-Cl3)RuAr]2C.
Five coordinate [Ru(pc)]2C with pc = phthalocyaninate was obtained from H[RuCl2(pc)] and CCl2 (in situ from KOH/HCCl3) [132]. The related pyridine adduct with six-coordinate Ru(IV) [(py)Ru(pc)]2C was obtained upon dissolution of [Ru(pc)]2C in warm pyridine. X-ray analysis revealed a Ru-C distance of 1.77(1) Å and a Ru-C-Ru angle of 174.5(8)° [136].
3.10.4. The Re=C=Re Core
The unique carbido complex [Re(CO)2Cp]2C in Figure 37 results from reaction of [Re(thf)(CO)2(η-C5H5)], CS2, and PPh3 (with the aim of the thiocarbonyl complex [Re(CS)(CO)(η-C5H5)]) as by-product in small amounts. X-ray analysis revealed Re-C distances of 1.882(14) and 1.881(14) Å and a Re-C-Re angle of 173.3(7)°. A 13C NMR shift for the bridging carbon atom at δ = 436.4 ppm was measured [141].
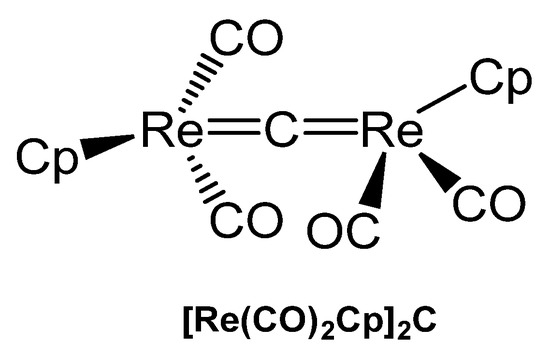
Figure 37.
Structural representation of the Re carbido complex [Re(CO)2Cp]2C.
3.10.5. The W=C=W Core
The oxo complex (tBu3SiO)2(O)W=C=WCl2(OSitBu3)2 in Figure 38 formed in high yield from thermolysis of [(siloxo)2Cl(CO)W]2 in toluene with loss of CO; in the 13C NMR spectrum the carbide C atom resonates at δ = 379.14 ppm (JWC = 200, 180 Hz). Degradation of the (silox)4C12W2(CNAr) complex afforded the imido μ-carbido compound (tBu3SiO)2(NR)W=C=WCl2(OSitBu3)2; the 13C NMR shift of the μ-C atom appears at δ = 406.25 ppm. X-ray analysis revealed a tetrahedral tungsten core with a W-C distance of 1.994(17) Å (W1) and a distorted square-pyramidal tungsten core with a shorter distance of 1.796(17) Å (W2). The W-C-W bond angle amounts to 176.0(12)° [142].
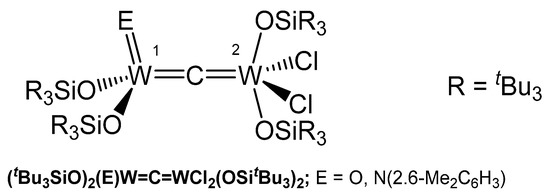
Figure 38.
Structural representation of the W carbido complexes (tBu3SiO)2(NR)W=C=WCl2(OSitBu3)2 and (tBu3SiO)2(O)W=C=WCl2(OSitBu3)2.
3.11. Asymmetrically Bridged Carbido Complex Fe=C=M
3.11.1. The Fe=C=Re Core
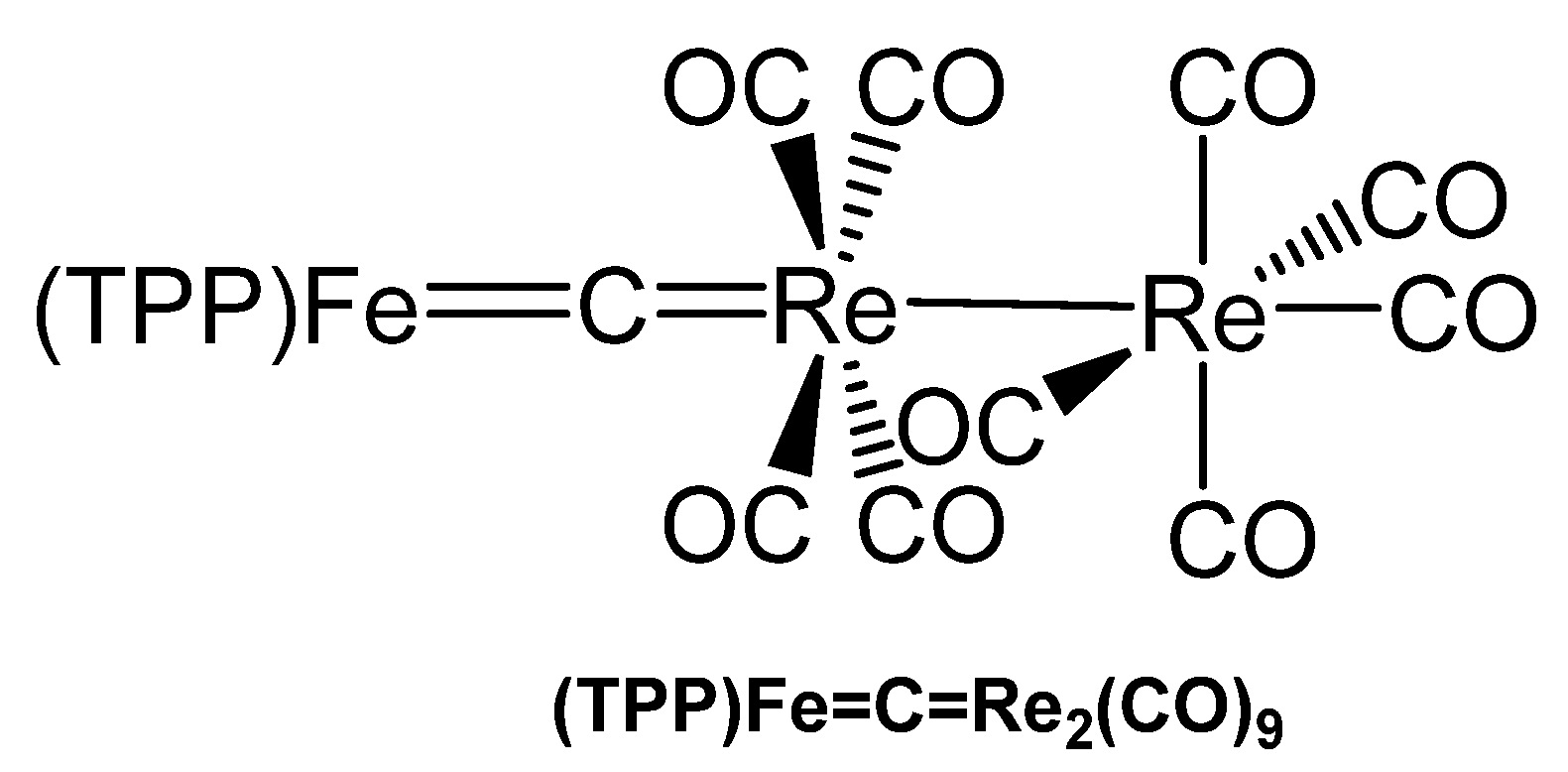
The asymmetrical carbido complex (TPP)Fe=C=Re(CO)4Re(CO)5 in Figure 39 was prepared upon reacting the dichlorocarbene complex (TPP)Fe=CCl2 with 2 eq of pentacarbonylrhenate, [Re(CO)5]−, under release of CO and 2 Cl−; TPP is tetraphenylporphyrin. Crystals were analyzed by X-ray diffraction and revealed a Fe=C distance of 1.605(13) Å and a C=Re distance of 1.957(12) Å. The Fe-C-Re angle amounts to 173.3(9)°; the Fe-C distance is somewhat smaller than in [(TPP)Fe]2C and the Re-C distance is appreciable longer than in [Re(CO)2Cp]2C. In the 13C NMR spectrum the central carbido C atom resonates at 211.7 ppm [143].
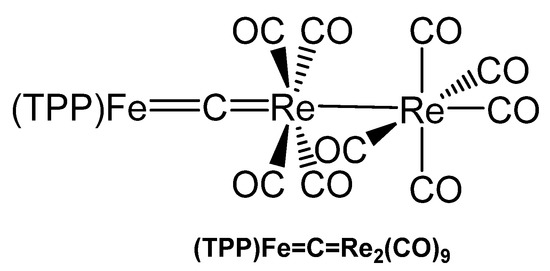
Figure 39.
Structural representation of the Fe=C=Re carbido complex (TPP)Fe=C=Re2(CO)9.
3.11.2. The Fe=C=Mn Core
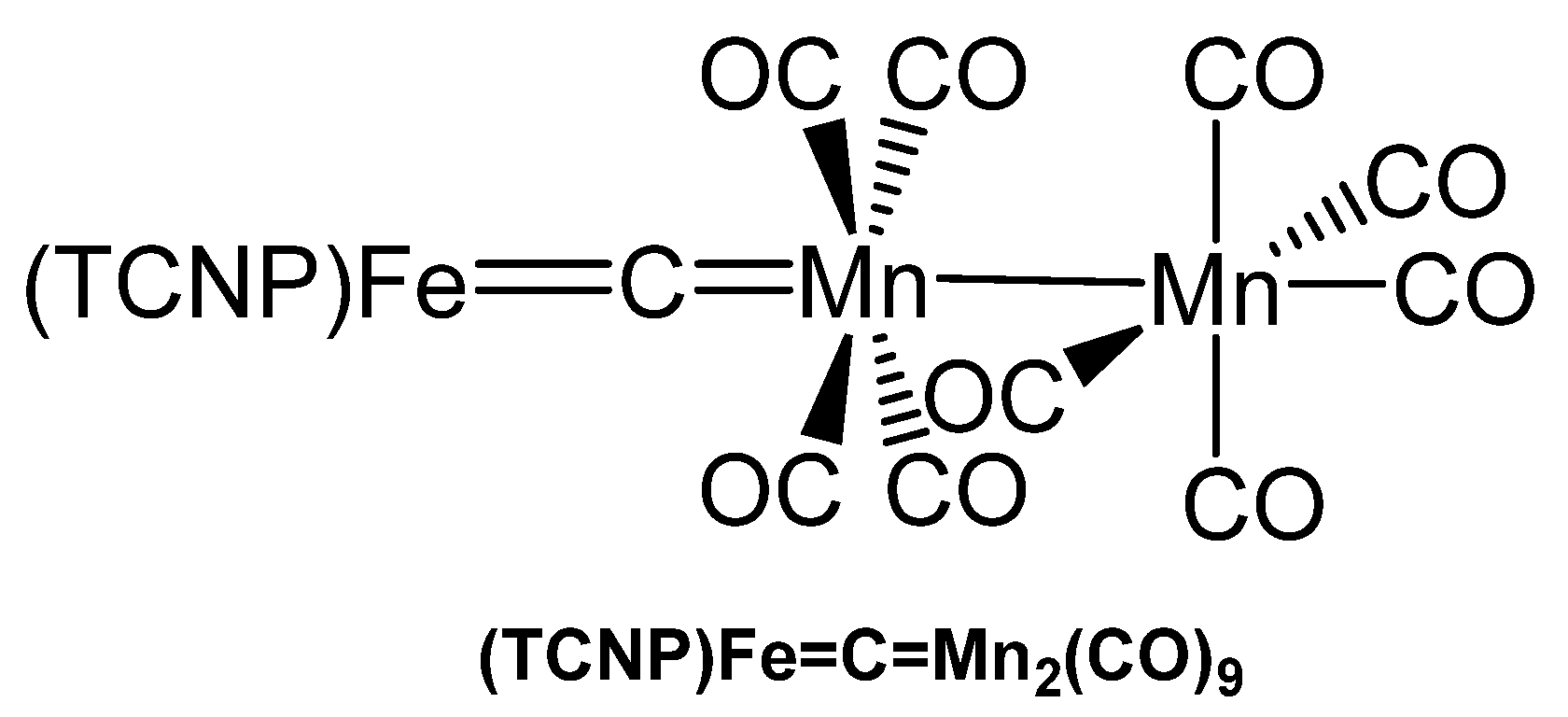
The carbido bridged di-manganese complex (TCNP)Fe=C=Mn2(CO)9 (TCNP = tetrakis(p-cyanophenyl)porphyrinate) (see Figure 40) was synthesized from [(TCNP)Fe=CCl2] and two eq. of Na(Mn(CO)5 in THF and characterized with elemental analysis, IR, and UV spectroscopy [121].
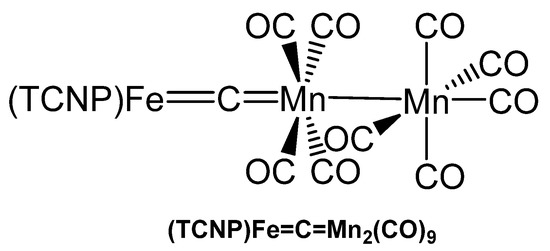
Figure 40.
Structural representation of the Fe=C=Mn carbido complex (TCNP)Fe=C=Mn2(CO)9.
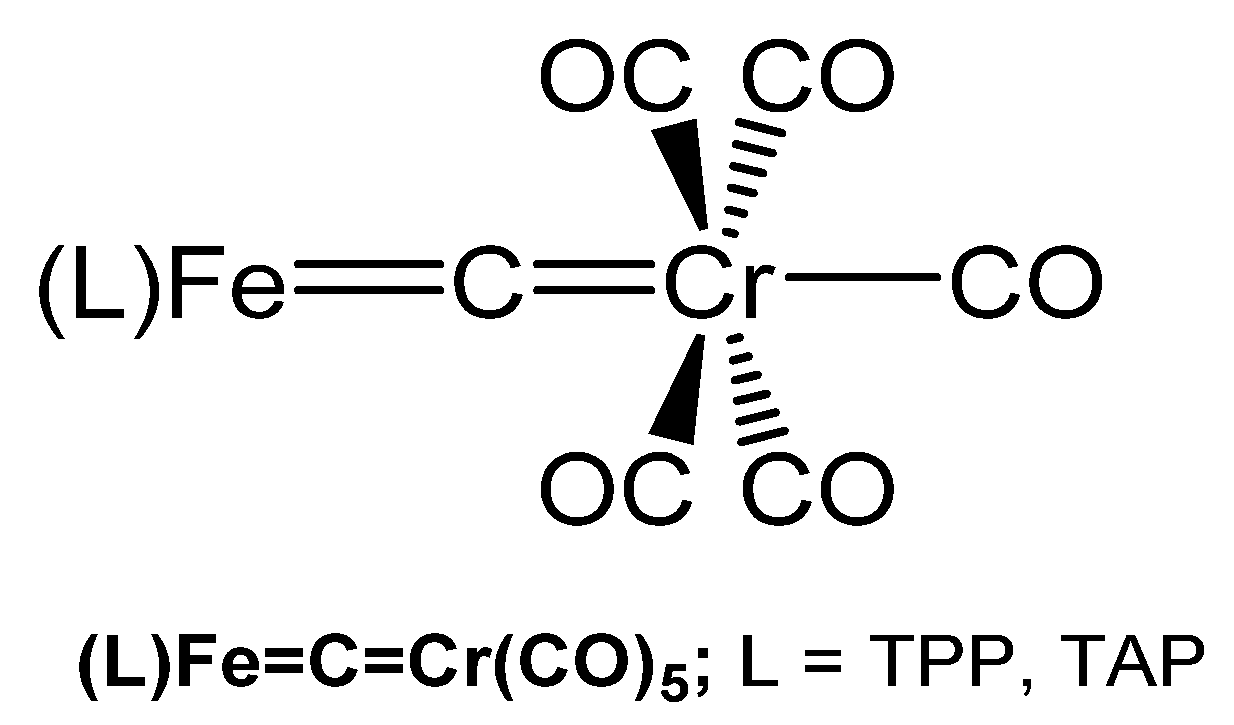
3.11.3. The Fe=C=Cr Core
Two compounds with the Fe=C=Cr core have been reported by the group of Beck and characterized by elemental analysis, IR, and UV spectroscopy. Thus, (TPP)Fe=C=Cr(CO)5 and (TAP)Fe=C=Cr(CO)5 (see Figure 41) were prepared upon reacting the related dichlorocarbene iron complexes [(L)Fe=CCl2] with Na2[Cr(CO)5] in THF (TAP = tetrakis(p-methoxyphenyl)porphyrinate) [121].
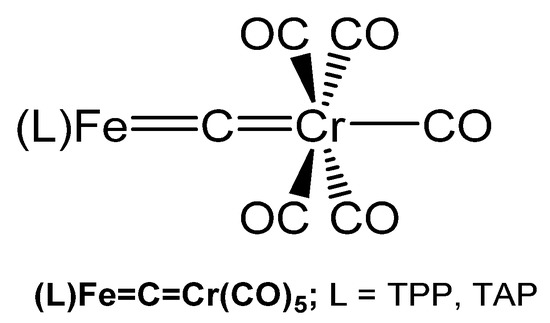
Figure 41.
Structural representation of the Fe=C=Cr carbido complexes (TPP)Fe=C=Cr(CO)5 and (TAP)Fe=C=Cr(CO)5.
4. Conclusions
The experimental and theoretical research with regard transition metal complexes with carbone ligands [M]-CL2 and carbido complexes [M]-C has blossomed in the recent past and it can be foreseen that it will remain a very active area of organometallic chemistry in the future. The well-known family of transition metal complexes with C1-bonded carbon ligands that comprise alkyl (CR3), carbene (CR2), and carbyne (CR) groups has been extended by carbones (CL2) and carbido (C) ligands. The summary of recent work, which is described in this review, indicates that carbone and carbido complexes are still largely terra incognita and that many new discoveries can be expected.
Author Contributions
Conceptualization and writing of the first draft, W.P. and G.F. Checking and partial visualization L.Z. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work at Marburg was financially supported by the Deutsche Forschungsgemeinschaft. L.Z. and G.F acknowledge the financial support from Nanjing Tech University (grant number 39837132 and 39837123), National Natural Science Foundation of China (Grant No. 21703099 and 21993044), Natural Science Foundation of Jiangsu Province for Youth (Grant No: BK20170964), and SICAM Fellowship from Jiangsu National Synergetic Innovation Center for Advanced Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frankland, E. Über die Isolierung der Organischen Radicale. Justus Liebigs Ann. Chem. 1849, 71, 171–213. [Google Scholar] [CrossRef]
- Seyferth, D. Zinc Alkyls, Edward Frankland, and the Beginnings of Main-Group Organometallic Chemistry. Organomet. Chem. 2001, 20, 2940–2955. [Google Scholar] [CrossRef]
- Fischer, E.O.M. A Zur Frage eines Wolfram-Carbonyl-Carben Komplexes. Angew. Chem. 1964, 76, 645. [Google Scholar] [CrossRef]
- Fischer, E.O.; Kreis, G.; Kreiter, C.G.; Müller, J.; Huttner, G.; Lorenz, H. Trans-Halogeno[alkyl(aryl)carbyne]tetracarbonyl Complexes of Chromium, Molybdenum, and Tungsten—A New Class of Compounds Having a Transition Metal-Carbon Triple Bond. Angew. Chem. Int. Ed. 1973, 12, 564–565. [Google Scholar] [CrossRef]
- Schrock, R.R. Alkylcarbene Complex of Tantalum by Intramolecular Alpha-Hydrogen Abstraction. J. Am. Chem. Soc. 1974, 96, 6796–6797. [Google Scholar] [CrossRef]
- McLain, S.J.; Wood, C.D.; Messerle, L.W.; Schrock, R.R.; Hollander, F.J.; Youngs, W.J.; Churchill, M.R. Multiple Metal-Carbon Bonds. Thermally Stable Tantalum Alkylidyne Complexes and the Crystal Structure of Ta(.eta.5-C5Me5)(CPh)(PMe3)2Cl. J. Am. Chem. Soc. 1978, 100, 5962–5964. [Google Scholar] [CrossRef]
- Dewar, M.J.S. A Review of Π Complex Theory. Bull. Soc. Chim. Fr. 1951, 18, C79. [Google Scholar]
- Chatt, J.; Duncanson, L.A. 586. Olefin Co-Ordination Compounds. Part III. Infra-Red Spectra and Structure: Attempted Preparation of Acetylene Complexes. J. Chem. Soc. 1953, 1, 2939–2947. [Google Scholar] [CrossRef]
- Vyboishchikov, S.F.; Frenking, G. Theoretical Studies of Organometallic Compounds, Part 29-Structure and Bonding of Low-Valent (Fischer-Type) and High-Valent (Schrock-Type) Transition Metal Carbene Complexes. Chem. Eur. J. 1998, 4, 1428–1438. [Google Scholar] [CrossRef]
- Vyboishchikov, S.F.; Frenking, G. Structure and Bonding of Low-Valent (Fischer-Type) and High-Valent (Schrock-Type) Transition Metal Carbyne Complexes. Chem. Eur. J. 1998, 4, 1439–1448. [Google Scholar] [CrossRef]
- Jerabek, P.; Schwerdtfeger, P.; Frenking, G. Dative and Electron-Sharing Bonding in Transition Metal Compounds. J. Comput. Chem. 2019, 40, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Frenking, G.; Tonner, R. Divalent Carbon(0) Compounds. Pure Appl. Chem. 2009, 81, 597–614. [Google Scholar] [CrossRef]
- Frenking, G.; Tonner, R.; Klein, S.; Takagi, N.; Shimizu, T.; Krapp, A.; Pandey, K.K.; Parameswaran, P. New Bonding Modes of Carbon and Heavier Group 14 Atoms Si–Pb. Chem. Soc. Rev. 2014, 43, 5106–5139. [Google Scholar] [CrossRef] [PubMed]
- Frenking, G.; Hermann, M.; Andrada, D.M.; Holzmann, N. Donor–Acceptor Bonding in Novel Low-Coordinated Compounds of Boron and Group 14 Atoms C–Sn. Chem. Soc. Rev. 2016, 45, 1129–1144. [Google Scholar] [CrossRef]
- Frenking, G.; Solà, M.; Vyboishchikov, S.F. Chemical bonding in transition metal carbene complexes. J. Organomet. Chem. 2005, 690, 6178–6204. [Google Scholar] [CrossRef]
- Tonner, R.; Heydenrych, G.; Frenking, G. First and Second Proton Affinities of Carbon Bases. Chem. Phys. Chem. 2008, 9, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Johns, J.W.C. The Infrared Spectrum of Carbon Suboxide in the ν6 Fundamental Region: Experimental Observation and Semirigid Bender Analysis. J. Mol. Spectrosc. 1986, 118, 248–266. [Google Scholar] [CrossRef]
- Koput, J. An ab Initio Study on the Equilibrium Structure and CCC Bending Energy Levels of Carbon Suboxide. Chem. Phys. Lett. 2000, 320, 237–244. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. Divalent Carbon(0) Chemistry, Part 1: Parent Compounds. Chem. Eur. J. 2008, 14, 3260–3272. [Google Scholar] [CrossRef]
- Frenking, G. Dative Bonds in Main-Group Compounds: A Case for More Arrows! Angew. Chem. Int. Ed. 2014, 53, 6040–6046. [Google Scholar] [CrossRef]
- Fustier-Boutignon, M.; Nebra, N.; Mézailles, N. Geminal Dianions Stabilized by Main Group Elements. Chem. Rev. 2019, 119, 855–8700. [Google Scholar] [CrossRef] [PubMed]
- Petz, W.; Frenking, G. Carbodiphosphoranes and Related Ligands. Top. Organomet. Chem. 2010, 30, 49–92. [Google Scholar]
- Petz, W. Addition Compounds between Carbones, CL2, and Main Group Lewis Acids: A New Glance at Old and New Compounds. Coord. Chem. Rev. 2015, 291, 1–27. [Google Scholar] [CrossRef]
- Alcarazo, M. Synthesis, Structure, and Reactivity of Carbidiphosphoranes, Carbodicarbenes and Related Species. Struct. Bond. 2018, 177, 25–50. [Google Scholar]
- Liu, S.; Chen, W.C.; Ong, T.G. Synthesis and Structure of Carbodicarbenes and Their Application in Catalysis. Struct. Bond. 2018, 177, 51–72. [Google Scholar]
- Peters, J.C.; Odom, A.L.; Cummins, C.C. A Terminal Molybdenum Carbide Prepared by Methylidyne Deprotonation. Chem. Commun. 1997, 20, 1995–1996. [Google Scholar] [CrossRef]
- Carlson, R.G.; Gile, M.A.; Heppert, J.A.; Mason, M.H.; Powell, D.R.; Velde, D.V.; Vilain, J.M. The Metathesis-Facilitated Synthesis of Terminal Ruthenium Carbide Complexes: A Unique Carbon Atom Transfer Reaction. J. Am. Chem. Soc. 2002, 124, 1580–1581. [Google Scholar] [CrossRef]
- Krapp, A.; Pandey, K.K.; Frenking, G. Transition Metal−Carbon Complexes. A Theoretical Study. J. Am. Chem. Soc. 2007, 129, 7596–7610. [Google Scholar] [CrossRef]
- Reinholdt, A.; Bendix, J. Platinum(ii) as an Assembly Point for Carbide and Nitride Ligands. Chem. Commun. 2019, 55, 8270–8273. [Google Scholar] [CrossRef]
- Ramirez, F.; Desai, N.B.; Hansen, B.; McKelvie, N. Hexaphenylcarbodiphosphorane, (C6H5)3PCP(C6H5)3. J. Am. Chem. Soc. 1961, 83, 3539–3540. [Google Scholar] [CrossRef]
- Hussain, M.S.; Schmidbaur, H. Ein Gemischt Methyl/Phenyl-Substituiertes Carbodiphosphoran. Darstellung, Reaktionen und verwandte Verbindungen. Z. Nat. B 1976, 31, 721–726. [Google Scholar]
- Kroll, A.; Steinert, H.; Scharf, L.T.; Scherpf, T.; Mallick, B.; Gessner, V.H. A Diamino-Substituted Carbodiphosphorane as Strong C-Donor and Weak N-Donor: Isolation of Monomeric Trigonal-Planar L·ZnCl2. Chem. Commun. 2020, 56, 8051–8054. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, P.J.; Parkin, G. Flexibility of the Carbodiphosphorane, (Ph3P)2C: Structural Characterization of a Linear Form. Inorg. Chem. 2017, 56, 5493–5497. [Google Scholar] [CrossRef]
- Böttger, S.; Gruber, M.; Münzer, J.E.; Bernard, G.M.; Kneusels, N.-J.H.; Poggel, C.; Klein, M.; Hampel, F.; Neumülöler, B.; Sundermeyer, J.; et al. Solvent-Induced Bond-Bending Isomerism in Hexaphenyl Carbodiphosphorane: Decisive Dispersion Interactions in the Solid State. Inorg. Chem. 2020, 59, 12054–12064. [Google Scholar]
- Vincent, A.T.; Wheatley, P.J. Crystal Structure of Bis(triphenylphosphoranylidene)methane[hexaphenylcarbodiphosphorane, Ph3P:C:PPh3]. J. Chem. Soc. Dalton Trans. 1972, 5, 617–622. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Hasslberger, G.; Deschler, U.; Schubert, U.; Kappenstein, C.; Frank, A. Problem of the Structure of Carbodiphosphoranes, R3PCPR3—New Aspects. Angew. Chem. Int. Ed. 1979, 18, 408–409. [Google Scholar] [CrossRef]
- Schubert, U.; Kappenstein, C.; Milewskimahrla, B.; Schmidbaur, H. Molecular and Crystal-Structures of 2 Carbodiphosphoranes with P-C-P Bond Angles near 120-Degrees. Chem. Ber. Recl. 1981, 114, 3070–3078. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Costa, T.; Milewskimahrla, B.; Schubert, U. Ring-Strained Carbodiphosphoranes. Angew. Chem. Int. Ed. 1980, 19, 555–556. [Google Scholar] [CrossRef]
- Petz, W.; Weller, F.; Uddin, J.; Frenking, G. Reaction of Carbodiphosphorane Ph3P=C=PPh3 with Ni(CO)4. Experimental and Theoretical Study of the Structures and Properties of (CO)3NiC(PPh3)2 and (CO)2NiC(PPh3)2. Organometallics 1999, 18, 619–626. [Google Scholar] [CrossRef]
- Flosdorf, K.; Jiang, D.D.; Zhao, L.L.; Neumüller, B.; Frenking, G.; Kuzu, I. An Experimental and Theoretical Study of the Structures and Properties of CDPMe-Ni(CO)3 and Ni-2(CO)4(µ2-CO)(µ2-CDPMe). Eur. J. Inorg. Chem. 2019, 2019, 4546–4554. [Google Scholar] [CrossRef]
- Petz, W.; Neumüller, B.; Klein, S.; Frenking, G. Syntheses and Crystal Structures of [Hg{C(PPh3)2}2][Hg2I6] and [Cu{C(PPh3)2}2]I and Comparative Theoretical Study of Carbene Complexes [M(NHC)2] with Carbone Complexes [M{C(PH3)2}2] (M = Cu+, Ag+, Au+, Zn2+, Cd2+, Hg2+). Organometallics 2011, 30, 3330–3339. [Google Scholar] [CrossRef]
- Petz, W.; Öxler, F.; Neumüller, B. Syntheses and Crystal Structures of Linear Coordinated Complexes of Ag+ with the Ligands C(PPh3)2 and (HC{PPh3}2)+. J. Organomet. Chem. 2009, 694, 4094–4099. [Google Scholar] [CrossRef]
- Sundermeyer, J.; Weber, K.; Peters, K.; von Schnering, H.G. Modeling Surface Reactivity of Metal Oxides: Synthesis and Structure of an Ionic Organorhenyl Perrhenate Formed by Ligand-Induced Dissociation of Covalent Re2O7. Organometallics 1994, 13, 2560–2562. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Zybill, C.E.; Müller, G.; Krüger, C. Coinage Metal Complexes of Hexaphenylcarbodiphosphorane–Organometallic Compounds with Coordination Number 2. Angew. Chem. Int. Ed. 1983, 22, 729–730. [Google Scholar] [CrossRef]
- Zybill, C.; Mueller, G. Mononuclear Complexes of Copper(I) and Silver(I) Featuring the Metals Exclusively Bound to Carbon. Synthesis and Structure of (.eta.5-Pentamethylcyclopentadienyl)[(triphenylphosphonio)(triphenylphosphoranylidene)methyl]Copper(I). Organometallics 1987, 6, 2489–2494. [Google Scholar] [CrossRef]
- Vicente, J.; Singhal, A.R.; Jones, P.G. New Ylide−, Alkynyl−, and Mixed Alkynyl/Ylide−Gold(I) Complexes. Organometallics 2002, 21, 5887–5900. [Google Scholar] [CrossRef]
- Kaska, W.C.; Reichelderfer, R.F. The Interaction of Hexaphenylcarbodiphosphorane with Iridium Olefin Cations. Metalation of Coordinated Ligands. J. Organomet. Chem. 1974, 78, C47–C50. [Google Scholar] [CrossRef]
- Münzer, J.E.; Kuzu, I. (Philipps-Universität Marburgdisabled, Marburg, Germany). Unpublished results. Private communication to WP.
- Pranckevicius, C.; Iovan, D.A.; Stephan, D.W. Three and Four Coordinate Fe Carbodiphosphorane Complexes. Dalton Trans. 2016, 45, 16820–16825. [Google Scholar] [CrossRef]
- Kneusels, N.J.H.; Münzer, J.E.; Flosdorf, K.; Jiang, D.; Neumüller, B.; Zhao, L.; Eichhöfer, A.; Frenking, G.; Kuzu, I. Double Donation in Trigonal Planar Iron–Carbodiphosphorane Complexes—A Aoncise Study on Their Spectroscopic and Electronic Properties. Dalton Trans. 2020, 49, 2537–2546. [Google Scholar] [CrossRef]
- Su, W.; Pan, S.; Sun, X.; Wang, S.; Zhao, L.; Frenking, G.; Zhu, C. Double Dative Bond between Divalent Carbon(0) and Uranium. Nat. Commun. 2018, 9, 4997. [Google Scholar] [CrossRef]
- Tonner, R.; Oexler, F.; Neumuller, B.; Petz, W.; Frenking, G. Carbodiphosphoranes: The Chemistry of Divalent Carbon(0). Angew. Chem. Int. Ed. 2006, 45, 8038–8042. [Google Scholar] [CrossRef] [PubMed]
- Petz, W.; Neumüller, B. Reaction of C(PPh3)2 with MI2 Compounds (M = Zn, Cd) - Formation and Crystal Structures of [I2Zn{C(PPh3)2}], [(I2Cd{C(PPh3)2})2] and the Salt-Like Compounds (HC{PPh3}2)[MI3(THF)] and (HC{PPh3}2)2[ZnI4]. Eur. J. Inorg. Chem. 2011, 31, 4889–4895. [Google Scholar] [CrossRef]
- Romeo, I.; Bardají, M.; Concepción Gimeno, M.; Laguna, M. Gold(I) Complexes Containing the Cationic Ylide Ligand Bis(methyldiphenylphosphonio)methylide. Polyhedron 2000, 19, 1837–1841. [Google Scholar] [CrossRef]
- Bruce, A.E.; Gamble, A.S.; Tonker, T.L.; Templeton, J.L. Cationic Phosphonium Carbyne and Bis(phosphonium) Carbene Tungsten Complexes: [Tp’(OC)2WC(PMe3)n][PF6] (n = 1, 2). Organometallics 1987, 6, 1350–1352. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Gasser, O. The Ambident Ligand Properties of Bis(trimethylphosphoranylidene)methan. Angew. Chem. Int. Ed. 1976, 15, 502–503. [Google Scholar] [CrossRef]
- Dorta, R.; Stevens, E.D.; Scott, N.M.; Costabile, C.; Cavallo, L.; Hoff, C.D.; Nolan, S.P. Steric and Electronic Properties of N-Heterocyclic Carbenes (NHC): A Detailed Study on Their Interaction with Ni(CO)4. J. Am. Chem. Soc. 2005, 127, 2485–2495. [Google Scholar] [CrossRef]
- Pugh, D.; Wright, J.A.; Freeman, S.; Danopoulos, A.A. ‘Pincer’ Dicarbene Complexes of Some Early Transition Metals and Uranium. Dalton Trans. 2006, 6, 775–782. [Google Scholar] [CrossRef]
- Gardner, B.M.; McMaster, J.; Liddle, S.T. Synthesis and Structure of a Dis-N-Heterocyclic Carbene Complex of Uranium Tetrachloride Exhibiting Short Cl⋯C Carbene Contacts. Dalton Trans. 2009, 35, 6924–6926. [Google Scholar] [CrossRef]
- Doddi, A.; Gemel, C.; Seidel, R.W.; Winter, M.; Fischer, R.A. Coordination Complexes of TiX4 (X = F, Cl) with a Bulky N-Heterocyclic Carbene: Syntheses, Characterization and Molecular Structures. Polyhedron 2013, 52, 1103–1108. [Google Scholar] [CrossRef]
- Datt, M.S.; Nair, J.J.; Otto, S. Synthesis and Characterisation of Two Novel Rh(I) Carbene Complexes: Crystal Structure of [Rh(acac)(CO)(L1)]. J. Organomet. Chem. 2005, 690, 3422–3426. [Google Scholar] [CrossRef]
- Stallinger, S.; Reitsamer, C.; Schuh, W.; Kopacka, H.; Wurst, K.; Peringer, P. Novel Route to Carbodiphosphoranes Producing a New P,C,P Pincer Carbene Ligand. Chem. Commun. 2007, 510–512. [Google Scholar] [CrossRef]
- Klein, M.; Demirel, N.; Schinabeck, A.; Yersin, H.; Sundermeyer, J. Cu(I) Complexes of Multidentate N,C,N- and P,C,P-Carbodiphosphorane Ligands and Their Photoluminescence. Molecules 2020, 25, 3990. [Google Scholar] [CrossRef]
- Klein, M.; Xie, X.; Burghaus, O.; Sundermeyer, J. Synthesis and Characterization of a N,C,N-Carbodiphosphorane Pincer Ligand and Its Complexes. Organometallics 2019, 38, 3768–3777. [Google Scholar] [CrossRef]
- Reitsamer, C.; Stallinger, S.; Schuh, W.; Kopacka, H.; Wurst, K.; Obendorf, D.; Peringer, P. Novel Access to Carbodiphosphoranes in the Coordination Sphere of Group 10 Metals: Template Synthesis and Protonation of PCP Pincer Carbodiphosphorane Complexes of C(dppm)2. Dalton Trans. 2012, 41, 3503–3514. [Google Scholar] [CrossRef]
- Maser, L.; Herritsch, J.; Langer, R. Carbodiphosphorane-Based Nickel Pincer Complexes and Their (de)Protonated Analogues: Dimerisation, Ligand Tautomers and Proton Affinities. Dalton Trans. 2018, 47, 10544–10552. [Google Scholar] [CrossRef] [PubMed]
- Reitsamer, C.; Hackl, I.; Schuh, W.; Kopacka, H.; Wurst, K.; Peringer, P. Gold(I) and Gold(III) Complexes of the [CH(dppm)2]+ and C(dppm)2 PCP Pincer Ligand Systems. J. Organomet. Chem. 2017, 830, 150–154. [Google Scholar] [CrossRef]
- Su, W.; Pan, S.; Sun, X.; Zhao, L.; Frenking, G.; Zhu, C. Cerium–Carbon Dative Interactions Supported by Carbodiphosphorane. Dalton Trans. 2019, 48, 16108–16114. [Google Scholar] [CrossRef]
- Kubo, K.; Jones, N.D.; Ferguson, M.J.; McDonald, R.; Cavell, R.G. Chelate and Pincer Carbene Complexes of Rhodium and Platinum Derived from Hexaphenylcarbodiphosphorane, Ph3PCPPh3. J. Am. Chem. Soc. 2005, 127, 5314–5315. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Okitsu, H.; Miwa, H.; Kume, S.; Cavell, R.G.; Mizuta, T. Carbon(0)-Bridged Pt/Ag Dinuclear and Tetranuclear Complexes Based on a Cyclometalated Pincer Carbodiphosphorane Platform. Organometallics 2017, 36, 266–274. [Google Scholar] [CrossRef]
- Petz, W.; Neumüller, B. New Platinum Complexes with Carbodiphosphorane as Pincer Ligand via Ortho Phenyl Metallation. Polyhedron 2011, 30, 1779–1784. [Google Scholar] [CrossRef]
- Lin, G.; Jones, N.D.; Gossage, R.A.; McDonald, R.; Cavell, R.G. A Tris(carbene) Pincer Complex: Monomeric Platinum Carbonyl with Three Bound Carbene Centers. Angew. Chem. Int. Ed. 2003, 42, 4054–4057. [Google Scholar] [CrossRef]
- Marrot, S.; Kato, T.; Gornitzka, H.; Baceiredo, A. Cyclic Carbodiphosphoranes: Strongly Nucleophilic σ-Donor Ligands. Angew. Chem. Int. Ed. 2006, 45, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Corberán, R.; Marrot, S.; Dellus, N.; Merceron-Saffon, N.; Kato, T.; Peris, E.; Baceiredo, A. First Cyclic Carbodiphosphoranes of Copper(I) and Gold(I) and Their Application in the Catalytic Cleavage of X−H Bonds (X = N and O). Organometallics 2009, 28, 326–330. [Google Scholar] [CrossRef]
- Yogendra, S.; Schulz, S.; Hennersdorf, F.; Kumar, S.; Fischer, R.; Weigand, J.J. Reductive Ring Opening of a Cyclo-Tri(phosphonio)methanide Dication to a Phosphanylcarbodiphosphorane: In Situ UV-Vis Spectroelectrochemistry and Gold Coordination. Organometallics 2018, 37, 748–754. [Google Scholar] [CrossRef]
- Alcarazo, M.; Radkowski, K.; Mehler, G.; Goddard, R.; Fürstner, A. Chiral Heterobimetallic Complexes of Carbodiphosphoranes and Phosphinidene–Carbene Adducts. Chem. Commun. 2013, 49, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Petz, W.; Kutschera, C.; Neumüller, B. Reaction of the Carbodiphosphorane Ph3PCPPh3 with Platinum(II) and -(0) Compounds: Platinum Induced Activation of C−H Bonds. Organometallics 2005, 24, 5038–5043. [Google Scholar] [CrossRef]
- Baldwin, J.C.; Kaska, W.C. The Interaction of Hexaphenylcarbodiphosphorane with the Trimethylplatinum(IV) Cation. Inorg. Chem. 1979, 18, 686–691. [Google Scholar] [CrossRef]
- Melaimi, M.; Parameswaran, P.; Donnadieu, B.; Frenking, G.; Bertrand, G. Synthesis and Ligand Properties of a Persistent, All-Carbon Four-Membered-Ring Allene. Angew. Chem. Int. Ed. 2009, 48, 4792–4795. [Google Scholar] [CrossRef]
- Hackl, L.; Petrov, A.R.; Bannenberg, T.; Freytag, M.; Jones, P.G.; Tamm, M. Dimerisation of Dipiperidinoacetylene: Convenient Access to Tetraamino-1,3-Cyclobutadiene and Tetraamino-1,2-Cyclobutadiene Metal Complexes. Chem. Eur. J. 2019, 25, 16148–16155. [Google Scholar] [CrossRef]
- Pranckevicius, C.; Liu, L.; Bertrand, G.; Stephan, D.W. Synthesis of a Carbodicyclopropenylidene: A Carbodicarbene Based Solely on Carbon. Angew. Chem. Int. Ed. 2016, 55, 5536–5540. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. C(NHC)2: Divalent Carbon(0) Compounds with N-Heterocyclic Carbene Ligands—Theoretical Evidence for a Class of Molecules with Promising Chemical Properties. Angew. Chem. Int. Ed. 2007, 46, 8695–8698. [Google Scholar] [CrossRef] [PubMed]
- Dyker, C.A.; Lavallo, V.; Donnadieu, B.; Bertrand, G. Synthesis of an Extremely Bent Acyclic Allene (A “Carbodicarbene”): A Strong Donor Ligand. Angew. Chem. Int. Ed. 2008, 47, 3206–3209. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Shen, J.S.; Lin, B.C.; Chen, W.C.; Chan, Y.T.; Ching, W.M.; Yap, G.P.A.; Hsu, C.P.; Ong, T.G. Synthesis and Isolation of an Acyclic Tridentate Bis(pyridine)carbodicarbene and Studies on Its Structural Implications and Reactivities. Angew. Chem. Int. Ed. 2015, 54, 2420–2424. [Google Scholar] [CrossRef]
- Chen, W.C.; Hsu, Y.C.; Lee, C.Y.; Yap, G.P.A.; Ong, T.G. Synthetic Modification of Acyclic Bent Allenes (Carbodicarbenes) and Further Studies on Their Structural Implications and Reactivities. Organometallics 2013, 32, 2435–2442. [Google Scholar] [CrossRef]
- Liberman-Martin, A.L.; Grubbs, R.H. Ruthenium Olefin Metathesis Catalysts Featuring a Labile Carbodicarbene Ligand. Organometallics 2017, 36, 4091–4094. [Google Scholar] [CrossRef]
- Chan, S.C.; Gupta, P.; Engelmann, X.; Ang, Z.Z.; Ganguly, R.; Bill, E.; Ray, K.; Ye, S.F.; England, J. Observation of Carbodicarbene Ligand Redox Noninnocence in Highly Oxidized Iron Complexes. Angew. Chem. Int. Ed. 2018, 57, 15717–15722. [Google Scholar] [CrossRef]
- Chen, W.C.; Shih, W.C.; Jurca, T.; Zhao, L.L.; Andrada, D.M.; Peng, C.J.; Chang, C.C.; Liu, S.K.; Wang, Y.P.; Wen, Y.S.; et al. Carbodicarbenes: Unexpected π-Accepting Ability during Reactivity with Small Molecules. J. Am. Chem. Soc. 2017, 139, 12830–12836. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.C.; Chiang, Y.T.; Wang, Q.; Wu, M.C.; Yap, G.P.A.; Zhao, L.; Ong, T.G. Invisible Chelating Effect Exhibited between Carbodicarbene and Phosphine through π–π Interaction and Implication in the Cross-Coupling Reaction. Organometallics 2017, 36, 4287–4297. [Google Scholar] [CrossRef]
- Chen, W.C.; Shen, J.S.; Jurca, T.; Peng, C.J.; Lin, Y.H.; Wang, Y.P.; Shih, W.C.; Yap, G.P.A.; Ong, T.G. Expanding the Ligand Framework Diversity of Carbodicarbenes and Direct Detection of Boron Activation in the Methylation of Amines with CO2. Angew. Chem. Int. Ed. 2015, 54, 15207–15212. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.A.; Melaimi, M.; Bertrand, G. Carbodicarbenes, Carbon(0) Derivatives, Can Dimerize. Chem. Asian J. 2013, 8, 2940–2942. [Google Scholar] [CrossRef]
- Pranckevicius, C.; Fan, L.; Stephan, D.W. Cyclic Bent Allene Hydrido-Carbonyl Complexes of Ruthenium: Highly Active Catalysts for Hydrogenation of Olefins. J. Am. Chem. Soc. 2015, 137, 5582–5589. [Google Scholar] [CrossRef]
- Goldfogel, M.J.; Roberts, C.C.; Meek, S.J. Intermolecular Hydroamination of 1,3-Dienes Catalyzed by Bis(phosphine)carbodicarbene–Rhodium Complexes. J. Am. Chem. Soc. 2014, 136, 6227–6230. [Google Scholar] [CrossRef]
- Roberts, C.C.; Matías, D.M.; Goldfogel, M.J.; Meek, S.J. Lewis Acid Activation of Carbodicarbene Catalysts for Rh-Catalyzed Hydroarylation of Dienes. J. Am. Chem. Soc. 2015, 137, 6488–6491. [Google Scholar] [CrossRef]
- Fürstner, A.; Alcarazo, M.; Goddard, R.; Lehmann, C.W. Coordination Chemistry of Ene-1,1-diamines and a Prototype “Carbodicarbene”. Angew. Chem. Int. Ed. 2008, 47, 3210–3214. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, A.M.; Pennesi, G.; Rossi, G.; Ercolani, C. A New Approach to Cofacially Assembled Partially Oxidized Metal Phthalocyanine Low Dimensional Solids: Synthesis, Structure, and Electrical Conductivity Properties of the Fe(IV) Containing Species [(PcFe)2C](l3)0.66 Obtained by I2 Doping of (m-Carbido)bis[phthalocyaninatoiron(IV)]. Inorg. Chem. 1995, 34, 4780–4784. [Google Scholar]
- Burzlaff, H.; Voll, U.; Bestmann, H.J. Die Kristall- und Molekülstruktur des (2,2-Diäthoxyvinyliden)-triphenylphosphorans. Chem. Ber. 1974, 107, 1949–1956. [Google Scholar] [CrossRef]
- Alcarazo, M.; Lehmann, C.W.; Anoop, A.; Thiel, W.; Furstner, A. Coordination Chemistry at Carbon. Nat. Chem. 2009, 1, 295–301. [Google Scholar] [CrossRef]
- Troadec, T.; Wasano, T.; Lenk, R.; Baceiredo, A.; Saffon-Merceron, N.; Hashizume, D.; Saito, Y.; Nakata, N.; Branchadell, V.; Kato, T. Donor-Stabilized Silylene/Phosphine-Supported Carbon(0) Center with High Electron Density. Angew. Chem. Int. Ed. 2017, 56, 6891–6895. [Google Scholar] [CrossRef] [PubMed]
- Morosaki, T.; Wang, W.W.; Nagase, S.; Fujii, T. Synthesis, Structure, and Reactivities of Iminosulfane- and Phosphane-Stabilized Carbones Exhibiting Four-Electron Donor Ability. Chem. Eur. J. 2015, 21, 15405–15411. [Google Scholar] [CrossRef] [PubMed]
- Pascual, S.; Asay, M.; Illa, O.; Kato, T.; Bertrand, G.; Saffon-Merceron, N.; Branchadell, V.; Baceiredo, A. Synthesis of a Mixed Phosphonium-Sulfonium Bisylide R3P=C=SR2. Angew. Chem. Int. Ed. 2007, 46, 9078–9080. [Google Scholar] [CrossRef]
- Morosaki, T.; Iijima, R.; Suzuki, T.; Wang, W.W.; Nagase, S.; Fujii, T. Synthesis, Electronic Structure, and Reactivities of Two-Sulfur-Stabilized Carbones Exhibiting Four-Electron Donor Ability. Chem. Eur. J. 2017, 23, 8694–8702. [Google Scholar] [CrossRef]
- Morosaki, T.; Suzuki, T.; Wang, W.W.; Nagase, S.; Fujii, T. Syntheses, Structures, and Reactivities of Two Chalcogen-Stabilized Carbones. Angew. Chem. Int. Ed. 2014, 53, 9569–9571. [Google Scholar] [CrossRef]
- Fujii, T.; Ikeda, T.; Mikami, T.; Suzuki, T.; Yoshimura, T. Synthesis and Structure of (MeN)Ph2S=C=SPh2(NMe). Angew. Chem. Int. Ed. 2002, 41, 2576–2578. [Google Scholar] [CrossRef]
- Morosaki, T.; Suzuki, T.; Fujii, T. Syntheses and Structural Characterization of Mono-, Di-, and Tetranuclear Silver Carbone Complexes. Organometallics 2016, 35, 2715–2721. [Google Scholar] [CrossRef]
- Chen, Y.; Petz, W.; Frenking, G. Is It Possible to Synthesize a Low-Valent Transition Metal Complex with a Neutral Carbon Atom as Terminal Ligand? A Theoretical Study of (CO)4FeC. Organometallics 2000, 19, 2698–2706. [Google Scholar] [CrossRef]
- Zhao, L.; von Hopffgarten, M.; Andrada, D.M.; Frenking, G. Energy Decomposition Analysis. Wires Comput. Mol. Sci. 2018, 8, e1345. [Google Scholar] [CrossRef]
- Takemoto, S.; Matsuzaka, H. Recent Advances in the Chemistry of Ruthenium Carbido Complexes. Coord. Chem. Rev. 2012, 256, 574–588. [Google Scholar] [CrossRef]
- Hejl, A.; Trnka, T.M.; Day, M.W.; Grubbs, R.H. Terminal Ruthenium Carbido Complexes as σ-Donor Ligands. Chem. Commun. 2002, 21, 2524–2525. [Google Scholar] [CrossRef]
- Caskey, S.R.; Stewart, M.H.; Kivela, J.E.; Sootsman, J.R.; Johnson, M.J.A.; Kampf, J.W. Two Generalizable Routes to Terminal Carbido Complexes. J. Am. Chem. Soc. 2005, 127, 16750–16751. [Google Scholar] [CrossRef]
- Reinholdt, A.; Bendix, J. Weakening of Carbide–Platinum Bonds as a Probe for Ligand Donor Strengths. Inorg. Chem. 2017, 56, 12492–12497. [Google Scholar] [CrossRef]
- Reinholdt, A.; Vibenholt, J.E.; Morsing, T.J.; Schau-Magnussen, M.; Reeler, N.E.A.; Bendix, J. Carbide Complexes as π-Acceptor Ligands. Chem. Sci. 2015, 6, 5815–5823. [Google Scholar] [CrossRef]
- Reinholdt, A.; Herbst, K.; Bendix, J. Delivering Carbide Ligands to Sulfide-Rich Clusters. Chem. Commun. 2016, 52, 2015–2018. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Day, M.W.; Grupps, R.H. Decomposition of a Key Intermediate in Ruthenium-Catalyzed Olefin Metatesis Reactions. J. Am. Chem. Soc. 2004, 126, 7414–7415. [Google Scholar] [CrossRef] [PubMed]
- Morsing, T.J.; Reinholdt, A.; Sauer, S.P.A.; Bendix, J. Ligand Sphere Conversions in Terminal Carbide Complexes. Organometallics 2016, 35, 100–105. [Google Scholar] [CrossRef]
- Stewart, M.H.; Johnson, M.J.A.; Kampf, J.W. Terminal Carbido Complexes of Osmium: Synthesis, Structure, and Reactivity Comparison to the Ruthenium Analogues. Organometallics 2007, 26, 5102–5110. [Google Scholar] [CrossRef]
- Etienne, M.; White, P.S.; Templeton, J.L. An Agostic.mu.-Methyne Molybdenum-Iron Complex from Protonation of a.mu.-Carbide Precursor, Tp’(CO)2Mo.tplbond.CFe(CO)2Cp. J. Am. Chem. Soc. 1991, 113, 2324–2325. [Google Scholar] [CrossRef]
- Cordiner, L.R.; Hill, A.F.; Wagler, J. Facil Generation of Lithiocarbyne Complexes; [M(≡CLi)(CO)2{HB(pzMe2)3}] (M = Mo, W; pz = Pyrazol-1-yl). Organometallics 2008, 27, 5177–5179. [Google Scholar] [CrossRef]
- Cade, I.A.; Hill, A.F.; McQueen, C.M.A. Iridium−Molybdenum Carbido Complex via C−Se Activation of a Selenocarbonyl Ligand: (μ-Se2)[Ir2{C≡Mo(CO)2(Tp*)}2(CO)2(PPh3)2](Tp* = Hydrotris(dimethylpyrazolyl)borate). Organometallics 2009, 28, 6639–6641. [Google Scholar] [CrossRef]
- Colebatch, A.L.; Cordiner, R.L.; Hill, A.F.; Nguyen, K.T.H.D.; Shang, R.; Willis, A.C. A Bis-Carbyne (Ethanediylidyne) Complex via the Catalytic Demercuration of a Mercury Bis(carbido) Complex. Organometallics 2009, 28, 4394–4399. [Google Scholar] [CrossRef]
- Knauer, W.; Beck, W. Carbide Bridged Complexes [HB(pz)3(OC)2Mo=C-Pt(PPh3)2Br], [(TPP)Fe=C-M(CO)4-M(CO)5] (M = Mn, Re), [(TPP)Fe=C=Cr(CO)5], TPP)Fe=C=Fe(CO)4] (pz- 3,5-dimethylpyrazol-1-yl; TPP Tetraphenylporphyrinate) from Halogeno-Carbyne and -Carbene Complexes. Z. Anorg. Allg. Chem. 2008, 634, 2241–2245. [Google Scholar] [CrossRef]
- Borren, E.S.; Hill, A.F.; Shang, R.; Sharma, M.; Willis, A.C. A Golden Ring: Molecular Gold Carbido Complexes. J. Am. Chem. Soc. 2013, 135, 4942–4945. [Google Scholar] [CrossRef]
- Buss, J.A.; Agapie, T. Mechanism of Molybdenum-Mediated Carbon Monoxide Deoxygenation and Coupling: Mono- and Dicarbyne Complexes Precede C–O Bond Cleavage and C–C Bond Formation. J. Am. Chem. Soc. 2016, 138, 16466–16477. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Sharma, M.; Willis, A.C. Heterodinuclear Bridging Carbido and Phosphoniocarbyne Complexes. Organometallics 2012, 31, 2538–2542. [Google Scholar] [CrossRef]
- Frogley, B.J.; Hill, A.F. Tungsten–Platinum μ-Carbido and μ-Methylidyne Complexes. Chem. Commun. 2019, 55, 12400–12403. [Google Scholar] [CrossRef] [PubMed]
- Agapie, T.; Diaconescu, P.L.; Cummins, C.C. Methine (CH) Transfer via a Chlorine Atom Abstraction/Benzene-Elimination Strategy: Molybdenum Methylidyne Synthesis and Elaboration to a Phosphaisocyanide Complex. J. Am. Chem. Soc. 2002, 124, 2412–2413. [Google Scholar] [CrossRef] [PubMed]
- Latesky, S.L.; Selegue, J.P. Preparation and Structure of [(Me3CO)3W.tplbond.C-Ru(CO)2(Cp)], a Heteronuclear,.mu.2-Carbide Complex. J. Am. Chem. Soc. 1987, 109, 4731–4733. [Google Scholar] [CrossRef]
- Mansuy, D.; Lecomte, J.P.; Chottard, J.C.; Bartoli, J.F. Formation of a Complex with a Carbide Bridge between Two Iron Atoms from the Reaction of (Tetraphenylporphyrin)iron(II) with Carbon Tetraiodide. Inorg. Chem. 1981, 20, 3119–3121. [Google Scholar] [CrossRef]
- Battioni, J.-P.; Dupre, D.; Mansuy, D. Reactions du Trichloromethyl-Trimethyl-Silane avec des Ferrotetra-Aryl-Porphyrines. J. Organometal. Chem. 1981, 414, 303–309. [Google Scholar] [CrossRef]
- Galich, L.; Kienast, A.; Hückstädt, H.; Homborg, H. Syntheses, Spectroscopical Properties, and Crystal Structures of Binuclear Homo- and Heteroleptic μ-Carbido Complexes of Iron(IV) with Phthalocyaninate and Tetraphenylporphyrinate Ligands. Z. Anorg. Allg. Chem. 1998, 624, 1235–1242. [Google Scholar] [CrossRef]
- Goedken, V.L.; Deakin, M.R.; Bottomley, L.A. Molecular Stereochemistry of a Carbon-Bridged Metalloporphyrin: µ-Carbido-Bid(5,10,15,20-tetraphenylporphinatoiron). J. Chem. Soc. Chem. Commun. 1982, 11, 607–608. [Google Scholar] [CrossRef]
- Kienast, A.; Galich, L.; Murray, K.S.; Moubaraki, B.; Lazarev, G.; Cashion, J.D.; Homborg, H. M-Carbido Dipophyrinates and Diphthalocyaninates of Iron and Ruthenium. J. Porphyr. Phthalocyanines 1997, 1, 141–157. [Google Scholar] [CrossRef]
- Kienast, A.; Bruhn, C.; Homborg, H. Synthesis, Properties, and Crystal Structure of μ-Carbidodi(pyridinephthalocyaninato(2-)Iron(IV)) and -Ruthenium(IV). Z. Anorg. Allg. Chem. 1997, 623, 967–972. [Google Scholar] [CrossRef]
- Rossi, G.; Goedken, V.L.; Ercolani, C. µ-Carbido-Bridged Iron Phthalocyanine Dimers: Synthesis and Characterization. J. Chem. Soc. Chem. Commun. 1988, 1, 46–47. [Google Scholar] [CrossRef]
- Kienast, A.; Homborg, H. Synthese und Eigenschaften von Bis(tetra(n-butyl)ammonium)-l-carbidodi(halogenophthalocyaninato(2-)ferraten(IV)); Kristallstruktur von Bis(tetra(n-butyl)ammonium)-μ-carbidodi(fluorophthalocyaninato(2-)ferrat(IV))-Trihydrat. Z. Anorg. Chem. 1998, 624, 107–112. [Google Scholar] [CrossRef]
- Ercolani, C.; Gardini, M.; Goedken, V.L.; Pennesi, G.; Rossi, G.; Russo, U.; Zanonato, P. High-Valent Iron Phthalocyanine Five- and Six-Coordinated μ-Carbido Dimers. Inorg. Chem. 1989, 28, 3097–3099. [Google Scholar] [CrossRef]
- Ahrens, T.; Schmiedecke, B.; Braun, T.; Herrmann, R.; Laubenstein, R. Activation of CS2 and COS at a Rhodium(I) Germyl Complex: Generation of CS and Carbido Complexes. Eur. J. Inorg. Chem. 2017, 3, 713–722. [Google Scholar] [CrossRef]
- Kalläne, S.I.; Braun, T.; Teltewskoi, M.; Braun, B.; Herrmann, R.; Laubenstein, R. Remarkable Reactivity of a Rhodium(i) Boryl Complex Towards CO2 and CS2: Isolation of a Carbido Complex. Chem. Commun. 2015, 51, 14613–14616. [Google Scholar] [CrossRef]
- Barnett, H.J.; Burt, L.K.; Hill, A.F. Simple Generation of a Dirhodium μ-Carbido Complex via Thiocarbonyl Reduction. Dalton Trans. 2018, 47, 9570–9574. [Google Scholar] [CrossRef]
- Solari, E.; Antonijevic, S.; Gauthier, S.; Scopelliti, R.; Severin, K. Formation of a Ruthenium mu-Carbide Complex with Acetylene as the Crbon Source. Eur. J. Inorg. Chem. 2007, 3, 367–371. [Google Scholar] [CrossRef]
- Young, R.D.; Hill, A.F.; Cavigliasso, G.E.; Stranger, R. [(μ-C){Re(CO)2(η-C5H5)}2]: A Surprisingly Simple Bimetallic Carbido Complex. Angew. Chem. Int. Ed. 2013, 52, 3699–3702. [Google Scholar] [CrossRef]
- Miller, R.L.; Wolczanski, P.T.; Rheingold, A.L. Carbide Formation via Carbon Monoxide Dissociation Across a W≡W Bond. J. Am. Chem. Soc. 1993, 115, 10422–10423. [Google Scholar] [CrossRef]
- Beck, W.; Knauer, W.; Robl, C. Synthesis and Structure of the Novel μ-Carbido Complex [(TPP)Fe=C=Re(CO)4Re(CO)5]. Angew. Chem. Int. Ed. 1990, 29, 318–320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).