[Bmim]Br Accelerated One-Pot Three-Component Cascade Protocol for the Construction of Spirooxindole–Pyrrolidine Heterocyclic Hybrids
Abstract
1. Introduction
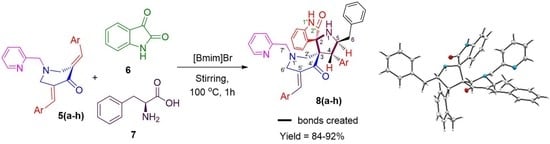
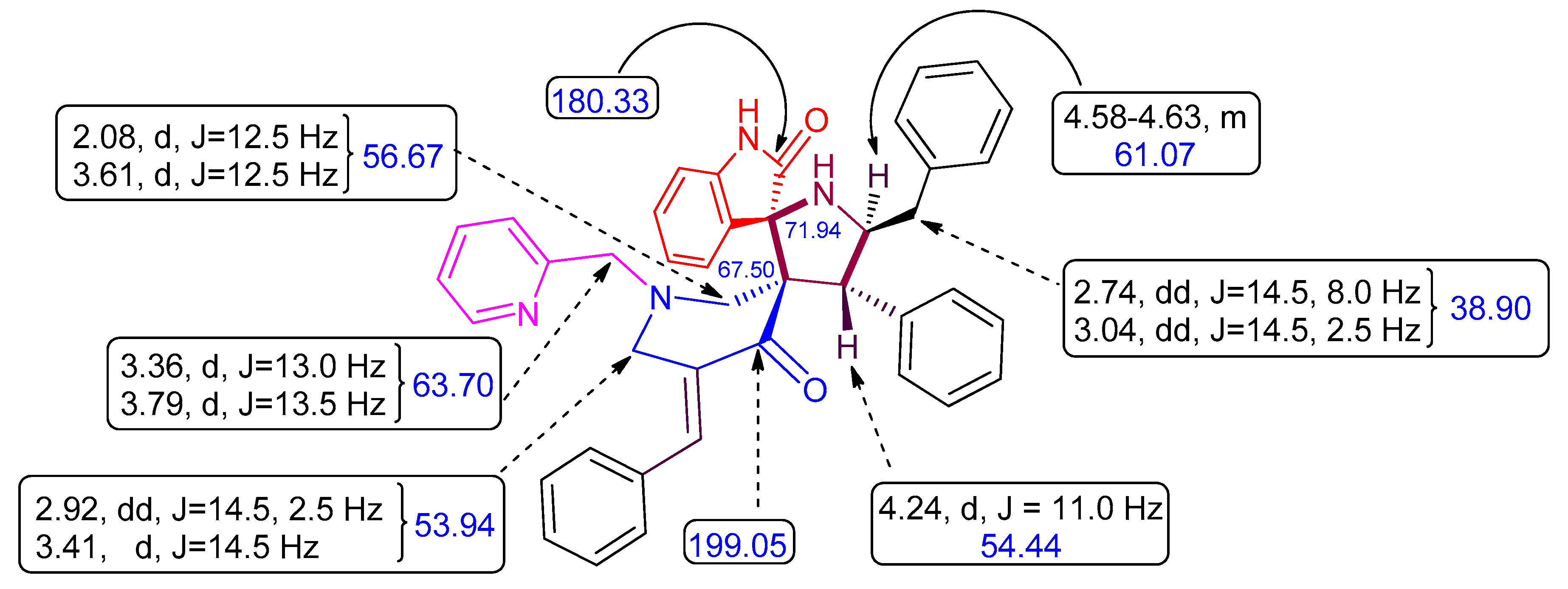
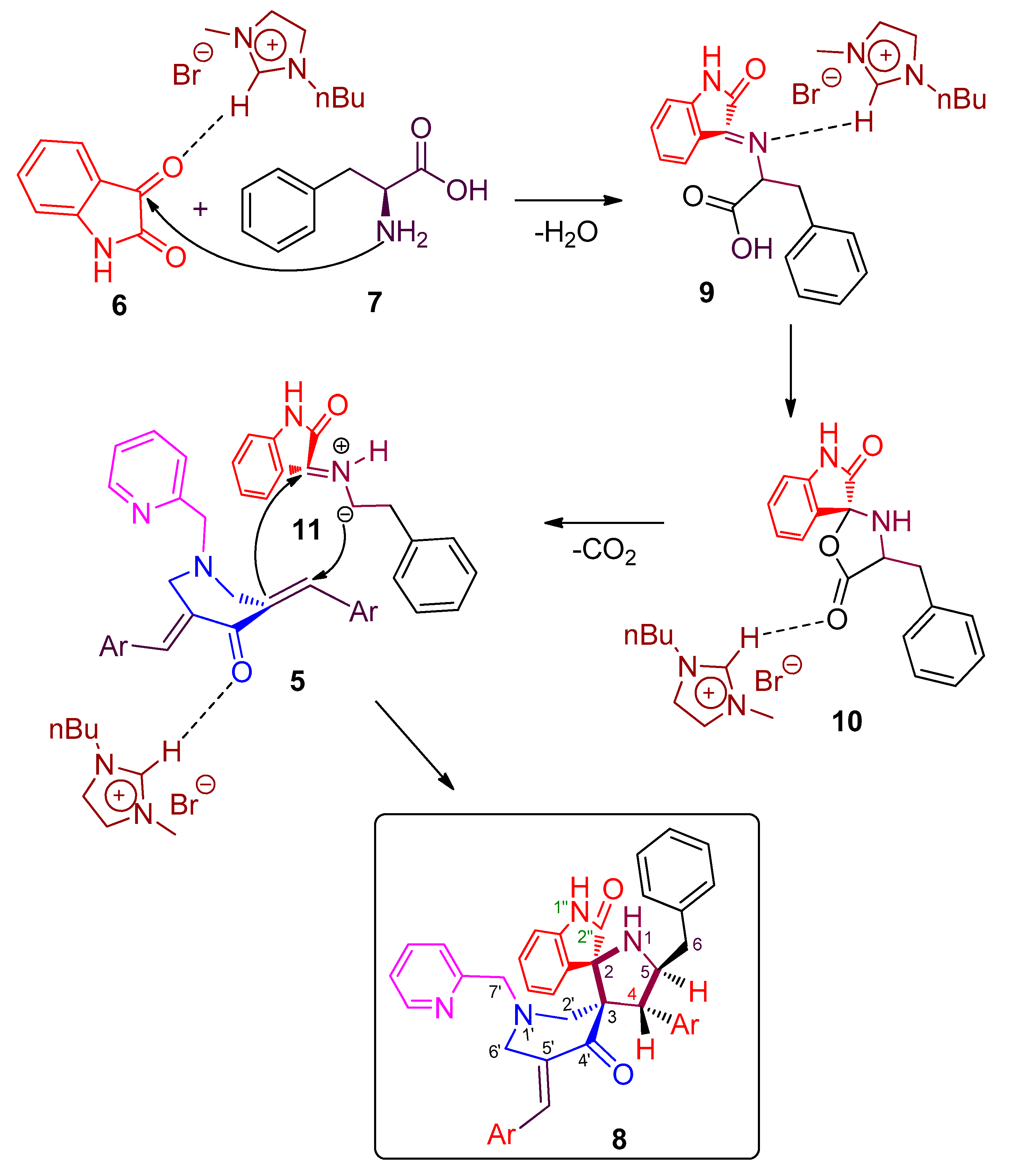
2. Results and Discussion
3. Materials and Methods
General Procedure for the Synthesis of 4-(Aryl)-5-benzylpyrrolo(spiro [2.3″]oxindole)-spiro[3.3′]-5′-(arylmethylidene)-1′-N-(pyridinylmethyl)piperidin-4′-one 8a–h
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, H.C.; Ma, J.A. Catalytic asymmetric tandem transformations triggered by conjugate additions. Angew. Chem. Int. Ed. 2006, 45, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Volla, C.M.R.; Atodiresei, I.; Rueping, M. Catalytic C-C bond-forming multi-component cascade or domino reactions: Pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 2014, 114, 2390–2431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ma, S. Efficient carbazole synthesis via Pd/Cu-cocatalyzed cross-coupling/isomerization of 2-allyl-3-iodoindoles and terminal alkynes. Org. Lett. 2014, 16, 1542–1545. [Google Scholar] [CrossRef]
- Kanbayashi, N.; Takenaka, K.; Okamura, T.; Onitsuka, K. Asymmetric auto-tandem catalysis with a planar-chiral ruthenium complex: Sequential allylic amidation and atom-transfer radical cyclization. Angew. Chem. Int. Ed. 2013, 52, 4897–4901. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Brasche, G.; Gericke, K. Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006; p. 672. [Google Scholar]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef]
- Kobayashi, S.; Jorgensen, A.K. Cycloaddition Reactions in Organic Synthesis; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Kanagaraju, G.; Thangamani, A. Design and synthesis of spiro derivatives containing a thiophene ring and evaluation of their anti-microbial activity. Orient. J. Chem. 2014, 30, 1619–1630. [Google Scholar] [CrossRef]
- Dhanalakshmi, P.; Sriram Babu, S.; Thimmarayaperumal, S.; Shanmugam, S. One-pot chemo/regio/stereoselective generation of a library of functionalized spiro-oxindoles/pyrrolizines/-pyrrolidines from α-aroylidineketene dithioacetals. RSC Adv. 2015, 5, 33705–33719. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Williams, R.M. A concise asymmetric synthesis of the ADE fragment of nakadomarin A. Org. Lett. 2004, 6, 4539–4541. [Google Scholar] [CrossRef] [PubMed]
- Boruah, M.; Konwar, D.; Sharma, S.D. KF/Al2O3 mediated 1,3-dipolar cycloaddition of azomethine ylides: A novel and convenient procedure for the synthesis of highly substituted pyrrolidines. Tetrahedron Lett. 2007, 48, 4535–4537. [Google Scholar] [CrossRef]
- Pandey, G.; Banerjee, P.; Gadre, S.R. Construction of enantiopure pyrrolidine ring system via asymmetric [3+2]-cycloaddition of azomethine ylides. Chem. Rev. 2006, 106, 4484–4517. [Google Scholar] [CrossRef]
- Zhang, Z.C. Catalysis in Ionic Liquids. Adv. Catal. 2006, 49, 153–237. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Application of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and application. Chem Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W.; Spande, T.F.; Whittaker, N.; Hignet, R.J.; Feigl, D.; Nishimori, N.; Tokuyama, T.; Myers, C.W. Alkaloids from dendrobatid frogs: Structures of two omega-hydroxy congeners of 3-butyl-5-propylindolizidine and occurrence of 2,5-disubstituted pyrrolidines and a 2,6-disubstituted piperidine. J. Nat. Prod. 1986, 49, 265–280. [Google Scholar] [CrossRef]
- Hilton, S.T.; Ho, T.C.T.; Pljevaljcic, G.; Jones, K. A new route to spirooxindoles. Org. Lett. 2000, 2, 2639–2641. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kotresha, D.; Balakrishna, J.P. Caspase dependent apoptotic activity of polycyclic cage-like heterocyclic hybrids. Saudi J. Biol. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N. In vitro mechanistic investigation of polycyclic cage-like heterocyclic hybrid possessing diverse pharmacophoric units. J. King Saud Univ. Sci. 2020, 32, 2406–2413. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kotresha, D.; Menendez, J.C. Spirooxindole-pyrrolidine heterocyclic hybrids promotes apoptosis through activation of caspase-3. Bioorg. Med. Chem. 2019, 27, 2487–2498. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Periyasami, G.; Athimoolam, S.; Ranjith Kumar, R.; Asad, M.; Asiri, A.M. Dipolar cycloaddition based multi-component reaction: Synthesis of spiro tethered acenaphthylene–indolizine–pyridinone hybrids. Tetrahedron Lett. 2018, 59, 3336–3340. [Google Scholar] [CrossRef]
- Almansour, A.I.; Kumar, R.S.; Beevi, F.; Shirazi, A.N.; Osman, H.; Ismail, R.; Choon, T.S.; Sullivan, B.; McCaffery, K.; Nahhas, A.; et al. Facile, Regio- and Diastereoselective Synthesis of Spiro-Pyrrolidine and Pyrrolizine Derivatives and Evaluation of Their Antiproliferative Activities. Molecules 2014, 19, 10033–10055. [Google Scholar] [CrossRef] [PubMed]
- Al-thamili, D.M.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kumar, R.S. Functionalized N-Pyridinylmethyl Engrafted Bisarylmethylidenepyridinones as Anticancer Agents. Processes 2020, 8, 1154. [Google Scholar] [CrossRef]
- Crystallographic Data for 8b has been Deposited with the Cambridge Crystallographic Data Centre as Supplementary Publication Number CCDC 1991074. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 17 March 2020).
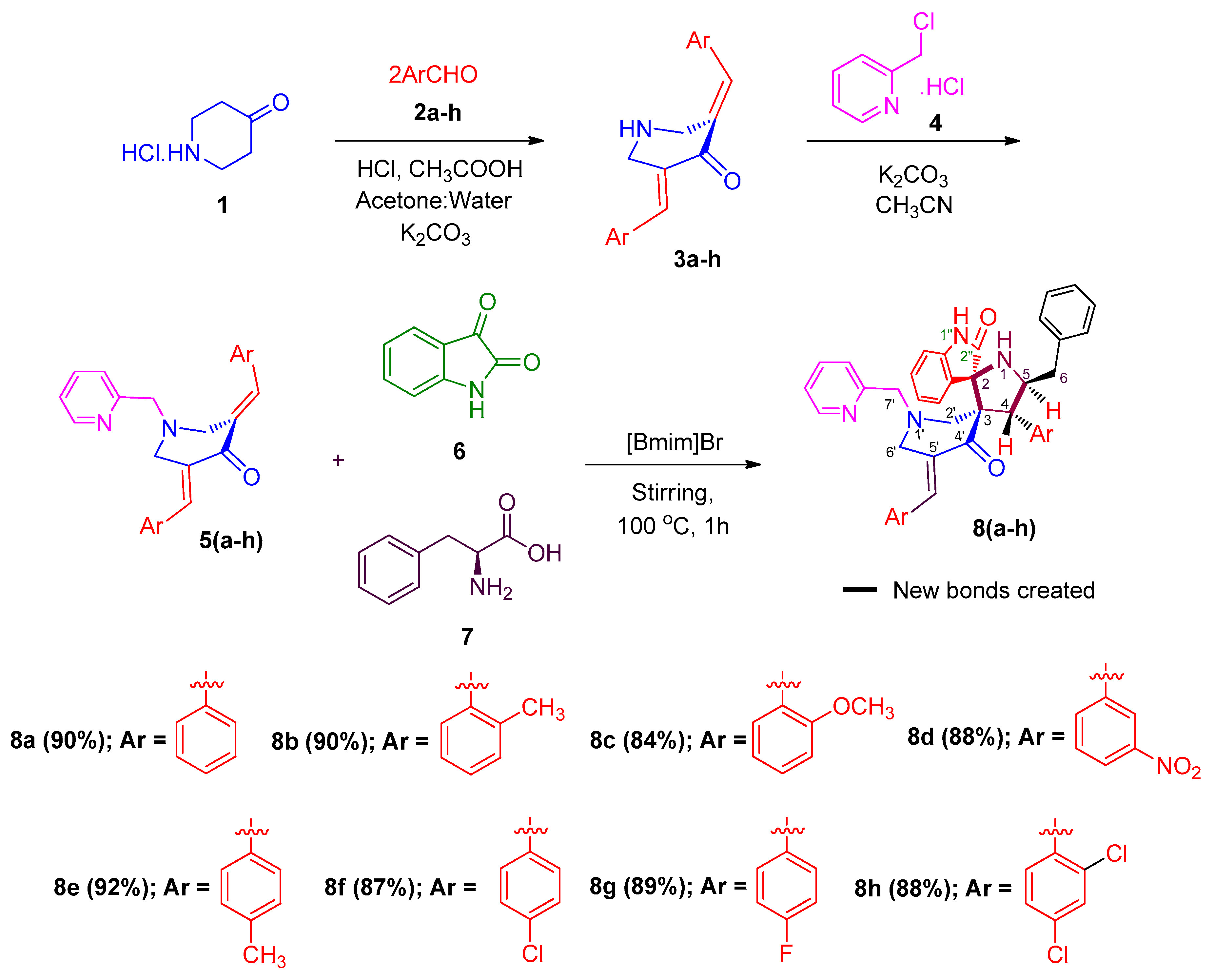

| Entry | Solvents | Temp (°C) | Time (h) | Yield a (%) |
|---|---|---|---|---|
| 1 | Ethanol | 80 | 6 | 67 |
| 2 | Methanol | 70 | 2 | 85 |
| 3 | Dioxane | 100 | 10 | 45 |
| 4 | Methanol: Dioxane | 100 | 10 | 51 |
| 5 | Acetonitrile | 80 | 10 | Traces |
| 6 | [bmim]Br | 100 | 1 | 92 |
Sample Availability: Samples of the compounds 8a–h are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.S.; M. Al-thamili, D.; Almansour, A.I.; Arumugam, N.; Dege, N. [Bmim]Br Accelerated One-Pot Three-Component Cascade Protocol for the Construction of Spirooxindole–Pyrrolidine Heterocyclic Hybrids. Molecules 2020, 25, 4779. https://doi.org/10.3390/molecules25204779
Kumar RS, M. Al-thamili D, Almansour AI, Arumugam N, Dege N. [Bmim]Br Accelerated One-Pot Three-Component Cascade Protocol for the Construction of Spirooxindole–Pyrrolidine Heterocyclic Hybrids. Molecules. 2020; 25(20):4779. https://doi.org/10.3390/molecules25204779
Chicago/Turabian StyleKumar, Raju Suresh, Dhaifallah M. Al-thamili, Abdulrahman I. Almansour, Natarajan Arumugam, and Necmi Dege. 2020. "[Bmim]Br Accelerated One-Pot Three-Component Cascade Protocol for the Construction of Spirooxindole–Pyrrolidine Heterocyclic Hybrids" Molecules 25, no. 20: 4779. https://doi.org/10.3390/molecules25204779
APA StyleKumar, R. S., M. Al-thamili, D., Almansour, A. I., Arumugam, N., & Dege, N. (2020). [Bmim]Br Accelerated One-Pot Three-Component Cascade Protocol for the Construction of Spirooxindole–Pyrrolidine Heterocyclic Hybrids. Molecules, 25(20), 4779. https://doi.org/10.3390/molecules25204779