Synthesis of Polyhedral Borane Cluster Fused Heterocycles via Transition Metal Catalyzed B-H Activation
Abstract
1. Introduction
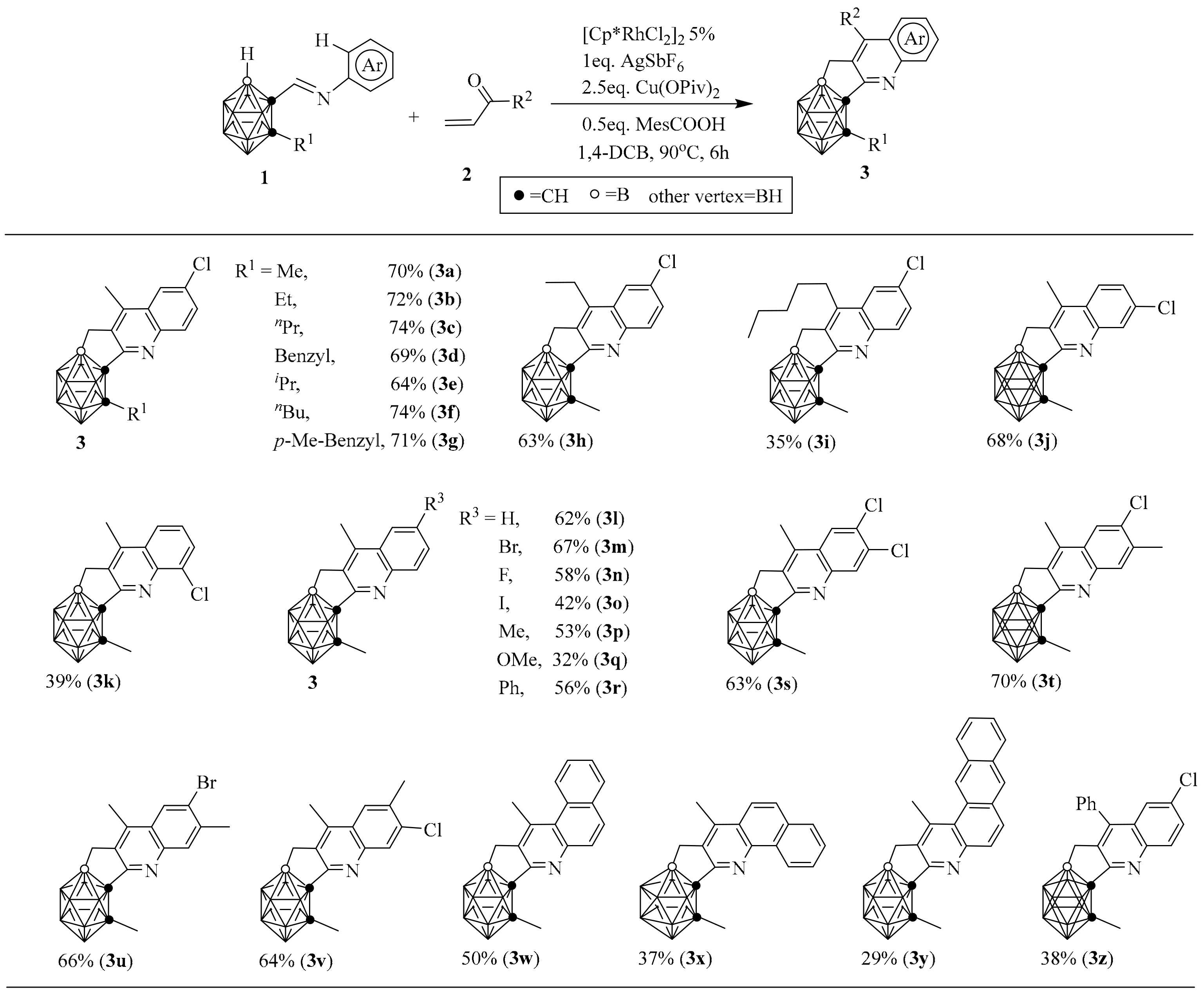
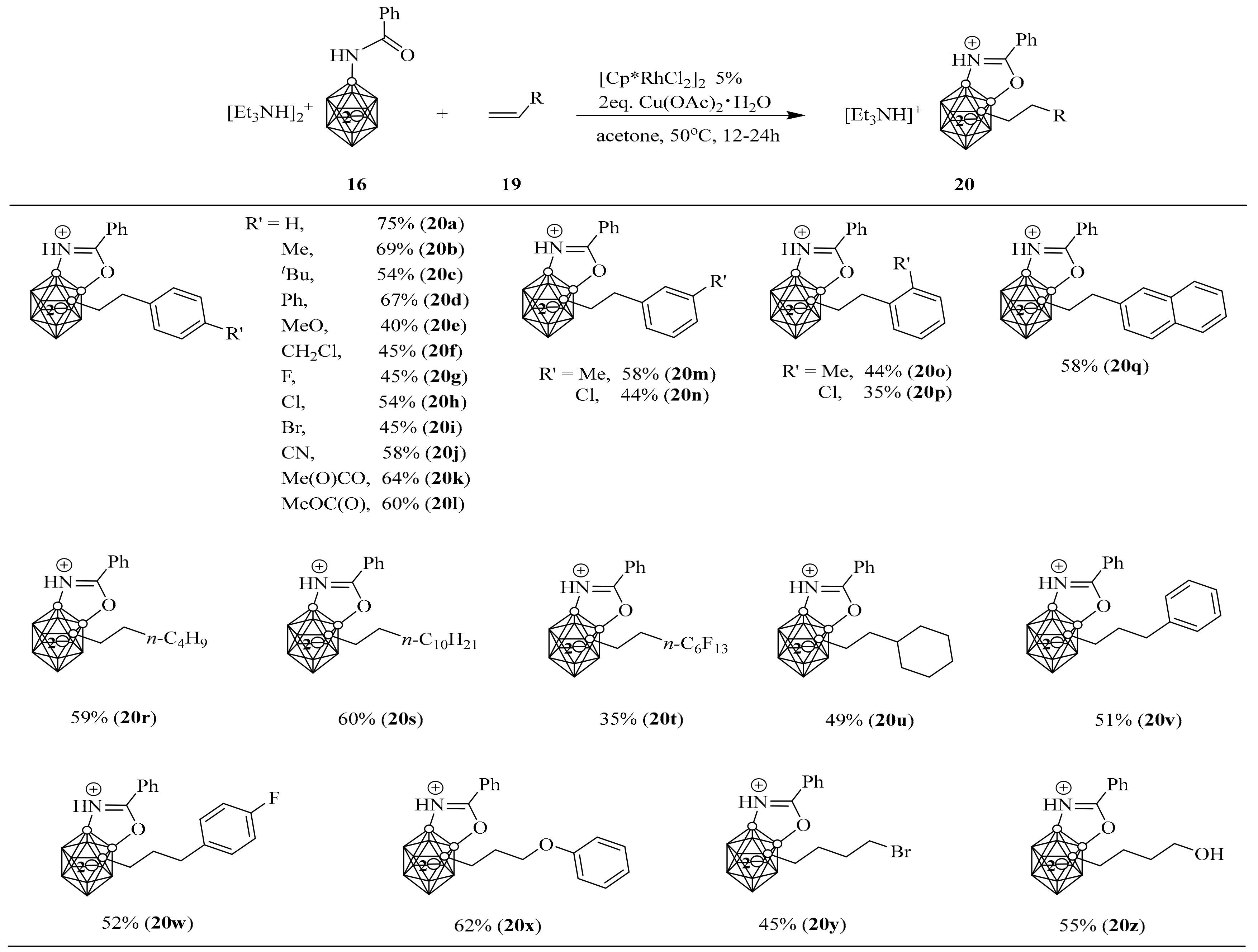
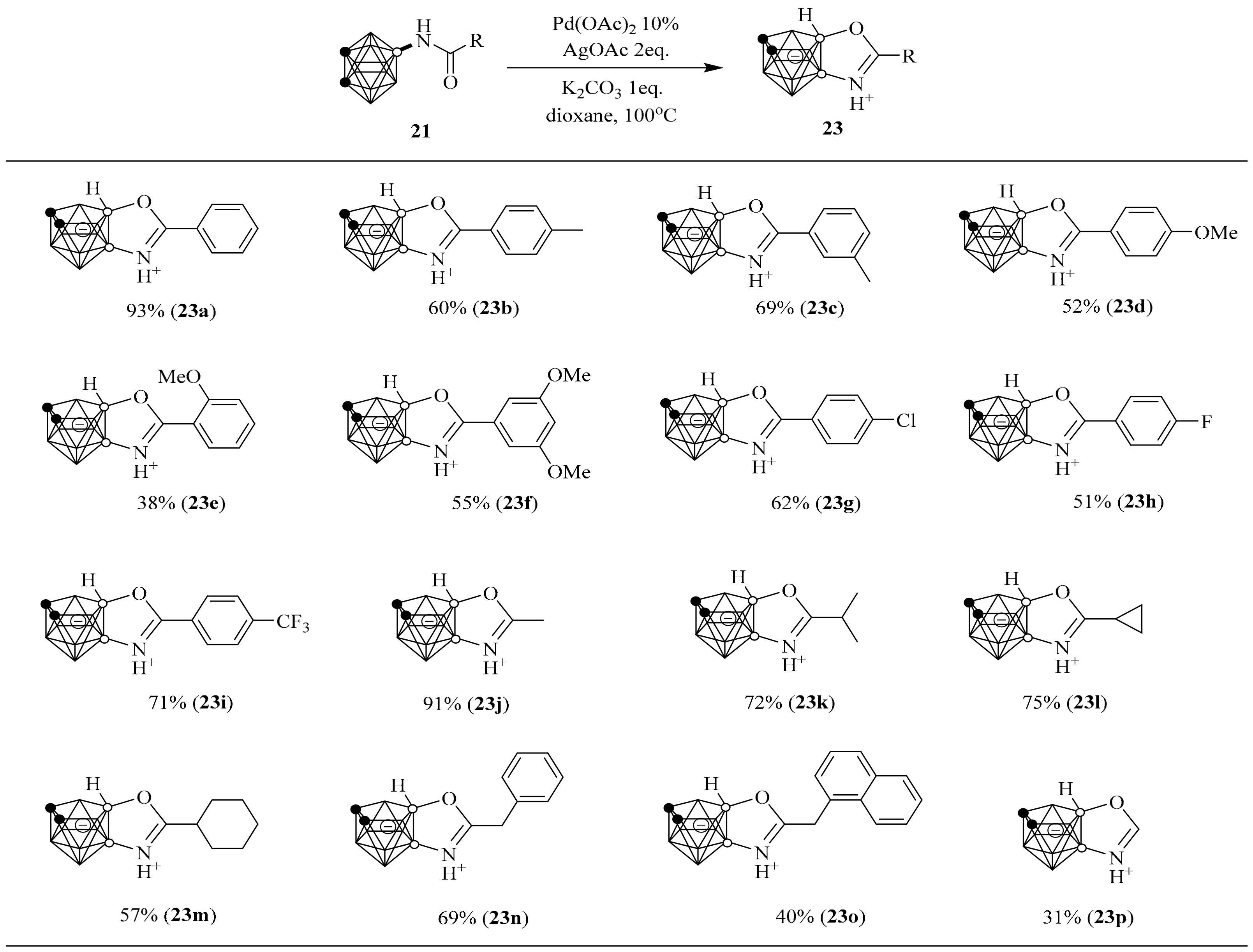
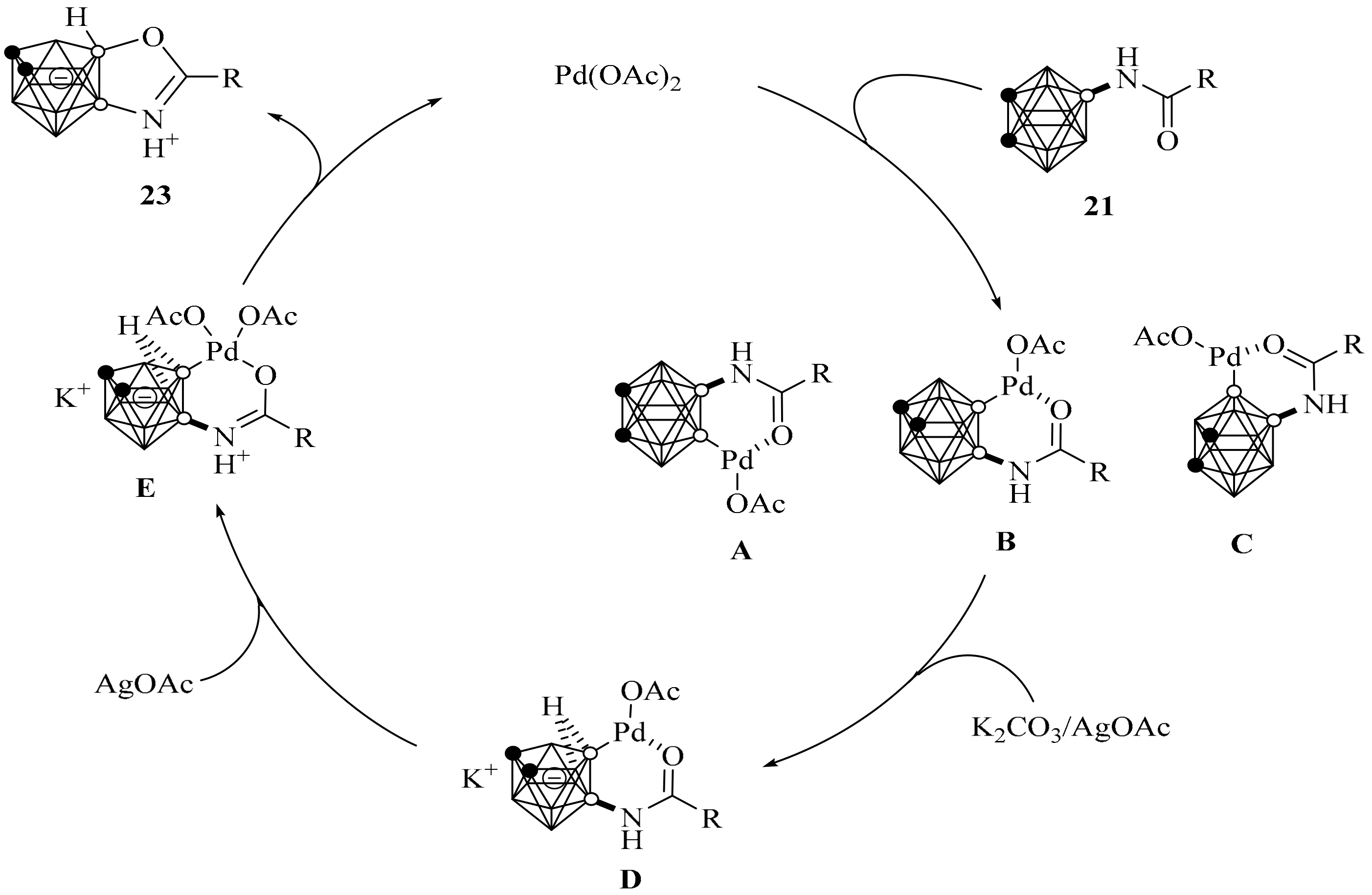
2. Synthesis of o-Carborane-Fused Heterocycles
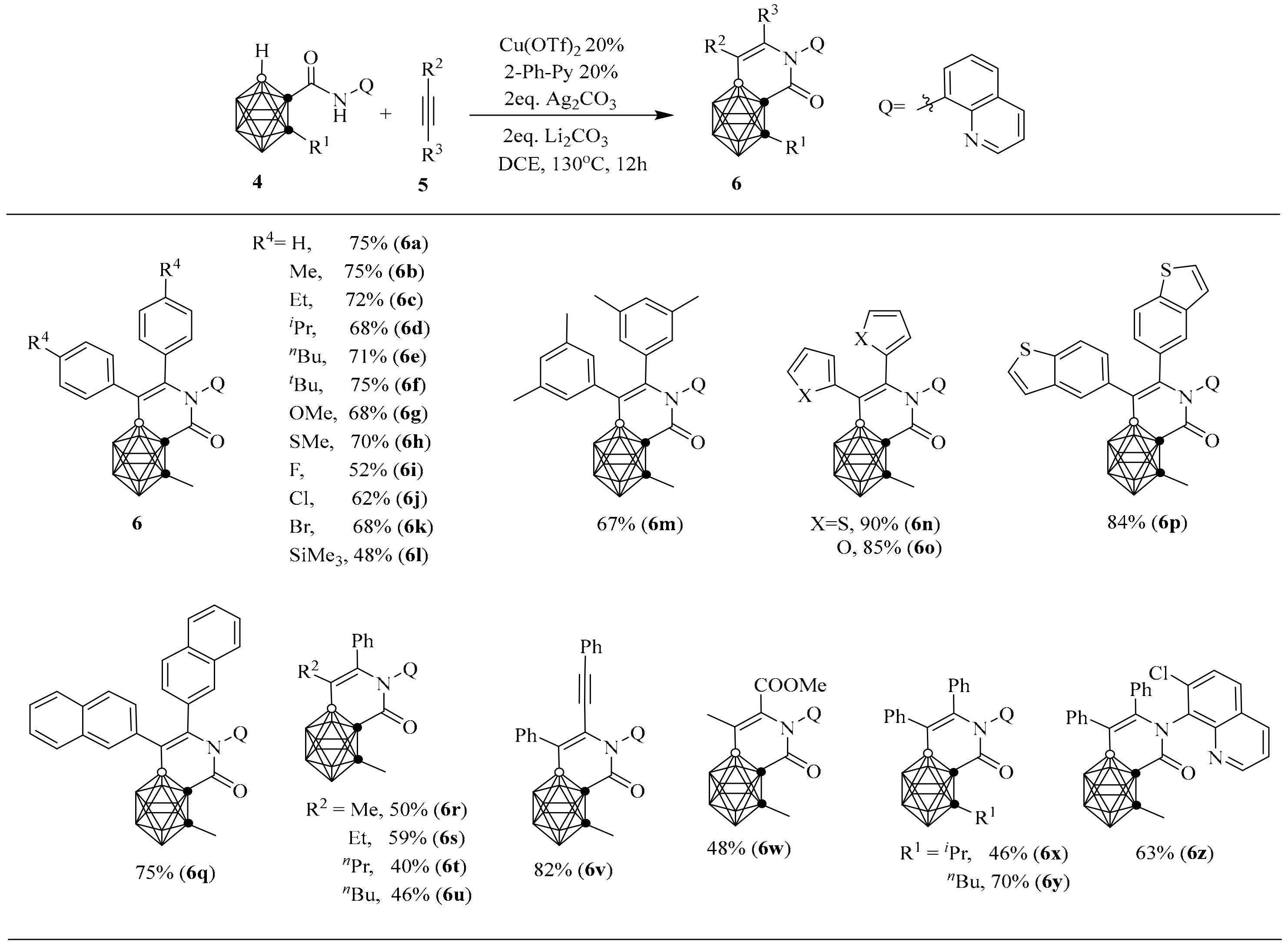
3. Synthesis of [CB11]−-Fused Heterocycle
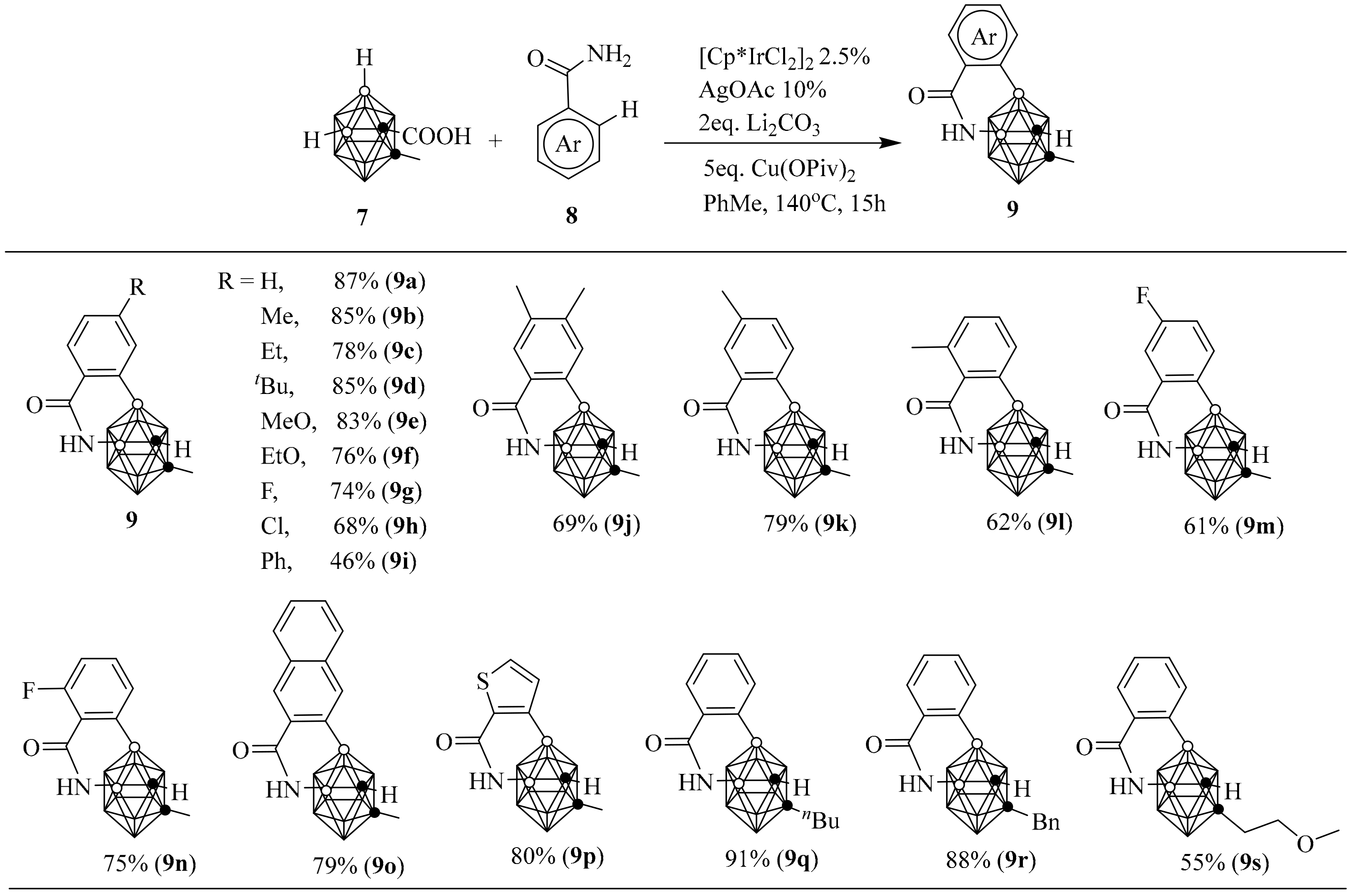
4. Synthesis of [B12]2−-Fused Heterocycles
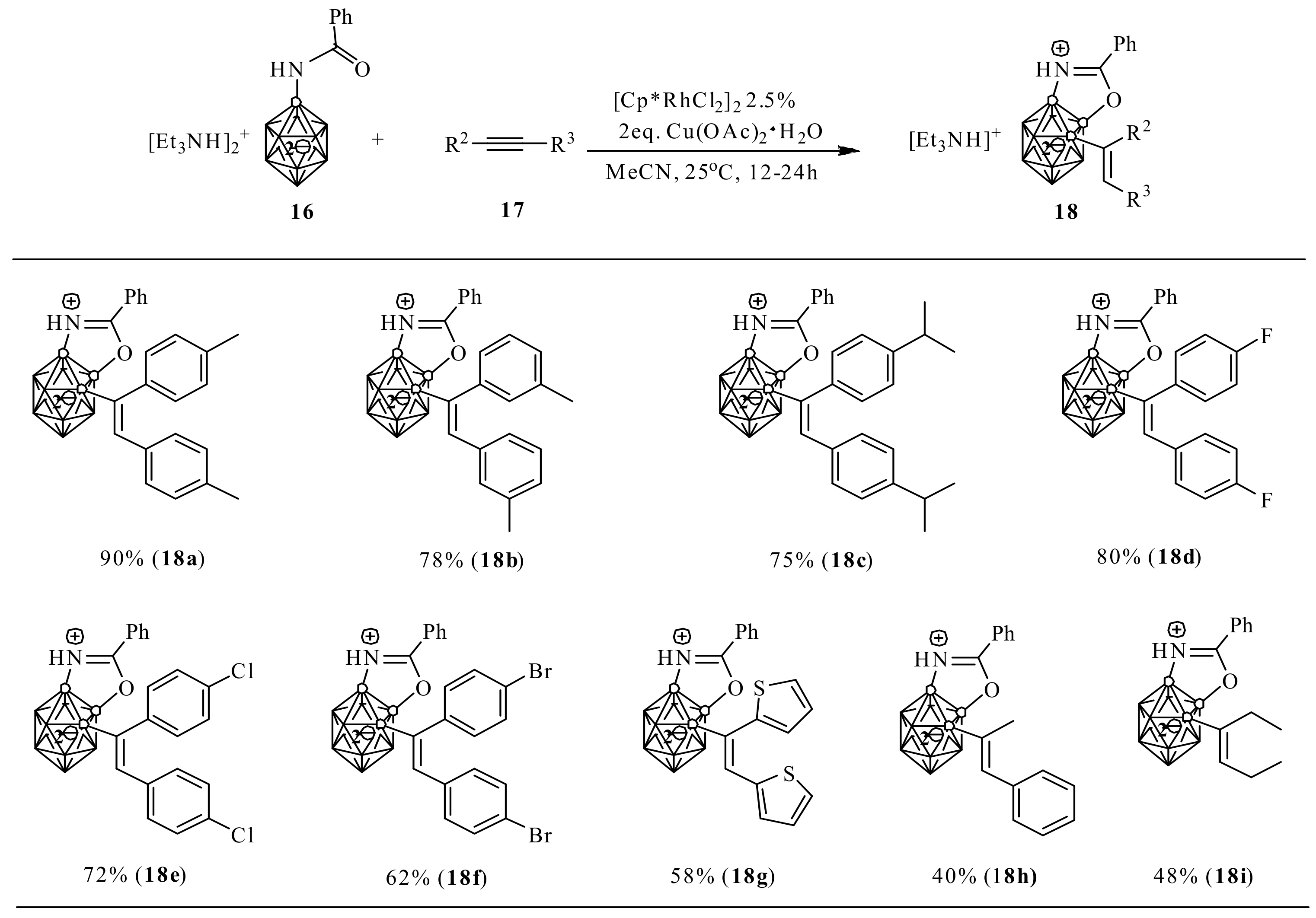
5. Synthesis of nido-Carborane-Fused Heterocycles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hosmane, N.S. Boron Science: New Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kirlikovali, K.O.; Axtell, J.C.; Gonzalez, A.; Phung, A.C.; Khan, S.I.; Spokoyny, A.M. Luminescent metal complexes featuring photophysically innocent boron cluster ligands. Chem. Sci. 2016, 7, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, J.; Cao, Y.; Zhao, J.; Jia, W.; Chen, Y.; Jia, D. Mechanically triggered reversible stepwise tricolor switching and thermochromism of anthracene-o-carborane dyad. Chem. Sci. 2018, 9, 5270–5277. [Google Scholar] [CrossRef] [PubMed]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 4240–4251. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Daou, A.; Barbu, E.; Tsibouklis, J. Towards carborane-functionalised structures for the treatment of brain cancer. Drug Discov. Today 2018, 23, 63–75. [Google Scholar] [CrossRef]
- Xie, Z. Cyclopentadienyl-carboranyl hybrid compounds: a new class of versatile ligands for organometallic chemistry. Acc. Chem. Res. 2003, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Xie, Z. Advances in the chemistry of carboranes and metallacarboranes with more than 12 vertices. Coord. Chem. Rev. 2007, 251, 2452–2476. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Jin, G.-X. Transition metal complexes based on carboranyl ligands containing N, P, and S donors: Synthesis, reactivity and applications. Coord. Chem. Rev. 2013, 257, 2522–2535. [Google Scholar] [CrossRef]
- Spokoyny, A.M. New ligand platforms featuring boron-rich clusters as organomimetic substituents. Pure Appl. Chem. 2013, 85, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Hosmane, N.S.; Maguire, J.A. Comprehensive Organometallic Chemistry III; Elsevier: Oxford, UK, 2007; Chapter 5; Volume 3. [Google Scholar]
- Qiu, Z. Recent advances in transition metal-mediated functionalization of o-carboranes. Tetrahedron Lett. 2015, 56, 963–971. [Google Scholar] [CrossRef]
- Duttwyler, S. Recent advances in B-H functionalization of icosahedral carboranes and boranes by transition metal catalysis. Pure Appl. Chem. 2018, 90, 733–744. [Google Scholar] [CrossRef]
- Quan, Y.; Qiu, Z.; Xie, Z. Transition-Metal-Catalyzed Selective Cage B-H Functionalization of o-Carboranes. Chem. Eur. J. 2018, 24, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, H. Transition metal-induced B-H functionalization of o-carborane. Coord. Chem. Rev. 2019, 378, 466–482. [Google Scholar] [CrossRef]
- Quan, Y.; Xie, Z. Controlled functionalization of o-carborane via transition metal catalyzed B-H activation. Chem. Soc. Rev. 2019, 48, 3660–3673. [Google Scholar] [CrossRef]
- Qiu, Z.; Quan, Y.; Xie, Z. Palladium-catalyzed selective fluorination of o-carboranes. J. Am. Chem. Soc. 2013, 135, 12192–12195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, H.; Li, J.; Xu, F.; Zhao, J.; Yan, H. Selective catalytic B-H arylation of o-carboranyl aldehydes by a transient directing Strategy. J. Am. Chem. Soc. 2017, 139, 14511–14517. [Google Scholar] [CrossRef]
- Wu, J.; Cao, K.; Xu, T.-T.; Zhang, X.-J.; Jiang, L.; Yang, J.; Huang, Y. Palladium catalyzed regioselective mono-alkenylation of o-carboranes via Heck type coupling reaction of a cage B-H bond. RSC Adv. 2015, 5, 91683–91685. [Google Scholar] [CrossRef]
- Cao, K.; Xu, T.-T.; Wu, J.; Jiang, L.; Yang, J.; Xu, T.-T.; Wu, J. Palladium catalyzed/silver tuned selective mono-/tetra-acetoxylation of o-carboranes via B-H activation. Chem. Commun. 2016, 52, 11446–11449. [Google Scholar] [CrossRef]
- Xu, T.-T.; Zhang, C.-Y.; Cao, K.; Wu, J.; Jiang, L.; Li, J.; Li, B.; Yang, J. Palladium-catalyzed selective mono-chlorination of o-carboranes: Changing the concept of FeCl3 from Lewis acid to chlorine source in carboranes. ChemistrySelect 2017, 2, 3396–3399. [Google Scholar] [CrossRef]
- Xu, T.-T.; Cao, K.; Wu, J.; Zhang, C.-Y.; Yang, J. Palladium-catalyzed selective mono-/tetra-acetoxylation of o-carboranes with acetic acid via cross dehydrogenative coupling of cage B-H/O-H Bonds. Inorg. Chem. 2018, 57, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-T.; Cao, K.; Zhang, C.-Y.; Wu, J.; Jiang, L.; Yang, J. Palladium catalyzed selective arylation of o-carboranes via B(4)-H activation: Amide induced regioselectivity reversal. Chem. Commun. 2018, 54, 13603–13606. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhang, J.; Yang, J.; Quan, Y.; Xie, Z. Catalytic regioselective cage B(8)-H arylation of o-carboranes via “cage-walking” strategy. J. Am. Chem. Soc. 2019, 141, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhang, C.-Y.; Xu, T.-T.; Wu, J.; Ding, L.-F.; Jiang, L.; Yang, J. Palladium catalyzed/counter ion tuned selective methylation of o-carboranes. J. Organomet. Chem. 2019, 902, 120956. [Google Scholar] [CrossRef]
- Wu, J.; Cao, K.; Zhang, C.-Y.; Xu, T.-T.; Ding, L.-F.; Li, B.; Yang, J. Catalytic Oxidative Dehydrogenative Coupling of Cage B-H/B-H Bonds for Synthesis of Bis(o-carborane)s. Org. Lett. 2019, 21, 5986–5989. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-T.; Cao, K.; Zhang, C.-Y.; Wu, J.; Jiang, L.-F.; Yang, J. Old Key Opens the Lock in Carborane: The in Situ NHC-Palladium Catalytic System for Selective Arylation of B(3,6)-H Bonds of o-Carboranes via B-H Activation. Org. Lett. 2019, 21, 9276–9279. [Google Scholar] [CrossRef]
- Quan, Y.; Xie, Z. Iridium catalyzed regioselective cage boron alkenylation of o-carboranes via direct cage B-H activation. J. Am. Chem. Soc. 2014, 136, 15513–15516. [Google Scholar] [CrossRef]
- Lyu, H.; Quan, Y.; Xie, Z. Palladium-catalyzed direct dialkenylation of cage B-H Bonds in o-carboranes through cross-coupling reactions. Angew. Chem. Int. Ed. 2015, 54, 10623–10626. [Google Scholar] [CrossRef]
- Lyu, H.; Quan, Y.; Xie, Z. Transition metal catalyzed direct amination of the cage B(4)-H Bond in o-carboranes: Synthesis of tertiary, secondary, and primary o-carboranyl amines. J. Am. Chem. Soc. 2016, 138, 12727–12730. [Google Scholar] [CrossRef]
- Lyu, H.; Quan, Y.; Xie, Z. Rhodium-catalyzed regioselective hydroxylation of cage B-H bonds of o-carboranes with O2 or Air. Angew. Chem. Int. Ed. 2016, 55, 11840–11844. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Tang, C.; Xie, Z. Palladium catalyzed regioselective B-C(sp) coupling via direct cage B-H activation: Synthesis of B(4)-alkynylated o-carboranes. Chem. Sci. 2016, 7, 5838–5845. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Xie, Z. Palladium-Catalyzed Regioselective Diarylation of o-Carboranes By Direct Cage B-H Activation. Angew. Chem. Int. Ed. 2016, 55, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Quan, Y.; Xie, Z. Transition metal catalyzed regioselective B(4)-halogenation and B(4,5)-diiodination of cage B-H bonds in o-carboranes. Chem. Eur. J. 2017, 23, 14866–14871. [Google Scholar] [CrossRef]
- Quan, Y.; Lyu, H.; Xie, Z. Dehydrogenative cross-coupling of o-carborane with thiophenes via Ir-catalyzed regioselective cage B-H and C(sp2)-H activation. Chem. Commun. 2017, 53, 4818–4821. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.; Li, Y.; Yan, H.; Bregadze, V.I. Cobalt-Mediated B-H Activation and Cyclopentadienyl-Participated Diels-Alder Addition in the Reaction of a 16e CpCo Complex Containing an o-Carborane-1,2-dithiolato Ligand with HC≡C-C(O)Ph. Inorg. Chem. 2009, 49, 4–6. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.; Zhang, X.; Li, Y.; Yan, H.; Bregadze, V.I. Stepwise and Selective Carborane Substitution in the B (3,6) Positions of a 16e CpCo Half-Sandwich Complex Containing a Chelating ortho-Carborane-1,2-dithiolate Ligand. Inorg. Chem. 2010, 49, 3911–3917. [Google Scholar] [CrossRef]
- Li, H.; Bai, F.; Yan, H.; Lu, C.; Bregadze, V.I. Iridium(III)-Catalyzed Selective Sulfonamidation of o-Carborane with Sulfonyl Azide by Carboxylic Acid-Assisted B(4)-H Bond Activation. Eur. J. Org. Chem. 2017, 1343–1352. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Maderna, A. Applications of radiolabeled boron clusters to the diagnosis and treatment of cancer. Chem. Rev. 1999, 99, 3421–3434. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Sivaev, I.B.; Glazun, S.A. Polyhedral boron compounds as potential diagnostic and therapeutic antitumor agents. Anti-Cancer Agents Med. Chem. 2006, 6, 75–109. [Google Scholar] [CrossRef]
- Potenza, J.A.; Lipscomb, W.N.; Vickers, G.D.; Schroeder, H. Order of Electrophilic Substitution in 1,2-Dicarbaclovododecaborane(12) and Nuclear Magnetic Resonance Assignment. J. Am. Chem. Soc. 1966, 88, 628–629. [Google Scholar] [CrossRef]
- Koetzle, T.F.; Lipscomb, W.N. Approximate wave functions for carboranes parametrized from self-consistent field model calculations. Inorg. Chem. 1970, 9, 2743–2748. [Google Scholar]
- Cao, K.; Huang, Y.; Yang, J.; Wu, J. Palladium catalyzed selective mono-arylation of o-carboranes via B-H activation. Chem. Commun. 2015, 51, 7257–7260. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Xie, Z. Palladium-Catalyzed Regioselective Intramolecular Coupling of o-Carborane with Aromatics via Direct Cage B-H Activation. J. Am. Chem. Soc. 2015, 137, 3502–3505. [Google Scholar] [CrossRef]
- Lyu, H.; Quan, Y.; Xie, Z. Rhodium catalyzed cascade cyclization featuring B-H and C-H activation: One-step construction of carborane-fused N-polyheterocycles. Chem. Sci. 2018, 9, 6390–6394. [Google Scholar] [CrossRef]
- Chen, Y.; Au, Y.K.; Quan, Y.; Xie, Z. Copper catalyzed/mediated direct B-H alkenylation/alkynylation in carboranes. Sci. China: Chem. 2019, 62, 74–79. [Google Scholar] [CrossRef]
- Au, Y.K.; Lyu, H.; Quan, Y.; Xie, Z. Catalytic Cascade Dehydrogenative Cross-Coupling of BH/CH and BH/NH: One-Pot Process to Carborano-Isoquinolinone. J. Am. Chem. Soc. 2019, 141, 12855–12862. [Google Scholar] [CrossRef]
- Lin, F.; Shen, Y.; Zhang, Y.; Sun, Y.; Liu, J.; Duttwyler, S. Fusing Carborane Carboxylic Acids with Alkynes: 3D Analogues of Isocoumarins via Regioselective B-H Activation. Chem. Eur. J. 2018, 24, 551–555. [Google Scholar] [CrossRef]
- Peymann, T.; Knobler, C.B.; Hawthorne, M.F. Synthesis of Alkyl and Aryl Derivatives of closo-B12H122- by the Palladium-Catalyzed Coupling of closo-B12H11I2- with Grignard Reagents. Inorg. Chem. 1998, 37, 1544–1548. [Google Scholar] [CrossRef]
- Himmelspach, A.; Finze, M.; Vöge, A.; Gabel, D. Cesium and Tetrabutylammonium Salt of the Ethynyl-closo-dodecaborate Dianion. Z. Anorg. Allg. Chem. 2012, 638, 512–519. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Lin, F.; Liu, J.; Duttwyler, S. Rhodium(III)-catalyzed alkenylation-annulation of closo-dodecaborate anions through double B-H activation at room temperature. Angew. Chem. Int. Ed. 2016, 55, 15609–15614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Wang, L.; Sun, Y.; Lin, F.; Liu, J.; Duttwyler, S. Rh(III)-Catalyzed Functionalization of closo-Dodecaborates via Selective B-H Activation: Bypassing Competitive C-H Activation. Chem. Eur. J. 2018, 24, 15812–15817. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Zhang, Y.; Liu, J.; van der Veen, S.; Duttwyler, S. The closo-dodecaborate dianion fused with oxazoles provides 3D diboraheterocycles with selective antimicrobial activity. Chem. Eur. J. 2018, 24, 10364–10371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Cao, K.; Xu, T.-T.; Wu, J.; Jiang, L.; Yang, J. A facile approach for the synthesis of nido-carborane fused oxazoles via one pot deboronation/cyclization of 9-amide-o-carboranes. Chem. Commun. 2019, 55, 830–833. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, K.; Zhang, C.-Y.; Xu, T.-T.; Wu, J.; Wen, X.-Y.; Jiang, W.-J.; Chen, M.; Yang, J. Synthesis of Polyhedral Borane Cluster Fused Heterocycles via Transition Metal Catalyzed B-H Activation. Molecules 2020, 25, 391. https://doi.org/10.3390/molecules25020391
Cao K, Zhang C-Y, Xu T-T, Wu J, Wen X-Y, Jiang W-J, Chen M, Yang J. Synthesis of Polyhedral Borane Cluster Fused Heterocycles via Transition Metal Catalyzed B-H Activation. Molecules. 2020; 25(2):391. https://doi.org/10.3390/molecules25020391
Chicago/Turabian StyleCao, Ke, Cai-Yan Zhang, Tao-Tao Xu, Ji Wu, Xin-Yu Wen, Wen-Jun Jiang, Mao Chen, and Junxiao Yang. 2020. "Synthesis of Polyhedral Borane Cluster Fused Heterocycles via Transition Metal Catalyzed B-H Activation" Molecules 25, no. 2: 391. https://doi.org/10.3390/molecules25020391
APA StyleCao, K., Zhang, C.-Y., Xu, T.-T., Wu, J., Wen, X.-Y., Jiang, W.-J., Chen, M., & Yang, J. (2020). Synthesis of Polyhedral Borane Cluster Fused Heterocycles via Transition Metal Catalyzed B-H Activation. Molecules, 25(2), 391. https://doi.org/10.3390/molecules25020391




