Abstract
Ionic liquids (ILs) are a class of pure ions with melting points lower than 100 °C. They are getting more and more attention because of their high thermal stability, high ionic conductivity and dielectric properties. The unique dielectric properties aroused by the ion motion of ILs makes ILs-contained inorganics or organics responsive to electric field and have great application potential in smart electrorheological (ER) fluids which can be used as the electro-mechanical interface in engineering devices. In this review, we summarized the recent work of various kinds of ILs-contained inorganic ionogels and poly(ionic liquid)s (PILs) as ER materials including their synthesis methods, ER responses and dielectric analysis. The aim of this work is to highlight the advantage of ILs in the synthesis of dielectric materials and their effects in improving ER responses of the materials in a wide temperature range. It is expected to provide valuable suggestions for the development of ILs-contained inorganics and PILs as electric field responsive materials.
1. Introduction
Stimuli-responsive materials are the materials that are able to sense and response to external stimuli, such as temperature, pH value, light, electric and magnetic field, by changing properties or structures in a controllable way [1,2,3,4,5]. For example, light-responsive molecules and temperature-responsive polymers are well known branches of stimuli-responsive materials [6,7,8,9]. Among various stimuli-responsive materials, electrorheological (ER) fluids are considerably attractive because of their rapid and reversible response to electric field. ER fluids are usually suspensions composed of dielectric particles from nanometers to microns dispersed in insulating liquids [10,11]. Without the stimuli of an electric field, the particles dispersed randomly that the suspensions perform like Newtonian fluids. However, they will be like Bingham fluids when an electric field is applied. This is because the particles are able to form chain or column structures in the direction of electric field (Figure 1) [12,13,14]. The electric field controlled liquid-like to solid-like mutual transition makes ER fluids attractive in many engineering applications, such as dampers, shock absorbers, finishing devices, brakes and tactile interfaces [15,16,17,18,19,20].
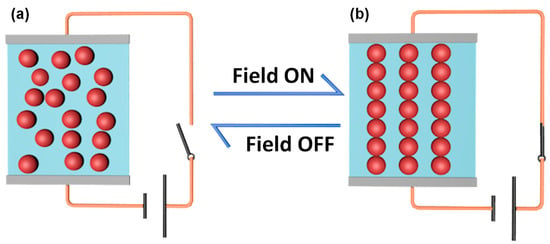
Figure 1.
The arrangement structure of the dispersed particles in an ER fluid before (a) and after (b) the application of an external electric field.
Properties of ER fluids are closely related to both solid particles and carrier liquids. Comparing with carrier liquids, solid particle materials are more concerned because of their diversity and designability of structures. Therefore, ER materials mainly refer to the solid component of ER suspensions. In the past several decades, researchers paid much attention to designing and developing solid particles with higher sensitivity to electric field and higher ER effect. In the early stage, hydrophilic and porous particles, such as silica, zeolite and titania are mostly used as the dispersed phase of ER fluids. The structures of these particles are easy to absorb water forming hydrous ER materials, which makes the ER effect of these materials depend on the water content absorbed in particles. On the one hand, the polar molecule, H2O, contributes to the ER effect of the solid particles. On the other hand, water molecules are easy to evaporate at high temperature which reduces the operating temperature of ER fluids [21]. Then, Block et al. reported semi-conducting polymers as anhydrous ER materials [22,23], which overcame the negative effect of water. After that, anhydrous ER materials won great interest from researchers and diverse kinds of anhydrous ER materials were developed, such as biopolymers, dielectric inorganics and hydrophobic polyelectrolytes [24,25,26,27]. Not only pure materials were studied, but also dual- or multi-component composite materials attracted considerable attention owing to the combined advantages of each component. The development of giant ER fluids by Wen et al. has been a great progress that it broke the upper limit of yield stress predicted in traditional ER fluids [28]. Because of the contribution of polar molecules and high concentration, giant ER fluids are able to exhibit much higher yield stress: tens of kPa or even hundreds of kPa [29,30,31]. However, high yield stress is not the only demand for industrial applications. Low zero field viscosity and long-term stability are also required, obviously which need further more research in the giant ER fluids. Therefore, it is still on the way to develop ER materials or ER fluids with high yield stress, low zero field viscosity, long term stability and wide operating temperature range.
Recently, ionic liquids (ILs) have been introduced in designing ER materials, creating a new approach to enhance the properties of ER fluids. ILs are a class of organic salts with melting points below 100 °C [32,33]. They usually consist of organic cations and inorganic or organic anions, the unique structures of which give ILs special physicochemical properties, such as low vapor pressure, high thermal stability, high dielectric constant and excellent electrochemical properties [34]. Some of the cations and anions of ILs are shown in Figure 2. Owing to the specific characteristics of ILs, they have been applied in variety of fields including gas absorption [35], catalysis [36,37,38], solar cells [39,40] and organic synthesis [41,42]. In materials synthesis, ILs have been used as not only solvents, but also reactants or templates for achieving inorganics with novel morphologies, structures and improved properties [43]. Sometimes, ILs are immobilized in inorganic frameworks to keep their properties in solids, which are called inorganic ionogels [44]. From this point of view, the matrix of ionogels also could be organics or hybrid materials. Poly(ionic liquid)s (PILs) that refer to a subclass of polyelectrolytes, can be considered one category of ionogels synthesized by polymerizing IL monomers. As a new hybrid material, ionogels considerably enlarge the application of ILs to solid electrolytes, drug delivery and sensors [44]. In recent study, both inorganic ionogels and PILs have been used as ER materials. Marins et al. reported ILs-assisted silica particles which presented higher permittivity and better ER effect than pure silica particles [45,46,47]. Yin et al. have done a series of work on designing PILs-based ER materials with various chemical structures in cations and anions [48,49,50]. Even though ILs have shown excellent physicochemical properties in other fields, there are still several key issues to be solved when applied as ER materials. For example, the counter ions of ILs are mobile at a certain electric field and the mobility of which is closely related to temperature. The result is that at higher temperature electric leakage usually occurs, which is more common in PILs-based ER fluids because of the low glass transition temperature of PILs and the plasticization of organic counter ions.
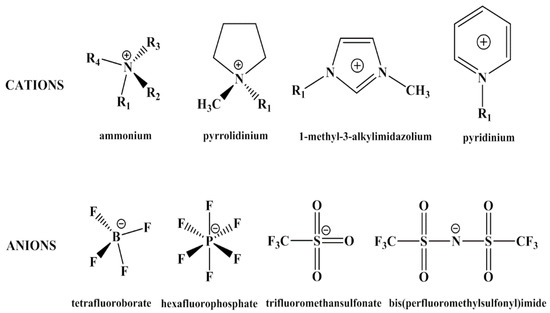
Figure 2.
Chemical structures of cations and anions of some ILs.
In this review, we mainly focus on the recent progress of ionogel-based ER materials, including their synthesis methods, ER characteristics and dielectric properties. The ILs-assisted synthesis not only modifies the chemical content of the matrix but also influences the physical properties of the colloidal particles, both of which will obviously affect the response of these particles under electric field [51]. The aim of this work is to highlight the advantage of ILs in synthesis of dielectric materials and their effect in improving the ER responses in a wide temperature range. It should be noted that there are still significant issues explored in the study of this kind of new ER materials. Possible solutions are also suggested to overcoming the problems in the present and further study.
2. ILs-Contained Inorganics
The tunability of anions and cations of ILs has shown great potential in the synthesis of materials, which can be used as a solvent and structure directing agent to improve the crystal form and morphology of inorganic materials in the synthesis of inorganic materials [52,53,54]. In addition, in the synthesis of organic materials, ILs can dissolve cellulose [55] and participate in organic synthesis [41,56] by taking advantage of its miscibility with organic matter and high solvation ability. As a green solvent and new type of reaction medium instead of traditional solvents, ILs are very easy to dissolve versatile precursors. It can be fixed or encapsulated in inorganic particles such as silica [57,58], titanium dioxide [59,60], carbon nanotubes [61] and so on, to generate inorganic ionogels [62,63]. Sol-gel processing is the mostly applied method in synthesizing inorganic ionogels. Herein, some examples of ILs-contained or ILs-tethered inorganics will be introduced. These candidates applied as electric field responsive materials will be highlighted.
2.1. SiO2-Based Ionogels
SiO2-based ionogels can be obtained either by dispersing SiO2 particles in ILs or adding ILs in the sol-gel process. A large number of studies have shown that nanoparticles are relatively high stable in ILs [64]. Shimano et al. [65] mechanically milled silica and 1-butyl-3-methylimidazole bis(trifluoromethylsulfonyl)imide ([BMIm][TFSI]) to obtain a solid electrolyte with a gel structure. Compared with liquid electrolytes, the high stability of ILs makes gel electrolytes show higher thermal stability, which makes the ionic gels have a wider working temperature range in lithium batteries [66,67]. In addition, within a wide temperature range, ionic gel electrolytes with liquid-like ion mobility may play an important role in electrochemical devices.
In the sol-gel preparation of inorganic materials, ILs can be used as solvents to participate in the synthesis of materials. Dai et al. [68] synthesized SiO2 aerogel by using 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ([EMIm][NTf2]) as the reaction solvent. On the one hand, the lower vapor pressure of the ILs can prevent the solvent from volatilizing and avoid the collapse of the gel pores caused by the solvent volatilization during the long-term aging process. On the other hand, the ILs are confined in the spatial network of silica, which can improve the stability of the aerogel. The new type of aerogel is expected to be applied as catalyst and insulating material. ILs also can be designed in the form of siliane coupling agent which finally produces a layer of ILs on the surface of SiO2 particles by hydrolysis reaction. Marins et al. [45,46,47] synthesized two kinds of SiO2-based ionogels by adding pristine ILs and silanized ILs in the sol-gel process. It is found that bare SiO2 tends to form a tightly packed structure in the presence of ILs [57], which means that the modification by ILs not only changes the chemical structure but also influences the morphology of the colloidal particles. As shown in Figure 3a, the addition of pristine IL (tri(n-butyl)-(tetradecyl)phosphonium dodecylbenzenesulfonate, IL201) during the sol-gel synthesis results in particles with aggregates, suggesting a strong influence of ILs on the morphology of the silica particles. In Figure 3b, silylated imidazolium-based IL is added in the later process of sol-gel synthesis to prepare organically modified silica particles (IL-ORMOSIL 1), which results in spherical particles with organic ILs on the surface.

Figure 3.
(a) SEM image of SiO2-based ionogels (SiO2/IL201), reprinted with permission from [46]. Copyright RSC, 2011. (b) TEM image of SiO2-based ionogels (IL-ORMOSIL 1), reprinted with permission from [47]. Copyright Elsevier, 2017.
2.2. TiO2-Based Ionogels
Because it acts not only as electrode material for lithium batteries but also as matrix for ionogel electrolytes, TiO2 is the most popular rechargeable battery material with high capacity [69]. Inorganic matrices that restrict ILs through physical effects usually have a unique mesoporous structure. TiO2 inorganic particles prepared by the sol-gel method are rich in 3D network structure. In the hydrolysis-condensation process forming the TiO2 inorganic matrix, a large amount of ILs is added. The wormhole nanostructure formed by co-condensation in TiO2 is used to confine partial ILs to the inside of the inorganic particles to form a TiO2-based inorganic ionogel. The first TiO2-based ionogels was synthesized by ultrasonic treatment with the mixture of inorganic titanium salts, methanol, FA, and [BMIm][TFSI] at room temperature [59]. Among many titanium precursors required for the formation of TiO2 by the sol-gel method, titanium alkoxide (TBOT) can be used to prepare halogen-free TiO2-based ionic gels [66,70]. The porous TiO2 matrix can provide ion transport channels and the high conductivity of ILs. Therefore, the electrical conductivity of the TiO2-based inorganic ionogel is greatly improved. This is also the reason that TiO2-based electrolyte has higher capacity and cycle times, which greatly optimizes the service life of the battery. Besides, the inorganic ionogels obtained by physical limitation has poor long-term stability. In order to compensate for this defect, functionalized ILs can be bonded to the TiO2 matrix by stable chemical bonds [71].
2.3. Other Inorganics-Based Ionogels
Due to their weakly coordinated molecular ions and high temperature stability, ILs greatly promote the synthesis of materials [72,73]. Here, the following is the application of ILs in metal-organic frameworks (MOFs) and carbon nanotubes (CNTs). MOFs are porous materials constructed from a variety of molecular complexes, which have good mechanical strength and adjustable host-guest interactions. At present, MOFs are widely used in gas storage and phase catalysis and as a new material, they can be used to confine ILs [74,75,76]. Under the condition of ionic thermal method, Ji et al. [77] synthesized a new type of 3D ferroelectric MOFs by using 1-ethyl-3-methylimidazolium bromide ([EMIm]Br) as a solvent and structure-directing agent. The asymmetric structure of the cation [EMIm]+, is the key factor in the formation of this helical chain, which further leads to a non-centrosymmetric polar stacking structure. The synthesis of ferroelectric MOF with a higher dielectric constant by ILs will be of great significance for further research on ferroelectric MOFs and general technical applications.
CNTs are usually assembled by tightly entangled graphene sheets with sp2 hybrid carbon atoms arranged in a hexagonal shape [78]. Although this structure provides CNTs with unique mechanical and electrical properties, it makes them difficult to dissolve and process [79,80]. By milling CNTs with 1-butyl-3methylimidazolium tetrafluoroborate ([BMIm][BF4]) to form a gel, Fukushima et al. [81] found that the cations of ILs will gather on the surface of nanotubes and separate the entangled nanotubes into smaller bundles. The bundles form CNTs gel through physical action. The large surface area of CNTs and the wider electrochemical window of ILs [82] expand the application of this material as electrodes [83].
3. Poly(Ionic Liquid)s and Their Composites
ILs can form organic ionic gels with low molecular weight adhesives or polymers [44,84]. As organic molecules, the low molecular weight adhesives are added to the liquids at high temperature and form physical gel when being cooled. When combining the ILs and the polymer to form a specific polymer or composite, they will be characterized with the high conductivity of the ILs and the mechanical properties of the polymer, which are widely used as an electrochemical device material [85,86,87]. Different from hydrophilic inorganic ionogels, the organic ones are hydrophobic that are able to avoid the negative effect of absorbed water of ER materials.
3.1. Poly(Ionic Liquid)s
Poly(ionic liquid)s (PILs) that use ILs as monomer and whose anionic or cationic center is restricted to the polymer backbone are a special type of polyelectrolyte [88]. The methods to prepare PILs include free radical polymerization, controlled polymerization and so on. From monomer to polymer, some special properties of ILs (such as negligible vapor pressure, thermal stability and wide electrochemical stability window) are transferred to the PILs chain and the diversity of ILs will enrich the characteristics and applications of PILs, which attract widespread attention in the study of polymers and other materials [32,89]. In practical applications, PILs always exhibit relative low conductivity because of the restriction effect of polymer backbone to mobile ions of the IL side groups. It is necessary to add highly conductive lithium salts to prepare electrolyte materials [34]. PILs are not only good candidates for electrolytes in batteries but also display stimuli-responsive properties, for example, the sensitivity to CO2 [90]. Interestingly, the low conductivity of PILs is just suitable for the application of electro-responsive ER materials, which makes PILs are attractive in the field of stimuli-responsive materials. Figure 4 shows the chemical structures of IL monomers and crosslinkers for synthesizing PILs that have been applied as ER materials.
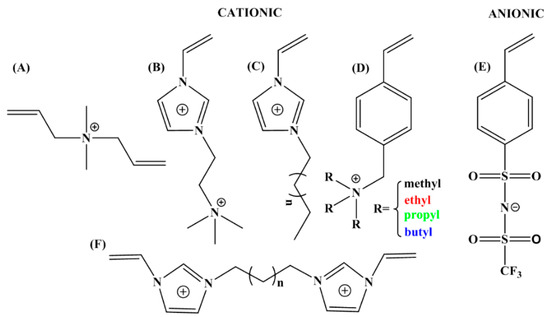
Figure 4.
Chemical structures of cationic and anionic IL monomers for PILs: (A) [DADMA]+; (B) [VIm]+ [BETA]+; (C) [CnVIm]+; (D) [VBTRA]+; (E) [STFSI]−; (F) [CnDVIM]2+.
When used as ER materials, PILs should be in the solid colloidal state with particle size ranging from submicrons to microns. A series of PILs with different chemical structures were prepared using either dispersion or solution polymerization method for this purpose [48,91]. Figure 5 shows a certain process of the microwave-assisted synthesis of poly[2-(methacryloyloxy)ethyltrimethylammonium bis(trifluoromethane sulfonylimide)] (P[MTMA][TFSI]). The reaction system transforms from transparent to opaque white because of the growth of P[MTMA][TFSI] particles. It has confirmed that the synthesis methods influences the morphology of the PILs particles significantly. As shown in Figure 6a, the spherical particles with an average size of 1.8 μm are prepared by the microwave-assisted dispersion polymerization method [91]. Figure 6b are the poly [(vinyl imidazole) bis(trifluoromethane sulfonylimide)] (P[VIm][TFSI]) particles prepared by a solution polymerization process using DMF as the solvent [92]. The particles from this process are irregular in shape and with much larger average size (~10 μm). Sometimes diethyl ether is used to make PILs precipitate in the original solvent (e.g., DMF). These precipitated PILs particles are also irregular in shape rather than spherical. In order to improve the thermal stability and mechanical properties of PILs, crosslinking agent is added in the polymerization process. It is also possible to prepare self-crosslinked PILs using bisvinylimidazolium-based monomers (Figure 4F). By varying the amount of crosslinking agent or the length of alkyl spacer, the mesh size of crosslinking network can be adjusted, which significantly influences the movement of backbones and counter ions of PILs. As shown in Figure 7, Liu et al. [93] prepared cross-linked PILs, poly[p-vinylbenzyltrimethylammonium bis(trifluoromethane sulfonimide)] (P[VBTMA][TFSI]). Comparing with the uncross-linked one, which shows enlarged free volume at higher temperature because of the uncoiling of polymer chain, the cross-linked P[VBTMA][TFSI] provides smaller cavities for ion transport at both room and elevated temperature.
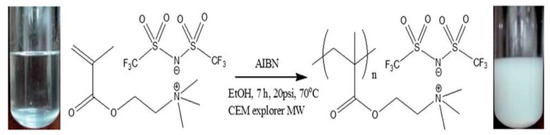
Figure 5.
The microwave-assisted synthesis process of P[MTMA][TFSI], reprinted with permission from [91]. Copyright RSC, 2014.
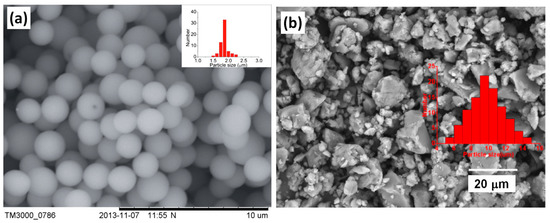
Figure 6.
(a) SEM image of P[MTMA][TFSI] particles, reprinted with permission from [91]. Copyright RSC, 2014. (b) SEM image of P[VIm][TFSI] particles, reprinted with permission from [92]. Copyright Elsevier, 2019.
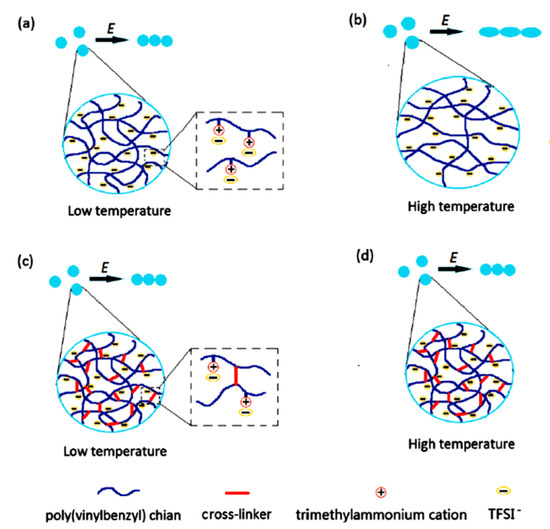
Figure 7.
Schematic diagram of chain formation of uncross-linked (a,b) and cross-linked (c,d) PILs particles at low and high temperatures, reprinted with permission from [93]. Copyright RSC, 2017.
3.2. Composites of P(Ionic Liquid)s
As introduced above, ion transport ability in uncross-linked PILs is accelerated at temperature higher than glass transition temperature (Tg), which could result in large current leakage and then deteriorating ER effect. Therefore, one of the issues for PILs-based ER materials is to improve their thermal stability or broaden their working temperature range. Introducing inorganic component to PILs to prepare PILs-inorganic composite is an effective method to improve the thermal stability of PILs. Both general composites and core-shell structured composites were prepared for this purpose. When a polymer is prepared on a inorganic substrate surface (e.g., SiO2), the strong interfacial interaction between the polymer and the inorganics will affect the Tg of the polymer by changing the stereoregularity of the polymer [94]. That is why core-shell particles with a SiO2 core and PIL shell were designed for the above issue [95]. Figure 8 is the schematic preparation process of SiO2@P[MTMA][TFSI] core-shell particles. In the first step, the surface of SiO2 particles is grafted by coupling agent (3-methacryloxypropyltrimethoxy-silane, MPS) via the hydrolyzation reaction. Then in the second step, IL monomer ([MTMA][TFSI]) is added and polymerized on the surface of SiO2 particles. Another way to prepare PILs-contained core-shell particles is the Pickering emulsion polymerization method [96,97,98,99,100]. In this process, inorganic nanoparticles rather than organic molecules are applied as emulsifier, producing inorganic nanoparticles-wrapped PILs particles. By using this way, Zhao et al. prepared PILs encapsulated nano-SiO2 composite, in which process nano-SiO2 was applied as a solid emulsifier [101]. It indicated that the SiO2 nanoparticles on the surface of the composite particles can largely decrease the leaking current density and improve the working temperature range of the ER fluid.
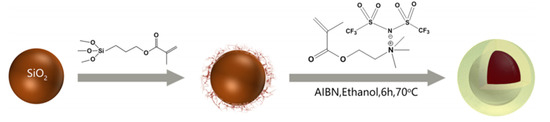
Figure 8.
Schematic preparation of SiO2@P[MTMA][TFSI] core-shell particles, reprinted with permission from [95]. Copyright ACS, 2018.
As a conductive polymer, polyaniline (PANI) has lower density, better processing properties, adjustable conductive properties, conjugate structure, and a wider potential for chemical functionalization [102]. It has been widely used in sensors, antistatic products as well as ER responsive materials [103,104,105,106,107,108]. Because of the excellent ER properties of PANI, it is applied to prepare composite materials with PILs to overcome the drawbacks of PILs. Zheng et al. [109] prepared PILs-encapsulated PANI composite particles. The synthesis process is a two-step method that the hydrophilic poly(vinylbenzyl)trimethylammonium chloride (P[VBTMA]Cl) is mixed with aniline firstly to obtain the PANI@P[VBTMA]Cl gel by oxidation polymerization and then the hydrophobic PANI@P[VBTMA][PF6] composite particles are obtained by ion exchange. The intrinsic conductivity of PANI suppresses the charged surface of P[VBTMA][PF6], which reduces the Zeta potential of PANI@P[VBTMA][PF6] to a lower level. In addition, core-shell structured PILs/PANI were also prepared using P[MTMA][TFSI] as core and semiconducting PANI as shell to limit the ion leakage of PILs [110]. The successful synthesis is mainly based on the good affinity between the P[MTMA][TFSI] core and the aniline monomer. Thus, interfacial polymerization of aniline could be realized by adding initiator or oxidant to the system. Low temperature polymerization at 0 °C is also required to slowdown the reaction rate of monomers and to avoid homogenous nucleation of aniline monomers.
Recently, graphene oxide (GO) has been widely used in electronic devices and capacitors due to its unique two-dimensional structure, high specific surface area, good polarizability and low conductivity [111,112]. However, for the application in ER fluids, the conductivity of GO is still higher than that of conventional ER materials, which easily causes electric breakdown in the electric field and reduces the ER effect of GO [27,113]. In order to improve the ER effect of GO, other inorganic substances or polymers are introduced to prepare composite particles [114]. By using a multi-layer composite method, Chen et al. [115] coated a polypyrrole (PPy) layer and a PILs layer on the surface of GO in sequence and obtained a multi-layered composite material with low conductivity and high ER effect. Firstly, the PPy layer is coated by an in situ polymerization process [116]. Then 1, 4-dibromobutan and 1-vinylimidazole are grafted on the surface of GO/PPy in turn and finally a PILs layer is formed by radical polymerization. The schematic synthesis process is shown in Figure 9.
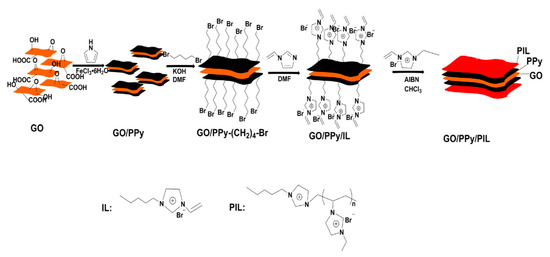
Figure 9.
Schematic diagram of fabrication of GO/PPy/PIL nanosheets, reprinted with permission from [115]. Copyright Elsevier, 2018.
4. ER Responses
As it has been introduced in Part 2, ILs and solid network can form ionic gel by restricting IL molecules in the solid network or on the surface of particles. The addition of ILs gives the ionic gel a unique responsiveness to electric field stimuli, which makes the ionic gel a good candidate for ER responsive materials. The high dielectric constant and ionic conductivity of ILs are expected to improve the ER response of the ionic gels. Besides, the ER effect of these materials will closely depend on the structures of both matrix and ILs.
4.1. ER Response of Inorganic Ionogels
By physical or chemical methods, ILs are confined in inorganic particles, which will enhance the responsiveness of inorganic particles in an electric field. Among the numerous inorganic ER materials, silica is widely used because of its easy synthesis, low cost and adjustable properties [117]. Although the ER effect of silica is not outstanding, adding a small amount of water or other polar molecules will enhance its ER effect [118,119,120,121]. According to the Stöber procedure, by adding different types of ILs during the hydrolysis of tetraethylorthosilicate (TEOS) [45,46], the ILs and silica can form ionic gel through physical interaction. Under an electric field, the ILs doped inside the ionic gel will produce charge migration, which affects the ion concentration and makes the gel possess a greater dielectric constant and a higher conductivity. The ionic gel shows obvious ER performance, which is several times that of pure silica. At the same time, according to the principle of “similar polarity”, when the anions and cations of the ILs doped physically have long alkyl chains, their wettability with the silicone oil will be increased, which causes partial anions and cations to leak out of the ionic gel. This effect increases the ionic conductivity of the insulating oil, reduces the dielectric constant of the ionic gel and eventually decreases the ER properties.
Compared with physical doping, chemical grafting by covalent bond is more effective in fixing ILs in inorganic matrix. ILs-grafted silica particles not only prevents its electric leakage during use but also provides a higher polarization rate and stronger ER performance [47]. In a word, during the synthesis process of silica, adding ILs can significantly improve the ER response of silica gels. However, other ILs-contained inorganics have not been applied as ER materials. There is still much work to do to explore the electric field responsive properties of inorganic ionogels.
4.2. ER Responses of PILs
PILs that are used as a new type of electrolyte material have a macromolecular structure composing of ILs as repeating units and connecting by a polymer backbone [34,85,88]. The formation of PILs reduces the number of movable ions of the system and the network structure limits the movement of chain segments and the long-distance migration of ions, which makes the conductivity of PILs significantly decrease to the range suitable for ER fluids. In ER fluids, proper conductivity helps to produce a stronger ER effect [122]. When PILs are used as the dispersed phase of ER fluids, the high density of anion and cation will generate stronger dielectric polarization, which leads to the enhancement of ER response [91]. In addition, due to the diverse chemical structures of ILs monomers, their ER response is able to be adjusted by modifying the chemical structures of ILs. It also means the chemical structures of cations, anions and even the backbones have significant effect on the ER response of PILs.
4.2.1. Chemical Structure of Anions
For ILs, the structures and properties of anion can affect the non-electrostatic interaction between ILs [123]. Among the anions of ILs, [TFSI]− is an hydrophobic one, whose large size and weak electrostatic interaction with cations lead to relatively large ion mobility and wide use in electrolytes [48,124]. Whereas it may be not the same in ER fluids. To explore the influence of ion mobility of anion on ER effect, Dong et al. [48,49] studied the ER effect of PILs with the same polymeric cations but different anions. In the characterization of ER performance, dynamic yield stress (τy) represents the response strength of suspension particles to the electric field. When comparing the ER responses of these PILs, it is found that these ER fluids have similar zero field viscosities due to the similar particle size of PILs. Under an electric field, the ER responses of the ER fluids of PILs with different anions are different and the order of τy is P[VBTMA][TfO] > P[VBTMA][BF4] > P[VBTMA][TFSI] > P[VBTMA][PF6]. Since they have the same cation, the different performance of ER response may be related to the anion. It seems that PILs with smaller anion size will exhibit a stronger ER effect. However, the inorganic or organic nature of the counter anions also has effect on the ER performance of PILs, for example, the temperature dependence of their ER performances. The PILs with inorganic anions show decreasing of static yield stress (τs) with temperature, on the contrary, increasing of τs with temperature for the PILs with organic anions (Figure 10A). The similar trend is observed for Δτ in Figure 10B. Note that τs used to evaluate the ER effect is the stress that drives the solidified ER fluid to flow and it can be approximately obtained by extrapolating the shear stress curve to zero shear rate. Δτ = τE − τ0 is the electric field induced increment of shear stress, where τE and τ0 represent the shear stress measured at applied electric field strength and zero electric field. For the cross-linked PILs, they have reduced free volume for counter anions to transport through. Therefore, mobility of anion is more sensitive to its own size, which was confirmed by Liu et al. [125] via studying the self-crosslinked PILs with different types of anions. They also found that the ER response of inorganic anion was significantly higher than that of organic anion. Inorganic anions usually have smaller size, the easy migration of which generates a greater interfacial polymerization and a stronger ER response. Therefore, it can be concluded that when PILs are used as suspension particles, the anion of PILs that has the relatively small size will lead to stronger ER effect.
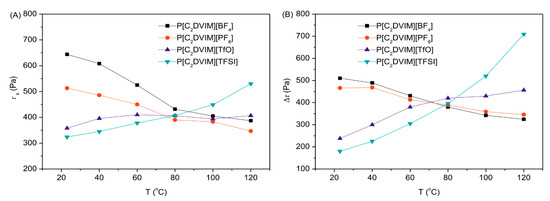
Figure 10.
Temperature dependence of static yield stress (τs) (A) and Δτ (B) for P[C2DVIM]X ER fluids (φ = 20 vol%). The electric field strength is 3 kV/mm and shear rate is 630 s−1. Reprinted with permission form [125]. Copyright Elsevier, 2019.
Typical low temperature ER effect is presented by an anionic PILs, which is considerably attractive because good low temperature performances of ER fluids are rarely reported using common ER materials. The poly[4-styrenesulfonyl (trifluoromethylsulfonyl) imide]-based anionic PILs containing counter cations with varied geometries from tetrahedron to plane (Figure 11) showed excellent ER effect with yield stress at 0 °C is about 1500 Pa (E = 3 kV/mm, φ = 20 vol%) [126]. The geometry of the counter cation has a significant effect on Tg of the PILs. As it varies from tetrahedron to plane, Tg of the PILs decreases, resulting in a inverse sequence of ER effect at 0 °C.
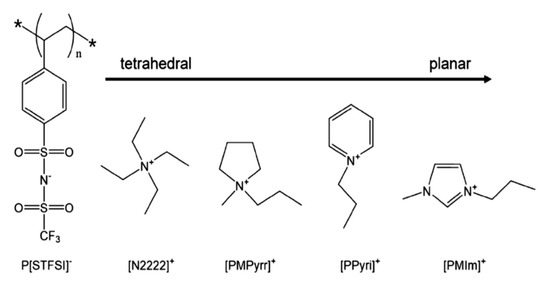
Figure 11.
Chemical structure of anionic PILs with different geometry of counter cations, reprinted with permission from [126]. Copyright Elsevier, 2020.
4.2.2. Chemical Structure of Cations
The size of cation and the interaction between anions will affect the conductivity of ILs and PILs [127,128]. In order to explore the effect of cations on the ER response of PILs, He et al. [129] synthesized PILs with different tethered cations dimethyldiallylammonium ([DADMA]+), benzylethyl trimethylammonium ([VBTMA]+) and 1-vinyl 4-ethylimidazolium ([C2VIm]+) and the same counter anion hexafluorophosphate (PF6−). The chemical structures of the cations are shown in Figure 4. Under an external electric field, suspensions of the three PILs show different ER response degrees and the trend of ER effect is P[DADMA][PF6] > P[VBTMA][PF6] > P[C2VIm][PF6]. Under the situation of similar particle morphology and zero field viscosity, the chemical nature of cation or the interaction between anion and cation may be the reason that leads to the different ER responses. The ion-pair interaction energy between [DADMA]+ and PF6− is the smallest, which results in higher ion mobility, then produces faster interfacial polarization and stronger ER response under an electric field. To compare the effect of ion number density on ER effect, Wang et al. [92] synthesized single-cation and double-cation PILs. Due to the higher ion number density in the dual-cation PILs, it exhibits higher polarizability and stronger ER response under an electric field. In addition, to study the influence of the chemical structure of the counter cation on the ER effect of anionic PILs, Zhao et al. [50,92,126,130] synthesized poly[4-styrenesulfonyl (trifluoromethylsulfonyl) imide (P[STFSI][X]) with anionic polymer backbone and cationic counterion. It is found that the size of cations did not show a monotonic influence on the ER effect of the PILs. Both lower activation energy for ion motion and a relative uniform amorphous structure are necessary for fast interfacial polarization rate and large polarization intensity. It means the ER effect is not only related the chemical structure of cations but also depends on the micromorphology of PILs.
4.2.3. Length of Alkyl Chain
When using molecular dynamics simulation to study the influence of cationic alkyl chain length on the properties of ILs [131,132,133,134], a local area of polar and non-polar will appear as the alkyl chain length increases. The local environment can affect the viscosity and ion conductivity of ILs. In order to understand the influence of side alkyl chain length of the immobile quaterammonium cation on ER effect, Dong et al. [48,135] synthesized PILs with styrene skeleton and quaternary ammonium cation with different lengths of alkyl chain on the counterpoint through free radical polymerization. The chemical structure of the monomers for PILs are shown in Figure 4D. Although the synthesized PILs have the similar particle size, morphology and type of anion and cation, they exhibited various ER responses. As the length of the alkyl chain increases, the volume of the cation becomes larger, which causes the local movement of the counterion to be restricted by the steric effect, thereby reducing the number of movable ions and the ion conductivity of the system. It leads to slower interfacial polarization rate and lower ER response of PILs at electric fields. Similar conclusion is obtained by Zhang et al. [136,137] upon the study of immidazolium-based PILs with different alkyl chain lengths on the immidazolium ring. It is found that as the alkyl chain becomes longer, the ER effect of the PILs becomes weaker. In other words, the shorter alkyl chain on the cation in PILs produces stronger ER performance.
However, when the alkyl chain acts on the cross-linked PILs, the opposite result will appear. In the study of the self-crosslinked PILs, divinylimidazolium monomers with different alkyl spacer lengths were used [138]. In the spatial structure of PILs, as the length of the alkyl spacer increases, the PILs have a larger cross-linked network mesh structure through the endoplasmic reticulum effect. The larger space structure reduces the energy required for ion diffusion and increases the ion mobility so that PILs with a large mesh structure have greater ionic conductivity and stronger ER response.
4.2.4. Crosslinking Structure
It has been demonstrated that due to the plasticizing effect of organic anions (such as [TFSI]¯) the Tg of PILs will decrease. Therefore, as temperature increases, the chain motion of PILs is intensified and the ion migration is promoted by coupling effect. Large leakage current will occur and limit the working temperature range of the PILs-based ER fluids [49,138]. As it has been mentioned above, the crosslink to PILs is used to solve this problem [93]. After cross-linking, the PILs form a high-density cavity and a rigid network, which limit the long-range migration of ions and let counter ions (TFSI¯) only move locally in the cross-linked network. In addition, the rigid structure after cross-linking increases the Tg of PILs, inhibiting chain movement and decreasing polymer free volume at high temperatures. When the temperature is increased, the cross-linked PILs are not easy to swell to become soft. Through a moderate crosslinking degree, it will slightly reduce the leakage current density and broaden the working temperature range of PILs, without sacrificing the ER effect of PILs significantly.
4.2.5. PILs-Based Composites
As it has been mentioned above, the preparation of PILs-based composite ER materials is to restrict the ion leakage of PILs in ER fluid. Other advantages achieved are the enhanced ER effect and broad working temperature range as well. Lei et al. [95] chose SiO2 particles as hard core and prepared SiO2@P[MTMA][TFSI] particles with core-shell structure. Because of the strong interaction between PILs shell and SiO2 core, the segmented movement of PILs is limited, leading to the increase in Tg of the PILs. The core-shell particles SiO2@ P[MTMA][TFSI] still have a strong ER response when the temperature is up to 120 °C. In addition, the SiO2@P[MTMA][TFSI] particles provide two interfaces: the interface between SiO2 core and the PILs shell where counter ions will gather, and the interface between the PIL shell and silicone oil. The core-shell structure of two interfaces is beneficial to increase the interfacial polarization, which makes SiO2@PILs have strong ER performance under the electric field. For the PILs encapsulated nano-SiO2 (PILs/SiO2) particles by Picking emulsion polymerization [101], nano-SiO2 on the surface of the composite particles seems like a cross-linking point which will increase the activation energy of ion migration of PILs and significantly inhibit the leakage current at high temperature. As a result, the leakage current of composite particles is small at 100 °C. Compared with pure PILs, PILs/SiO2 has outstanding temperature stability and stronger ER effect. Therefore, the introduction of inorganic SiO2 into the PILs can significantly reduce the leakage current density and improve the temperature stability, which provides a new idea for developing ER fluids suitable for in high temperature environments.
In order to overcome the charged nature of the PILs surface after ion exchange, PANI is used as either a filler or a shell material to prepare PANI filled PILs or PANI coated PILs (PANI@PILs) [109,110]. The change of surface potential makes the composites behave stronger ER response under the electric field and the yield stress obtained under an electric field of 4 kV/mm is about 2.3 kPa, which is twice that of pure PILs and 2.5 times that of PANI. In order to study the effect of reducing ion leakage and the degree of polarization matching between PANI and PILs, PANI was treated by alkali to obtain different forms [110,139]. After PANI undergoing different treatments, PILs/PANI (NH3) particles exhibit a stable shear range at room temperature, while PILs/PANI (N2H4) particles have stable shear at high temperatures. These differences are because PANI treated with ammonia and hydrazine have different dielectric polarization ratios. When PANI and PILs have similar dielectric matching, they will exhibit stable shear flow curves. Therefore, PANI@PILs can exhibit a strong ER response by changing the charge state of the particle surface. The coating of PANI on the surface of PILs effectively inhibits the positively charged surface state and produces a stronger ER effect.
5. Dielectric Analysis
It is generally accepted that the response of ER materials to the electric field is from the interfacial polarization of dispersed particles [140] and the dielectric properties of particles affect the polarization strength which will affect ER performance of the particle suspensions [141,142]. In the research of ER fluids, to analyze the rheological behavior of ER fluids, the dielectric constant and relaxation time are two important parameters [113]. ER fluids with good performance should have a large dielectric relaxation strength and a suitable dielectric relaxation peak in the frequency range of 102–105 Hz [143]. Dielectric spectrum analysis method has advantages such as broad measurement frequency, non-invasive and rapid measurement. Nowadays, more and more ER researches are using dielectric analysis method to understand the mechanism of the ER phenomena.
In the study of silica-based ionogels, which were synthesized by adding 11-carboxyundecyltriphenylphosphonium bromide (IL1) and octadecyltriphenylphosphonium iodide (IL2) in the sol-gel process, it is found that both relative dielectric constant (Figure 12a) and conductivity (Figure 12b) of silica/ILs are higher than pure silica [46], corresponding to the better ER response than that of pure silica as well. Significantly, among the three samples, the silica/IL1 exhibits the highest relative permittivity (~1100 at 10 Hz). It would be related the chemical structure of IL1 which contains a carboxylic acid tail in the triphenylphosphonium cation. Similarly, ILs organically modified silica particle also exhibits higher ER response and higher dielectric properties [47].
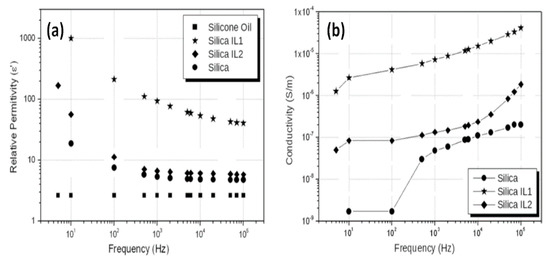
Figure 12.
Dependence of relative permittivity (a) and conductivity (b) on frequency for the silica, silica/IL1, and silica/IL2 suspensions, reprinted with permission from [45]. Copyright Elsevier, 2013.
To fit the dielectric data of ER materials, a relaxation Equation, which includes a Cole-Cole’s term, a DC conductivity term and an electrode polarization (EP) term, was used to analyze the dielectric spectra [139]:
where is the dielectric strength ( and are the limit values of the relative permittivity at the frequencies below and above the relaxation frequencies, respectively), λ = 1/ωmax the relaxation time, ωmax is the local angular frequency of dielectric loss peak, β is the Cole-Cole parameter indicating the distribution of the relaxation time, σ is the DC conductivity, n is related to the slope of EP’s high frequency tail, and A is related to the amplitude of EP.
If the dispersed phase of ER fluid consists of two components, two Cole-Cole’s terms can be used to analyze the dielectric properties. The equations of ε′ and ε″ are shown below, where subscript 1 and 2 are for each component respectively [139].
The above Equations are suitable for the ER systems containing composite as dispersed phase, such as the core-shell particles. Compared with hydrous ER fluid, the anhydrous PILs-based ER fluid has incomparable advantages, but it also faces the irreversible leakage of mobile ions from particles into carrier liquid under high electric field, which will increase energy consumption. In order to solve this problem, Zheng et al. [110] coated a layer of PANI on the surface of PILs particles, which successfully limits the ion leakage, reduces the energy consumption and enhances the interface polarization due to the more charge accumulation at the interface between PANI and PILs. Figure 13 shows the dielectric spectra of the ER fluids based on P[MTMA][TFSI], P[MTMA][TFSI]@PANI (thin) and P[MTMA][TFSI]@PANI (thick) paritcles. In Figure 13a, it is observed that both ε′ and ε″ increase rapidly in the low frequency regime, which is caused by the high DC conductivity of of the PILs and the electrode polarization. When the PILs particles are coated by PANI, it is found in Figure 13b,c that the rapid increase in both ε′ and ε″ at the low frequency regime is suppressed, indicating the critical effect of PANI in isolating the ion leakage of the PILs core. It is possible to obtain dielectric relaxation information of each component (P[MTMA][TFSI] and PANI) by using Equaitons (2) and (3) to fit the dielectric spectra of the P[MTMA][TFSI]@PANI-based ER fluids. Two relaxation process are observed: one is for the P[MTMA][TFSI] core and the other is for the PANI shell.
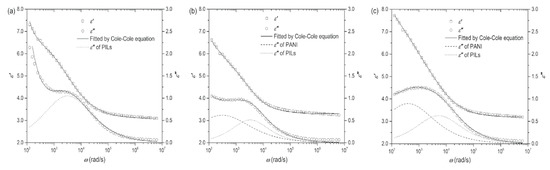
Figure 13.
Dielectric spectra of the suspensions of P[MTMA][TFSI] microspheres (a) P[MTMA][TFSI]@PANI (thin) microspheres (b) and P[MTMA][TFSI]@PANI (thick) microspheres (c) (φ = 20 vol%, T = 23 °C), reprinted with permission from [110]. Copyright John Wiley & Sons, 2018.
Moreover, the plasticizing effect of polyatomic fluorinated counter ions ([TFSI]¯) reduces the Tg of PILs, which decreases the thermal stability of the PILs-based ER fluids. In order to improve the thermal stability of the ER fluids, PILs encapsulated nano-SiO2 composite particles and SiO2@P[MTMA][TFSI] core-shell particles were designed and synthesized [95,101], which limit the movement of chain segments and the ionic transport of PILs. After analyzing dielectric properties of the SiO2@P[MTMA][TFSI] core-shell particles, it is found that the hard SiO2 core not only increases the dielectric strength of the core-shell particles but also restrains the segment relaxation of the P[MTMA][TFSI] shell. As shown in Table 1, the SiO2@P[MTMA][TFSI] ER fluid exhibits higher value of Δε′ but longer relaxation time (λ) due to the effect of hard core. Chemical crosslinking to PILs is also an effective way to broaden the working temperature of PILs-base ER fluid by restricting the segment movement of PILs with cross-linked networks. However, these networks also provide mobile counter ions with smaller cavities which decrease the mobility of the ions, resulting in an obvious increase in relaxation time.

Table 1.
Dielectric characteristics of the suspensions of P[MTMA][TFSI] and SiO2@P[MTMA][TFSI] particles at room temperature (φ = 12 vol%), reprinted with permission from [95]. Copyright ACS, 2018.
By studying the dielectric spectra of the ER materials at different temperatures, it is found that the dielectric relaxation of the ER fluids moves to high frequency region due to the faster relaxation at higher temperature. In addition, both ε’ and ε” rise up to higher value in the low frequency region because of the electrode polarization. It is also noted that for the composite particles, the relaxation peak is broader than that shown by the single component particles, resulted by the two relaxation processes of the composite. It means temperature affects the migration of ions in ILs or PILs which is related to the relaxation time. It is found that the reciprocal of the dielectric relaxation time (λ−1) follows the Arrhenius equation [139]:
where Ea is the activation energy, R is the molar gas constant, and T is the absolute temperature. Upon the study of the Arrhenius dependence of the PILs-based ER fluids, it is indicated that the ion motions in PILs or its composites are from the thermal diffusion, and not coupled with the chain segment below Tg of the PILs. The situation is much different when temperature is higher than Tg, the activation of segment relaxation drives λ−1 to depart from the Arrhenius line [95].
The activation energy Ea is actually the potential energy barrier for ion transport, which is closely related to the chemical and physical structure of PILs. For the core-shell particles, for example the P[MTMA][TFSI]@PANI particles, they showed higher Ea than pure P[MTMA][TFSI]. Chemical crosslinking to PILs rise the Ea to higher value as well [93]. With the increase of crosslinking degree, both the relaxation time and Ea of the ER fluid increased. Figure 14a shows the temperature dependence of the relaxation time (τ) of the cross-linked PILs, in which the slope of the fitting line is Ea. By comparing the slopes of different PILs, it is found that the higher the crosslinking degree of PILs is, the greater the Ea is. It means the crosslinking hinders the ion migration, which increases the Ea and prolongs the relaxation time. Another way to adjust the crosslinking degree or mesh size of the crosslinking network of PILs is to apply cross-linkers with different length of alkyl spacer. As shown in Figure 14b, as the alkyl spacer becomes longer, the Ea of the ER fluid decreases from 107.5 to 93.5 kJ/mol. In addition, comparing the ER response and dielectric spectra of the suspensions of P[MTMA][TFSI] and SiO2@P[MTMA][TFSI], we found that the hard SiO2 core can influence the ion dynamics, restrain the segment softening of P[MTMA][TFSI] shell and increase the interfacial polarization strength of SiO2@P[MTMA]-[TFSI]-based ER fluid by substrate confinement effect.
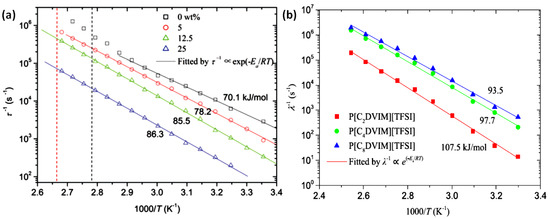
Figure 14.
(a) Temperature dependence of the relaxation time of the ER fluids based on of P[VBTMA][TFSI] and cross-linked P[VBTMA][TFSI] particles with different weight ratios of the cross-linker (a), reprinted with permission from [93]. Copyright RSC, 2017. (b) The self-crosslinked PILs with different alkyl space length, reprinted with permission from [138]. Copyright Elsevier 2019. The solid lines in (a,b) are fitted by the Arrhenius equation.
6. Conclusions
ILs-contained ionogels and PILs have showed combined characteristics of both ILs and the solid matrix, which implying many applications in various fields. According to the ion motion induced dielectric properties in ionogels and PILs, they have been applied as ER materials. Upon the review of the synthesis, ER and dielectric response of both inorganic ionogels and oPILs, it indicated that ILs which are physically or chemically restricted either in the matrix or on the surface of the matrix contribute to the interfacial polarization of the whole particle and then enhance the ER effect of the system. PILs received intensive study due to their accessible hydrophobic nature and the structure diversity in cations and anions both of which have significant effect on the ER response of PILs. The main issue in PILs-based ER materials is the narrow operating temperature caused by the low glass transition temperature of PILs. By introducing inorganic particles or semiconducting polymer to PILs, the ion motion in PILs is reduced, which successfully limits the electric leakage of the ER fluid. The same effect was found in the cross-linked PILs. For the lower temperature limit, anionic PILs with proper counter cations showed much excellent ER effect at 0 °C. On the side of inorganic ionogels, ILs have been widely used in synthesizing inorganics, however, which are not effectively used as electroresponsive ER materials. Therefore, there is still much work to do to understand the ER properties of inorganic ionogels. To avoid large leakage current and broaden the operating temperature are both challenges on the way to real application of ER fluids. Furthermore, long term stability of this kind of ER materials should be also concerned for their application in electric field controlled devices.
Author Contributions
Conceptualization, Y.D.L.; Formal Analysis, Z.Z. and Y.D.L.; Methodology, Z.Z., G.Z. and Y.D.L.; Resources, Z.Z., G.Z., C.D. and Y.Y.; Writing—Original Draft Preparation, Z.Z.; Writing—Review & Editing, Y.Y. and Y.D.L.; Supervision and Funding Acquisition, Y.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (Grant nos. 21872118 and 21403186).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-responsive conductive nanocomposite hydrogels with high stretchability, self-healing, adhesiveness, and 3D printability for human motion sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zheng, H.; Cao, Y.; Che, S. Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release. Adv. Mater. 2012, 24, 6433–6437. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.J.; Li, S.; Lee, W.W.; Guo, H.; Cai, Y.; Wang, C.; Tee, B.C.K. Self-healing electronic skins for aquatic environments. Nat. Electron. 2019, 2, 75–82. [Google Scholar] [CrossRef]
- Yun, G.; Tang, S.Y.; Sun, S.; Yuan, D.; Zhao, Q.; Deng, L.; Yan, S.; Du, H.; Dickey, M.D.; Li, W. Liquid metal-filled magnetorheological elastomer with positive piezoconductivity. Nat. Commun. 2019, 10, 1300. [Google Scholar] [CrossRef]
- Baroncini, M.; Groppi, J.; Corra, S.; Silvi, S.; Credi, A. Light-responsive (Supra) molecular architectures: Recent advances. Adv. Opt. Mater. 2019, 7, 1900392. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, J.; Zhu, X.X. Temperature-, light-, and host-molecule-responsive polymers with UCST behavior for aqueous sensing applications. ACS Appl. Polym. Mater. 2020, 2, 256–262. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zuo, Y.; Wang, R.; Xiong, Y. Preparation of thermo-responsive poly(ionic liquid)s-based nanogels via one-step cross-linking copolymerization. Molecules 2015, 20, 17378–17392. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Lim, K.H.; Gonuguntla, S.; Lim, K.W.; Pranantyo, D.; Yong, W.P.; Yam, W.J.T.; Low, Z.; Teo, W.J.; et al. The pathway to intelligence: Using stimuli-responsive materials as building blocks for constructing smart and functional systems. Adv. Mater. 2019, 31, 1804540. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological fluids: Mechanisms, dynamics, and microfluidics applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef]
- Liu, Y.D.; Choi, H.J. Electrorheological fluids: Smart soft matter and characteristics. Soft Matter 2012, 8, 11961–11978. [Google Scholar] [CrossRef]
- Martin, J.E.; Odinek, J.; Halsey, T.C.; Kamien, R. Structure and dynamics of electrorheological fluids. Phys. Rev. E 1998, 57, 756–775. [Google Scholar] [CrossRef]
- Choi, H.J.; Jhon, M.S. Electrorheology of polymers and nanocomposites. Soft Matter 2009, 5, 70–72. [Google Scholar] [CrossRef]
- Seo, Y.P.; Han, S.; Kim, J.; Choi, H.J.; Seo, Y. Analysis of the flow behavior of electrorheological fluids containing polypyrrole nanoparticles or polypyrrole/silica nanocomposite particles. Rheol. Acta 2020, 59, 415–423. [Google Scholar] [CrossRef]
- Coulter, J.P.; Weiss, K.D.; Carlson, J.D. Engineering applications of electrorheological materials. J. Intell. Mater. Syst. Struct. 1993, 4, 248–259. [Google Scholar] [CrossRef]
- Han, Y.-M.; Choi, S.-B.; Oh, J.-S. Tracking controls of torque and force of 4-degree-of-freedom haptic master featuring smart electrorheological fluid. J. Intell. Mater. Syst. Struct. 2015, 27, 915–924. [Google Scholar] [CrossRef]
- Lu, Q.; Han, W.J.; Choi, H.J. Smart and functional conducting polymers: Application to electrorheological fluids. Molecules 2018, 23, 2854. [Google Scholar] [CrossRef]
- Tonazzini, A.; Sadeghi, A.; Mazzolai, B. Electrorheological valves for flexible fluidic actuators. Soft Robot. 2016, 3, 34–41. [Google Scholar] [CrossRef]
- Weiss, K.D.; Carlson, J.D.; Coulter, J.P. Review: Material aspects of electrorheological systems. J. Intell. Mater. Syst. Struct. 2016, 4, 13–34. [Google Scholar] [CrossRef]
- Su, J.; Cheng, H.; Feng, Y.; Tam, H.Y. Study of a wheel-like electrorheological finishing tool and its applications to small parts. Appl. Opt. 2016, 55, 638. [Google Scholar] [CrossRef]
- Stangroom, J.E. Electrorheological fluids. Phys. Technol. 1983, 14, 290–296. [Google Scholar] [CrossRef]
- Block, H.; Kelly, J.P.; Qin, A.; Watson, T. Materials and mechanisms in electrorheology. Langmuir 1990, 6, 6–14. [Google Scholar] [CrossRef]
- Filisko, F.E.; Radzilowski, L.H. An intrinsic mechanism for the activity of alumino-silicate based electrorheological materials. J. Rheol. 1990, 34, 539–552. [Google Scholar] [CrossRef]
- Yin, J.B.; Xia, X.; Xiang, L.Q.; Qiao, Y.P.; Zhao, X.P. The electrorheological effect of polyaniline nanofiber, nanoparticle and microparticle suspensions. Smart Mater. Struct. 2009, 18, 095007. [Google Scholar] [CrossRef]
- Lee, Y. Synthesis and electrorheological characteristics of microencapsulated polyaniline particles with melamine–formaldehyde resins. Polymer 2001, 42, 8277–8283. [Google Scholar] [CrossRef]
- Fang, F.F.; Cho, M.S.; Choi, H.J.; Yoon, S.S.; Ahn, W.-S. Electrorheological characteristics of conducting polypyrrole/swollen MCM-41 nanocomposite. J. Ind. Eng. Chem. 2008, 14, 18–21. [Google Scholar] [CrossRef]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Core-shell structured semiconducting PMMA/polyaniline snowman-like anisotropic microparticles and their electrorheology. Langmuir 2010, 26, 12949–12954. [Google Scholar] [CrossRef]
- Wen, W.; Huang, X.; Yang, S.; Lu, K.; Sheng, P. The giant electrorheological effect in suspensions of nanoparticles. Nat. Mater. 2003, 2, 727–730. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, K.; Rao, G.; Tian, Y.; Zhang, S.; Liang, J. Electrorheological fluid with an extraordinarily high yield stress. Appl. Phys. Lett. 2002, 80, 888–890. [Google Scholar] [CrossRef]
- Wu, J.; Jin, T.; Liu, F.; Guo, J.; Cui, P.; Cheng, Y.; Xu, G. Preparation of rod-like calcium titanyl oxalate with enhanced electrorheological activity and their morphological effect. J. Mater. Chem. C 2014, 2, 5629–5635. [Google Scholar] [CrossRef]
- Wu, J.; Song, Z.; Liu, F.; Guo, J.; Cheng, Y.; Ma, S.; Xu, G. Giant electrorheological fluids with ultrahigh electrorheological efficiency based on a micro/nano hybrid calcium titanyl oxalate composite. NGP Asia Mater. 2016, 8, e322. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Chemistry. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, S.; Hou, Y.; Zhang, K.; Wu, W. Highly efficient absorption of NO by dual functional ionic liquids with low viscosity. Ind. Eng. Chem. Res. 2019, 58, 13313–13320. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Sureshkumar, K.; Dupont, J.; Ramakrishnappa, T.; Nagaraju, G. Ionic liquid-assisted hydrothermal synthesis of TiO2nanoparticles and its applications towards the photocatalytic activity and electrochemical sensor. J. Exp. Nanosci. 2015, 10, 1358–1373. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.-J. Ionic liquid mediated synthesis of grapheme—TiO2 hybrid and its photocatalytic activity. Mater. Sci. Eng. B 2014, 180, 38–45. [Google Scholar] [CrossRef]
- Nagaraju, G.; Manjunath, K.; Ravishankar, T.N.; Ravikumar, B.S.; Nagabhushan, H.; Ebeling, G.; Dupont, J. Ionic liquid-assisted hydrothermal synthesis of TiO2 nanoparticles and its application in photocatalysis. J. Mater. Sci. 2013, 48, 8420–8426. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, W.; Liu, Q.; Zhou, P.; Chen, T.; Lu, Y.; Qiao, Q.; Yang, S. Solution-processable ionic liquid as an independent or modifying electron transport layer for high-efficiency perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 34464–34473. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Matsui, T.; Luo, J.; Correa-Baena, J.-P.; Giordano, F.; Saliba, M.; Schenk, K.; Ummadisingu, A.; Domanski, K.; Hadadian, M.; et al. Ionic liquid control crystal growth to enhance planar perovskite solar cells efficiency. Adv. Energy Mater. 2016, 6, 1600767. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R.; Adams, C.J.; Roberts, G. Friedel–Crafts reactions in room temperature ionic liquids. Chem. Commun. 1998, 19, 2097–2098. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, A. Diels-Alder reactions are faster in water than in ionic liquids at room temperature. Angew. Chem. Int. Ed. 2006, 45, 4824–4825. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, L.; Schmitzer, A.R. Supramolecular effects involving the incorporation of guest substrates in imidazolium ionic liquid networks: Recent advances and future developments. Supramol. Chem. 2009, 21, 245–263. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G.; Silva, A.A.; Hurtado, M.G.; Livi, S. Electrorheological and dielectric behavior of new ionic liquid/silica systems. J. Colloid Interface Sci. 2013, 405, 64–70. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G.; Silva, A.A.; Livi, S. Silica prepared in the presence of alkylphosphonium-based ionic liquids and its performance in electrorheological fluids. RSC Adv. 2014, 4, 50925–50931. [Google Scholar] [CrossRef]
- Marins, J.A.; Soares, B.G. Ionic liquid-based organically modified silica for the development of new electrorheological fluids. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 311–319. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Yin, J.B.; Yuan, J.H.; Zhao, X.P. Microwave-assisted synthesis and high-performance anhydrous electrorheological characteristic of monodisperse poly(ionic liquid) particles with different size of cation/anion parts. Polymer 2016, 97, 408–417. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Liu, Y.; Wang, B.; Xiang, L.Q.; Zhao, X.P.; Yin, J.B. Influence of counterion type on dielectric and electrorheological responses of poly(ionic liquid)s. Polymer 2017, 132, 273–285. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Interfacial polarization and electroresponsive electrorheological effect of anionic and cationic poly(ionic liquids). ACS Appl. Polym. Mater. 2019, 1, 2862–2874. [Google Scholar] [CrossRef]
- Vioux, A.; Viau, L.; Volland, S.; Bideau, J.L. Use of ionic liquids in sol-gel; ionogels and applications. Comptes Rendus Chimie 2010, 13, 242–255. [Google Scholar] [CrossRef]
- Ding, K.; Miao, Z.; Hu, B.; An, G.; Sun, Z.; Han, B.; Liu, Z. Study on the anatase to rutile phase transformation and controlled synthesis of rutile nanocrystals with the assistance of ionic liquid. Langmuir 2010, 26, 10294–10302. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Miao, Z.; Hu, B.; An, G.; Sun, Z.; Han, B.; Liu, Z. Shape and size controlled synthesis of anatase nanocrystals with the assistance of ionic liquid. Langmuir 2010, 26, 5129–5134. [Google Scholar] [CrossRef] [PubMed]
- Voepel, P.; Seitz, C.; Waack, J.M.; Zahn, S.; Leichtweiß, T.; Zaichenko, A.; Mollenhauer, D.; Amenitsch, H.; Voggenreiter, M.; Polarz, S.; et al. Peering into the mechanism of low-temperature synthesis of bronze-type TiO2 in ionic liquids. Cryst. Growth Des. 2017, 17, 5586–5601. [Google Scholar] [CrossRef]
- Hermanutz, F.; Vocht, M.P.; Panzier, N.; Buchmeiser, M.R. Processing of cellulose using ionic liquids. Macromol. Mater. Eng. 2019, 304, 1800450. [Google Scholar] [CrossRef]
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Diels-Alder reactions in room-temperature ionic liquids. Tetrahedron Lett. 1999, 40, 793–796. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Comte, P.; Exnar, I.; Gratzel, M. Gelation of ionic liquid-based electrolytes with silica nanoparticles for quasi-solid-state dye-sensitized solar cells. J. Am. Chem. Soc. 2003, 125, 1166–1167. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Gorller-Walrand, C.; Binnemans, K.; Bellayer, S.; Viau, L.; Le Bideau, J.; et al. Lanthanide-doped luminescent ionogels. Dalton Trans. 2009, 2, 298–306. [Google Scholar] [CrossRef]
- Lee, U.H.; Kudo, T.; Honma, I. High-ion conducting solidified hybrid electrolytes by the self-assembly of ionic liquids and TiO2. Chem. Commun. 2009, 21, 3068–3070. [Google Scholar] [CrossRef]
- Verma, Y.; Tripathi, A.K.; Shalu; Singh, V.; Balo, L.; Gupta, H.; Singh, S.; Singh, R. Preparation and properties of titania based ionogels synthesized using ionic liquid 1-ethyl-3-methyl imidazolium thiocyanate. Mater. Sci. Eng. B 2017, 220, 37–43. [Google Scholar] [CrossRef]
- Chen, S.; Wu, G.; Sha, M.; Huang, S. Transition of ionic liquid [bmim][PF6] from liquid to high-melting-point crystal when confined in multiwalled carbon nanotubes. J. Am. Chem. Soc. 2007, 129, 2416–2417. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.C.; Ma, J.M.; Lian, J.B.; Zheng, W.J. The art of using ionic liquids in the synthesis of inorganic nanomaterials. Crystengcomm 2014, 16, 2550–2559. [Google Scholar] [CrossRef]
- Selvam, T.; Machoke, A.; Schwieger, W. Supported ionic liquids on non-porous and porous inorganic materials—A topical review. Appl. Catal. A Gen. 2012, 445–446, 92–101. [Google Scholar] [CrossRef]
- Torimoto, T.; Tsuda, T.; Okazaki, K.-I.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Shimano, S.; Zhou, H.; Honma, I. Preparation of nanohybrid solid-state electrolytes with liquidlike Mobilities by solidifying ionic liquids with silica particles. Chem. Mater. 2007, 19, 5216–5221. [Google Scholar] [CrossRef]
- Wu, F.; Chen, N.; Chen, R.; Zhu, Q.; Tan, G.; Li, L. Self-Regulative nanogelator solid electrolyte: A new option to improve the safety of lithium battery. Adv. Sci. 2016, 3, 1500306. [Google Scholar] [CrossRef]
- Rodrigues, M.T.F.; Kalaga, K.; Gullapalli, H.; Babu, G.; Reddy, A.L.M.; Ajayan, P.M. Hexagonal boron nitride—Based electrolyte composite for Li-ion battery operation from room temperature to 150 °C. Adv. Energy Mater. 2016, 6, 1600218. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Gao, H.J.; Lin, J.S.; Pennycook, S.J.; Barnes, C.E. Preparation of silica aerogel using ionic liquids as solvents. Chem. Commun. 2000, 3, 243–244. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Li, L.; Chen, R.; Guo, S. Ionogel electrolytes for high-performance lithium batteries: A review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, Z.X.; Yang, L.; Tachibana, K.; Hirano, S. TiO2-based ionogel electrolytes for lithium metal batteries. J. Power Sources 2015, 293, 831–834. [Google Scholar] [CrossRef]
- Moganty, S.S.; Srivastava, S.; Lu, Y.; Schaefer, J.L.; Rizvi, S.A.; Archer, L.A. Ionic liquid-tethered nanoparticle suspensions: A novel class of ionogels. Chem. Mater. 2012, 24, 1386–1392. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Freudenmann, D.; Wolf, S.; Wolff, M.; Feldmann, C. Ionic liquids: New perspectives for inorganic synthesis? Angew. Chem. Int. Ed. 2011, 50, 11050–11060. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Z.; Dekrafft, K.E.; Lin, W. Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wu, T.; Feng, P.; Bu, X. Multiroute synthesis of porous anionic frameworks and size-tunable extraframework organic cation-controlled gas sorption properties. J. Am. Chem. Soc. 2009, 131, 16027–16029. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Gu, Z.; Chen, C.; Wu, Z.; Que, Y.; Wang, Q.; Wan, H.; Guan, G. Efficient confinement of ionic liquids in MIL-100(Fe) frameworks by the “impregnation-reaction-encapsulation” strategy for biodiesel production. RSC Adv. 2016, 6, 37110–37117. [Google Scholar] [CrossRef]
- Ji, W.J.; Zhai, Q.G.; Li, S.N.; Jiang, Y.C.; Hu, M.C. The first ionothermal synthesis of a 3D ferroelectric metal-organic framework with colossal dielectric constant. Chem. Commun. 2011, 47, 3834–3836. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Itkis, M.E.; Borondics, F.; Yu, A.; Haddon, R.C. Bolometric infrared photoresponse of suspended single-walled carbon nanotube films. Science 2006, 312, 413–416. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Ionic liquid for in situ Vis/NIR and Raman spectroelectrochemistry: Doping of carbon nanostructures. Chem. Phys. Chem. 2003, 4, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Biomolecule-functionalized carbon nanotubes: Applications in nanobioelectronics. Chem. Phys. Chem. 2004, 5, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Li, Y.; Yan, B.; Yin, C.; Ran, R.; Shi, L.-Y. Transparent stretchable dual-network ionogel with temperature tolerance for high-performance flexible strain sensors. ACS Appl. Mater. Interfaces 2020, 12, 37597–37606. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Mao, H.; Liang, J.; Zhang, H.; Pei, Q.; Liu, D.; Wu, S.; Zhang, Y.; Song, X.M. Poly(ionic liquids) functionalized polypyrrole/graphene oxide nanosheets for electrochemical sensor to detect dopamine in the presence of ascorbic acid. Biosens. Bioelectron. 2015, 70, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Qiu, L.; Lu, J.; Yan, F. Cross-Linked alkaline ionic liquid-based polymer electrolytes for alkaline fuel cell applications. Chem. Mat. 2010, 22, 6718–6725. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Guo, J.; Sun, Z.; Qian, W.; Zhang, Y.; Yan, F. CO2 responsive imidazolium-type poly(ionic liquid) gels. Macromol. Rapid Commun. 2016, 37, 1194–1199. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, J.; Zhao, X. Microwave-synthesized poly(ionic liquid) particles: A new material with high electrorheological activity. J. Mater. Chem. A 2014, 2, 9812–9819. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhao, J.; Zheng, C.; Liu, Y.; Zhao, X.P.; Yin, J.B. Enhanced interfacial polarization and electro-responsive characteristic of di-ionic poly(ionic liquid)s. Polymer 2019, 182, 121847. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.; Dong, Y.; Zhao, X.; Yin, J. Enhanced temperature effect of electrorheological fluid based on cross-linked poly(ionic liquid) particles: Rheological and dielectric relaxation studies. Soft Matter 2017, 13, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Grohens, Y.; Brogly, M.; Labbe, C.; David, M.-O.; Schultz, J. Glass transition of stereoregular poly(methyl methacrylate) at interfaces. Langmuir 1998, 14, 2929–2932. [Google Scholar] [CrossRef]
- Lei, Q.; Zheng, C.; He, F.; Zhao, J.; Liu, Y.; Zhao, X.; Yin, J. Enhancing electroresponsive electrorheological effect and temperature dependence of poly(ionic liquid) particles by hard core confinement. Langmuir 2018, 34, 15827–15838. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Zhang, W.L.; Choi, H.J. Pickering emulsion-fabricated polystyrene–graphene oxide microspheres and their electrorheology. J. Mater. Chem. C 2014, 2, 7541–7546. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, R.; Qi, W.; Su, R.; He, Z. Interfacial polymerization of dopamine in a pickering emulsion: Synthesis of cross-linkable colloidosomes and enzyme immobilization at Oil/Water interfaces. ACS Appl. Mater. Interfaces 2015, 7, 14954–14964. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhang, W.L.; Choi, H.J. Pickering emulsion polymerization of core–shell-structured polyaniline@SiO2 nanoparticles and their electrorheological response. Colloid Polym. Sci. 2012, 290, 855–860. [Google Scholar] [CrossRef]
- Huo, J.; Marcello, M.; Garai, A.; Bradshaw, D. MOF-Polymer composite microcapsules derived from pickering emulsions. Adv. Mater. 2013, 25, 2717–2722. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, Y.D.; Seo, Y.; Choi, H.J. Pickering-emulsion-polymerized polystyrene/Fe2O3 composite particles and their magnetoresponsive characteristics. Langmuir 2013, 29, 4959–4965. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Zheng, C.; Lei, Q.; Dong, Y.Z.; Zhao, X.P.; Yin, J.B. Pickering emulsion polymerization of poly(ionic liquid)s encapsulated nano-SiO2 composite particles with enhanced electro-responsive characteristic. Polymer 2018, 146, 109–119. [Google Scholar] [CrossRef]
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Polyaniline nanofibers: Broadening applications for conducting polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.; Shepherd, H.; Faltens, T.; Haussmann, P.C.; Kaner, R.B.; Tolbert, S.H.; Huang, J.; Virji, S.; Weiller, B.H. Construction of a polyaniline nanofiber gas sensor. J. Chem. Educ. 2008, 85, 1102–1104. [Google Scholar] [CrossRef]
- Baker, C.O.; Shedd, B.; Innis, P.C.; Whitten, P.G.; Spinks, G.M.; Wallace, G.G.; Kaner, R.B. Monolithic actuators from flash-welded polyaniline nanofibers. Adv. Mater. 2008, 20, 155–158. [Google Scholar] [CrossRef]
- Pramanik, S.; Hazarika, J.; Kumar, A.; Karak, N. Castor oil based hyperbranched poly(ester amide)/polyaniline nanofiber nanocomposites as antistatic materials. Ind. Eng. Chem. Res. 2013, 52, 5700–5707. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Singh, N.; Venkatachalam, S. Shielding effectiveness of conducting polyaniline coated fabrics at 101 GHz. Synth. Met. 2001, 125, 389–393. [Google Scholar] [CrossRef]
- Liu, Y.D.; Fang, F.F.; Choi, H.J.; Seo, Y. Fabrication of semiconducting polyaniline/nano-silica nanocomposite particles and their enhanced electrorheological and dielectric characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2011, 381, 17–22. [Google Scholar] [CrossRef]
- Liu, Y.D.; Choi, H.J. Electrorheological response of polyaniline and its hybrids. Chem. Pap. 2013, 67, 849–859. [Google Scholar] [CrossRef]
- Zheng, C.; Dong, Y.; Liu, Y.; Zhao, X.; Yin, J. Enhanced stimuli-responsive electrorheological property of poly(ionic liquid)s-capsulated polyaniline particles. Polymers 2017, 9, 385. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Dong, Y.; He, F.; Zhao, X.; Yin, J. Low-temperature interfacial polymerization and enhanced electro-responsive characteristic of poly(ionic liquid)s@polyaniline core-shell microspheres. Macromol. Rapid Commun. 2019, 40, 1800351. [Google Scholar] [CrossRef]
- Wang, X.; Qian, X.; Jiang, X.; Lu, Z.; Hou, L. Tunable electrorheological characteristics and mechanism of a series of graphene-like molybdenum disulfide coated core–shell structured polystyrene microspheres. RSC Adv. 2016, 6, 26096–26103. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Plachy, T.; Moucka, R.; Pavlinek, V.; Mosnacek, J. Tunable electrorheological performance of silicone oil suspensions based on controllably reduced graphene oxide by surface initiated atom transfer radical polymerization of poly(glycidyl methacrylate). J. Ind. Eng. Chem. 2018, 57, 104–112. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J.; Kim, S.G. Electrorheology of graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 2267–2672. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, O.N.; Fernando, K.A.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Cheng, Q.Q.; Wang, L.M.; Liu, Y.D.; Choi, H.J. Fabrication of dual-coated graphene oxide nanosheets by polypyrrole and poly(ionic liquid) and their enhanced electrorheological responses. J. Ind. Eng. Chem. 2019, 69, 106–115. [Google Scholar] [CrossRef]
- Seo, Y.P.; Choi, H.J.; Lee, J.R.; Seo, Y. Modeling and analysis of an electrorheological flow behavior containing semiconducting graphene oxide/polyaniline composite particles. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 363–367. [Google Scholar] [CrossRef]
- Otsubo, Y.; Sekine, M.; Katayama, S. Electrorheological properties of silica suspensions. J. Rheol. 1992, 36, 479–496. [Google Scholar] [CrossRef]
- Otsubo, Y.; Sekine, M.; Katayama, S. Effect of adsorbed water on the electrorheology of silica suspensions. J. Colloid Interface Sci. 1992, 150, 324–330. [Google Scholar] [CrossRef]
- Gehin, C.; Persello, J.; Charraut, D.; Cabane, B. Electrorheological properties and microstructure of silica suspensions. J. Colloid Interface Sci. 2004, 273, 658–667. [Google Scholar] [CrossRef]
- Hao, B.N.; Guo, Y.X.; Liu, Y.D.; Wang, L.-M.; Choi, H.J. Highly transparent electrorheological fluids of silica nanoparticles: The effect of urea modification. J. Mater. Chem. C 2016, 4, 7875–7882. [Google Scholar] [CrossRef]
- Park, D.E.; Choi, H.J.; Vu, C.M. Stimuli-responsive polyaniline coated silica microspheres and their electrorheology. Smart Mater. Struct. 2016, 25, 055020. [Google Scholar] [CrossRef]
- Fan, F.; Wang, W.; Holt, A.P.; Feng, H.; Uhrig, D.; Lu, X.; Hong, T.; Wang, Y.; Kang, N.-G.; Mays, J.; et al. Effect of molecular weight on the ion transport mechanism in polymerized ionic liquids. Macromolecules 2016, 49, 4557–4570. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zhang, S.; Xuan, X. Structural effects of anions and cations on the aggregation behavior of ionic liquids in aqueous solutions. J. Phys. Chem. B 2008, 112, 16682–16689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.; Dong, Y.; Zhao, X.; Yin, J. Distinctly different electroresponsive electrorheological effect in low-molecular-weight and polymerized ionic liquids: Rheological and dielectric relaxation studies. J. Phys. Chem. B 2018, 122, 12184–12193. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; He, F.; Zheng, C.; Lei, Q.; Zhao, X.P.; Yin, J.B. Ion transport, polarization and electro-responsive elelctrorheological effect of self-crosslinked poly(ionic liquid)s with different counterions. Polymer 2019, 177, 149–159. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Zhao, X.; Yin, J. Influence of geometry of mobile countercations on conductivity, polarization and electrorheological effect of polymeric anionic liquids at ice point temperature. Polymer 2020, 205, 122826. [Google Scholar] [CrossRef]
- Ogihara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochim. Acta 2006, 51, 2614–2619. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, B.; Zhao, J.; Zhao, X.; Yin, J. Influence of tethered ions on electric polarization and electrorheological property of polymerized ionic liquids. Molecules 2020, 25, 2896. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Nonmonotonic influence of size of quaternary ammonium countercations on micromorphology, polarization, and electroresponse of anionic poly(ionic liquid)s. J. Phys. Chem. B 2020, 124, 2920–2929. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Yan, T.; Voth, G.A. A multiscale coarse-graining study of the liquid/vacuum interface of room-temperature ionic liquids with alkyl substituents of different lengths. J. Phys. Chem. C 2008, 112, 1132–1139. [Google Scholar] [CrossRef]
- Lopes, J.N.C.; Pádua, A.A.H. Nanostructural organization in ionic liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Voth, G.A. Tail aggregation and domain diffusion in ionic liquids. J. Phys. Chem. B 2006, 110, 18601–18608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Voth, G.A. Unique spatial heterogeneity in ionic liquids. J. Am. Chem. Soc. 2005, 127, 12192–12193. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, B.; Xiang, L.; Liu, Y.; Zhao, X.; Yin, J. Influence of side chain sizes on dielectric and electrorheological responses of poly(ionic liquid)s. J. Phys. Chem. B 2017, 121, 6226–6237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Zhang, Z.G.; Hao, B.N.; Zhang, H.Y.; Wang, M.; Liu, Y.D. Fabrication of imidazolium-based poly(ionic liquid) microspheres and their electrorheological responses. J. Mater. Sci. 2017, 52, 5778–5787. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, H.Y.; Hao, B.N.; Wang, M.; Zhang, H.Q.; Liu, Y.D.; Zhang, Z.L. Preparation and electrorheological properties of imidazolium-based poly (ionic liquid) microspheres. Chin. J. Mater. Res. 2017, 31, 679–686. [Google Scholar]
- Liu, Y.; Zhao, J.; He, F.; Zheng, C.; Zhao, X.P.; Yin, J.B. Influence of alkyl spacer length on ion transport, polarization and electro-responsive electrorheological effect of self-crosslinked poly(ionic liquid)s. Polymer 2019, 171, 161–172. [Google Scholar] [CrossRef]
- Zheng, C.; Lei, Q.; Zhao, J.; Zhao, X.; Yin, J. The effect of dielectric polarization rate difference of filler and matrix on the electrorheological responses of poly(ionic liquid)/polyaniline composite particles. Polymers 2020, 12, 703. [Google Scholar] [CrossRef]
- Parthasarathy, M.; Klingenberg, D. Electrorheology: Mechanisms and models. Mater. Sci. Eng. R-Rep. 1996, 17, 57–103. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Pavlinek, V.; He, Y.; Lengalova, A.; Li, C.Z.; Saha, P. Structural and electrorheological properties of mesoporous silica modified with triethanolamine. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 169–174. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, M.S.; Choi, H.J.; Jhon, M.S. Effect of polymerization temperature on polyaniline based electrorheological suspensions. Colloid Polym. Sci. 1999, 277, 73–76. [Google Scholar] [CrossRef]
- Hao, T. Electrorheological suspensions. Adv. Colloid Interface Sci. 2002, 97, 1–35. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).