A Stable Coordination Polymer Based on Rod-Like Silver(I) Nodes with Contiguous Ag-S Bonding
Abstract
1. Introduction
2. Experimental Materials
2.1. Instrumentation and Methods
2.2. Linker Synthesis
2.3. [Ag2(ASBDC)] Synthesis
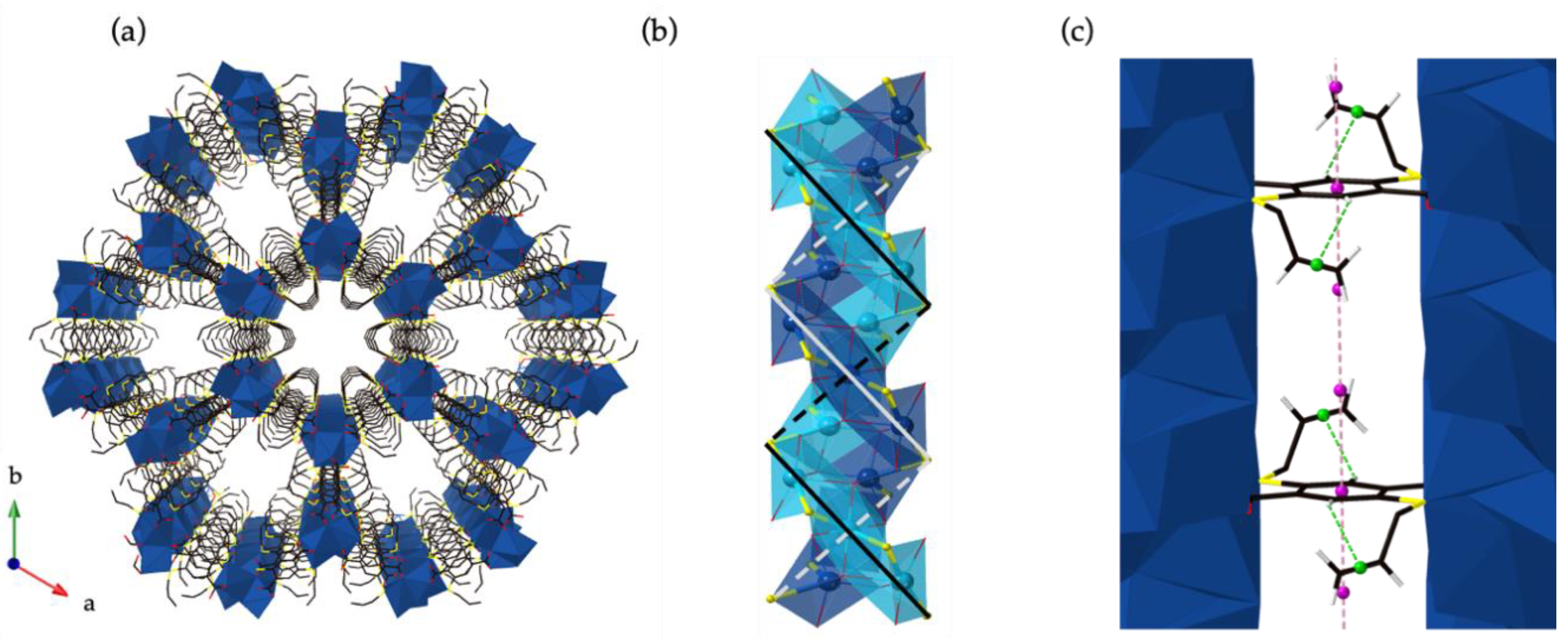
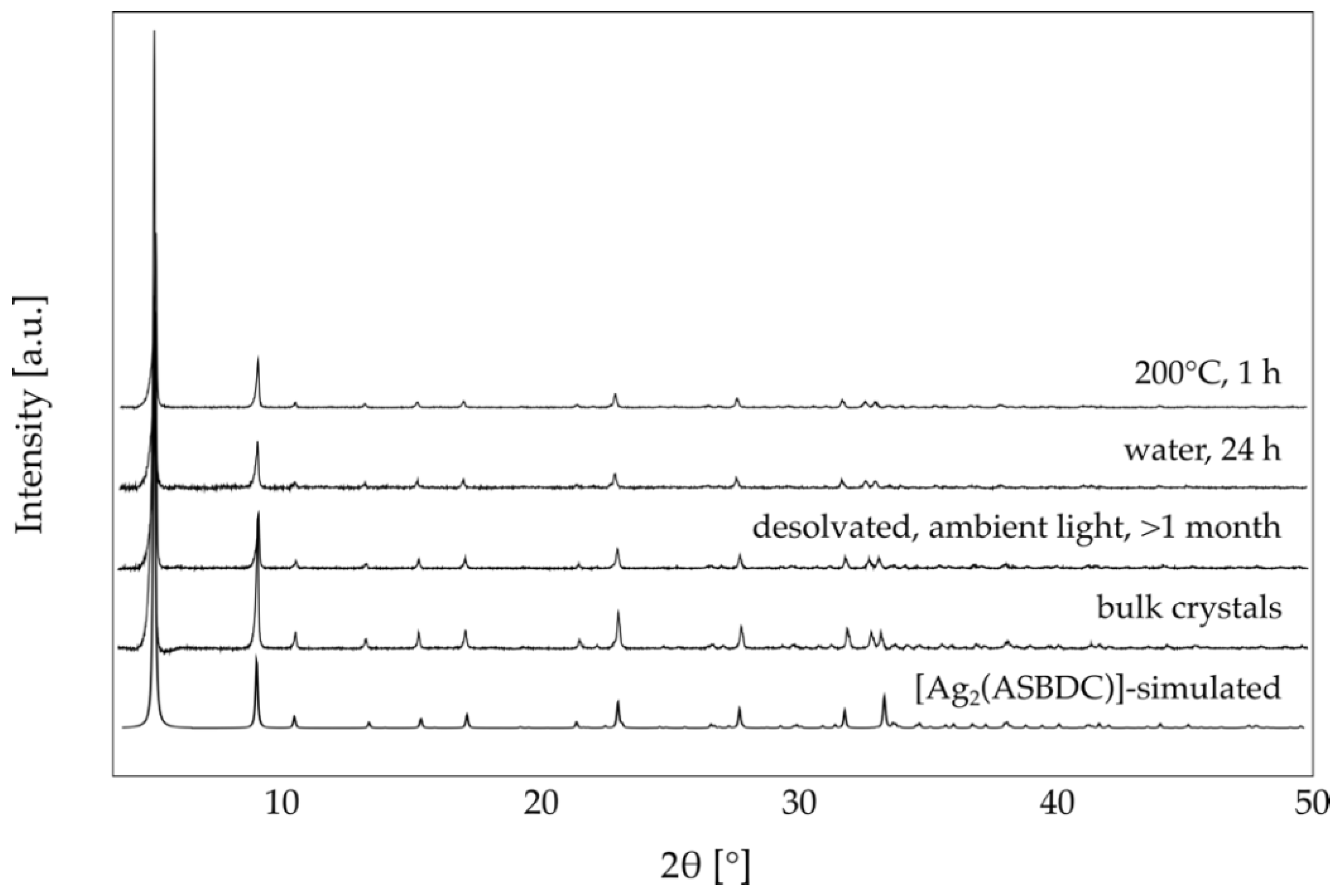
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Cheng, Y.; Zhao, D. Silver-decorated hafnium metal-organic framework for ethylene/ethane separation. Ind. Eng. Chem. Res. 2017, 56, 4508–4516. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef]
- Taylor-Edinbyrd, K.; Li, T.; Kumar, R. Effect of chemical structure of S-nitrosothiols on nitric oxide release mediated by the copper sites of a metal organic framework based environment. Phys. Chem. Chem. Phys. 2017, 19, 11947–11959. [Google Scholar] [CrossRef]
- Li, H.; Hill, M.R. Low-energy CO2 release from metal-organic frameworks triggered by external stimuli. Acc. Chem. Res. 2017, 50, 778–786. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Li, A.; Yang, P.; Zhang, C.; Tang, B. H2S-activable MOF nanoparticle photosensitizer for effective photodynamic therapy against cancer with controllable singlet-oxygen release. Angew. Chem. Int. Ed. 2017, 56, 13752–13756. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate metal-organic frameworks for dialing—In the binding and programming the release of drug molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Jhung, S.H. Zr-MOF with free carboxylic acid for storage and controlled release of caffeine. J. Mol. Liq. 2019, 296, 112060. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Porous iron-carboxylate metal-organic framework: A novel bioplatform with sustained antibacterial efficacy and nontoxicity. Appl. Mater. Interfaces 2017, 9, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, J.; Gu, J. Immobilization of silver nanoparticles in Zr-based MOFs: Induction of apoptosis in cancer cells. J. Nanoparticle Res. 2018, 20, 77. [Google Scholar] [CrossRef]
- Young, R.J.; Begg, S.L.; Coghlan, C.J.; McDevitt, C.A.; Sumby, C.J. Exploring the use of structure and polymer incorporation to tune silver ion release and antibacterial activity of silver coordination polymers. Eur. J. Inorg. Chem. 2018, 2018, 3512–3518. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal-organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef]
- Robson, R. A net-based approach to coordination polymers. J. Chem. Soc. Dalton Trans. 2000, 3735–3744. [Google Scholar] [CrossRef]
- Bloch, W.M.; Sumby, C.J. Guest-induced crystal-to-crystal expansion and contraction of a 3-D porous coordination polymer. Chem. Commun. 2012, 48, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.M.; Sumby, C.J. Probing solid-state breathing and structural transformations in a series of silver(I) porous coordination polymers. Eur. J. Inorg. Chem. 2015, 2015, 3723–3729. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sato, M.; Amano, A.; Hara, A.; Tsuruta, S.; Sugie, A.; Nomiya, K. Light-stable and antimicrobial active silver(I) complexes composed of triphenylphosphine and amino acid ligands: Synthesis, crystal structure, and antimicrobial activity of silver(I) complexes constructed with hard and soft donor atoms (n∞{[Ag(L)(PPh3)]2} with L = α-ala or asn and n = 1 or 2). Inorg. Chim. Acta 2008, 361, 1267–1273. [Google Scholar]
- Kasuga, N.C.; Yamamoto, R.; Hara, A.; Amano, A.; Nomiya, K. Molecular design, crystal structure, antimicrobial activity and reactivity of light-stable and water-soluble Ag–O bonding silver(I) complexes, dinuclear silver(I) N-acetylglycinate. Inorg. Chim. Acta 2006, 359, 4412–4416. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Yoshikawa, R.; Sakai, Y.; Nomiya, K. Syntheses, structures, and antimicrobial activities of remarkably light-stable and water-soluble silver complexes with amino acid derivatives, silver(I) N-acetylmethioninates. Inorg. Chem. 2012, 51, 1640–1647. [Google Scholar] [CrossRef]
- Nomiya, K.; Yokoyama, H. Syntheses, crystal structures and antimicrobial activities of polymeric silver(i) complexes with three amino-acids [aspartic acid (H2asp), glycine (Hgly) and asparagine (Hasn)]. J. Chem. Soc. Dalton Trans. 2002, 2483–2490. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Li, W.-H.; Wei, Z.; Zhang, C.; Li, Y.-H.; Dong, X.-Y.; Xu, G.; Zang, S.-Q. A hydrophobic semiconducting metal-organic framework assembled from silver chalcogenide wires. Chem. Commun. 2020, 56, 2091–2094. [Google Scholar] [CrossRef]
- Houlihan, J.C.C.; Moratti, S.; Hanton, L.R. Formation of a robust, double-walled LiMOF from an L-shaped di-substituted N-heterocyclic adamantane-based ligand. Dalton Trans. 2020, 49. [Google Scholar] [CrossRef]
- Li, R.-J.; Li, M.; Zhou, X.-P.; Li, D.; O’Keeffe, M. A highly stable MOF with a rod SBU and a tetracarboxylate linker: Unusual topology and CO2 adsorption behaviour under ambient conditions. Chem. Commun. 2014, 50, 4047–4049. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of metal-organic frameworks with rod secondary building units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Skowron, P.-T.; Dumartin, M.; Jeamet, E.; Perret, F.; Gourlaouen, C.; Baudouin, A.; Fenet, B.; Naubron, J.-V.; Fotiadu, F.; Vial, L.; et al. On-demand cyclophanes: Substituent-directed self-assembling, folding, and binding. J. Org. Chem. 2016, 81, 654–661. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zha, M.; Cui, J.; Zeller, M.; Hunter, A.D.; Yiu, S.M.; Lee, S.T.; Xu, Z. Convenient detection of Pd(II) by a metal-organic framework with sulfur and olefin functions. J. Am. Chem. Soc. 2013, 135, 7807–7810. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Liu, J.; Wong, Y.-L.; Xu, Z. Extraction of palladium from nuclear waste-like acidic solutions by a metal-organic framework with sulfur and alkene functions. J. Mater. Chem. A 2015, 3, 3928–3934. [Google Scholar] [CrossRef]
- Sun, L.; Hendon, C.H.; Dincă, M. Coordination-induced reversible electrical conductivity variation in the MOF-74 analogue Fe2(DSBDC). Dalton Trans. 2018, 47, 11739–11743. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.-K.; Reimer, N.; Liu, J.; Cheng, S.-Y.; Yiu, S.-M.; Weber, J.; Stock, N.; Xu, Z. Effective mercury sorption by thiol-laced metal-organic frameworks: In strong acid and the vapor phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef]
- Joseph, J.; Jemmis, E. Red-, blue-, or no-shift in hydrogen bonds: A unified explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, E.J.; Song, D.; Jeong, N.C. High proton mobility with high directionality in isolated channels of MOF-74. Appl. Mater. Interfaces 2018, 10, 35354–35360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Liu, D. The most advanced synthesis and a wide range of applications of MOF-74 and its derivatives. Microporous Mesoporous Mater. 2019, 283, 88–103. [Google Scholar] [CrossRef]
- Britt, D.; Furukawa, H.; Wang, B.; Glover, T.G.; Yaghi, O.M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci. USA 2009, 106, 20637. [Google Scholar] [CrossRef]
- Gung, B.W.; Emenike, B.U.; Alverez, C.N.; Rakovan, J.; Kirschbaum, K.; Jain, N. Relative substituent position on the strength of pi-pi stacking interactions. Tetrahedron Lett. 2010, 51, 1648–1650. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betts, H.D.; Linder-Patton, O.M.; Sumby, C.J. A Stable Coordination Polymer Based on Rod-Like Silver(I) Nodes with Contiguous Ag-S Bonding. Molecules 2020, 25, 4548. https://doi.org/10.3390/molecules25194548
Betts HD, Linder-Patton OM, Sumby CJ. A Stable Coordination Polymer Based on Rod-Like Silver(I) Nodes with Contiguous Ag-S Bonding. Molecules. 2020; 25(19):4548. https://doi.org/10.3390/molecules25194548
Chicago/Turabian StyleBetts, Harley D., Oliver M. Linder-Patton, and Christopher J. Sumby. 2020. "A Stable Coordination Polymer Based on Rod-Like Silver(I) Nodes with Contiguous Ag-S Bonding" Molecules 25, no. 19: 4548. https://doi.org/10.3390/molecules25194548
APA StyleBetts, H. D., Linder-Patton, O. M., & Sumby, C. J. (2020). A Stable Coordination Polymer Based on Rod-Like Silver(I) Nodes with Contiguous Ag-S Bonding. Molecules, 25(19), 4548. https://doi.org/10.3390/molecules25194548





