Abstract
A series of dinuclear copper(I) N,C,N- and P,C,P-carbodiphosphorane (CDP) complexes using multidentate ligands CDP(Py)2 (1) and (CDP(CH2PPh2)2 (13) have been isolated and characterized. Detailed structural information was gained by single-crystal XRD analyses of nine representative examples. The common structural motive is the central double ylidic carbon atom with its characteristic two lone pairs involved in the binding of two geminal L-Cu(I) fragments at Cu–Cu distances in the range 2.55–2.67 Å. In order to enhance conformational rigidity within the characteristic Cu–C–Cu triangle, two types of chelating side arms were symmetrically attached to each phosphorus atom: two 2-pyridyl functions in ligand CDP(Py)2 (1) and its dinuclear copper complexes 2–9 and 11, as well as two diphenylphosphinomethylene functions in ligand CDP(CH2PPh2)2 (13) and its di- and mononuclear complexes 14–18. Neutral complexes were typically obtained via the reaction of 1 with Cu(I) species CuCl, CuI, and CuSPh or via the salt elimination reaction of [(CuCl)2(CDP(Py)2] (2) with sodium carbazolate. Cationic Cu(I) complexes were prepared upon treating 1 with two equivalents of [Cu(NCMe)4]PF6, followed by the addition of either two equivalents of an aryl phosphine (PPh3, P(C6H4OMe)3) or one equivalent of bisphosphine ligands bis[(2-diphenylphosphino)phenyl] ether (DPEPhos), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (XantPhos), or 1,1′-bis(diphenyl-phosphino) ferrocene (dppf). For the first time, carbodiphosphorane CDP(CH2PPh2)2 (13) could be isolated upon treating its precursor [CH(dppm)2]Cl (12) with NaNH2 in liquid NH3. A protonated and a deprotonated derivative of ligand 13 were prepared, and their coordination was compared to neutral CDP ligand 13. NMR analysis and DFT calculations reveal that the most stable tautomer of 13 does not show a CDP (or carbone) structure in its uncoordinated base form. For most of the prepared complexes, photoluminescence upon irradiation with UV light at room temperature was observed. Quantum yields (ΦPL) were determined to be 36% for dicationic [(CuPPh3)2(CDP(Py)2)](PF6)2 (4) and 60% for neutral [(CuSPh)2(CDP(CH2PPh2)2] (16).
1. Introduction
In 1961, hexaphenyl-carbodiphosphorane, the first carbodiphosphorane (CDP), was synthesized by Ramirez et al. [1]. Despite this early discovery, the interest in such double ylide carbon (or carbone) compounds is still evolving. One reason for attracting interest is the bonding description of carbodiphosphoranes. Next to a classical ylide valence bond description, the bonding in carbodiphosphoranes can be decribed as a formal carbon(0) atom stabilized by two dative phosphine ligands with C–P retro dative bonding components, which is a model discussed earlier but quantified by a theoretical approach of Frenking and co-workers [2,3,4,5,6]. The central carbon atom is best described in its excited singlet (1D) state [7]. It acts as an acceptor and is stabilized by the σ donating phosphine ligands. The two characteristic occupied lone pairs (HOMO and HOMO+1) centered at this carbon atom (therefore named “carbone”) are either capable of binding two metals via two σ bonds in a close to tetrahedral configuration P2CM2 or one metal in a trigonal–planar P2CM configuration via a σ- and a π dative bond of very strong π,σ-donor character [8]. For this reason, the coordination chemistry of carbodiphosphoranes has experienced a renaissance [9,10,11]. A topic of current interest is introducing secondary ligand functions into the CDP frame: Cyclometalation with noble metals rhodium and platinum gave rise to the characterization of C,C,C-pincer ligand complexes with two cyclometalated phenyl rings [12,13,14,15,16,17], and an ortho-directed double lithiation of hexaphenyl-carbodiphosphorane leads to lithium complexes that are capable of transfering the C,C,C-pincer ligand synthon [CDP]2− to any other element of the periodic table [17]. P,C,P-chelate complexes of a phosphine functionalized CDP ligand CDP(CH2PPh2)2 (13), formally a carbone C(dppm)2 (dppm = bis-diphenylphosphinomethane), were characterized, but the free ligand 13 was not isolated so far [18,19,20,21,22,23,24]. Only recently, complexes of 2-pyridyl functionalized N,C,N-carbodiphosphorane CDP(Py)2 (1) have been reported [25,26]. The isolation of the free ligand base 1 [25] enabled the synthesis of Cu(I) CPD complexes, which are discussed in this work. Cu(I) complexes [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] can be used as cost-efficient luminescent materials, which potentially can replace highly phosphorescent Ir [44,45,46,47,48,49,50] or Pt [47,51,52,53,54,55,56,57,58] complexes in OLED technology. For example, OLED devices with internal quantum efficiencies of up to 100% could be realized based on the thermally activated delayed fluorescence (TADF) singlet-harvesting mechanism [28,29,30,31,41]. According to this mechanism, both the singlet and triplet excitons formed in an OLED emission layer can be harvested, and emission occurs via the S1 state.
Very frequently, Cu(I) complexes exhibit low-lying metal-to-ligand charge transfer (MLCT) transitions that are related to small energy separations ΔE(S1–T1) between the lowest singlet S1 and the lowest triplet T1 state due to small HOMO–LUMO overlap. As a consequence, efficient up-intersystem crossing (T1→S1), also designated as reverse intersystem crossing RISC, can occur at near ambient temperature [28,41,45,59,60], thus resulting in thermally activated delayed fluorescence (TADF). This is also related to a small transition dipole moment, and thus, a small radiative rate kr(S1→S0) [31,32]. The described MLCT formally corresponds to the oxidation of Cu(I) to Cu(II) and leads to photo-induced structural rearrangements in the excited state(s) being connected to large Franck–Condon factors [61], and as a consequence, to competing non-radiative relaxations. Therefore, the design of rigid structures with small reorganization energy between the ground state and excited states is essential.
While the first luminescent behavior of an Au(I) N-heterocyclic carbene (NHC) complex was already described in 1999 [62], it took another 10 years until the first photoluminescent Cu(I) NHC complexes were characterized [63] followed by further studies more recently [64,65,66,67,68,69,70]. In contrast to the π-acidic NHCs ligands, the π-donating CDP ligands have not yet been considered in luminescent materials. Herein, we report such luminescent Cu(I) CDP complexes, their synthesis, X-ray structure data, and photoluminescence properties. We demonstrate, that high emission quantum yields can be obtained with selected materials of this class.
2. Results
2.1. Synthesis and Characterization of N,C,N-CDP Complexes
The N,C,N-carbodiphosphorane pincer ligand CDP(Py)2 (1) was synthesized as reported previously [25] and used as a ligand in order to synthesize neutral and cationic dinuclear copper (I) complexes. Complexes 2, 3, and 9 were conveniently prepared by stirring ligand 1 with two equivalents of the respective copper(I) salts CuX in THF at room temperature for 18 h. Moderate yields of 86% and 63% for 2 and 3, as well as 27% for 9 were achieved in form of orange powders. Dicationic complexes 4–8 were prepared in an in situ two-step protocol by the reaction of CDP(Py)2 (1) with tetrakis(acetonitrile)copper(I) hexafluorophosphate (2 eq.) in THF, followed by the addition of either two equivalents of monodentate triaryl phosphine or one equivalent of a bisphosphine ligand: triphenylphosphine, tris(o-methoxyphenyl)phosphine, bis[(2-diphenylphosphino)phenyl] ether (DPEPhos), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (XantPhos), and 1,1’-bis(diphenyl-phosphino) ferrocene (dppf) were chosen as ligands. The dicationic Cu(I) complexes were isolated and crystallized in yields of 47–90% (Scheme 1). Additionally, a neutral Cu(I) CDP complex was obtained via the deprotonation of carbazole (10) in THF using sodium tert-butoxide and the addition of [(CuCl)2(CDP(Py)2)] (2) to this solution. [(CuCarb)2(CDP(Py)2)] (11) was obtained as light orange powder in a yield of 56%. Complexes 2–9 and 11 have been characterized via 31P{1H} NMR, 1H-NMR, 13C{1H} NMR, and by elemental analyses. Due to the typically poor volatility of ionic and zwitterionic Cu(I) complexes 2–9 and 11, no mass spectra with molecular ions were obtained under EI, FD, and ESI ionization techniques.
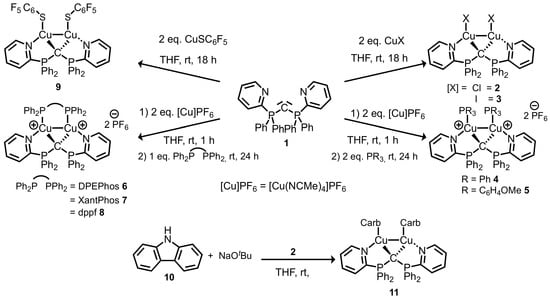
Scheme 1.
Synthesis of a wide variety of novel dinuclear N,C,N-carbodiphosphorane complexes 2–9 and 11.
Single crystals suitable for X-ray diffraction analysis were obtained upon layering THF or DCM solutions of the complexes with n-pentane. Crystal structures for 2, 4, 6, 7, 9, and 11 are shown in Figure 1, selected bond distances and angles are shown in Table 1. Further details of the XRD analyses of 3, 5, and 8 are described in the Supplementary Materials. The molecular structures of 2–9 reveal that the central carbon atom within the CDP ligand is capable of coordinating two copper atoms in a geminal fashion. Each copper atom is additionally coordinated by one 2-pyridyl unit of ligand 1. If the Cu–Cu interaction is disregarded, the two copper atoms per molecule are coordinated in a planar fashion, which is more T-shaped than trigonal planar. Each copper atom is interacting with one of the two carbone lone pairs of the central carbon atom C1, each by one nitrogen atom of a 2-pyridyl chelate ring and by the variable neutral ligand L or anionic ligand X. The strongest ligand interactions (C and X/phosphine) define a Cu(I) archetypical close to the linear axis. The geminal nature of both copper(I) centers leads Cu–Cu distances in the range of 2.55–2.67 Å (Table 1). These distances are smaller than twice the size of the covalent radius of Cu (1.32 Å) [71] or twice the size of the van der Waals radius of Cu (1.4 Å) [72]. Twice the size of the Cu(I) covalent radius (1.27 Å) [73] is close to the observed Cu–Cu distance. Similar trends are observed in dinuclear Cu(I) CDP complexes without any constraints of additional chelating CDP functions [74]. The Cu–Cu interaction leads to a formally coordinatively saturated pseudo tetrahedral coordination around each copper atom. This dinuclear entity is intramolecularly stabilized by a neutral 4-electron donor carbone ligand bridging the two Cu atoms. This rather rigid ligand template is characterized by characteristic torsion angles X–Cu–Cu–X in the range 41.9° (2)–76.0° (3) for anionic ligands X (X = Cl, I, S(C6F6) or L–Cu–Cu–L in the range 62.4° (8)–82.9° (4) for phosphine and the bridging bisphosphine ligands. The rather rigid frame of this N,C,N-ligand backbone seems to be privileged to stabilize this 8-electron-5-center inner Cu2CN2 core.
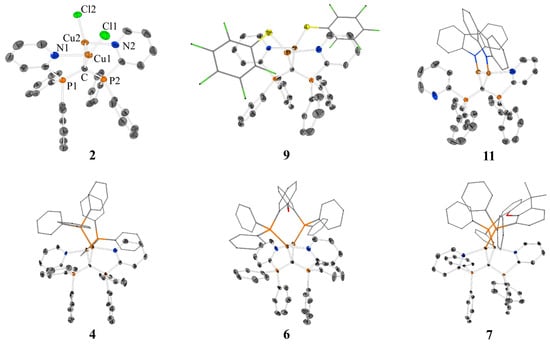
Figure 1.
XRD molecular structures of [(CuCl)2(CDP(Py)2)] (2), [Cu2(PPh3)(CDP(Py)2)](PF6)2 (4), [Cu2(DPEPhos)(CDP(Py)2)](PF6)2 (6), [Cu2(XantPhos) (CDP(Py)2)](PF6)2 (7), [(CuS(C6F5))2 (CDP(Py)2)] (9), and [(CuCarb)2(CDP(Py)2)] (11). Hydrogen atoms and solvent molecules have been omitted for clarity; thermal ellipsoids are given at 50% probability. For 4, 6, and 7, the counter anions [PF6]− are omitted for clarity. The labeling of 2 is identical for all species. For details and further XRD molecular structures of 3, 5, and 8, see the Supplementary Materials.

Table 1.
Selected bond distances [Å] and angles [°] for 2–9 and 11.
Representative parent complex 2 crystalizes in a triclinic crystal system with a crystallographic point group of P-1 and with four units and two unique molecules in the unit cell. One of the two independent molecules is slightly disordered, and both have very similar geometric parameters. The angles (°) around copper are almost identical for the two Cu atoms, but crystallographically, they are not strictly identical: C–Cu–Cl 162.11(8)°, C–Cu–N 89.45(9)°, and N–Cu–Cl 106.95(6)°. Each copper atom deviates only marginally from the plane defined by C, N, and X = Cl to which copper(I) is bound. Cu–Cu distances, which indicate weak Cu–Cu interactions, e.g., 2.5525(5) Å for 2. The C–Cu–Cu angles of 2 and related species are typically sharp, e.g., 49.98(7)° in case of 2. A comparable coordination scenario can be found for the other complexes 3–9. Only small differences for the C–P distances as well for the Cu–C–Cu and the P–C–C angles are observed within the series 2–9.
Complex 11 crystalizes in a monoclinic crystal system with a space group of P21/n and four units in its unit cell. In contrast to the described XRD molecular structures of 2–9, the neutral complex 11 shows only one pyridine copper interaction, while the remaining pyridyl unit stays in a dangling nonbonding situation. The carbazolyl anions display a perpendicular orientation with respect to each other. Both steric and electronic factors are probably responsible for the dangling pyridyl unit in 11. As expected, the Cu–Ncarb distance 1.911(3) Å for copper with the higher coordination number due to additional pyridine interaction is longer than Cu–Ncarb 1.861(2) Å for the other one. According to NMR spectroscopy, there is a dynamic exchange process of bonded and dangling pyridine ligands in solution.
2.2. Synthesis and Characterization of P,C,P–CDP Complexes
Peringer et al. developed P,C,P–CDP pincer complexes of a formal carbone ligand C(dppm)2, which was not isolated and characterized, but trapped in the form of its complexes [18,19,20,21,22,23,24]. The synthetic strategy involved complex redox reactions. It is limited to the characterization of Ni(II), Pd(II), Pt(II), or Au(III) complexes so far. Our synthetic approach was to isolate the free CDP base. Thus, [CH(dppm)2]Cl (12) [18,19] was treated with an excess of sodium amide (6.5 eq.) in liquid ammonia at −78 °C. Since the basicity of sodium amide leads to the deprotonation of only one proton, CDP(CH2PPh2)2 (13) could be isolated in 98% yield as an intense yellow powder. No further deprotonation products and no adduct formation with lithium salts were observed as in the case of using organolithium bases. The isolation of 13 was the precondition to access the coordination chemistry of Cu(I) with this P,C,P–CDP ligand base. Dinuclear copper complexes 14–16 were synthesized and characterized via NMR spectroscopy and mass spectrometry (Scheme 2).
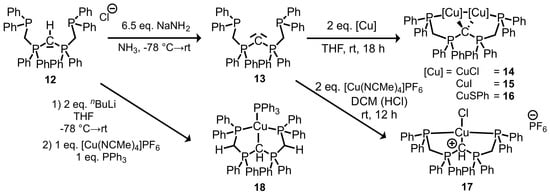
Scheme 2.
Synthesis of dinuclear P,C,P–CDP complexes 14–16 via the isolation of previously non-characterized CDP(CH2PPh2)2 (13). A monoprotonated form of 13 was trapped and characterized in 17 and a monodeprotonated form of 13 was trapped and characterized in 18.
Upon treating 13 with tetrakis(acetonitrile)copper(I) hexafluorophosphate in DCM, a cationic complex [CuCl(H-CDP(CH2PPh2)2]PF6 (17) was obtained. The enhanced basicity of alkyl-substituted CDP 13 compared to pyridyl-substituted CDP 1 leads to a protonation of a Lewis acid-activated acetonitrile ligand. Therefore, monoprotonated 13 is acting as a ligand in mononuclear copper complex 17 with hexafluorophosphate as a counter ion. While searching for adequate bases for the deprotonation of 12, we observed the ability of n-BuLi (2 eq.) to further deprotonate CDP 13, generating an anionic CDP ligand 20 (Scheme 3) as lithium salt. Trapping this anion with one equivalent of tetrakis(acetonitrile)copper(I) hexafluorophosphate and one equivalent of triphenylphosphine leads to neutral copper(I) complex 18 as a light yellow powder in 73% yield. 18 was characterized via 31P{1H} NMR, 1H-NMR, and elemental and XRD analysis.
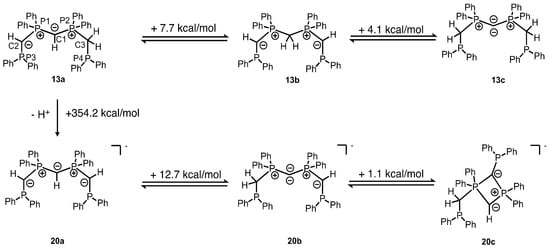
Scheme 3.
Results of quantum chemical calculations on the deprotonation of [H-CDP(CH2PPh2)2]Cl (12) and formation of different more or less stable tautomers of CDP(CH2PPh2)2 (13). The positive value of the energy corresponds to the energy that has to be applied in order to convert one molecule into the other. The most stable tautomer 13a and its deprotonation product 20a are shown on the left side of the scheme.
After the deprotonation of symmetric protonated CDP form 12, 31P{1H} NMR spectra of the product (or products) become temperature and solvent-dependent. We presumed that this observation could be an indication of the presence of more than one tautomer, at least two with definitely chemically non-equivalent 31P nuclei of monodeprotonated base 13 (see Figure S-28). As there were no literature data available on this particular carbodiphosphorane 13, even though it was used as a ligand in several publications, we decided to investigate the tautomeric forms of 13 via computational methods (Scheme 3). Geometry optimizations were performed at the PBE-D3(BJ)/def2-TZVPP level of theory, which were followed by single-point calculations and a natural bond orbital (NBO) analysis at the PBE0-D3(BJ)/def2-TZVPP level of theory. Interestingly, the results reveal that the free ligand base 13 cannot be acknowledged as a carbodiphosphorane, but rather as tautomer 13a. Due to the high first proton affinity (PA) and drastically lower second PA of the alkyl-substituted central CDP carbon atom and due to the enhanced CH acidity of the methylene group placed in between a phosphanyl and a phosphionio functionality, the ground state of 13 is not represented by tautomer 13c or 13b but by asymmetric tautomer 13a. This equilibrium explains the highly complex 31P{1H} NMR spectra obtained from solutions of pure 13. Symmetric tautomer 13b is 4.1 kcal/mol more stable than 13c, but asymmetric 13a is 7.7 kcal/mol more stable than 13b. Therefore, 13b seems to be observable at very low concentration in a dynamic equilibrium ratio next to 13a but not symmetric carbodiphosphorane form 13c.
Our results from solution and gas phase investigation and very clear results from XRD solid-state investigations of ligand 13 complexes indicate that the equilibrium of tautomers displayed in Scheme 3 is shifted toward 13c, if the free base 13 is trapped by coordination with two Cu(I) ions. The further deprotonation of 13a leads to symmetric carbanion 20 as the most stable tautomer: 20a with equally CH-functionalized C1, C2, and C3 is 12.7 kcal/mole more stable than asymmetric tautomer 20b retaining a carbodiphosphorane structure. A hypothetical 1λ5,3λ3 diphosphete derivate 20c is just 1.1 kcal/mole less stable than 20b in the gas phase. The charge distribution of the tautomers can be monitored via NBO analysis. While the atomic partial charge q(C) of C1 of 13a is −1.38 e, which corresponds to q(C) of the protonated hexaphenyl-carbodiphosphorane (−1.33 e) [6], the one of 13c reveals as −1.45 e and therefore is in the same order of magnitude as for the hexaphenyl-carbodiphosphorane (−1.43 e) [6]. For 20a, the q(C) values of C1, C2, and C3 are –1.39 e, 1.37 e, and 1.37 e, while the q(C) values of P1, P2, P3 and P4 are 1.68 e, 1.68 e, 0.83 e and 0.83 e. For more information regarding the atomic partial charges and for a detailed deprotonation of 12, see Tables S-1–S-8, as well as Scheme S-1 in the Supplementary Materials.
Single crystals suitable for X-ray diffraction analysis were obtained upon layering a THF or a DCM solution of the complexes 14–18 with n-pentane. The XRD molecular structures are depicted in Figure 2, while selected bond distances and angles are shown in Table 2 and Table 3. For dinuclear complexes 14–16, a very similar trend is observed, as discussed in Chapter 2.1. The central CDP carbon atom acts as 4-electron donor involving two geminal copper atoms into a Cu–C–Cu triangle. Each copper atom is further coordinated to one chelating phosphine group. While 14 and 16 crystalize in a triclinic crystal system with space group P-1 and two units in the unit cell, 15 crystalizes in a monoclinic crystal system with space group C2/c and four units in the unit cell. In contrast to dinuclear Cu(I) complexes of pyridyl-CDP 1, complexes 14–16 of phosphanyl-CDP 13 reveal significantly longer Cu–Cu distances (Å). 2.8681(5) (14), 2.8816(12) (15), and 2.989(2) (16) compared to 2.5525(5) (2) and 2.671(2) (11). This is in accord with the higher steric demand of the phosphine and an increased freedom of motion in CDP ligand 13 compared to the more rigid and compact CDP 1 (also compare the XYZ.file of the SI). In contrast to 2–9, disregarding the Cu–Cu interaction, a less pronounced T-shape but more trigonal planar coordination sphere of the copper(I) ions is observed for 14–16. This is probably due to the fact that phosphines, carbones, and the anions X are more similar in their donor strength and Cu(I) affinity compared to weaker pyridine ligands in the first series of compounds. For 14, the angles (°) around copper are 128.57(7) (C–Cu–Cl), 99.71(7) (C–Cu–P) and 129.61(3) (P–Cu–Cl) and therefore closer to the ideal 120° of a trigonal coordination sphere compared to 2. This rather rigid ligand template is characterized by characteristic torsion angles X–Cu–Cu–X in the range 119.9° (15)–140.2 (16) and are therefore larger compared to the complexes of 2. The less rigid frame of this P,C,P ligand backbone stabilizes an 8-electron-5-center inner Cu2CP2 core.
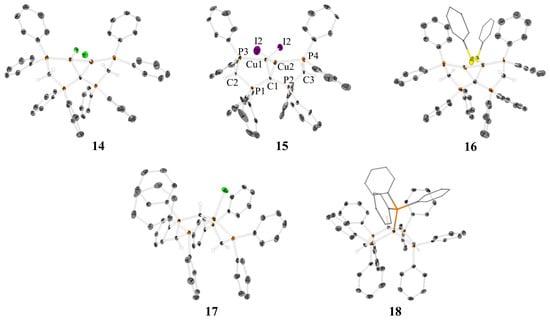
Figure 2.
XRD molecular structures of [(CuCl)2(CDP(CH2PPh2)2] (14), [(CuI)2(CDP(CH2PPh2)2] (15), [(CuSPh)2(CDP(CH2PPh2)2] (16), [CuCl(H-CDP(CH2PPh2)2]PF6 (17), and [CuPPh3(CH(PPh2CHPPh2)2] (18). Hydrogen atoms and solvent molecules have been omitted for clarity; thermal ellipsoids are given at 50% probability. For 17, the counter anion [PF6]− is omitted for clarity. The labeling of 15 is identical for all species. For more details, see the Supplementary Materials.

Table 2.
Selected bond distances [Å] and angles [°] for 14–16.

Table 3.
Selected bond distances [Å] and angles [°] for 14, 17 and 18.
Selected bond distances and angles of 14–16 can be found in Table 2, which demonstrates an increase of the Cu–C–Cu angle of about 10° in addition to the increased Cu–Cu distances relating to the increasing freedom of motion of 13 compared to 1. The P–C–P angles of the CDP complexes 14–16 are comparable to the ones of ligand 1.
Selected bond distances and angles of 17 and 18 are displayed in Table 3 and are compared to the ones of complex 14. While [CuCl(H-CDP(CH2PPh2)2]PF6 (17) can be considered as a complex of a cationic ligand, [CuPPh3(CH(PPh2CHPPh2)2] (18) has to be considered as an example of a complex with the deprotonated, anionic form of ligand 13. The charge distribution of the corresponding ligand is also reflected in the C–P distances within the complexes 14, 17, and 18. While C1–P1 and C1–P2 are distinctly shorter for 14, an increase in C–P bond distance is observed for 17 and 18 due to the protonation of C1. Furthermore, the deprotonation of C2 and C3 of complex 18 leads to a shortening of the distances C2–P1, C2–P3 and C3–P2, C3–P4 compared to 14 and 17, where C2 and C3 are considered as methylene groups. This also corresponds to the P1–C2–P3 and P2–C3–P4 angles, which are significantly larger for the anionic ligand complex 18 compared to 14 and 17.
2.3. Photophysical Characterization of Selected CDP Complexes
Since the photophysical properties of carbodiphosphorane Cu(I) complexes have not yet been considered, the first investigations were performed in this report. The Cu(I) complexes 2–7, 9 and 14–16 show photoluminescence upon irradiation with UV light at room temperature. As proof of concept, we investigated emission spectra and quantum yields of [(CuPPh3)2(CDP(Py)2)](PF6)2 (4) and [(CuSPh)2(CDP(CH2PPh2)2] (16). Figure 3 illustrates the normalized room-temperature emission spectra of these materials. Compound 4 shows an emission maximum at 541 nm, corresponding to green/yellow color, along with a quantum yield (ΦPL) of 36% for the powder sample. The emission maximum of [(CuSPh)2(CDP(CH2PPh2)2] (16) (powder) is found at 510 nm (green color) showing ΦPL = 60%. The high quantum yields indicate the relatively high rigidity of the complexes in powder form. Moreover, these materials are chemically robust: After exposing the complexes to air for two months, the compounds still show their characteristic photoluminescence upon irradiation with UV light at room temperature.
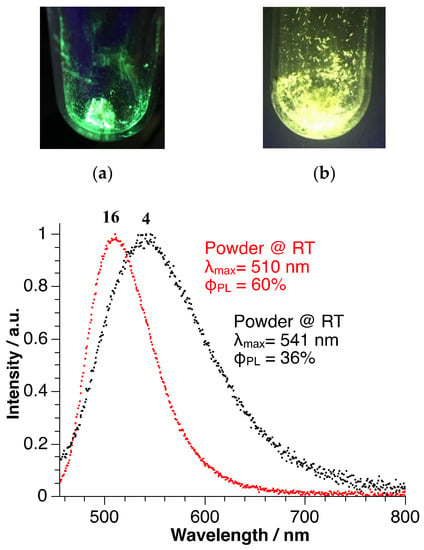
Figure 3.
Normalized room-temperature emission spectra for [(CuPPh3)2(CDP(Py)2)](PF6)2 (4) and [(CuSPh)2(CDP(CH2PPh2)2] (16). (a) illustrates the photoluminescence upon irradiation with UV light at room temperature of 16, (b) of complex 4.
First insight in the electronic structure of the emitting compounds 4 and 16 is obtained from consideration of the HOMO and LUMO distributions. Figure 4 displays that the HOMO shows for both compounds significant participation of metal d character as well as a marginal contribution of the central carbon. The LUMO, on the other hand, is primarily localized at the pyridyl units of the ligand backbone as well as on the phenyl groups attached to P1 and P2 for 16. Considering HOMO→LUMO transitions, the excitations can be ascribed to metal-to-ligand charge transfer (MLCT) transitions. According to the relatively small HOMO-LUMO overlap, it is indicated that the energy separations ΔE(S1–T1) between the lowest singlet S1 and triplet T1 states are small enough to allow for up-inter-system crossing at ambient temperature [28,31,32]. Therefore, we tentatively assign the emission observed as TADF emission. Details will be reported in a subsequent study.
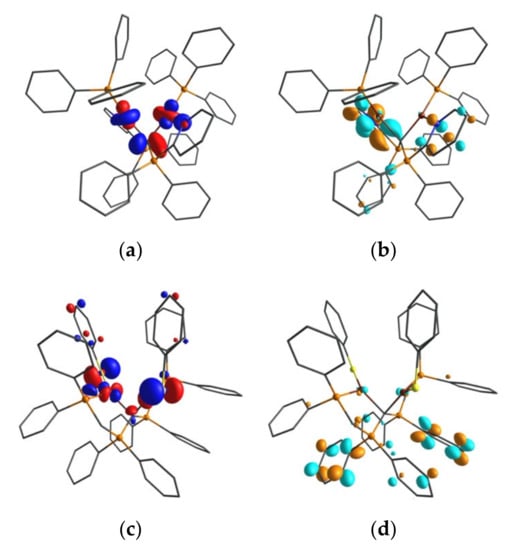
Figure 4.
Kohn–Sham orbitals of HOMO (a), LUMO (b) of [(CuPPh3)2(CDP(Py)2)](PF6)2 (4) and HOMO (c), LUMO (d) of [(CuSPh)2(CDP(CH2PPh2)2] (16) calculated for the optimized S0 state geometry (isovalue = 0.05). Calculations were performed at the PBE-D3(BJ)/def2-TZVPP level of theory. For more details of the MOs, compare Figure S-43 and Figure S-44 in the Supplementary Materials.
For completeness, it is mentioned that also complexes 17 and 18 exhibit photoluminescence upon irradiation with UV light at room temperature. This was not the case for [Cu2(dppf)(CDP(Py)2)](PF6)2 (8) and [(CuCarb)2(CDP(Py)2)] (11). For 8, quenching of the ferrocenyl ligand could be responsible for the lack of photoluminescence. In case of 11, a reason could be the asymmetric coordination found in the crystal structure. The reduced rigidity could lead to larger geometry rearrangement after excitation and thus to quenching.
3. Conclusions
We successfully isolated and characterized a series of dinuclear copper(I) complexes of two so far poorly investigated, multidentate pyridyl and phosphanyl functionalized N,C,N- and P,C,P- carbodiphosphorane ligands. A series of neutral complexes of CDP(Py)2 (1) with anionic coligands X and a series of dicationic complexes with monodentate and bridging bidentate bisphosphine ligands DPEPhos, XantPhos, and dppf were fully characterized, including their XRD molecular structures. In order to prepare unprecedented dinuclear copper complexes with a previously discovered P,C,P-carbodiphosphorane ligand backbone, it was necessary to isolate the free ligand base CDP(CH2PPh2)2 (13), which has not been demonstrated before. 13 can be obtained from [CH(dppm)2]Cl (12) and an excess of sodium amide in liquid ammonia. DFT calculations reveal that the ground state of 13 has no CDP structure in the gas phase, but rather an unsymmetric tautomer form 13a. However, upon reaction with CuX, the CDP tautomer is trapped from the tautomeric equilibrium and neutral dinuclear Cu(I) CDP complexes are isolated and fully characterized. In addition, a protonated and a deprotonated ligand form of 13 was characterized in mononuclear complexes [CuCl(H-CDP(CH2PPh2)2]PF6 (17) and [CuPPh3(CH(PPh2CHPPh2)2] (18). With the exception of [Cu2(dppf)(CDP(Py)2)](PF6)2 (8) and [(CuCarb)2(CDP(Py)2)] (11)), the complexes studied show photoluminescence upon irradiation with UV light at room temperature. Photophysical measurements reveal quantum yields ΦPL of 36% and 60% for [(CuPPh3)2(CDP(Py)2)](PF6)2 (4) and [(CuSPh)2(CDP(CH2PPh2)2] (16). As found in the crystal structure, the formal central carbon(0) atom is capable of coordinating two copper atoms relatively close to each other. They are further coordinated in a chelating manner to the chelating side arms of the CDPs. This rigid ligand design leads to high-emission quantum yields and makes the CDP complexes relatively stable under air. Therefore, it is proposed to test the compound’s OLED suitability.
Supplementary Materials
The following are available online. Experimental section, NMR spectra, IR spectra, crystal data tables of 2 (2017394), 3 (2017392), 4 (2017399), 5 (2017397), 6 (2017398), 7 (2017400), 8 (2017406), 9 (2017395), 11 (2017393), 14 (2017408), 15 (2017410), 16 (2017409), 17 (2017407) and 18 (2017411) and DFT calculations (PDF). Cartesian coordinates of calculated structures (XYZ).
Author Contributions
Conceptualization, M.K. and J.S.; Formal analysis, H.Y. and J.S.; Structural Analysis and DFT Calculations, M.K.; Synthesis, M.K. and N.D.; Luminescence Investigation and Methology, A.S. and H.Y.; Project administration, J.S.; Writing—original draft, M.K. and H.Y.; Writing—review & editing, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support by TransMIT GmbH, Gesellschaft für Technologietransfer (Gießen, Germany) is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramirez, F.; Desai, N.B.; Hansen, B.; McKelvie, N. HEXAPHENYLCARBODIPHOSPHORANE, (C6H5)3PCP(C6H5)3. J. Am. Chem. Soc. 1961, 83, 3539–3540. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. Divalent Carbon(0) Chemistry, Part 1: Parent Compounds. Chem. Eur. J. 2008, 14, 3260–3272. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. Divalent Carbon(0) Chemistry, Part 2: Protonation and Complexes with Main Group and Transition Metal Lewis Acids. Chem. Eur. J. 2008, 14, 3273–3289. [Google Scholar] [CrossRef] [PubMed]
- Tonner, R.; Frenking, G. C(NHC)2: Divalent Carbon(0) Compounds with N-Heterocyclic Carbene Ligands-Theoretical Evidence for a Class of Molecules with Promising Chemical Properties. Angew. Chem. Int. Ed. 2007, 46, 8695–8698. [Google Scholar] [CrossRef] [PubMed]
- Frenking, G.; Tonner, R. Divalent carbon(0) compounds. Pure Appl. Chem. 2009, 81, 597–614. [Google Scholar] [CrossRef]
- Tonner, R.; Öxler, F.; Neumüller, B.; Petz, W.; Frenking, G. Carbodiphosphoranes: The Chemistry of Divalent Carbon(0). Angew. Chem. Int. Ed. 2006, 45, 8038–8042. [Google Scholar] [CrossRef]
- Appel, R.; Knoll, F.; Schöler, H.; Wihler, H.-D. Vereinfachte Synthese von Bis(triphenylphosphoranyliden)methan. Angew. Chem. 1976, 88, 769–770. [Google Scholar] [CrossRef]
- Sundermeyer, J.; Weber, K.; Peters, K.; Von Schnering, H.G. Modeling Surface Reactivity of Metal Oxides: Synthesis and Structure of an Ionic Organorhenyl Perrhenate Formed by Ligand-Induced Dissociation of Covalent Re2O7. Organometallics 1994, 13, 2560–2562. [Google Scholar] [CrossRef]
- Petz, W.; Frenking, G. ChemInform Abstract: Carbodiphosphoranes and Related Ligands. J. Nat. Prod. 2010, 42, 49–92. [Google Scholar] [CrossRef]
- Petz, W. Addition compounds between carbones, CL2, and main group Lewis acids: A new glance at old and new compounds. Coord. Chem. Rev. 2015, 291, 1–27. [Google Scholar] [CrossRef]
- Gessner, V.H. Modern Ylide Chemistry, 1st ed.; Springer International Publishing AG: Oxford, UK, 2018. [Google Scholar]
- Kubo, K.; Jones, N.D.; Ferguson, M.J.; McDonald, R.; Cavell, R.G. Chelate and Pincer Carbene Complexes of Rhodium and Platinum Derived from Hexaphenylcarbodiphosphorane, Ph3PCPPh3. J. Am. Chem. Soc. 2005, 127, 5314–5315. [Google Scholar] [CrossRef] [PubMed]
- Cavell, R.G. Pincer and Chelate Carbodiphosphorane Complexes of Noble Metals. In The Chemistry of Pincer Compounds, 1st ed.; Morales-Morales, D., Jensen, C.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 347–355. [Google Scholar]
- Kubo, K.; Okitsu, H.; Miwa, H.; Kume, S.; Cavell, R.G.; Mizuta, T. Carbon(0)-Bridged Pt/Ag Dinuclear and Tetranuclear Complexes Based on a Cyclometalated Pincer Carbodiphosphorane Platform. Organometallics 2017, 36, 266–274. [Google Scholar] [CrossRef]
- Petz, W.; Neumuüller, B.; Klein, S.; Frenking, G. Syntheses and Crystal Structures of [Hg{C(PPh3)2}2][Hg2I6] and [Cu{C(PPh3)2}2]I and Comparative Theoretical Study of Carbene Complexes [M(NHC)2] with Carbone Complexes [M{C(PH3)2}2] (M = Cu+, Ag+, Au+, Zn2+, Cd2+, Hg2+). Organometallics 2011, 30, 3330–3339. [Google Scholar] [CrossRef]
- Petz, W.; Neumüller, B. New platinum complexes with carbodiphosphorane as pincer ligand via ortho phenyl metallation. Polyhedron 2011, 30, 1779–1784. [Google Scholar] [CrossRef]
- Böttger, S.C.; Poggel, C.; Sundermeyer, J. Ortho-directed Dilithiation of Hexaphenyl- carbodiphosphorane. Preprints 2020. [Google Scholar] [CrossRef]
- Stallinger, S.; Reitsamer, C.; Schuh, W.; Kopacka, H.; Wurst, K.; Peringer, P. Novel route to carbodiphosphoranes producing a new P,C,P pincer carbene ligand. Chem. Commun. 2007, 5, 510–512. [Google Scholar] [CrossRef]
- Reitsamer, C.; Schuh, W.; Kopacka, H.; Wurst, K.; Peringer, P. Synthesis and Structure of the First Heterodinuclear PCP−Pincer−CDP Complex with a Pd−Au d8−d10Pseudo-Closed-Shell Interaction. Organometallics 2009, 28, 6617–6620. [Google Scholar] [CrossRef]
- Reitsamer, C.; Schuh, W.; Kopacka, H.; Wurst, K.; Ellmerer, E.P.; Peringer, P. The First Carbodiphosphorane Complex with Two Palladium Centers Attached to the CDP Carbon: Assembly of a Single-Stranded di-Pd Helicate by the PCP Pincer ligand C(dppm)2. Organometallics 2011, 30, 4220–4223. [Google Scholar] [CrossRef]
- Reitsamer, C.; Stallinger, S.; Schuh, W.; Kopacka, H.; Wurst, K.; Obendorf, D.; Peringer, P. Novel access to carbodiphosphoranes in the coordination sphere of group 10 metals: Template synthesis and protonation of PCP pincer carbodiphosphorane complexes of C(dppm)2. Dalton Trans. 2012, 41, 3503. [Google Scholar] [CrossRef]
- Reitsamer, C.; Hackl, I.; Schuh, W.; Kopacka, H.; Wurst, K.; Peringer, P. Gold(I) and Gold(III) complexes of the [CH(dppm)2]+ and C(dppm)2 PCP pincer ligand systems. J. Organomet. Chem. 2017, 830, 150–154. [Google Scholar] [CrossRef]
- Maser, L.; Herritsch, J.; Langer, R. Carbodiphosphorane-based nickel pincer complexes and their (de)protonated analogues: Dimerisation, ligand tautomers and proton affinities. Dalton Trans. 2018, 47, 10544–10552. [Google Scholar] [CrossRef]
- Maser, L.; Vondung, L.; Langer, R. The ABC in pincer chemistry—From amine- to borylene- and carbon-based pincer-ligands. Polyhedron 2018, 143, 28–42. [Google Scholar] [CrossRef]
- Klein, M.; Xie, X.; Burghaus, O.; Sundermeyer, J. Synthesis and Characterization of a N,C,N-Carbodiphosphorane Pincer Ligand and Its Complexes. Organometallics 2019, 38, 3768–3777. [Google Scholar] [CrossRef]
- Su, W.; Pan, S.; Sun, X.; Zhao, L.; Frenking, G.; Zhu, C. Cerium–carbon dative interactions supported by carbodiphosphorane. Dalton Trans. 2019, 48, 16108–16114. [Google Scholar] [CrossRef]
- Kwon, J.H.; Yoo, S.; Lampande, R.; Kim, S. Vacuum Deposition. In Handbook of Organic Light-Emitting Diodes; Adachi, C., Hattori, R., Kaji, H., Tsujimura, T., Eds.; Springer: Tokyo, Japan, 2019. [Google Scholar] [CrossRef]
- Yersin, H. Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; WILEY-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Deaton, J.C.; Switalski, S.C.; Kondakov, D.Y.; Young, R.H.; Pawlik, T.D.; Giesen, D.J.; Harkins, S.B.; Miller, A.J.M.; Mickenberg, S.F.; Peters, J.C. E-Type Delayed Fluorescence of a Phosphine-Supported Cu2(μ-NAr2)2 Diamond Core: Harvesting Singlet and Triplet Excitons in OLEDs‖. J. Am. Chem. Soc. 2010, 132, 9499–9508. [Google Scholar] [CrossRef]
- Leitl, M.J.; Zink, D.M.; Schinabeck, A.; Baumann, T.; Volz, D.; Yersin, H. Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties. Top. Curr. Chem. 2016, 374, 25. [Google Scholar] [CrossRef] [PubMed]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes. Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Yersin, H.; Czerwieniec, R.; Shafikov, M.Z.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on CuI and AgI Complexes. Chem. Phys. Chem. 2017, 18, 3508–3535. [Google Scholar] [CrossRef]
- Chen, J.; Yu, R.; Zhang, Q.-K.; Zhou, L.-J.; Wu, X.-Y.; Zhang, Q.; Lu, C.-Z. Rational Design of Strongly Blue-Emitting Cuprous Complexes with Thermally Activated Delayed Fluorescence and Application in Solution-Processed OLEDs. Chem. Mater. 2013, 25, 3910–3920. [Google Scholar] [CrossRef]
- Gneuß, T.; Leitl, M.J.; Finger, L.H.; Yersin, H.; Sundermeyer, J. A new class of deep-blue emitting Cu(I) compounds—Effects of counter ions on the emission behavior. Dalton Trans. 2015, 44, 20045–20055. [Google Scholar] [CrossRef]
- Gneuß, T.; Leitl, M.J.; Finger, L.H.; Rau, N.; Yersin, H.; Sundermeyer, J. A new class of luminescent Cu(I) complexes with tripodal ligands—TADF emitters for the yellow to red color range. Dalton Trans. 2015, 44, 8506–8520. [Google Scholar] [CrossRef] [PubMed]
- Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Fléchon, C.; Zink, D.M.; Friedrichs, J.; Flügge, H.; Steininger, R.; Göttlicher, J.; et al. Bridging the Efficiency Gap: Fully Bridged Dinuclear Cu(I)-Complexes for Singlet Harvesting in High-Efficiency OLEDs. Adv. Mater. 2015, 27, 2538–2543. [Google Scholar] [CrossRef]
- Salehi, A.; Ho, S.; Chen, Y.; Peng, C.; Yersin, H.; So, F. Highly Efficient Organic Light-Emitting Diode Using A Low Refractive Index Electron Transport Layer. Adv. Opt. Mater. 2017, 5, 1700197. [Google Scholar] [CrossRef]
- Igawa, S.; Hashimoto, M.; Kawata, I.; Yashima, M.; Hoshino, M.; Osawa, M. Highly efficient green organic light-emitting diodes containing luminescent tetrahedral copper(i) complexes. J. Mater. Chem. C 2013, 1, 542–551. [Google Scholar] [CrossRef]
- Hashimoto, M.; Igawa, S.; Yashima, M.; Kawata, I.; Hoshino, M.; Osawa, M. Highly Efficient Green Organic Light-Emitting Diodes Containing Luminescent Three-Coordinate Copper(I) Complexes. J. Am. Chem. Soc. 2011, 133, 10348–10351. [Google Scholar] [CrossRef]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet Exciton Confinement in Green Organic Light-Emitting Diodes Containing Luminescent Charge-Transfer Cu(I) Complexes. Adv. Funct. Mater. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Yu, J.; Yersin, H. Blue-Light Emission of Cu(I) Complexes and Singlet Harvesting. Inorg. Chem. 2011, 50, 8293–8301. [Google Scholar] [CrossRef]
- Chen, J.; Yu, R.; Wu, X.-Y.; Liang, D.; Jia, J.-H.; Lu, C.-Z. A strongly greenish-blue-emitting Cu4Cl4 cluster with an efficient spin–orbit coupling (SOC): Fast phosphorescence versus thermally activated delayed fluorescence. Chem. Commun. 2016, 52, 6288–6291. [Google Scholar] [CrossRef]
- Schinabeck, A.; Rau, N.; Klein, M.; Sundermeyer, J.; Yersin, H. Deep blue emitting Cu(i) tripod complexes. Design of high quantum yield materials showing TADF-assisted phosphorescence. Dalton Trans. 2018, 47, 17067–17076. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.-E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M. Highly phosphorescent bis-cyclometalated iridium complexes: Synthesis, photophysical characterization, and use in organic light emitting diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar] [CrossRef]
- Yersin, H. Triplet Emitters for OLED Applications. Mechanisms of Exciton Trapping and Control of Emission Properties. Top. Curr. Chem. 2012, 241, 1–26. [Google Scholar] [CrossRef]
- Yersin, H.; Finkenzeller, W.J. Triplet Emitters for Organic Light-Emitting Diodes: Basic Properties. In Highly Efficient OLEDs; Wiley: Weinheim, Germany, 2008; pp. 1–97. [Google Scholar]
- Adachi, C.; Baldo, M.A.; Thompson, M.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Deaton, J.C.; Castellano, F.N. Archetypal Iridium(III) Compounds for Optoelectronic and Photonic Applications. In Iridium(III) in Optoelectronic and Photonics Applications; Wiley: Hoboken, NJ, USA, 2017; Volume 83, pp. 1–69. [Google Scholar]
- Liang, X.; Zhang, F.; Yan, Z.-P.; Wu, Z.-G.; Zheng, Y.-X.; Cheng, G.; Wang, Y.; Zuo, J.-L.; Pan, Y.; Che, C.-M. Fast Synthesis of Iridium(III) Complexes Incorporating a Bis(diphenylphorothioyl)amide Ligand for Efficient Pure Green OLEDs. ACS Appl. Mater. Interfaces 2019, 11, 7184–7191. [Google Scholar] [CrossRef]
- Cheng, G.; Chow, P.-K.; Kui, S.C.F.; Kwok, C.-C.; Che, C.-M. High-Efficiency Polymer Light-Emitting Devices with Robust Phosphorescent Platinum(II) Emitters Containing Tetradentate Dianionic O∧N∧C∧N Ligands. Adv. Mater. 2013, 25, 6765–6770. [Google Scholar] [CrossRef]
- Li, G.; Fleetham, T.; Li, J. Efficient and Stable White Organic Light-Emitting Diodes Employing a Single Emitter. Adv. Mater. 2014, 26, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R. Organometallic Emitters for OLEDs: Triplet Harvesting, Singlet Harvesting, Case Structures, and Trends. In Physics of Organic Semiconductors; Wiley: Hoboken, NJ, USA, 2013; pp. 371–424. [Google Scholar]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Che, C.-M.; Kwok, C.-C.; Lai, S.-W.; Rausch, A.; Finkenzeller, W.; Zhu, N.; Yersin, H. Photophysical Properties and OLED Applications of Phosphorescent Platinum(II) Schiff Base Complexes. Chem. Eur. J. 2010, 16, 233–247. [Google Scholar] [CrossRef]
- Williams, J.A.G. Photochemistry and Photophysics of Coordination Compounds: Platinum. In Photochemistry and Photophysics of Coordination Compounds II; Balzani, V., Campagna, S., Eds.; Springer: Berlin, Germany, 2007; pp. 205–268. [Google Scholar]
- Murphy, L.; Williams, J.A.G. Luminescent Platinum Compounds: From Molecules to OLEDs. In Molecular Organometallic Materials for Optics; Bozec, H., Guerchais, V., Eds.; Springer: Berlin, Germany, 2010; pp. 75–111. [Google Scholar]
- Cheng, G.; Kui, S.C.F.; Ang, W.-H.; Ko, M.-Y.; Chow, P.-K.; Kwong, C.-L.; Kwok, C.-C.; Ma, C.; Guan, X.; Low, K.-H.; et al. Structurally robust phosphorescent [Pt(O^N^C^N)] emitters for high performance organic light-emitting devices with power efficiency up to 126 lm W−1 and external quantum efficiency over 20%. Chem. Sci. 2014, 5, 4819–4830. [Google Scholar] [CrossRef]
- Cui, L.; Nomura, H.; Geng, Y.; Kim, J.U.; Nakanotani, H.; Adachi, C. Controlling Singlet–Triplet Energy Splitting for Deep-Blue Thermally Activated Delayed Fluorescence Emitters. Angew. Chem. Int. Ed. 2017, 56, 1571–1575. [Google Scholar] [CrossRef]
- Samanta, P.K.; Kim, D.; Coropceanu, V.; Brédas, J.-L. Up-Conversion Intersystem Crossing Rates in Organic Emitters for Thermally Activated Delayed Fluorescence: Impact of the Nature of Singlet vs Triplet Excited States. J. Am. Chem. Soc. 2017, 139, 4042–4051. [Google Scholar] [CrossRef] [PubMed]
- Turro, N.J. Modern Molecular Photochemistry of Organic Molecules; Benjamin/Cummings Pub. Co.: Melon Park, CA, USA, 1978. [Google Scholar]
- Wang, H.M.J.; Chen, C.Y.L.; Lin, I.J.B. Synthesis, Structure, and Spectroscopic Properties of Gold(I)-Carbene Complexes. Organometallics 1999, 18, 1216–1223. [Google Scholar] [CrossRef]
- Matsumoto, K.; Matsumoto, N.; Ishii, A.; Tsukuda, T.; Hasegawa, M.; Tsubomura, T. Structural and spectroscopic properties of a copper(I)–bis(N-heterocyclic)carbene complex. Dalton Trans. 2009, 6795. [Google Scholar] [CrossRef] [PubMed]
- Krylova, V.A.; Djurovich, P.I.; Whited, M.T.; Thompson, M. Synthesis and characterization of phosphorescent three-coordinate Cu(i)–NHC complexes. Chem. Commun. 2010, 46, 6696. [Google Scholar] [CrossRef]
- Catalano, V.J.; Munro, L.B.; Strasser, C.E.; Samin, A.F. Modulation of Metal–Metal Separations in a Series of Ag(I) and Intensely Blue Photoluminescent Cu(I) NHC-Bridged Triangular Clusters. Inorg. Chem. 2011, 50, 8465–8476. [Google Scholar] [CrossRef]
- Krylova, V.A.; Djurovich, P.I.; Aronson, J.W.; Haiges, R.; Whited, M.T.; Thompson, M. Structural and Photophysical Studies of Phosphorescent Three-Coordinate Copper(I) Complexes Supported by an N-Heterocyclic Carbene Ligand. Organometallics 2012, 31, 7983–7993. [Google Scholar] [CrossRef]
- Hamze, R.; Shi, S.; Kapper, S.C.; Sylvinson, D.; Estergreen, L.; Jung, M.; Tadle, A.; Haiges, R.; Djurovich, P.I.; Peltier, J.L.; et al. “Quick-Silver” from a Systematic Study of Highly Luminescent, Two-Coordinate, d10 Coinage Metal Complexes. J. Am. Chem. Soc. 2019, 141, 8616–8626. [Google Scholar] [CrossRef]
- Shi, S.; Jung, M.C.; Coburn, C.; Tadle, A.; Sylvinson, M.R.D.; Djurovich, P.I.; Forrest, S.R.; Thompson, M. Highly Efficient Photo- and Electroluminescence from Two-Coordinate Cu(I) Complexes Featuring Nonconventional N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2019, 141, 3576–3588. [Google Scholar] [CrossRef]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime. Sciences 2019, 363, 601–606. [Google Scholar] [CrossRef]
- Leitl, M.J.; Krylova, V.A.; Djurovich, P.I.; Thompson, M.; Yersin, H. Phosphorescence versus Thermally Activated Delayed Fluorescence. Controlling Singlet–Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds. J. Am. Chem. Soc. 2014, 136, 16032–16038. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Soloveichik, G.L.; Eisenstein, O.; Poulton, J.T.; Streib, W.E.; Huffman, J.C.; Caulton, K.G. Multiple structural variants of LnCuI(.mu.-X)2CuILn (n = 1, 2). Influence of halide on a “soft” potential energy surface. Inorg. Chem. 1992, 31, 3306–3312. [Google Scholar] [CrossRef]
- Ma, G.; Ferguson, M.J.; McDonald, R.; Cavell, R.G. Rare, Hexatomic, Boat-Shaped, Cross-Linked Bis(iminodiphenylphosphorano)methanediide Pincer Carbon Bridged Photoluminescent Copper Clusters Capped with Methyl or Halide Bridges. Organometallics 2010, 29, 4251–4264. [Google Scholar] [CrossRef]
Sample Availability: Samples of the selected compounds might be available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).