Abstract
The design of novel metal complexes with N-heterocyclic carbene (NHC) ligands that display biological activity is an active research field in organometallic chemistry. One of the possible approaches consists of the use of NHC ligands functionalized with a carbohydrate moiety. Two novel Au(I)–Au(I) dinuclear complexes were synthesized; they present a neutral structure with one bridging diNHC ligand, having one or both heterocyclic rings decorated with a carbohydrate functionality. With the symmetric diNHC ligand, the dicationic dinuclear complex bearing two bridging diNHC ligands was also synthesized. The study was completed by analyzing the antiproliferative properties of these complexes, which were compared to the activity displayed by similar mononuclear Au(I) complexes and by the analogous bimetallic Au(I)–Au(I) complex not functionalized with carbohydrates.
1. Introduction
Cisplatin was used as an anticancer drug for many years, and it is still one of the most used ones, despite its numerous issues with drug resistance and side effects [1,2]. These issues require more research to find a valid transition metal-based alternative to cisplatin. In this regard, metal complexes with N-heterocyclic carbene ligands (NHC) [3,4,5] are attracting increasing attention from the bioinorganic scientific community; these complexes are especially interesting due to their high stability imparted by the strength of the M–NHC bond, such that it appears reasonable that the structure of these complexes remains unchanged and stable under physiological conditions [6,7,8,9,10,11,12].
In particular, the interest in gold-based drugs received great impulse from the discovery of the anti-cancer properties of Auranofin, originally used as an antiarthritic drug. Compared to cisplatin, Auranofin presents better activity against difficult to treat tumors, better selectivity, and less cell resistance [13,14]. As Auranofin presents a phosphine ligand, it is reasonable to assume that this ligand could be substituted with an NHC ligand, as NHCs are rapidly substituting phosphine ligands given the higher stability of the resulting complexes; furthermore, as for phosphine ligands, for NHC ones it is also possible to easily and independently modify their steric and electronic properties [15]. This NHC versatility, obtained for example by simply changing the substituents on the nitrogen atoms of the heterocyclic ring, also allows a better fine-tuning of the lipophilic/hydrophilic balance of the molecule, thus enhancing the selectivity of the drug [16]. Many gold(I) and gold(III) complexes with NHC ligands show biological activity, and several reviews appeared on this topic in recent years [10,14,17].
The decoration of the carbene ligand with a carbohydrate can be of interest for a variety of reasons; sugars are abundant in nature and present an extremely varied structure, they can enhance the water solubility of the complex, and finally the presence of a sugar residue in the molecule can enhance the drug selectivity as a result of the increased carbohydrate uptake of cancer cells [18,19,20,21,22,23]. The bioactivity of mononuclear gold(I) complexes with carbohydrate-functionalized NHCs were recently studied by some of us [18]. In this manuscript, we report on the synthesis of dinuclear Au(I)–NHC complexes with incorporated acetylated glucopyranose moieties and on their antiproliferative activity. The performances of the dinuclear complexes, in terms of both activity and selectivity, were compared to their corresponding mononuclear counterparts and to dinuclear complexes not having carbohydrate-functionalized NHCs. Dinuclear Au(I) complexes with one or two bridging diNHC carbene ligands were reported to present anticancer properties, acting by inhibiting the thioredoxin reductase TrxR or by leading to mitochondria-induced apoptosis [24,25,26,27,28,29,30,31].
2. Results and Discussion
2.1. Synthesis of the Bis(Imidazolium) Salts
Compound L1∙2HPF6 was synthesized following a three-step process (Scheme 1), in which the carbohydrate-functionalized imidazole (a) [32] reacts with 1,3-dibromopropane to obtain the imidazolium salt (b). This reaction does not yield the bis(imidazolium) symmetric product, possibly due either to the limited nucleophilicity of the imidazole (a) or to the low reactivity of dibromopropane under the reaction conditions. In fact, by reacting product (b) with N-methylimidazole, compound L1∙2HBr can be isolated. The final Br−/PF6− anion metathesis step is usually required to isolate an azolium salt more soluble in organic solvents, such as acetonitrile, and to prevent interferences of the counter anions during the synthesis of the Au(I) complexes, especially the dinuclear dicationic ones.
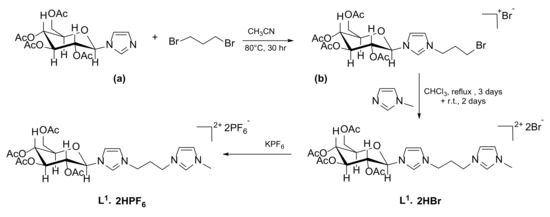
Scheme 1.
Synthesis of the bis(imidazolium) salt L1∙2HPF6; (a) carbohydrate-functionalized imidazole; (b) imidazolium salt.
In order to obtain the symmetric bis(imidazolium) salt L2∙2HPF6, imidazole (a) was reacted with 1,3-propylenebistriflate, a substrate more activated than 1,3-dibromopropane for the nucleophilic substitution, following a procedure reported by Anneser et al. (Scheme 2) [33]. Also in this case, the final step is the anion metathesis.
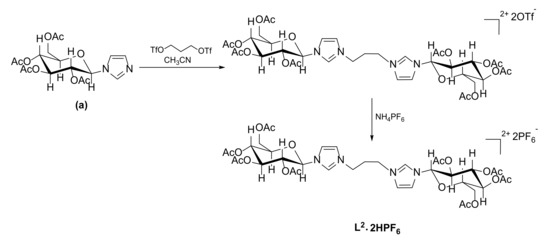
Scheme 2.
Synthesis of the bis(imidazolium) salt L2∙2HPF6; (a) carbohydrate-functionalized imidazole.
Both L1∙2HPF6 and L2∙2HPF6 were characterized by 1H- and 13C{1H}-NMR spectroscopy, as well as electrospray ionization mass spectrometry (ESI-MS), and both salts appear to be spectroscopically pure. In the case of compound L1∙2HPF6, the acidic protons of the imidazole C2-H hydrogens give two signals in the 1H-NMR spectrum at 8.72 and 8.73 ppm, indicating the lack of symmetry of the system. Conversely, the 1H-NMR spectrum of symmetric L2∙2HPF6 presents only one peak at 8.82 ppm. Anomerization processes were reported in the literature during the quaternization of the imidazole ring or during the synthesis of carbene complexes with carbohydrate functionalized NHC [34]. In both compounds, the signal relative to the anomeric proton, which is found around 5.7–5.8 ppm, has a coupling constant value of around 9 Hz. Comparing this 3JHHvalue with data found in literature relative to the anomeric proton in glucopyranose rings, it is reasonable to state that the carbohydrate remains present in the β anomer form in both diazolium salts; the coupling constant of the same peak for the α anomer is, in fact, much lower, around 2–4 Hz [35].
2.2. Synthesis of the [Au2Br2L] Complexes
Compounds 1 and 2 were synthesized following a single-step procedure already reported in the literature [36] in which the proper bis(imidazolium) salt reacts with the gold precursor AuCl(SMe2) in the presence of LiBr and K2CO3 as a base to deprotonate the bis(imidazolium) salt. The addition of LiBr prevents the formation of the analogous chloro complexes [Au2Cl2L]; furthermore, it was reported by Nolan and co-workers that the anion which usually coordinates to gold(I) in the complexes is that of the starting azolium salt [37]. The neutral [Au2Br2L] complexes (Scheme 3) were then characterized by 1H- and 13C{1H}-NMR spectroscopy, as well as ESI-MS. In particular, an indication that the complexes formed is provided by the disappearance of the peak relative to the acidic C2-Hs, supporting the deprotonation of the diazolium salt. Further proof comes from the 13C-NMR spectrum, in which the peak relative to the imidazole C2 is present at around 170–175 ppm, in the range of values found in the literature for carbene carbons coordinated to a gold(I) center trans to a bromide ligand [36,38]. In both complexes, the carbohydrate is present in the β anomeric form as suggested by the value (9 Hz) of the 3JHH coupling constant of the anomeric proton in the glucopyranose ring [35]. From the ESI-MS spectra, the most prominent peaks are the [Au2BrL]+ (at m/z 993 and 1311 for compounds 1 and 2, respectively) and the [Au2Br2LK]+ cations (m/z 1113 and 1429 for compounds 1 and 2, respectively).
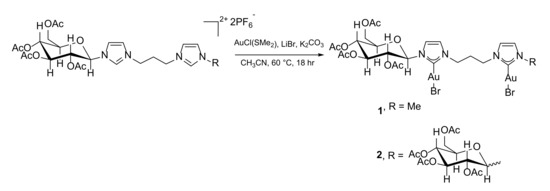
Scheme 3.
Synthesis of the dinuclear gold(I) complexes 1 and 2.
2.3. Synthesis of the [Au2L2](PF6)2 Complexes
With this type of dicarbene ligand, in addition to the neutral complexes described in the previous section, it is possible to also isolate dinuclear dicationic complexes with the general formula [Au2L2]2+ having two ligands bridging the two gold centers. Considering the asymmetric nature of the proligand L1∙2HPF6, at least two configurational isomers can be isolated: one with the carbohydrate-imidazoles facing each other and one with each carbohydrate-NHC facing a methylimidazole-2-ylidene. Furthermore, we recently reported that, in similar [Au2L2]2+ complexes with heteroditopic ligands, the number of possible products is also increased by the different conformations of the propylene linkers between the carbene units [39,40]. For this reason, we investigated only the reaction with the symmetric ligand L2. Compound 3 was synthesized following the same procedure described for complex 2 but using a 1:1 L2∙2HPF6:AuCl(SMe2) molar ratio and without adding LiBr to the reaction mixture (Scheme 4). Once again, the formation of the complex is supported by the disappearance in the 1H-NMR spectrum of the peak associated to the C2-H on the imidazole rings. The stoichiometry of the complex was confirmed by high-resolution mass spectrometry measurements where a peak relative to the dicationic [Au2L22]2+ fragment is present at 1033.2594 m/z. Another indication that this complex is cationic comes from the 13C{1H}-NMR spectrum; the peak of the carbene carbon is found at 183.2 ppm, downfield shifted by ca. 10 ppm with respect to the signal observed in the corresponding neutral complex 2 (174.6 ppm). This chemical shift value is coherent with the values reported in literature for carbene carbons with another carbene carbon in trans position [41,42,43,44,45], and this geometry is frequently observed in cationic gold(I) complexes with two carbene ligands coordinated to the same metal center.
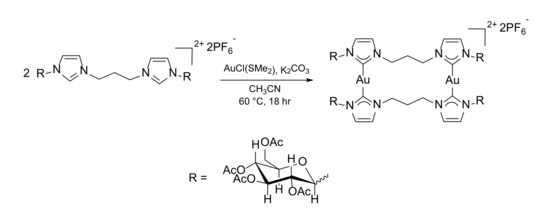
Scheme 4.
Synthesis of the dinuclear gold(I) complex 3.
2.4. Reactivity of the Gold(I) Complex 3 toward Oxidative Addition of Halogens
The reactivity of the gold(I) complex 3 in the oxidative addition of halogens to gold was investigated; the reactions were performed in an NMR tube at room temperature in deuterated acetonitrile as solvent, using a slight excess of oxidant (I2:[Au] = 1.2:1 and PhICl2:[Au] = 1.5:1) (Scheme 5). Many possible products can be obtained from the oxidative addition of halogens to dinuclear diNHC gold(I) complexes: the fully oxidized gold(III)–gold(III) product, the mixed-valence gold(I)–gold(III) complex, and the gold(II)–gold(II) species (Figure 1) [46]. Therefore, the reactions were followed by recording 1H-NMR spectra before the addition of the oxidant, immediately after, and three hours and 24 h later in order to monitor any possible changes in the product distribution. With both halogens, the dinuclear gold(III) complex was immediately formed, and no further evolution of the product was detected.
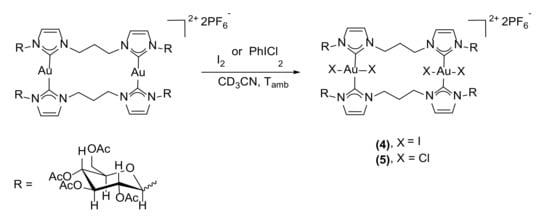
Scheme 5.
Synthesis of the dinuclear Au(III) complexes by oxidative addition to 3.
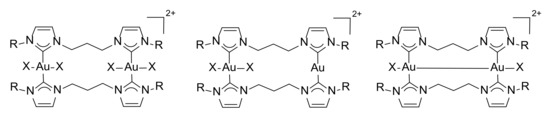
Figure 1.
Possible products obtained from the oxidative addition of halogens to the dinuclear gold(I)–gold(I) complexes.
In both cases, the symmetry of the complex is maintained, as shown by the 1H-NMR spectra which present only one set of peaks relative to the sugars and imidazole rings. This suggests that the two metal centers are equivalent and, therefore, present the same oxidation state. The 13C-NMR spectra show more definite proof that the oxidation state of the gold centers changed from gold(I) to gold(III); the carbene carbons present, in fact, a peak at 145.6 ppm for complex 4 and 154.6 ppm for 5. These values of chemical shifts are 20–30 ppm lower than the value (δ 183.2 ppm) found for complex 3. This is usually explained taking into consideration the more pronounced Lewis acidic behavior of gold(III), causing an extended delocalization of the π electron density of the imidazole C=C double bond toward the carbene carbon [47,48]. The difference between the 13C-NMR carbene chemical shifts in 4 and 5 is ca. 10 ppm and is due to the different nature of the two halide ligands [46]. The definitive proof that both complexes are dinuclear gold(III) complexes comes from the high-resolution mass spectra; for both complexes, the most prominent signal is the one given by the [Au2X4L2]2+ ion, at m/z 1287.0697 for 4 and 1103.1974 for 5, both for monoisotopic peaks.
2.5. Biological Activity of the Gold(I) Complexes
The biological activity of the gold(I) complexes 1–3 and 6 was tested on different eukaryotic cell lines. Complex 6 (Figure 2), bearing only methyl groups as wingtip substituents, was chosen for the absence of any carbohydrate moiety as comparison.
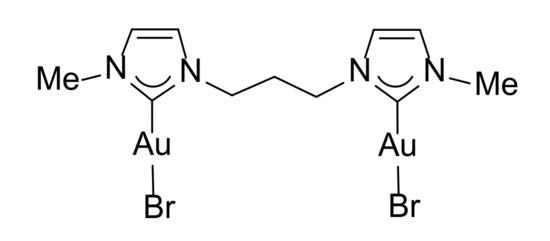
Figure 2.
Structure of complex 6.
The assay was performed to study the importance of the functionalization with a carbohydrate moiety in determining the antiproliferative activity. We did not test the antiproliferative activity of gold(III) complexes 4 and 5, as it is well known that similar dicationic gold(III) complexes easily undergo reduction to the corresponding gold(I) species in a physiological environment [26,48]. In particular, two cancer cell lines, A431 and SVT2, were tested in the presence of increasing amount of each compound. Immortalized cell lines, HaCaT and BALB/c-3T3, were analyzed as well to study the selectivity of the newly synthesized compounds. Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the results, after 48 h of incubation, are reported in Figure 3. All the complexes were cytotoxic on all cell lines analyzed, and they showed a dose-dependent toxicity. Interestingly, the analyzed compounds induced an increase in cell proliferation at very low concentration (5–10 µg/mL). The IC50 values, i.e., the complex concentration required to induce 50% of cell death, is reported in Table 1. Complex 3 was the compound with the lowest toxicity with respect to the other tested molecules.
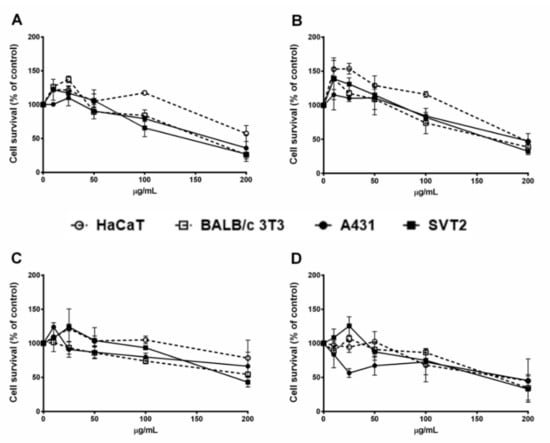
Figure 3.
Effect of complexes 1, 2, 3, and 6 on the survival of different cell lines. Immortalized human cells (HaCaT, dashed line with empty circles), immortalized murine cells (BALB/c-3T3, dashed line with empty squares), human epidermoid carcinoma (A431, black line with black circles), and murine fibroblast transformed with simian virus 40 (SV40) (SVT2, black line with black squares) were incubated with increasing amounts of each compound (10–200 µg/mL) for 48 h. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and expressed as described in the Section 3. A, complex 1; B, complex 2; C, complex 3; D, complex 6. Values are given as means ± SD (n ≥ 3).

Table 1.
IC50 values (µM) obtained for 1, 2, 3, and 6 on HaCaT, BALB/c 3T3, A431, and SVT2 cells line after 48 h of incubation.
Even if the IC50 values for the reported dinuclear complexes are very high (>100 μM), these results are not totally unexpected, as this inertness was also observed for the mononuclear complex [Au(magi)Cl] (magi = 1-methyl-3-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)imidazole-2-ylidene) [18] and was attributed to the low mitochondrial penetration of the complex. Comparing the performances of complexes 1, 2, and 6, it is evident that the introduction of a sugar in the carbene moiety only slightly improves the cytotoxic activity. Complex 3 shows lower IC50 values than those of the neutral complex 2, having the same diNHC ligand.
In general, halo-substituted neutral gold(I) complexes turn out to be less effective than their corresponding cationic bis(NHC) complexes. The lability of the Au–X (X = halogen) bond, compared to the relative inertness of the Au–NHC bonds, makes the halide derivatives less stable in biologically relevant conditions, favoring the occurrence of deactivation reactions by different cellular components. Another parameter that can explain the lower activity of the neutral gold(I) complexes is their lower solubility in water, which possibly reduces the drug uptake by cells [49]. Finally, the higher activity of complex 3 can be also attributed to its dicationic nature, which allows its classification as a delocalized lipophilic cation (DLC) [50]. Indeed, the difference in the mitochondrial membrane potential between cancerous and healthy cells could explain the higher penetration of DLCs into the mitochondrial membrane of tumor cells, which in turn would lead to cell apoptosis [51].
3. Materials and Methods
3.1. General Comments
All commercially available reagents (Sigma-Aldrich, Darmstadt, Germany) were used as received without additional purification steps. The reagents a [32], b [52], 1,3-propylenebistriflate [53], and complex 6 [36] were prepared according to literature procedures. The NMR spectra were recorded on a Bruker Avance 300 (Bruker, Billerica, MA, USA; 300.1 MHz for 1H and 75.5 MHz for 13C) at 298 K unless otherwise stated; chemical shifts (δ) are reported in units of ppm relative to the residual solvent signals. ESI-MS analyses of compounds L1∙2HPF6, L1∙2HPF6, 1, and 2 were performed using an LCQ-Duo (Thermo Fisher Scientific, Waltham, Massachusetts, USA) operating in positive ion mode; sample solutions were prepared by dissolving the compounds in acetonitrile and were directly infused into the ESI source by a syringe pump at 8 μL/min flow rate. The HRMS measures of complexes 3–5 were performed using a Q-Exactive hybrid quadrupole-Orbitrap™ mass spectrometer (Thermo Fisher Scientific). MS conditions were as follows: electrospray ionization in positive mode, resolution 70,000, automatic gain control (AGC) target 1 × 106, max injection time of 50 ms, scan range 500–2000 amu, capillary voltage 3.5 kV and radiofrequency (RF) voltage 50 V, capillary temperature 320 °C and probe temperature 350 °C; nitrogen was used as sheath gas at 11 psi. Samples were prepared using acetonitrile as solvent and injected for analysis at a flow rate of 10 μL/min. Calibration was performed with a standard solution purchased from Thermo Fisher Scientific (Pierce® ESI positive Ion Calibration Solution). The software for analysis of MS data was Xcalibur 3.1 (Thermo Fisher Scientific). Elemental analyses were carried out by the microanalytical laboratory of Chemical Sciences Department (University of Padova) with a Thermo Scientific FLASH 2000 apparatus. The recorded NMR and ESI-MS spectra of the reported compounds can be found in the Supplementary Materials section.
In the characterization of the imidazolium salts and gold complexes, the following notation (Figure 4) was adopted for the sugar substituent:
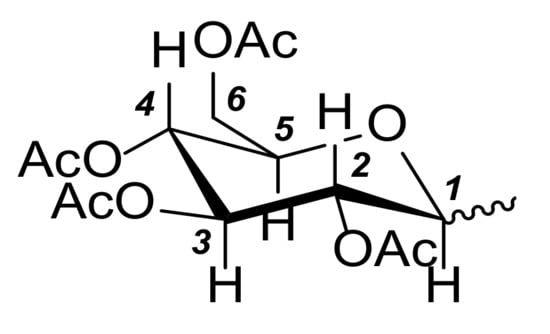
Figure 4.
Numbering of the carbon atoms in the sugar substituent for NMR assignments.
3.2. Synthesis of the Bis(Imidazolium) Salts
3.2.1. Synthesis of the Bis(Imidazolium) Salt L1∙2HPF6
Compound b (0.19 g, 0.32 mmol), N-methylimidazole (91 μL, 1.14 mmol), and 10 mL of CHCl3 were added to an Ace pressure tube. The solution was stirred at 65 °C for three days, then for two additional days at room temperature. The solvent was evaporated under reduced pressure to give a white solid. The solid product was dissolved in 5 mL of a saturated aqueous KPF6 solution and left stirring overnight. The formation of an oil was observed. The oily residue was separated from the aqueous solution and dried under vacuum. The residue was treated with 20 mL of diethyl ether under stirring for three hours and the formation of a white solid was observed. The solid was isolated by filtration (yield 44%). 1H-NMR (300 MHz, CD3CN) δ 8.76 (s, 1H, NCHN-GluIm), 8.41 (s, 1H, NCHN-MeIm), 7.64 (s, 1H, GluIm), 7.47 (s, 1H, GluIm), 7.38 (m, 2H, MeIm), 5.74 (d, 3J = 9.0 Hz, 1H, 1-Glu), 5.50 (m, 1H, 3-Glu), 5.38–5.21 (m, 2H, 2,4-Glu), 4.32–4.18 (m, 3H, 5,6-Glu), 4.18–4.09 (m, 4H, NCH2), 3.84 (s, 3H, CH3-MeIm), 2.38 (quint, 2H, 3J = 7.2 Hz, 2H, CH2), 2.07 (s, 3H, CH3-Ac), 2.03 (s, 3H, CH3-Ac), 1.99 (s, 3H, CH3-Ac), 1.94 (s, 3H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 171.16 (CO), 170.59 (CO), 170.48 (CO), 170.43 (CO), 136.91 (NCHN-GluIm), 136.31 (NCHN-MeIm), 124.78 (GluIm), 124.12 (GluIm), 123.01 (MeIm), 121.61 (MeIm), 85.28 (1-Glu), 75.56 (Glu), 72.13 (Glu), 71.87 (Glu), 67.97 (Glu), 62.08 (6-Glu), 47.64 (NCH2), 46.76 (NCH2), 36.67 (CH3-MeIm), 30.46 (CH2), 20.63 (CH3-Ac), 20.56 (CH3-Ac), 20.33 (CH3-Ac). ESI-MS (positive ions, CH3CN): m/z 667 [H2L1PF6]+, 983 [H2L2PF6]+, 1479 [(H2L1)2(PF6)3]+. Elemental analysis C24H34N4O9P2F12. Calculated: C, 35.48%; H, 4.22%; N, 6.90%. Found: C, 34.96%; H, 3.73%; N 5.57%.
3.2.2. Synthesis of the Bis(Imidazolium) Salt L2∙2HPF6
Compound a (231 mg, 0.58 mmol) was dissolved in 50 mL of acetonitrile at 0 °C. A solution of 1,3-propylenebistriflate (98 mg, 0.29 mmol) in 7 mL of acetonitrile was prepared and added dropwise, over one hour, to the solution containing a. The resulting solution was warmed to room temperature and left stirring for 16 h. The solvent was removed and the obtained solid was dissolved in 5 mL of distilled water. A saturated aqueous NH4PF6 solution (5 mL) was added, and the final solution was left stirring for an hour until the formation of a white solid precipitate was observed. The solid was filtered, washed with distilled water, and then dried under reduced pressure (yield 48%). 1H-NMR (300 MHz, CD3CN) δ 8.82 (s, 2H, NCHN-Im), 7.67 (t, 3J = 1.9 Hz, 2H, Im), 7.50 (t, 3J = 1.9 Hz, 2H, Im), 5.78 (d, 3J = 9.0 Hz, 2H, 1-Glu), 5.52 (m, 2H, 3-Glu), 5.38–5.25 (m, 4H, 2,4-Glu), 4.28–4.22 (m, 6H, 5,6-Glu), 4.22–4.17 (m, 4H, NCH2), 2.47–2.36 (m, 2H, CH2), 2.05 (s, 6H, CH3-Ac), 2.05 (s, 6H, CH3-Ac), 2.01 (s, 6H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 171.2 (CO), 170.6 (CO), 170.5 (CO), 136.5 (NCHN-Im), 124.2 (Im), 121.8 (Im), 85.4 (1-Glu), 75.7 (3-Glu), 72.2 (Glu), 71.9 (Glu), 68.0 (Glu), 62.1 (Glu), 47.6 (NCH2), 30.4 (CH2), 20.7 (CH3-Ac), 20.7 (CH3-Ac), 20.5 (CH3-Ac). ESI-MS (positive ions, CH3CN): m/z 983 [H2L2PF6]+, 507 [H2L2-Glu]+, 419 [H2L2]2+. Elemental analysis C37H50N4O18P2F12. Calculated: C, 39.37%; H, 4.46%; N, 4.96%. Found: C, 39.07%; H, 4.24%; N, 4.36%.
3.3. General Procedure for the Synthesis of Complexes [Au2Br2L]
The proper bis(imidazolium) salt (0.086 mmol), AuCl(SMe2) (0.172 mmol), K2CO3 (1.89 mmol), LiBr (0.86 mmol), and 40 mL of acetonitrile were added to a round-bottom flask. The mixture was heated to 60 °C and left stirring for 18 h, then filtered on Celite to remove excess salts. The solvent was removed under reduced pressure. The solid was finally recrystallized with chloroform/n-hexane (for 1) or acetonitrile/diethyl ether (for 2), obtaining a white solid product which was filtered and dried under vacuum.
1. White solid, yield 59%. 1H-NMR (300 MHz, CD3CN) δ 7.43 (d, 3J = 2.1 Hz, 1H, GluIm), 7.29 (d, 3J = 2.1 Hz, 1H, GluIm), 7.20 (d,3J = 1.9 Hz, 1H, MeIm), 7.17 (d, 3J = 1.9 Hz, 1H, MeIm), 6.12 (d,3J = 8.9 Hz, 1H, 1-Glu), 5.52 (m, 1H, 3-Glu), 5.33–5.19 (m, 2H, 2,4-Glu), 4.27–4.17 (m, 3H, 5,6-Glu), 4.17–4.02 (m, 4H, NCH2), 3.85 (s, 3H, CH3-MeIm), 2.50–2.35 (m, 2H,CH2), 2.06 (s, 3H, CH3-Ac), 2.04 (s, 3H, CH3-Ac), 1.98 (s, 3H, CH3-Ac), 1.93 (s, 3H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 175.07 (NCN-MeIm), 172.91 (NCN-GluIm), 171.27 (CO), 170.66 (CO), 170.58 (CO), 169.99 (CO), 123.93 (MeIm), 122.30 (GluIm), 120.73 (MeIm), 119.62 (GluIm), 86.64 (1-Glu), 75.13 (3-Glu), 72.64 (Glu), 72.24 (Glu), 68.51 (Glu), 62.34 (Glu), 48.31 (NCH2), 47.50 (NCH2), 38.75 (CH3-MeIm), 30.96 (CH2), 21.08 (CH3-Ac), 20.95 (CH3-Ac), 20.82 (CH3-Ac), 20.75 (CH3-Ac). ESI-MS (positive ions, CH3CN): m/z 993 [Au2L1Br]+, 1113 [Au2L1Br2K]+.
2. White solid, yield 89%. 1H-NMR (300 MHz, CD3CN) δ 7.45 (d, 3J = 2.0 Hz, 2H, Im), 7.30 (d, 3J = 2.0 Hz, 2H, Im), 6.14 (d, 3J = 8.9 Hz, 2H, 1-Glu), 5.54 (m, 2H, 3-Glu), 5.26 (m, 4H, 2,4-Glu), 4.34–4.18 (m, 6H, 5,6-Glu), 4.18–3.98 (m, 4H, NCH2), 2.49–2.35 (m, 2H, CH2), 2.06 (s, 6H, CH3-Ac), 2.04 (s, 6H, CH3-Ac), 1.98 (s, 6H, CH3-Ac), 1.92 (s, 6H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 174.64 (NCN-Im), 171.24 (CO), 170.65 (CO), 170.59 (CO), 170.01 (CO), 122.34 (Im), 119.67 (Im), 86.76 (1-Glu), 75.20 (Glu), 72.65 (Glu), 72.33 (Glu), 68.55 (Glu), 62.36 (6-Glu), 48.22 (NCH2), 21.05 (CH3-Ac), 20.97 (CH3-Ac), 20.85 (CH3-Ac), 20.78 (CH3-Ac). ESI-MS (positive ions, CH3CN): m/z 1311 [Au2L2Br]+, 1429 [Au2L2Br2K]+.
3.4. Synthesis of the Complex [Au2L22](PF6)2, 3
The salt L2∙2HPF6 (76 mg, 0.067 mmol), AuCl(SMe2) (20 mg, 0.067 mmol), K2CO3 (203 mg, 1.47 mmol), and 40 mL of acetonitrile were added to a round-bottom flask; the mixture was heated to 60 °C and left stirring for 18 h, then filtered on Celite to remove excess salts. The solvent was removed at reduced pressure. The residue was recrystallized with acetonitrile/diethyl ether, obtaining a white solid which was filtered and dried under vacuum (yield 69%). 1H-NMR (300 MHz, CD3CN) δ 7.58 (d, 3J = 1.9 Hz, 4H, Im), 7.42 (d, 3J = 1.9 Hz, 4H, Im), 6.08 (d, 3J = 8.9 Hz, 4H, 1-Glu), 5.63 (m 4H, 3-Glu), 5.35 (m, 8H, 2,4-Glu), 4.47–4.31 (m, 8H, NCH2), 4.31–4.13 (m, 12H, 5,6-Glu), 2.54–2.38 (m, 4H,CH2), 2.05 (s, 12H, CH3-Ac), 2.01 (s, 12H, CH3-Ac), 2.01 (s, 12H, CH3-Ac), 1.92 (s, 12H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 183.17 (Im), 171.16 (CO), 170.73 (CO), 170.55 (CO), 170.09 (CO), 126.84 (Im), 123.80 (Im), 87.14 (1-Glu), 75.51 (Glu), 72.70 (Glu), 72.58 (Glu), 68.29 (Glu), 62.26 (Glu), 50.11 (NCH2), 32.53 (CH2), 21.04 (CH3-Ac), 20.83 (CH3-Ac), 20.80 (CH3-Ac). HRMS (positive ions, monoisotopic peak): m/z 991.2506 [Au2L22(-2CH2CO)]2+ (calculated for C70H92Au2N8O342+ = 991.2518), 1012.2554 [Au2L22(-CH2CO)]2+ (calculated for C72H94Au2N8O352+ = 1012.2571), 1033.2594 [Au2L22]2+ (calculated for C74H96Au2N8O362+ = 1033.2624).
3.5. Reactivity of Complex 3 in Halogen Oxidative Addition: Characterization of the Dinuclear Au(III) Complexes [Au2L22X4](PF6)2, 4 and 5
Complex 3 was added to an NMR tube and dissolved in CD3CN; the oxidant (I2 or PhICl2) was then added in a [I2]/[Au] = 1.2 and [Cl2]/[Au] = 1.5 ratio. NMR spectra were recorded before the addition of the oxidant, immediately after, and three hours and 24 hours later to monitor the reaction. In the case of the [Au2L22Cl4](PF6)2 complex, the product was isolated as a white solid by removal of the deuterated solvent and by treatment of the residue with diethyl ether to remove the coproduct of the oxidant (PhI). These reactivity tests were run on NMR scale and, for this reason, the characterization of the complexes involved only NMR and MS analysis.
4. 1H-NMR (300 MHz, CD3CN) δ 7.77 (d, 3J = 2.1 Hz, 4H, Im), 7.62 (d,3J = 2.1 Hz, 4H, Im), 5.90 (d, 3J = 9.3 Hz, 4H, 1-Glu), 5.66 (m, 4H, 3-Glu), 5.40 (m, 4H, 2-Glu), 5.24 (m, 4H, 4-Glu), 4.28–4.00 (m, 20H, 5,6-Glu and NCH2), 2.66–2.43 (m, 4H, CH2), 1.96 (s, 12H, CH3-Ac), 1.94 (s, 12H, CH3-Ac), 1.89 (s, 12H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 171.06 (CO), 171.01 (CO), 170.66 (CO), 170.64 (CO), 145.63 (NCN-Im), 126.58 (Im), 123.81 (Im), 85.81 (1-Glu), 75.96 (Glu), 72.69 (Glu), 72.29 (Glu), 68.11 (Glu), 62.43 (Glu), 50.02 (NCH2), 30.45 (CH2), 22.13 (CH3-Ac), 20.96 (CH3-Ac), 20.80 (CH3-Ac), 20.75 (CH3-Ac). HRMS (positive ions, monoisotopic peak): m/z 1160.1664 [Au2L22I2]2+ (calculated for C74H96Au2I2N8O362+ = 1160.1668), 1287.0697 [Au2L22I4]2+ (calculated for C74H96Au2I4N8O362+ = 1287.0713), 1266.0660 [Au2L22I4(-CH2CO)]2+ (calculated for C72H94Au2I4N8O352+ = 1266.0660).
5. 1H-NMR (300 MHz, CD3CN) δ 7.84 (s, 4H, Im), 7.68 (s, 4H, Im), 6.39 (d, 3J = 9.3 Hz, 4H, 1-Glu), 5.80 (t, 3J = 9.6 Hz 4H, 3-Glu), 5.45–5.19 (m, 8H, 2,4-Glu), 4.61–4.42 (m, 4H, NCH2), 4.42–4.27 (m, 4H, NCH2), 4.27–4.05 (m, 12H, 5,6-Glu), 2.71–2.48 (m, 4H, CH2), 2.05 (s, 12H, CH3-Ac), 2.01 (s, 12H, CH3-Ac), 1.93 (s, 12H, CH3-Ac), 1.90 (s, 12H, CH3-Ac). 13C{1H}-NMR (75 MHz, CD3CN) δ 171.37 (CO), 171.01 (CO), 170.66 (CO), 170.63 (CO), 154.57 (NCN-Im), 126.14 (Im), 122.99 (Im), 85.68 (1-Glu), 76.16 (Glu), 73.56 (Glu), 72.41 (Glu), 68.10 (Glu), 62.40 (6-Glu), 49.46 (NCH2), 31.56 (CH2), 21.50 (CH3-Ac), 20.80 (CH3-Ac), 20.76 (CH3-Ac). HRMS (positive ions, monoisotopic peak): m/z 1033.2615 [Au2L22]2+ (calculated for C74H96Au2N8O362+ = 1033.2624), 1103.1970 [Au2L22Cl4]2+ (calculated for C74H96Au2Cl4N8O362+ = 1103.2001).
3.6. Cytotoxicity Assay
To assess the cytotoxicity of the compounds, both immortalized and tumorigenic cells were chosen. Immortalized human keratinocytes (HaCaT, from Innoprot, Derio, Spain), immortalized murine fibroblasts (BALB/c 3T3, from ATCC, Manassas, Vi, USA), human epidermoid carcinoma cells (A431, from ATCC), and BALB/c-3T3 transformed with simian virus 40 (SV40) (SVT2, from ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 2 mM l-glutamine and antibiotics. Cells were grown in a 5% CO2 humidified atmosphere at 37 °C and seeded in 96-well plates at a density of 2 × 103 cells per well. Cells were incubated with increasing concentrations of each compound (from 10 to 200 µg·mL−1). After 4 h of incubation, cell viability was measured using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, which measures mitochondrial functionality. Briefly, the MTT reagent was dissolved in DMEM in the absence of phenol red (Sigma-Aldrich) and added to the cells (0.5 mg·mL-1 final concentration). Following 4 h of incubation at 37 °C, the culture medium was removed and the resulting formazan salts were dissolved by adding isopropanol containing 0.01 mol·L−1 HCl (100 μL per well). Absorbance values were determined at 570 nm using an automatic plate reader (Microbeta Wallac 1420, PerkinElmer, Waltham, MA, USA).
4. Conclusions
In this work, we reported two novel diNHC precursors with one or both the heterocyclic rings functionalized with a carbohydrate moiety. The corresponding neutral complexes of the type Au2Br2(diNHC) and, with the symmetric ligand, also the dicationic complex [Au2(diNHC)2](PF6)2 were synthesized. The antiproliferative properties of these complexes were investigated. Results suggest that the complexes appear rather inert and the introduction of a carbohydrate moiety does not significantly improve their performance. The investigation of the coordinating properties of the new ligands described in this work will be extended to other metal centers in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/17/3850/s1: NMR and ESI-MS spectra of the reported compounds.
Author Contributions
Conceptualization, C.T. and A.B.; methodology, C.T. and D.M.M.; validation, C.T., A.A., L.D., and D.M.M.; formal analysis, F.T., M.R., and P.S.; investigation, F.T., V.S., M.R., S.B., P.S., M.B., A.A., L.D., F.R., and D.M.M.; data curation, F.T., C.T., S.B., A.A., L.D., and D.M.M..; writing—original draft preparation, F.T., C.T., D.M.M., and F.R.; writing—review and editing, all authors; visualization, F.T.; supervision, C.T.; project administration, A.B.; funding acquisition, A.B. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Chemical Sciences (University of Padova), grant number P-DiSC #03BIRD2017-UNIPD.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin−DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, 2nd ed.; RSC Catalysis Series; RSC: Cambridge, UK, 2017. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Huynh, H.V. The Organometallic Chemistry of N-heterocyclic Carbenes; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2017. [Google Scholar]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903. [Google Scholar] [CrossRef]
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Update on metal N-heterocyclic carbene complexes as potential anti-tumor metallodrugs. Co-ord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Teyssot, M.-L.; Jarrousse, A.-S.; Manin, M.; Chevry, A.; Roche, S.; Norre, F.; Beaudoin, C.; Morel, L.; Boyer, D.; Mahiou, R.; et al. Metal-NHC complexes: A survey of anti-cancer properties. Dalton Trans. 2009, 6894–6902. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Tong, K.-C.; Hu, D.; Wan, P.-K.; Lok, C.-N.; Che, C.-M. Anti-cancer gold, platinum and iridium compounds with porphyrin and/or N-heterocyclic carbene ligand(s). Adv. Inorg. Chem. 2020, 75, 87–119. [Google Scholar] [CrossRef]
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. Adv. Inorg. Chem. 2020, 75, 121–148. [Google Scholar] [CrossRef]
- Marzano, C.; Gandin, V.; Folda, A.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free. Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Baker, M.V.; Barnard, P.J.; Berners-Price, S.J.; Brayshaw, S.K.; Hickey, J.L.; Skelton, B.W.; White, A.H. Synthesis and structural characterisation of linear Au(I) N-heterocyclic carbene complexes: New analogues of the Au(I) phosphine drug Auranofin. J. Organomet. Chem. 2005, 690, 5625–5635. [Google Scholar] [CrossRef]
- Boselli, L.; Ader, I.; Carraz, M.; Hemmert, C.; Cuvillier, O.; Gornitzka, H. Synthesis, structures, and selective toxicity to cancer cells of gold(I) complexes involving N-heterocyclic carbene ligands. Eur. J. Med. Chem. 2014, 85, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cucciolito, M.E.; Trinchillo, M.; Iannitti, R.; Palumbo, R.; Tesauro, D.; Tuzi, A.; Ruffo, F.; D’Amora, A. Sugar-Incorporated N-Heterocyclic-Carbene-Containing Gold(I) Complexes: Synthesis, Characterization, and Cytotoxic Evaluation. Eur. J. Inorg. Chem. 2017, 4955–4961. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; D’Amora, A.; De Feo, G.; Ferraro, G.; Giorgio, A.; Petruk, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-Coordinate Platinum(II) Compounds Containing Sugar Ligands: Synthesis, Characterization, Cytotoxic Activity, and Interaction with Biological Macromolecules. Inorg. Chem. 2018, 57, 3133–3143. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; Bossa, F.D.L.; Esposito, R.; Ferraro, G.; Iadonisi, A.; Petruk, G.; D’Elia, L.; Romanetti, C.; Traboni, S.; Tuzi, A.; et al. C-Glycosylation in platinum-based agents: A viable strategy to improve cytotoxicity and selectivity. Inorg. Chem. Front. 2018, 5, 2921–2933. [Google Scholar] [CrossRef]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Imbimbo, P.; Petruk, G.; Ferraro, G.; Pinto, V.; Tuzi, A.; Monti, D.M.; Merlino, A.; et al. A highly efficient and selective antitumor agent based on a glucoconjugated carbene platinum(II) complex. Dalton Trans. 2019, 48, 7794–7800. [Google Scholar] [CrossRef]
- Zhao, W.; Ferro, V.; Baker, M.V. Carbohydrate–N-heterocyclic carbene metal complexes: Synthesis, catalysis and biological studies. Coord. Chem. Rev. 2017, 339, 1–16. [Google Scholar] [CrossRef]
- Annunziata, A.; Amoresano, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Iacobucci, I.; Imbimbo, P.; Lucignano, R.; Melchiorre, M.; Monti, M.; et al. Pt(II) versus Pt(IV) in Carbene Glycoconjugate Antitumor Agents: Minimal Structural Variations and Great Performance Changes. Inorg. Chem. 2020, 59, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Day, D. Mitochondrial permeability transition induced by dinuclear gold(I)–carbene complexes: Potential new antimitochondrial antitumour agents. J. Inorg. Biochem. 2004, 98, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lum, C.T.; Lok, C.-N.; To, W.-P.; Low, K.-H.; Che, C.-M. A Binuclear Gold(I) Complex with Mixed Bridging Diphosphine and Bis(N-Heterocyclic Carbene) Ligands Shows Favorable Thiol Reactivity and Inhibits Tumor Growth and Angiogenesis In Vivo. Angew. Chem. Int. Ed. 2014, 53, 5810–5814. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Bellemin-Laponnaz, S.; Tubaro, C.; Basato, M.; Bogialli, S.; Dolmella, A. Synthesis and biological assays on cancer cells of dinuclear gold complexes with novel functionalised di(N-heterocyclic carbene) ligands. J. Inorg. Biochem. 2014, 141, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.B.; Bernd, M.A.; Schütz, M.; Oberkofler, J.; Pöthig, A.; Reich, R.M.; Kühn, F.E. Synthesis, characterization, and biological studies of multidentate gold(I) and gold(III) NHC complexes. Dalton Trans. 2019, 48, 16615–16625. [Google Scholar] [CrossRef]
- Barnard, P.J.; Wedlock, L.E.; Baker, M.V.; Berners-Price, S.J.; Joyce, D.A.; Skelton, B.W.; Steer, J.H. Luminescence Studies of the Intracellular Distribution of a Dinuclear Gold(I) N-Heterocyclic Carbene Complex. Angew. Chem. Int. Ed. 2006, 45, 5966–5970. [Google Scholar] [CrossRef]
- Wedlock, L.E.; Barnard, P.J.; Filipovska, A.; Skelton, B.W.; Berners-Price, S.J.; Baker, M.V. Dinuclear Au(I) N-heterocyclic carbene complexes derived from unsymmetrical azolium cyclophane salts: Potential probes for live cell imaging applications. Dalton Trans. 2016, 45, 12221–12236. [Google Scholar] [CrossRef]
- Mullick, A.B.; Chang, Y.M.; Ghiviriga, I.; Abboud, K.A.; Tan, W.; Veige, A.S. Human cancerous and healthy cell cytotoxicity studies of a chiral μ-dicarbene–digold(I) metallamacrocycle. Dalton Trans. 2013, 42, 7440. [Google Scholar] [CrossRef]
- Rieb, J.; Dominelli, B.; Mayer, D.; Jandl, C.; Drechsel, J.; Heydenreuter, W.; Sieber, S.A.; Kühn, F.E. Influence of wing-tip substituents and reaction conditions on the structure, properties and cytotoxicity of Ag(I)– and Au(I)–bis(NHC) complexes. Dalton Trans. 2017, 46, 2722–2735. [Google Scholar] [CrossRef]
- Smiataczowa, K.; Kosmalski, J.; Nowacki, A.; Czaja, M.; Warnke, Z. Proton-acceptor properties and capability for mutarotation of some glucosylamines in methanol. Carbohydr. Res. 2004, 339, 1439–1445. [Google Scholar] [CrossRef]
- Anneser, M.R.; Haslinger, S.; Pöthig, A.; Cokoja, M.; Basset, J.-M.; Kühn, F.E. Synthesis and Characterization of an Iron Complex Bearing a Cyclic Tetra-N-heterocyclic Carbene Ligand: An Artificial Heme Analogue? Inorg. Chem. 2015, 54, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Bortolamiol, E.; Rizzolio, F.; Demitri, N.; Visentin, F. Allyl palladium complexes bearing carbohydrate-based N-heterocyclic carbenes: Anticancer agents for selective and potent in vitro cytotoxicity. Appl. Organomet. Chem. 2020, e5876. [Google Scholar] [CrossRef]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Baron, M.; Battistel, E.; Tubaro, C.; Biffis, A.; Armelao, L.; Rancan, M.; Graiff, C. Single-Step Synthesis of Dinuclear Neutral Gold(I) Complexes with Bridging Di(N-heterocyclic carbene) Ligands and Their Catalytic Performance in Cross Coupling Reactions and Alkyne Hydroamination. Organometallics 2018, 37, 4213–4223. [Google Scholar] [CrossRef]
- Collado, A.; Suárez, A.G.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward synthesis of [Au(NHC)X] (NHC = N-heterocyclic carbene, X = Cl, Br, I) complexes. Chem. Commun. 2013, 49, 5541–5543. [Google Scholar] [CrossRef]
- Gil Rubio, J.; Cámara, V.; Bautista, D.; Vicente, J. Dinuclear Alkynyl Gold(I) Complexes Containing Bridging N-Heterocyclic Dicarbene Ligands: New Synthetic Routes and Luminescence. Organometallics 2012, 31, 5414–5426. [Google Scholar] [CrossRef]
- Monticelli, M.; Baron, M.; Tubaro, C.; Bellemin-Laponnaz, S.; Graiff, C.; Bottaro, G.; Armelao, L.; Orian, L. Structural and Luminescent Properties of Homoleptic Silver(I), Gold(I), and Palladium(II) Complexes with nNHC-tzNHC Heteroditopic Carbene Ligands. ACS Omega 2019, 4, 4192–4205. [Google Scholar] [CrossRef]
- Longhi, A.; Baron, M.; Rancan, M.; Bottaro, G.; Armelao, L.; Sgarbossa, P.; Tubaro, C. Possible Synthetic Approaches for Heterobimetallic Complexes by Using nNHC/tzNHC Heteroditopic Carbene Ligands. Molecules 2019, 24, 2305. [Google Scholar] [CrossRef]
- Baron, M.; Tubaro, C.; Biffis, A.; Basato, M.; Graiff, C.; Poater, A.; Cavallo, L.; Armaroli, N.; Accorsi, G. Blue-Emitting Dinuclear N-heterocyclic Dicarbene Gold(I) Complex Featuring a Nearly Unit Quantum Yield. Inorg. Chem. 2012, 51, 1778–1784. [Google Scholar] [CrossRef]
- Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Skelton, B.W.; White, A.H. Dinuclear gold(I) complexes of bridging bidentate carbene ligands: Synthesis, structure and spectroscopic characterisation. Dalton Trans. 2004, 1038–1047. [Google Scholar] [CrossRef]
- Tubaro, C.; Baron, M.; Costante, M.; Basato, M.; Biffis, A.; Gennaro, A.; Isse, A.A.; Graiff, C.; Accorsi, G. Dinuclear gold(I) complexes with propylene bridged N-heterocyclic dicarbene ligands: Synthesis, structures, and trends in reactivities and properties. Dalton Trans. 2013, 42, 10952–10963. [Google Scholar] [CrossRef] [PubMed]
- Hemmert, C.; Poteau, R.; Dominique, F.J.-B.D.; Ceroni, P.; Bergamini, G.; Gornitzka, H. Amide-Functionalized Bis(NHC) Systems: Anion Effect on Gold-Gold Interactions. Eur. J. Inorg. Chem. 2012, 3892–3898. [Google Scholar] [CrossRef]
- Cure, J.; Poteau, R.; Gerber, I.C.; Gornitzka, H.; Hemmert, C. Dimeric Gold Bis(carbene) Complexes by Transmetalation in Water. Organometallics 2012, 31, 619–626. [Google Scholar] [CrossRef]
- Baron, M.; Tubaro, C.; Basato, M.; Isse, A.A.; Gennaro, A.; Cavallo, L.; Graiff, C.; Dolmella, A.; Falivene, L.; Caporaso, L. Insights into the Halogen Oxidative Addition Reaction to Dinuclear Gold(I) Di(NHC) Complexes. Chem. - A Eur. J. 2016, 22, 10211–10224. [Google Scholar] [CrossRef] [PubMed]
- De Frémont, P.; Singh, R.; Stevens, E.D.; Petersen, J.L.; Nolan, S.P. Synthesis, Characterization and Reactivity of N-Heterocyclic Carbene Gold(III) Complexes. Organometallics 2007, 26, 1376–1385. [Google Scholar] [CrossRef]
- Baron, M.; Tubaro, C.; Basato, M.; Biffis, A.; Natile, M.M.; Graiff, C. Dinuclear N-Heterocyclic Dicarbene Gold Complexes in I–III and III–III Oxidation States: Synthesis and Structural Analysis. Organometallics 2011, 30, 4607–4615. [Google Scholar] [CrossRef]
- Zhang, C.; Hemmert, C.; Gornitzka, H.; Cuvillier, O.; Zhang, M.; Sun, R.W. Cationic and Neutral N -Heterocyclic Carbene Gold(I) Complexes: Cytotoxicity, NCI-60 Screening, Cellular Uptake, Inhibition of Mammalian Thioredoxin Reductase, and Reactive Oxygen Species Formation. ChemMedChem 2018, 13, 1218–1229. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Barnard, P.J.; Berners-Price, S.J. Targeting the mitochondrial cell death pathway with gold compounds. Co-ord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, J.; Xie, L.; Du, F.; Xu, G.; Xie, Y.; Ling, Q. Synthesis of Chiral Imidazolium Salts from a Carbohydrate and Their Application in Pd-Catalyzed Suzuki–Miyaura Reaction. Catal. Lett. 2014, 144, 1911–1918. [Google Scholar] [CrossRef]
- Salomon, M.F.; Salomon, R.G. The peroxide transfer reaction. J. Am. Chem. Soc. 1979, 101, 4290–4299. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).