Abstract
The cooperative binding behavior of a face-directed octahedral metal-organic supercontainer featuring one endo cavity and six exo cavities was thoroughly examined in chloroform solution through ultraviolet-visible (UV-Vis) titration technique using two representative drug molecules as the guests. The titration curves and their nonlinear fit to Hill equation strongly suggest the efficient encapsulation of the guest molecules by the synthetic host, which exhibit interesting cooperative and stepwise binding behavior. Based on the control experiments using tetranuclear complex as a reference, it is clear that two equivalents of the guest molecules are initially encapsulated inside the endo cavity, followed by the trapping of six additional equivalents of the drug molecules through six exo cavities (1 eq. per exo cavity), and the remaining guests are entrapped by the external pockets. The results provide an in-depth understanding of the cooperative binding behavior of metal-organic supercontainers, which opens up new opportunities for designing synthetic receptors for truly biomimetic functional applications.
1. Introduction
Binding cooperativity effects are a well-documented phenomenon and an essential principle prevalent in the fields of biology and supramolecular chemistry [1,2,3,4,5,6,7]. Well-known examples include allosteric binding of oxygen by hemoglobin in biology and metal ions chelation process by ethylenediaminetetraacetic acid (EDTA) in chemistry. Cooperative binding has been proven to be a very important mechanism that regulates molecular recognition behavior [2,3] and self-assembly processes [4,8,9] in biology and supramolecular chemistry. To date, a cooperative effect has been widely explored in biologic systems, and different basic principles have been developed to understand a wide range of biological phenomena [2]. However, cooperative modulation of macromolecular assemblies and guest binding behaviors in synthetic supramolecular systems remains poorly illustrated and understood.
Over the years, considerable attention has been paid to coordination containers possessing a well-defined hollow space that is suitable for guest encapsulation and allows them to be widely applied in a number of applications, such as guest recognition [10,11,12], drug delivery [13,14], and supramolecular catalysis [15,16]. A new class of coordination containers, namely, metal-organic supercontainers (MOSCs), incorporating macrocyclic container thia- or sulfonyl- calix[4]arenes as precursors [17], have been well demonstrated by us and others [18,19,20,21,22,23,24,25,26,27,28]. The MOSCs are demonstrated to possess several unique features such as their modular synthesis, robust architecture, and multiple binding domains (containing both endo and exo cavities). The unique multicavity structures allow MOSCs to be promising in various applications including guest regulation [21,22,23] and electrocatalysis [24,29]. Although encapsulation of multiple guests by MOSCs through their multiple binding domains has been demonstrated [20], the exact mechanism of binding cooperativity and/or binding regulation of those synthetic receptors remains unclear.
In the present study, we report a systematic study of the cooperative binding behavior of a biomimetic MOSC by using the two well-known gastric proton pump inhibitors, (R)-(+)-rabeprazole sodium and (S)-(−)-pantoprazole sodium, as representative guests. The cooperative binding profiles of the guests by the MOSC were carefully examined through ultraviolet-visible (UV-Vis) spectroscopic titration technique, which clearly revealed that stepwise encapsulation of the guests occurred sequentially, first within the endo cavity, then the exo cavities, and finally the external pockets of the MOSC. Each of these steps featured different degrees of binding affinity and cooperativity. Our results thus illustrate a rare example of stepwise, cooperative binding, and provide an in-depth understanding of such unique binding properties of a biomimetic receptor molecule that features multiple binding domains.
2. Results and Discussion
The metal-organic supercontainer MOSC-1-Co18, labelled here as H1, is constructed from six tetranuclear complex units [30] connected by eight units of the 1,3,5-benzenetricarboxylate (BTC) linker (Scheme 1). It features a dual-pore architecture containing two types of well-defined cavities: an endo cavity (with an inner diameter of ca. 1.4 nm and an estimated internal volume of 0.55 nm3) and six open-ended exo cavities (with an opening of ca. 0.8 nm × 0.8 nm and a depth of ca. 0.7 nm) (Figure 1). There are also eight external pockets defined by the BTC linker and three adjacent TBSC units. The multiple binding domains make the H1 an intriguing and effective synthetic receptor for investigating efficient guest regulation and/or encapsulation, as well as binding cooperativity. To elucidate the guest binding behavior of supercontainer H1, the host–guest interaction profiles in solutions were carefully examined using the UV-Vis titration technique [31].31 Two drug molecules, namely, (R)-(+)-rabeprazole sodium (D1) and (S)-(−)-pantoprazole sodium (D2) (Figure 1), were selected as the guests. The synthetic precursor of H1, that is, a tetranuclear complex, labelled as H2, which mimics the structure of the exo cavities of H1, was chosen as a control host molecule to simulate the binding behavior of the exo cavities of the H1.
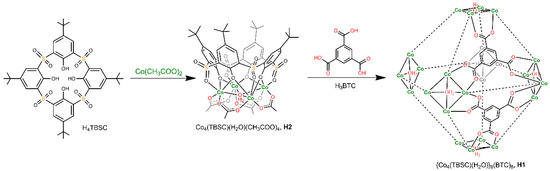
Scheme 1.
The self-assembly of host molecules H118 and H230 from p-tert-butylsulfonylcalix[4]arene (H4TBSC); the TBSC units in H1 molecule are omitted for clarity.
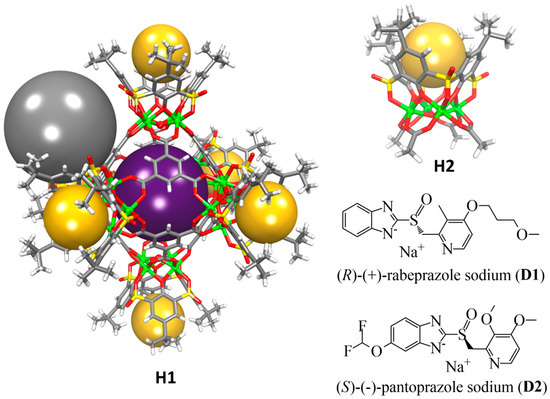
Figure 1.
The crystal structures of host molecules (H1 and H2) based on the CIF files reported in the original literature [18,30] and chemical structure diagrams of guest molecules (D1 and D2) used in this study. The spheres serve to guide the eyes representing the endo cavity (purple), six exo cavities (yellow), and one of the eight external pockets (gray).
The absorption spectrum of H1 in CHCl3 features an intense and characteristic absorption band centered at 350 nm (Figure 2), ascribable to the combination of π→π* transitions of organic ligands and intramolecular charge transfer (ICT) involving the tetranuclear units [18,20,21]. As shown in Figure 3a, upon gradual addition of D1 to H1 solution in CHCl3, the intensity of the maximum absorption at 350 nm increases gradually along with the appearance of a shoulder centered at 370 nm. The changes observed in the absorption spectra during the titration indicated the encapsulation of D1 guest molecules within the H1.
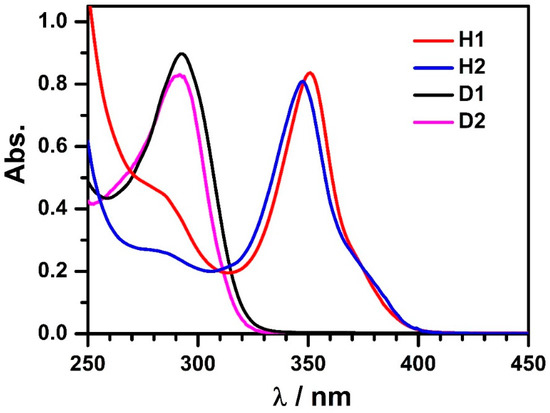
Figure 2.
The ultraviolet-visible (UV-Vis) spectra of free hosts (H1 and H2) and guests (D1 and D2) in chloroform solution.
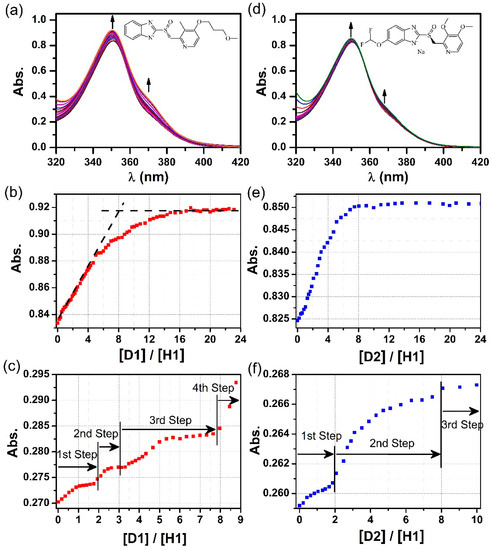
Figure 3.
(a) UV-Vis spectra of host H1 upon titration with D1, and plots of D1/H1 molar ratio vs. absorbance at (b) 350 nm and (c) 370 nm based on the titration experiment. (d) UV-Vis spectra of host H1 upon titration with D2, and plots of D2/H1 molar ratio vs. absorbance at (e) 350 nm and (f) 370 nm based on the titration experiment.
A plot of absorbance at 350 nm vs. D1 equivalents (Figure 3b) exhibits a characteristic sigmoidal kinetic profile typically observed in the binding of substrates by enzymes or receptors containing multiple binding sites [20,32]. At low guest concentrations, the D1 molecules added bind strongly to the H1, causing an initially flat and then sudden increase of the absorbance intensity. As the D1 equivalents continue to increase, binding of D1 guest to H1 appears to become weaker, leading to a deviation of the titration curve from the initial tangent and a much more slow and gradual increase of the absorbance until reaching a saturation plateau. The capability of D1 binding with H1 can be estimated to be ~8 equivalents according to the intersection point of the initial tangent and the asymptote.
Notably, a plot of the absorbance at 370 nm vs [D1]/[H1] ratio displays a rare profile of well-defined multiple incremental steps, indicating sequential and cooperative host–guest interactions (Figure 3c). The stepwise increases in absorbance are observed in the [D1]/[H1] ranges of 0–2, 2–3, 3–8, and 8–23, respectively, which is ascribable to the stepwise encapsulation of D1 guest molecules within different binding domains of the H1 host, including the endo-cavity, exo-cavities, and the external pockets.
The binding behavior of the D2 guest with H1 was similarly investigated by the UV-Vis titration (Figure 3d–f). Similar sigmoidal kinetic profile was observed via a plot of absorbance at 350 nm vs. [D2]/[H1], but with a faster increase in the absorbance as the D2 concentration increases at lower guest loadings. In the meanwhile, only three incremental steps in the [D2]/[H1] ranges of 0–2, 2–8, and 8–23 were observed. It is plausible that the smaller size of guest molecule (for example, the D2 molecule bears a shorter side chain relative to D1) is beneficial to enhance the binding kinetic of guest encapsulation.
In order to understand how the guest molecules bind to the H1 host, control experiments employing the tetranuclear complex H2 as the host were carried out under similar UV-Vis titration conditions (Figure 4). The binding of the D1 guest molecules with H2 is confirmed by the red shift of the H2 absorption maxima from 347 nm to 360 nm upon gradual increase of the guest equivalents. The binding stoichiometry of D1 or D2 with H2 is estimated to be ~1 as evidenced by the plots of absorbance vs. guest equivalents. This suggests that each exo cavity of the H1 host tends to accommodate one molecule of the D1 or D2 guest. Therefore, it is plausible that the H1 encapsulates six molecules of D1 or D2 through its six exo cavities, and two molecules of D1 or D2 within its endo cavity, while the remaining guest molecules are likely aggregating around the external triangular pockets.
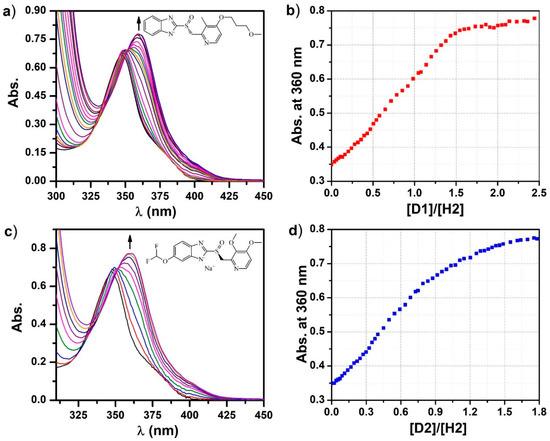
Figure 4.
UV-Vis spectra of host H2 upon titration with (a) D1 and (c) D2, and plots of (b) D1/H2 and (d) D2/H2 molar ratio vs. absorbance at 360 nm based on the titration experiment.
Finally, the UV-Vis titration data was further analyzed using the well-known Hill equation33 in order to quantitatively understand the guest binding cooperativity. The binding association constants and the Hill coefficient values based on the nonlinear fit are listed in Table 1. The Hill equation is widely utilized for estimating the degree of cooperativity of the guest(s) binding to the receptor. The value of Hill coefficient provides a means to quantify the extent of cooperativity among multiple ligand binding stites. A Hill coefficient of one suggests independent binding, while the value different from one indicates multiple ligand binding corresponding to negatively (n < 1) or positively (n > 1) cooperative binding. As shown in Figure 5, the overall apparent association constants of guest molecules binding with H1 were calculated to be 3.58 × 104 M−1 and 6.69 × 104 M−1 for D1 and D2, respectively, compared with the corresponding values (4.11 × 104 M−1 for D1 and 5.55 × 104 M−1 for D2) observed in the tetranuclear host H2 (Figure 6). Taking into account the given Hill coefficient value (n > 1), the results suggested the positively cooperative and relatively strong overall binding between the host and guest [20,23].

Table 1.
Association constant and Hill coefficient determined by the nonlinear fit of the titration data to Hill equation.
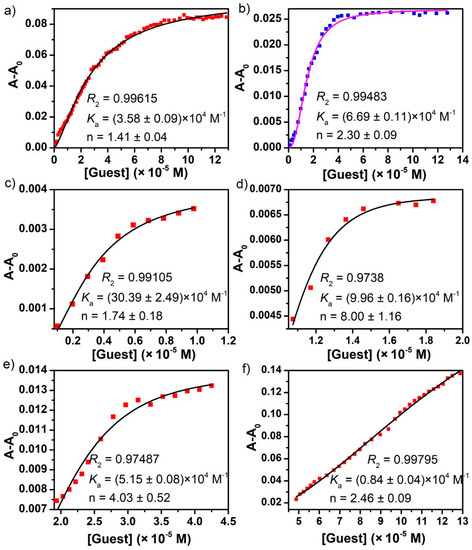
Figure 5.
The nonlinear fits to Hill equation of UV-Vis titration experiments based on the absorption band centered at 350 nm for (a) D1 ≅ H1 and (b) D2 ≅ H1, and at 370 nm in the [D1]/[H1] ranges of (c) 0−2, (d) 2−3, (e) 3−8, and (f) 8−23.
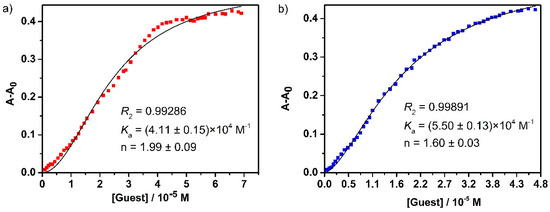
Figure 6.
The nonlinear fits to Hill equation of UV-Vis titration experiments based on the absorption band centered at 360 nm for (a) D1 ≅ H2 and (b) D2 ≅ H2.
It is worth noting that the stepwise guest encapsulation process revealed remarkable differences in the binding affinity of individual steps. The strongest binding affinity (i.e., 3.04 × 105 M−1 for D1 or 2.81 × 105 M−1 for D2) was established in the first stage of the guest binding process involving two molecules of the guest, attributed to the encapsulation of the guest molecules inside the circumscribed endo cavity of H1 through π···π interaction and hydrogen bonding between the host and guest molecules. A medium association constant found in the range of 5.15 × 104–9.60 × 104 M−1 in the following stage clearly suggested the entrapment of six molecules of guest by the six open, bowl-shaped exo cavites of H1 through hydrogen bonding and hydrophobic interaction. Additional binding of the guest by the external pockets was supported by the weakest binding constant (i.e., 0.84 × 104 M−1 for D1 or 1.69 × 104 M−1 for D2); however, the exact binding stoichiometry could not be determined, likely due to the fast exchange with the free guests.
3. Materials and Methods
3.1. General Information
Starting materials and solvents were obtained from commercial suppliers (Fisher Scientific, TCI, Alfa Aesar, etc.) and used without further purification. The metal-organic supercontainer H1 [18] and a related reference compound, known as tetranuclear complex H2 [30], were synthesized as described in the literature. The host materials were dried on a Schlenk line under vacuum at 120 °C for 4 h. UV-Vis absorption spectra were collected on a Perkin–Elmer Lambda 35 UV-Vis spectrophotometer at room temperature.
3.2. Solution UV-Vis Titration Experiments
Stock solutions of the hosts (H1 and H2), called the titrand, were prepared in CHCl3 at a concentration of ~5 × 10−6 M. 25.00 mL of the host’s stock solution was then used to dissolve an accurately known mass of guest molecule, (R)-(+)-rabeprazole sodium (D1) or (S)-(−)-pantoprazole sodium (D2), called the titrant, wherein the guest concentration was 50~100 times greater than that of the host. 2.00 mL of the host solution (the titrand) was placed in a 10.0 mm quartz cell, upon which 0.01 to 2 mL of the titrant was added gradually using a syringe. After each addition, the cell was stoppered and inverted, and the UV-Vis spectrum was collected (at 25 °C) after 3 min to ensure complete mixing and reaching equilibration.
3.3. Calculation of Binding Constants from UV-Vis Titration Data
In order to evaluate the overall binding strength, the titration results were fitted to the nonlinear form of Hill equation [33,34].
where ΔA (= Aobs − A0) is the change in absorbance, ΔAmax is the maximum change of absorbance, [L]0 is initial guest concentration, n is the Hill coefficient, and Ka is the association constant. A plot of against [L]0 can be used to estimate ΔAmax and Ka. The titration data were fit to this model using the nonlinear regression method within the Origin 9 software.
4. Conclusions
The guest binding behaviour of metal-organic supercontainer H1 with two drug molecules, D1 and D2, was investigated in chloroform solution using the UV-Vis titration technique. The results revealed highly intriguing cooperative and stepwise binding of the drug molecules with the multiple binding domains of H1. Taking into account the control experiments with the H1 replaced by the tetranuclear complex H2, which represents the exo cavities of H1, it is suggested that the guest molecules were likely encapsulated sequentially by the different cavities of H1: two equivalents of the guest molecule were encapsulated inside the endo cavity of H1, followed by the subsequent entrapment of six molecules of the guest in the six exo cavities of H1, and finally, immobilization of additional guest molecules by the external pockets of H1 was evidenced. The present study affords a thorough understanding of the cooperative binding behavior of metal-organic supercontainers featuring multiple binding domains, thus facilitating their potential biological applications such as drug delivery. We are currently addressing exciting opportunities along these lines.
Author Contributions
Data curation, T.-P.S.; Formal analysis, G.-Z.Z. and F.-R.D.; Funding acquisition, F.-R.D. and Z.-N.C.; Investigation, T.-P.S. and X.-X.F.; Project administration, G.-Z.Z., F.-R.D., and Z.-N.C.; Supervision, F.-R.D. and Z.-N.C.; Validation, T.-P.S.; Visualization, F.-R.D.; Writing—original draft, T.-P.S. and F.-R.D.; Writing—review and editing, F.-R.D. and Z.-N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (21673239, 21501179 and 21531008) and Natural Science Foundation of Fujian Province (2017J06008), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB20000000).
Acknowledgments
We thank Zhenqiang Wang (USD) for helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fersht, A. Structure and Mechanism in Protein Science; W. H. Freeman and Company: New York, NY, USA, 1999. [Google Scholar]
- Whitty, A. Cooperativity and biological complexity. Nat. Chem. Biol. 2008, 4, 435–439. [Google Scholar] [CrossRef]
- Williamson, J.R. Cooperativity in macromolecular assembly. Nat. Chem. Biol. 2008, 4, 458–465. [Google Scholar] [CrossRef]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem. Int. Ed. 2015, 6400–6441. [Google Scholar] [CrossRef]
- Ercolani, G.; Schiaffino, L. Allosteric, Chelate, and Interannular Cooperativity: A Mise au Point. Angew. Chem. Int. Ed. 2011, 50, 1762–1768. [Google Scholar] [CrossRef]
- Hunter, C.A.; Anderson, H.L. What is Cooperativity? Angew. Chem. Int. Ed. 2009, 48, 7488–7499. [Google Scholar] [CrossRef]
- Cui, Q.; Karplus, M. Allostery and cooperativity revisited. Protein Sci. A Publ. Protein Soc. 2008, 17, 1295–1307. [Google Scholar] [CrossRef]
- Badjic, J.D.; Nelson, A.; Cantrill, S.J.; Turnbull, W.B.; Stoddart, J.F. Multivalency and cooperativity in supramolecular chemistry. Acc. Chem. Res. 2005, 38, 723–732. [Google Scholar] [CrossRef]
- Thordarson, P.; Coumans, R.G.E.; Elemans, J.A.A.W.; Thomassen, P.J.; Visser, J.; Rowan, A.E.; Nolte, R.J.M. Allosterically driven multicomponent assembly. Angew. Chem. Int. Ed. 2004, 43, 4755–4759. [Google Scholar] [CrossRef]
- Mendez-Arroyo, J.; Barroso-Flores, J.; Lifschitz, A.M.; Sarjeant, A.A.; Stern, C.L.; Mirkin, C.A. A multi-state, allosterically-regulated molecular receptor with switchable selectivity. J. Am. Chem. Soc. 2014, 136, 10340–10348. [Google Scholar] [CrossRef]
- Mirtschin, S.; Slabon-Turski, A.; Scopelliti, R.; Velders, A.H.; Severin, K. A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc. 2010, 132, 14004–14005. [Google Scholar] [CrossRef]
- Ronson, T.K.; Giri, C.; Beyeh, N.K.; Minkkinen, A.; Topic, F.; Holstein, J.J.; Rissanen, K.; Nitschke, J.R. Size-selective encapsulation of hydrophobic guests by self-assembled M4L6 cobalt and nickel cages. Chem. Eur. J. 2013, 19, 3374–3382. [Google Scholar] [CrossRef]
- Therrien, B. Drug Delivery by Water-Soluble Organometallic Cages. Top. Curr. Chem. 2012, 319, 35–55. [Google Scholar]
- Du, S.; Yu, T.-Q.; Liao, W.; Hu, C. Structure modeling, synthesis and X-ray diffraction determination of an extra-large calixarene-based coordination cage and its application in drug delivery. Dalton Trans. 2015, 44, 14394–14402. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. Supramolecular nanoreactors for catalysis. Coord. Chem. Rev. 2016, 324, 106. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Tamura, M.; Fujita, M. Diels-alder in aqueous molecular hosts: Unusual regioselectivity and efficient catalysis. Science 2006, 312, 251–254. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef]
- Dai, F.-R.; Wang, Z. Modular Assembly of Metal–Organic Supercontainers Incorporating Sulfonylcalixarenes. J. Am. Chem. Soc. 2012, 134, 8002–8005. [Google Scholar] [CrossRef]
- Netzer, N.L.; Dai, F.-R.; Wang, Z.; Jiang, C. pH-Modulated Molecular Assemblies and Surface Properties of Metal–Organic Supercontainers at the Air–Water Interface. Angew. Chem. Int. Ed. 2014, 53, 10965–10969. [Google Scholar] [CrossRef]
- Dai, F.-R.; Sambasivam, U.; Hammerstrom, A.J.; Wang, Z. Synthetic Supercontainers Exhibit Distinct Solution versus Solid State Guest-Binding Behavior. J. Am. Chem. Soc. 2014, 136, 7480–7491. [Google Scholar] [CrossRef]
- Dai, F.-R.; Becht, D.C.; Wang, Z. Modulating guest binding in sulfonylcalixarene-based metal-organic supercontainers. Chem. Commun. 2014, 50, 5385–5387. [Google Scholar] [CrossRef]
- Dai, F.-R.; Qiao, Y.; Wang, Z. Designing structurally tunable and functionally versatile synthetic supercontainers. Inorg. Chem. Front. 2016, 3, 243–249. [Google Scholar] [CrossRef]
- Bhuvaneswari, N.; Dai, F.-R.; Chen, Z.N. Sensitive and Specific Guest Recognition through Pyridinium-Modification in Spindle-Like Coordination Containers. Chem. A Eur. J. 2018, 24, 6580–6585. [Google Scholar] [CrossRef]
- Bhuvaneswari, N.; Annamalai, K.P.; Dai, F.-R.; Chen, Z.-N. Pyridinium functionalized coordination containers as highly efficient electrocatalysts for sustainable oxygen evolution. J. Mater. Chem. A 2017, 5, 23559–23565. [Google Scholar] [CrossRef]
- Cheng, L.-J.; Fan, X.-X.; Li, Y.-P.; Wei, Q.-H.; Dai, F.-R.; Chen, Z.-N.; Wang, Z. Engineering solid-state porosity of synthetic supercontainers via modification of exo-cavities. Inorg. Chem. Commun. 2017, 78, 61–64. [Google Scholar] [CrossRef]
- Bi, Y.; Du, S.; Liao, W. Thiacalixarene-based nanoscale polyhedral coordination cages. Coord. Chem. Rev. 2014, 276, 61–72. [Google Scholar] [CrossRef]
- Liu, M.; Liao, W.; Hu, C.; Du, S.; Zhang, H. Calixarene-Based Nanoscale Coordination Cages. Angew. Chem. Int. Ed. 2012, 51, 1585–1588. [Google Scholar] [CrossRef]
- Xiong, K.; Jiang, F.; Gai, Y.; Yuan, D.; Chen, L.; Wu, M.; Su, K.; Hong, M. Truncated octahedral coordination cage incorporating six tetranuclear-metal building blocks and twelve linear edges. Chem. Sci. 2012, 3, 2321–2325. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Hang, X.; Zhu, X.; Han, H.; Liao, W.; Chen, W. Ultrafine Pt Nanoclusters Confined in a Calixarene-Based {Ni24} Coordination Cage for High-Efficient Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2016, 138, 16236–16239. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kobashi, T.; Shinagawa, R.; Ito, T.; Takaishi, S.; Yamashita, M.; Iki, N. Highly symmetrical tetranuclear cluster complexes supported by p-tert-butylsulfonylcalix[4]arene as a cluster-forming ligand. Eur. J. Inorg. Chem. 2006, 9, 1765–1770. [Google Scholar] [CrossRef]
- Schalley, C.A. Analytical Methods in Supramolecular Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bisswanger, H. Enzyme Kinetics: Principles and Methods, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hill, A.V. The possible effects of the aggregation of the molecules of hæmoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Chenprakhon, P.; Sucharitakul, J.; Panijpan, B.; Chaiyen, P. Measuring Binding Affinity of Protein-Ligand Interaction Using Spectrophotometry: Binding of Neutral Red to Riboflavin-Binding Protein. J. Chem. Educ. 2010, 87, 829–831. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds H1 and H2 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).