Structural and Photophysical Properties of 2,1,3-Benzothiadiazole-Based Phosph(III)azane and Its Complexes
Abstract
1. Introduction
2. Results and Discussion
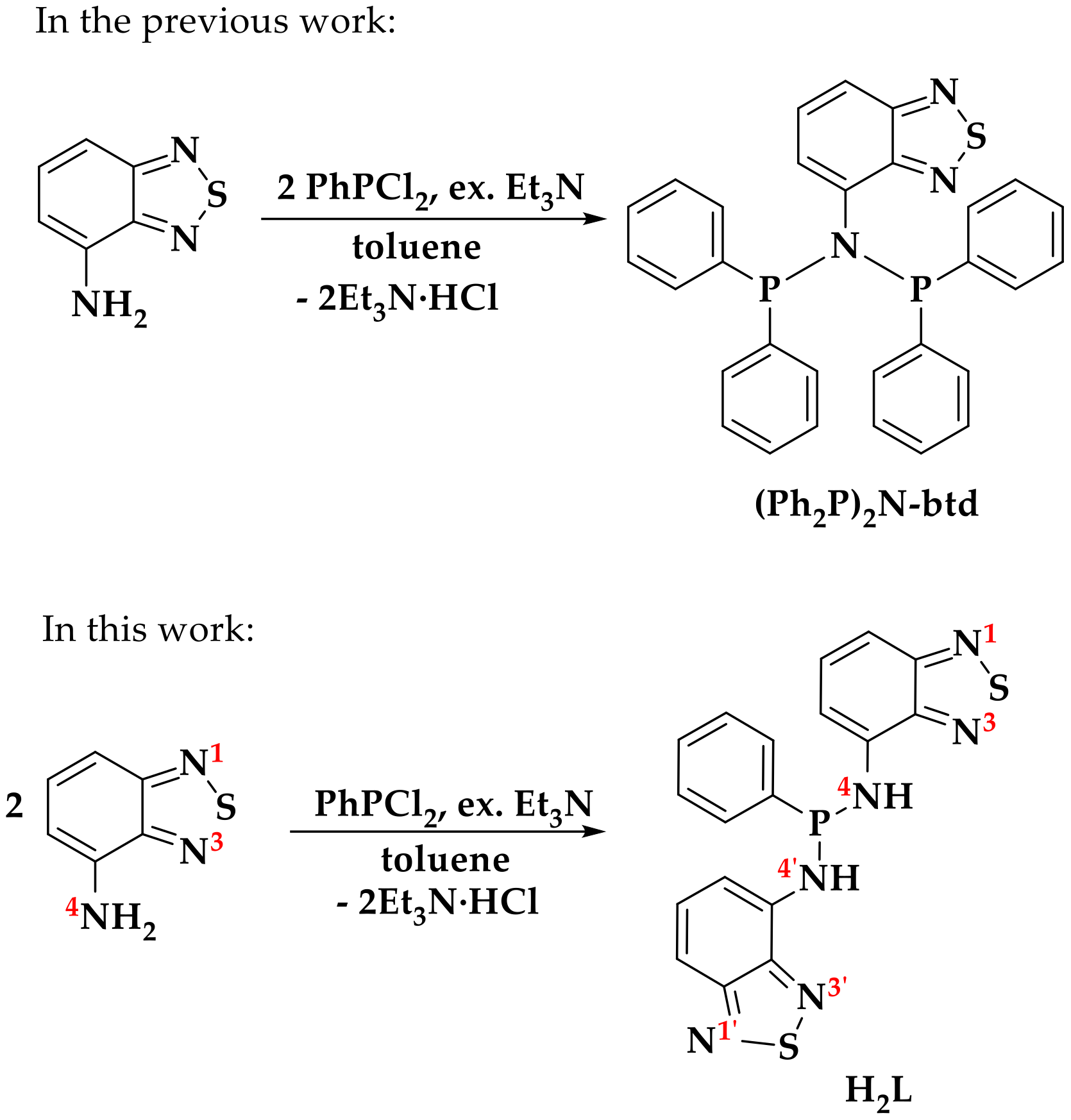
2.1. Synthesis
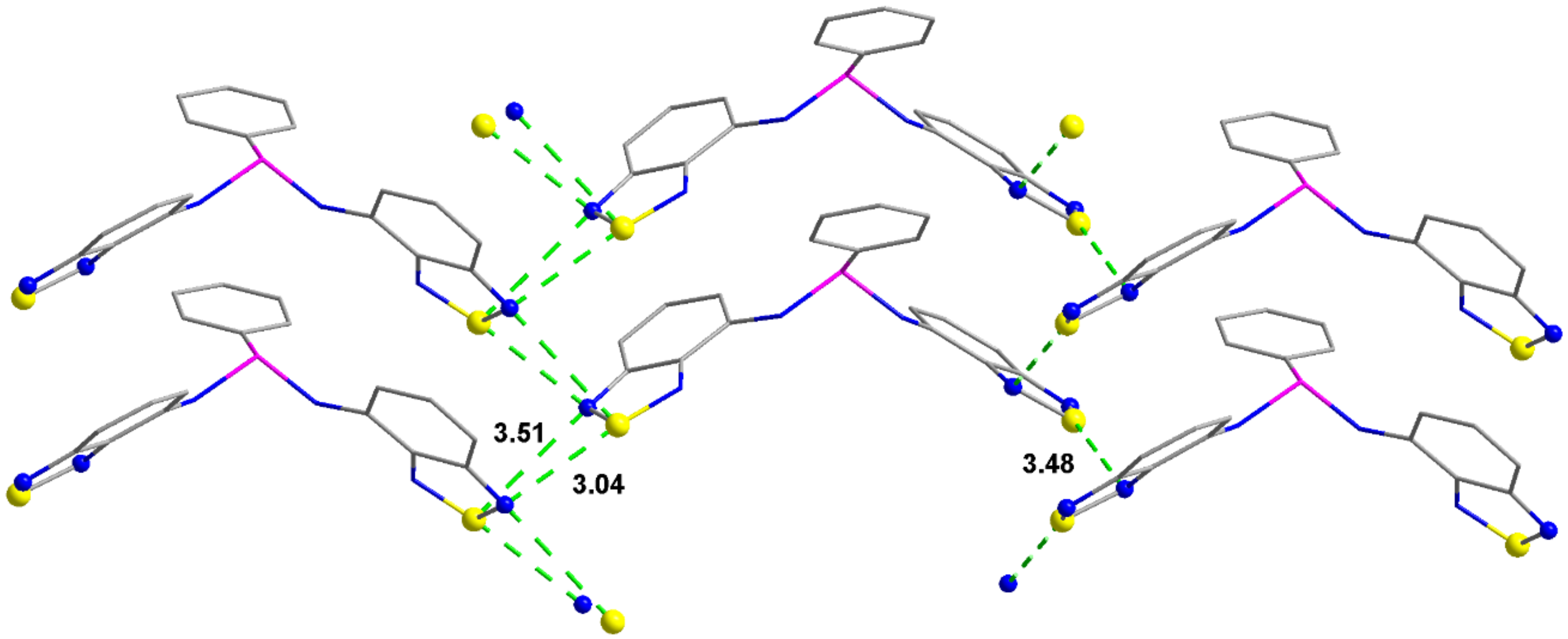
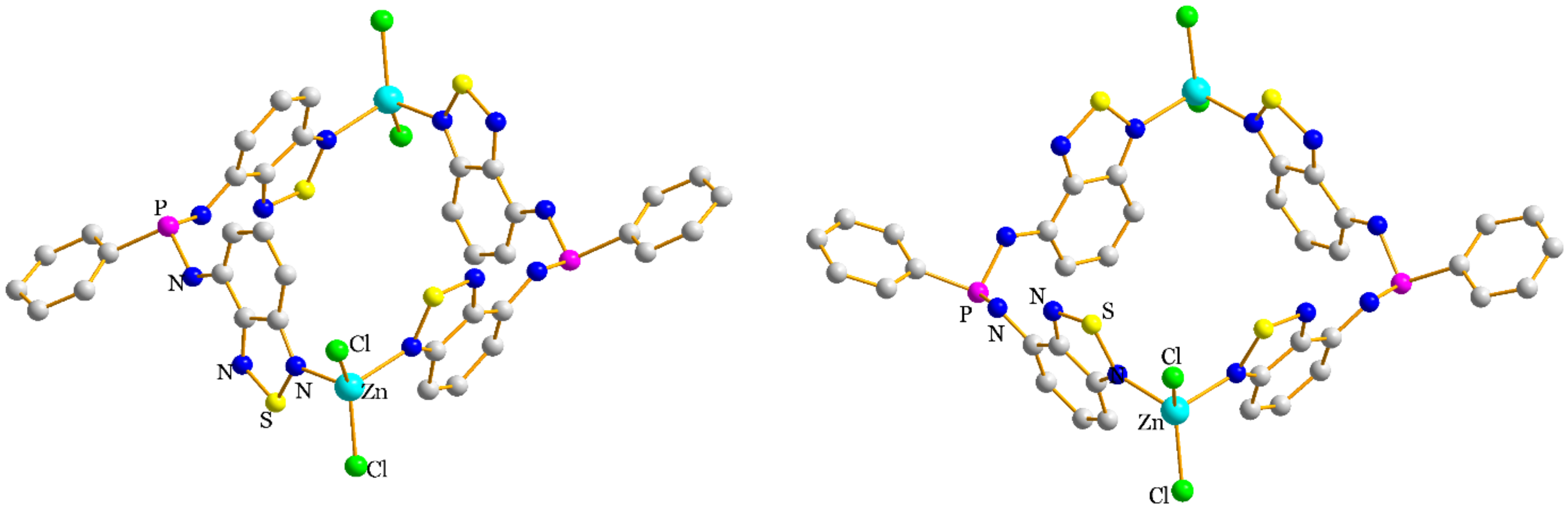
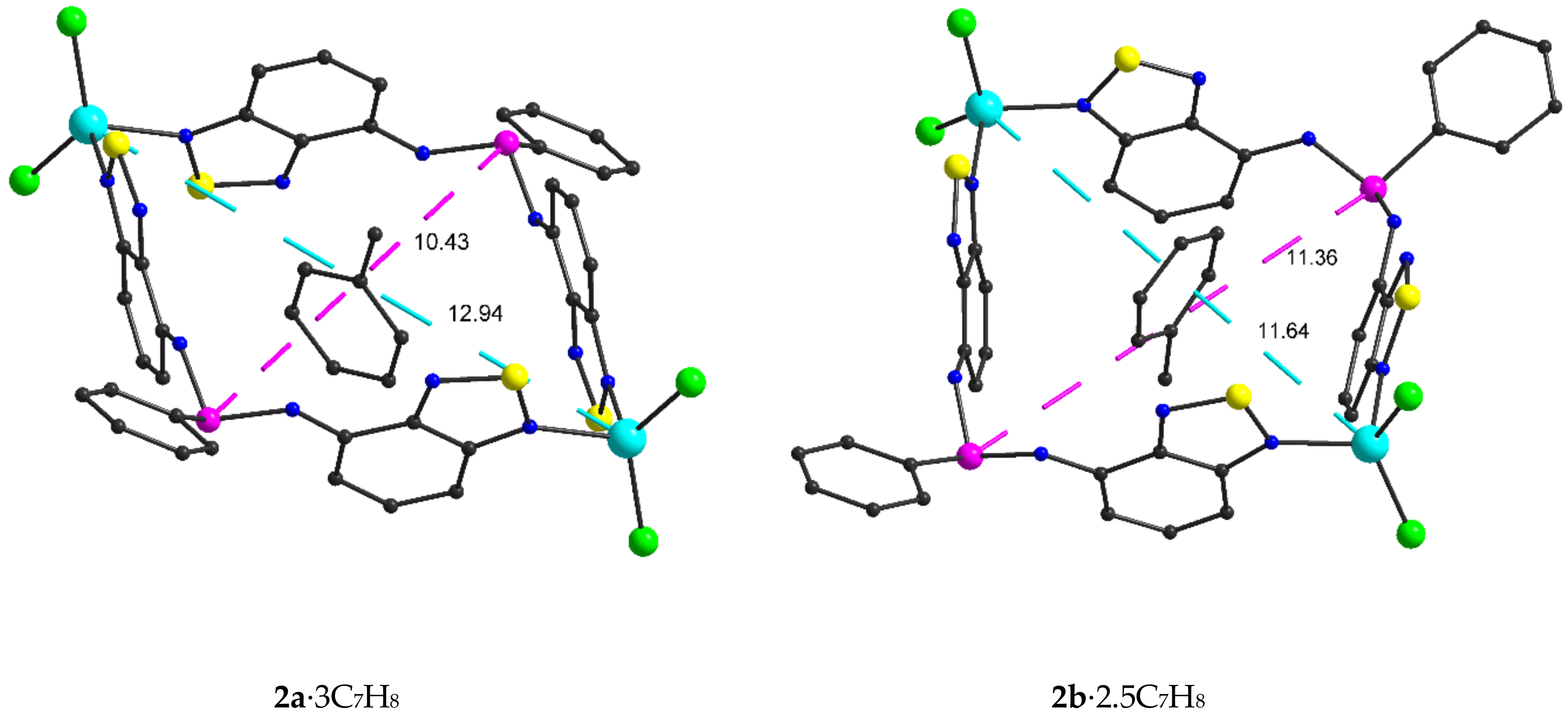
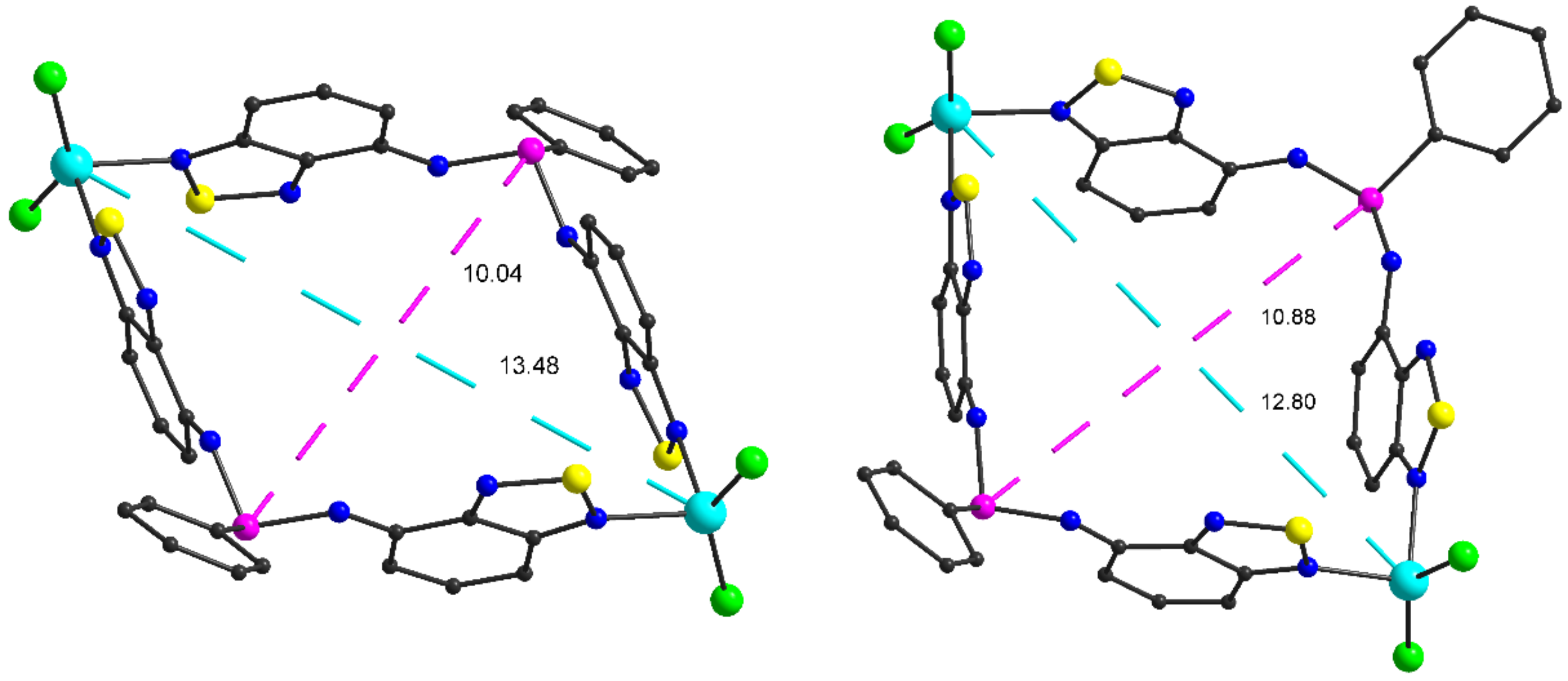
2.2. Structural Characterization
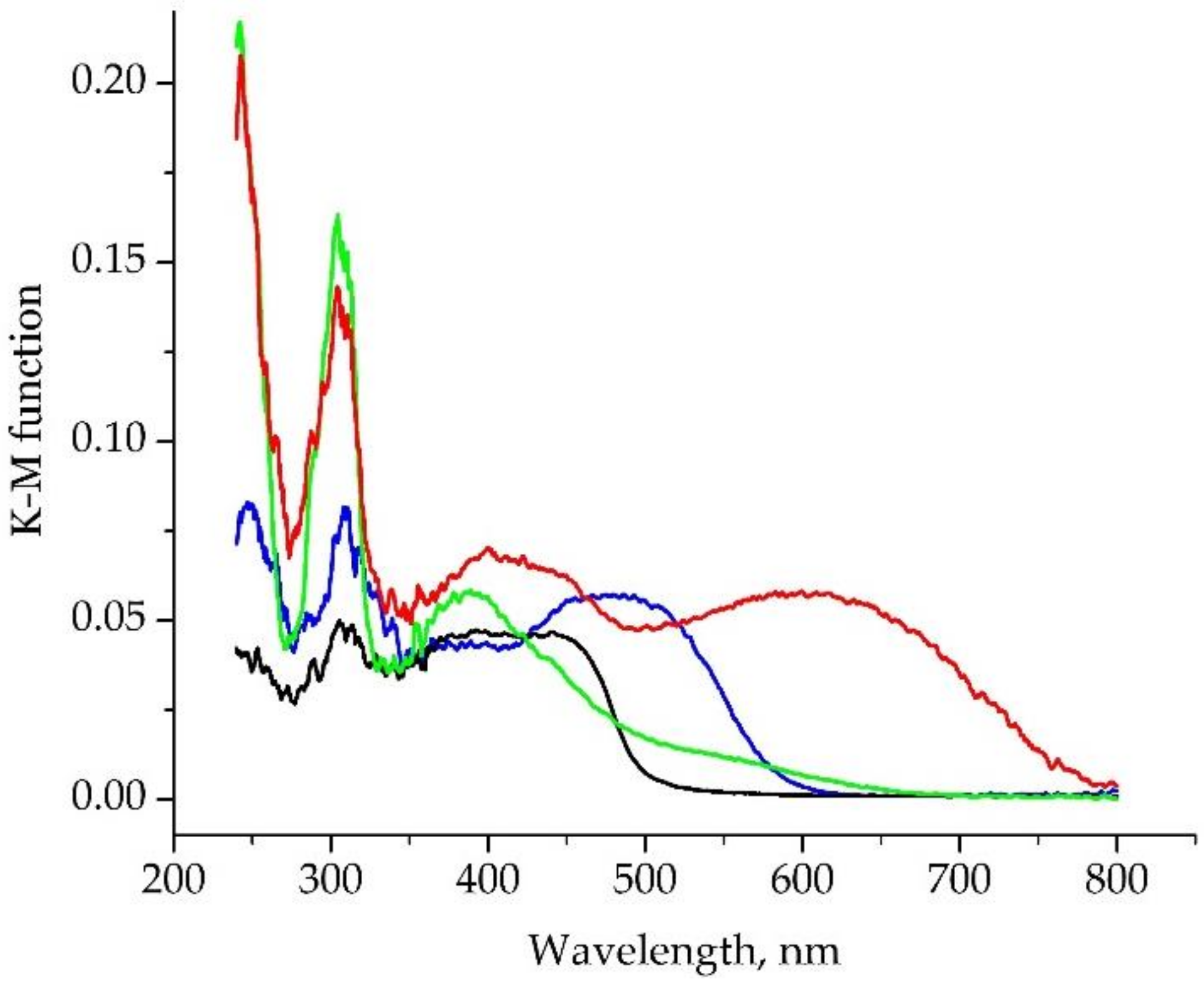
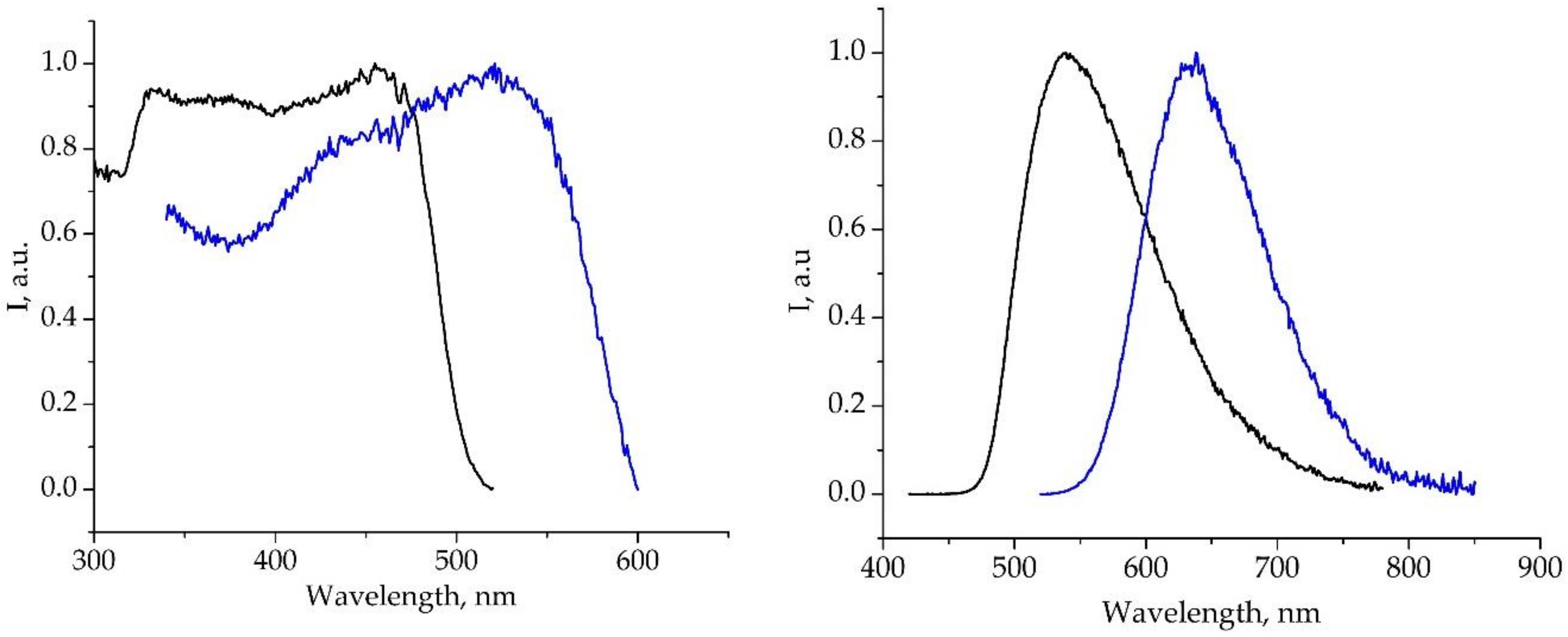
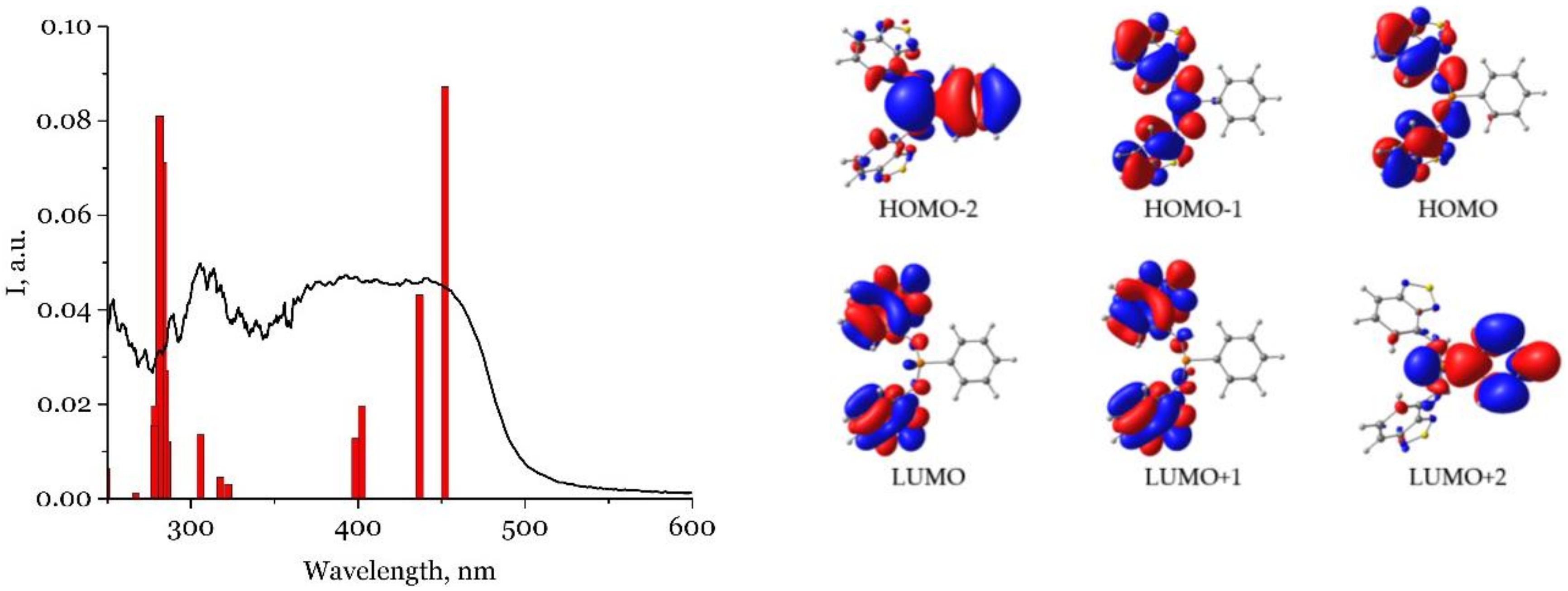
2.3. Photophysical Properties
3. Materials and Methods
3.1. General Methods
3.2. Quantum Chemical Calculations
3.3. X-ray Structure Determination
3.4. Syntheses
3.4.1. Synthesis of H2L
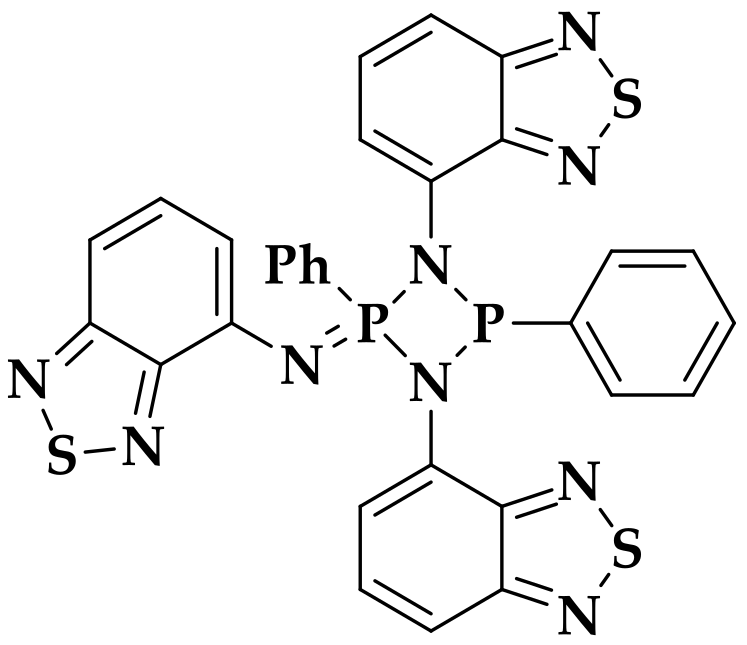
3.4.2. [Zn2L2]·nC7H8 (1)
3.4.3. [Zn2(H2L)2Cl4]·nC7H8 (2a·nC7H8 and 2b·nC7H8)
3.4.4. [Cu(H2L)Cl]n nTHF (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutierrez, G.D.; Sazama, G.T.; Wu, T.; Baldo, M.A.; Swager, T.M. Red Phosphorescence from Benzo[2,1,3]thiadiazoles at Room Temperature. J. Org. Chem. 2016, 81, 4789–4796. [Google Scholar] [CrossRef]
- Paisley, N.R.; Tonge, C.M.; Mayder, D.M.; Thompson, K.A.; Hudson, Z.M. Tunable benzothiadiazole-based donor–acceptor materials for two-photon excited fluorescence. Mater. Chem. Front. 2020, 4, 555–566. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Carvalho, P.H.P.R.; Correa, J.R. Benzothiadiazole Derivatives as Fluorescence Imaging Probes: Beyond Classical Scaffolds. Acc. Chem. Res. 2015, 48, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Schill, J.; Ferrazzano, L.; Tolomelli, A.; Schenning, A.P.H.J.; Brunsveld, L. Fluorene benzothiadiazole co-oligomer based aqueous self-assembled nanoparticles. RSC Adv. 2020, 10, 444–450. [Google Scholar] [CrossRef]
- Kini, G.P.; Choi, J.Y.; Jeon, S.J.; Suh, I.S.; Moon, D.K. Effect of mono alkoxy-carboxylate-functionalized benzothiadiazole-based donor polymers for non-fullerene solar cells. Dye. Pigment. 2019, 164, 62–71. [Google Scholar] [CrossRef]
- Prima, D.O.; Makarov, A.G.; Bagryanskaya, I.Y.; Kolesnikov, A.E.; Zargarova, L.V.; Baev, D.; Eliseeva, T.F.; Politanskaya, L.V.; Makarov, A.Y.; Slizhov, Y.G.; et al. Fluorine-Containing n-6 and Angular and Linear n-6-n′ (n, n′ = 5, 6, 7) Diaza-Heterocyclic Scaffolds Assembled on Benzene Core in Unified Way. Chemistry 2019, 4, 2383–2386. [Google Scholar] [CrossRef]
- Qian, G.; Wang, X.; Wang, S.; Zheng, Y.; Wang, S.; Zhu, W.; Wang, Y. Polymorphous Luminescent Materials Based on ’T’-Shaped Molecules Bearing 4,7-Diphenylbenzo[c][1,2,5]thiadiazole Skeletons: Effect of Substituents on the Photophysical Properties. Chem. A Eur. J. 2019, 25, 15401–15410. [Google Scholar] [CrossRef]
- Mikhailov, M.S.; Gudim, N.S.; Knyazeva, E.A.; Tanaka, E.; Zhang, L.; Mikhalchenko, L.V.; Robertson, N.; Rakitin, O.A. 9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole—A new donor building-block in the design of sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2020, 391, 112333. [Google Scholar] [CrossRef]
- Ansell, J.; Wills, M. Enantioselective catalysis using phosphorus-donor ligands containing two or three P-N or P-O bonds. Chem. Soc. Rev. 2002, 31, 259–268. [Google Scholar] [CrossRef]
- Martín, R.; Prieto, P.; Carrillo, J.R.; Rodríguez, A.M.; De Cozar, A.; Boj, P.G.; Díaz-García, M.A.; Ramírez, M.G. Design, synthesis and amplified spontaneous emission of 1,2,5-benzothiadiazole derivatives. J. Mater. Chem. C 2019, 7, 9996–10007. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Konchenkoa, S.N. Luminescent complexes of 2,1,3-benzothiadiazole derivatives. Russ. Chem. Bull. 2019, 68, 651–661. [Google Scholar] [CrossRef]
- Ishi-I, T.; Tanaka, H.; Youfu, R.; Aizawa, N.; Yasuda, T.; Kato, S.-I.; Matsumoto, T. Mechanochromic fluorescence based on a combination of acceptor and bulky donor moieties: Tuning emission color and regulating emission change direction. N. J. Chem. 2019, 43, 4998–5010. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zibarev, A.V. Synthesis and Applications of 5-Membered Chalcogen-Nitrogen π-Heterocycles with Three Heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Islam, A.; Akhtaruzzaman, M.; Chowdhury, T.H.; Qin, C.; Han, L.; Bedja, I.M.; Stalder, R.; Schanze, K.; Reynolds, J.R. Enhanced Photovoltaic Performances of Dye-Sensitized Solar Cells by Co-Sensitization of Benzothiadiazole and Squaraine-Based Dyes. ACS Appl. Mater. Interfaces 2016, 8, 4616–4623. [Google Scholar] [CrossRef] [PubMed]
- Page, Z.A.; Liu, Y.; Puodziukynaite, E.; Russell, T.P.; Emrick, T. Hydrophilic Conjugated Polymers Prepared by Aqueous Horner–Wadsworth–Emmons Coupling. Macromolecules 2016, 49, 2526–2532. [Google Scholar] [CrossRef]
- Boucard, J.; Boudjemaa, R.; Steenkeste, K.; Jacqueline, C.; Stephant, N.; Lefèvre, F.-X.; Laurent, A.D.; Lartigue, L.; Hulin, P.; Nedellec, S.; et al. Phosphonic Acid Fluorescent Organic Nanoparticles for High-Contrast and Selective Staining of Gram-Positive Bacteria. ACS Omega 2018, 3, 17392–17402. [Google Scholar] [CrossRef]
- Ren, Y.; Sezen, M.; Guo, F.; Jäkle, F.; Loo, Y.-L. [d]-Carbon–carbon double bond engineering in diazaphosphepines: A pathway to modulate the chemical and electronic structures of heteropines. Chem. Sci. 2016, 7, 4211–4219. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, Z.; Fu, W.; Shi, M.; Chen, H. Phosphate ester side-chain-modified conjugated polymer for hybrid solar cells. J. Appl. Polym. Sci. 2017, 134, 134. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.-Y.; Zhao, Y.-D.; Cao, Y.-C. Recent advancements of high efficient donor–acceptor type blue small molecule applied for OLEDs. Mater. Today 2017, 20, 258–266. [Google Scholar] [CrossRef]
- Duan, C.; Li, J.; Han, C.; Ding, D.; Yang, H.; Wei, Y.; Xu, H. Multi-dipolar Chromophores Featuring Phosphine Oxide as Joint Acceptor: A New Strategy toward High-Efficiency Blue Thermally Activated Delayed Fluorescence Dyes. Chem. Mater. 2016, 28, 5667–5679. [Google Scholar] [CrossRef]
- Li, H.; Hong, M.; Scarpaci, A.; He, X.; Risko, C.; Sears, J.S.; Barlow, S.; Winget, P.; Marder, S.R.; Kim, D.; et al. Chemical Stabilities of the Lowest Triplet State in Aryl Sulfones and Aryl Phosphine Oxides Relevant to OLED Applications. Chem. Mater. 2019, 31, 1507–1519. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Shi, H.; An, Z.; Huang, W. Manipulating the Ultralong Organic Phosphorescence of Small Molecular Crystals. Chem. A Eur. J. 2020, 26, 4437–4448. [Google Scholar] [CrossRef] [PubMed]
- Joly, D.; Bouit, P.-A.; Hissler, M. Organophosphorus derivatives for electronic devices. J. Mater. Chem. C 2016, 4, 3686–3698. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Bashirov, D.A.; Komarov, V.Y.; Molokeev, M.S.; Ryadun, A.A.; Benassi, E.; Konchenko, S.N. Tuning of the Coordination and Emission Properties of 4-Amino-2,1,3-Benzothiadiazole by Introduction of Diphenylphosphine Group. Cryst. Growth Des. 2020. [Google Scholar] [CrossRef]
- Burford, N.; Cameron, T.S.; Conroy, K.D.; Ellis, B.; Lumsden, M.; Macdonald, C.L.B.; McDonald, R.; Phillips, A.D.; Ragogna, P.J.; Schurko, R.W.; et al. Transformations between Monomeric, Dimeric, and Trimeric Phosphazanes: Oligomerizing NP Analogues of Olefins. J. Am. Chem. Soc. 2002, 124, 14012–14013. [Google Scholar] [CrossRef]
- Ams, M.; Trapp, N.; Schwab, A.; Milić, J.V.; Diederich, F. Chalcogen Bonding “2S–2N Squares” versus Competing Interactions: Exploring the Recognition Properties of Sulfur. Chem. A Eur. J. 2018, 25, 323–333. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Bashirov, D.; Shuvaev, S.; Komarov, V.; Kuratieva, N.; Konchenko, S.; Benassi, E. Noncovalent interactions and photophysical properties of new Ag(I) complexes with 4-amino-2,1,3-benzothiadiazole. Polyhedron 2018, 141, 77–86. [Google Scholar] [CrossRef]
- CrystalExplorer, version 17; University of Western: Perth, Australia, 2017.
- Bond, A.D.; Doyle, E.L.; García, F.; Kowenicki, R.A.; Moncrieff, D.; McPartlin, M.; Riera, L.; Woods, A.D.; Wright, D.S. Thermodynamic/Kinetic Control in the Isomerization of the[{tBuNP(μ-NtBu)}2]2− Ion. Chem. A Eur. J. 2004, 10, 2271–2276. [Google Scholar] [CrossRef]
- Tirreé, J.; Gudat, D.; Nieger, M.; Niecke, E. Reversible Tautomeric Transformation between a Bis(amino)cyclodiphosph(V)azene and a Bis(imino)cyclodiphosph(V)azane. Angew. Chem. Int. Ed. 2001, 40, 3025–3028. [Google Scholar] [CrossRef]
- Moser, D.F.; Carrow, C.J.; Stahl, L.; Staples, R.J. Titanium complexes of bis(1°-amido)cyclodiphosph(III)azanes and bis(1°-amido)cyclodiphosph(V)azanes: Facial versus lateral coordination. J. Chem. Soc. Dalton Trans. 2001, 1246–1252. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Kurat’Eva, N.V.; Smolentsev, A.I.; Konchenko, S.N. Samarium, Europium, and Gadolinium Complexes with 4-(2,1,3-Benzothiadiazol-4-ylamino)pent-3-en-2-onate. Russ. J. Coord. Chem. 2019, 45, 30–35. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Prashad, M.; Repič, O.; Blacklock, T.J. A Practical and Chemoselective Reduction of Nitroarenes to Anilines Using Activated Iron. Adv. Synth. Catal. 2005, 347, 217–219. [Google Scholar] [CrossRef]
- Kubelka, P. New contributions to the optics intensely light scattering materials. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 2, 73–78. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Bruker Apex3 Software Suite: Apex3, SADABS-2017/2 and SAINT, version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. Struct. Science 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples used are available from the authors. |

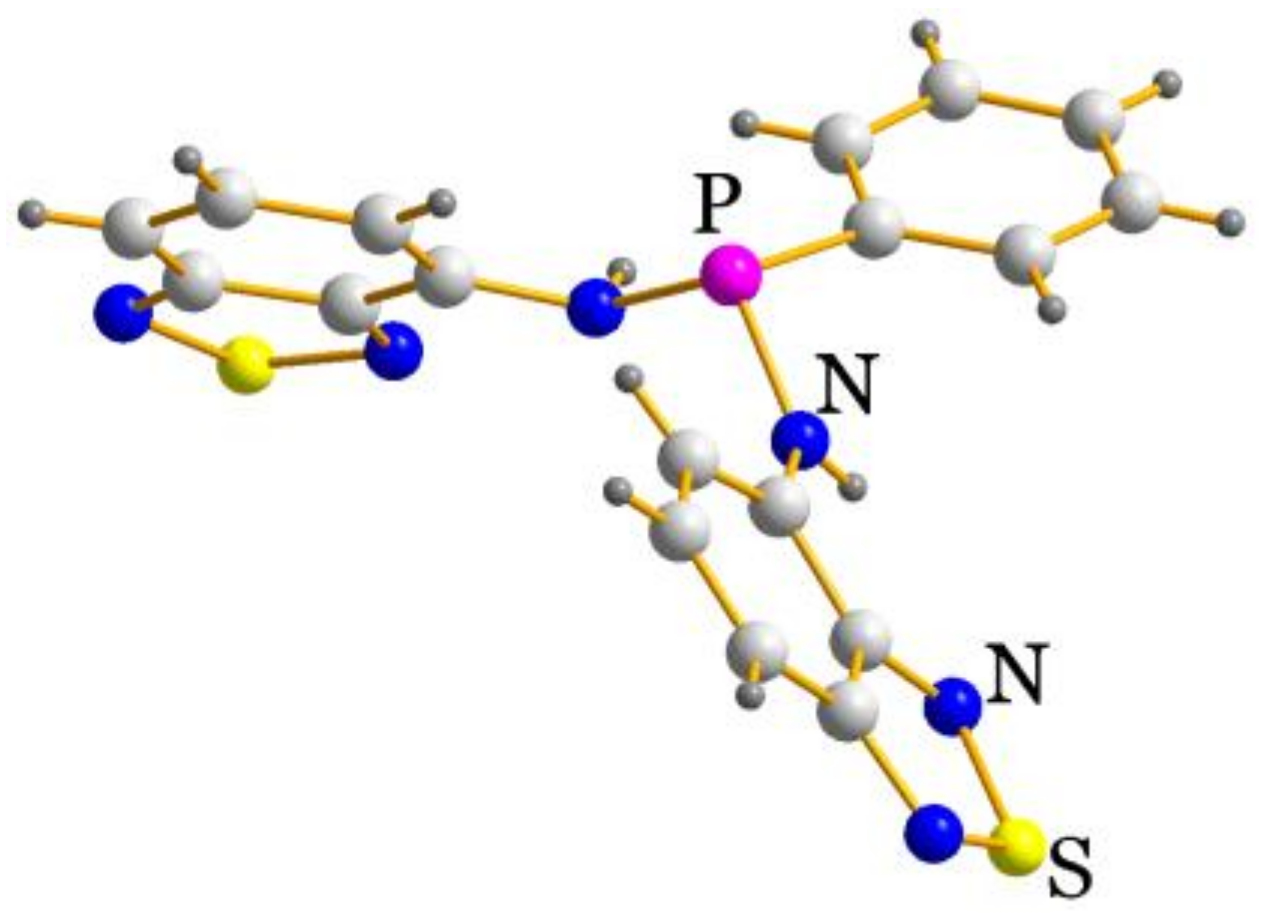

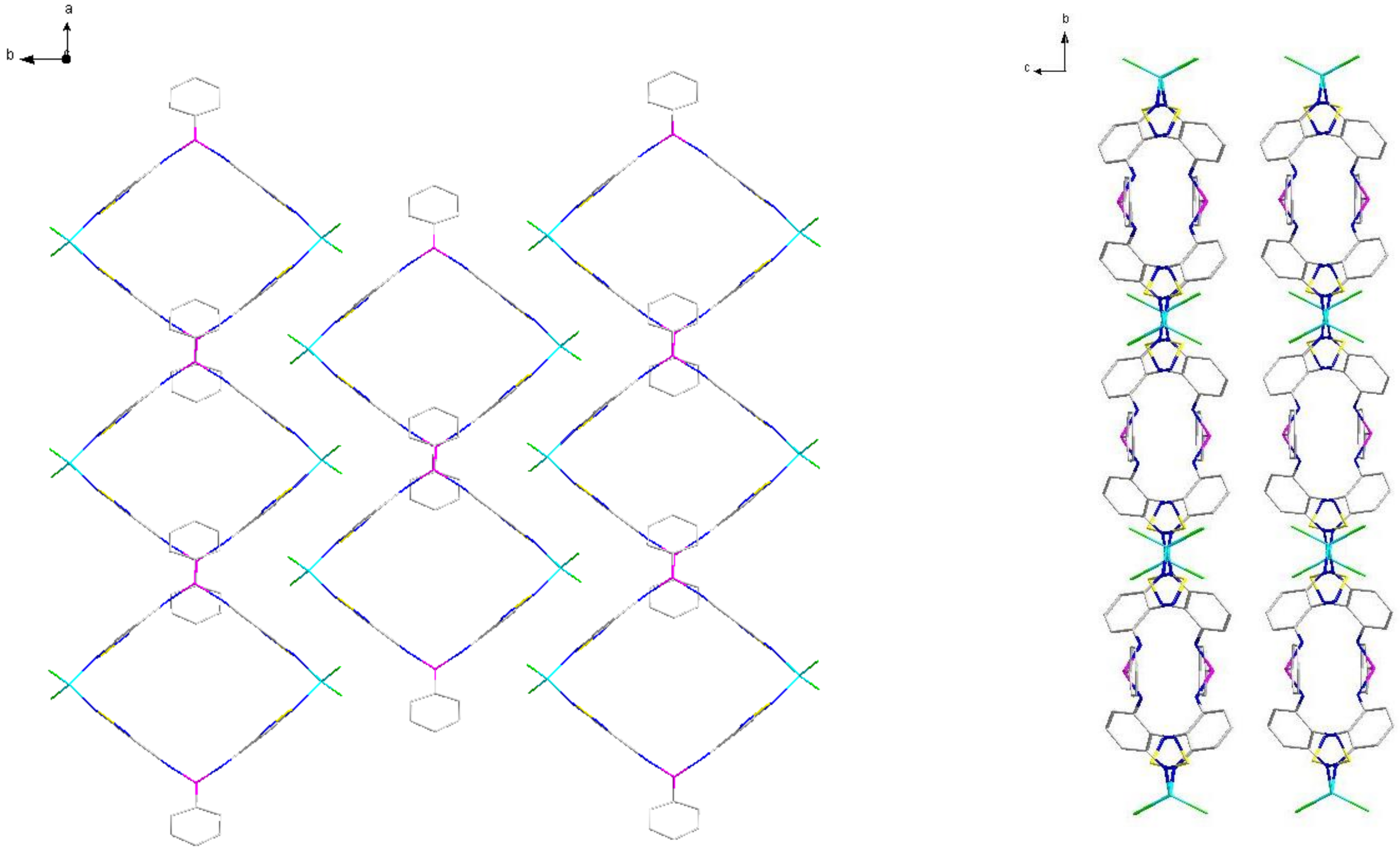
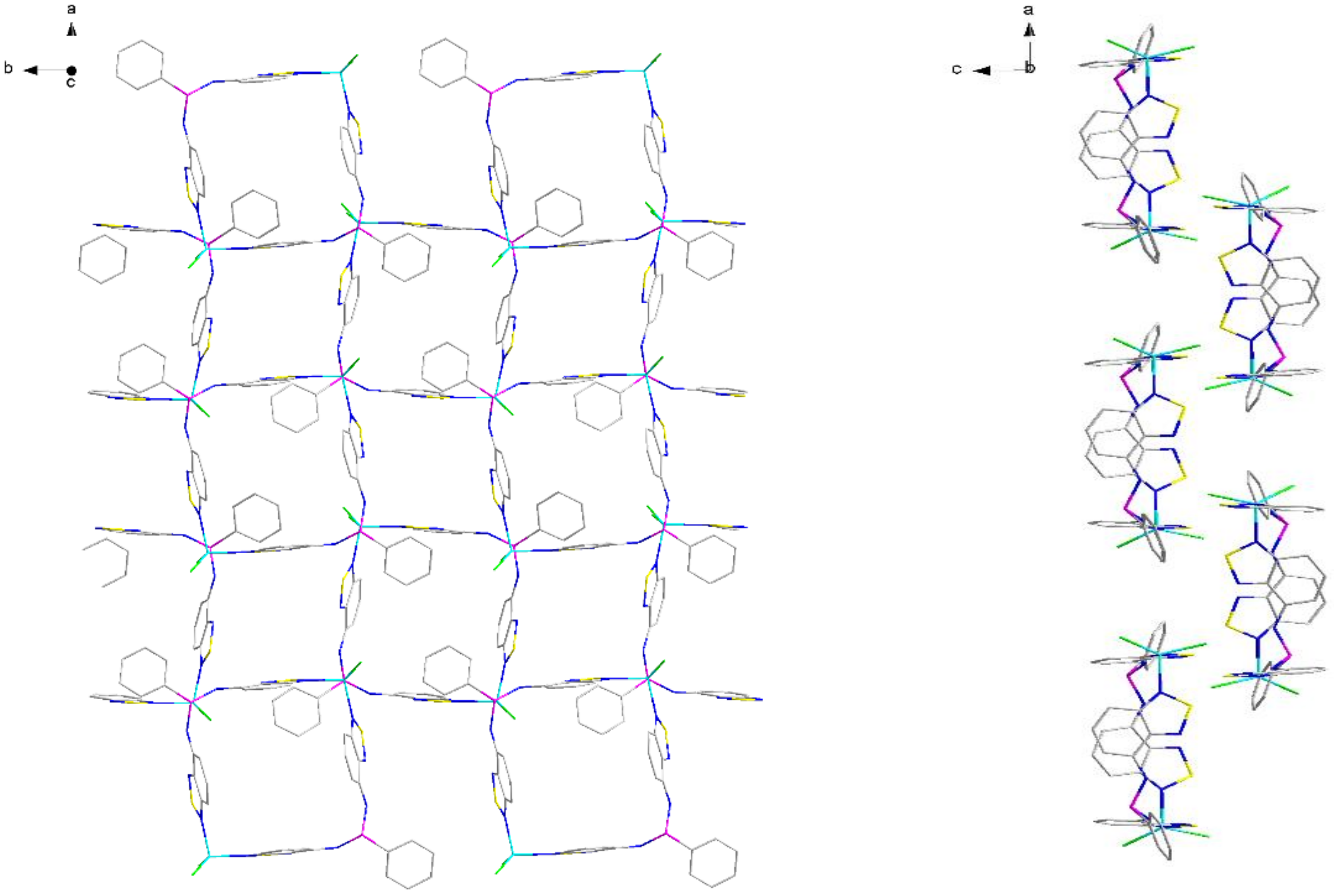
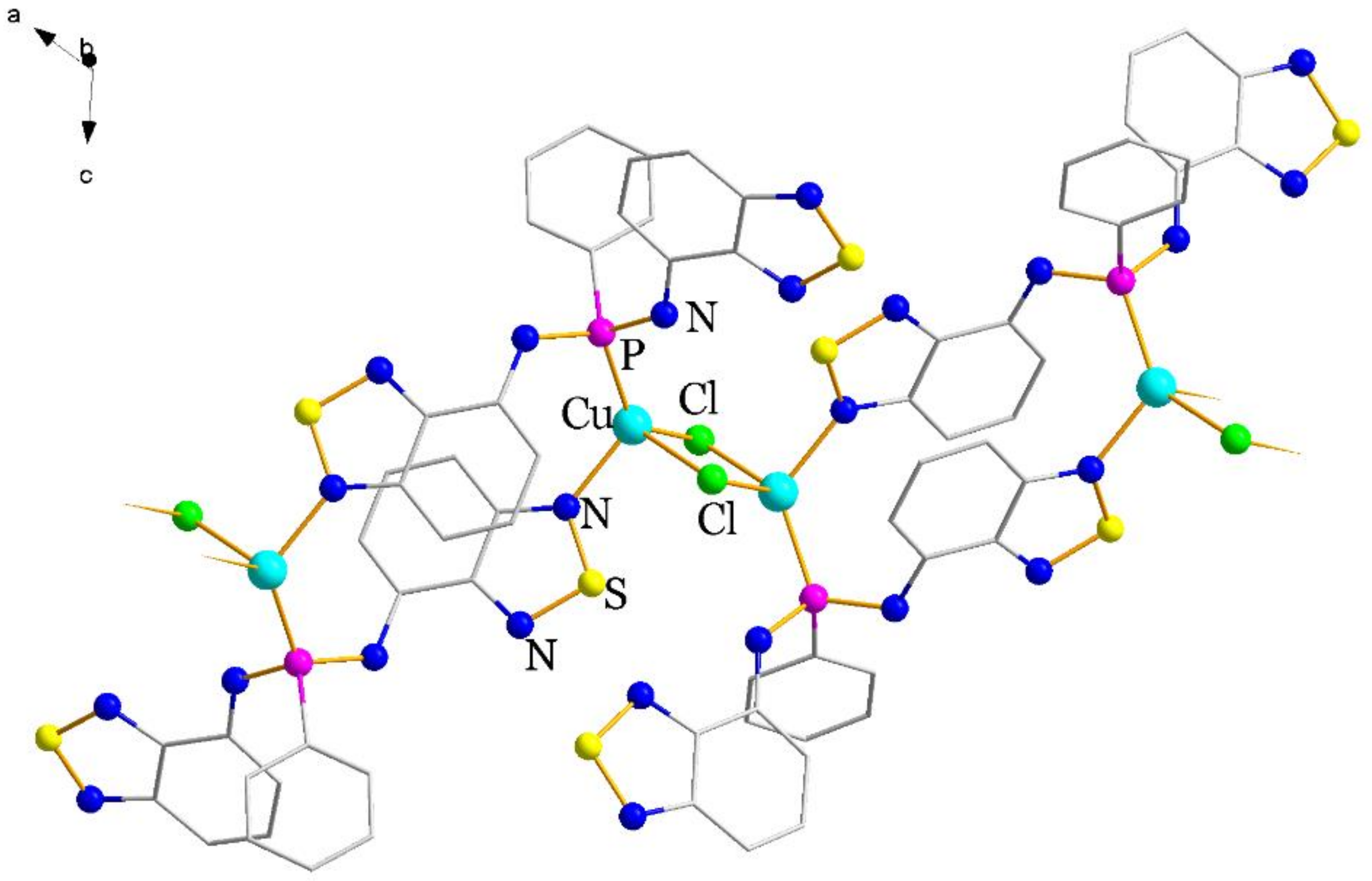
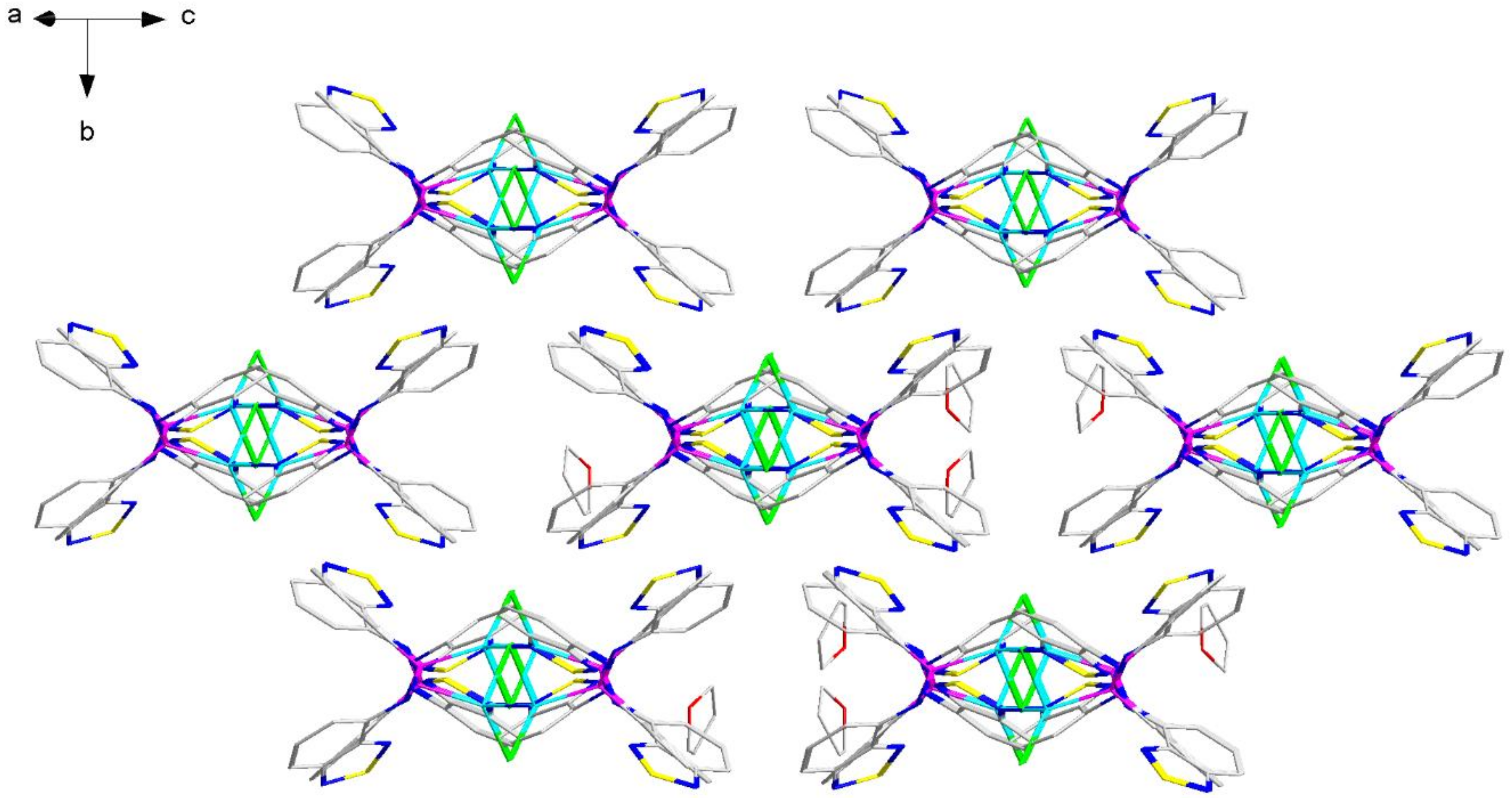
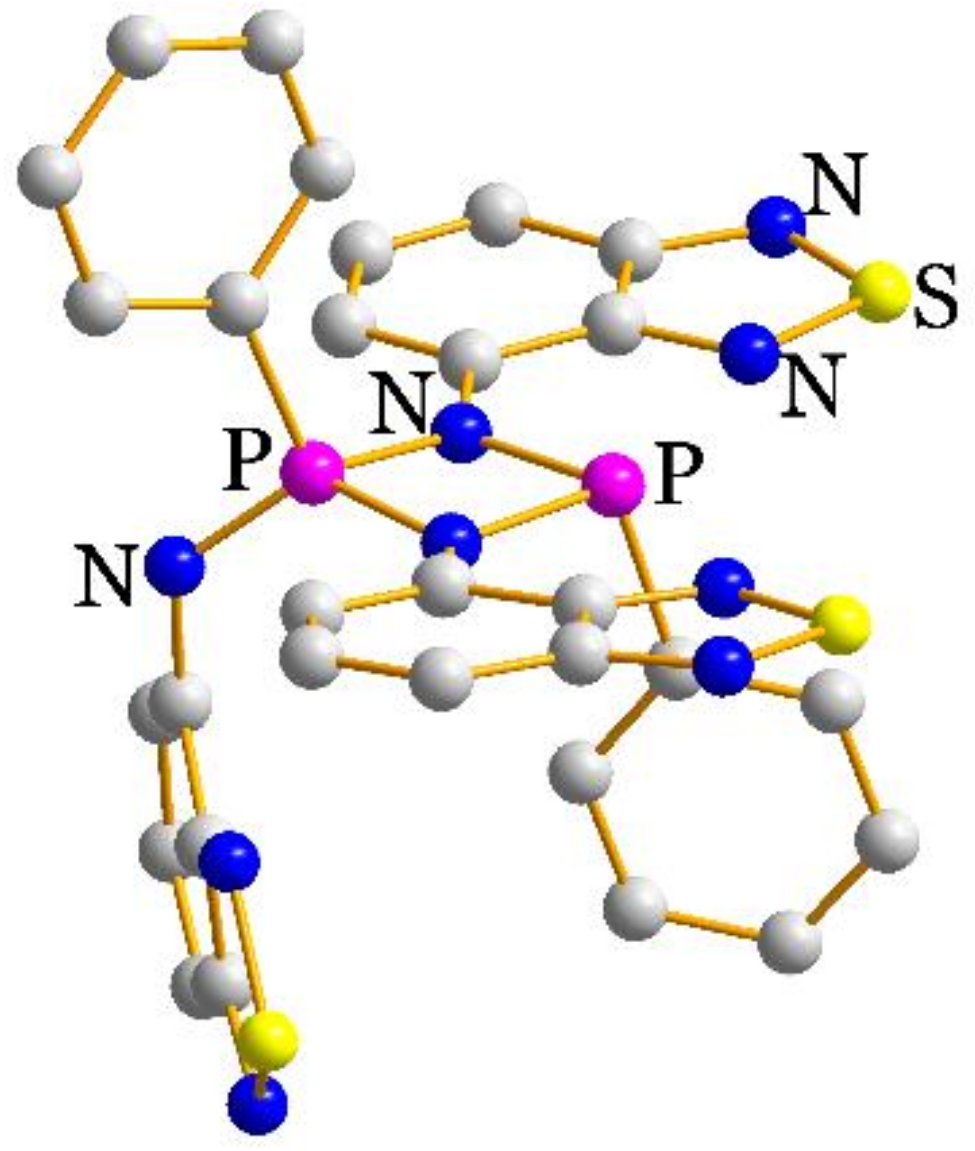

| P–N–C | N–P–N | C–P–N–C | P–N–C–C(N) | N–Zn–N | |
|---|---|---|---|---|---|
| H2L | 125.7, 127.3, | 107.4 | 151.4, 145.7 | 176.0, 171.8 | |
| H2L (optimized) | 125.6, 126.0 | 105.5 | 154.9, 156.4 | 170.2, 164.9 | |
| 1·C7H8 | 122.0, 122.1 | 97.9 | 80.0, 145.9 | 166.7, 177.8 | 84.3, 84.7 |
| 2a·3C7H8 | 124.1 | 106.2 | 150.0 | 177.4 | 96.2 |
| 2a (optimized) | 126.4, 126.4 | 104.8 | 152.0, 152.0 | 176.5 | 92.6 |
| 2b·2.5C7H8 | 122.2, 124.5 | 105.2 | 156.3, 154.9 | 160.0, 165.9 | 101.5 |
| 2b (optimized) | 125.4, 126.2 | 104.5 | 156.6, 151.2 | 171.9, 178.5 | 94.6 |
| 3·THF | 120.9, 129.9 | 109.1 | 156.9, 49.2 | 137.9, 169.2 |
| Compound | λabs, nm | λEm, nm | τ | QY, % |
|---|---|---|---|---|
| H2L | 305, 390-440 | 540 | 4.3 ns | 8 |
| 1·nC7H8 | 304, 400, 600 | |||
| 2·nC7H8 | 309, 480 | 635 | 3.1 ns | 3 |
| 3·THF | 304, 385, 550(sh) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khisamov, R.; Sukhikh, T.; Bashirov, D.; Ryadun, A.; Konchenko, S. Structural and Photophysical Properties of 2,1,3-Benzothiadiazole-Based Phosph(III)azane and Its Complexes. Molecules 2020, 25, 2428. https://doi.org/10.3390/molecules25102428
Khisamov R, Sukhikh T, Bashirov D, Ryadun A, Konchenko S. Structural and Photophysical Properties of 2,1,3-Benzothiadiazole-Based Phosph(III)azane and Its Complexes. Molecules. 2020; 25(10):2428. https://doi.org/10.3390/molecules25102428
Chicago/Turabian StyleKhisamov, Radmir, Taisiya Sukhikh, Denis Bashirov, Alexey Ryadun, and Sergey Konchenko. 2020. "Structural and Photophysical Properties of 2,1,3-Benzothiadiazole-Based Phosph(III)azane and Its Complexes" Molecules 25, no. 10: 2428. https://doi.org/10.3390/molecules25102428
APA StyleKhisamov, R., Sukhikh, T., Bashirov, D., Ryadun, A., & Konchenko, S. (2020). Structural and Photophysical Properties of 2,1,3-Benzothiadiazole-Based Phosph(III)azane and Its Complexes. Molecules, 25(10), 2428. https://doi.org/10.3390/molecules25102428