Abstract
A universally applicable method for the prediction of the isobaric heat capacities of the liquid and solid phase of molecules at 298.15 K is presented, derived from their “true” volume. The molecules’ “true” volume in A3 is calculated on the basis of their geometry-optimized structure and the Van-der-Waals radii of their constituting atoms by means of a fast numerical algorithm. Good linear correlations of the “true” volume of a large number of compounds encompassing all classes and sizes with their experimental liquid and solid heat capacities over a large range have been found, although noticeably distorted by intermolecular hydrogen-bond effects. To account for these effects, the total amount of 1303 compounds with known experimental liquid heat capacities has been subdivided into three subsets consisting of 1102 hydroxy-group-free compounds, 164 monoalcohols/monoacids, and 36 polyalcohols/polyacids. The standard deviations for Cp(liq,298) were 20.7 J/mol/K for the OH-free compunds, 22.91 J/mol/K for the monoalcohols/monoacids and 16.03 J/mol/K for the polyols/polyacids. Analogously, 797 compounds with known solid heat capacities have been separated into a subset of 555 OH-free compounds, 123 monoalcohols/monoacids and 119 polyols/polyacids. The standard deviations for Cp(sol,298) were calculated to 23.14 J/mol/K for the first, 21.62 J/mol/K for the second, and 19.75 J/mol/K for the last subset. A discussion of structural and intermolecular effects influencing the heat capacities as well as of some special classes, in particular hydrocarbons, ionic liquids, siloxanes and metallocenes, has been given. In addition, the present method has successfully been extended to enable the prediction of the temperature dependence of the solid and liquid heat capacities in the range between 250 and 350 K.
1. Introduction
The heat capacity is a fundamental extensive property characterizing a molecule’s thermophysical response towards the addition of heat energy, knowledge of which is important e.g., in connection with the adjustment calculation of further thermodynamic properties such as the heats and entropies of sublimation, vaporization or solvation to a defined temperature. The theoretical approach for the evaluation of the heat capacity Cp at constant pressure and at a given physical phase state and temperature is feasible for single atoms and basically only for very small molecules due to the rapid increase of the degrees of freedom of translational, rotational, vibrational and electronic motion with the number of atoms in a molecule and the question as to which of these degrees of freedom are excited at all at a certain temperature. Based on these findings, it is clear that the heat capacity of a molecule is temperature-dependent, being zero at 0K and increasing with temperature. As for larger molecules, various experimental calorimetric methods have shown to provide an easy experimental access to their values. Among these, the most popular instruments are the adiabatic calorimetry (AC) [1], the differential scanning calorimetry (DSC) [2], and the modulated DSC (MDSC) [3,4]. These methods have successfully been applied to all kinds of molecules besides ordinary organic compounds: literature references of experimental data for inorganic and organic salts, liquid crystals, ionic liquids (ILs) as well as metal-organics have been found and will be cited later on.
Based on these experimental data, a large number of methods for the prediction of the heat capacities of as yet unknown compounds have been developed, the most prominent of which are founded on the group-additivity (GA) approach [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Chickos et al. [5] described a GA method for the prediction of the heat capacities of 810 liquids and 446 solids using 47 functional groups, which take account of the carbon atoms’ substitution and hybridization states, yielding a standard error of 19.5 J/mol/K for liquids and of 26.9 J/mol/K for solids. They compared these standard deviations with the experimental standard errors of 8.12 and 23.4 J/mol/K respectively, derived from the numerous experimental data variations for 219 liquids and 102 solids cited by several independent sources. Ruzicka and Domalski [6] developed a second-order GA method for the prediction of the heat capacity of 265 liquid hydrocarbons as a function of temperature between their melting and normal boiling point, introducing a polynomial expression, taking into account the temperature-dependence of the group parameters. The mean percentage deviation (MPD) of their prediction was given as 1.9%. The same authors extended their GA method to liquid compounds carrying halogens, nitrogen, oxygen and sulfur [7], enabling the estimation of the heat capacity of a total of 558 liquids with an MPD of 2.9%. Later on, Zàbransky and Ruzicka [8] presented an amended GA method based on the one published by Ruzicka and Domalski [6,7], which extended the number of functional groups to 130, including cis, trans as well as ortho and meta corrections in the GA parameters calculations, reporting deviation errors of between 1 and 2%. A setback was the observation that the results deteriorated if the compound contained functional groups from more than one family, as e.g., in N,N’-diethanolamine or 1-chloro-2-propanol. Another approach was chosen by Goodman et al. [9] in that they tested two different equations to account for the temperature dependence of the heat capacity Cp, the simple power-law form Cp = ATm, where A and m are empirical coefficients and m is less than 1, and a more elaborate form based on the Einstein-Debye partition function for crystals applying a modified frequency-distribution function. The MPD for 455 compounds in the training set amounted to 6.8% for the former formula and 8% for the latter function. A novel three-level GA method was introduced by Kukal et al. [10], the levels being defined as increasingly complex molecule fragments. In addition, the temperature dependence of the heat capacity in the range between the melting and the normal boiling point was included in a polynomial formula for the contributions of these fragments. Based on 549 compounds, an MPD of 1.2% over the complete set over the mentioned temperature range was found. At 298.15K, an MPD of 1.5% resulted for 404 compounds of the basis set and 2.5% for 149 compounds for an independent test set. In recent years the GA approach has found particular interest in the estimation of the heat capacity of ILs [11,12,13,14,15,16]. Gardas and Coutinho [11] presented the results of the predictions of the heat capacity and its temperature relation for a series of 19 ILs consisting of imidazolium, pyridinium and pyrrolidinium cations and several mono- and polyatomic anions, based on the second-order GA method described by Ruzicka and Domalski [6,7]. The groups therein represented the complete anions and the cations minus the alkyl chains, while their methylene and methyl functions were treated separately, which limits the method’s applicability to just this kind of ILs. Accordingly, the Cp predictions deviated for 90.2% by less than 1% from the experimental data. They also observed an interesting correlation of the heat capacities with the ILs’ molar and molecular volumes, which will be the subject of further discussion. Ceriani et al. [12] used a GA method for the prediction of the heat-capacities and their temperature dependence of 1395 fatty compounds contained in edible oils and biofuels, encompassing acids, alcohols, esters, triacylglycerols as well as hydrocarbons, applying only seven functional groups, whereby each group contribution was represented by a temperature-dependent linear function. They reported an MPD of only 2.6%. In contrast to Gardas and Coutinho’s [11] paper, Valderrama et al. [13] refined the functional groups constituting a set of 32 ionic liquids into 42 smaller fragments and added the heat-capacity data of 126 further ordinary compounds to the database in order to achieve a more general applicability. Beyond this, they added a term called mass connectivity index, introduced by Randic [14] and extended by Valderrama and Rojas [15], representing the degree of branching to the GA calculation. The MPD for the 32 ILs was calculated to 2.8%. In order to find the most effective functional groups in the GA method for the heat-capacity prediction of ILs, Sattari et al. [16] used a genetic function approach, which required the investigation of a series of models with increasing numbers of functional groups. They showed that a model with the 13 most effective groups produces sufficiently reliable results, yielding an overall correlation coefficient R2 of 0.99 and standard deviation error of 18.42 J/mol/K and an MPD of 1.68% for a total of 82 ILs. Albert and Müller [17] applied a GA model including a polynomial function with a degree of two for the temperature dependence of the heat capacities of ILs based on 36 functional groups and seven heterocyclic and heteroaromatic rings as corrective groups, yielding a standard deviation of 36 J/mol/K (MPD = 2.58%) for the training set of 86 ILs and 47 J/mol/K (MPD = 5.44%) for the test set of 20 ILs. A critical evaluation of the previous GA methods for the prediction of the heat capacities of ILs has been presented by Nancarrow et al. [18], whereby the methods have been distinguished into two approaches, the Meccano and the Lego approach. The former one describes a GA method wherein the functional groups consist of the complete building blocks of cations and ions, e.g., as presented by Gardas and Coutinho [11], whereas in the latter one the groups represent single atoms or small molecular fragments, exemplified by the method of Valderrama et al. [13]. Based on two sets of ILs for the respective GA methods they reported the obvious: while the Meccano approach was found to provide slightly lower deviations with experiment for the 45 test ILs, but was of limited applicability, the Lego approach, applied on 92 ILs, produced less accurate results but was more widely usable.
Besides the GA approach, a number of further methods for the prediction of the heat capacities have been used [19,20,21,22,23,24,25,26,27,28,29]. Morad et al. [19] estimated the heat capacities of fatty acids, triacylglycerols and vegetable oils by means of the Rowlinson-Bondi equation [20], which evaluates the difference between the liquid specific heat capacity and the ideal gas liquid specific heat capacity, based on the reduced temperature and an acentric factor. The heat capacity of the ideal gas again was calculated on the basis of the GA method of Rihany and Doraisamy [21]. An experimental approach based on the vibrational spectra and a direct calculation of the spectra using density functional theory was used for the prediction of the heat capacity of polynuclear aromatic solids by Sallamie and Shaw [22]. A method especially designed for the heat-capacity prediction of ILs was provided by Müller and Albert [23], in that they evaluated the contribution of each of 39 cations and 32 anions and their temperature dependence to the IL’s heat capacity. They reported a standard deviation of 13.2 J/mol/K for the training set and 30.1 J/mol/K for the test set. Barati-Harooni et al. [24] compared three Cp-prediction models, one called coupled simulated annealing least square support vector machine (CSA-LSSVM), a second one being a gene expression programming algorithm (GEP), and a third one called adaptive neuro fuzzy inference system (ANFIS). Applied on 56 ILs with 2940 data points, the results showed the best results with CSA-LSSVM, yielding a correlation coefficient R2 of 0.9933 and a standard deviation of 9.99 J/mol/K. An interesting approach was presented by Preiss et al. [25] in that they correlated the molecular volumes of ILs with their experimental heat capacities. They defined an IL’s molecular volume (in nm3) as the sum of the molecular volumes of its constituting cation and anion. Since these volumes have been derived from the unit cell dimensions of crystal structures obtained from x-ray measurements, this method’s scope of use was limited to the sets of ILs only consisting of the named anions and cations. Nevertheless, the fact that the results showed a linear relationship between the ILs’ volumes and their heat capacities over a Cp range of between ca. 300 and 1300 J/mol/K with a correlation coefficient R2 of ca. 0.97 and an MPD of ca. 4–5% (based on 34 ILs) not only confirmed the observation of Gardas et al. [11], but also indicated that the prediction of any molecules’ heat capacities via their molecular volumes should be feasible, on condition that a reliable and general method for the calculation of the molecular volumes is available. A similar linear correlation was found by Paulechka et al. [26] between the molar volumes (in m3/mol) and the heat capacities for 19 ILs, for which the authors calculated a standard deviation of 6.7 J/mol/K at 298.15K. Again, due to the requirement of the knowledge of the ILs’ density, the calculation of the heat capacity via the density would be limited. Later called “volume-based thermodynamics” (VBT) [27], Glasser and Jenkins [28] extended this approach to minerals and ionic solids and liquids, demonstrating the validity of Neumann–Kopp’s rule [29] of the additivity of the heat capacity contribution per atom.
All the presented methods for the prediction of the heat capacity of organic molecules, despite their usefulness and reliability within their designed range, have the disadvantage of not being generally applicable. In principle, the GA approach can solve this deficiency, e.g., following the GA approach of Ruzicka and Domalski [6,7], provided that the number of published experimental data is large and the molecules structurally versatile enough. Unfortunately, this is not the case. In the ongoing project ChemBrain, a recent extension of its versatile GA method for the prediction of a large number of molecular descriptors [30,31,32,33], now also enabling the prediction of the liquid and solid heat capacities (due for publication), has revealed that, due to the limited number of experimental data, the liquid heat capacity of only ca. 62% and the solid heat capacity of only ca. 65% of the compounds have been evaluable in its database of currently ca. 31’600, the structural diversity of which can be viewed as representative for the entire space of chemical structures. Therefore, a method was sought which preferably required only one molecular property, reliably computable for each and every molecule, from which its heat capacities could be derived. Gardas et al. [11] and Glasser and Jenkins [28] opened a possible solution in that the VBT approach refers to a property that is inherent in each molecule: its volume. However, although there are various experimental methods for the evaluation of a molecule’s volume, it would not make any sense to derive it from experimental data, e.g., x-ray or density, as these data are also limited in number. The present paper offers a reliable and reproducible way for the calculation of the “true” molecular volume, which only depends on the molecules’ 3-dimensional geometry and the Van-der-Waals (vdw) radii of their constituting atoms. It will demonstrate that these volumes excellently linearly correlate with the heat capacity of the solid and liquid phase of any class, size and structure of molecules, not only at standard conditions but also over a large temperature range. Thanks to the unlimited scope of its applicability, this study will also provide an awareness of the forces influencing the heat capacities, in particular the structural or intermolecular effects that are often camouflaged by other methods, and it will enable a reliable estimate of their magnitude. The limitless range of its applicability, on the other hand, forestalls a direct comparison of the quality of the present method with any of the results cited above, as none of these was based on a similarly comprehensive set of compounds, which encompasses molecules as diverse as alkanes, ionic liquids, siloxanes and metal complexes, to name a few. Therefore, the reliability of the present results has been put in relation to the scatter of the experimental heat capacities, the amount of which can best be assessed by the Cp values of several homologous series of compounds for which the increase between the consecutive elements should be approximately constant, examples of which will be presented.
2. General Procedure
The present method for the calculation of the molecular heat capacities is based on a database of currently ca. 31’600 molecules, each of them stored as geometry-optimized 3D structure in an object-oriented datafile, together with a number of experimental and routinely calculated thermodynamic and further descriptors, among which the experimental heat-capacity Cp(liq,298) data at 298.15K for 1303 liquids and the Cp(sol,298) data for 800 solids are to be mentioned in this context. In the following, the two decisive preconditions for a reliable heat-capacity prediction will be outlined in detail, the optimization of the molecular 3D geometry as the first step in the process, followed by the calculation of the “true” molecular volume. It will be shown in the results section that a third step is required, distinguishing between OH-group-containing and OH-free molecules, to enable the selection of the correct parameters for the linear equation leading to a reliable prediction of the heat capacities of their liquid and solid phases.
2.1. Geometry Optimization
The goal of the geometry optimization process is to ensure that the final bond lengths and angles in a molecule’s 3D presentation correspond to standard bond lengths and angles, as e.g., listed in the CRC Handbook of Chemistry and Physics [34], and that the intramolecular interactions of vdw radii [35] have been minimized. This optimization process in the ongoing project ChemBrain IXL is carried out by means of a force-field method called “steepest descent”, e.g., described by O. Ermer [36], meaning that each step of the iterative process leading to the energy minimum is controlled by the largest value of the first derivatives in x-, y- and z- direction of Equation (1) at each atom, evaluated by moving its position in each direction by a tiny amount and calculating the corresponding energy change.
In this equation, the term E denotes the total energy which is defined as the sum of all bond stretching (Estr), bond angle (Eang), torsional angle (Etor), vdw interaction (Evdw) and out-of-plane bending (Eoop) energies. By default, during the optimization process the algorithm periodically scans the energy hyper-surface for the molecule structure’s global energy minimum, which e.g., results in the linearization of long-chain alkyl groups. As will be shown, however, achievement of the global energy minimum is not required, as the energy itself of the optimized structure is irrelevant for the present task.
2.2. Calculation of the “True” Molecular Volume
A number of publications [35,37,38,39,40,41] have been dealing with the calculation of the molecular volume. Several of them based the calculation on a physical property of the molecule such as the density, e.g., Kurtz and Sankin [37] and Ye and Shreeve [38], or the crystal structure, e.g., Jenkins et al. [39]. The disadvantage of these approaches lies in the fact that they require an examined physical property as a starting point. Beyond this, the evaluated volumes are not really “true” molecular volumes as they also include parts of the empty space around them. A purely analytical approach was chosen by Conolly [40], in that he constructed the molecular volume from a collection of spheres, consisting of partitions of the atomic vdw volumes and of solvent-excluded volumes. Although not dependent on any experimentally determined property, a computational algorithm based on this procedure seems far from easily generalizable. Gavezotti [41], in contrast, suggested a numerical method which starts from a molecule-enveloping space, containing a very large number of spatially randomly distributed probe points, counting all of those points inside any of the atomic vdw spheres and dividing this number by the total number of all probes. Multiplication of this fraction with the volume of the envelope space yielded the molecular volume. It will be evident that this approach fairly closely resembles the one which will now be outlined here.
The evaluation of the “true” molecular volume is carried out in the following way: at first, a rectangular box is defined, the sidelengths of which in x-, y- and z-direction are defined by the corresponding extensions of the molecule including the atomic vdw radii. Then a cube of a defined sidelength is systematically scanned through the box in all three directions in steps of the cube’s sidelength. Whenever the cube center is within the range of any of the atoms’ vdw radii, the cube’s volume is added to a container, which constitutes the “true” molecular volume. In order to speed up this procedure, the algorithm in each consecutive scan in x- and y-direction first sets up a list of those atoms whose vdw radii are within the cube’s reach in both directions, before scanning the third, i.e., the z-direction. In other words, the scan in the z-direction is reduced to those cubes that are within the molecule’s projection onto the xy-surface of the box. The molecular volume of salts with both anions and cations consisting of polyatomic structures, such as ILs, are calculated just like ordinary neutral molecules. However, in this case it is important to ensure that the two ions are well separated in order to prevent intermolecular overlap. As this procedure does not include monoatomic ions occurring in organic salts and ILs, because they are added separately to the formula, the scanning procedure in this case is completed by the addition of the ions’ individual vdw volume [42] to the container.
The cube’s sidelength has been defined in this project as 0.05 Angstroms (5 pm), resulting in a cube volume of 0.000125 A3 (125 pm3). Accordingly, the “true” molecular volume is given in A3. Despite the cube’s small size, the evaluation of the molecular volume of even a large molecule is carried out within a split second on a desktop computer.
3. Results
3.1. General Remarks
Both the lists of molecules used in the correlation calculations of the heat capacities of the solid and liquid phase are collected in standard SDF files and are stored in the Supplementary Material, ready to be imported by external chemistry software. The Supplementary Material also provides the molecules lists used for each of the following correlation diagrams containing molecule names, experimental and prediction values. Additionally, it also contains the lists of outliers in the solid and liquid heat-capacity calculations.
3.2. “True” Molecular Volumes
As mentioned in the prior section, the present procedure for the calculation of the molecular volume largely corresponds to the one of Gavezotti [41]. Correspondingly, one would expect similar results. This is indeed the case as is demonstrated on Table 1.

Table 1.
Comparison of “true” molecular volumes in A3 with literature references.
The values of the molecular volumes are not dependent on the geometry having the lowest global energy minimum, as is demonstrated in Figure 1, where tristearin, an unsaturated longchain glyceryl triester, is compared in a stretched and a randomly folded structural form. Their molecular volumes differ by less than 1%, the difference being owed to some intramolecular vdw overlaps.
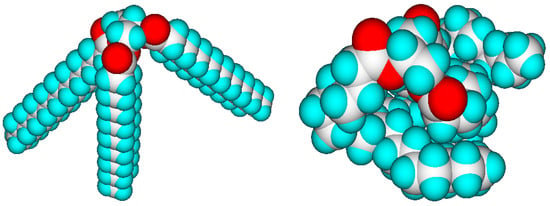
Figure 1.
Energy-minimized forms of tristearin (graphics by ChemBrain IXL). (Left) stearyl chains stretched, Vm = 997.6 A3; (right) stearyl chains randomly folded, Vm = 992.9 A3.
The first tentative rounds of heat-capacity calculations revealed that for ionic liquids—when using standard bonds and vdw radii—the predicted heat capacities were generally too low by ca. 20 J/mol/K, i.e., by ca. one standard deviation. This deficiency was remedied by modifying the bond lengths of the bonds between the central, formally charge-carrying, atom in the ions and its neighbors, in that in the case of the anions the bonds have been extended by ca. 10% and in the cations they have been shortened by the same amount. In addition, for polyatomic anions the central atom’s vdw radius has been enlarged as suggested in [42] (for monoatomic anions the vdw values are given as listed in [42]).
Taking these modifications into account, the calculation of the molecular volumes of ILs as well as their constituting cations and anions is straightforward. In order to enable readers interested in the “true” molecular volumes of ILs and subsequently their heat capacities, Table 2 lists a number of anions and cations and their calculated molecular volume which proved to be the most popular in the heat-capacity studies. Thus, the “true” molecular volume of an IL consisting of any of the cations and anions of Table 2 is simply the sum of their partial volumes. Minor differences with the directly calculated molecular volumes of corresponding ILs have to be ascribed to slight differences in the optimized geometry as discussed above.

Table 2.
Molecular volumes of popular cations and anions used in ionic liquids.
3.3. Sources of Heat-Capacity Data
The majority of experimental heat-capacity data has been found in several collective papers, of which Zabransky et al. collected a series of liquid homologous linear alkanes [43] and 1-alkanols [44], while Costa et al. [45] complemented these series by further homologues of liquid linear 1-alkenes, 1-alkynes, 1-alkylthiols, 1-alkylamines, 1-nitro- and 1-haloalkanes. Domalski and Hearing [46] contributed heat-capacity data of liquid and solid, saturated and unsaturated, linear and cyclical hydrocarbons. The same authors provided a large compilation of further data of liquid and solid molecules possessing from one to 1077 carbons [47,48]. Another large collection of data for liquids and solids was provided in the section “Standard Thermodynamic Properties of Chemical Substances” of ref. [34]. Beyond these compilations a large number of recent papers presenting the heat-capacity data of several classes of compounds and of individual molecules of any kind as well as their temperature dependence have been published up to the present. Special mention shall be given to the hydrocarbons [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65], halogenated hydrocarbons [66,67,68,69,70,71], unsubstituted and substituted alcohols and polyols including sugar derivatives [72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107], phenol derivatives [108,109], carboxylic acids [110,111,112,113,114,115,116,117,118], esters [119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138], anhydrides [139,140,141], aldehydes and ketones [142,143,144,145,146,147,148,149,150], ethers [151,152,153,154,155], amines [156,157,158,159,160,161,162], imines [163], oximes [164,165], anilines [166,167], amides [168,169,170,171], imides [172], barbiturates [173,174,175,176], ureas [177,178,179,180,181], hydantoins [182,183], carbamates [184,185], isocyanates [186], thiols, thio ethers and disulfides [187,188,189,190,191], sulfonamides [192,193,194], nitriles [195], metal complexes [196,197,198,199], silanes and siloxanes [200,201,202,203,204], nucleic bases and nucleosides [205,206,207], amino acids and peptides [208,209,210,211,212], unsubstituted and substituted hetarenes and heterocycles [213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243], high-energy nitro compounds [244,245] and various more [246,247,248,249,250,251,252,253,254,255,256,257,258,259,260].
In 2010, as the ILs gained increasing interest, Paulechka [261] sampled the room-temperature heat capacities of 102 ILs, covering the time from 1998 to 2010. In addition, a number of further IL data have been provided by the National Institute of Standards and Technology [262]. In the meantime, a number of recent papers have been published presenting further heat-capacity data of ILs [263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287].
3.4. Heat Capacity of Liquids
As mentioned in the introductory section, the VBT approach of Gardas et al. [11] and Glasser and Jenkins [28] pointed to a possible way to overcome the GA models’ disadvantage of not being globally applicable. Since ChemBrain’s database enables the search for correlations between any two molecular descriptors, it was obvious to try to find one—in accordance with the VBT approach—between the heat capacities and the “true” molecular volume, calculated as described in Section 3.2. The correlation between the “true” molecular volume and the experimentally measured liquid heat capacity of 1303 compounds of all classes indeed revealed a surprisingly good linearity over a large volume range, as shown in Figure 2, with a correlation coefficient of 0.9785 after removal of the worst outliers, despite a fairly large standard error of ca. 28 J/mol/K and a mean absolute percentage deviation (MAPD) of 8.23%.
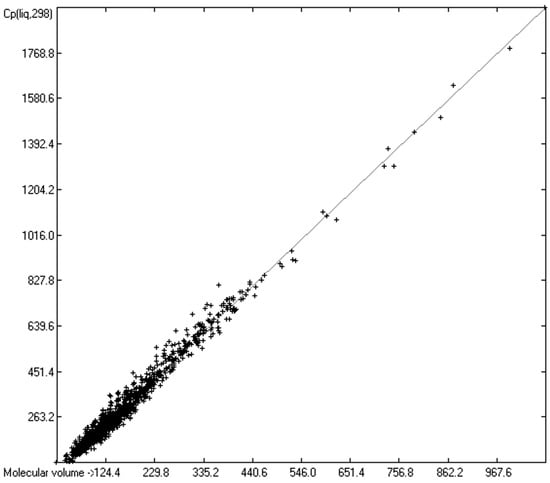
Figure 2.
Correlation of molecular volumes with Cp(liq) at 298.15 K. (N = 1303, R2 = 0.9785, σ = 27.84 J/mol/K, MAPD = 8.23%, regression line: intercept = 1.7887, slope = 1.8211).
However, a thorough analysis of the corresponding histogram (Figure 3) pointed to a severe deficiency of this preliminary correlation attempt, reflected in its apparent asymmetry with an overweight to the right side and a shift of the maximum to the left: for molecules carrying hydroxy groups, i.e., alcohols, carboxylic acids and strong acids, the predictions were systematically well below the experimentally determined heat capacities by up to ca. 130 J/mol/K! This deficiency evidently resulted from the neglect of the strong polarity of the OH group in alcohols and acids, apparently increasing the liquid heat capacity by the additional intermolecular O-H interaction, which is to be the subject of detailed discussion in Section 3.4.2.
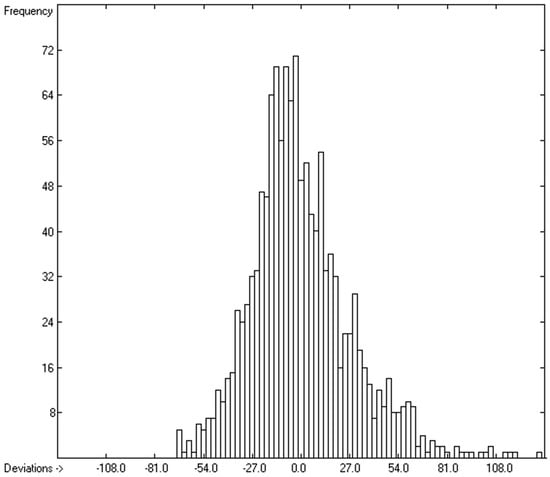
Figure 3.
Histogram of molecular volumes with Cp(liq) at 298.15 K. Values range from 75.32 to 1956.10 J/mol/K.
3.4.1. Heat Capacity of Liquids of Molecules Free of Hydroxy Groups
The intermolecular hydrogen-bridge effect found in alcohols and acids evidently required a separation of these molecules from those without this effect in the heat-capacity prediction. This task was easily achieved by a simple algorithm which scanned each molecule for the first single bond having an oxygen atom at one end and a hydrogen atom at the other and, if found, skipped it. The exclusion of these molecules from the list of liquids yielded a distinctly better compliance with the experimental data as is demonstrated in the correlation diagram (Figure 4). The corresponding histogram (Figure 5) also shows a more symmetrical deviation distribution about the zero point. The good accordance of the experimental with the calculated Cp(liq.298) values, with a correlation coefficient R2 of 0.9890 and a MAPD of 6.51% based on a large variety of chemical structures within the 1102 compounds, allows great confidence in Equation (2), which translates the “true” volume Vm of a molecule free of hydroxy groups into its liquid heat capacity at 298.15 K.
Cp(liq.298)OH-free = −5.3055 + 1.8183 × Vm
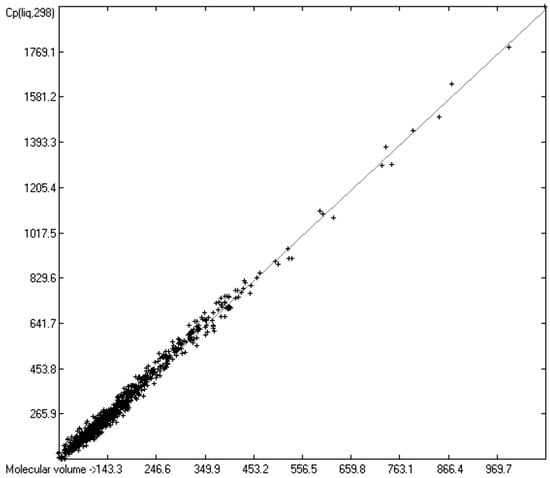
Figure 4.
Correlation of molecular volumes with Cp(liq) at 298.15 K, excluding alcohols and acids. (N = 1102, R2 = 0.9890, σ = 20.70 J/mol/K, MAPD = 6.51%, regression line: intercept = −5.3055, slope = 1.8183).
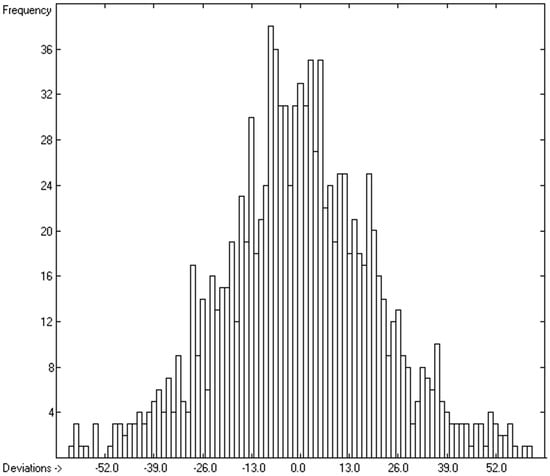
Figure 5.
Histogram of molecular volumes with Cp(liq) at 298.15 K, excluding alcohols and acids. Values range from 78.7 to 1956.10 J/mol/K.
Apart from the many ordinary compounds, for which the Cp(liq) at 298.15 K have been predicted in great accordance with experiment, the ILs have gained particular interest as a class of polar solvents, for which the knowledge of the liquid heat capacity is especially important. Hence, a list of the experimental and calculated data of the 145 ILs, which were included in the calculations of the parameters of Equation (2), are presented in Table 3. The mean values of the deviations shown at the bottom of the list indicate that Equation (2) tends to slightly underestimate liquid heat capacities by ca. 1.6%; however, in view of the relatively large “local” standard deviation of 24.49 J/mol/K or 5.14% (evaluated by means of the experimental and Equation (2)-predicted Cp values of Table 3), this deficiency seems acceptable. Beyond this, an independent direct correlation calculation with the molecular volumes and the experimental Cp(liq,298) of the ILs of Table 3 revealed a correlation coefficient of 0.9873 and a standard deviation R2 of 22.64 J/mol/K, values that are very similar to the ones received by means of Equation (2), confirming its applicability. In addition, in order to assess the cause of the fairly large “local” standard deviation of 24.49 J/mol/K for this class of compounds, an analogous correlation calculation with the homologous series of 1-CH3(CH2)n-3-methylimidazolium bis(trifluoromethanesulfonyl) amide, with n = 1–7, 9 and 11, was carried out, which should yield a steady increase of their liquid heat capacities by ca. 32.4 units per methylene group. The resulting standard deviation of 14.0 J/mol/K clearly points to experimental inaccuracy as the main cause of the large standard error.

Table 3.
Experimental and Equation (2)-calculated Cp(liq.298) of 145 OH-free ionic liquids in J/mol/K.
A further drawback concerning this class of compounds is the relatively large number of outliers, defined as molecules, for which the difference between the experimental and predicted Cp value exceeds three times the standard deviation: more than half of the total number of 80 Cp(liq,298) outliers are ILs. In some cases, their experimental value is closer to—or even below—the predicted value for the heat capacity of their solid phase. The list of Cp(liq) outliers has been added to the Supplementary Material.
Siloxanes are a class of compounds that have become increasingly important for their thermodynamic stability at high temperatures as working fluids in the field of organic rankine cycle processes [204]. Table 4 compares the experimental liquid heat capacities at 298.15 K of 23 siloxanes with the values predicted by Equation (2). The mean deviation between the experimental and the calculated values, shown at the bottom of Table 4, exhibits a general underestimation by Equation (2) by 1.5%. An independent correlation calculation with the molecular volumes of the siloxanes of Table 4 and their experimental Cp(liq,298) values yielded a correlation coefficient R2 of 0.9866 and a standard deviation of 29.09 J/mol/K. While R2 is very similar to that resulting from Equation (2) (see Figure 2), the corresponding deviation is only moderately better than that listed at the bottom of Table 4. This, on the one hand, again confirms the general applicability of the present method on the calculation of the liquid heat capacities. The relatively large standard deviation, on the other hand, requires an answer as to its origin. Therefore, an analogous correlation calculation using the (incomplete) homologous series tetramethyoxy-, tetraethoxy-, tetrapropoxy-, tetrabutoxy-, tetraheptoxy-, tetraoctoxy- and tetradecoxysilane alone, which should show a correspondingly steady increase of their liquid heat capacities by ca. 123.8 units per four methylene groups, was carried out. This calculation indeed revealed a high correlation coefficient R2 of 0.9948, but still a large scatter with a standard deviation of 27.96 J/mol/K, allowing for the assumption that the large standard deviation listed in Table 4 is essentially caused by questionable experimental data.

Table 4.
Experimental and Equation (2)-calculated Cp(liq.298) of 23 siloxanes in J/mol/K.
Finally, in Table 5, the experimental liquid heat capacities of 222 hydrocarbons are compared with the predicted ones, evaluated by means of Equation (2). The mean values of the deviations added at the bottom show that the “global” Equation (2) on average overestimates the Cp(liq,298) values of the hydrocarbons by 6.34%. The predicted liquid heat capacities of the (incomplete) homologous series of 21 linear alkanes from butane to unatriacontane, on the other hand, are in excellent agreement with the experimental values, with the exception of eicosane and unatriacontane. Therefore, the reason for the general overestimation of the hydrocarbons and their standard deviation of 22.1 J/mol/K had to be found elsewhere.

Table 5.
Experimental and Equation (2)-calculated Cp(liq.298) of 222 hydrocarbons in J/mol/K.
A careful scan through Table 5 exhibits a distinct discrepancy of the deviations between the predictions and experimental Cp(liq,298) values of the noncyclic and the cyclic hydrocarbons: the deviations of the latter are nearly always much more negative, i.e., Equation (2) systematically and distinctly overestimates their liquid heat capacity. This is particularly clear on comparison of the linear alkanes as excellent benchmarks with the cycloalkanes having the same number of carbon atoms: the predicted values of the corresponding cyclic alkanes are systematically too high. A few examples may illustrate the difference in the deviation of the predictions: butane: 2.84% vs. cyclobutane: −13.26%; pentane: −0.01% vs. cyclopentane: −15.3%; hexane: 0.08% vs. cyclohexane: −12.14%; heptane: −1.1% vs. cycloheptane −13.23%; octane: −0.99% vs. cyclooctane: −10.29%. The same observation has been made with branched cycloalkanes, e.g., methylcyclopentane vs. hexane or dimethylcyclohexane vs. octane. As these deviations are systematic and therefore cannot be ascribed to experimental inaccuracies, they indicate an important limitation of the present prediction method: the “true” molecular volume does not adequately reflect the decrease of the rotational degrees of freedom within the cyclic moiety of a molecule in relation to a ring-open one, although the volume of a cyclic structure is necessarily smaller than a non-cyclic one with the same number of carbon atoms due to the deduction of the partial volume of two hydrogen atoms per cycle which, however, only corresponds to a difference of 17–23 J/mol/K per cycle between cyclic and non-cyclic alkanes, depending on the molecule size.
A weaker trend of this kind can be found when comparing linear with branched alkanes of the same chemical formula in that the branched species have systematically and significantly lower experimental heat capacities than their linear relatives, although their molecular volumes are all of nearly equal size, and thus Equation (2) would suggest very similar Cp values. Some examples of the differences of deviation may be given: pentane: −0.01% vs. 2,2-dimethylpropane (neopentane): −9.01%; heptane: −1.10% vs. 3,3-dimethylpentane: −5.40%. Hence, it seems that branching also lowers the number of effective rotational and possibly vibrational degrees of freedom. Nevertheless, these prediction errors rarely exceed the methods’ standard deviation but should be kept in mind when applying Equation (2).
3.4.2. Heat Capacity of Liquid Alcohols and Acids
The exceptionally high polarity of the hydroxy group has been shown in the previous subsection to exhibit a decisive, enhancing effect on the heat capacity of a molecule. Figure 6 demonstrates the correlation of the molecular volume with the experimental liquid heat capacity of the 194 alcohols and acids extracted from the complete set used in Figure 2, revealing, apart from a fairly large scatter, some peculiarities in the volume range of 135 to 190 A3 that require an explanation. Beyond this, the obvious asymmetry of the corresponding histogram (Figure 7) hints at a similar deficiency of this simple prediction method as in the previous subsection, this time disclosing a systematic underestimation of the molecules carrying two or more OH groups. This inadequacy was resolved by separating the molecules carrying a single OH group from those carrying two or more. The results are shown in the correlation diagrams in Figure 8 and Figure 9. The corresponding lists of molecules with their molecular volumes, experimental and predicted data as well as their deviations are added as Table 6 and Table 7.
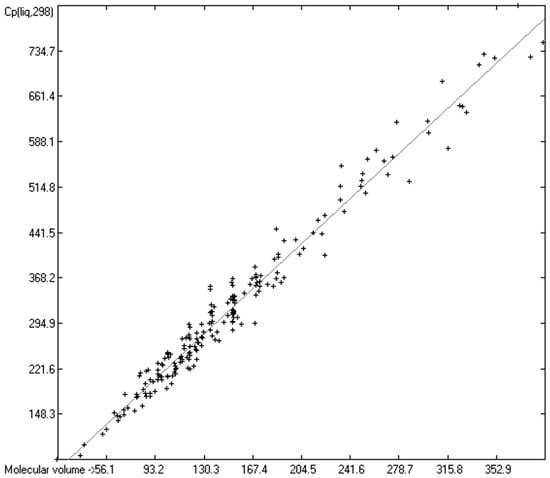
Figure 6.
Correlation of molecular volumes with Cp(liq) at 298.15 K of mono- and polyols and –acids. (N = 201, R2 = 0.9724, σ = 23.24 J/mol/K, MAPD = 6.11%, regression line: intercept = 20.9141, slope = 1.9676).
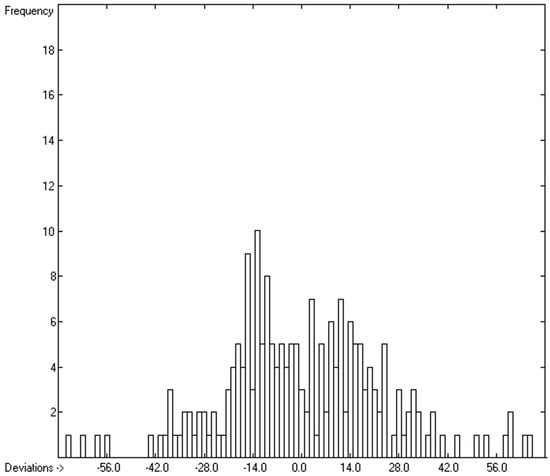
Figure 7.
Histogram of molecular volumes with Cp(liq) at 298.15 K of 202 mono- and polyols and –acids. Value range from 75.32 to 807.5 J/mol/K.
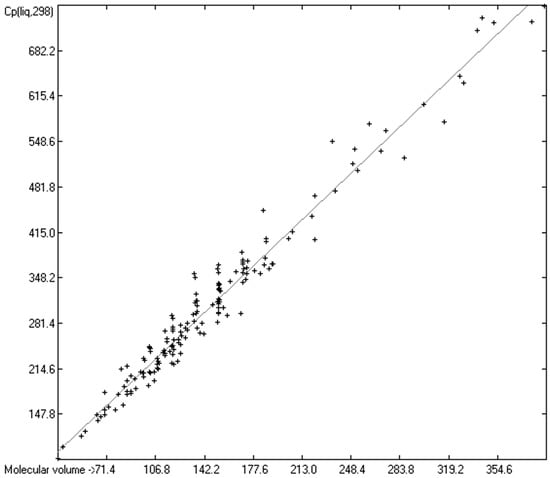
Figure 8.
Correlation of molecular volumes with Cp(liq) at 298.15 K of monools and monoacids. (N = 164, R2 = 0.9685, σ = 22.91 J/mol/K, MAPD = 6.04%, regression line: intercept = 23.3101, slope = 1.9282, Value range from 81.92 to 748.0 J/mol/K).
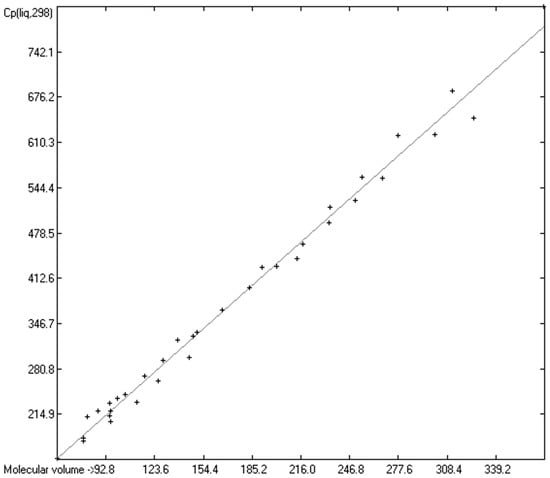
Figure 9.
Correlation of molecular volumes with Cp(liq) at 298.15 K of polyols and polyacids. (N = 36, R2 = 0.9910, σ = 16.03 J/mol/K, MAPD = 3.77%, regression line: intercept = 23.5782, slope = 2.0422, Value range from 149.8 to 807.5 J/mol/K).

Table 6.
Molecular volumes Vm (in A3), experimental and Equation (3)-calculated Cp(liq.298) (in J/mol/K) of 164 monools and monoacids.

Table 7.
Molecular volumes Vm (in A3), experimental and Equation (4)-calculated Cp(liq.298) (in J/mol/K) of 36 polyols and polyacids.
The corresponding Equation (3) for the heat capacity (Cp(liq.298)OH1) of the monoalcohols and –acids and Equation (4) for the heat capacity (Cp(liq.298)OH>1) of the polyalcohols and –acids are therefore as follows, wherein Vm is the “true” molecular volume:
Cp(liq.298)OH1 = 23.3101+ 1.9282 × Vm
Cp(liq.298)OH>1 = 23.5782 + 2.0422 × Vm
As mentioned, at the beginning of this subsection, Figure 6 and Figure 8 reveal an abnormality in the volume range at ca. 135 and 190 A3, in that the experimental Cp(liq,298) values of clusters of compounds with similar molecular volume distinctly deviate from the predicted values, hinting at a systematic deficiency of the present prediction method as already discussed with the OH-free compounds. In order to assess the importance of these deviations, it is helpful to start from a common base. In this case, in analogy to the linear alkanes used in the prior subsection, the linear alcohols may serve as the starting point. Scanning Table 6, the experimental values of this group of alcohols systematically deviate from the predictions by, on average, −10.75 J/mol/K (the value for 1-hexadecanol has been omitted as being an obvious outlier with −54.06 J/mol/K), i.e., Equation (3) systematically overestimates their Cp(liq,298) values. A similar overestimation is found with the branched 1-alkanols in Table 6, e.g., 2-methyl-1-propanol, 2-ethyl-1-butanol, 3,3-dimethyl-1-butanol and 2-methyl-1-pentanol. An even larger overestimation of, on average, −18.58 J/mol/K is found for the four cyclic alcohols cyclobutanol, cyclopentanol, cyclohexanol and cycloheptanol. On the other hand, with the exception of 2-propanol the experimental Cp(liq,298) values of all the 16 unbranched secondary alcohols of Table 6, i.e., 2-butanol, 3-pentanol, 2- and 3-hexanol, 2-, 3- and 4-heptanol, 2-, 3- and 4-octanol, 2-, 3-, 4- and 5-nonanol and 5-decanol, are higher by an average of +13.16 J/mol/K than the predicted ones. Even worse is the deviation of the 17 branched secondary alcohols in Table 6 with an average of +14.51 J/mol/K. However, the largest underestimation by Equation (3) with an average deviation of +35.82 J/mol/K was found for the 8 tertiary alcohols 2-methyl-2-propanol, 2-methyl-2-butanol, 2-methyl-2-pentanol, 3-methyl-3-pentanol, 2-Methyl-2-hexanol, 2-methyl-2-heptanol, 4-methyl-4-heptanol 4-propyl-4-heptanol of Table 6.
Comparison of these findings with those discussed with the alkanes of the prior subsection yields one accordance and one striking contradiction: accordance is found with the cyclic compounds in that in both cases Equation (3) overestimates the experimental Cp(liq,298) values. On the other hand, the results of the unbranched and branched secondary and tertiary alcohols are counterintuitive in that they all may be viewed as analoga of the branched alkanes and, thus should also have lower liquid heat capacities than their linear relatives. Serra et al. [73] explained the discrepancies of the heat capacities of a series of C7 alcohols by the strength of the hydrogen bridges, which they assumed to be dependent on the steric hindrance of the hydroxy group. This steric hindrance is lowest with primary alcohols and highest with tertiary alcohols, as is demonstrated in Figure 10. Hence, the hydrogen bridging potential should increase in the order tertiary < secondary < cyclical < primary alcohols. The findings in Table 6 obviously oppose this order because it is common knowledge that the stronger the hydrogen bridges, the higher the heat capacity, e.g., compare water ((Cp(liq,298) = 75.32 J/mol/K) with ammonia ((Cp(liq,298) = 33 J/mol/K). The resolution for this conflict might have been provided by the theoretical studies of Huelsekopf and Ludwig [288], who demonstrated, by means of the quantum cluster equilibrium theory (QCE), exemplified on two primary (ethanol and benzyl alcohol) and a tertiary alcohol (2,2-dimethyl-3-ethyl-3-pentanol), that the primary alcohols principally exist as cyclic tetramers and pentamers in the liquid phase, whereas the tertiary alcohol only forms monomers and dimers. In other words, the higher liquid heat capacity of the secondary and tertiary alcohols could be owed to the formation of smaller clusters, which overall leads to a higher number of rotational and translational degrees of freedom. This could explain the systematic overestimation of the liquid heat capacity of the 1-alkanols by Equation (3) in that in these cases the experiments were probably carried out on cyclical tetra- or pentamers exhibiting lower translational and rotational freedoms than smaller clusters or even monomers. It could also explain the exceptionally low heat capacity of the saturated cyclic alcohols: as demonstrated in Figure 10, the hydroxy group in cyclic alcohols is sterically hardly hindered and, thus could also form cyclic tri-, tetra- or pentamers, reducing the number of motional degrees of freedom in addition to the reduction of the degrees of freedom due to cyclic skeleton of the compound itself as discussed for the cycloalkanes. Carboxylic acids are known to build dimers in the liquid phase. Hence, one would expect—following the arguments for the linear alcohols—that their liquid heat capacity should again be generally lower than calculated. This is indeed the case: the mean deviation from the calculated values is −19.57 J/mol/K for the 11 linear monocarboxylic acids from acetic acid to dodecanoic (lauric) acid listed in Table 6. All these systematic deviations between experiment and prediction in the classes of alcohols and acids demonstrate a fundamental limit of the present prediction method in that it can principally not consider intermolecular effects on the liquid heat capacity. This reflects the findings of Ruzicka and Domalski [7], who stated that the “group of oxygen compounds includes families, such as alcohols and aldehydes, that exhibit the largest prediction error of all families of organic compounds” by their second-order GA method. However, they did not elaborate on the reason.
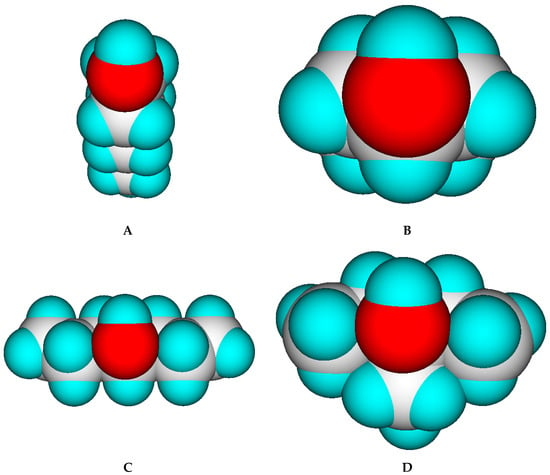
Figure 10.
Top view of energy-minimized forms of four C7 alcohols (graphics by ChemBrain IXL). (A): 1-heptanol. (B): 4-methylcyclohexanol, (C): 4-heptanol. (D): 3-ethyl-3-pentanol.
For comparison, the correlation statistics of the OH-free compounds (Figure 4) with those of the alcohols and acids before separation (Figure 6), and those of the mono-alcohols and –acids (Figure 8) as well as those of the poly-alcohols and –acids (Figure 9) have been collected in Table 8. The difference in the heat capacities of the OH-free molecules and the alcohols and acids appears very prominently in the values of the intercepts and the slopes. Comparison of these two values clearly shows the large enhancing effect of the OH groups on the liquid heat capacity of the alcohols and acids. In addition, Table 8 also reveals a close similarity between the set of alcohols and acids before separation and that of the monoalcohols and –acids. Finally, the largest intercept and slope for the polyalcohols and –acids indicate that this group generally exhibits the highest Cp(liq,298) values. The exceptionally high correlation coefficient of 0.991 for this group, however, should not be overrated, as it mostly consists of linear, α,o-substituted, primary alcohols and acids. (The sum of the mono- and polyalcohols/acids only adds up to 200; the missing 201st compound of Figure 6 is water, which has not been included in the separated sets).

Table 8.
Statistics of the correlations between molecular volumes and liquid heat capacities at 298.15K.
3.5. Heat Capacity of Solids
While true liquid phases of molecules are isotropic and thus appear as only one single phase in the measurement of their heat capacity, solids require special care with respect to the association phase in which the molecules are arranged at room temperature. In many cases molecules crystallize in several, energetically different phases, which not only have different heat capacities but can also change from one phase into another one at the time of the heat capacity measurement. Beyond this, quite often the molecules, although in a seemingly solid form, have not really crystallized but are in truth a supercooled melt. These uncertainties may be a major reason for the larger scatter of the Cp(sol,298) compared to the Cp(liq,298) values measured by independent sources as referenced by Chickos et al. [5]. Irrespective of these difficulties, the heat capacity of solids should always exhibit a lower value than that of liquids due to the inhibition of the translational and rotational freedoms of motion of the molecules in the crystalline phase. In order to compare the heat capacities of solids with those of liquids, the total of 797 compounds with known experimental Cp(sol,298) data have been separated into an OH-free set and one that encompasses all alcohols, sugars and acids. The correlation of the former set is shown in Figure 11, that of the latter in Figure 12. The OH-containing 241 compounds have been further separated into 123 monools (Table 9) and monoacids and into 118 polyols and polyacids (Table 10). Their corresponding correlations are visualized in Figure 13 and Figure 14.
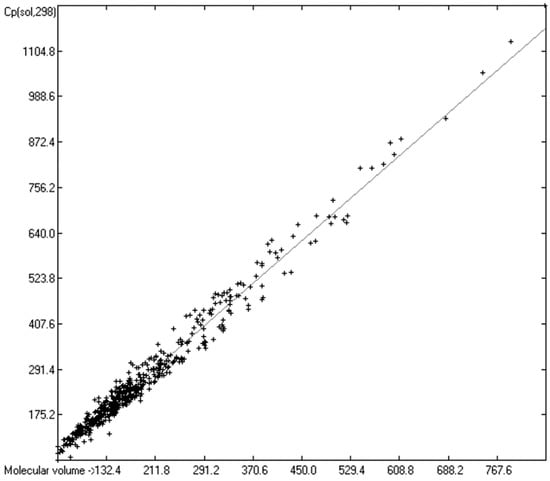
Figure 11.
Correlation of molecular volumes with Cp(sol) at 298.15 K of OH-free compounds. (N = 555, R2 = 0.9766, σ = 23.14 J/mol/K, MAPD = 7.19%, regression line: intercept = 2.8899, slope = 1.3669, value range from 59.4 to 1220.9 J/mol/K).
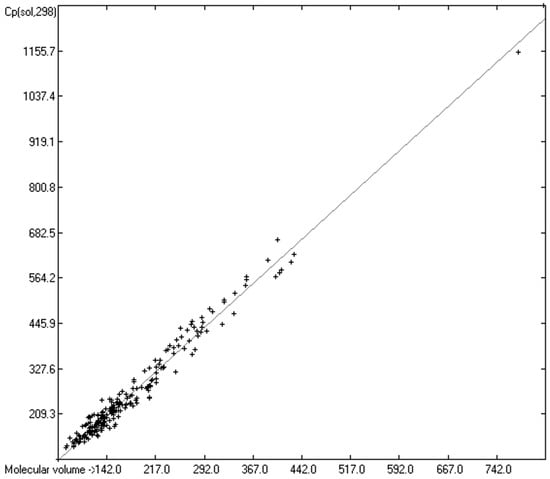
Figure 12.
Correlation of molecular volumes with Cp(sol) at 298.15 K of mono- and polyols and -acids. (N = 242, R2 = 0.9792, σ = 20.87 J/mol/K, MAPD = 6.72%, regression line: intercept = −14.7861, slope = 1.5364, value range from 91 to 1273 J/mol/K).

Table 9.
Molecular volumes, experimental and calculated Cp(sol.298) of 123 monools and monoacids in J/mol/K.

Table 10.
Molecular volumes Vm (in A3), experimental and calculated Cp(sol.298) (in J/mol/K) of 119 polyols and polyacids.
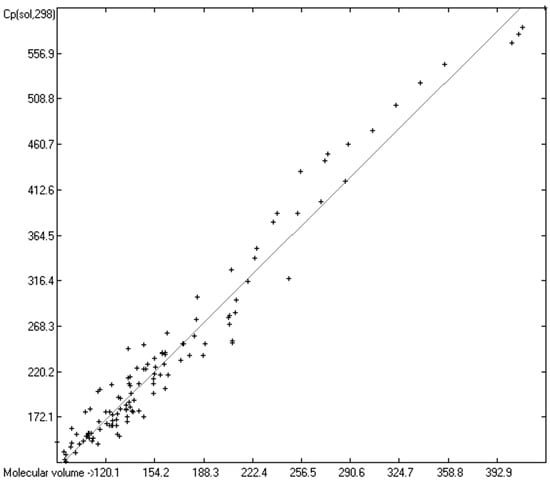
Figure 13.
Correlation of molecular volumes with Cp(sol) at 298.15 K of monools and monoacids. (N = 123, R2 = 0.9613, σ = 21.62 J/mol/K, MAPD = 7.34%, regression line: intercept = −11.2179, slope = 1.5050, value range from 124.7 to 604.8 J/mol/K).
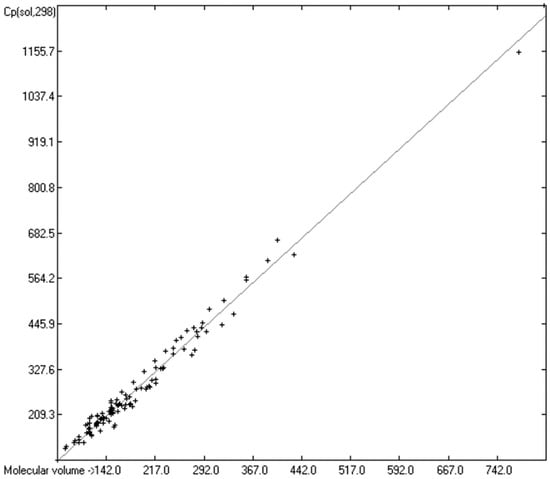
Figure 14.
Correlation of molecular volumes with Cp(sol) at 298.15 K of polyols and polyacids. (N = 119, R2 = 0.9866, σ = 19.75 J/mol/K, MAPD = 5.93%, regression line: intercept = −14.9656, slope = 1.5462, value range from 91 to 1273 J/mol/K).
The parameters for the final Equations (5)–(7) for the prediction of the solid heat capacities correspond to the intercepts and the slopes of the regression lines resulting from the correlations in Figure 11, Figure 13 and Figure 14. In these equations the variable Vm and the subscripts at the heat capacities Cp(sol,298) have the same meanings as given for Equations (2)–(4):
Cp(sol.298)OH-free = 2.8899 + 1.3669 × Vm
Cp(sol.298)OH1 = −11.2179 + 1.5050 × Vm
Cp(sol.298)OH>1 = −14.9656 + 1.5462 × Vm
Figure 11 demonstrates the good correlation of the “true” molecular volume with the experimental solid heat capacity of compounds of all OH-free classes, i.e., excluding alcohols, sugars and acids. Among these classes, the metallocenes [196,197,198,199] in Table 11 are especially interesting in that they demonstrate the inertness of the heat capacity towards the central atom and its McGowan-vdw radius [42], which varies from 0.74 A for Fe2+ to 0.99 A for Mn2+, because the metal ions are nearly completely encapsulated by the two cyclopentadienyl ligands.

Table 11.
Experimental and Equation (5)-calculated Cp(sol.298) of 9 metallocenes in J/mol/K.
In Table 12, the solid heat capacities of the siloxanes [201,202,203,204] are collected for comparison with the values of their liquid heat capacities in Table 4. The experimental Cp(sol,298) values are on average underestimated by 10.64 J/mol/K by Equation (5). An independent Vm vs. Cp(sol,298) correlation calculation of these 16 siloxanes yielded a correlation coefficient of 0.9778 and a standard deviation of 38.29 J/mol/K, confirming the fairly large scatter.

Table 12.
Experimental and Equation (5)-calculated Cp(sol.298) of 16 siloxanes in J/mol/K.
Table 13 presents a list of the hydrocarbons for which experimental solid heat capacities were available. Their mean deviation, shown at the bottom of Table 13, again indicates a general underestimation of the Cp(sol,298) values for the hydrocarbons by Equation (5) by ca. 4%. (An independent calculation, based on the hydrocarbons of Table 13 only, resulted in a correlation coefficient of 0.9779, a standard deviation of 28.35 J/mol/K and a MAPD of 6.96%. The intercept of the regression line was calculated to −21.9433 and the slope to 1.4291). The standard deviation of their experimental values from the predicted ones is much larger than that of their liquid heat capacity shown in Table 5, which could be ascribed to uncertainties such as various crystal forms mentioned at the beginning of this subsection. Some examples may shed some light on their impact: the three structural isomers o-, m- and p-quinquephenyl have nearly the same molecular volume of 364.1 A3, resulting in a predicted Cp(sol,298) value of 500.6 J/mol/K. Yet, the experimental values are given as 444.3, 443.7 and 455.5 J/mol/K, respectively, i.e., their values deviate by up to 11.9 units. Similarly, for the three isomers o-, m- and p-terphenyl, a solid heat capacity was calculated to 308.7 ± 0.7 J/mol/K; the experimental values are 274.75, 281.0 and 279.6 J/mol/K, respectively, i.e., a difference of up to 6.25 units. Evidently, in both cases the ortho-isomer has a helical structure, in contrast to the more planar structure of the m- and p-isomers, which probably leads to a crystal structure that differs from that of the m- and p-isomer. For anthracene and phenanthrene, two very closely related compounds, the present prediction method suggests Cp(sol,298) values of 234.9 and 233.8 J/mol/K; the experimental values are 210.5 and 220.3, respectively, i.e., a difference of 9.8 units. Finally, for the three isomers 2,3-, 2,6- and 2,7-dimethylnaphthalene Cp(sol,298) values of 219.8, and twice 220.5 J/mol/K, respectively, have been predicted; the experimental values are 216.47, 204.39 and 204.20 J/mol/K, a difference of up to 12.27 units. Nevertheless, these deviations never exceeded the standard deviation of 23.66 J/mol/K.

Table 13.
Experimental and Equation (5)-calculated Cp(sol.298) of 107 hydrocarbons in J/mol/K.
In Table 14, the correlation statistics data of the heat capacities of solids have been collected. In this table, the differences between the heat capacities of the solid OH-free compounds and those of the solid alcohols, acids and sugars are again centered on the parameters of the regression lines, i.e., the intercepts and slopes, analogous to the ones discussed in the case of the liquids (Table 8). Comparing the corresponding parameters in Table 8 for liquids with those in Table 14 for solids and applying them in the respective Equations (2)–(7), it is immediately evident that the heat capacities of solids calculated in this way are indeed always smaller than those of liquids. A rough calculation, however, comparing the results of Equations (5)–(7), e.g., using an average molecular volume, reveals much smaller differences of the solid heat capacities between the OH-free compounds and the alcohols or acids than when applying Equations (2)–(4) in the case of their liquid heat capacities. This is in accord with the notion that hydrogen bridges in crystals have no significantly additional effect on the inherently restricted freedoms of motion. Their contribution is essentially of the vibrational type.

Table 14.
Statistics of the correlations between molecular volumes and solid heat capacities at 298.15K.
Equations (2)–(7) enable an estimate of the heat-capacity differences between the liquid and solid phase for the three classes of compounds by simply subtracting the respective equations for the liquid phase from the ones for the solid phase. Thus, for the OH-free class of compounds, the rounded difference ΔCp(298)Oh-free is calculated by Equation (8), and for the other classes the corresponding differences are defined by the Equations (9) and (10):
ΔCp(298)OH-free = −8.20 + 0.45 × Vm
ΔCp(298)OH1 = 34.53 + 0.42 × Vm
ΔCp(298)OH>1 = 38.54 + 0.50 × Vm
The comparison of Equation (8) with Equation (9) immediately shows the large median additional effect of a hydroxy group on the heat capacity upon the phase change of a molecule from the solid into the liquid phase, exemplified by two compounds of similar molecular volume: anisole (109.1 A3)) and o-cresol (108.5A3). For both compounds a solid heat capacity of ca. 152 J/mol/K was predicted (for o-cresol the experimental value is 154.56 J/mol/K [48]). For anisole the calculated liquid Cp value was 193.1, for o-cresol 232.7 J/mol/K. These values have been confirmed by the experimental data: for anisole a value of 199 J/mol/K [47] was published, for o-cresol 229.75 J/mol/K [48]. Equation (10) indicates that further hydroxy groups provide a substantially lower contribution.
At the beginning of this subsection, the subject of the definition of the solid phase of a molecule under experimental conditions has been mentioned, which adds an uncertainty to the experimental value of the solid heat capacities. Beyond this, since the present calculation method allows the prediction of both the solid and liquid heat capacity of any molecule, it can be demonstrated that in several cases the experimental value of the alleged solid heat capacity of a molecule is much more closely related to that of its liquid phase. This may be illustrated by two examples: for benzylideneaniline, a Cp value of 302.67 J/mol/K was measured [163]; the solid heat capacity was predicted at 244.5 J/mol/K and the liquid one at 316.1 J/mol/K. In view of its melting point of 54 °C which is fairly close to the experimental conditions, the assumption is not unrealistic that the compound could at least be partially molten. For 2-methyl-3-amino-4-methoxymethyl-5-aminomethylpyridine, the experimental—supposedly solid—heat capacity was published as 307.1 J/mol/K [48]; the corresponding solid and liquid Cp values have been calculated to 240.3 and 310.5 J/mol/K, respectively. A melting point has not been given. A number of outliers, which had to be excluded from the solid heat-capacity calculations and are separately listed in the Supplementary Material, would have suited very well in the liquid Cp calculations and vice versa. In fact, for several molecules the experimental solid Cp value was even higher than their calculated liquid Cp value. To set the experimental values in relation to the predicted ones, both the calculated solid and liquid Cp data have been added to the liquid and solid outliers lists.
3.6. Temperature Dependence of the Heat Capacities
The great majority of the publications cited in Section 3.3, particularly the more recent ones, not only present heat capacity data at specific temperatures but also provide temperature profiles over a certain range, e.g., from below the molecule’s melting up to its boiling point. Considering the evident linearity of the correlations between the “true” molecular volumes and the Cp values at the ultimately arbitrary 298.15 K, it was obvious to try to find analogous correlations at different temperatures. In order to receive a representative picture of the influence of the temperature on the correlations, two temperatures below and two above the standard value have been chosen at a space of 25 K, resulting in a set of the four temperatures at 250, 275, 325 and 350 K. Cp values not directly listed at these temperatures in the publications have been linearly interpolated by means of values listed at the nearest two temperatures. This set was then applied on the group of OH-free and OH-carrying compounds in their liquid and solid phase. (It quickly turned out that a separation of the OH-carrying group of compounds into a mono- and a polyhydroxy subgroup was either not feasible due to the insufficient number of examples or had a negligible impact on the results). Figure 15, Figure 16, Figure 17 and Figure 18 demonstrate the correlation diagrams at the mentioned temperatures for the liquid and solid phase of the OH-free and OH-carrying sets of compounds. In Table 15, Table 16, Table 17 and Table 18 their respective statistical data have been collected and combined with the statistics data of the correlation at the standard temperature.
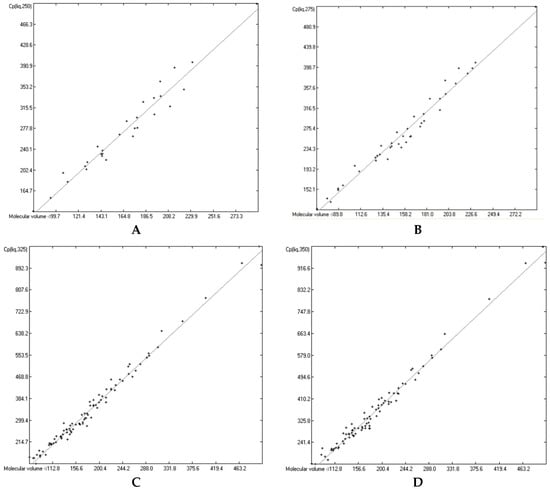
Figure 15.
Correlation of molecular volumes with liquid heat capacities of OH-free compounds at various temperatures. (A): 250 K. (B): 275 K. (C): 325 K. (D): 350 K.
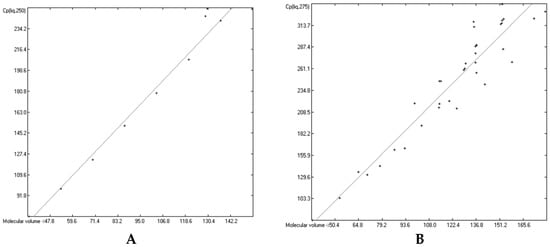
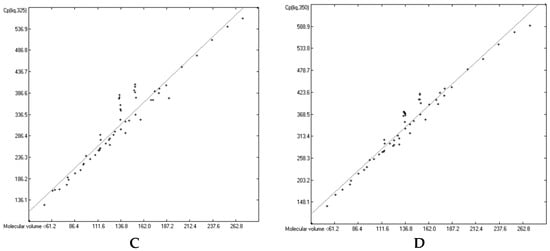
Figure 16.
Correlation of molecular volumes with liquid heat capacities of OH-carrying compounds at various temperatures. (A): 250 K. (B): 275 K. (C): 325 K. (D): 350 K.
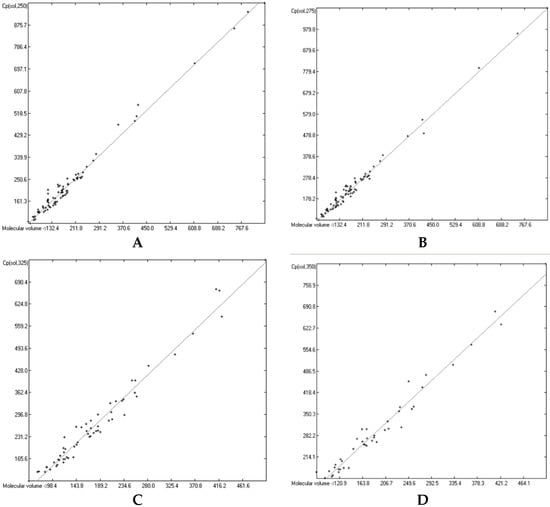
Figure 17.
Correlation of molecular volumes with solid heat capacities of OH-free compounds at various temperatures. (A): 250 K. (B): 275 K. (C): 325 K. (D): 350 K.
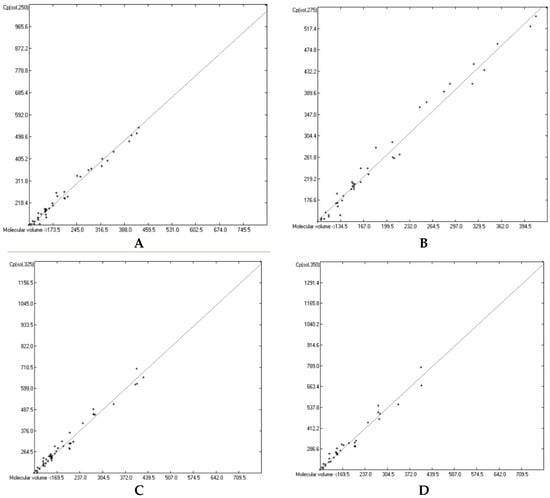
Figure 18.
Correlation of molecular volumes with solid heat capacities of OH-carrying compounds at various temperatures. (A): 250 K. (B): 275 K. (C): 325 K. (D): 350 K.

Table 15.
Statistics of liquid heat capacities of OH-free compounds at various temperatures.

Table 16.
Statistics of liquid heat capacities of OH-carrying compounds at various temperatures.

Table 17.
Statistics of solid heat capacities of OH-free compounds at various temperatures.

Table 18.
Statistics of solid heat capacities of OH-carrying compounds at various temperatures.
Figure 15, Figure 16, Figure 17 and Figure 18 prove that linearity of the Vm vs. Cp correlations is independent of temperature. Figure 16B–D, showing the liquid heat capacities of OH-carrying compounds at 275, 325 and 350 K, exhibit the largest scatter of data and thus yielded the poorest correlation coefficients (see Table 16), reflecting the observation of the strong effect on the intermolecular hydrogen bonds on going from primary to secondary and tertiary alcohols discussed earlier. Looking at Table 15, Table 16, Table 17 and Table 18, a common feature is immediately apparent: the slope of the regression lines always increases with the increasing temperature. This seems logical considering that compounds of any molecular volume have no heat capacity at all at 0 K, and thus the slope would have a value of zero, and that, on the other hand, with both the growing molecular volume as well as the rising temperature the number of degrees of freedom of motion and vibration increases, thus multiplying their increasing effect on the heat capacities. Analogous to the Equations (2)–(7), Table 15, Table 16, Table 17 and Table 18 enable the calculation of the solid and liquid heat capacities at 250, 275, 325 and 350 K, using the corresponding general Equation (11), wherein T is the selected temperature in K, the values of the intercept and slope are given for this temperature in Table 15, Table 16, Table 17 and Table 18, and Vm is the “true” molecular volume:
Cp(T) = intercept + slope × Vm
In Table 19 and Table 20 a number of examples demonstrates the expandability of the present Cp-prediction method to a range of temperatures. For many compounds, nearly perfect linearity of the temperature dependence of the heat capacity in the range between 250 and 350 K has been graphically demonstrated (specifically for hexatriacontane [51], alkylsubstituted adamantanes [53], neopentylbenzene [54], 4,4′-disubstituted biphenyls [59], tetracene and pentacene [62], 2-propenol and cyclohexylalcohols [76], adamantanols [77], monoterpenoids [85], a,ω-alkanediols [88], 1,2-cyclohexanediol [99], ribose and mannose [100], ketohexoses [101], glucose [102], sugar alcohols [103], 3,5-di-t-butylsalicylic acid [111], 2-pyrazinecarboxylic acid [116], vitamin B3 [118], 2,4-dinitrobenzaldehyde [145], various monoterpenes [147,148], 2-pyridinealdoxime [164], chloroanilines and chloronitrobenzenes [166], linear alkyldiamides [170], 2-thiobarbituric acids [175], monuron [179], 1,3,5-trithiane [189], ferrocene derivatives [198,199], cyclic siloxanes [202], adenosine [206], tryptophan [210], carnitine [211], 2-(chloromethylthio)benzothiazole [231], 2-amino-5-nitropyridine [233], 2-aminopyridine [234], 4-dimethylaminopyridine [236], 8-hydroxyquinoline [237], caffeine [238], 4′-bromomethyl-2-cyanobiphenyl [249], myclobutanil [250], fenoxycarb [251], methylprednisolone [254], N-methylnorephedrine [255], N,N-dimethylnorephedrine hydrochloride [256], risperidone [258], and vitamin B2 [259]). Table 19 and Table 20 have made use of this linearity in that the predicted values of some intermediate temperatures have been linearly interpolated using the two values of the nearest temperatures, calculated by means of Equation (11) and the corresponding parameters of Table 15, Table 16, Table 17 and Table 18.


Table 19, presenting 1-phenylpyrazole as an example of the OH-free compounds and 1-octanol representing the OH-carrying molecules class for the calculation of their liquid heat capacities, very clearly shows the difference in the reliability of the correlations of these two classes: the Cp predictions for the alcohol reveal larger deviations over all temperatures than for the OH-free compound, reflecting the larger scatter in the Figure 16D and the correspondingly lower correlation coefficients in Table 16. The representative of the OH-free class in Table 20, 3,4-dimethoxyphenylacetonitrile, has a melting point of 311.82 K (38.67 °C). Hence, the calculated solid heat capacity at 325 K is fictional, because at this temperature the compound has probably mostly changed to the liquid phase. However, its calculation enabled the interpolation of the Cp(sol) data at 310 and 320 K, which reveals by the increasing deviation from the experimental values that these are already increasingly “contaminated” by the energy absorption caused by the phase change process.
4. Conclusions
This paper has presented a universally applicable method for the calculation of the heat capacities of the solid and liquid phase of all molecules, based on a property that is common to every molecule, its molecular volume. The main advantage of the present approach lies in its absence of any requirement of further experimental data to evaluate a molecule’s “true” volume, which allows the extension of the correlation results between molecular volumes and known experimental heat capacities for liquids and solids for the heat-capacity prediction of any further imaginable molecule. Therefore, in project ChemBrain IXL, the predicted Cp data for liquids and solids have been routinely added to all of the presently ca. 31’600 compounds. The enablement to predict both the heat capacities of the liquid as well as the solid phase even for molecules for which at standard conditions only one of them is experimentally accessible, e.g., in borderline cases, allows an assessment as to whether an examined compound is really present in a defined crystalline form or e.g., rather a super-cooled melt. The possibility to cover the entire scope of compounds by means of just two parameters of a linear regression line has also enabled the discovery of structural effects that have a strong influence on the accuracy of the prediction, two of which have been outlined in detail: (1) Cyclisation and branching, demonstrated with alkanes, diminishes the heat capacity of liquids. (2) The strong influence on the liquid heat capacity of the intermolecular hydrogen bonds with alcohols and acids as well as the restrictive effects of steric hindrance on the hydroxy group has been demonstrated. Despite the observation that these structural influences and the direction and magnitude of their effect are generally within the range of one or two standard deviations, they have to be taken into account on assessing the predicted values. Furthermore, it has been shown that the linearity of the correlation between the molecular volume and the heat capacity is not limited to the standard temperature, which also enables a reasonably reliable prediction of the temperature dependence of the heat capacities, at least in the vicinity of the standard temperature. The presented prediction approach evidently does not allow for a simple paper-and-pencil Cp calculation as described for other thermodynamic and further properties [30,31,32,33]. However, the computer algorithm for the calculation of the “true” molecular volume and the subsequent evaluation of the heat capacities by means of one of the Equations (2)–(7) is very simple and thus easily integrable in software dealing with 3D-molecular structures.
The present work is part of an ongoing project called ChemBrain IXL available from Neuronix Software (www.neuronix.ch, Rudolf Naef, Lupsingen, Switzerland).
Supplementary Materials
Supplementary materials can be accessed at: https://www.mdpi.com/1420-3049/24/8/1626/s1. The lists of 3D structures of the compounds and their experimental values used for the liquid and solid heat-capacities calculations are available online as standard SDF files under the names “S01. Compounds List for Cp(liq,298) calculations.sdf” and “S02. Compounds List for Cp(sol,298) calculations.sdf”, respectively. The compounds used in the correlation diagrams of Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17 and Figure 18D as well as their experimental and calculated heat-capacities data are collected under the self-explanatory file names numbered as S03 to S27. The lists of outliers of the respective heat-capacities calculations are available as Excel files under the names “S28. Outliers of Cp(liq,298) by Vm approach.xls” and “S29. Outliers of Cp(sol,298) by Vm approach.xls”. The figures are available as tif files and the tables as doc files under the names given in the text.
Funding
This research received no external founding.
Acknowledgments
Ruaolf Naef is deeply indebted to W. E. Acree for providing the majority of literature references and for many valuable discussions. He is also indebted to the library of the University of Basel for allowing full and free access to the electronic literature database.
Conflicts of Interest
The author declares no conflict of interest.
References
- Karasz, F.E. Adiabatic calorimetry. Contemp. Phys. 1963, 4, 321–330. [Google Scholar] [CrossRef]
- Watson, E.; O’Neill, S. Differential Microcalorimeter. U.S. Patent 3,263,484, 2 August 1966. [Google Scholar]
- Simon, S.L. Temperature-modulated differential scanning calorimetry: Theory and application. Thermochim. Acta 2001, 374, 55–71. [Google Scholar] [CrossRef]
- Danley, R.L. New modulated DSC measurement technique. Thermochim. Acta 2003, 402, 91–98. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hesse, D.G.; Liebman, J.F. A Group Additivity Approach for the Estimation of Heat Capacities of Organic Liquids and Solids. Struct. Chem. 1993, 4, 261–269. [Google Scholar] [CrossRef]
- Ruzicka, V.; Domalski, E.S. Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature using Group Additivity. I. Hydrocarbon Compounds. J. Phys. Chem. Ref. Data 1993, 22, 597–618. [Google Scholar] [CrossRef]
- Ruzicka, V.; Domalski, E.S. Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature using Group Additivity. II. Compounds of Carbon, Hydrogen, Halogens, Nitrogen, Oxygen, and Sulfur. J. Phys. Chem. Ref. Data 1993, 22, 619–657. [Google Scholar] [CrossRef]
- Zàbransky, M.; Ruzicka, V. Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature using Group Additivity: An Amendment. J. Phys. Chem. Ref. Data 2004, 33, 1071–1081. [Google Scholar] [CrossRef]
- Goodman, B.T.; Wilding, W.V.; Oscarson, J.L.; Rowley, R.L. Use of the DIPPR Database for Development of Quantitative Structure-Property Relationship Correlations: Heat Capacity of Solid Organic Compounds. J. Chem. Eng. Data 2004, 49, 24–31. [Google Scholar] [CrossRef]
- Kolskà, Z.; Kukal, J.; Zàbransky, M.; Ruzicka, V. Estimation of the Heat Capacity of Organic Liquids as a Function of Temperature by a Three-Level Group Contribution Method. Ind. Eng. Chem. Res. 2008, 47, 2075–2085. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. A Group Contribution Method for Heat Capacity Estimation of Ionic Liquids. Ind. Eng. Chem. Res. 2008, 47, 5751–5757. [Google Scholar] [CrossRef]
- Ceriani, R.; Gani, R.; Meirelles, A.J.A. Prediction of heat capacities and heats of vaporization of organic liquids by group contribution methods. Fluid Phase Equil. 2009, 283, 49–55. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Toro, A.; Rojas, R.E. Prediction of the heat capacity of ionic liquids using the mass connectivity index and a group contribution method. J. Chem. Thermodyn. 2011, 43, 1068–1073. [Google Scholar] [CrossRef]
- Randic, M. Characterization of molecular branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Rojas, R.E. Mass connectivity index, a new molecular parameter for the estimation of ionic liquid properties. Fluid Phase Equil. 2010, 297, 107–112. [Google Scholar] [CrossRef]
- Sattari, M.; Gharagheizi, F.; Ilani-Kashkouli, P.; Mohammadi, A.H.; Ramjugernath, D. Development of a group contribution method for the estimation of heat capacities of ionic liquids. J. Therm. Anal. Calorim. 2014, 115, 1863–1882. [Google Scholar] [CrossRef]
- Albert, J.; Müller, K. A Group Contribution Method for the Thermal Properties of Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 17522–17526. [Google Scholar] [CrossRef]
- Nancarrow, P.; Lewis, M.; AbouChakra, L. Group Contribution Methods for Estimation of Ionic Liquid Heat Capacities: Critical Evaluation and Extension. Chem. Eng.Technol. 2015, 38, 632–644. [Google Scholar] [CrossRef]
- Morad, N.A.; Kamal, A.A.M.; Panau, F.; Yew, T.W. Liquid Specific Heat Capacity Estimation for Fatty Acids, Triacylglycerols, and Vegetable Oils Based on Their Fatty Acid Composition. J. Am. Oil Chem. Soc. 2000, 77, 1001–1005. [Google Scholar] [CrossRef]
- Reid, R.C.; Praustnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 4th ed.; McGraw Hill: Singapore, 1988. [Google Scholar]
- Rihany, D.N.; Doraisamy, L.K. Estimation of Heat Capacity, of Organic Compounds from Group Contributions. Ind. Eng. Chem. Fundam. 1965, 4, 17–21. [Google Scholar] [CrossRef]
- Sallamie, N.; Shaw, J.M. Heat capacity prediction for polynuclear aromatic solids using vibration spectra. Fluid Phase Equil. 2005, 237, 100–110. [Google Scholar] [CrossRef]
- Müller, K.; Albert, J. Contribution of the Individual Ions to the Heat Capacity of Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 10343–10346. [Google Scholar] [CrossRef]
- Barati-Harooni, A.; Najafi-Marghmaleki, A.; Arabloo, M.; Mohammadi, A.H. Chemical structural models for prediction of heat capacities of ionic liquids. J. Mol. Liq. 2017, 232, 113–122. [Google Scholar] [CrossRef]
- Preiss, U.P.R.M.; Slattery, J.M.; Krossing, I. In Silico Prediction of Molecular Volumes, Heat Capacities, and Temperature-Dependent Densities of Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2290–2296. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Kabo, A.G.; Blokhin, A.V.; Kabo, G.J.; Shevelyova, M.P. Heat Capacity of Ionic Liquids: Experimental Determination and Correlations with Molar Volume. J. Chem. Eng. Data 2010, 55, 2719–2724. [Google Scholar] [CrossRef]
- Glasser, L.; Jenkins, H.D.B. Volume-Based Thermodynamics: A Prescription for Its Application and Usage in Approximation and Prediction of Thermodynamic Data. J. Chem. Eng. Data 2011, 56, 874–880. [Google Scholar] [CrossRef]
- Glasser, L.; Jenkins, H.D.B. Ambient isobaric heat capacities, C(p,m), for ionic solids and liquids: An application of volume-based thermodynamics (VBT). Inorg. Chem. 2011, 5, 8565–8569. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Vonka, P.; Sedmidubsky, D.; Svoboda, P. Application of Neumann–Kopp rule for the estimation of heat capacity of mixed oxides. Thermochim. Acta 2010, 497, 7–13. [Google Scholar] [CrossRef]
- Naef, R. A Generally Applicable Computer Algorithm Based on the Group Additivity Method for the Calculation of Seven Molecular Descriptors: Heat of Combustion, LogPO/W, LogS, Refractivity, Polarizability, Toxicity and LogBB of Organic Compounds; Scope and Limits of Applicability. Molecules 2015, 20, 18279–18351. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E. Calculation of Five Thermodynamic Molecular Descriptors by Means of a General Computer Algorithm Based on the Group-Additivity Method: Standard Enthalpies of Vaporization, Sublimation and Solvation, and Entropy of Fusion of OrdinaryOrganic Molecules and Total Phase-Change Entropy of Liquid Crystals. Molecules 2017, 22, 1059. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E. Application of a General Computer Algorithm Based on the Group-Additivity Method for the Calculation of two Molecular Descriptors at both Ends of Dilution: Liquid Viscosity and Activity Coefficient in Water at infinite Dilution. Molecules 2018, 23, 5. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E., Jr. Calculation of the Surface Tension of Ordinary Organic and Ionic Liquids by Means of a Generally Applicable Computer Algorithm Based on the Group-Additivity Method. Molecules 2018, 23, 1224. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Van der Bondi, A. Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Ermer, O. Calculation of molecular properties using force fields. Applications in organic chemistry. Springer Link Ser. Struct. Bond. 2005, 27, 161–211. [Google Scholar]
- Kurtz, S.S., Jr.; Sankin, A. Calculation of Molecular Volumes of Hydrocarbons. Ind. Eng. Chem. 1954, 46, 2186–2191. [Google Scholar] [CrossRef]
- Ye, C.; Shreeve, J.M. Rapid and Accurate Estimation of Densities of Room-Temperature Ionic Liquids and Salts. J. Phys. Chem. A 2007, 111, 1456–1461. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Roobottom, H.K.; Passmore, J.; Glasser, L. Relationships among Ionic Lattice Energies, Molecular (Formula Unit) Volumes, and Thermochemical Radii. Inorg. Chem. 1999, 38, 3609–3620. [Google Scholar] [CrossRef] [PubMed]
- Conolly, M.L. Computation of Molecular Volume. J. Am. Chem. Soc. 1985, 107, 1118–1124. [Google Scholar] [CrossRef]
- Gavezzotti, A. The Calculation of Molecular Volumes and the Use of Volume Analysis in the Investigation of Structured Media and of Solid-state Organic Reactivity. J. Am. Chem. Soc. 1983, 105, 5220–5225. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Zissimos, A.M. Determination of McGowan Volumes for Ions and Correlation with van der Waals Volumes. J. Chem. Inf. Comput. Sci. 2003, 43, 1848–1854. [Google Scholar] [CrossRef]
- Ruzicka, V.; Zabransky, M.; Majer, V. Heat Capacities of Organic Compounds in the Liquid State II. C1 to C18 1-Alkanes. J. Phys. Chem. Ref. Data 1991, 20, 405–444. [Google Scholar] [CrossRef]
- Zabransky, M.; Ruzicka, V.; Majer, V. Heat Capacities of Organic Compounds in the Liquid State, I. C1 to C18 1-Alkanols. J. Phys. Chem. Ref. Data 1990, 19, 719–762. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Mendes, A.; Santos, L.M.N.B.F. Chain Length Dependence of the Thermodynamic Properties of n-Alkanes and their Monosubstituted Derivatives. J. Chem. Eng. Data 2018, 63, 1–20. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15K. J. Phys. Chem. Ref. Data 1988, 17, 1637–1678. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Heat Capacities and Entropies of Organic Compounds in the Condensed Phase Volume II. J. Phys. Chem. Ref. Data 1990, 19, 881–1047. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Heat Capacities and Entropies of Organic Compounds in the Condensed Phase Volume III. J. Phys. Chem. Ref. Data 1996, 25, 1–523. [Google Scholar] [CrossRef]
- Jin, Y.; Wunderlich, B. Heat Capacities of Parafflns and Polyethylene. J. Phys. Chem. 1991, 95, 9000–9007. [Google Scholar] [CrossRef]
- Korotkovskii, V.I.; Lebedev, A.V.; Ryshkova, O.S.; Bolotnikov, M.F.; Shevchenko, Y.E.; Neruchev, Y.A. Thermophysical Properties of Liquid Squalane C30H62 within the Temperature Range of 298.15-413.15 K at Atmospheric Pressure. High Temp. 2012, 50, 471–474. [Google Scholar] [CrossRef]
- Wang, L.; Tan, Z.-C.; Meng, S.-H.; Liang, D.-B. Low-temperature heat capacity and phase transition of n-hexatriacontane. Thermochim. Acta 1999, 342, 59–65. [Google Scholar] [CrossRef]
- Tkachenko, E.S.; Varushchenko, R.M.; Druzhinina, A.I.; Reshetova, M.D.; Borisova, N.E. Heat Capacity and Thermodynamic Functions of Diphenylacetylene. J. Chem. Eng. Data 2011, 56, 4700–4709. [Google Scholar] [CrossRef]
- Varushchenko, R.M.; Druzhinina, A.I.; Senyavin, V.M.; Sarkisova, V.S. The low-temperature heat capacities, phase transitions and thermodynamic properties of 1,3-dimethyladamantane and 1-ethyladamantane. J. Chem. Thermodyn. 2005, 37, 141–151. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Letyanina, I.A.; Zakharova, Y.A.; Pimerzin, A.A.; Vishnevskaya, E.E. Thermodynamic properties of neopentylbenzene over the range from T -> (0 to 350) K. J. Chem. Thermodyn. 2012, 48, 118–122. [Google Scholar] [CrossRef]
- Cheda, J.A.R.; Westrum, E.F., Jr. Subambient-Temperature Thermophysics of Acenaphthene and Acenaphthylene: Molecular Disorder in the Latter. J. Phys. Chem. 1994, 98, 2482–2488. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Lapuk, S.E.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Schick, C.; Solomonov, B.N. Application of fast scanning calorimetry to the fusion thermochemistry of low-molecular-weight organic compounds: Fast-crystallizing m-terphenyl heat capacities in a deeply supercooled liquid state. Thermochim. Acta 2018, 668, 96–102. [Google Scholar] [CrossRef]
- Rodrigues, A.S.M.C.; Rocha, M.A.A.; Santos, L.M.N.B.F. Isomerization effect on the heat capacities and phase behavior of oligophenyls isomers series. J. Chem. Thermodyn. 2013, 63, 78–83. [Google Scholar] [CrossRef]
- Varushchenko, R.M.; Efimova, A.A.; Druzhinina, A.I.; Tkachenko, E.S.; Nesterov, I.A.; Nesterova, T.N.; Verevkin, S.P. The heat capacities and thermodynamic functions of 4-methylbiphenyl and 4-tert-butylbiphenyl. J. Chem. Thermodyn. 2010, 42, 1265–1272. [Google Scholar] [CrossRef]
- Efimova, A.A.; Varushchenko, R.M.; Druzhinina, A.I.; Chelovskaya, N.V.; Tkachenko, E.S.; Nesterov, I.A.; Nesterova, T.N. Heat Capacity and Thermodynamic Functions of 4,4′-Dimethylbiphenyl and 4,4′-Di-tert-butylbiphenyl. Russ. J. Phys. Chem. A 2010, 84, 343–349. [Google Scholar] [CrossRef]
- Tkachenko, E.S.; Druzhinina, A.I.; Varushchenko, R.M.; Tarazanov, S.V.; Nesterova, T.N.; Reshetova, M.D.; Polyakova, O.V. Heat Capacity and Thermodynamic Functions of 2-Methylbiphenyl and 3,3′-Dimethylbiphenyl in the Range of 6 to 372 K. Russ. J. Phys. Chem. A 2013, 87, 705–713. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Santos, L.M.N.B.F.; Lima, L.M.S.S. Thermodynamic study of 1,2,3-triphenylbenzene and 1,3,5-triphenylbenzene. J. Chem. Thermodyn. 2010, 42, 134–139. [Google Scholar] [CrossRef]
- Fulem, M.; Lastovka, V.; Straka, M.; Ruzicka, K.; Shaw, J.M. Heat Capacities of Tetracene and Pentacene. J. Chem. Eng. Data 2008, 53, 2175–2181. [Google Scholar] [CrossRef]
- Torres, L.A.; Campos, M.; Martinez, M.; Rojas, A. The thermochemistry of coronene revisited. J. Chem. Thermodyn. 2009, 41, 957–965. [Google Scholar] [CrossRef]
- Rojas-Aguilar, A.; Martinez-Herrera, M. Enthalpies of combustion and formation of fullerenes by micro-combustion calorimetry in a Calvet calorimeter. Thermochim. Acta 2005, 437, 126–133. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, J.; Varma-Nair, M.; Liang, G.; Fu, Y.; Wunderlich, B.; Xiang, X.-D.; Mostovoy, R.; Zettl, A.K. Thermodynamic Characterization of C60 by Differential Scanning Calorimetry. J. Phys. Chem. 1992, 96, 5151–5156. [Google Scholar] [CrossRef]
- Korotkovskii, V.I.; Ryshkova, O.S.; Neruchev, Y.A.; Goncharov, A.L.; Postnikov, E.B. Isobaric heat capacity, isothermal compressibility and fluctuational properties of 1-bromoalkanes. Int. J. Thermophys. 2016, 37, 58–71. [Google Scholar] [CrossRef]
- Chorazewski, M.; Tkaczyk, M. Heat Capacity, Speed of Ultrasound, and Density for 1,5-Dibromopentane + Heptane within the Temperature Range from 293.15 K to 313.15 K. J. Chem. Eng. Data 2006, 51, 1825–1831. [Google Scholar] [CrossRef]
- Straka, M.; Ruzicka, K.; Fulem, M.; Cervinka, C. Heat capacity of selected chlorohydrocarbons. Fluid Phase Equil. 2012, 336, 128–136. [Google Scholar] [CrossRef]
- Melent’ev, V.V.; Postnikov, E.B.; Polishuk, I. Experimental Determination and Modeling Thermophysical Properties of 1-Chlorononane in a Wide Range of Conditions: Is It Possible To Predict a Contribution of Chlorine Atom? Ind. Eng. Chem. Res. 2018, 57, 5142–5150. [Google Scholar] [CrossRef]
- Bazyleva, A.B.; Blokhin, A.V.; Kabo, G.J.; Kabo, A.G.; Paulechka, Y.U. Thermodynamic properties of 1-bromoadamantane in the condensed state and molecular disorder in its crystals. J. Chem. Thermodyn. 2005, 37, 643–657. [Google Scholar] [CrossRef]
- Van der Linde, P.R.; van Miltenburg, J.C.; van den Berg, G.J.K.; Oonk, H.A.J. Low-Temperature Heat Capacities and Derived Thermodynamic Functions of 1,4-Dichlorobenzene, 1,4-Dibromobenzene, 1,3,5-Trichlorobenzene, and 1,3,5-Tribromobenzene. J. Chem. Eng. Data 2005, 50, 164–172. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Schick, C. Vapour pressures and heat capacity measurements on the C7–C9 secondary aliphatic alcohols. J. Chem. Thermodyn. 2007, 39, 758–766. [Google Scholar] [CrossRef]
- Serra, P.B.P.; Ruzicka, K.; Fulem, M.; Vlk, O.; Krakovsky, I. Calorimetric and FTIR study of selected aliphatic heptanols. Fluid Phase Equil. 2016, 423, 43–54. [Google Scholar] [CrossRef]
- Serra, P.B.P.; Krakovsky, I.; Fulem, M.; Ruzicka, K. Calorimetric and FTIR study of selected aliphatic octanols. J. Therm. Anal. Calor. 2018, 134, 2157–2170. [Google Scholar] [CrossRef]
- Stejfa, V.; Fulem, M.; Ruzicka, K.; Matejka, P. Vapor pressures and thermophysical properties of selected hexenols and recommended vapor pressure for hexan-1-ol. Fluid Phase Equil. 2015, 402, 18–29. [Google Scholar] [CrossRef]
- Straka, M.; Ruzicka, K.; Fulem, M. Heat capacities of 2-propenol and selected cyclohexylalcohols. Thermochim. Acta 2014, 587, 67–71. [Google Scholar] [CrossRef]
- Charapennikau, M.B.; Blokhin, A.V.; Kabo, A.G.; Kabo, G.J. The heat capacities and parameters of solid phase transitions and fusion for 1- and 2-adamantanols. J. Chem. Thermodyn. 2003, 35, 145–157. [Google Scholar] [CrossRef]
- Svoboda, V.; Zabransky, M.; Barta, M. Molar heat capacities of 2-methoxyethanol, 2-ethoxyethanol, and 2-propoxyethanol in the temperature range from 298 K to 330 K. J. Chem. Thermodyn. 1991, 23, 711–712. [Google Scholar] [CrossRef]
- Atake, T.; Kawaji, H.; Tojo, T.; Kawasaki, K.; Ootsuka, Y.; Katou, M.; Koga, Y. Heat Capacities of Isomeric 2-Butoxyethanols from 13 to 300 K: Fusion and Glass Transition. Bull. Chem. Soc. Jpn. 2000, 73, 1987–1991. [Google Scholar] [CrossRef]
- Francesconi, R.; Bigi, A.; Rubini, K.; Comelli, F. Excess Enthalpies, Heat Capacities, Densities, Viscosities and Refractive Indices of Dimethyl Sulfoxide + Three Aryl Alcohols at 308.15 K and Atmospheric Pressure. J. Chem. Eng. Data 2005, 50, 1932–1937. [Google Scholar] [CrossRef]
- Ruzicka, K.; Fulem, M.; Serra, P.B.P.; Vlk, O.; Krakovsky, I. Heat capacities of selected cycloalcohols. Thermochim. Acta 2014, 596, 98–108. [Google Scholar] [CrossRef]
- Feng, J.; Shang, Y.; Zhang, Y. Research on synthesis and thermodynamic properties of 2-methoxycyclohexanol. J. Therm. Anal. Calorim. 2018, 131, 2197–2203. [Google Scholar] [CrossRef]
- Caceres-Alonso, M.; Costas, M.; Andreoli-Ball, L.; Patterson, D. Steric effects on the self-association of branched and cyclic alcohols in inert solvents. Apparent heat capacities of secondary and tertiary alcohols in hydrocarbons. Can. J. Chem. 1988, 66, 989–998. [Google Scholar] [CrossRef]
- Steja, V.; Bazyleva, A.; Fulem, M.; Rohlicek, J.; Skorepova, E.; Ruzicka, K.; Blokhin, A.V. Polymorphism and thermophysical properties of L- and DL-menthol. J. Chem. Thermodyn. 2019, 131, 524–543. [Google Scholar] [CrossRef]
- Steifa, V.; Dergal, F.; Mokbel, I.; Fulem, M.; Jose, J.; Ruzicka, K. Vapor pressures and thermophysical properties of selected monoterpenoids. Fluid Phase Equil. 2015, 406, 124–133. [Google Scholar] [CrossRef]
- Hu, J.; Tamura, K.; Murakami, S. Excess thermodynamic properties of binary mixtures of ethyl acetate with benzene, ethanol, and 2,2,2-trifluoroethan-1-ol at 298.15 K. Fluid Phase Equil. 1997, 134, 239–253. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Lima, C.F.R.A.C.; Mendes, A.; Santos, L.M.N.B.F. Fluorination effect on the thermodynamic properties of long-chain hydrocarbons and alcohols. J. Chem. Thermodyn. 2016, 102, 378–385. [Google Scholar] [CrossRef]
- Goralski, P.; Tkaczik, M. Heat Capacities of Some Liquid α,ω-Alkanediols within the Temperature Range between (293.15 and 353.15) K. J. Chem. Eng. Data 2008, 53, 1932–1934. [Google Scholar] [CrossRef]
- Zemankova, K.; Troncoso, J.; Romani, L. Excess volumes and excess heat capacities for alkanediol + watersystems in the temperature interval (283.15–313.15) K. Fluid Phase Equil. 2013, 356, 1–10. [Google Scholar] [CrossRef]
- Pietrzak, A.; Ludzik, K. Excess volumes and excess heat capacities of {1,2-alkanediol + methanol} mixtures and ionic volumes in these systems. Fluid Phase Equil. 2015, 401, 56–63. [Google Scholar] [CrossRef]
- Zorebski, E.; Goralski, P. Molar heat capacities for (1-butanol + 1,4-butanediol, 2,3-butanediol, 1,2-butanediol, and 2-methyl-2,4-pentanediol) as function of temperature. J. Chem. Thermodyn. 2007, 39, 1601–1607. [Google Scholar] [CrossRef]
- Pietrzak, A.; Piekarski, H. Molar heat capacities for {isomer of butanediol + methanol} as function of mixture composition and temperature. J. Chem. Thermodyn. 2014, 79, 171–177. [Google Scholar] [CrossRef]
- Della Gatta, G.; Jozwiak, M.; Ferloni, P. Heat capacities near room temperature of ten solid alkane-α,ω-diols HO–(CH2)n–OH where n = 6 and 8 <= n <= 16. J. Chem. Thermodyn. 1999, 31, 537–546. [Google Scholar] [CrossRef]
- Schrödle, S.; Hefter, G.; Buchner, R. Effects of hydration on the thermodynamic properties of aqueous ethylene glycol ether solutions. J. Chem. Thermodyn. 2005, 37, 513–522. [Google Scholar] [CrossRef]
- Svärd, M.; Krishna, G.R.; Rasmuson, A.C. Synthesis, crystallisation and thermodynamics of two polymorphs of a new derivative of meglumine: 1-(2,2,3-trimethyl-1,3-oxazolidin-5-yl)-butane-1,2,3,4-tetrol. Cryst. Eng. Comm. 2018, 20, 88. [Google Scholar] [CrossRef]
- Svärd, M.; Hjorth, T.; Bohlin, M.; Rasmuson, A.C. Calorimetric Properties and Solubility in Five Pure Organic Solvents of N-Methyl-D-Glucamine (Meglumine). J. Chem. Eng. Data 2016, 61, 1199–1204. [Google Scholar] [CrossRef]
- Lian, Y.-N.; Chen, A.-T.; Suurkuusk, J.; Wadsö, I. Polyol – Water Interactions as Reflected by Aqueous Heat Capacity Values. Acta Chem. Scand. A 1982, 36, 735–739. [Google Scholar] [CrossRef]
- Wasiak, M.; Komudzinska, M.; Piekarski, H.; Tkaczyk, M. Heat capacity and phase behaviour of {pentaethylene glycolmonoheptyl ether + water} system. Two-point scaling approach. J. Mol. Liq. 2018, 266, 781–788. [Google Scholar] [CrossRef]
- Maria, T.M.R.; Eusebio, M.E.S. Molar Heat Capacity of 1,2-Cyclohexanediol Isomers From (173 to 428) K. J. Chem. Eng. Data 2008, 53, 1316–1320. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Zhang, Y.; Zhang, J.; Wang, S.; Tan, Z.; Shi, Q. Heat capacities and thermodynamic functions of D-ribose and D-mannose. J. Therm. Anal. Calor. 2018, 133. [Google Scholar] [CrossRef]
- Yamamura, Y.; Iwagaki, S.; Hishida, M.; Nagatomo, S.; Fukada, K.; Saito, K. Heat capacity and standard thermodynamic functions of three ketohexoses in monosaccharides including rare sugars: D-fructose, D-psicose, and D-tagatose. J. Chem. Thermodyn. 2019, 131, 420–430. [Google Scholar] [CrossRef]
- Boerio-Goates, J. Heat-capacity measurements and thermodynamic functions of crystalline a-D-glucose at temperatures from 10 K to 340 K. J. Chem. Thermodyn. 1991, 23, 403–409. [Google Scholar] [CrossRef]
- Jia, R.; Sun, K.; Li, R.; Zhang, Y.; Wang, W.; Yin, H.; Fang, D.; Shi, Q.; Tan, Z. Heat capacities of some sugar alcohols as phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2017, 115, 233–248. [Google Scholar] [CrossRef]
- Hernandez-Segura, G.O.; Campos, M.; Costas, M.; Torres, L.A. Temperature dependence of the heat capacities in the solid state of 18 mono-, di-, and poly-saccharides. J. Chem. Thermodyn. 2009, 41, 17–20. [Google Scholar] [CrossRef]
- Magon, A.; Wurm, A.; Schick, C.; Pangloli, P.; Zivanovic, S.; Skotnicki, M.; Pyda, M. Reprint of “Heat capacity and transition behavior of sucrose by standard, fast scanning and temperature-modulated calorimetry. Thermochim. Acta 2015, 603, 149–161. [Google Scholar] [CrossRef]
- Kabo, G.J.; Paulechka, Y.U.; Voitkevich, O.V.; Blokhin, A.V.; Stepurko, E.N.; Kohut, S.V.; Voznyi, Y.V. Experimental and theoretical study of thermodynamic properties of levoglucosan. J. Chem. Thermodyn. 2015, 85, 101–110. [Google Scholar] [CrossRef]
- Diot, M.; de Braver, C.; Germain, P. Calorimetric Behaviour of Anhydrous β-Cyclodextrin at Very Low Temperature. J. Incl. Phenom. Mol. Recogn. Chem. 1998, 30, 143–150. [Google Scholar] [CrossRef]
- Bhatia, S.C.; Rani, R.; Bhatia, R. Densities, Speeds of Sound, and Refractive Indices of Binary Mixtures of Decan-1-ol with Anisole, o-Cresol, m-Cresol, and p-Cresol at T = (298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 2011, 56, 1669–1674. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhao, X.; Zhu, L.; Yang, L.; Sha, Z. Determination and thermodynamic modeling of solid–liquid phase equilibrium for the 2,4,6-trimethylphenol and 2,5-dimethylphenol binary system. J. Therm. Anal. Calorim. 2018, 132, 1923–1931. [Google Scholar] [CrossRef]
- Schaake, R.C.F.; van Miltenburg, J.C.; de Kruif, C.G. Thermodynamic properties of the normal alkanoic acids I I. Molar heat capacities of seven even-numbered normal alkanoic acids. J. Chem. Thermodvn. 1982, 14, 771–778. [Google Scholar] [CrossRef]
- Yu, H.-G.; Tan, Z.-C.; Liu, Y.; Lan, X.-Z.; Xu, F.; Huang, X.-M.; Sun, L.-X. Standard enthalpy of formation and heat capacities of 3,5-di-tert-butylsalicylic acid. Thermochim. Acta 2003, 404, 89–95. [Google Scholar] [CrossRef]
- Vecchio, S.; Brunetti, B. Vapor pressures, standard molar enthalpies, entropies Gibbs energies of sublimation and heat capacities of 2,5- and 3,5-dibromobenzoic acids. Fluid Phase Equil. 2013, 338, 148–154. [Google Scholar] [CrossRef]
- Camarillo, E.A.; Flores, H.; Santiago, O. Energy Content of Cinnamic Acid Derivatives Found in Coffee Bean by Combustion Calorimetry. J. Chem. Biol. Phy. Sci. Sec. C 2016, 6, 1223–1229. [Google Scholar]
- Garcia-Castro, M.A.; Amador, P.; Rojas, A.; Hernandez-Perez, J.M.; Solano-Altamirano, J.M.; Flores, H.; Salas-Lopez, K. Experimental and computational thermochemistry of 3- and 4-nitrophthalic acids. J. Chem. Thermodyn. 2018, 127, 117–125. [Google Scholar] [CrossRef]
- Bernardes, C.E.S.; Simoes, R.G.; Diogo, H.D.; Minas de Piedade, M.E. Thermochemistry of 2,2,5,7,8-pentamethylchroman-6-ol (PMC) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox). J. Chem. Thermodyn. 2014, 73, 140–147. [Google Scholar] [CrossRef]
- Kong, Y.-X.; Di, Y.-Y.; Yang, W.-W.; Gao, S.-L.; Tan, Z.-C. Low Temperature Heat Capacities and Standard Molar Enthalpy of Formation of 2-Pyrazinecarboxylic Acid (C5H4N2O2)(s). Acta Chim. Slov. 2010, 57, 370–375. [Google Scholar]
- Joseph, A.; Bernardes, C.E.S.; Minas da Piedade, M.E. Heat capacity and thermodynamics of solid and liquid pyridine-3-carboxylic acid (nicotinic acid) over the temperature range 296 K to 531 K. J. Chem. Thermodyn. 2012, 55, 23–28. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Smirnova, N.N.; Shipilova, A.S.; Shushunov, A.N.; Gusarova, E.V.; Knyazeva, S.S. Thermodynamic properties and low-temperature X-ray diffraction of vitamin B3. Thermochim. Acta 2015, 604, 115–121. [Google Scholar] [CrossRef]
- Agafonova, L.E.; Varushchenko, R.M.; Druzhinina, A.I.; Polyakova, O.V.; Kolesov, Y.S. Heat Capacity, Saturation Vapor Pressure, and Thermodynamic Functions of Ethyl Esters of C3–C5 and C18 Carboxylic Acids. Russ. J. Phys. Chem. A 2011, 85, 1516–1527. [Google Scholar] [CrossRef]
- Noel, J.A.; White, M. A: Heat Capacities of Potential Organic Phase Change Materials. J. Chem. Thermodyn. 2019, 128, 127–133. [Google Scholar] [CrossRef]
- Bogatishcheva, N.S.; Faizullin, M.Z.; Nikitin, E.D. Heat capacities and thermal diffusivities of some n-alkanoic acid methyl esters. J. Chem. Thermodyn. 2019, 130, 33–37. [Google Scholar] [CrossRef]
- Dzida, M.; Jezak, S.; Sumara, J.; Zarska, M.; Goralski, P. High-Pressure Physicochemical Properties of Ethyl Caprylate and Ethyl Caprate. J. Chem. Eng. Data 2013, 58, 1955–1962. [Google Scholar] [CrossRef]
- Dzida, M.; Jezak, S.; Sumara, J.; Zarska, M.; Goralski, P. High pressure physicochemical properties of biodiesel components used for spray characteristics in diesel injection systems. Fuel 2013, 111, 165–171. [Google Scholar] [CrossRef]
- Kozyro, A.A.; Blokhin, A.V.; Kabo, G.J.; Paulechka, Y.U. Thermodynamic properties of some cyclohexyl esters in the condensed state. J. Chem. Thermodyn. 2001, 33, 305–331. [Google Scholar] [CrossRef]
- Liu, X.; Su, C.; Qi, X.; He, M. Isobaric heat capacities of ethyl heptanoate and ethyl cinnamate at pressures up to 16.3 Mpa. J. Chem. Thermodyn. 2016, 93, 70–74. [Google Scholar] [CrossRef]
- Kulagina, T.G.; Samosudova, Y.S.; Latyanina, I.A.; Sevast’anov, E.V.; Smirnova, N.N.; Smirnova, L.A.; Mochalova, A.E. The Thermodynamic Properties of 2-Ethylhexyl Acrylate over the Temperature Range from T → 0 to 350 K. Russ. J. Phys. Chem. A 2012, 86, 747–751. [Google Scholar]
- Blokhin, A.V.; Paulechka, Y.U.; Kabo, G.J.; Kozyro, A.A. Thermodynamic properties of methyl esters of benzoic and toluic acids in the condensed state. J. Chem. Thermodyn. 2002, 34, 29–55. [Google Scholar] [CrossRef]
- Ledo, J.M.; Flores, H.; Hernandez-Perez, J.M.; Ramos, F.; Camarillo, E.A.; Solano-Altamirano, J.M. Gas-phase enthalpies of formation of ethyl hydroxybenzoates: An experimental and theoretical approach. J. Chem. Thermodyn. 2018, 116, 176–184. [Google Scholar] [CrossRef]
- Navarro, A.M.; Garcia, B.; Hoyuelos, F.J.; Penacoba, I.A.; Ibeas, S.; Leal, J.M. Heat Capacity Behavior and Structure of Alkan-1-ol/Alkylbenzoate Binary Solvents. J. Phys. Chem. B 2012, 116, 9768–9775. [Google Scholar] [CrossRef]
- Inaba, A.; Suzuki, H.; Massalska-Arodz, M.; Rozwadowski, T. Polymorphism and thermodynamic functions of liquid crystalline material 4-cyano-3-fluorophenyl 4-butylbenzoate. J. Chem. Thermodyn. 2012, 54, 204–210. [Google Scholar] [CrossRef]
- Ledo, J.M.; Flores, H.; Solano-Altamirano, J.M.; Ramos, F.; Hernandez-Perez, J.M.; Camarillo, E.A.; Rabell, B.; Amador, M.P. Experimental and theoretical study of methyl n-hydroxybenzoates. J. Chem. Thermodyn. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Lugo, L.; Segovia, J.J.; Carmen Martin, M.; Fernandez, J.; Villamanan, M.A. An experimental setup for isobaric heat capacities for viscous fluids at high pressure: Squalane, bis(2-ethylhexyl) sebacate and bis(2-ethylhexyl) phthalate. J. Chem. Thermodyn. 2012, 49, 75–80. [Google Scholar] [CrossRef]
- Pokomy, V.; Steifa, V.; Fulem, M.; Cervinka, C.; Ruzicka, K. Vapor Pressures and Thermophysical Properties of Dimethyl Carbonate, Diethyl Carbonate, and Dipropyl Carbonate. J. Chem. Eng. Data 2017, 62, 3206–3215. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Toktonov, A.V.; Chernyak, Y.; Schäffner, B.; Börner, A. Cyclic alkylene carbonates. Experiment and first principle calculations for prediction of thermochemical properties. J. Chem. Thermodyn. 2008, 40, 1428–1432. [Google Scholar] [CrossRef]
- Mansson, M. Enthalpies of combustion and formation of ethyl propionate and diethyl carbonate. J. Chem. Thermodyn. 1972, 4, 865–871. [Google Scholar] [CrossRef]
- Shen, C.; Li, W.; Zhou, C. Investigation on molar heat capacity, standard molar enthalpy of combustion for guaiacol and acetyl guaiacol ester. Chin. J. Chem. Eng. 2016, 24, 1772–1778. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Emel’yanenko, V.N.; Smirnova, N.N.; Stepanova, O.V.; Shipilova, A.S.; Markin, A.V.; Samosudova, Y.S.; Gusarova, E.V.; Knyazeva, S.S.; Verevkin, S.P. Thermodynamic properties of methylprednisolone aceponate. J. Chem. Thermodyn. 2016, 103, 244–248. [Google Scholar] [CrossRef]
- Comelli, F.; Francesconi, R.; Bigi, A.; Rubini, K. Excess Molar Enthalpies, Molar Heat Capacities, Densities, Viscosities, and Refractive Indices of Dimethyl Sulfoxide + Esters of Carbonic Acid at 308.15 K and Atmospheric Pressure. J. Chem. Eng. Data 2006, 51, 665–670. [Google Scholar] [CrossRef]
- Lv, X.-C.; Tan, Z.-C.; Shi, Q.; Zhang, H.-T.; Sun, L.-X.; Zhang, T. Molar Heat Capacity and Thermodynamic Properties of 4-Methyl-4-cyclohexene-1,2-dicarboxylic Anhydride [C9H10O3]. J. Chem. Eng. Data 2005, 50, 932–935. [Google Scholar] [CrossRef]
- Lv, X.-C.; Tan, Z.-C.; Di, Y.-Y.; Shi, Q.; Sun, L.-X.; Zhang, T. Molar heat capacity and thermodynamic properties of 1-cyclohexene-1,2-dicarboxylic anhydride [C8H8O3]. J. Chem. Thermodyn. 2004, 36, 787–792. [Google Scholar] [CrossRef]
- Garcia-Castro, M.A.; Amador, P.; Hernandez-Perez, J.M.; Medina-Favela, A.E.; Flores, H. Experimental and Computational Thermochemistry of 3- and 4-Nitrophthalic Anhydride. J. Phys. Chem. A 2014, 118, 3820–3826. [Google Scholar] [CrossRef]
- Fuchs, R. Heat capacities of liquid ketones and aldehydes at 298 K. Can. J. Chem. 1980, 58, 2305–2306. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Chickos, J.S. Some Thermophysical Properties of Several Solid Aldehdes. J. Therm. Anal. Calorim. 2008, 94, 257–262. [Google Scholar] [CrossRef]
- Sharma, V.K.; Malik, S.; Solanki, S. Thermodynamic Studies of Molecular Interactions in Mixtures Containing Tetrahydropyran, 1,4-Dioxane, and Cyclic Ketones. J. Chem. Eng. Data 2017, 62, 623–632. [Google Scholar] [CrossRef]
- Wang, S.-X.; Tan, Z.-C.; Shi, Q.; Di, Y.-Y.; Zhang, H.-T.; Xu, F.; Sun, L.-X.; Zhang, T. Calorimetric study and thermal analysis of crystalline 2,4-dinitrobenzaldehyde (C7H4N2O5). J. Chem. Thermodyn. 2005, 37, 349–355. [Google Scholar] [CrossRef]
- Nagumo, T.; Matsuo, T.; Suga, H. Thermodynamic Study on Camphor Crystals. Thermochim. Acta 1989, 139, 121–132. [Google Scholar] [CrossRef]
- Stejfa, V.; Fulem, M.; Ruzicka, K.; Cervinka, C. Thermodynamic study of selected monoterpenes II. J. Chem. Thermodyn. 2014, 79, 272–279. [Google Scholar] [CrossRef]
- Stejfa, V.; Fulem, M.; Ruzicka, K.; Cervinka, C. Thermodynamic study of selected monoterpenes III. J. Chem. Thermodyn. 2014, 79, 280–289. [Google Scholar] [CrossRef]
- De Kruif, C.G.; van Miltenburg, J.C.; Blok, J.G. Molar heat capacities and vapour pressures of solid and liquid benzophenone. J. Chem. Thermodyn. 1983, 15, 129–136. [Google Scholar] [CrossRef]
- Rojas-Aguilar, A.; Flores-Lara, H.; Martinez-Herrera, M.; Ginez-Carbajal, F. Thermochemistry of benzoquinones. J. Chem. Thermodyn. 2004, 36, 453–463. [Google Scholar] [CrossRef]
- Sanchez-Bulas, T.; Cruz-Vasquez, O.; Hernandez-Obregon, J.; Rojas, A. Enthalpies of fusion, vaporisation and sublimation of crown ethers determined by thermogravimetry and differential scanning calorimetry. Thermochim. Acta 2017, 650, 123–133. [Google Scholar] [CrossRef]
- Bykova, T.A.; Lebedev, B.V. Thermodynamic Properties of Dibenzo-24-crown-8 in the Range from T -> 0 to 500 K. Russ. J. Gen. Chem. 2004, 74, 250–255. [Google Scholar] [CrossRef]
- Rodriguez, S.; Lafuente, C.; Artigas, H.; Royo, F.M.; Urieta, J.S. Densities, speeds of sound, and isentropic compressibilities of a cyclic ether with chlorocyclohexane, or bromocyclohexane at the temperatures 298.15K and 313.15K. J. Chem. Thermodyn. 1999, 31, 139–149. [Google Scholar] [CrossRef]
- Druzhinina, A.I.; Pimenova, S.M.; Lukyanova, V.A. Low temperature heat capacity and thermodynamic parameters of phase transitions of 4-nitro-4’-tert-butyl-diphenyl oxide. J. Chem. Thermodyn. 2017, 105, 312–316. [Google Scholar] [CrossRef]
- Druzhinina, A.I.; Pimenova, S.M.; Tarazanov, S.V.; Nesterova, T.N.; Varushchenko, R.M. Thermodynamic properties of 4-tert-butyl-diphenyl oxide. J. Chem. Thermodyn. 2015, 87, 69–77. [Google Scholar] [CrossRef]
- Goralski, P.; Wasiak, M.; Bald, A. Heat Capacities, Speeds of Sound, and Isothermal Compressibilities of Some n-Amines and Tri-n-amines at 298.15 K. J. Chem. Eng. Data 2002, 47, 83–86. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemistry of amines: Strain in six-membered rings from experimental standard molar enthalpies of formation of morpholines and piperazines. J. Chem. Thermodyn. 1998, 30, 1069–1079. [Google Scholar] [CrossRef]
- Verevkin, S.P. Thermochemistry of Amines: Experimental Standard Molar Enthalpies of Formation of N-Alkylated Piperidines. Struct. Chem. 1998, 9, 113–119. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Xu, K.; Ren, Z.; Song, J.; Zhao, F. Studies on Thermodynamic Properties of FOX-7 and Its Five Closed-Loop Derivatives. J. Chem. Eng. Data 2015, 60, 2057–2061. [Google Scholar] [CrossRef]
- Verevkin, S.P. Relationships among strain energies of monoand poly-cyclic cyclohexanoid molecules and strain of their component rings. J. Chem. Thermodyn. 2002, 34, 263–275. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Santos, L.M.N.B.F. Hole Transport Materials Based Thin Films: Topographic Structures and Phase Transition Thermodynamics of Triphenylamine Derivatives. J. Phys. Chem. C 2013, 117, 10919–10928. [Google Scholar] [CrossRef]
- Domanska, U.; Marciniak, M. Experimental solid–liquid equilibria for systems containing alkan-1-ol + 1,3-diaminopropane Heat capacities of alkan-1-ols and amines—Thermodynamic functions of dissociation and enthalpies of melting of the congruently melting compounds for the systems (alkan-1-ol + amine). Fluid Phase Equil. 2005, 235, 30–41. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Morgenthaler, J.; Rüchardt, C. Thermochemistry of imines: Experimental standard molar enthalpies of formation. J. Chem. Thermodyn. 1997, 29, 1175–1183. [Google Scholar] [CrossRef]
- Shi, Q.; Tan, Z.-C.; Tong, B.; Di, Y.-Y.; Zhang, Z.-H.; Zeng, J.-L.; Sun, L.-X.; Li, Y.-S. Low-temperature heat capacity and standard molar enthalpy of formation of crystalline 2-pyridinealdoxime (C6H6N2O). J. Chem. Thermodyn. 2007, 39, 817–821. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yan, B.; Guan, Y.-L.; Ma, H.-X.; Song, J.-R.; Zhao, F.-Q. Thermodynamic properties of 4-amino-3-furazanecarboxamidoxime. J. Chem. Thermodyn. 2015, 90, 87–91. [Google Scholar] [CrossRef]
- Straka, M.; Ruzicka, K.; Ruzicka, V. Heat Capacities of Chloroanilines and Chloronitrobenzenes. J. Chem. Eng. Data 2007, 52, 1375–1380. [Google Scholar] [CrossRef]
- Tian, Q.-F.; Tan, Z.-C.; Shi, Q.; Xu, F.; Sun, L.-X.; Zhang, T. Heat capacity and thermodynamic properties of N-(2-cyanoethyl)aniline (C9H10N2). Thermochim. Acta 2005, 430, 53–58. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitseva, K.V.; Nagrimanov, R.N.; Solomonov, B.N.; Verevkin, S.P. Benchmark Thermodynamic Properties of Methyl- and Methoxy-Benzamides: Comprehensive Experimental and Theoretical Study. J. Phys. Chem. A 2016, 120, 8419–8429. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-H.; Liu, Y.-F.; Tan, Z.-C.; Ji, P.-J.; Wang, M.-H. Heat Capacity and Thermodynamic Properties of Crystalline 2-Chloro-N,N-dimethylnicotinamide. Chin. J. Chem. 2005, 23, 1490–1494. [Google Scholar] [CrossRef]
- Abate, L.; Badea, E.; Blanco, I.; D’Angelo, D.; Della Gatta, G. Heat capacities of a series of terminal linear alkyldiamides determined by DSC. J. Therm. Anal. Calorim. 2007, 90, 575–580. [Google Scholar] [CrossRef]
- Di, Y.-Y.; Tan, Z.-C.; Wu, X.-M.; Meng, S.-H.; Qu, S.-S. Heat capacity and thermochemical study of trifluoroacetamide (C2H2F3NO). Thermochim. Acta 2000, 356, 143–151. [Google Scholar] [CrossRef]
- Salas-Lopez, K.; Amador, P.; Rojas, A.; Melendez, F.J.; Flores, H. Experimental and Theoretical Thermochemistry of the Isomers 3- and 4-Nitrophthalimide. J. Phys. Chem. A 2017, 121, 5509–5519. [Google Scholar] [CrossRef]
- Notario, R.; Roux, M.V.; Ros, F.; Emel’yanenko, V.N.; Zaitsau, D.H.; Verevkin, S.P. Thermochemistry of 1,3-diethylbarbituric and 1,3-diethyl-2-thiobarbituric acids: Experimental and computational study. J. Chem. Thermodyn. 2014, 77, 151–158. [Google Scholar] [CrossRef]
- Roux, M.V.; Notario, R. Experimental and Computational Thermochemical Study of 2-Thiobarbituric Acid: Structure−Energy Relationship. J. Phys. Chem. A 2012, 116, 4639–4645. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.V.; Notario, R.; Segura, M.; Chickos, J.S. Thermophysical Study of 2-Thiobarbituric Acids by Differential Scanning Calorimetry. J. Chem. Eng. Data 2012, 57, 249–255. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.M.C.; Ribeiro da Silva, M.A.V.; Freitas, V.L.S.; Roux, M.V.; Jimenez, P.; Temprado, M.; Davalos, J.Z.; Cabildo, P.; Claramunt, R.M.; Elguero, J. Structural studies of cyclic ureas: 3. Enthalpy of formation of barbital. J. Chem. Thermodyn. 2009, 41, 1400–1407. [Google Scholar] [CrossRef]
- Kabo, G.J.; Kozyro, A.A.; Diky, V.V.; Simirsky, V.V. Additivity of Thermodynamic Properties of Organic Compounds in Crystalline State. 2. Heat Capacities and Enthalpies of Phase Transition of Alkyl Derivatives of Urea in Crystalline State. J. Chem. Eng. Data 1996, 40, 371–393. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.M.C.; Ribeiro da Silva, M.A.V.; Freitas, V.L.S.; Roux, M.V.; Jimenez, P.; Davalos, J.Z.; Cabildo, P.; Claramunt, R.M.; Pinilla, E.; Torres, M.R.; et al. Energetic studies of urea derivatives: Standard molar enthalpy of formation of 3,4,4’-trichlorocarbanilide. J. Chem. Thermodyn. 2010, 42, 536–544. [Google Scholar] [CrossRef]
- Kong, L.-G.; Tan, Z.-C.; Mei, J.-T.; Sun, L.-X.; Bao, X.-H. Thermodynamic studies of monuron. Thermochim. Acta 2004, 414, 131–135. [Google Scholar] [CrossRef]
- Yu, P.; Tan, Z.C.; Meng, S.H.; Lu, S.W.; Lan, X.Z.; Sun, L.X.; Xu, F.; Zhang, T.; Hu, S.X. Low-temperature heat capacities and thermodynamic properties of crystalline isoproturon. J. Therm. Anal. Calorim. 2003, 74, 867–874. [Google Scholar] [CrossRef]
- Svärd, M.; Valavi, M.; Khamar, D.; Kuhs, M.; Rasmuson, A.C. Thermodynamic Stability Analysis of Tolbutamide Polymorphs and Solubility in Organic Solvents. J. Pharm. Sci. 2016, 105, 1901–1906. [Google Scholar] [CrossRef]
- Ramos, F.; Ledo, J.M.; Flores, H.; Camarillo, E.A.; Carvente, J.; Amador, M.P. Evaluation of sublimation enthalpy by thermogravimetry: Analysis of the diffusion effects in the case of methyl and phenyl substituted hydantoins. Thermochim. Acta 2017, 655, 181–193. [Google Scholar] [CrossRef]
- Ledo, J.M.; Camarillo, E.A.; Flores, H.; Ramos, F.; Rojas, A. Energies of combustion and enthalpies of formation of 5-methyl-5- phenylhydantoin and 5,5-diphenylhydantoin. J. Therm. Anal. Calorim. 2016, 123, 2391–2396. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Yu, S.-B.; Tomg, B.; Sun, L.-X.; Tan, Z.-C.; Cao, Z.; Yang, D.-W.; Zhang, J.-N. Heat capacities and thermodynamic properties of (S)-tert-butyl 1-phenylethylcarbamate. J. Therm. Anal. Calorim. 2011, 103, 1087–1093. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Parameswar, A.R.; Demchenko, A.V.; Chickos, J.S.; Liebman, J.F. Thermophysical properties in medium temperature range of several thio and dithiocarbamates. J. Therm. Anal. Calorim. 2008, 91, 471–475. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Kandeev, K.V.; Bykova, T.A.; Kulagina, T.G. Thermodynamics of 1,4-diisocyanatobutane in the range from T ->(0 to 360) K at standard pressure. J. Chem. Thermodyn. 2006, 38, 376–382. [Google Scholar] [CrossRef]
- Good, W.D. Enthalpies of Combustion of 18 Organic Sulfur Compounds Related to Petroleum. J. Chem. Eng. Data 1972, 17, 158–162. [Google Scholar] [CrossRef]
- Roux, M.V.; Davalos, J.Z.; Jimenez, P.; Flores, H.; Saiz, J.-L.; Abboud, J.-L.M. Structural effects on the thermochemical properties of sulfur compounds: I. Enthalpy of combustion, vapour pressures, enthalpy of sublimation, and standard molar enthalpy of formation in the gaseous phase of 1,3-dithiane. J. Chem. Thermodyn. 1999, 31, 635–646. [Google Scholar] [CrossRef]
- Van Miltenburg, J.C.; van Ekeren, P.J. Thermodynamic properties of 1,3,5-trithiane derived from solid phase heat capacity measurements by adiabatic calorimetry and DSC measurements in the melting range. Thermochim. Acta 2002, 385, 11–17. [Google Scholar] [CrossRef][Green Version]
- Ramos, F.; Flores, H.; Hernandez-Perez, J.M.; Sandoval-Lira, J.; Camarillo, E.A. The Intramolecular Hydrogen Bond N−H···S in 2,2′-Diaminodiphenyl Disulfide: Experimental and Computational Thermochemistry. J. Phys. Chem. A 2018, 122, 239–248. [Google Scholar] [CrossRef]
- Wang, S.-X.; Tan, Z.-C.; Li, Y.-S.; Li, Y.; Shi, Q.; Tong, B. Heat capacity and thermodynamic properties of benzyl disulfide (C14H14S2). Thermochim. Acta 2007, 463, 21–25. [Google Scholar] [CrossRef]
- Flores, H.; Camarillo, E.A.; Amador, P. Enthalpies of combustion and formation of benzenesulfonamide and some of its derivatives. J. Chem. Thermodyn. 2012, 47, 408–411. [Google Scholar] [CrossRef]
- Camarillo, E.A.; Flores, H. Determination of the energies of combustion and enthalpies of formation of nitrobenzenesulfonamides by rotating-bomb combustion calorimetry. J. Chem. Thermodyn. 2010, 42, 425–428. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Volkova, T.V. Sublimation thermodynamics aspects of adamantane and memantine derivatives of sulfonamide molecular crystals. Phys. Chem. Chem. Phys. 2018, 20, 19784–19791. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.-F.; Zhang, J.-N.; Tan, Z.-C.; Dong, Y.-P.; Li, W.; Shi, Q. Heat Capacities and Thermodynamic Properties of (3,4-Dimethoxyphenyl) Acetonitrile (C10H11NO2). J. Chem. Eng. Data 2009, 54, 232–235. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Verevkin, S.P. Ferrocene: Temperature adjustments of sublimation and vaporization enthalpies. Fluid Phase Equil. 2018, 472, 196–203. [Google Scholar] [CrossRef]
- Sorai, M.; Kaneko, Y.; Hashiguchi, T. Heat capacity of paramagnetic nickelocene: Comparison with diamagnetic ferrocene. J. Phys. Chem. Solids 2014, 75, 656–661. [Google Scholar] [CrossRef]
- Krol, O.V.; Druzhinina, A.I.; Varushchenko, R.M.; Dorofeeva, O.V.; Reshetova, M.D.; Borisova, N.E. The heat capacities and thermodynamic functions of some derivatives of ferrocene. J. Chem. Thermodyn. 2008, 40, 549–557. [Google Scholar] [CrossRef]
- Domracheva, I.G.; Karyakin, N.V.; Sheiman, M.S.; Kamelova, G.V.; Larina, V.N.; Suvorova, O.N.; Domrachev, G.A. Thermodynamics and molecular dynamics of some ferrocene derivatives. Russ. Chem. Bull. 1999, 48, 1647–1655. [Google Scholar] [CrossRef]
- Lebedev, B.; Smirnova, N.N.; Novosyolova, N.; Makovetskii, K.; Ostrovskaya, I. Calorimetric study of 5-trimethylsilyl-2-norbornene, of its polymerization process and of poly(5-trimethylsilyl-2-norbornene) from 5 to 600 K at standard pressure. Macromol. Chem. Phys. 1994, 195, 1807–1822. [Google Scholar] [CrossRef]
- Palczewska-Tulinska, M.; Oracz, P. Selected Physicochemical Properties of Hexamethylcyclotrisiloxane, Octamethylcyclotetrasiloxane, and Decamethylcyclopentasiloxane. J. Chem. Eng. Data 2005, 50, 1711–1719. [Google Scholar] [CrossRef]
- Bykova, T.A.; Lebedev, B.V. Thermodynamic Properties of Tetraphenyl-tetrahydroxycyclotetrasiloxane, Octaphenyltetrahydroxytricyclooctasiloxane, Octaphenyl-pentacyclosilsesquioxane, and Polyphenylsilsesquioxane in the Range 0–300 K. Russ. J. Gen. Chem. 2003, 73, 1077–1085. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Tsvetkova, L.Y.; Lebedev, B.; Zavin, B.G.; Kotov, V.M. Thermodynamics of polymethylhydrosiloxane based on 1,3,5,7-tetramethyl-1,3,5,7-tetrahydrocyclotetrasiloxane. J. Therm. Anal. Calorim. 2007, 89, 217–222. [Google Scholar] [CrossRef]
- Abbas, R.; Schedemann, A.; Ihmels, C.; Enders, S.; Gmehling, J. Measurement of Thermophysical Pure Component Properties for a Few Siloxanes Used as Working Fluids for Organic Rankine Cycles. Ind. Eng. Chem. Res. 2011, 50, 9748–9757. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Shoifet, E.; Meurer, F.; Verevkin, S.P.; Schick, C.; Held, C. Benchmark Thermochemistry for Biologically Relevant Adenine and Cytosine. A Combined Experimental and Theoretical Study. J. Phys. Chem. A 2015, 119, 9680–9691. [Google Scholar] [CrossRef] [PubMed]
- Boerio-Goates, J.; Francis, M.R.; Goldberg, R.N.; Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.D.M.C.; Tewari, Y.B. Thermochemistry of adenosine. J. Chem. Thermodyn. 2001, 33, 929–994. [Google Scholar] [CrossRef]
- Boerio-Goates, J.; Hopkins, S.D.; Monteiro, R.A.R.; Ribeiro da Silva, M.D.M.C.; Ribeiro da Silva, M.A.V.; Goldberg, R.N. Thermochemistry of inosine. J. Chem. Thermodyn. 2005, 37, 1239–1249. [Google Scholar] [CrossRef]
- Lukyanova, V.A.; Druzhinina, A.I.; Pimenova, S.M.; Ioutsi, V.A.; Buyanovskaya, A.G.; Takazova, R.U.; Sagadeev, E.V.; Gimadeev, A.A. Thermodynamic properties of L-threonine. J. Chem. Thermodyn. 2018, 116, 248–252. [Google Scholar] [CrossRef]
- Di, Y.-Y.; Yang, W.-W.; Kong, Y.-X.; Shi, Q.; Tan, Z.-C. Low-Temperature Heat Capacities and Standard Molar Enthalpy of Formation of L-3-(3,4-Dihydroxyphenyl) Alanine (C9H11NO4). J. Chem. Eng. Data 2008, 53, 900–904. [Google Scholar] [CrossRef]
- Lukyanova, V.A.; Druzhinina, A.I.; Pimenova, S.M.; Ioutsi, V.A.; Buyanovskaya, A.G.; Takazova, R.U.; Sagadeyev, E.V.; Gimadeev, A.A. Thermodynamic properties of L-tryptophan. J. Chem. Thermodyn. 2017, 105, 44–49. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Emel’yanenko, V.N.; Shipilova, A.S.; Zaitsau, D.H.; Smirnova, N.N.; Knyazeva, S.S.; Gulenova, M.V. Thermodynamic investigation of L-carnitine. J. Chem. Thermodyn. 2019, 131, 495–502. [Google Scholar] [CrossRef]
- Markin, A.V.; Markhasin, E.; Sologubov, S.S.; Smirnova, N.N.; Griffin, R.G. Standard Thermodynamic Functions of Tripeptides N-Formyl-L-methionyl-L-leucyl-L-phenylalaninol and N-Formyl-L-methionyl-L-leucyl-L-phenylalanine Methyl Ester. J. Chem. Eng. Data 2014, 59, 1240–1246. [Google Scholar] [CrossRef]
- Brunetti, B.; Lapi, A.; Ciprioti, S.V. Thermodynamic study on six tricyclic nitrogen heterocycliccompounds by thermal analysis and effusion techniques. Thermochim. Acta 2016, 636, 71–84. [Google Scholar] [CrossRef]
- Bonicelli, M.G.; Catalani, A.; Mariano, G.; Vecchio, S. Heat capacities and molar enthalpies and entropies of fusion for anhydrous 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline. Thermochim. Acta 2007, 466, 69–71. [Google Scholar] [CrossRef]
- Chirico, R.D.; Kazakov, A.F.; Steele, W.V. Thermodynamic properties of three-ring aza-aromatics. 2. Experimental results for 1,10-phenanthroline, phenanthridine, and 7,8-benzoquinoline, and mutual validation of experiments and computational methods. J. Chem. Thermodyn. 2010, 42, 581–590. [Google Scholar] [CrossRef]
- Chirico, W.V. Thermodynamic Properties of 2-Methylquinoline and 8-Methylquinoline. J. Chem. Eng. Data 2005, 50, 697–708. [Google Scholar] [CrossRef]
- Camarillo, E.A.; Mentado, J.; Flores, H.; Hernandez-Perez, J.M. Standard enthalpies of formation of 2-aminobenzothiazoles in the crystalline phase by rotating-bomb combustion calorimetry. J. Chem. Thermodyn. 2014, 73, 269–273. [Google Scholar] [CrossRef]
- Calvin, J.J.; Rosen, P.F.; Smith, S.J.; Woodfield, B.F. Heat capacities and thermodynamic functions of the ZIF organic linkers imidazole, 2-methylimidazole, and 2-ethylimidazole. J. Chem. Thermodyn. 2018, in press. [Google Scholar] [CrossRef]
- Mo, O.; Yanez, M.; Roux, M.V.; Jimenez, P.; Davalos, J.Z.; Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.M.C.; Matos, M.A.R.; Amaral, L.M.P.F.; Sanchez-Migallon, A.; et al. Enthalpies of Formation of N-Substituted Pyrazoles and Imidazoles. J. Phys. Chem. A 1999, 103, 9336–9344. [Google Scholar] [CrossRef]
- Perdomo, G.; Flores, H.; Ramos, F.; Notario, R.; Freitas, V.L.S.; Ribeiro da Silva, M.D.M.C.; Camarillo, E.A.; Davalos, J.Z. Thermochemistry of R-SH group in gaseous phase: Experimental and theoretical studies of three sulfur imidazole derivatives. J. Chem. Thermodyn. 2018, 122, 65–72. [Google Scholar] [CrossRef]
- Ramos, F.; Flores, H.; Rojas, A.; Hernandez-Perez, J.M.; Camarillo, E.A.; Amador, M.P. Experimental and computational thermochemical study of benzofuran, benzothiophen and indole derivatives. J. Chem. Thermodyn. 2016, 97, 297–306. [Google Scholar] [CrossRef]
- Mentado, J.; Flores, H.; Amador, P. Combustion energies and formation enthalpies of 2-SH-benzazoles. J. Chem. Thermodyn. 2008, 40, 1106–1109. [Google Scholar] [CrossRef]
- Flores, H.; Ledo, J.M.; Hernandez-Perez, J.M.; Camarillo, E.A.; Sandoval-Lira, J.; Amador, M.P. Thermochemical and theoretical study of 2-oxazolidinone and 3-acetyl-2-oxazolidinone. J. Chem. Thermodyn. 2016, 102, 386–391. [Google Scholar] [CrossRef]
- McCullough, J.P.; Sunner, S.; Finke, H.L.; Hubbard, W.N.; Gross, M.E.; Pennington, R.E.; Messerly, J.F.; Good, W.D.; Waddington, G. The Chemical Thermodynamic Properties of 3-Methylthiophene from 0 to 1O0O-K. J. Am. Chem. Soc. 1953, 75, 5075–5081. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Jimenez, P.; Foces-Foces, C.; Notario, R.; Verevkin, S.P.; Liebman, J.F. Thermochemistry of 2,5-Thiophenedicarboxylic Acid. J. Phys. Chem. A 2006, 110, 12477–12483. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Jimenez, P.; Davalos, J.Z.; Notario, R. Experimental and Computational Thermochemistry of 2- and 3-Thiophenecarboxylic Acids. J. Phys. Chem. A 2002, 106, 11173–11180. [Google Scholar] [CrossRef]
- Temprado, M.; Roux, M.V.; Jimenez, P.; Foces-Foces, C.; Notario, R. Thermochemistry of 2- and 3-Thiopheneacetic Acids: Calorimetric and Computational Study. J. Phys. Chem. A 2008, 112, 10378–10385. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Hart, E.; Ji, Y.; Sadowski, G. Solubility and Caloric Properties of Cinnarizine. J. Chem. Eng. Data 2015, 60, 2256–2261. [Google Scholar] [CrossRef]
- Gudino, R.; Torres, L.A.; Campos, M.; Santillan, R.L.; Farfan, N. The standard molar enthalpies of combustion and sublimation of benzothiazino!benzothiazine and benzoxazino!benzoxazine. J. Chem. Thermodyn. 1997, 29, 565–574. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Blokhin, A.V.; Bazyleva, A.B.; Emel’yanenko, G.J. Thermodynamics of Ionic Liquids Precursors: 1-Methylimidazole. J. Phys. Chem. B 2011, 115, 4404–4411. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-H.; Tan, Z.-C.; Sun, X.-H.; Zhang, H.-T.; Liu, B.-P.; Sun, L.-X.; Zhang, T. Determination of Heat Capacities and Thermodynamic Properties of 2-(Chloromethylthio)benzothiazole by an Adiabatic Calorimeter. J. Chem. Eng. Data 2005, 50, 270–273. [Google Scholar] [CrossRef]
- Shi, Q.; Tan, Z.-C.; Di, Y.-Y.; Lv, X.-C.; Tong, B.; Zhang, Z.-H.; Sun, L.-X.; Zhang, T. Heat capacity and standard molar enthalpy of formation of crystalline 2,6-dicarboxypyridine (C7H5NO4). J. Chem. Thermodyn. 2006, 38, 1701–1705. [Google Scholar] [CrossRef]
- Shi, Q.; Tan, Z.-C.; Di, Y.-Y.; Tong, B.; Wang, S.-X.; Li, Y.-S. Thermodynamic studies of crystalline 2-amino-5-nitropyridine (C5H5N3O2). Thermochim. Acta 2007, 463, 6–9. [Google Scholar] [CrossRef]
- Zhou, C.-S.; Chen, F.-Y.; Di, Y.-Q.; Liu, Y.-L.; Liu, Y.-F. Low-Temperature Heat Capacities and Thermodynamic Properties of 2-Aminopyridine. Asian J. Chem. 2016, 28, 529–534. [Google Scholar] [CrossRef]
- Kulagina, T.G.; Smirnova, N.N.; Bykova, T.A.; Lebedev, B.V. Thermodynamics of 4,4’-Bipyridine in the Range 0-330 K. Russ. J. Gen. Chem. 2000, 70, 841–845. [Google Scholar]
- Shi, Q.; Tan, Z.-C.; Di, Y.-Y.; Tong, B.; Li, Y.-S.; Wang, S.-X. Thermal Analysis and Calorimetric Study of 4-Dimethylaminopyridine. J. Chem. Eng. Data 2007, 52, 941–947. [Google Scholar] [CrossRef]
- Wang, S.-X.; Tan, Z.-C.; Li, Y.-S.; Tong, B.; Li, Y.; Shi, Q.; Zhang, J.-N. Thermodynamic Study of 8-Hydroxyquinoline by Adiabatic Calorimetry and Thermal Analysis. Chin. J. Chem. 2008, 26, 2016–2020. [Google Scholar] [CrossRef]
- Dong, J.-X.; Li, Q.; Tan, Z.-C.; Zhang, Z.-H.; Liu, Y. The standard molar enthalpy of formation, molar heat capacities, and thermal stability of anhydrous caffeine. J. Chem. Thermodyn. 2007, 39, 108–114. [Google Scholar] [CrossRef]
- Steele, W.V.; Chirico, R.D.; Nguyen, A.; Knipmeyer, S.E. The thermodynamic properties of 2-methylaniline and trans-(R,S)-decahydroquinoline. J. Chem. Thermodyn. 1994, 26, 515–544. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.M.C.; Matos, M.A.R.; Jimenez, P.; Roux, M.V.; Martin-Luengo, M.A.; Elguero, J.; Claramunt, R.; Cabildo, P. Enthalpies of combustion, heat capacities, and enthalpies of vaporisation of 1-phenylimidazole and 1-phenylpyrazole. J. Chem. Thermodyn. 2000, 32, 237–245. [Google Scholar] [CrossRef]
- Chirico, R.D.; Knipmeyer, S.E.; Steele, W.V. Heat capacities, enthalpy increments, and derived thermodynamic functions for pyrazine between the temperatures 5K and 380K. J. Chem. Thermodyn. 2003, 35, 1059–1072. [Google Scholar] [CrossRef]
- Flores, H.; Ramos, F.; Camarillo, E.A.; Santiago, O.; Perdomo, G.; Notario, R.; Cabrera, S. Isothermal Thermogravimetric Study for Determining Sublimation Enthalpies of Some Hydroxyflavones. J. Chem. Eng. Data 2018, 63, 1925–1936. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Ribeiro da Silva, M.M.C.; Amaral, L.M.P.F.; Elguero, J.; Jimenez, P.; Roux, M.V.; Davalos, J.Z.; Temprado, M.; Cabildo, P.; Claramunt, R.M.; et al. Thermochemical properties of two benzimidazole derivatives: 2-Phenyl- and 2-benzylbenzimidazole. J. Chem. Thermodyn. 2005, 37, 1168–1176. [Google Scholar] [CrossRef]
- Kazakov, A.I.; Dalinger, I.L.; Zyuzin, I.N.; Lempert, D.B.; Plishkin, N.A.; Sheremetev, A.B. Enthalpies of formation of 3,4- and 3,5-dinitro-1-trimethyl-1H-pyrazoles. Russ. Chem. Bull. Int. Ed. 2016, 65, 2783–2788. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Liu, R.; Ji, T.-Z.; Ren, J.-C.; Guo, Q.; Wang, B.-Z.; Hu, R.-Z. Thermal behavior and thermal safety of 6bnitrohexahydro-2H-1,3,5-trioxacyclopenta[cd]-pentalene-2,4,6-triyltrinitrate. R. Soc. Chem. Adv. 2017, 7, 30747–30754. [Google Scholar]
- Masuda, N.; Nagano, Y.; Kimura, T. Standard molar enthalpy of formation of CH3(CH3SCH2)SO, Methyl Methylthiomethyl sulfoxide. J. Therm. Anal. Calorim. 2005, 81, 533–535. [Google Scholar] [CrossRef]
- Rayer, A.V.; Henni, A.; Tontiwachwuthikul, P. Molar Heat Capacities of Solvents Used in CO2 Capture: A Group Additivity and Molecular Connectivity Analysis. Can. J. Chem. Eng. 2012, 90, 367–376. [Google Scholar] [CrossRef]
- Malik, S.; Gupta, H.; Sharma, V.K. Topological investigation of ternary mixtures: Excess heat capacities. J. Mol. Liq. 2017, 233, 319–325. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.; Wang, Y.; Luan, Q.; Zhang, J.; Wang, G.; Hao, H. Thermodynamics of 4’-bromomethyl-2-cyanobiphenyl in different solvents. J. Chem. Thermodyn. 2015, 83, 77–84. [Google Scholar] [CrossRef]
- Yan, B.; Li, J.; Gao, J.; Wang, A.-M.; Ren, G.-Y.; Ma, H.-X. Crystal structure and thermodynamic properties of myclobutanil. J. Chem. Thermodyn. 2016, 101, 44–48. [Google Scholar] [CrossRef]
- Sun, X.-H.; Liu, Y.-F.; Tan, Z.-C.; Jia, Y.-Q.; Yang, J.-W.; Wang, M.-H. Heat Capacity and Enthalpy of Fusion of Fenoxycarb. Chin. J. Chem. 2005, 23, 501–505. [Google Scholar]
- Sun, X.-H.; Liu, Y.-F.; Tan, Z.-C.; Di, Y.-Y.; Wang, H.-F.; Wang, M.-H. Heat capacity and enthalpy of fusion of pyrimethanil laurate (C24H37N3O2). J. Chem. Thermodyn. 2004, 36, 895–899. [Google Scholar] [CrossRef]
- Di, Y.-Y.; Ye, C.-T.; Tan, Z.-C.; Zhang, G.-D. Low-temperature heat capacity and standard molar enthalpy of formation of crystalline (S)-(+)-Ibuprofen (C13H18O2)(s). Ind. J. Chem. 2007, 46A, 947–951. [Google Scholar]
- Knyazev, A.V.; Emel’yanenko, V.N.; Smirnova, N.N.; Zaitsau, D.H.; Stepanova, O.V.; Shipilova, A.S.; Markin, A.V.; Gusarova, E.V.; Knyazeva, S.S.; Verevkin, S.P. Comprehensive thermodynamic study of methylprednisolone. J. Chem. Thermodyn. 2017, 107, 37–41. [Google Scholar] [CrossRef]
- Di, Y.-Y.; Wang, D.-Q.; Shi, Q.; Tan, Z.-C. Low-temperature heat capacities and standard molar enthalpy of formation of N-methylnorephedrine C11H17NO(s). Chin. Phys. B 2008, 17, 2859–2866. [Google Scholar]
- Di, Y.-Y.; Kong, Y.-X.; Yang, W.-W.; Wang, D.-Q.; Tan, Z.-C. Crystal Structure and Thermodynamic Properties of N, N-dimethylnorephedrine Hydrochloride (C11H18NOCl) (s). Acta Chim. Slov. 2009, 56, 392–398. [Google Scholar]
- Simoes, R.G.; Bernardes, C.E.S.; Diogo, H.P.; Agapito, F.; Minas de Piedade, M.E. Energetics and Structure of Simvastatin. Mol. Pharm. 2013, 10, 2713–2722. [Google Scholar] [CrossRef]
- Mealy, D.; Svärd, M.; Rasmuson, A. Thermodynamics of Risperidone and Solubility in Pure Organic Solvents. Fluid Phase Equil. 2014, 373, 73–79. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Letyanina, I.A.; Plesovskikh, A.S.; Smirnova, N.N.; Knyazeva, S.S. Thermodynamic properties of vitamin B2. Thermochim. Acta 2014, 575, 12–16. [Google Scholar] [CrossRef]
- Becker, L.; Gmehling, J. Measurement of Heat Capacities for 12 Organic Substances by Tian-Calvet Calorimetry. J. Chem. Eng. Data 2001, 46, 1638–1642. [Google Scholar] [CrossRef]
- Paulechka, Y.U. Heat Capacity of Room-Temperature Ionic Liquids: A Critical Review. J. Phys. Chem. Ref. Data 2010, 39, 033108-1-23. [Google Scholar] [CrossRef]
- NIST National Institute of Standards and Technology Data Gateway. Available online: http://srdata.nist.gov/gateway/ (accessed on 1 May 2015).
- Hanaya, M.; Shibazaki, H.; Oguni, M.; Nemoto, T.; Ohashi, Y. Orientational ordering/disordering of ions accompanied by phase transitions in pyridinium tetrafluoroborate crystal. J. Phys. Chem. Solids 2000, 61, 651–657. [Google Scholar] [CrossRef]
- Anouti, M.; Caillon-Caravanier, M.; Dridi, Y.; Jacquemin, J.; Hardacre, C.; Lemordant, D. Liquid densities, heat capacities, refractive index and excess quantities for {protic ionic liquids + water} binary system. J. Chem. Thermodyn. 2009, 41, 799–808. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Tsvetkova, L.Y.; Bykova, T.A.; Ruchenin, V.A.; Marcus, Y. Thermodynamic properties of tetrabutylammonium iodide and tetrabutylammonium tetraphenylborate. Thermochim. Acta 2009, 483, 15–20. [Google Scholar] [CrossRef]
- Tong, B.; Liu, Q.-S.; Tan, Z.-C.; Weiz-Biermann, U. Thermochemistry of Alkyl Pyridinium Bromide Ionic Liquids: Calorimetric Measurements and Calculations. J. Phys. Chem. A 2010, 114, 3782–3787. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical Characterization of Ionic Liquids Able To Dissolve Biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Bastos, M.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Heat capacities at 298.15 K of the extended [CnC1im][Ntf2] ionic liquid series. J. Chem. Thermodyn. 2012, 53, 140–143. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Simoes, P.N.; Ferreira, A.G.M. Quaternary phosphonium-based ionic liquids: Thermal stability and heat capacity of the liquid phase. J. Chem. Thermodyn. 2012, 45, 16–27. [Google Scholar] [CrossRef]
- Domanska, U.; Skiba, K.; Zawadski, M.; Paduszynski, K.; Krolikowski, M. Synthesis, physical, and thermodynamic properties of 1-alkyl-cyanopyridinium bis{(trifluoromethyl)sulfonyl}imide ionic liquids. J. Chem. Thermodyn. 2013, 56, 153–161. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Tan, Z.-C.; Weiz-Biermann, U.; Liu, X.-X. Molar heat capacity and thermodynamic properties of N-alklypyridinium hexafluorophosphate salts, [Cnpy][PF6] (n = 2, 3, 5). J. Chem. Thermodyn. 2014, 68, 82–89. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Emel’yanenko, V.N.; Heintz, A.; Verevkin, S.P.; Schick, C.; Berdzinski, S.; Strehmel, V. Structure–property relationships in ILs: Vaporization enthalpies of pyrrolidinium based ionic liquids. J. Mol. Liq. 2014, 192, 171–176. [Google Scholar] [CrossRef]
- Yan, B.; Li, H.-Y.; Zhao, N.-N.; Ma, H.-X.; Song, J.-R.; Zhao, F.-Q.; Hu, R.-Z. Thermodynamic properties, detonation characterization and free radical of N-20,40-dinitrophenyl-3,3-dinitroazetidine. J. Chem. Thermodyn. 2014, 69, 152–156. [Google Scholar] [CrossRef]
- Talavera-Prieto, N.M.C.; Ferreira, A.G.M.; Simoes, P.N.; Carvalho, P.J.; Mattedi, S.; Coutinho, J.A.P. Thermophysical characterization of N-methyl-2-hydroxyethylammonium carboxilate ionic liquids. J. Chem. Thermodyn. 2014, 68, 221–234. [Google Scholar] [CrossRef]
- Paulechka, E.; Blokhin, A.V.; Rodrigues, A.S.M.C.; Rocha, M.A.A.; Santos, L.M.N.B.F. Thermodynamics of long-chain 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ionic liquids. J. Chem. Thermodyn. 2016, 97, 331–340. [Google Scholar] [CrossRef]
- Serra, P.B.P.; Ribeiro, F.M.S.; Rocha, M.A.A.; Fulem, M.; Ruzicka, K.; Santos, L.M.N.B.F. Phase Behavior and Heat Capacities of the 1-Benzyl-3-methylimidazolium Ionic Liquids. J. Chem. Thermodyn. 2016, 100, 124–130. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Vilas, M.; Rodrigues, A.S.M.C.; Tojo, E.; Santos, L.M.N.B.F. Physicochemical properties of 2-alkyl-1-ethylpyridinium based ionic liquids. Fluid Phase Equil. 2016, 428, 112–120. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Varfolomeev, M.A.; Verevkin, S.P.; Stanton, A.D.; Hindman, M.S.; Bara, J.E. Structure–property relationships in ionic liquids: Influence of branched and cyclic groups on vaporization enthalpies of imidazolium-based Ils. J. Chem. Thermodyn. 2016, 93, 151–156. [Google Scholar] [CrossRef]
- Yan, B.; Li, H.-Y.; Guan, Y.-L.; Ma, H.-X.; Song, J.-R.; Zhao, F.-Q. Thermodynamic properties of 3,3-dinitroazetidinium nitrate. J. Chem. Thermodyn. 2016, 103, 206–211. [Google Scholar] [CrossRef]
- Zheng, L.; Li, L.; Guo, Y.-F.; Guan, W.; Fang, D.-W. The isobaric heat capacities and thermodynamic properties of ionic liquid 1- ethylpyridinium bis(trifluoromethylsulfonyl)imide. J. Therm. Anal. Calorim. 2017, 131. [Google Scholar] [CrossRef]
- Tian, T.; Hu, X.; Guan, P.; Wang, S.; Ding, X. Density and thermodynamic performance of energetic ionic liquids based on 1-alkyl/esteryl-4-amino-1,2,4-triazolium. J. Mol. Liq. 2017, 248, 70–80. [Google Scholar] [CrossRef]
- Gupta, H.; Malik, S.; Chandrasekhar, M.; Sharma, V.K. Thermodynamic investigations of excess heat capacities of ternary liquid mixtures containing [Bmmim][BF4] + [Bmim][BF4] or [Emim][BF4] + cyclopentanone or cyclohexanone. J. Therm. Anal. Calorim. 2018, 131, 1653–1669. [Google Scholar] [CrossRef]
- Serra, P.B.P.; Ribeiro, F.M.S.; Rocha, M.A.A.; Fulem, M.; Ruzicka, K.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Solid-liquid equilibrium and heat capacity trend in the alkylimidazolium PF6 series. J. Mol. Liq. 2017, 248, 678–687. [Google Scholar] [CrossRef]
- Dong, Y.; Shah, S.N.; Pranesh, M.; Prokkola, H.; Kärkkäinen, J.; Leveque, J.-M.; Lassi, U.; Lethesh, K.C. Azepanium based protic ionic liquids: Synthesis, thermophysical properties and COSMO-RS study. J. Mol. Liq. 2018, 264, 24–31. [Google Scholar] [CrossRef]
- Bendova, M.; Canji, M.; Wagner, Z.; Bogdanov, M.G. Ionic Liquids as Thermal Energy Storage Materials: On the Importance of Reliable Data Analysis in Assessing Thermodynamic Data. J. Solution Chem. 2018. [Google Scholar] [CrossRef]
- Oster, K.; Jacquemin, J.; Hardacre, C.; Ribeiro, A.P.C.; Elsinawi, A. Further development of the predictive models for physical properties of pure ionic liquids: Thermal conductivity and heat capacity. J. Chem. Thermodyn. 2018, 118, 1–15. [Google Scholar] [CrossRef]
- Gomez, E.; Calvar, N.; Dominguez, A.; Macedo, E.A. Thermal behavior and heat capacities of pyrrolidinium-based ionic liquids by DSC. Fluid Phase Equil. 2018, 470, 51–59. [Google Scholar] [CrossRef]
- Huelsekopf, M.; Ludwig, R. Temperature Dependence of Hydrogen Bonding. J. Mol. Liq. 2000, 85, 105–125. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).