Secondary Metabolites and the Risks of Isaria fumosorosea and Isaria farinosa
Abstract
1. Introduction
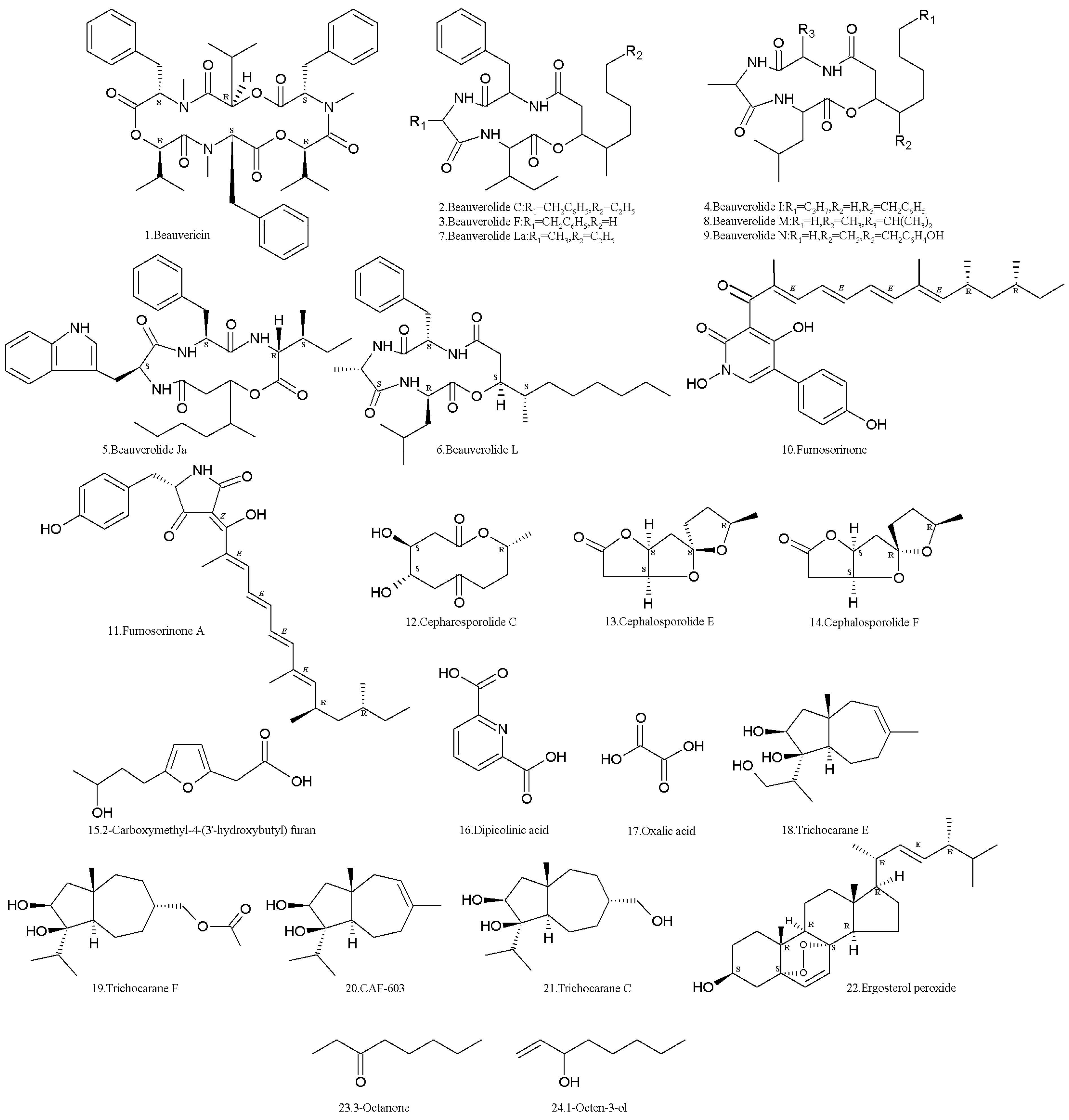
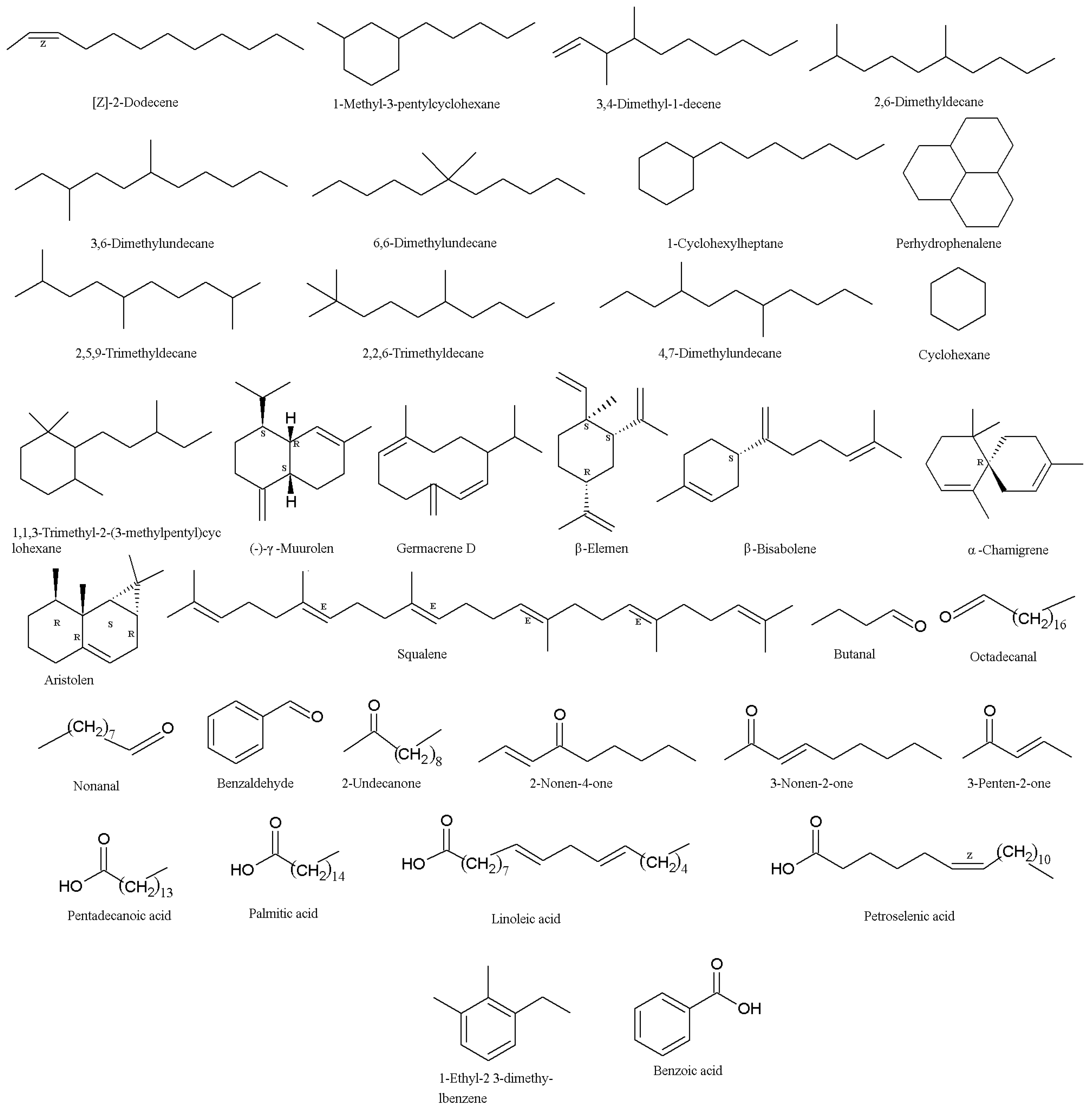
2. Secondary Metabolites (SMs) from Isaria fumosorosea
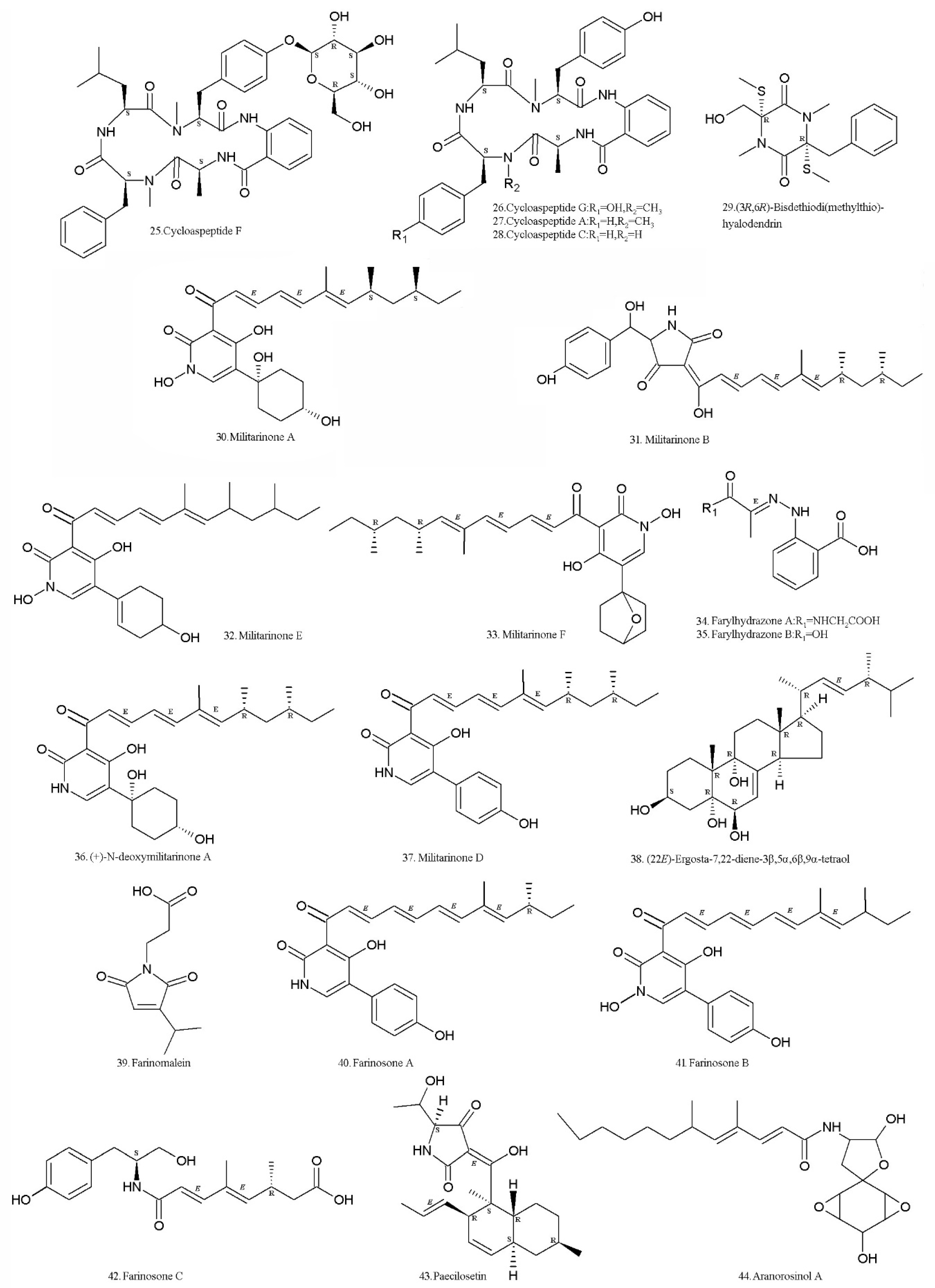
3. Secondary Metabolites (SMs) from Isaria farinosa
4. Risks of the Secondary Metabolites (SMs) from both Isaria Myco-Insecticides
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Liu, S.; Yin, F.; Cai, S.; Zhong, G.; Ren, S. Diversity and virulence of soil-dwelling fungi Isaria spp. and Paecilomyces spp. against Solenopsis invicta (hymenoptera: Formicidae). Biocontrol Sci. Technol. 2011, 21, 225–234. [Google Scholar] [CrossRef]
- Lopes, R.D.; de Lima, G.; Correia, M.T.D.; da Costa, A.F.; Lima, E.A.D.A.; Lima, V.L.D. The potential of Isaria spp. as a bioinsecticide for the biological control of nasutitermes corniger. Biocontrol Sci. Technol. 2017, 27, 1038–1048. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Huang, Y.; Keyhani, N.O.; Huang, Z. Lack of resistance development in Bemisia tabaci to Isaria fumosorosea after multiple generations of selection. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, E.; Hoc, N.; Sycz, J.; Urbaniak, M.; Dymarska, M.; Grzeszczuk, J.; Kostrzewa-Suslow, E.; Stepien, L.; Plaskowska, E.; Janeczko, T. Biotransformation of steroids by entomopathogenic strains of Isaria farinosa. Microb. Cell Fact. 2018, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Suslow, E. Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the genus Isaria. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Grzeszczuk, J.; Urbaniak, M.; Janeczko, T.; Plaskowska, E.; Stepien, L.; Kostrzewa-Suslow, E. Glycosylation of 6-methylflavone by the strain Isaria fumosorosea KCH J2. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): Biology, ecology and use in biological control. Biocontrol Sci. Technol. 2008, 18, 865–901. [Google Scholar] [CrossRef]
- Majeed, M.Z.; Fiaz, M.; Ma, C.-S.; Afzal, M. Entomopathogenicity of three muscardine fungi, Beauveria bassiana, Isaria fumosorosea and Metarhizium anisopliae, against the asian citrus psyllid, Diaphorina citri (Kuwayama) (Hemiptera: Psyllidae). Egypt. J. Biol. Pest Control 2017, 27, 211–215. [Google Scholar]
- Jessica, J.J.; Peng, T.L.; Sajap, A.S.; Lee, S.H.; Syazwan, S.A. Evaluation of the virulence of entomopathogenic fungus, Isaria fumosorosea isolates against subterranean termites Coptotermes spp. (Isoptera: Rhinotermitidae). J. For. Res. (Harbin, China) 2018. [Google Scholar] [CrossRef]
- Usanmaz-Bozhuyuk, A.; Kordali, S.; Kesdek, M.; Simsek, D.; Altinok, M.A.; Altinok, H.H.; Komaki, A. Mortality effects of six different entomopathogenic fungi strains on rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Fresen. Environ. Bull. 2018, 27, 4373–4380. [Google Scholar]
- Montemayor, C.O.; Avery, P.B.; Cave, R.D. Infection and mortality of Microtheca ochroloma (Coleoptera: Chrysomelidae) by Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Biocontrol Sci. Technol. 2016, 26, 605–616. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Shakeel, M.; Li, S.; Wang, S.; Zhou, X.; Yu, J.; Xu, X.; Yu, X.; Jin, F. The entomopathogenic fungi Isaria fumosorosea plays a vital role in suppressing the immune system of Plutella xylostella: Rna-seq and dge analysis of immunity-related genes. Front. Microbiol. 2017, 8, 1421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zou, C.; Hu, Q. Effects of Isaria fumosorosea on TYLCV (tomato yellow leaf curl virus) accumulation and transmitting capacity of Bemisia tabaci. PLoS ONE 2016, 11, e0164356. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Li, Z. Insect Mycology, 1st ed.; Anhui Science and Technology Press: Hefei, China, 1996. [Google Scholar]
- Lappa, N.V.; Goral, V.M.; Drozda, V.P. Effectiveness of boverin and paecilomin in the control of the codling moth. Zashchita Rastenii 1977, 24, 13–18. (in Russian). [Google Scholar]
- Samsinakova, A.; Kalalova, S.; Vlcek, V.; Kybal, J. Mass production of Beauveria bassiana for regulation of Leptinotarsa decemlineata populations. J. Invertebr. Pathol. 1981, 38, 16–174. [Google Scholar] [CrossRef]
- Mochi, D.A.; Monteiro, A.C.; Machado, A.C.; Yoshida, L. Entomopathogenic fungal activity against pupae and adult Haematobia irritans (Diptera: Muscidae). Vet. Parasitol. 2010, 168, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Mustu, M.; Demirci, F.; Koksal, M.; Serbes, C.; Armagan, B. Mortality effects of Isaria farinosa and Purpureocillium lilacinum (Sordariomycetes: Hypocreales) on the two spotted spider mite Tetranychus urticae (Acari: Tetranychidae) and its predator Neoseiulus californicus (Acari: Phytoseiidae) under controlled conditions. Entomol. Gen. 2016, 35, 243–252. [Google Scholar]
- Mustu, M.; Demirci, F.; Kaydan, M.B.; Ulgenturk, S. Laboratory assay of the effectiveness of the entomopathogenic fungus Isaria farinosa (Holmsk.) fries (Sordariomycetes: Hypocreales) against the vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae), even under the use of fungicides. Int. J. Pest Manag. 2015, 61, 264–271. [Google Scholar] [CrossRef]
- Mustu, M.; Demirci, F.; Kocak, E. Mortality of Isaria farinosa and Beauveria bassiana on sunn pests Eurygaster integriceps and Eurygaster austriaca. Phytoparasitica 2014, 42, 93–97. [Google Scholar] [CrossRef]
- Johny, S.; Kyei-Poku, G.; Gauthier, D.; van Frankenhuyzen, K. Isolation and characterisation of Isaria farinosa and Purpureocillium lilacinum associated with emerald ash borer, Agrilus planipennis in canada. Biocontrol Sci. Technol. 2012, 22, 723–732. [Google Scholar] [CrossRef]
- Mustu, M.; Demirci, F.; Kocak, E. Mortality effects of Isaria farinosa (Holm.) and Beauveria bassiana (Balsamo) Vuillemin (Sordariomycetes: Hypocreales) on Aelia rostrata Boh. (Hemiptera: Pentatomidae). Turkiye Entomoloji Dergisi-Turkish 2011, 35, 559–568. [Google Scholar]
- Yang, S.; Zhuang, H.; Li, Y.; Kuang, R. Insecticidal efficacy of Isaria farinosa in different life stages of Pissodes punctatus (Coleoptera: Curculionidae). J. Pest Sci. 2009, 82, 321–325. [Google Scholar] [CrossRef]
- Liu, F.; Wu, X.L.; Liu, Y.; Chen, D.X.; Zhang, D.L.; Yang, D.J. Progress on molecular biology of Isaria farinosa, pathogen of host of ophiocordyceps sinensis during the artificial culture. China J. Chin. Mater. Med. 2016, 41, 403–409. [Google Scholar]
- Lv, Y.; Xia, J.; Zhang, Z.; Li, Q.; Dong, C.; Li, W. Symptoms, infection and histopathology of Hepialus sp. larvae parasitized by Isaria farinosa. Mycosystema 2018, 37, 314–324. [Google Scholar]
- Liu, F.; Xiang, M.; Guo, Y.; Wu, X.; Lu, G.; Yang, Y.; Liu, X.; Chen, S.; Zhang, G.; Shi, W. Culture conditions and nutrition requirements for the mycelial growth of Isaria farinosa (Hypocreales: Cordycipitaceae) and the altitude effect on its growth and metabolome. Sci. Rep. 2018, 8, 15623. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Brunner-Mendoza, C.; Navarro-Barranco, H.; Leon-Mancilla, B.; Perez-Torres, A.; Toriello, C. Biosafety of an entomopathogenic fungus Isaria fumosorosea in an acute dermal test in rabbits. Cutan. Ocul. Toxicol. 2017, 36, 12–18. [Google Scholar] [CrossRef]
- Avery, P.B.; Hunter, W.B.; Hall, D.G.; Jackson, M.A.; Powell, C.A. Efficacy of topical application, leaf residue or soil drench of blastospores of Isaria fumosorosea for citrus root weevil management: Laboratory and greenhouse investigations. Insects 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, L.-L.; Wei, J.-J.; Fan, P.; Liu, S.-S.; Yuan, W.-Y.; Zhao, Y.-X.; Luo, D.-Q. PTP1B inhibitors from the entomogenous fungi Isaria fumosorosea. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Wang, Q.G.; Xu, L.J. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Lin, H.-I.; Chen, B.-F.; Jow, G.-M. Beauvericin-induced cell apoptosis through the mitogen-activated protein kinase pathway in human nonsmall cell lung cancer A549 cells. J. Toxicol. Sci. 2016, 41, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.W.; Lin, Y.C.; She, Z.G.; Lin, M.T.; Chen, P.X.; Zhang, J.Y. Anticancer activity and mechanism investigation of beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anticancer Agents Med. Chem. 2015, 15, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Patocka, J.; Wu, Q. Biological activities of beauvericin, a mini review. Mini. Rev. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and levels of regulated and emerging mycotoxins in silage in poland. Mycotoxin Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ojuri, O.T.; Ezekiel, C.N.; Sulyok, M.; Ezeokoli, O.T.; Oyedele, O.A.; Ayeni, K.I.; Eskola, M.K.; Sarkanj, B.; Hajslova, J.; Adeleke, R.A.; et al. Assessing the mycotoxicological risk from consumption of complementary foods by infants and young children in nigeria. Food Chem. Toxicol. 2018, 121, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Mallebrera, B.; Prosperini, A.; Font, G.; Ruiz, M.J. In vitro mechanisms of beauvericin toxicity: A review. Food Chem. Toxicol. 2018, 111, 537–545. [Google Scholar] [CrossRef]
- Madariaga-Mazon, A.; Gonzalez-Andradeb, M.; Toriello, C.; Navarro-Barranco, H.; Mata, R. Potent anti-calmodulin activity of cyclotetradepsipeptides isolated from Isaria fumosorosea using a newly designed biosensor. Nat. Prod. Commun. 2015, 10, 113–116. [Google Scholar]
- Jegorov, A.; Sedmera, P.; Matha, V.; Simek, P.; Zahradnickova, H.; Landa, Z.; Eyal, J. Beauverolides L and La from Beauveria tenella and Paecilomyces fumosoroseus. Phytochemistry 1994, 37, 1301–1303. [Google Scholar] [CrossRef]
- Vilcinskas, A.; Jegorov, A.; Landa, Z.; Gotz, P.; Matha, V. Effects of beauverolide L and cyclosporin A on humoral and cellular immune response of the greater wax moth, Galleria mellonella. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 122, 83–92. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Chen, C.; Teng, J.; Wang, C.; Luo, D. Structure and biosynthesis of fumosorinone, a new protein tyrosine phosphatase 1B inhibitor firstly isolated from the entomogenous fungus Isaria fumosorosea. Fungal Genet. Biol. 2015, 81, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xue, T.; Fan, P.; Meng, L.; Wei, J.; Luo, D. Cytotoxic activity of SHP2 inhibitor fumosorinone in human cancer cells. Oncol. Lett. 2018, 15, 10055–10062. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Pramjit, S.; Pakawatchai, C.; Isaka, M.; Supothina, S. 10-membered macrolides from the insect pathogenic fungus Cordyceps militaris BCC 2816. J. Nat. Prod. 2004, 67, 1953–1955. [Google Scholar] [CrossRef] [PubMed]
- Asaff, A.; Cerda-Garcia-Rojas, C.; de la Torre, M. Isolation of dipicolinic acid as an insecticidal toxin from Paecilomyces fumosoroseus. Appl. Microbiol. Biotechnol. 2005, 68, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Asaff, A.; Cerda-Garcia-Rojas, C.M.; Viniegra-Gonzalez, G.; de la Torre, M. Carbon distribution and redirection of metabolism in Paecilomyces fumosoroseus during solid-state and liquid fermentations. Process. Biochem. (Amst., The Neth.) 2006, 41, 1303–1310. [Google Scholar] [CrossRef]
- Martinez-Espla, A.; Serrano, M.; Martinez-Romero, D.; Valero, D.; Zapata, P.J. Oxalic acid preharvest treatment increases antioxidant systems and improves plum quality at harvest and during postharvest storage. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillen, F.; Martinez-Romero, D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.M.; Yin, W.Q.; Liu, M.M.; Wang, C.L.; Guo, S.X. Oxalic acid and sclerotial differentiation of Polyporus umbellatus. Sci. Rep. 2015, 5, 10759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and quality control of Polyporus umbellatus (Pers.) fries: A review. J. Ethnopharmacol. 2013, 149, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.S.; Yuan, W.Y.; Wei, J.J.; Zhao, Y.X.; Luo, D.Q. Carotane-type sesquiterpenes from cultures of the insect pathogenic fungus Isaria fumosorosea. J. Asian Nat. Prod. Res. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Hu, F.; Wang, X.; Wang, X.; Li, Z.; Fan, M. Isolation and purification of peroxy-ergosterol from Paecilomyces fumosoroseus by high-speed-counter-current chromatography and identification by ESI-MS. Food Ferment. Ind. 2009, 35, 14–17. [Google Scholar]
- Sheu, J.H.; Chang, K.C.; Duh, C.Y. A cytotoxic 5alpha,8alpha-epidioxysterol from a soft coral Sinularia species. J. Nat. Prod. 2000, 63, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Yoshida, M.; Ohnishi-Kameyama, M.; Kobori, M. Ergosterol peroxide, an apoptosis-inducing component isolated from Sarcodon aspratus (berk.) s. Ito. Biosci. Biotechnol. Biochem. 2005, 69, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Y.; Liu, X.; Liang, Z. Screening of vitamin a-praducing fungal resources in soil. Guizhou Agric. Sci. 2013, 41, 99–101. [Google Scholar]
- Yanagawa, A.; Imai, T.; Akino, T.; Toh, Y.; Yoshimura, T. Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus. J. Chem. Ecol. 2015, 41, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Bland, J.M.; Gu, W.X. Behavioral and electrophysiological responses of Coptotermes formosanus Shiraki towards entomopathogenic fungal volatiles. Biol. Control 2010, 55, 166–173. [Google Scholar] [CrossRef]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Golebiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Liu, H.; Liu, X.; Che, Y. Cycloaspeptides F and G, cyclic pentapeptides from a Cordyceps-colonizing isolate of Isaria farinosa. J. Nat. Prod. 2009, 72, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Hormazabal, E.; Rodriguez, J.A.; Theoduloz, C. Cycloaspeptide a and pseurotin a from the endophytic fungus penicillium janczewskii. Z Naturforsch C 2008, 63, 383–388. [Google Scholar] [CrossRef]
- de Mattos-Shipley, K.M.J.; Greco, C.; Heard, D.M.; Hough, G.; Mulholland, N.P.; Vincent, J.L.; Micklefield, J.; Simpson, T.J.; Willis, C.L.; Cox, R.J.; et al. The cycloaspeptides: Uncovering a new model for methylated nonribosomal peptide biosynthesis. Chem. Sci. 2018, 9, 4109–4117. [Google Scholar] [CrossRef]
- Prachyawarakorn, V.; Mahidol, C.; Sureram, S.; Sangpetsiripan, S.; Wiyakrutta, S.; Ruchirawat, S.; Kittakoop, P. Diketopiperazines and phthalides from a marine derived fungus of the order pleosporales. Planta Med. 2008, 74, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-hydroxypyridones, phenylhydrazones, and a quinazolinone from Isaria farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Gunther, W.; Stoyanova, S.; Schubert, B.; Li, Z.Z.; Hamburger, M. Militarinone a, a neurotrophic pyridone alkaloid from Paecilomyces militaris. Organ. Lett. 2002, 4, 197–199. [Google Scholar] [CrossRef]
- Tsang, T.H.; Gubler, D.A. Total synthesis of farylhydrazones a and b. Tetrahedron Lett. 2012, 53, 4243–4244. [Google Scholar] [CrossRef]
- Cheng, Y.; Schneider, B.; Riese, U.; Schubert, B.; Li, Z.; Hamburger, M. (+)-n-deoxymilitarinone a, a neuritogenic pyridone alkaloid from the insect pathogenic fungus Paecilomyces farinosus. J. Nat. Prod. 2006, 69, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; William, R.; Leow, M.L.; Chai, H.; Fong, J.Z.; Liu, X.W. Directed orthometalation and the asymmetric total synthesis of n-deoxymilitarinone A and torrubiellone B. Organ. Lett. 2014, 16, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.P.; Kinoshita, H.; Ihara, F.; Igarashi, Y.; Nihira, T. Farinomalein, a maleimide-bearing compound from the entomopathogenic fungus Paecilomyces farinosus. J. Nat. Prod. 2009, 72, 1544–1546. [Google Scholar] [CrossRef] [PubMed]
- Miles, W.H.; Yan, M. Synthesis of farinomalein. Tetrahedron Lett. 2010, 51, 1710–1712. [Google Scholar] [CrossRef]
- Putri, S.P.; Ishido, K.; Kinoshita, H.; Kitani, S.; Ihara, F.; Sakihama, Y.; Igarashi, Y.; Nihira, T. Production of antioomycete compounds active against the phytopathogens Phytophthora sojae and Aphanomyces cochlioides by clavicipitoid entomopathogenic fungi. J. Biosci. Bioeng. 2014, 117, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.X.; Schneider, B.; Riese, U.; Schubert, B.; Li, Z.Z.; Hamburger, M. Farinosones A-C, neurotrophic alkaloidal metabolites from the entomogenous Deuteromycete Paecilomyces farinosus. J. Nat. Prod. 2004, 67, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Blunt, J.W.; Cummings, N.J.; Cole, A.L.; Munro, M.H. Paecilosetin, a new bioactive fungal metabolite from a new zealand isolate of Paecilomyces farinosus. J. Nat. Prod. 2005, 68, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Vijayakumar, E.K.; Mukhopadhyay, T.; Chatterjee, S.; Bhat, R.G.; Blumbach, J.; Ganguli, B.N. Aranorosinol A and aranorosinol B, two new metabolites from pseudoarachniotus roseus: Production, isolation, structure elucidation and biological properties. J. Antibiot. (Tokyo) 1992, 45, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Lee, Y.H.; Nanthakumar, K.; Kamala-Kannan, S.; Dufosse, L.; Mapari, S.A.S.; Oh, B.-T. Water-soluble red pigments from Isaria farinosa and structural characterization of the main colored component. J. Basic Microbiol. 2010, 50, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef]
- Genthner, F.J.; Chancy, C.A.; Couch, J.A.; Foss, S.S.; Middaugh, D.P.; George, S.E.; Warren, M.A.; Bantle, J.A. Toxicity and pathogenicity testing of the insect pest control fungus Metarhizium anisopliae. Arch. Environ. Contam. Toxicol. 1998, 35, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, E.A.; Murza, V.I. Investigation of the safety of industrial strains of microorganisms and microbial insecticides. J. Hyg. Epidemiol. Microbiol. Immunol. 1980, 24, 425–431. [Google Scholar]
- Smagghe, G.; De Meyer, L.; Meeus, I.; Mommaerts, V. Safety and acquisition potential of Metarhizium anisopliae in entomovectoring with bumble bees, Bombus terrestris. J. Econ. Entomol. 2013, 106, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Haas-Costa, J.; Alves, L.F.A.; Daros, A.A. Safety of Beauveria bassiana (Bals.) Vuill. to Gallus domesticus L. Braz. Arch. Biol. Technol. 2010, 53, 465–471. [Google Scholar] [CrossRef]
- Hu, Q.; Li, F.; Zhang, Y. Risks of mycotoxins from mycoinsecticides to humans. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liang, L.; Hao, C.; Ma, R. The study on the acute toxicity and dermal sensitization of Isaria fumosorosea. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2013, 33, 144–146. [Google Scholar]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic fungi: New insights into host-pathogen interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar] [PubMed]
- Mantzoukas, S.; Chondrogiannis, C.; Grammatikopoulos, G. Effects of three endophytic entomopathogens on sweet sorghum and on the larvae of the stalk borer Sesamia nonagrioides. Entomol. Exp. Appl. 2015, 154, 78–87. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Scorsetti, A.C. Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, hypocreales). Biocontrol Sci. Technol. 2015, 25, 475–480. [Google Scholar] [CrossRef]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. Jove-J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Yang, L.; Qiu, X.; Liu, Y.G.; Zhou, W.; Wan, Y.J. Diversity analysis of Beauveria bassiana isolated from infected silkworm in southwest china based on molecular data and morphological features of colony. World J. Microbiol. Biotechnol. 2013, 29, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Reynaldi, F.J.; Lucia, M.; Genchi Garcia, M.L. Ascosphaera apis, the entomopathogenic fungus affecting larvae of native bees (xylocopa augusti): First report in south america. Rev. Iberoam. Micol. 2015, 32, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Hernandez, R.A.; Ruiz-Toledo, J.; Toledo, J.; Sanchez, D. Effect of three entomopathogenic fungi on three species of stingless bees (Hymenoptera: Apidae) under laboratory conditions. J. Econ. Entomol. 2016. [Google Scholar] [CrossRef]
- Rannback, L.M.; Cotes, B.; Anderson, P.; Ramert, B.; Meyling, N.V. Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J. Invertebr. Pathol. 2015, 124, 78–86. [Google Scholar] [CrossRef]
- Yousef, M.; Alba-Ramirez, C.; Garrido Jurado, I.; Mateu, J.; Raya Diaz, S.; Valverde-Garcia, P.; Quesada-Moraga, E. Metarhizium brunneum (Ascomycota; Hypocreales) treatments targeting olive fly in the soil for sustainable crop production. Front. Plant Sci. 2018, 9, 1. [Google Scholar] [CrossRef]
- Herrick, N.J.; Cloyd, R.A. Direct and indirect effects of pesticides on the insidious flower bug (Hemiptera: Anthocoridae) under laboratory conditions. J. Econ. Entomol. 2017, 110, 931–940. [Google Scholar] [CrossRef]
- Fan, M.; Li, J.; Guo, C.; Hashan, E. Persistent forms and survival potential of Metarhizium anisopliae in soil. J. Northwestern Coll. For. 1991, 6, 48–54. [Google Scholar]
- Li, Y.; Hu, Z.; Hu, Z.; Xiao, H.; Li, H. Survival dynamics of Beauveria bassiana BB09 in the muscardine cadaver of Hyphantria cunea and surrounding soil. For. Ecol. Sci. 2018, 33, 172–177. [Google Scholar]
- Zhang, Y.; Wu, X.; Ye, B.; Wang, H.; Shu, J. Inhibition effects of soil bacteria on conidium germination of Metarhizium pingshaense. Chin. J. Biol. Control 2017, 33, 788–795. [Google Scholar]
- Wu, X.; Zhang, Y.; Wu, P.; Ye, B.; Wang, H.; Shu, J. Influence of temperature, moisture, and soil type on conidium germination of Metarhizium pingshaense in soil. Chin. J. Biol. Control 2014, 30, 766–771. [Google Scholar]
- Biswas, C.; Dey, P.; Gotyal, B.S.; Satpathy, S. A method of multiplex pcr for detection of field released Beauveria bassiana, a fungal entomopathogen applied for pest management in jute (Corchorus olitorius). World J. Microbiol. Biotechnol. 2015, 31, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Tai, M.H.; Santos, A.H.; Rocha, L.F.; Albernaz, D.A.; Silva, H.H. Ovicidal activity of entomopathogenic hyphomycetes on Aedes aegypti (Diptera: Culicidae) under laboratory conditions. J. Med. Entomol. 2007, 44, 799–804. [Google Scholar] [CrossRef]
- Falvo, M.L.; Albornoz Medina, P.; Rodrigues, J.; Lopez Lastra, C.C.; Garcia, J.J.; Fernandes, E.K.K.; Luz, C. Effect of UV-B irradiation on water-suspended Metarhizium anisopliae s.l. (Hypocreales: Clavicipitaceae) conidia and their larvicidal activity in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2018, 55, 1330–1333. [Google Scholar] [CrossRef]
- Milner, R.J.; Lim, R.P.; Hunter, D.M. Risks to the aquatic ecosystem from the application of Metarhizium anisopliae for locust control in australia. Pest Manag. Sci. 2002, 58, 718–723. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L. (Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef]
- Raya-Diaz, S.; Sanchez-Rodriguez, A.R.; Segura-Fernandez, J.M.; Del Campillo, M.D.C.; Quesada-Moraga, E. Entomopathogenic fungi-based mechanisms for improved fe nutrition in sorghum plants grown on calcareous substrates. PLoS ONE 2017, 12, e0185903. [Google Scholar] [CrossRef] [PubMed]
- Skrobek, A.; Shah, F.A.; Butt, T.M. Destruxin production by the entomogenous fungus Metarhizium anisopliae in insects and factors influencing their degradation. Biocontrol 2008, 53, 361–373. [Google Scholar] [CrossRef]
- Rios-Moreno, A.; Garrido-Jurado, I.; Raya-Ortega, M.C.; Quesada-Moraga, E. Quantification of fungal growth and destruxin a during infection of Galleria mellonella larvae by Metarhizium brunneum. J. Invertebr. Pathol. 2017, 149, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Oyedele, O.A.; Ezekiel, C.N.; Sulyok, M.; Adetunji, M.C.; Warth, B.; Atanda, O.O.; Krska, R. Mycotoxin risk assessment for consumers of groundnut in domestic markets in nigeria. Int. J. Food Microbiol. 2017, 251, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Miller, J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Schenzel, J.; Forrer, H.R.; Vogelgsang, S.; Hungerbuhler, K.; Bucheli, T.D. Mycotoxins in the environment: I. Production and emission from an agricultural test field. Environ. Sci. Technol. 2012, 46, 13067–13075. [Google Scholar] [CrossRef] [PubMed]
 indicates an existing pathway, while
indicates an existing pathway, while  indicates a pathway that has not been found to date. (SMs = secondary metabolites) (modified based on Hu et al. (2016) [81]).
indicates a pathway that has not been found to date. (SMs = secondary metabolites) (modified based on Hu et al. (2016) [81]).
 indicates an existing pathway, while
indicates an existing pathway, while  indicates a pathway that has not been found to date. (SMs = secondary metabolites) (modified based on Hu et al. (2016) [81]).
indicates a pathway that has not been found to date. (SMs = secondary metabolites) (modified based on Hu et al. (2016) [81]).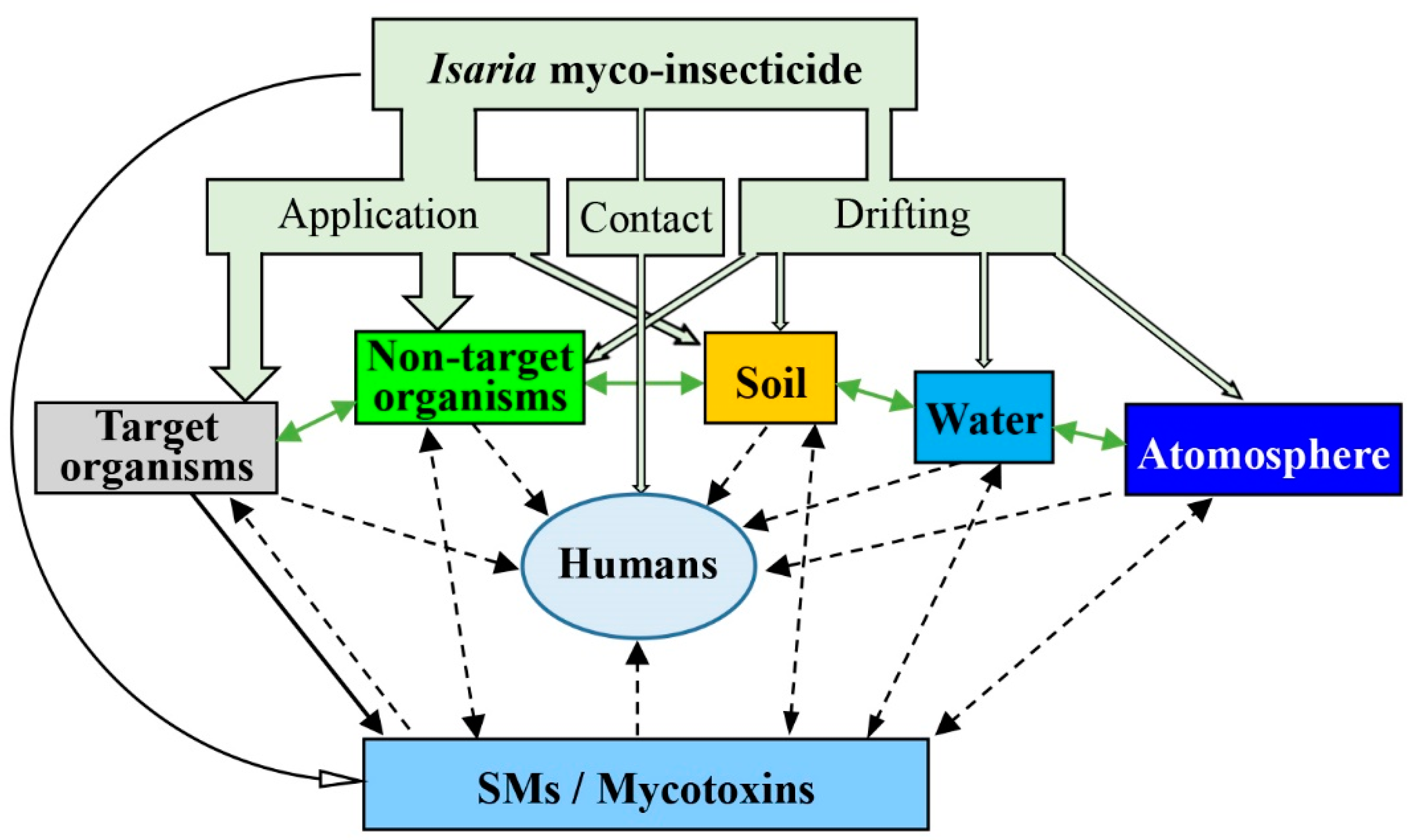
| Metabolite | CAS no. | Strain | Biological activity | References |
|---|---|---|---|---|
| Beauvericin (1) | 26048-05-5 | ACCC37775 (Hebei University, Baoding, China) | Inhibiting HepG2 cells with an IC50 of 2.40 μM. Cytotoxicity to multiple drug-resistant HepG2 cell lines with an IC50 value 25-fold more than that of doxorubicin. Inhibitor of PTP1B with an IC50 of 0.59 μM. | [32,33,34,35,36] |
| Beauverolide C (2) | 75899-64-8 | BMFM-UNAM 834 (Universidad Nacional Autonoma de Mexico, Mexico City, Mexico). | Calmodulin (CaM) inhibitor | [40] |
| Beauverolide F (3) | 75947-00-1 | Same as above | Calmodulin (CaM) inhibitor | [40] |
| Beauverolide I (4) | 62995-91-9 | Same as above | Calmodulin (CaM) inhibitor | [40] |
| Beauverolide Ja (5) | 76265-41-3 | Same as above | Calmodulin (CaM) inhibitor | [40] |
| Beauverolide L (6) | 154491-56-2 | BMFM-UNAM 834 (Universidad Nacional Autonoma de Mexico, Mexico City, Mexico); PFR97-Apopka (ATCC 20874) (WR Grace & Co, Conn, MD, USA) | Calmodulin (CaM) inhibitor Anti-immunity activity against the greater wax moth, Galleria mellonella | [40,41,42] |
| Beauverolide La (7) | 160825-68-3 | PFR97-Apopka (ATCC 20874) (WR Grace & Co, Conn, MD, USA) | [41] | |
| Beauverolide M (8) | BMFM-UNAM 834 (Universidad Nacional Autonoma de Mexico, Mexico City, Mexico). | Calmodulin (CaM) inhibitor | [40] | |
| Beauverolide N (9) | Same as above | Calmodulin (CaM) inhibitor | [40] | |
| Fumosorinone (10) | 1879030-70-2 | ACCC37775 (Hebei University, Baoding, China) | Inhibitor of PTP1B (IC50 of 14.04 μM) | [43,44] |
| Fumosorinone A (11) | 2241028-99-7 | Same as above | Inhibitor of PTP1B (IC50 of 3.24 μM) | [32] |
| Cepharosporolide C (12) | 97344-02-0 | Same as above | No activities to malaria Plasmodium falciparum K1, and PTP1B | [32,45] |
| Cepharosporolide E (13) | 97373-15-4 | Same as above | [32,45] | |
| Cepharosporolide F (14) | 97344-04-2 | Same as above | [32,45] | |
| 2-carboxymethyl-4-(3′-hydroxybutyl)furan (15), | Same as above | [32,45] | ||
| Dipicolinic acid (16) | 499-83-2 | Pfrd (Centro Nacional de Referencia de Control Biológico, Tecomán, Colima, Mexico) | Insecticidal activity against third-instar whitefly nymphs | [47,48,49,50] |
| Oxalic acid (OXA) (17) | 144-62-7 | Same as above | Insecticidal activity against third-instar whitefly nymphs | [47,48,49,50] |
| Trichocarane E (18) | ACCC37775 (Hebei University, Baoding, China) | Cytotoxicity to six tumor cell lines (i.e., MDA, MCF-7, SKOV-3, Hela, A549, and HepG2) with an IC50 of 0.1–6.0 μg/mL. | [52] | |
| Trichocarane F (19) | Same as above | Cytotoxicity to six tumor cell lines (i.e., MDA, MCF-7, SKOV-3, Hela, A549, and HepG2, with an IC50 of 0.1–6.0 μg/mL. | [52] | |
| CAF-603 (20) | Same as above | Cytotoxicity to six tumor cell lines (i.e., MDA, MCF-7, SKOV-3, Hela, A549, and HepG2, with an IC50 of 0.1–6.0 μg/mL. | [52] | |
| Trichocarane C (21) | Same as above | [52] | ||
| Ergosterol peroxide (22) | 2061-64-5 | RCEF1253 (Anhui Agricultural University, Hefei, China) | Cytotoxic to cancer cells P-388, KB, A549, and HT-29 (with ED50 values of 0.4, 2.1, 2.7, and 1.4 μg/mL) and human leukemia cell, HL-60 | [53,54,55,56] |
| 3-octanone (23) | 106-68-3 | Conidia of strain K3 (Kyoto University, Kyoto, Japan). | Repellent to termites | [57] |
| 1-octen-3-ol (24) | 3391-86-4 | Same as above | Repellent to termites | [57] |
| Metabolite | CAS no. | Strain | Biological Activity | References |
|---|---|---|---|---|
| Cycloaspeptide F (25) | 1174132-23-0 | XJC04-CT-303 (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) | Cytotoxic to HeLa and MCF7 cell lines | [60] |
| Cycloaspeptide G (26) | 1174132-24-1 | Same as above | Cytotoxic to HeLa and MCF7 cell lines | [60] |
| Cycloaspeptide A (27) | 109171-13-3 | Same as above | Cytotoxicity to human lung fibroblasts | [60,61] |
| Cycloaspeptide C (28) | 109171-15-5 | Same as above | [60] | |
| (3R,6R)-Bisdethiodi (methylthio) hyalodendrin (29) | 52080-06-5 | Same as above | Weak cytotoxic activity | [60,63] |
| Militarinone A (30) | 400604-05-9 | Same as above | Cytotoxicity to A549 cells. Neurotrophic effects on PC-12 cells | [64,65] |
| Militarinone B (31) | 503584-83-6 | RCEF0097 (Anhui Agricultural University, Hefei, China); XJC04-CT-303 (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) | Anti-microbes to Staphylococcus aureus, Streptococcus pneumoniae, and Candida albicans | [64] |
| Militarinone E (32) | 1261060-55-2 | XJC04-CT-303 (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) | Cytotoxicity to A549 cells | [64] |
| Militarinone F (33) | 1261060-56-3 | Same as above | [64] | |
| Farylhydrazone A (34) | 1261060-57-4 | Same as above | [66] | |
| Farylhydrazone B (35) | 1261060-58-5 | Same as above | [66] | |
| (+)-N-deoxymilitarinone A (36) | 881376-40-5 | RCEF0097 (Anhui Agricultural University, Hefei, China) | Induce neurite sprouting in PC 12 cells when tested at 33 and 100 μM concentrations. Cytotoxic to human neurons (IMR-32) at a concentration of 100 μM. | [67] |
| Militarinone D (37) | 503584-82-5 | RCEF 0097 (Anhui Agricultural University, Hefei, China); XJC04-CT-303 (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) | [67] | |
| (22E)-Ergosta-7,22-diene-3β,5α,6β,9α-tetraol (38) | 88191-06-4 | Same as above | [67] | |
| Farinomalein (39) | 1175521-35-3 | HF599 (National Institute of Fruit Tree Science, Tsukuba, Japan) | Antifungal to phytopathogenic Phytophthora sojae | [69,70,71] |
| Farinosone A (40) | 816431-89-7 | RCEF 0101 (Anhui Agricultural University, Hefei, China) | Neuritogenic in the PC-12 cell model | [72] |
| Farinosone B (41) | 816431-94-4 | Same as above | Inhibitory to Bacillus subtilis and Staphylococcus aureus. Moderate cytotoxicity to brine shrimp larvae (Artemia salina) | [72] |
| Farinosone C (42) | 816431-98-8 | Same as above | Induced neurite outgrowth in the PC-12 cell line at concentrations of 50 μM | [72] |
| Paecilosetin (43) | 856258-89-4 | CANU TE108 (University of Canterbury, Christchurch, New Zealand). HF511 (National Institute of Fruit Tree Science, Tsukuba, Japan.) | Antioomycete activity against both Phytophthora sojae and Aphanomyces cochlioides | [71,73] |
| Aranorosinol A (44) | 145147-04-2 | HF511 (National Institute of Fruit Tree Science, Tsukuba, Japan.) | Antioomycete to both Phytophthora sojae and Aphanomyces cochlioides | [71,74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Q.; Zhang, X.; Chen, W.; Hu, Q. Secondary Metabolites and the Risks of Isaria fumosorosea and Isaria farinosa. Molecules 2019, 24, 664. https://doi.org/10.3390/molecules24040664
Weng Q, Zhang X, Chen W, Hu Q. Secondary Metabolites and the Risks of Isaria fumosorosea and Isaria farinosa. Molecules. 2019; 24(4):664. https://doi.org/10.3390/molecules24040664
Chicago/Turabian StyleWeng, Qunfang, Xiaofeng Zhang, Wei Chen, and Qiongbo Hu. 2019. "Secondary Metabolites and the Risks of Isaria fumosorosea and Isaria farinosa" Molecules 24, no. 4: 664. https://doi.org/10.3390/molecules24040664
APA StyleWeng, Q., Zhang, X., Chen, W., & Hu, Q. (2019). Secondary Metabolites and the Risks of Isaria fumosorosea and Isaria farinosa. Molecules, 24(4), 664. https://doi.org/10.3390/molecules24040664





