Abstract
Base-catalyzed annulation reactions of 5,6-dihydro-2(1H)-pyridones with Nazarov-type reagents are reported. The effect of the solvent polarity and the concentration of the reagents is studied. The process involves two successive Michael additions and stereoselectively provides functionalized cis-perhydroisoquinolin-1-ones.
1. Introduction
Nitrogen heterocycles exhibit a broad range of significant biological and pharmacological activities, and many of them have been developed as therapeutic drugs [1,2].
In particular, the partially or totally reduced isoquinoline ring system is present in a large number of biologically active natural products (such as the alkaloids of the yohimbine [3,4], manzamine [5], and madangamine [6] groups) and medicinally useful synthetic compounds (e.g., the HIV protease inhibitors saquinavir and nelfinavir [7,8], the antimigraine drugs tezampanel and LY466195 [9], and the antiobesity agent AMG 076 [10]) (Figure 1).
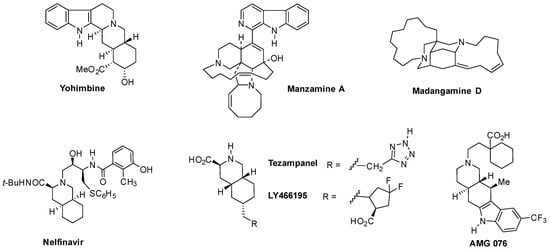
Figure 1.
Hydroisoquinoline-containing bioactive natural products and synthetic compounds.
Together with the intramolecular Diels−Alder cyclization of suitable azatrienes [11,12], one of the most straightforward approaches for the construction of the hydroisoquinoline ring system involves the generation of the carbocyclic ring by an annulation reaction from appropriate 5,6-dihydro-2(1H)-pyridone derivatives. The latter strategy was developed some years ago in our laboratory using the classical Diels−Alder methodology with a variety of dienes [13]. Bearing in mind that Nazarov reagents (γ,δ-unsaturated β-keto esters) are able to participate in double Michael addition reactions with α,β-unsaturated carbonyl derivatives, we envisaged an alternative annulation procedure to directly access functionalized hydroisoquinolines from 5,6-dihydro-2(1H)-pyridones.
Nazarov reagents are versatile annulating agents, extensively used in a variety of Robinson-type and double Michael addition annulations [14,15,16]. In the former, the reagent sequentially acts as an electrophilic Michael acceptor and as a nucleophile to promote an aldol condensation. In the latter, however, it successively acts as a nucleophilic Michael donor and an electrophilic Michael acceptor, a reactivity pattern that has been successfully applied to assemble pentacyclic yohimbine-type derivatives from unsaturated indolo[2,3-a]quinolizidine-derived lactams [17,18,19].
2. Results and Discussion
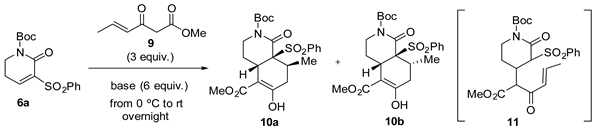
Compounds 6a,b,c and 8, which incorporate an additional phenylsulfonyl or ethoxycarbonyl activating electron-withdrawing group conjugated to the carbon-carbon double bond, were selected as the starting dihydropyridones.
Lactams 6a,b, bearing an easily removable phenylsulfonyl group, were prepared in acceptable overall yield from 2-piperidone (1a) by bis-sulfenylation, followed by reaction with either (Boc)2O or MeI, and subsequent stepwise m-CPBA oxidation of the resulting N-substituted piperidones 3a,b via unsaturated sulfenyl and sulfinyl derivatives 4a,b and 5a,b. A similar reaction sequence from N-tosyl-2-piperidone 3c led to unsaturated lactam 6c in low overall yield. Due to their instability, lactams 6a,b,c were used in the annulation step without purification. In turn, ethoxycarbonyl lactam 8 was prepared in high yield from N-Boc-2-piperidone (1b) via seleno derivative 7, as outlined in Scheme 1.
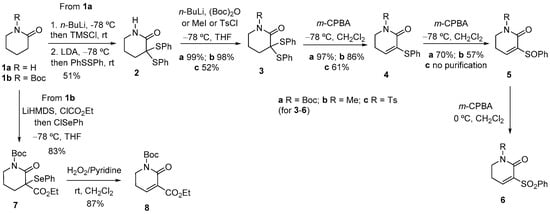
Scheme 1.
Preparation of the starting unsaturated lactams.
To study the annulation reactions, we initially used unsaturated lactam 6a and the Nazarov reagent 9 [14], which incorporates a methyl group at the terminal olefinic carbon, in the presence of Cs2CO3, the most commonly used base for the generation of Nazarov enolates [20]. Reagent 9 has been used extensively by Deslongchamps to generate cis-decalin derivatives [20,21]. The reaction was carried out at room temperature, using an excess (6 equiv.) of Cs2CO3 in CH2Cl2 at different concentrations (from 50 mM to 5 mM). In all cases, the double Michael addition reaction occurred satisfactorily, although with only moderate stereoselectivity, to give 3:1 C-8 stereoisomeric mixtures of cis-hydroisoquinolones 10a (cis Me/SO2Ph) and 10b (trans Me/SO2Ph), the chemical yield increasing (79% yield at 5 mM) with the dilution (Table 1, entries 1–3). Trace amounts of the monoaddition product 11 were detected by NMR, indicating that the annulation involves two successive Michael addition reactions. In contrast, when acetonitrile was used as the solvent the yield was very low (entry 4). Remarkably, the use of KF as the base in a polar solvent such as methanol (entry 5) resulted in a reversal of the stereoselectivity, leading to a mixture of bicyclic lactams 10a and 10b, in which the trans Me/SO2Ph isomer predominated (56% yield, ratio 1:2). The influence of the solvent polarity on the stereoselectivity of annulation reactions of 9 with β-keto esters has previously been observed [21]. The facial selectivity when using Cs2CO3 as the base can be attributed to the coordination of the Cs+ cation to the oxygen atoms of both the Nazarov reagent and the starting lactam [19].

Table 1.
Reaction of unsaturated lactam 6a with Nazarov reagent 9.
The relative Me/SO2Ph cis configuration of bicyclic lactam 10a was unambiguously established by X-ray crystallographic analysis (Figure 2).
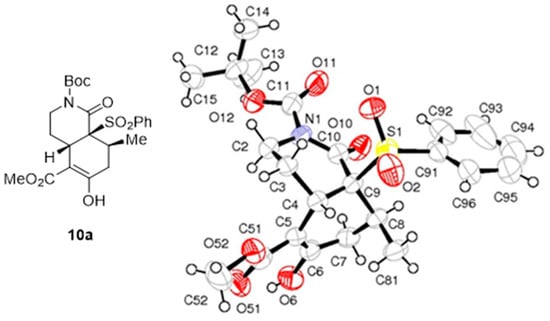
Figure 2.
ORTEP plot of the X-ray structure of bicyclic lactam 10a.
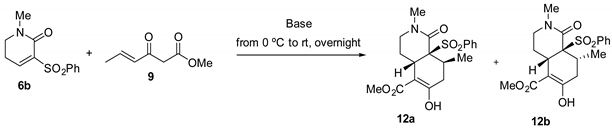
Unsaturated lactam 6b behaved similarly to lactam 6a in the annulation reaction with Nazarov reagent 9, although the yields were slightly lower, probably due to the lower electrophilicity of the Michael acceptor as a consequence of the absence of an electron-withdrawing group on the piperidone nitrogen. cis-Hydroisoquinolone 12a, with a cis Me/SO2Ph relationship, was stereo-selectively formed (4:1 12a/12b ratio) when the reaction was performed in CH2Cl2 solution using Cs2CO3 as the base (Table 2, entries 1 and 2), the yield once again being higher with increasing dilution (40% yield at 5 mM). As before, a reversal of the stereoselectivity was observed and the trans Me/SO2Ph isomer predominated when using polar solvents, either MeOH in the presence of KF (1:4 ratio; entry 3) or DMF in the presence of Cs2CO3 (1:5 ratio; entry 4). The Me/SO2Ph cis relationship of the adducts 10a and 12a was maintained unchanged after an additional treatment (20 h, rt) with Cs2CO3 in CH2Cl2 or KF in MeOH, thus suggesting the non-reversibility of the cyclization step.

Table 2.
Reaction of unsaturated lactam 6b with Nazarov reagent 9.
The relative configuration of both hydroisoquinolones, 12a and 12b, was unambiguously established by X-ray crystallographic analysis (Figure 3).
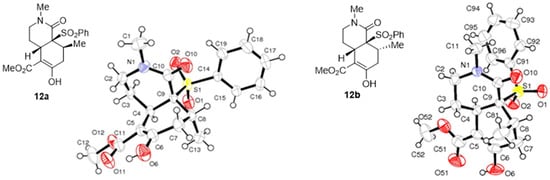
Figure 3.
ORTEP plots of the X-ray structures of bicyclic lactams 12a and 12b.
The activating phenylsulfonyl group of bicyclic lactams 10a, 12a, and 12b was stereoselectively removed, with retention of configuration, by treatment with sodium amalgam [22] to give the respective cis-hydroisoquinolones 13a, 14a, and 14b (Scheme 2). Alternatively, removal of the N-Boc protecting group of hydroisoquionolones 10a and 13a quantitatively afforded the potentially useful N-unsubstituted derivatives 15 and 16, respectively. The cis ring function was evident from the observation of a positive NOE effect between the 4a and 8a methine protons.
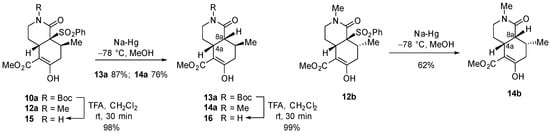
Scheme 2.
Desulfonylation reactions and removal of the N-Boc protecting group.
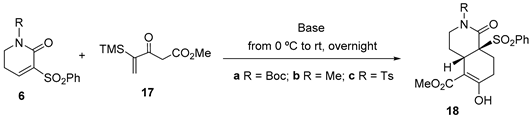
To expand the scope of the methodology and access hydroisoquinolones lacking the methyl substituent on the B ring, we decided to study double Michael annulations using the silylated Nazarov reagent 17 [17], which constitutes a stable synthetic equivalent of the original Nazarov reagent (methyl or ethyl 3-oxo-4-pentenoate) [15] that avoids the polymerization problems associated with the latter under basic conditions.
As expected, the Cs2CO3-promoted annulation of the Nazarov reagent 17 with unsaturated lactam 6a under the usual reaction conditions (5 mM in CH2Cl2 as the solvent) stereoselectively afforded cis-hydroisoquinolone 18a, in which protodesilylation had occurred, in acceptable yield (Table 3, entry 1). The yield was not improved by increasing the excess of reagent and base (entry 2) and was lower when operating at a higher concentration (entry 3) or when using KF in MeOH as the solvent (entry 4). Similar moderate yields were obtained in the generation of cis-hydroisoquinolones 18b and 18c from lactams 6b (entries 5–7) and 6c (entry 8) under a variety of conditions.

Table 3.
Reactions of unsaturated lactams 6a-c with Nazarov reagent 17.
Remarkably, the yield of the annulation with the silylated Navarov reagent 17 was higher when using unsaturated lactam 8, which incorporates an ester group as an additional activating substituent. Operating under the previously optimized reaction conditions, cis-hydroisoquinolone 19 was obtained in 60% yield (Scheme 3).
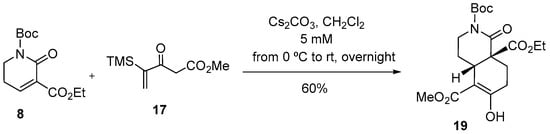
Scheme 3.
Reaction of unsaturated lactam 8 with the silylated Nazarov reagent 17.
In conclusion, base-promoted annulation reactions of Nazarov reagents 9 and 17 with 5,6-dihydro-2(1H)-pyridones bearing an additional activating electron-withdrawing group α to the lactam carbonyl constitute a straightforward procedure for the stereoselective synthesis of highly substituted cis-hydroisoquinolin-2-ones. In the reactions with the methyl-substituted reagent 9, leading to 8-substituted derivatives, the use of Cs2CO3 in CH2Cl2 leads to cis-hydroisoquinolones with a cis 8-Me/8a-SO2Ph relationship as the major stereoisomers. The stereoselectivity is reversed in a polar solvent such as DMF or when the annulation is performed using KF in MeOH.
The methodology developed here provides access to polyfunctionalized bicyclic scaffolds with potential use as precursors of bioactive hydroisoquinoline-containing natural products and synthetic derivatives.
3. Materials and Methods
3.1. General Information
All air sensitive manipulations were carried out under a dry argon or nitrogen atmosphere. THF and CH2Cl2 were dried using a column solvent purification system. Analytical thin-layer chromatography was performed on SiO2 (silica gel 60A 35–70 μm, Carlo Erba, Val de Reuil Cedex, France), and the spots were located with 1% aqueous KMnO4. Chromatography refers to flash chromatography and was carried out on SiO2 (SDS silica gel 60 ACC, 35–75 mm, 230-240 mesh ASTM). NMR spectra were recorded at 300 or 400 MHz (1H) and 100.6 MHz (13C), and chemical shifts are reported in δ values downfield from TMS or relative to residual chloroform (7.26 ppm, 77.0 ppm) as an internal standard. Data are reported in the following manner: chemical shift, multiplicity, coupling constant (J) in hertz (Hz), integrated intensity, and assignment (when possible). Assignments and stereochemical determinations are given only when they are derived from definitive two-dimensional NMR experiments (HSQC-COSY). IR spectra were performed in an Avatar 320 FT-IR spectrophotometer (Thermo Nicolet, Madison, WI, USA) and only noteworthy IR absorptions (cm−1) are listed. High resolution mass spectra (HMRS; LC/MSD TOF, Agilent Technologies, Santa Clara, CA, USA) were performed by Centres Científics i Tecnològics de la Universitat de Barcelona.
3.2. Preparation of the Starting Unsaturated Lactams
3,3-Bis(phenylthio)-2-piperidone (2). n-BuLi (25.5 mL of a 1.6 M solution in hexane, 40.8 mmol) was added under an argon atmosphere at −78 °C to a solution of δ-valerolactam (1a, 4 g, 40.82 mmol) in anhydrous THF (100 mL). The solution was stirred for 30 minutes, and then TMSCl (5.2 mL, 40.8 mmol) was added. The solution was stirred at room temperature for 30 minutes, and then re-cooled to −78 °C. LDA (45 mL, 2 M solution in THF/heptane/ethylbenzene, 89.8 mmol) was added, and the solution was stirred for 30 minutes. Then, PhSSPh (19.5 g, 89.8 mmol) in anhydrous THF (50 mL) was added, and the resulting solution was stirred at room temperature for 1 h. After this time, NH4Cl was added, and the organic layer was extracted with EtOAc. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (9:1 hexane-EtOAc) of the residue gave lactam 2 (6.61 g, 51% yield): IR (ATR Pike) ν (cm−1): 3260 (NH), 1661 (CO); 1H-NMR (400 MHz, CDCl3): δ = 1.72-1.90 (m, 2H, H-5), 1.97 (t, J = 6.2 Hz, 2H, H-4), 3.18 (dt, J = 2.4, 6.0 Hz, 2H, H-6), 6.26 (br. s, 1H, NH), 7.32–7.34 (m, 6H, HAR), 7.63–7.65 (m, 4H, HAR); HRMS (ESI) calcd for [C17H18NOS2 + H+]: 316.0824, found: 316.062714b (detailed data in supplementary materials).
3,3-Bis(phenylthio)-1-(tert-butoxycarbonyl)-2-piperidone (3a). n-BuLi (6.1 mL of a 1.4 M solution in hexane, 8.54 mmol) was added under an argon atmosphere at −78 °C to a solution of lactam 2 (2.25 g, 7.12 mmol) in anhydrous THF (100 mL), and the resulting solution was stirred for 1 h. Then, Boc2O (2.30 g, 10.68 mmol) was added, and the mixture was stirred at −78 °C for 1 h. After this time, saturated NH4Cl was added, and the mixture was extracted with EtOAc. The combined organic extracts were dried over Na2SO4 and filtered, and the solvent was removed under reduced pressure. Flash chromatography (9:1 hexane-EtOAc) of the residue gave N-Boc derivative 3a (2.92 g, 99% yield): IR (ATR Pike) ν (cm−1): 1717 (CO); 1H-NMR (400 MHz, CDCl3): δ = 1.53 [s, 9H, (CH3)3C], 1.94 (m, 2H, H-5), 2.05 (m, 2H, H-4), 3.60 (t, J = 6.1 Hz, 2H, H-6), 7.33–7.42 (m, 6H, HAR), 7.68 (m, 4H, HAR); 13C-NMR (400 MHz, CDCl3): δ = 20.1 (C-5), 28.0 [(CH3)3C], 35.1 (C-4), 46.7 (C-6), 68.6 (C-3), 83.2 [(CH3)3C], 128.7 (4CHAR), 129.6 (2CHAR), 130.9 (2CAR), 136.8 (4CHAR), 153.8 (CO), 168.3 (CO); HRMS (ESI) calcd for [C22H25NO3S2 + Na+]: 438.1168, found: 438.117.
3,3-Bis(phenylthio)-1-methyl-2-piperidone (3b). n-BuLi (8.54 mL of a 1.6 M solution in hexane, 13.67 mmol) was added under an argon atmosphere at −78 °C to a solution of lactam 2 (3.60 g, 11.39 mmol) in anhydrous THF (200 mL), and the resulting mixture was stirred at −78 °C for 1 h. Then, MeI (1.06 mL, 17.09 mmol) was added, and the solution was stirred at room temperature for 1 h. The reaction was quenched by addition of saturated NH4Cl, and the mixture was extracted with EtOAc. The combined organic extracts were dried over Na2SO4, filtered, and concentrated under reduced pressure. Flash chromatography (9:1 hexane-EtOAc) of the residue afforded N-methyl derivative 3b (3.7 g, 98% yield): IR (ATR Pike) ν (cm−1): 1642 (NCO); 1H-NMR (400 MHz, CDCl3): δ = 1.86–1.91 (m, 2H, H-5), 1.97–1.99 (m, 2H, H-4), 2.92 (s, 3H, CH3), 3.16 (t, J = 6.4 Hz, 2H, H-6), 7.31–7.38 (m, 6H, HAR), 7.63–7.65 (m, 4H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 19.6 (C-5), 34.0 (C-4), 35.8 (CH3), 50.1 (C-6), 66.0 (C-3), 128.6 (4CHAR), 129.3 (2CHAR), 131.6 (2CAR), 136.6 (4CHAR); HRMS (ESI) calcd for [C18H19NOS2 + H+]: 330.0981, found: 330.0977.
3,3-Bis(phenylthio)-1-(p-toluenesulfonyl)-2-piperidone (3c). n-BuLi (1.2 mL of a 1.6 M solution in hexane, 1.9 mmol) was added at −78 °C to a solution of lactam 2 (500 mg, 1.58 mmol) in anhydrous THF (25 mL), and the resulting mixture was stirred at −78 °C for 1 h. Then, TsCl (452 mg, 2.37 mmol) was added, and the solution was stirred at room temperature overnight. The reaction was quenched by addition of saturated NH4Cl, and the mixture was extracted with EtOAc. The combined organic extracts were dried over Na2SO4, filtered, and concentrated under reduced pressure. Flash chromatography (9:1 hexane-EtOAc) afforded N-tosyl derivative 3c (264 mg, 52% yield): IR (ATR Pike) ν (cm−1): 1683 (NCO), 1169 (SO2); 1H-NMR (400 MHz, CDCl3): δ = 1.55 (s, 2H, H-5), 2.02 (s, 2H, H-4), 2.49 (s, 3H, CH3), 3.46 (br. s, 2H, H-6), 7.19–7.40 (m, 12H, HAR), 7.93 (d, J = 8.4 Hz, 2H, HAR).
1-(tert-Butoxycarbonyl)-3-(phenylthio)-5,6-dihydro-2(1H)-pyridone (4a). m-Chloroperbenzoic acid (1.09 g, technical grade 70%, 766 mg pure oxidant, 6.33 mmol) in CH2Cl2 (100 mL) was added to a solution of lactam 3a (2.64 g, 6.33 mmol) in CH2Cl2 (200 mL) at −78 °C. The solution was stirred at this temperature for 2 h and then for 30 minutes at room temperature. After this time, a saturated solution of NaHCO3 was added, the mixture was extracted with CH2Cl2, and the organic solvent was removed under reduced pressure. The crude product was purified by flash chromatography (95:5 hexane-EtOAc) to afford unsaturated lactam 4a (1.88 g, 97% yield): IR (ATR Pike) ν (cm−1): 1762 (CO), 1715 (CO); 1H-NMR (300 MHz, CDCl3): δ = 1.54 [s, 9H, (CH3)3C], 2.39 (td, J = 6.6, 4.8 Hz, 2H, H-5), 3.84 (t, J = 6.6 Hz, 2H, H-6), 6.08 (t, J = 4.8 Hz, 1H, H-4), 7.33–7.41 (m, 2H, HAR), 7.47–7.50 (m, 2H, HAR), 7.66–7.70 (m, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 25.3 (C-5), 28.0 [(CH3)3C], 43.7 (C-6), 83.4 [(CH3)3C], 128.8 (CHAR), 129.5 (2CHAR), 131.0 (C-3, CAR), 134.8 (2CHAR), 135.5 (C-4), 152.8 (CO), 161.7 (CO); HRMS (ESI) calcd for [C16H19NO3S + Na+]: 328.0978, found: 328.0975.
1-Methyl-3-(phenylthio)-5,6-dihydro-2(1H)-pyridone (4b). Operating as described for the preparation of compound 4a, from a solution of lactam 3b (280 mg, 0.85 mmol) in CH2Cl2 (20 mL) and m-CPBA (210 mg, technical grade 70%, 0.85 mmol) in CH2Cl2 (20 mL), unsaturated lactam 4b was obtained (160 mg, 86% yield) as a yellow foam: 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 2.34 (td, J = 7.2, 4.8 Hz, 2H, H-5), 3.04 (s, 1H, CH3), 3.40 (t, J = 7.2 Hz, 2H, H-6), 5.81 (t, J = 4.8 Hz, 1H, H-4), 7.35–7.39 (m, 3H, HAR), 7.48–7.50 (m, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 24.6 (C-5), 34.9 (CH3), 47.6 (C-6), 128.5 (CHAR), 129.4 (2CHAR), 130.8 (C-4), 132.0, 134.4 (C-3, CAR), 134.6 (2CHAR), 162.9 (CO).
3-(Phenylthio)-1-(p-toluenesulfonyl)-5,6-dihydro-2(1H)-pyridone (4c). Operating as described for the preparation of compound 4a, from a solution of lactam 3c (264 mg, 0.58 mmol) in CH2Cl2 (15 mL) and m-CPBA (142 mg, technical grade 70%, 0.58 mmol) in CH2Cl2 (15 mL), unsaturated lactam 4c was obtained (123 mg, 61% yield) as a yellow foam: IR (ATR Pike) ν (cm−1): 1683 (CO), 1166 (SO2); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 2.43 (s, 3H, CH3), 2.48 (td, J = 6.3, 4.8 Hz, 2H, H-5), 4.04 (t, J = 6.3 Hz, 2H, H-6), 6.08 (t, J = 4.8 Hz, 1H, H-4), 7.30–7.37 (m, 7H, HAR), 7.93 (d, J = 8.4 Hz, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 21.6 (CH3), 25.9 (C-5), 44.1 (C-6), 128.7 (CHAR), 128.9 (CHAR), 129.4 (CHAR), 129.6 (CHAR), 130.7 (CAR), 134.5 (CHAR), 135.6 (C-3, CAR), 136.2 (C-4), 144.9 (CHAR), 161.1 (NCO); HRMS (ESI) calcd for [C18H17NO3S2 + H+]: 360.0723, found: 360.0720.
1-(tert-Butoxycarbonyl)-3-(phenylsulfinyl)-5,6-dihydro-2(1H)-pyridone (5a). m-Chloroperbenzoic acid (1.51 g, technical grade 70%, 1.06 g pure oxidant, 6.14 mmol) in CH2Cl2 (100 mL) was added to a solution of phenylthio derivative 4a (1.884 g, 6.14 mmol) in CH2Cl2 (200 mL) at −78 °C. The solution was stirred at this temperature for 1.5 h. Then, a saturated solution of NaHCO3 was added, the mixture was extracted with CH2Cl2, and the organic solvent was removed under reduced pressure. The crude product was purified by flash chromatography (95:5 hexane-EtOAc) to afford sulfinyl derivative 5a (1.39 g, 70% yield): IR (ATR Pike) ν (cm−1): 1762 (CO), 1719 (CO), 1099, 1049 (SO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.51 [s, 9H, (CH3)3C], 2.55–2.75 (m, 2H, H-5), 3.59 (ddd, J = 13.2, 10.4, 5.2 Hz, 1H, H-6), 4.05 (dtd, J = 13.2, 5.2, 1.1 Hz, H-6), 7.45-7.47 (m, 3H, HAR), 7.57 (td, J = 3.6, 1.2 Hz, 1H, H-4), 7.79–7.81 (m, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 24.9 (C-5), 27.9 [(CH3)3C], 43.2 (C-6), 83.8 [(CH3)3C)], 125.6 (2CHAR), 129.1 (2CHAR), 131.3 (CHAR), 142.3 (C-4), 142.6, 143.7 (CAR, C-3), 151.9 (CO), 160.2 (CO); HRMS (ESI) calcd for [C16H19NO4S + Na+]: 344.0927, found: 344.0927.
1-(Methyl)-3-(phenylsulfinyl)-5,6-dihydro-2(1H)-pyridone (5b). Operating as described for the preparation of compound 5a, from a solution of phenylthio derivative 4b (163 mg, 0.74 mmol) in CH2Cl2 (15 mL) and m-CPBA (183 mg, technical grade 70%, 0.74 mmol) in CH2Cl2 (20 mL), sulfinyl derivative 5b was obtained (100 mg, 57% yield): IR (ATR Pike) ν (cm−1): 1652 (CO), 1043 (SO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 2.53–2.74 (2m, 2H, H-5), 2.90 (s, 3H, CH3), 3.38–3.46 (dd, J = 8.8, 6.8 Hz, 2H, H-6), 7.31 (t, J = 4.8 Hz, 1H, H-4), 7.44–7.45 (m, 3H, HAR), 7.78–7.81 (m, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 24.0 (C-5), 33.7 (CH3), 46.8 (C-6), 125.5 (2CHAR), 128.8 (2CHAR), 131.0 (CHAR), 138.1 (C-4), 140.6, 143.8 (CAR, C-3), 160.1 (NCO); HRMS (ESI) calcd for [C12H13NO2S + H+]: 236.074, found: 236.0741.
3-(Phenylsulfinyl)-1-(p-toluenesulfonyl)-5,6-dihydro-2(1H)-pyridone (5c). Operating as described for the preparation of compound 5a, from a solution of phenylthio derivative 4c (55 mg, 0.12 mmol) in CH2Cl2 (3 mL) and m-CPBA (30 mg, technical grade 70%, 0.12 mmol) in CH2Cl2 (3 mL), sulfinyl derivative 5c was obtained. This compound was used in the next step without further purification: IR (ATR Pike) ν (cm−1): 1683, (NCO), 1362 (SO2), 1170 (SO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 2.43 (s, 3H, CH3), 2.67-2.81 (m, 2H, H-5), 3.78–3.86 (m, 1H, H-6), 4.11–4.20 (m, 1H, H-6), 7.27 (d, J = 7.6 Hz, 2H, HAR), 7.36–7.41 (m, 3H, HAR), 7.52 (dd, J = 4.4, 3.6 Hz, 1H, H-4), 7.63–7.66 (m, 2H, HAR), 7.82 (d, J = 8.0 Hz, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 21.6 (CH3), 25.3 (C-5), 43.8 (C-6), 125.1 (2CHAR), 128.3 (2CHAR), 129.1 (2CHAR), 129.4 (2CHAR), 131.3 (CHAR), 135.1 (CAR), 142.8 (C-4), 143.2, 145.1 (CAR, C-3), 159.7 (NCO).
1-(tert-Butoxycarbonyl)-3-(phenylsulfonyl)-5,6-dihydro-2(1H)-pyridone (6a). m-Chloroperbenzoic acid (1.06 mg, technical grade 70%, 745 mg pure oxidant, 4.32 mmol) in CH2Cl2 (150 mL) was added to a solution of sulfinyl derivative 5a (1.39 g, 4.32 mmol) in CH2Cl2 (60 mL) at −78 °C. The solution was stirred at room temperature for 6 h. Then, a saturated solution of NaHCO3 was added, the mixture was extracted with CH2Cl2, and the organic solvent was removed under reduced pressure to afford sulfonyl derivative 6a, which was used in the next step without purification: IR (ATR Pike) ν (cm−1): 1721 (CO), 1689 (CO), 1446 (SO2), 1143 (SO2); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.49 [s, 9H, (CH3)3C], 2.67 (td, J = 6.0, 4.0 Hz, 2H, H-5), 3.87 (t, J = 6.8 Hz, 2H, H-6), 7.51–7.53 (m, 2H, HAR), 7.60 (t, J = 7.6 Hz, 1H, HAR), 8.05 (dm, J = 7.2 Hz, 2H, HAR), 8.08 (t, J = 4.4 Hz, 1H, H-4); 13C-NMR (100.6 MHz, CDCl3): δ = 25.0 (C-5), 27.9 [(CH3)3C], 42.9 (C-6), 84.2 [(CH3)3C], 128.7 (2CHAR), 129.3 (2CHAR), 133.5 (CHAR), 138.7, 139.5 (C-3, CAR), 151.4 (CO), 152.2 (C-4), 157.8 (CO); HRMS (ESI) calcd for [C16H19NO5S + H+]: 338.1057, found: 338.1066.
1-Methyl-3-(phenylsulfonyl)-5,6-dihydro-2(1H)-pyridone (6b). Operating as described for the preparation of compound 6a (reaction time 20 h), from a solution of sulfinyl derivative 5b (100 mg, 0.42 mmol) in CH2Cl2 (10 mL) and m-CPBA (104 mg, technical grade 70%, 0.42 mmol) in CH2Cl2 (10 mL), sulfonyl derivative 6b was obtained. This compound was used in the next step without purification: IR (ATR Pike) ν (cm−1): 1662 (NCO), 1306 (SO2), 1151 (SO2); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 2.67 (td, J = 7.2, 4.4 Hz, 2H, H-5), 2.93 (s, 3H, CH3), 3.46 (t, J = 7.2 Hz, 2H, H-6), 7.51 (tm, J = 7.6 Hz, 2H, HAR), 7.57 (m, 1H, HAR), 7.87 (t, J = 4.4 Hz, 1H, H-4), 8.05 (dm, J = 7.6 Hz, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 24.3 (C-5), 34.4 (CH3), 46.6 (C-6), 128.6 (2CHAR), 129.1 (2CHAR), 133.4 (CHAR), 140.0 (C-3, CAR), 148.4 (C-4), 159.0 (CO); HRMS (ESI) calcd for [C12H13NO3S + H+]: 252.0689, found: 252.0679.
3-(Phenylsulfonyl)-1-(p-toluenesulfonyl)-5,6-dihydro-2(1H)-pyridone (6c). Operating as described for the preparation of compound 6a (reaction time 20 h), from a solution of sulfinyl derivative 5c (45 mg, 0.12 mmol) in CH2Cl2 (6 mL) and m-CPBA (30 mg, technical grade 70%; 21 mg pure oxidant, 0.12 mmol) in CH2Cl2 (6 mL), sulfonyl derivative 6c was obtained. This compound was used in the next step without purification: IR (ATR Pike) ν (cm−1): 1695 (NCO), 1362 (SO2), 1157 (SO2); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 2.42 (s, 1H, CH3), 2.79 (td, J = 6.4, 4.4 Hz, 2H, H-5), 4.04 (t, J = 6.0 Hz, 2H, H-6), 7.29 (d, J = 8.0 Hz, 2H, HAR), 7.47–7.51 (m, 2H, HAR), 7.57 (tt, J = 7.6, 1.2 Hz, 1H, HAR), 7.85 (d, J = 8.0 Hz, 2H, HAR), 7.96 (d, J = 8.0 Hz, 2H, HAR), 8.06 (t, J = 4.4 Hz, 1H, H-4); 13C-NMR (100.6 MHz, CDCl3): δ = 21.6 (CH3), 25.7 (C-5), 43.5 (C-6), 128.4 (2CHAR), 128.8 (2CHAR), 129.1 (2CHAR), 129.6 (2CHAR), 133.7 (CHAR), 134.9 (CAR), 137.8 (CHAR), 139.0 (C-3), 145.3 (CAR), 153.2 (C-4), 157.2 (NCO).
1-(tert-Butoxycarbonyl)-3-(ethoxycarbonyl)-3-(phenylselenyl)-2-piperidone (7). Lithium bis(trimethyl silyl)amide (11.1 mL of a 1 M solution in THF, 11.1 mmol) was slowly added at −78 °C under an argon atmosphere to a solution of lactam 1b (1.0 g, 5.05 mmol) in anhydrous THF (100 mL), and the solution was stirred for 1 h. Then, ethyl chloroformate (532 μl, 5.56 mmol) and, after 1 h of continuous stirring at −78 °C, a solution of PhSeCl (1.48 g, 7.58 mmol) in anhydrous THF (10 mL) were added to the solution. The mixture was stirred for a further 1 h and poured into saturated aqueous NH4Cl. The resulting mixture was extracted with EtOAc, and the combined organic extracts were dried, filtered, and concentrated under reduced pressure. Flash chromatography (8:2 hexane-EtOAc) of the resulting oil gave the seleno derivative 7 (1.79 g, 83% yield) as a yellow foam: IR (ATR Pike) ν (cm−1): 1718 (CO); 1H-NMR (300 MHz, CDCl3): δ = 1.27 (t, J = 7.2 Hz, 3H, CH2CH3), 1.54 [s, 9H, (CH3)3C], 1.73 (m, 1H, H-5), 1.84 (m, 1H, H-5), 2.01 (ddd, J = 13.8, 10.2, 5.7 Hz, 1H, H-4), 2.28 (dt, J = 13.8, 6.0 Hz, 1H, H-4), 3.52 (m, 1H, H-6), 3.61 (ddd, J = 13.2, 8.4, 5.4 Hz, 1H, H-6), 4.22 (qd, J = 7.2, 3.3 Hz, 2H, CH2CH3), 7.31 (t, J = 7.2, 2H, HAR), 7.41 (t, J = 7.2 Hz, 1H, HAR), 7.65 (t, J = 7.2 Hz, 2H, HAR); HRMS (ESI) calcd for [C19H25NO5Se + Na+]: 450.0787, found: 450.0788.
1-(tert-Butoxycarbonyl)-3-(ethoxycarbonyl)-5,6-dihydro-2(1H)-pyridone (8). H2O2 (900 μl, 29.35 mmol) and pyridine (405 μl, 5.03 mmol) were added at room temperature to a solution of seleno lactam 7 (1.79 g, 4.19 mmol) in CH2Cl2 (300 mL), and the resulting solution was stirred at room temperature for 30 minutes. Water was then added, and the mixture was extracted with CH2Cl2. The combined organic extracts were dried, filtered, and concentrated under reduced pressure. Flash chromatography (8:2 hexane-EtOAc) of the resulting oil gave unsaturated lactam 8 (981 mg, 87% yield) as a yellow foam: IR (ATR Pike) ν (cm−1): 1715 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.33 (t, J = 6.8 Hz, 3H, CH2CH3), 1.54 [s, 9H, (CH3)3C], 2.52 (td, J = 6.4, 4.4 Hz, 2H, H-5), 3.88 (t, J = 6.4 Hz, 2H, H-6), 4.29 (q, J = 6.8 Hz, 2H, CH2CH3), 7.48 (t, J =.4 Hz, 1H, H-4); 13C-NMR (100.6 MHz, CDCl3): δ = 14.1 (CH2CH3), 24.7 (C-5), 28.0 [(CH3)3C], 43.0 (C-6), 61.5 (CH2CH3), 83.6 [(CH3)3C], 130.9 (C-3), 148.5 (C-4), 160.0 (CO), 164.0 (CO); HRMS (ESI) calcd for [C13H19NO5 + Na+]: 292.1155, found: 292.1166.
3.3. General Procedure for the Double Michael Addition Reactions
A solution of unsaturated lactam 6a,b,c or 8 (1 equiv.) in anhydrous CH2Cl2, MeCN or MeOH was added at 0 °C under an argon atmosphere to a solution of the Nazarov reagent (9 or 15) and Cs2CO3 or KF in anhydrous CH2Cl2, MeCN or MeOH, and the resulting mixture was allowed to warm slowly to room temperature. After 20 h of stirring at room temperature, the mixture was concentrated under reduced pressure. Flash chromatography (9:1 hexane-EtOAc) of the residue afforded the corresponding adduct/s (10a,b, 12a,b, 16a,b,c or 17) as yellow foams (detailed data in supplementary materials).
cis-2-(tert-Butoxycarbonyl)-6-hydroxy-5-(methoxycarbonyl)-8-methyl-1-oxo-8a-(phenylsulfonyl)-1,2,3,4,4a, 7,8,8a-octahydroisoquinolines10a and 10b. Operating as described above in the general procedure, from unsaturated lactam 6a (800 mg, 2.28 mmol) in anhydrous CH2Cl2 (200 mL), Nazarov reagent 9 (1.07 g, 7.5 mmol), and Cs2CO3 (4.9 g, 15 mmol) in anhydrous CH2Cl2 (250 mL), cis-hydroisoquinolones 10a (cis Me/SO2Ph isomer) and 10b (trans Me/SO2Ph isomer) were obtained (ratio 3:1, 890 mg, 79% yield). Compound 10a: IR (ATR Pike) ν (cm−1): 1727 (CO), 1654 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.08 (d, J = 6.4 Hz, 3H, CH3), 1.50 [s, 9H, (CH3)3C], 1.89 (m, 1H, H-7), 2.32 (dm, J = 14.8 Hz, 1H, H-4), 2.40 (m, 2H, H-8, H-7), 2.70 (dddd, J = 14.8, 13.6, 5.2, 3.6 Hz, 1H, H-4), 3.23 (td, J = 12.8, 4.0 Hz, 1H, H-3), 3.74 (dm, J = 12.8 Hz, 1H, H-3), 3.82 (s, 3H, CH3O) 3.92 (br. s, 1H, H-4a), 7.49 (td, J = 7.6, 2.0 Hz, 2H, HAR), 7.63 (td, J = 7.6, 1.2 Hz, 1H, HAR), 7.96–7.99 (dd, J = 7.6, 1.2 Hz, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 14.0 (CH3), 23.3 (C-4), 27.8 [(CH3)3C], 29.3 (C-4a), 30.8 (C-8), 35.0 (C-7), 42.9 (C-3), 51.8 (CH3O), 77.4 (C-8a), 83.6 [(CH3)3C], 96.6 (C-5), 128.3 (2CHAR), 130.9 (2CHAR), 134.1 (CHAR), 137.0 (CAR), 151.8 (CO), 167.1 (CO), 171.5, 171.6 (CO, C-6); HRMS (ESI) calcd for [C23H29NO8S + Na+]: 502.1506, found: 502.1511. Compound 10b: 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.06 (d, J = 6.8 Hz, 3H, CH3), 1.53 [s, 9H, (CH3)3C], 1.69 (ddd, J = 13.2, 3.6, 1H, H-4), 2.17 (d, J = 18.4 Hz, 1H, H-7), 2.44 (dd, J = 14.0, 2.8 Hz, 1H, H-4), 2.79 (m, 1H, H-8), 3.15 (dd, J = 18.0, 6.0 Hz, 1H, H-7), 3.39 (td, J = 12.8, 3.2 Hz, 1H, H-3), 3.71 (dd, J = 12.8, 1.2, 1H, H-4a), 3.86 (s, 3H, CH3O) 3.94 (dt, J = 12.4, 2.4 Hz, H-3), 7.54 (t, J = 7.2 Hz, 2H, HAR), 7.68 (td, J = 8.0, 1.2 Hz, 1H, HAR), 7.89 (d, J = 8.0 Hz, 2H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 20.4 (CH3), 27.7 (C-4), 27.9 [(CH3)3C], 30.1 (C-8), 33.2 (C-7), 33.2 (C-4a), 45.7 (C-3), 51.8 (CH3O), 77.9 (C-8a), 83.5 [(CH3)3C], 96.8 (C-5), 128.5 (2CHAR), 131.0 (2CHAR), 134.3 (CHAR), 134.9 (CAR), 151.5 (CO), 166.3 (CO), 171.0, 171.8 (CO, C-6).
cis-6-Hydroxy-5-(methoxycarbonyl)-2,8-dimethyl-1-oxo-8a-(phenylsulfonyl)-1,2,3,4,4a,7,8,8a-octahydroiso- quinolines12a and 12b. Operating as described in the general procedure, from unsaturated lactam 6b (220 mg, 0.87 mmol) in anhydrous MeOH (160 mL), Nazarov reagent 9 (372 mg, 2.62 mmol), and KF (304 mg, 5.24 mmol) in anhydrous MeOH (10 mL), cis-hydroisoquinolones 12a (cis Me/SO2Ph isomer) and 12b (trans Me/SO2Ph isomer) were obtained (ratio 1:5, 175 mg, 51% yield). Compound 12a: IR (ATR Pike) ν (cm−1): 1646 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.09 (d, J = 6.8 Hz, 3H, CH3), 1.90 (dd, J = 18.5, 2.0 Hz, 1H, H-7), 2.30 (dq, J = 17.6, 2.4 Hz, 1H, H-4), 2.39 (dm, J = 18.5 Hz, 1H, H-7), 2.48 (m, 1H, H-8), 2.67-2.73 (m, 1H, H-4), 2.96 (s, 3H, CH3N), 3.21 (m, 2H, H-3), 3.84 (s, 3H, CH3O), 7.53 (t, J = 7.6 Hz, 2H, HAR), 7.65 (t, J = 7.2 Hz, 1H, HAR), 8.07 (dd, J = 7.6 Hz, 1H, HAR), 12.34 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 13.8 (CH3), 23.0 (C-4), 29.6 (C-4a), 30.5 (C-8), 35.3 (C-7), 35.8 (NCH3), 46.3 (C-3), 51.8 (CH3O), 75.5 (C-8a), 96.6 (C-5), 128.3 (2CHAR), 131.0 (2CHAR), 133.9 (CHAR), 138.0, (CAR), 165.0 (CO), 171.9 (C-6, CO); HRMS (ESI) calcd for [C19H23NO6S + H+]: 394.1319, found: 394.1323. Compound 12b: 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.02 (d, J = 7.2 Hz, 3H, CH3), 1.69 (qd, J = 12.8, 4.4 Hz, 1H, H-4), 2.16 (dd, J = 18.0, 1.6 Hz, 1H, H-7), 2.32 (dm, J = 12.8 Hz, 1H, H-4), 2.77 (s, 3H, CH3N), 3.04 (t, J = 6.8 Hz, 1H, H-8), 3.04–3.10 (m, 2H, H-7, H-3), 3.25 (td, J = 13.2, 4.0 Hz, 1H, H-3), 3.60 (dd, J = 12.8, 4.0 Hz, 1H, H-4a), 3.81 (s, 3H, CH3O), 7.51 (t, J = 7.6 Hz, 2H, HAR), 7.62 (t, J = 7.2 Hz, 1H, HAR), 7.90 (t, J = 7.2 Hz, 1H, HAR), 12.2 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 20.6 (CH3), 28.1 (C-4), 29.7 (C-8), 33.2 (C-7), 33.9 (C-4a), 35.8 (CH3N), 48.8 (C-3), 51.8 (CH3O), 75.8 (C-8a), 97.4 (C-5), 128.3 (CHAR), 130.8 (CHAR), 133.9 (C-3), 136.7, (CAR), 164.9 (CO), 171.1 (C-6), 171.9 (CO).
cis-2-(tert-Butoxycarbonyl)-6-hydroxy-5-(methoxycarbonyl)-1-oxo-8a-(phenylsulfonyl)-1,2,3,4,4a,7,8,8a-octahydroisoquinoline (18a). Operating as described in the general procedure, from unsaturated lactam 6a (150 mg, 0.44 mmol) in anhydrous CH2Cl2 (65 mL), Nazarov reagent 17 (268 mg, 1.33 mmol), and Cs2CO3 (865 mg, 2.66 mmol) in anhydrous CH2Cl2 (20 mL), cis-hydroisoquinolone 18a was obtained (96 mg, 47% yield): IR (ATR Pike) ν (cm−1): 1733 (C=O), 1653 (NCO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.55 [s, 9H, (CH3)3C)], 1.64 (m, 1H, H-4), 2.12–2.21 (m, 3H, 2H-7, H-8), 2.46-2.54 (m, 2H, H-8, H-4), 3.62-3.74 (m, 2H, 2H-3), 3.85 (s, 3H, CH3O), 3.96 (dd, J = 9.6, 3.6 Hz, H-4a), 7.53 (t, J = 7.6 Hz, 2H, HAR), 7.68 (t, J = 7.6, 1.2 Hz, 1H, HAR), 7.86 (d, J = 7.6, 1.2 Hz, 2H, HAR), 12.34 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 25.8 (C-8), 26.4 (C-7), 27.9 [(CH3)3C], 28.3 (C-4), 32.6 (C-4a), 44.3 (C-3), 51.9 (CH3O), 74.1 (C-8a), 83.6 [(CH3)3C], 98.4 (C-5), 128.5 (2CHAR), 130.8 (2CHAR), 134.3 (CHAR), 135.0 (CAR), 151.8 (CO), 166.7 (CO), 171.5, 173.9 (CO, C-6); HRMS (ESI) calcd for [C22H27NO8S + NH4+]: 483.1796, found: 483.1789.
cis-6-Hydroxy-5-(methoxycarbonyl)-2-methyl-1-oxo-8a-(phenylsulfonyl)-1,2,3,4,4a,7,8,8a-octahydroisoqui- noline (18b). Operating as described in the general procedure, from unsaturated lactam 6b (60 mg, 0.24 mmol) in anhydrous CH2Cl2 (35 mL), Nazarov reagent 17 (144 mg, 0.71 mmol), and Cs2CO3 (469 mg, 1.44 mmol) in anhydrous CH2Cl2 (15 mL), cis-hydroisoquinolone 18b was obtained (32 mg, 35% yield): IR (ATR Pike) ν (cm−1): 1653 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.97 (m, 1H, H-4), 2.00-2.15 (m, 3H, H-7, 2H-8), 2.35 (m, 1H, H-7), 2.46-2.54 (ddt, J = 13.8, 8.8, 4.2 Hz, 1H, H-4), 2.93 (s, 3H, CH3N), 3.17 (ddt, J = 12.8, 9.4, 3.8 Hz, 1H, H-3), 3.27 (m, 1H, H-3), 3.84 (s, 3H, CH3O), 4.01 (dd, J = 6.4, 3.6 Hz, H-4a), 7.51 (t, J = 7.6, Hz, 2H, HAR), 7.64 (t, J = 7.6 Hz, 1H, HAR), 7.88 (d, J = 7.6 Hz, 2H, HAR), 12.40 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 26.1 (C-7), 26.2 (C-4), 27.2 (C-8), 32.1 (C-4a), 35.8 (CH3N), 46.9 (C-3), 51.8 (CH3O), 71.6 (C-8a), 97.7 (C-5), 128.6 (2CHAR), 130.1 (2CHAR), 133.8 (CHAR), 136.7 (CAR), 164.5 (CO), 171.8, 172.9 (CO, C-6); HRMS calcd for [C18H21NO6S + H+]: 380.1162, found: 380.1158.
cis-6-Hydroxy-5-(methoxycarbonyl)-1-oxo-8a-(phenylsulfonyl)-2-(p-toluenesulfonyl)-1,2,3,4,4a,7,8,8a-octa- hydroisoquinoline (18c). Operating as described in the general procedure, from unsaturated lactam 6c (50 mg, 0.12 mmol) in anhydrous CH2Cl2 (20 mL), Nazarov reagent 17 (73 mg, 0.36 mmol) and Cs2CO3 (234 mg, 0.72 mmol) in anhydrous CH2Cl2 (5 mL), cis-hydroisoquinolone 18c was obtained (20 mg, 32% overall yield for the three steps). IR (ATR Pike) ν (cm−1): 1653 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.85−1.93 (m, 2H, H-4, H-7), 1.99-2.04 (m, 2H, H-7, H-8), 2.29-2.35 (m, 1H, H-8), 2.50 (s, 3H, CH3), 2.75-2.70 (m, 1H, H-4), 3.84 (s, 3H, CH3O) 3.91 (m, 1H, H-3), 4.01 (m, 1H, H-3), 7.22 -7.34 (m, 6H, HAR), 7.60 (td, J = 7.2, 1.2 Hz, 1H, HAR), 7.91 (d, J = 8.8 Hz, 2H, HAR), 12.34 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 21.7 (CH3), 25.7 (C-8), 27.0 (C-7), 28.3 (C-4), 31.9 (C-4a), 43.8 (C-3), 52.0 (CH3O), 73.1 (C-8a), 97.8 (C-5), 128.3-130.7 (CHAR), 134.3 (CHAR), 134.6, 135.2 (CAR, CHAR), 145.1 (CAR), 165.5 (CO), 171.4, 172.9 (CO, C-6); HRMS (ESI) calcd for [C24H25NO8S2 + H+]: 520.1094, found: 520.1108.
cis-2-(tert-Butoxycarbonyl)-8a-(ethoxycarbonyl)-6-hydroxy-5-(methoxycarbonyl)-1-oxo-1,2,3,4,4a,7,8,8a- octahydroisoquinoline (19). Operating as described in the general procedure, from unsaturated lactam 8 (90 mg, 0.33 mmol) in anhydrous CH2Cl2 (60 mL), Nazarov reagent 17 (202 mg, 1 mmol), and Cs2CO3 (652 mg, 2 mmol) in anhydrous CH2Cl2 (5 mL), cis-hydroisoquinolone 19 was obtained (87 mg, 60% yield): IR (ATR Pike) ν (cm−1): 1725 (CO), 1654 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.09 (d, J = 7.2 Hz, 3H, CH2CH3), 1.36 [s, 9H, CH3)3C], 1.51 (ddd, J = 14.0, 12.0, 4.8 Hz, 1H, H-4), 1.90 (ddd, J = 14.0, 11.2, 7.2 Hz, 1H, H-8), 2.03 (dq, J = 14.0, 3.6 Hz, 1H, H-4), 2.18 (ddd, J = 18.4, 7.2, 2.0 Hz, 1H, H-7), 2.32 (dd, J = 14.0, 7.2 Hz, 1H, H-8), 2.63 (ddd, J = 18.4, 10.4, 8.0 Hz, 1H, H-7), 3.31 (dd, J = 11.6, 3.2 Hz, 1H, H-4a), 3.40 (td, J = 12.4, 3.6 Hz, 1H, H-3), 3.64 (s, 3H, CH3O), 3.66 (ddd, J = 12.4, 4.8, 3.2 Hz, 1H, H-3), 4.05 (qd, J = 7.2, 2.4 Hz, 2H, CH2CH3), 12.18 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 13.9 (CH2CH3), 24.6 (C-8), 26.3 (C-7), 27.5 (C-4), 27.9 [(CH3)3C], 34.7 (C-4a), 45.7 (C-3), 51.7 (CH3O), 56.1 (C-8a), 61.7 (CH2CH3), 83.4 [(CH3)3C], 98.0 (C-5), 152.7 (C-6), 170.4 (CO), 170.5 (CO), 171.9 (CO), 172.7 (CO); HRMS (ESI) calcd for [C19H27NO8 + Na+]: 420.1629, found: 420.1645.
3.4. General Procedure for the Desulfonylation Reactions
Na2HPO4 (50 equiv.) and sodium amalgam (25 equiv.) were added at −78 ˚C under an argon atmosphere to a solution of the sulfonyl derivative 10a, 12a or 12b (1 equiv.) in anhydrous methanol (0.025 M), and the mixture was stirred at −78 ˚C for 2 h. The solution was then filtered and quenched with H2O at low temperature. The mixture was concentrated under reduced pressure, and the resulting aqueous solution was extracted with EtOAc. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (9:1 hexane-EtOAc) of the residue afforded the desulfurated compounds 13a, 14a or 14b (detailed data in supplementary materials).
cis-2-(tert-Butoxycarbonyl)-6-hydroxy-5-(methoxycarbonyl)-8-methyl-1-oxo-1,2,3,4,4a,7,8,8a-octahydroiso- quinoline (H-8/H-8a trans, 13a). Operating as described in the above general desulfonylation procedure, from a solution of sulfonyl derivative 10a (580 mg, 1.21 mmol) in anhydrous methanol (50 mL), Na2HPO4 (8.60 g, 60.6 mmol), and sodium amalgam (6.77 g, 30.3 mmol), compound 13a was obtained (357 mg, 87% yield): 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.05 (d, J = 6.0 Hz, 3H, CH3), 1.53 [s, 9H, (CH3)3C], 1.79 (dddd, J = 19.2, 12.8, 11.2, 4.8 Hz, 1H, H-4), 2.04 (m, 1H, H-7), 2.10 (m, 1H, H-4), 2.30 (m, 2H, H-8, H-8a), 2,46 (dd, J = 19.2, 4.8 Hz, 1H, H-7), 2.98 (dt, J = 11.2, 4.0 Hz, 1H, H-4a), 3.41 (td, J = 12.8, 4.0 Hz, 1H, H-3), 3.70-3.75 (m, 1H, H-3), 3.79 (s, 3H, CH3O), 12.23 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 18.8 (CH3), 26.9 (C-8), 27.1 (C-4), 27.9 [(CH3)3C], 32.0 (C-4a), 36.8 (C-7), 45.2 (C-3), 50.5 (C-8a), 51.6 (CH3O), 82.7 [(CH3)3C], 99.3 (C-5), 153.3 (CO), 169.0 (CO), 172.2, 173.1 (CO, C-6); HRMS (ESI) calcd for [C17H25NO6 + Na+]: 362.1574, found: 362.1579.
cis-6-Hydroxy-5-(methoxycarbonyl)-2-methyl-1-oxo-1,2,3,4,4a,7,8,8a-octahydroisoquinoline (H-8/H-8a trans, 14a). Operating as described in the general desulfonylation procedure, from a solution of sulfone 12a (100 mg, 0.25 mmol) in anhydrous MeOH (10 mL), Na2HPO4 (1.81 g, 12.7 mmol), and sodium amalgam (1.42 g, 6.36 mmol), compound 14a was obtained (48 mg, 76% yield) as a white foam: IR (ATR Pike) ν (cm−1): 1645 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.09 (d, J = 7.2 Hz, 3H, CH3), 1.90 (m, 1H, H-4), 1.94–2.10 (m, 2H, H-4, H-7), 2.24 (m, 2H, H-8, H-8a), 2.38 (d, J = 18.4, 4.4 Hz, 1H, H-7), 2.90 (m, 1H, H-4a), 2.92 (s, 3H, CH3N), 3.21–3.25 (m, 2H, H-3), 3.79 (s, 3H, CH3O), 12.30 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 19.2 (CH3), 26.5 (C-4), 27.4 (C-8a), 31.3 (C-4a), 34.8 (CH3N), 37.1 (C-7), 47.2 (C-8), 48.8 (C-3), 51.5 (CH3O), 99.1 (C-5), 170.7 (CO), 172.3 (C-6), 172.6 (CO); HRMS (ESI) calcd for [C13H20NO4 + H+]: 254.1387, found: 254.1387.
cis-6-Hydroxy-5-(methoxycarbonyl)-2-methyl-1-oxo-1,2,3,4,4a,7,8,8a-octahydroisoquinoline (H-8/H-8a cis, 14b). Operating as described in the general desulfonylation procedure, from a solution of sulfone 12b (340 mg, 0.87 mmol) in anhydrous MeOH (10 mL), Na2HPO4 (6.134 g, 43.2 mmol), and sodium amalgam (4.836 g, 21.6 mmol), compound 14b was obtained (137 mg, 62% yield) as a white foam: 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.05 (d, J = 7.2 Hz, 3H, CH3), 1.86 (qd, J = 12.0, 5.2, 1H, H-4), 2.10 (dm, J = 12.0 Hz, 1H, H-4), 2.19 (dd, J = 18.0, 2.0 Hz, 1H, H-7), 2.657-2.64 (m, 12H, H-8, H-7), 2.67 (m, 1H, H-8a), 2.85–2.90 (m, 1H, H-4a), 3.00 (s, 3H, NCH3), 3.24 (dq, J = 12.0, 3.2 Hz, 1H, H-3), 3.39 (td, J = 12.0, 4.4 Hz, 1H, H-3), 3.79 (s, 3H, CH3O), 12.48 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 18.8 (CH3), 26.6 (C-4), 29.4 (C-8a), 31.9 (C-4a), 34.9 (CH3N), 36.1 (C-7), 43.6 (C-8), 49.2 (C-3), 51.5 (CH3O), 98.0 (C-5), 171.2 (CO), 172.6 (C-6, CO).
3.5. General Procedure for the Removal of the N-Boc Substituent
TFA (1:3 TFA/CH2Cl2 ratio) was added to a solution of the N-Boc derivative 10a or 13a in anhydrous CH2Cl2 (0.05-0.06 M), and the mixture was stirred for 30 minutes at room temperature. Toluene (1 mL) was added to the resulting solution, and the mixture was concentrated under reduced pressure. After a second addition of toluene (1 mL) and concentration of the mixture, flash chromatography (7:3 to 3:7 hexane-EtOAc) of the residue afforded the deprotected compound 15 or 16.
cis-6-Hydroxy-5-(methoxycarbonyl)-8-methyl-1-oxo-8a-(phenylsulfonyl)-1,2,3,4,4a,7,8,8a-octahydroisoqui- noline (Me/SO2Ph cis, 15). Operating as described in the above general procedure, from a solution of the N-Boc derivative 10a (23 mg, 0.048 mmol) in anhydrous CH2Cl2 (750 μL) and TFA (250 μL), compound 15 was obtained (18 mg, 98% yield): IR (ATR Pike) ν (cm−1): 3352 (NH), 1668 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.11 (d, J = 6.8 Hz, 3H, CH3), 1.94 (dd, J = 17.6, 2.0 Hz, H-7), 2.32 (dm, J = 14.8 Hz, 1H, H-4), 2.45 (m, 1H, H-8), 2.50 (dm, J = 17.6 Hz, 1H, H-7), 2.65 (dddd, J = 14.8, 12.4, 5.6, 3.6 Hz, 1H, H-4), 3.16 (td, J = 12.4, 4.4 Hz, 1H, H-3), 3.27 (m, 1H, H-3), 3.85 (s, 3H, CH3O), 3.97 (br.s, 1H, H-4a), 6.29 (br.s, 1H, NH), 7.53 (t, J = 7.2, Hz, 2H, HAR), 7.65 (t, J = 7.2 Hz, 1H, HAR), 8.06 (d, J = 7.2 Hz, 2H, HAR), 12.33 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 13.7 (CH3), 22.4 (C-4), 29.2 (C-4a), 30.1 (C-8), 35.2 (C-7), 38.7 (C-3), 51.8 (CH3O), 75.1 (C-8a), 96.5 (C-5), 128.3 (2CHAR), 130.6 (2CHAR), 134.0 (CHAR), 137.7 (CAR), 167.0 (CO), 171.6, 171.8 (CO, C-6); HRMS calcd for [C18H21NO6S + H+]: 380.1162, found: 380.1164.
cis-6-Hydroxy-5-(methoxycarbonyl)-8-methyl-1-oxo-1,2,3,4,4a,7,8,8a-octahydroisoquinoline (H-8/H-8a trans, 16). Operating as described in the above general procedure, from a solution of the N-Boc derivative 13a (10 mg, 0.03 mmol) in anhydrous CH2Cl2 (600 μL) and TFA (200 μL), compound 16 was obtained (7 mg, 99% yield): IR (ATR Pike) ν (cm−1): 3340 (NH), 1659 (CO); 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.12 (d, J = 6.8 Hz, 3H, CH3), 1.83 (m, 1H, H-4), 1.98 (dd, J = 13.6, 3.6 Hz, 1H, H-4), 2.06 (dd, J = 18.4, 8.8 Hz, H-7), 2.23 (m, 2H, H-8, H-8a), 2.42 (dd, J = 18.4, 4.8 Hz, 1H, H-7), 2.93 (m, 1H, H-4a), 3.23-3.40 (m, 2H, H-3), 3.79 (s, 3H, CH3O), 6.22 (br.s, 1H, NH), 12.29 (s, 1H, OH); 13C-NMR (100.6 MHz, CDCl3): δ = 19.1 (CH3), 25.7 (C-4), 27.3 (C-8, C-8a), 31.0 (C-4a), 37.1 (C-7), 41.1 (C-3), 51.6 (CH3O), 99.1 (C-5), 172.2, 172.4 (CO, C-6); HRMS calcd for [C12H17NO4 + H+]: 240.1230, found: 240.1232.
Supplementary Materials
The following are available online. Copies of 1H, 13C, and NOE NMR spectra, and crystallographic data for 10a (CCDC 1890851), 12a (CCDC 1890850), and 12b (CCDC 1890852). CCDC 1890850-1890852 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Author Contributions
M.A. designed and planned the research; M.P. supervised the experimental work; F.A. and C.A. performed the experimental work and characterized the compounds; E.M. carried out the X-ray analysis; J.B. discussed the results and prepared the manuscript for publication.
Funding
This research was funded by the MINECO/FEDER, Spain (Project CTQ2015-65384-R).
Acknowledgments
Financial support from the MINECO/FEDER, Spain (Project CTQ2015-65384-R) is gratefully acknowledged. Thanks are also due to the MINECO (Spain) for a fellowship to F. A. (AP2010-1663). We also acknowledge the networking contribution from the COST Action CM1407.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taylor, D.R.; MacCoss, M.; Lawson, A.D.G. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.T. The yohimbine group. Monoterpenoid Indole Alkaloids. In The Chemistry of Heterocyclic Compounds; Saxton, J.E., Ed.; John Wiley and Sons: New York, NY, USA, 1983; Volume 25, pp. 147–200. [Google Scholar]
- Szántay, C.; Honty, K. The yohimbine group. Monoterpenoid Indole Alkaloids. In The Chemistry of Heterocyclic Compounds; Saxton, J.E., Ed.; John Wiley and Sons: New York, NY, USA, 1994; Volume 25, pp. 161–216. [Google Scholar]
- Peng, J.; Rao, K.V.; Choo, Y.-M.; Hamann, M.T. Manzamine alkaloids. In Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Fattorusso, E., Taglialatela-Scafati, O., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 189–232. [Google Scholar]
- Amat, M.; Perez, M.; Ballette, R.; Proto, S.; Bosch, J. The alkaloids of the madangamine group. In The Alkaloids; Knölker, H.-J., Ed.; Elsevier: Oxford, UK, 2015; Volume 74, pp. 159–199. [Google Scholar]
- Kaldor, S.W.; Kalish, V.J.; Davies, J.F., II; Shetty, B.V.; Fritz, J.E.; Appelt, K.; Burgess, J.A.; Campanale, K.M.; Chirgadze, N.Y.; Clawson, D.K.; et al. Viracept (nelfinavir mesylate, AG1343): A potent, orally bioavailable inhibitor of HIV-1 protease. J. Med. Chem. 1997, 40, 3979–3985. [Google Scholar] [CrossRef] [PubMed]
- Arusksakunwong, O.; Promsri, S.; Witta, K.; Nimmanpipug, P.; Lee, V.S.; Wijitkosoom, A.; Sompornpisut, P.; Hannongbua, S. Current development on HIV-1 protease inhibitors. Curr. Comput. Aided Drug Des. 2007, 3, 201–213. [Google Scholar] [CrossRef]
- Weiss, B.; Alt, A.; Ogden, A.M.; Gates, M.; Dieckman, D.K.; Clemens-Smith, A.; Ho, K.H.; Jarvie, K.; Rizkalla, G.; Wright, R.A.; et al. Pharmacological characterization of the competitive GLUK5 receptor antagonist decahydroisoquinoline LY466195 in vitro and in vivo. Pharmacol. Exp. Ther. 2006, 318, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Motani, A.S.; Luo, J.; Liang, L.; Mihalic, J.T.; Chen, X.; Tang, L.; Li, L.; Jaen, J.; Chen, J.-L.; Dai, K. Evaluation of AMG 076, a potent and selective MCHR1 antagonist, in rodent and primate obesity models. Pharmacol. Res. Perspect. 2013, 1, e00003. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F.; Williamson, S.A.; Gist, R.P.; Smith, K.M. Aspects of the intramolecular Diels-Alder reactions of some 1,3,9-trienic amides, amines, and esters. An approach to the pentacyclic skeleton of the yohimboid alkaloids. J. Org. Chem. 1983, 48, 5170–5180. [Google Scholar] [CrossRef]
- Moriwake, T.; Hamano, S.; Saito, S.; Torii, S.; Kashino, S. Synthesis of the chiral (8S)-7-aza-1,3(E),9-decatriene system from natural. α-Amino acids and its intramolecular Diels-Alder reaction directed toward chiral trans-hydroisoquinolones. J. Org. Chem. 1989, 54, 4114–4120. [Google Scholar] [CrossRef]
- Casamitjana, N.; López, V.; Jorge, A.; Bosch, J.; Molins, E.; Roig, A. Diels-Alder reactions of 5,6-dihydro-2(1H)-pyridones. Tetrahedron 2000, 56, 4027–4042. [Google Scholar] [CrossRef]
- Zibuck, R. t-Butyl (E)-3-oxo-4-hexenoate. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L.A., Ed.; Wiley: Chichester, UK, 1995; Volume 2, pp. 936–937. [Google Scholar]
- Zibuck, R. Methyl 3-oxo-4-pentenoate. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L.A., Ed.; Wiley: Chichester, UK, 1995; Volume 5, pp. 3558–3559. [Google Scholar]
- Audran, G.; Brémond, P.; Feuerstein, M.; Marque, S.R.A.; Santelli, M. Nazarov reagents and their use in organic synthesis. Tetrahedron 2013, 69, 8325–8348. [Google Scholar] [CrossRef]
- Amat, M.; Arioli, F.; Pérez, M.; Molins, E.; Bosch, J. Preparation and double Michael addition reactions of a synthetic equivalent of the Nazarov reagent. Org. Lett. 2013, 15, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Arioli, F.; Pérez, M.; Are, C.; Estarellas, C.; Luque, F.J.; Bosch, J.; Amat, M. Stereocontrolled annulations of indolo[2,3-a]quinolizidine-derived lactams with a silylated Nazarov reagent. Access to allo and epiallo yohimbine-type derivatives. Chem. Eur. J. 2015, 21, 13382–13389. [Google Scholar] [CrossRef] [PubMed]
- Estarellas, C.; Arioli, F.; Pérez, M.; Are, C.; Hevia, D.; Molins, E.; Luque, F.J.; Bosch, J.; Amat, M. Origin of the base-dependent facial selectivity in annulation reactions of Nazarov-type reagents with unsaturated indolo[2,3-a]quinolizidine lactams. Eur. J. Org. Chem. 2017, 3969–3979. [Google Scholar] [CrossRef]
- Lavallée, J.-F.; Deslongchamps, P. Synthesis of cis-decalin via Diels-Alder and double Michael cycloaddition with substituted Nazarov reagent. Tetrahedron Lett. 1988, 29, 5117–5118. [Google Scholar] [CrossRef]
- Lavallée, J.-F.; Spino, C.; Ruel, R.; Hogan, K.T.; Deslongchamps, P. Stereoselective synthesis of cis-decalins via Diels-Alder and double Michael addition of substituted Nazarov reagents. Can. J. Chem. 1992, 70, 1406–1426. [Google Scholar] [CrossRef]
- Jung, Y.C.; Yoon, C.H.; Turos, E.; Yoo, K.S.; Jung, K.J. Total Syntheses of (−)-α-kainic acid and (+)-α-allokainic acid via stereoselective C-H insertion and efficient 3,4-stereocontrol. J. Org. Chem. 2007, 72, 10114–10122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).