Abstract
The dried seeds of Aesculus chinensis Bge. var. chekiangensis (Hu et Fang) Fang, called “Suo Luo Zi”, have been used in traditional Chinese medicine. Nevertheless, most studies have been focused on components of less polarity fractions. In this research, twelve indoles, including six new indole glycosides (1–6) as well as six known analogs were isolated from the polar portion which has been seldom studied. This is the first description of N-glucosylated indoles obtained from the genus of Aesculus. Structures of the new compounds (1–6) were elucidated based on comprehensive interpretation of HRESIMS, 1D and 2D NMR. Additionally, the neuroprotective activities of the N-glucosylated indoles were evaluated for the first time indicating that compounds 1–5 and 9–10 exhibited moderate neuroprotective activities. Further cytotoxicity tests of isolates 1–10 on three human tumor cell lines suggested that none of these compounds were cytotoxic (IC50 > 50 μM).
1. Introduction
Aesculus chinensis Bge. var. chekiangensis (Hu et Fang) Fang belonging to the Hippocastanaceae family is a species endemic to China. Its dried seeds together with Aesculus chinensis Bge. and Aesculus wilsonii Rehd have been used in traditional Chinese medicine for treating chest and abdomen pain, dysentery and ague [1,2]. Recently, many studies have demonstrated that Aesculus chinensis have beneficial effects involving their antitumor, cardio-protective, anti-inflammatory, and neuroprotective activities [3,4]. Previous investigation of its chemical constituents resulted in various types of compounds, such as triterpenoids [5,6,7], flavonoids [8,9], coumarins [10] and steroids. So far, most studies have been focused on components of less polarity fractions and little has been known about polar fractions consists. As an extension study on biologically active compounds from polar portion, six new indole glycosides (1–6) and six known analogs (7–12) were obtained (Figure 1) and their neuroprotective activities and cytotoxic activities were also evaluated.
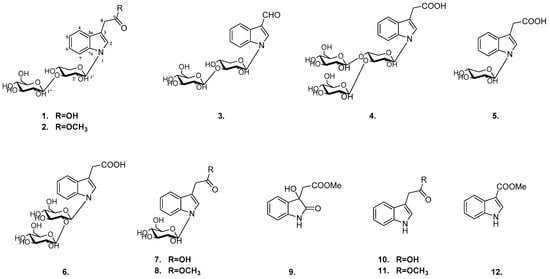
Figure 1.
The structures of compounds 1–12.
2. Results
Compound 1 was isolated as a yellow, amorphous powder (MeOH). The UV spectrum showed absorption at 290 nm, and its pseudomolecular ion [M+H]+ at m/z 470.1655, indicating the presence of odd number of nitrogen atom in compound 1. The HRMS analysis (m/z 470.1655 [M + H]+) and the NMR data (Table 1 and Table 2) indicated the molecular formula C21H27NO11.

Table 1.
1H-NMR spectroscopic data (δ) for compounds 1–6a (δ in ppm, J in Hz).

Table 2.
13C-NMR spectroscopic data (δ) for compounds 1–6a (δ in ppm).
The 13C-NMR spectrum exhibited resonances of 21 carbon signals composed of nine unsaturated carbons (δC 176.0, 138.2, 129.8, 126.4, 122.9, 120.9, 119.8, 111.7, 109.8), eleven sugar carbons (δC 105.0, 86.1, 81.1, 79.0, 77.7, 77.4, 75.8, 70.9, 70.8, 69.4, 62.0) and one additional aliphatic carbons (δC 31.9). The 1H-NMR spectrum displayed a spin coupling system of four aromatic protons [δH 7.53 (1H, d, J = 7.8 Hz), 7.48 (1H, d, J = 8.3 Hz), 7.16 (1H, ddd, J = 8.2, 7.0, 1.2 Hz), 7.08 (1H, ddd, J = 7.5, 7.0, 0.9 Hz)] indicating an ortho-substituted aromatic ring; an additional aromatic proton signal at δH 7.41 (1H, s), indicative of a 3-substituted indole moiety, an isolated methylene protons at δH 3.72 (2H, s) and two anomeric protons at δH 5.45 (1H, d, J = 9.0 Hz) and 4.35 (1H, d, J = 7.8 Hz).
All of the protons and carbons were unambiguously assigned by HSQC experiment.
The HMBC spectrum correlations between H-4 (δH 7.53, 1H, d, J = 7.8 Hz) and C-3 (δC 109.8), C-6 (δC 122.9), C-7a (δC 138.2); between H-5 (δH 7.08, 1H, ddd, J = 7.5, 7.0, 0.9 Hz) and C-7 (δC 111.7), C-3a (δC 129.8); between H-6 (δH 7.16, 1H, ddd, J = 8.2, 7.0, 1.2 Hz) and C-4 (δC 119.8), C-7a (δC 138.2) and between H-7 (δH 7.48, 1H, d, J = 8.3 Hz) and C-5 (δC 120.9), C-3a (δC 129.8) further confirm the existence of the ortho-substituted aromatic ring in compound 1. The substituent at C(3) was deduced to be a CH2COOH group, supported by the HMBC correlations between H-2 (δH 7.41, 1H, s) and C-3a (δC 129.8), C-7a (δC 138.2), C-8 (δC 31.9) and between H-8 (δH 3.72) and C-2 (δC 126.4), C-3 (δC 109.8), C-3a (δC 129.8), C9 (δC 176.0). Thus, the aglycone of compound 1 was established as indole-3-acetic acid.
The two anomeric protons at δ 5.45 (1H, d, J = 9.0 Hz), 4.35 (1H, d, J = 7.8 Hz) correlated with carbons at δ 86.1 and 105.0 in HSQC spectrum, respectively, indicated disaccharide residues. Acid hydrolysis of 1 with 2M HCl produced d-glucose and d-xylose, which was identified with HPLC analysis by comparing with authentic sugar samples after derivatization [11]. The β-configuration of the glycosidic linkages were deduced from the large coupling constants. In addition, the HMBC correlations of H-1′ (δ 5.45) with C-2, C-7a and H-1″ (δ 4.35) with C-3′ revealed the sequence glc-(1→3)-xyl to be linked at nitrogen of aglycone portion (Figure 2). Based on the above analyses, the structure of 1 was identified as N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-acetic acid.
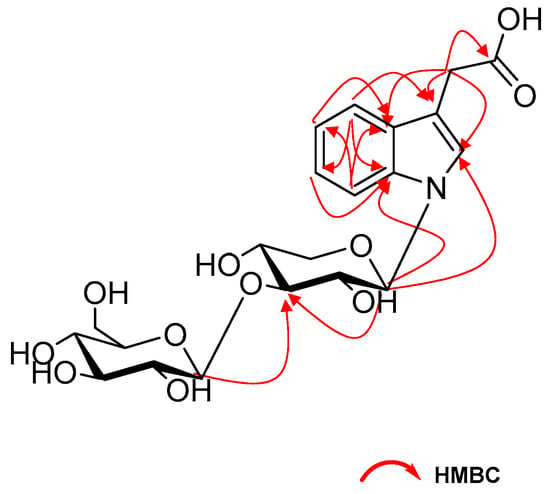
Figure 2.
The selected HMBC (H→C) correlations of compound 1.
Compound 2 was obtained as a yellow amorphous powder with the elemental formula C22H29NO11 (HR-ESI-MS m/z: 484.1811 [M + H]+; calculated for C22H30NO11, 484.1819). Acid hydrolysis of 2 yielded the same sugar units as 1. Its NMR spectra were closely similar to those of 1 with the only difference of an extra methoxy signals (δH 3.71 and δC 52.5). The HMBC cross-peaks of H-10 (δH 3.71) with C-9 (δC 174.4) implied the -COOH in 1 was replaced by -COOCH3 in 2, which was further confirmed by their formulas. Thus, compound 2 was assigned as N-[β-d-glucopyranosyl(1→3)]-β-d- xylopyranosyl-indole-3-methyl acetate.
Compound 3, a yellow, amorphous powder, was assigned the molecular formula C20H25NO10 (HRESIMS m/z 440.1549 [M + H]+; calculated for C20H26NO10, 440.1557). The sugar chain of 3 was the same as that of 2 by comparing their 1H and 13C-NMR data (Table 1 and Table 2) and analysis of the hydrolysis result. The similar NMR spectra of 3 to those of 1 and 2 indicated that compound 3 is a structural analogue of these compounds. The 1H-NMR spectra showed proton resonances corresponding to a 3-substituted indole group [δH 8.18 (1H, d, J = 7.7 Hz), 7.62 (1H, d, J = 8.1 Hz), 7.31 (1H, ddd, J = 8.1, 7.3, 1.1 Hz), 7.26 (1H, ddd, J = 7.7, 7.3, 0.9 Hz), 8.37 (1H, s)]. The substituent at C-3 was deduced as a formyl moiety, established by HSQC correlations between H-8 (δH 3.71) and C-8 (δC 187.7) together with the HMBC correlations between H-8 (δH 3.71) and C-3 (δC 119.7) as well as C-3a (δC 126.4). Hence, 3 was defined as N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-carbaldehyde.
Compound 4 was assigned the molecular formula of C27H37NO16 from HRESIMS (m/z 632.2150 [M + H]+, calculated for 632.2191) and its NMR data. The MS and NMR spectra were closely parallel to those of 1, revealing the same aglycone and sugar residues with the difference of an additional hexose unit (162 Da) in compound 4. This conclusion was further ensured by hydrolysis, conversion to chiral diastereomers and HPLC analysis. The HMBC correlations from H-1′ (δ 5.46) to C-2 (δ 126.4), C-7a (δ138.2), from H-1″ (δ 4.37) to C-3′ (δ 80.5) and from H-1‴ (δ 4.43) to C-4′ (δ 77.6) confirmed the linkage of the trisaccharide moiety in 4. Consequently, it was assigned as N-[β-d-glucopyranosyl(1→3)-[β-d-glucopyranosyl (1→4)]-β-d-xylopyranosyl-indole-3-acetic acid.
For compound 5, isolated as a yellowish powder, established the molecular formula was C15H17NO6 by HRESIMS (m/z 308.1136 [M + H]+; calculated for C15H18NO6, 308.1134). Acid hydrolysis of 5 yielded d-xylose, which was identified using the same method as 1–4. Analysis of the 1H- and 13C-NMR spectroscopic data (Table 1 and Table 2) of 5 displayed a close structural resemblance to 1, except for the absence of a d-glucose. This deduction was supported by the key HMBC correlations from H-1′ (δ 5.31) to C-2 (δ 125.0), C-7a (δ 138.3) and from H-8 (δ 3.66) to C-2 (δ 125.0), C-3 (δ112.2), C3a (δ130.2), C9 (δ180.1). Accordingly, 5 was unambiguously established as N-β-d-xylopyranosyl-indole-3-acetic acid.
Compound 6 had a molecular formula of C22H29NO12, which was established from the [M+H]+ ion at m/z 500.1762 (calculated for 500.1768) in the positive HR-ESI-MS, 30 mass units more than that of 1. Comparison of the NMR data of 6 with those of 1 showed that both isolates are closely related, but only differed at the disaccharide group. Acid hydrolysis suggested that only d-glucose existed in 6. As observed in the HMBC spectrum, the long-range correlations of H-1′ (δ 5.56) with C-2 (δ 126.4), C-7a (δ138.4) and H-1″ (δ 4.38) with C-2′ (δ 81.2) provided definitive evidences that the linkage glc-(1→2)-glc was bound to nitrogen of aglycone portion. Therefore, compound 6 was defined as N-[β-d-glucopyranosyl (1→2)]-β-d-xylopyranosyl-indole-3-acetic acid.
The known indoles were identified as N-β-d-glucopyranosyl-indole-3-acetic acid (7) [12], N-β-d-glucopyranosyl-indole-3-methyl acetate (8) [13], methyl dioxindole-3-acetate (9) [14], indole-3-acetic acid (10) [15], indole-3-methyl acetate (11) [15], indole-3-carboxylic acid methyl ester (12) [16] by NMR analysis and comparison with literature data.
It has been reported that Aesculus chinensis showed neuroprotective activities [3]. In the present study, the isolated compounds 1−10 were also evaluated for their neuroprotective effects against CoCl2-induced PC12 cell damage. As shown in Figure S43, all substances showed no obvious cytotoxic effects on PC12 cells at a dose of 10 μM. Then, 10 μM samples were bioassayed for neuroprotective activities against CoCl2-induced toxicity in PC12 cells with Trolox (10 μM) as the positive control. According to Figure 3, compared with the Trolox, compounds 1–5 and 9–10 show similar effect on increasing the cell viabilities in CoCl2-treated PC12 cells, indicating that compounds 1–5 and 9–10 exhibited statistically significant neuroprotective activities.
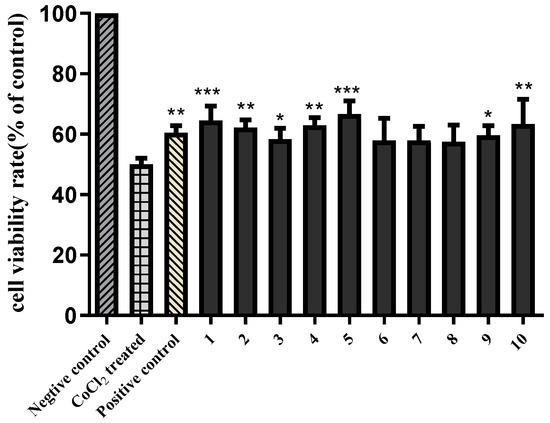
Figure 3.
Neuroprotective activities of compounds 1–10 (10 μM) against COCl2-induced cell death in PC12 cells. The data (cell viability, measured by MTT assay) are expressed as means ± SD. Three independent experiments were performed. Trolox was used as the positive control at 10 μM. Compared with CoCl2 treated group, * p < 0.05, ** p < 0.01, *** p < 0.001.
The cytotoxic activities against three human cancer cell lines (Hep G2, HCT-116, and MGC-803) of compounds 1–10 were assayed using the MTT method, with 5-fluorouracil (5-FU) as the positive control. None of these compounds displayed cytotoxic activity (IC50 > 50 μM) (Table S1).
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded on a Rudolph (Hackettstown, NJ, USA) Autopol V automatic polarimeter. The UV spectra were acquired on a UNICO 2102PCS spectrophotometer (Dayton, NJ, USA). The IR spectra were obtained in a KBr-disc (cm−1) on a Brucker Tensor II spectrometer (Billerica, MA, USA). NMR spectra were carried out on a Bruker (Billerica, MA, USA) AM-600 spectrometer at 25 °C referencing to the residuals of CD3OD. High-Resolution-ESI-MS (HR-ESI-MS) was performed on a Waters (Milford, MA, USA) Xevo G2-S UPLC-Q/TOF equipped with an ACQUITY UPLC BEH C18 (2.1 × 50 mm, Waters 1.7 μm, Milford, MA, USA). Analytical HPLC were performed on a Waters e2695 system equipped with a 2998 PDA detector (Waters, Milford, MA, USA) using a YMC-Pack-ODS-A column (250 × 4.6 mm, 5 μm, YMC, Tokyo, Japan). Semi-preparative HPLC was performed using a Shimadzu LC-6AD Series instrument equipped with a YMC Packed C18 column (250 × 10.0 mm, 5 μm, YMC, Tokyo, Japan) and detected with a DAD detector (Shimadzu, Tokyo, Japan) set at 205 and 230 nm. Column chromatography (CC) was done with Sephadex LH-20 (GE Healthcare Co. Ltd., Marlborough, MA, USA), ODS RP-C18 (40–75 μm Merck, Darmstadt, Germany), macroporous resin D101 (Chemical Plant of Nankai University, Tianjin, China), and silica gel (200–400 mesh, Qingdao Haiyang Chemical, Qingdao, China). All reagents used were of analytical grade (Concord Technology Co. Ltd., Tianjin, China).
3.2. Plant Material
Seeds of Aesculus chinensis Bge. var. chekiangensis (Hu et Fang) Fang were purchased from An guo (Hebei Province, China) in August 2015, and authenticated by professor Lijuan Zhang (Tianjin University of Traditional Chinese Medicine, Tianjin, China). A voucher specimen was deposited at the School of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine.
3.3. Extraction and Isolation
The dried seeds of A. chinensis Bge. (8.8 kg) were cut into small pieces and were extracted with 70% ethanol three times (3 h) under reflux. After removal of the solvent under reduced pressure, a dark residue (2100 g) was afforded. The residue was suspended in H2O and subjected to D101 resin and then sequentially eluted with H2O, a gradient of EtOH in water to yield the corresponding fractions. The 20% EtOH−H2O part was further fractionated with a silica gel column, eluting with a gradient of 0–100% CH2Cl2/CH3OH to yield 4 fractions (A–D).
Fraction A (8.0 g) was applied to an RP C18 CC (MeOH−H2O, from 0:100 to 50:50) to give four subfractions (A1−A4). Subfraction A2 was purified by an RP-HPLC (MeCN−H2O, 8:92, 3.0 mL/min) to obtain compounds 9 (6.7 mg, tR 11.2 min) and 12 (5.4 mg, tR 14.7 min). Further purification of subfraction A3 using preparative RP-HPLC (MeCN−H2O, 8:92, 3.0 mL/min) yielded compounds 10 (4.8 mg, tR 16.5 min) and 11 (2.3 mg, tR 17.8 min).
Fraction C (22.0 g) was applied to an ODS MPLC column eluting with gradient MeOH−H2O from 10:90 to 100: 0 to afford five major subfractions (C1–C5). Compound 4 (9.2 mg, tR 31.5 min) was purified by preparative HPLC with 10% MeCN/H2O from subfraction C1. Compounds 1 (7.1 mg, tR 8.9 min), 2 (13.5 mg, tR 9.7 min), 3 (11.0 mg, tR 13.4 min) and 6 (9.6 mg, tR 16.5 min) were obtained from Fr. C2 using Sephadex LH-20 column and further purified by RP-HPLC (MeCN−H2O 15: 85, v/v, 3.0 mL/min). Subfraction C3 was purified by preparative HPLC to afford compounds 7 (7.0 mg, tR 16.5 min) and 8 (9.1 mg, tR 18.2 min) using 15% MeCN/H2O. Subfraction C4 was chromatographed on a Sephadex LH-20 column and then purified through preparative HPLC with 15% MeCN/H2O to yield compound 5 (9.8 mg, tR 20.3 min).
3.3.1. N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-acetic Acid (1)
3.3.2. N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-methyl Acetate (2)
3.3.3. N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-carbaldehyde (3)
3.3.4. N-[β-d-glucopyranosyl(1→3)-[β-d-glucopyranosyl(1-4)]-β-d-xylopyranosyl-indole-3-acetic Acid (4)
3.3.5. N-β-d-xylopyranosyl-indole-3-acetic Acid (5)
3.3.6. N-[β-d-glucopyranosyl(1→2)]-β-d-xylopyranosyl-indole-3-acetic Acid (6)
3.3.7. Hydrolysis and Determination of Absolute Configuration of Sugars
A solution of 1–6 (1.0 mg, respectively) dissolved in 2 M HCl (4.0 mL) was heated at 90 °C for 2 h. The reaction mixture was extracted two times with EtOAc (4 mL), and the aqueous layer was evaporated to dryness under N2 atmosphere. Then l-cysteine methyl ester (1.0 mg) was added to the residues dissolved in pyridine (1.0 mL) and heated at 60 °C. One hour later, o-tolyisothiocyanate (1.0 mL) was added and heated for another hour. Then each reaction mixture was analyzed by the Waters e2695 HPLC system (YMC- Pack-ODS-A column, 1.0 mL/min, 250 nm) eluting with A (0.1% formic acid): B (acetonitrile) = 80: 20 (v/v). By comparison of the retention times with the standards, the absolute configuration of sugars in 1–6 was established [11,17].
3.4. Neuroprotective Effect Assay
The neuroprotective effects of compounds 1–10 was evaluated on CoCl2 damaged PC12 cells model [18,19]. Rat pheochromocytoma cell line (PC12) were cultured in RPMI-1640 medium with 10% (v/v) inactivated fetal bovine serum and 100 U/mL penicillin/streptomycin. The cells were grown and treated at 37 °C in 5% CO2 and 95% humidified air incubator. Cells were placed into a 96-well plate at a density of 2 × 104 cells/well and kept there for 24 h for the adherence of the cells. Cells were treated with the compounds at concentrations of 10 μM for 2 h. After incubation, 1 mM CoCl2 was added and incubated for 24 h. After a 24 h treatment, the supernatant was changed with MTT solution (5 mg/mL). After incubation at 37 °C for 4 h, cells were finally lysed with 150 μL of DMSO. The absorbance was measured at 490 nm with a microplate reader. The cell viability was indicated as a percentage of the live control cells. The results were expressed as means ± SD of the indicated numbers from three independent experiments. Statistical analysis was performed by one-way analysis of variance (ANOVA) and Student’s Dunnett test using the SPSS statistical software (version 19 for Windows, IBM Corp., Armonk, NY, USA). P values below 0.05 were considered statistically significant.
3.5. Cytotoxicity Assay
The human cancer cell lines, HepG2, HCT-116, and MGC-803 were purchased from ATCC. The in vitro cytotoxicity of compounds 1–10 was tested by MTT assay [20,21] with 5-fluorouracil as the positive control. The tested cell lines were cultured in 96-well plates at a density of 1 × 104 cells/well and incubated for 24 h. Subsequently, cells were treated with compounds 1–10 at a dosage of 3.125–50 μM, respectively. After 24 h, the supernatant was changed with MTT solution (5 mg/mL) and incubated for another 4 h. Then cells were finally lysed with 150 μL of DMSO and the absorbance was measured at 490 nm with a microplate reader. The cell viability was indicated as a percentage of the live control cells.
4. Conclusions
In summary, this is the first study of the water-soluble portion of Aesculus chinensis Bge. var. chekiangensis (Hu et Fang) Fang, six new indole glycosides (1–6) along with six known analogs were isolated and characterized. What is more, this is the first report of N-glucosylated indoles from Aesculus genus, which largely enriched its chemical diversity. In addition, the neuroprotective activities of the N-glucosylated indoles were evaluated for the first time and compounds 1–5, 9–10 exhibited statistically significant neuroprotective activities.
Supplementary Materials
The following are available online: antitumor activities (IC50 μM, n = 3) of compounds 1–10 and 5-Fu (Table S1), UV, IR, HR-ESI-MS, 1D- and 2D-NMR spectra of compounds 1–6 (Figures S1–S42), cytotoxic activities of compounds 1–10 on PC12 cells at 10 μM (Figure S43).
Author Contributions
Writing—original draft preparation and writing—review and editing, N.Z.; supervision, L.D. and F.Q.; validation, S.C., W.H., P.L. and N.K.
Funding
This research was funded by the State Key Program of National Natural Science of China, grant number 81430095.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Z.; Li, S.; Zhang, S.; Gorenstein, D. Triterpenoid saponins from the fruits of Aesculus pavia. Phytochemistry 2006, 67, 784–794. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Cui, Y.; Liu, X.; Ma, C.; Hattori, M.; Zhang, L. Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J. Nat. Prod. 1999, 62, 1510–1513. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, F.; Zhang, X.; Zhu, M.; Wang, T.; Fan, H. Escin attenuates cognitive deficits and hippocampal injury after transient global cerebral ischemia in mice via regulating certain inflammatory genes. Neurochem. Int. 2010, 57, 119–127. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, S.J.; Zhou, Y.N.; Pan, S.H.; Sun, B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κb and nuclear factor-κb-regulated gene products in pancreatic cancer both in vitro and in vivo. J. Cancer Res. Clin. 2012, 138, 785–797. [Google Scholar] [CrossRef]
- Zhao, J.; Yangi, X.W.; Cul, Y.X.; Liu, X.H.; Ouyangz, S.H. A New Triterpenoid Oligoglycoside Escin IVe from the Seeds of Aesculus Chinensis. Chin. Chem. Lett. 1999, 6, 473–476. [Google Scholar]
- Jie, G.; XiuWei, Y. Studies on Triterpenoid Saponins of Seeds of Aesculus chinensis Bunge var. chekiangensis (Hu et Fang) Fang. J. Chin. Pharm. Sci. 2004, 13, 87–91. [Google Scholar]
- Voutquenne, L.; Guinot, P.; Froissard, C.; Thoison, O.; Litaudon, M.; Lavaud, C. Haemolytic acylated triterpenoid saponins from Harpullia austro-caledonica. Phytochemistry 2005, 59, 825–832. [Google Scholar] [CrossRef]
- Ireneusz, K.; Bogdan, J.; Barbara, S.; Anna, S.; Sonia, P.; Cosimo, P.; Federico, F.; Chlodwig, F.; Wieslaw, O. Flavonoids in horse chestnut (Aesculus hippocastanum) seeds and powdered waste water byproducts. J. Agric. Food Chem. 2007, 55, 8485–8490. [Google Scholar]
- Wei, F.; Ma, S.; Ly, M.; But, P.P.; Lin, R.C.; Khan, I.A. Antiviral flavonoids from the seeds of Aesculus chinensis. J. Nat. Prod. 2004, 67, 650–653. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Y.; Li, W.; Zhang, H.; Wang, X.; Mu, Q.; He, Z.; Yao, H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015, 29, 779–786. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Kai, K.; Kyo, W.; Hisashi, M. Metabolism of indole-3-acetic acid in rice: Identification and characterization of N-beta-D-glucopyranosyl indole-3-acetic acid and its conjugates. Phytochemistry 2007, 68, 2512–2522. [Google Scholar] [CrossRef]
- Bernd, S.; Thomas, H. Isolation, structure determination, and sensory activity of mouth-drying and astringent nitrogen-containing phytochemicals isolated from red currants (Ribes rubrum). J. Agric. Food Chem. 2007, 55, 1405–1410. [Google Scholar]
- Lee, M.Y.; Lin, H.Y.; Cheng, F.; Chiang, W.; Kuo, Y.H. Isolation and characterization of new lactam compounds that inhibit lung and colon cancer cells from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran. Food Chem. Toxicol. 2008, 46, 1933–1939. [Google Scholar] [CrossRef]
- Elsayed, Y.; Refaat, J.; Abdelmohsen, U.R.; Ahmed, S.; Fouad, M.A. Retraction Note to: Rhodozepinone, a new antitrypanosomal azepino-diindole alkaloid from the marine sponge-derived bacterium Rhodococcus sp. UA13. Med. Chem. Res. 2019, 28, 105. [Google Scholar] [CrossRef]
- Bano, S.; Ali, M.S.; Ahmad, V.U. Marine Natural Products; VI. A Halogenated Chamigrene Epoxide from the Red Alga Laurencia pinnatifida. Planta Med. 1987, 53, 508. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, W.; Xia, G.; Oppong, M.B.; Ding, L.; Li, P.; Qiu, F. Methods for determination of absolute configuration of monosaccharides. Chin. Herb. Med. 2018, 10, 14–22. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Yong, Y.; Dong, Y.H.; Wang, R.J.; Li, H.X.; Zhang, H.; Guo, D.L.; Zhang, S.J.; Dong, X.P.; Xie, X.F. A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem Lett. 2016, 17, 177–180. [Google Scholar] [CrossRef]
- Zou, W.; Yan, M.; Xu, W.; Huo, H.; Sun, L.; Zheng, Z.; Liu, X. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J. Neurosci. Res. 2001, 64, 646–653. [Google Scholar] [CrossRef]
- Elreadi, M.Z.; Eid, S.; Ashour, M.L.; Tahrani, A.; Wink, M. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef]
- Xia, Y.Z.; Yang, L.; Wang, Z.D.; Guo, C.; Zhang, C.; Geng, Y.D.; Kong, L.Y. Schisandrin A enhances the cytotoxicity of doxorubicin by the inhibition of nuclear factor-kappa B signaling in a doxorubicin-resistant human osteosarcoma cell line. RSC Adv. 2015, 5, 13972–13984. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).