Abstract
Biotransformations were performed on eight selected yeast strains, all of which were able to selectively hydrogenate the chalcone derivatives 3-(2”-furyl)- (1) and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3) into 3-(2”-furyl)- (2) and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-propan-1-one (4) respectively. The highest efficiency of hydrogenation of the double bond in the substrate 1 was observed in the cultures of Saccharomyces cerevisiae KCh 464 and Yarrowia lipolytica KCh 71 strains. The substrate was converted into the product with > 99% conversion just in six hours after biotransformation started. The compound containing the sulfur atom in its structure was most effectively transformed by the Yarrowia lipolytica KCh 71 culture strain (conversion > 99%, obtained after three hours of substrate incubation). Also, we observed that, different strains of tested yeasts are able to carry out the bioreduction of the used substrate with different yields, depending on the presence of induced and constitutive ene reductases in their cells. The biggest advantage of this process is the efficient production of one product, practically without the formation of side products.
1. Introduction
In recent years, there has been growing interest from the food industry in sweeteners. Within this group of substances, dihydrochalcones are increasingly gaining attention. The increase in interest is due to the fact that dihydrochalcones are synthesized by plant cells, and they are a daily part of our diet [,]. They are present in citrus, apples, tomatoes, potatoes, bean sprouts, and other plants []. Dihydrochalcones have a wide spectrum of activity, such as antiviral (the ability to inhibit dengue virus proteases or herpes simplex virus), and anti-inflammatory and antioxidant activity [,,,]. They are also known for their activity against pathogenic microorganisms, including gram-positive and gram-negative bacteria, as well as fungi [] and antimalarial or anti-tuberculosis activity []. Dihydrochalcone (phlorizin) is an active inhibitor of fungal tyrosinase [,]. Dihydrochalcones are also used in chemical synthesis to obtain biologically active compounds. 2′-Hydroxydihydrochalcon is used as a building block in the synthesis of propafenone—the active substance of anti-arrhythmic drugs [,,].
Schallenberger’s hypothesis explains the sensation of sweet taste, according to which the flavor substance and the receptor create contact by hydrogen bonding []. This is due to the proton donor groups (AH) and an electron donor (B), which are also present in taste receptors and the structure of sweet substance. The sweetness of dihydrochalcones is related to their structure. The most important element of the structure of these compounds is the substitution of the B-ring in the meta or para position with at least one -OH group. The compounds that have three adjacent substituents on the B-ring do not have a sweet taste []. It is suspected that the structure of the B-ring also affects the perception of non-sweet aftertaste in dihydrochalcones, which do not always correspond to human preferences []. Commonly used in the food industry, neohesperidin dihydrochalcone is characterized by a licorice flavor and a cool feeling on the tongue []. There is also a group of dihydrochalcone analogs, that have a heteroatom in the B-ring and having non-sweet flavors []. This group also describes 3-(2”-furyl)-1-(2’-hydroxyphenyl)-propan-1-one (2), which, depending on the concentration, may have different taste sensations: A concentration of 0.01 ppm does not show any flavor properties; 1 ppm, soapy; umami, bitter; 10 ppm, licorice, soapy, slightly sweet, bitter, vegetable, terpenic; a particularly interesting impression is noted in higher concentrations – 100 ppm, bitter, licorice, light-sweet, celery, umami, and broth [].
A method for obtaining 3-(2”-furyl)-1-(2’-hydroxyphenyl)-propan-1-one (2) with a conversion of 75%, as a result of the chemical synthesis of 2’-hydroxyacetophenone and furfuryl alcohol with NaOH, in the presence of an iridium catalyst, has been identified in []. The direct chemical hydrogenation of chalcones to dihydrochalcones requires the use of metal salts (complexes) of iridium, palladium, ruthenium, or nickel, and the entire reaction (due to its high flammability) must be conducted under strictly controlled conditions [,,,]. There are no reports in the literature regarding the use of biotechnological methods to obtain both, 3-(2”-furyl)- (2) as well 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-propan-1-one (4).
The majority of living organisms are able to hydrogenate the double bond [,]. Yeasts possess their specific enzymes, capable of hydrogenating chalcones [,,]. Such activity is also described in relation to other microorganisms, including bacteria, such as Gordonia sp. and Rhodococcus sp. [], the entomopathogenic fungus, Aspergillus flavus [], and the cyanobacterium, Spirulina platensis []. The reduction of activated alkenes by ente reductases [EC 1.3.1.31], which are flavoproteins from the old yellow enzyme (OYE) family, has been investigated in great detail [,,]. The substrates in these reactions are predominantly hydroxy and methoxy derivatives of chalcones. In this study, we aimed to assess whether the heteroatom-containing substrate in the B-ring would also be accepted by the double-binding dehydrogenase, present in the tested biocatalysts.
The main aim of the study was to assess the capability of yeast strains for the biotransformation of a compound, containing a furan (1) and thiophene (3) substituent. Yeast strains, used in this experiment, are the subject of many years of research conducted in the Department of Chemistry of Wrocław University of Environmental and Life Sciences, regarding their catalytic capabilities [,,], and have been selected as having a high ability in reduceing the double bond in the chalcone and its derivatives [,,]. An additional goal was to optimize the biotechnological production of dihydrochalcone (2, 4), which would enable the development of the method of obtaining the product on an increased scale.
2. Results and Discussion
Each of the eight tested microorganisms (Rhodotorula rubra KCh 4, Yarrowia lipolytica KCh 71, Rhodotorula marina KCh 77, Rhodotorula rubra KCh 82, Candida viswanathii KCh 120, Rhodotorula glutinis KCh 242, Saccharomyces cerevisiae KCh 464, Candida parapsilosis KCh 909) was able to transform both substrates into the expected products, but the efficiency of the described process differs significantly between the strains (Table 1 and Figure 1). High regioselectivity of the biocatalysts’ ability to reduce the double bond was observed, which was described before on the example of other compounds, e.g., chalcone containing no substituents and 2′-hydroxychalcone [,,].

Table 1.
Relationship between transformation of substrates 1 and 3 [%] and time in the cultures of tested strains.
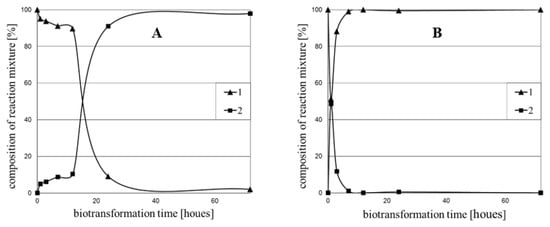
Figure 1.
Time dependence of the transformation of chalcone (1) in the culture of: (A) Rhodotorula rubra KCh 4; (B) Yarrowia lipolytica KCh 71.
In the experiment, 3-(2”-furyl)- (1) and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3) were obtained by chemical synthesis, and then converted to 3-(2”-furyl)- (2) and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-propan-1-one (4) using biotransformation methods on a semi-preparative scale (Figure 2 and Figure 3). The obtained products 1–4 were purified (isolated yield > 70%) and their structures were determined on the basis of NMR analysis (1H-NMR, 13C-NMR and correlation spectra – HMBC; HMQC, COSY) as well as by gas chromatography (GC) and thin-layer chromatography (TLC) analysis (Supplementary Materials).
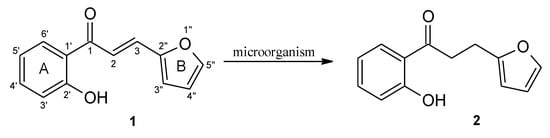
Figure 2.
Biotransformation of 3-(2”-furyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (1) by selected yeast cultures. The ring designations and the numbering of carbon atoms have been placed on compound 1.

Figure 3.
Biotransformation of 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3) by selected yeast cultures.
The reduction of the double bond was the most efficient in the Saccharomyces cerevisiae KCh 464 strain, where over 99% of the product 2 was observed in the reaction medium after six-hour substrate incubation (Table 2). On the first day of the reaction, the four tested microorganisms exceeded the threshold of 90% of the substrate 1 conversion: R. rubra KCh 4, R. rubra KCh 82, Y. lipolytica KCh 71, R. marina KCh 77. In the culture of C. viswanathii KCh 120, the 90% limit was exceeded after three days of the biotransformation process. A surprisingly low conversion was observed for the strains R. glutinis KCh 242 and C. parapsilosis KCh 909 (Table 1). High hydrogenation of the double bond in the cultures of these strains was observed for chalcone and its methoxy derivatives [,].

Table 2.
Transformation of the substrate 1 by selected strains.
Substrate 3 was most effectively transformed by the Y. lipolytica KCh 71 strain. The conversion above 99% was recorded after the third hour of biotransformation. A high efficiency of reduction of the double bond of chalcone derivatives was also confirmed for Saccharomyces cerevisiae KCh 464 strain (conversion > 98% after three hours of transformation). For most of the tested strains, a lower conversion of the substrate, containing thiophene (3) in its, compared to the furan-containing substrate (1), was observed. Interestingly, the strain Rhodotorula rubra KCh 4 after twelve hours showed only 10% of substrate 1 conversion, and after 24h it was already 91% (Figure 1), which may indicate that ene reductases are not present in the cell, but their production begins as a response to the stimulus (substrate induction) from the environment. An analogous induction of dehydrogenases (ene reductases) was observed for the strain C. viswanathii KCh 120. In the culture of this strain, substrate 3 was converted faster. However, conversion above 98% was achieved for both substrates at the same time in three days. Based on the obtained results, it can be concluded that the constitutive enzyme is present in Y. lipolytica KCh 71 and S. cerevisiae KCh 464 strains (observations for both substrates 1 and 3). Biotransformations with these microorganisms show the fastest conversion progress, compared to other used microorganisms for both substrate 1 and 3 (Table 1, Table 2 and Table 3). Similar observations were obtained during the biotransformation of chalcone and described earlier [].

Table 3.
Transformation of the substrate 3 by selected strains.
The strains of the species Saccharomyces cerevisiae are widely described as biocatalysts, effectively reducing the double bond in various compounds – derivatives of chalcones [], containing methyl, methoxy, hydroxy substituents [], and even bromine or chlorine [,], in both A and B-rings. On the basis of the results obtained in this study, it can be concluded that a chalcone derivative, containing a heteroatom in the B-ring, is effectively transformed by this strain. In previous studies during biotransformation of chalcones, in addition to the hydrogenation product, a carbonyl reduction product was also observed [,,,]. In this study, additional products were not observed in any of the reactions carried out for the test compounds.
Biotransformation
The obtained product 3-(2”-furyl)-1-(2’-hydroxyphenyl)-propan-1-one (2) was characterized by the following NMR spectrum: 1H-NMR (600 MHz) (CDCl3) δ (ppm): 3.07-3.12 (m, 2H, H-3), 3.34-3.41 (m, 2H, H-2), 6.06 (dq, 1H, J = 3.2, 0.8 Hz, H-3”), 6.29 (dd, 1H, J = 3.1, 1.9 Hz, H-4”), 6.90 (ddd, 1H, J = 8.2, 7.1, 1.2 Hz, H-5’), 7.02 (dd, 1H, J = 8.4, 0.9 Hz, H-3’), 7.32 (dd, 1H, J = 1.8, 0.8 Hz, H-5”), 7.49 (ddd, 1H, J = 8.5, 7.2, 1.6 Hz, H-4’), 7.92 (dd, 1H, J = 8.0, 1.6 Hz, H-6’), 12.23 (s, 1H, -OH).
13C-NMR (151 MHz, CDCl3) δ = 22.50 (C-3), 36.73 (C-2), 105.69 (C-3”), 110.44 (C-4”), 118.72 (C-3’), 119.12 (C-5’), 119.40 (C-1’), 129.94 (C-6’), 136.56 (C-4’), 141.41 (C-5’’), 154.33 (C-2”), 162.56 (C-2’), 204.90 (C-1).
The obtained product 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-propane-1-one (4) was characterized by the following NMR spectrum: 1H-NMR (600 MHz) (CDCl3) δ (ppm): 3.27-3.33 (m, 2H, H-3), 3.37-3.43 (m, 2H, H-2), 6.87 (dq, 1H, J = 3.4, 1.0 Hz, H-3”), 6.90 (ddd, 1H, J = 7.4, 7.1, 1.0 Hz, H-5’), 6.29 (dd, 1H, J = 5.1, 3.4 Hz, H-4”), 6.99 (ddd, 1H, J = 8.4, 1.1, 0.4 Hz, H-3’), 7.14 (dd, 1H, J = 5.1, 1.2 Hz, H-5”), 7.47 (ddd, 1H, J = 8.4, 7.3, 1.5 Hz, H-4’), 7.76 (dd, 1H, J = 8.1, 1.6 Hz, H-6’), 12.24 (s, 1H, -OH).
13C-NMR (151 MHz, CDCl3) δ = 24.16 (C-3), 40.26 (C-2), 118.73 (C-3’), 119.13 (C-5’), 119.38 (C-1’), 123.72 (C-5”), 124.99 (C-3”), 127.06 (C-4”), 129.90 (C-6’), 136.60 (C-4’), 143.40 (C-2”), 162.56 (C-2’), 204.76 (C-1).
3. Materials and Methods
3.1. Substrate
The substrates, used for biotransformation, were obtained by Claisen-Shmidt condensation reaction of 2-hydroxyacetophenone (5) with furfural (6) or aldehyde 7 [purchased from Sigma-Aldrich (St. Louis, MO, USA)] dissolved in methanol in an alkaline environment at high temperature (Figure 4 and Figure 5) according to the procedure described previously [,]. The resulting 3-(2”-furyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (1) and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3) were used as a substrates for the biotransformation.
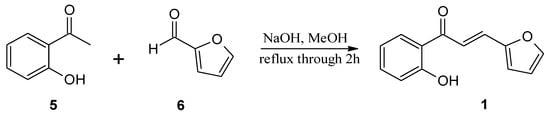
Figure 4.
Synthesis of 3-(2”-furyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (1).
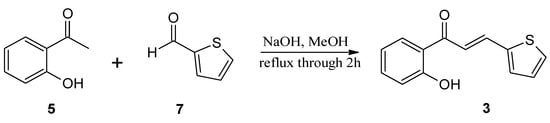
Figure 5.
Synthesis of 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3).
The resulting compound (1) was characterized by the following NMR spectral data:
1H-NMR (600 MHz) (CDCl3) δ (ppm): 6.54 (dd, 1H, J = 3.4, 1.8 Hz, H-3”), 6.77 (d, 1H, J = 3.4 Hz, H-4”), 6.93 (ddd, 1H, J = 8.2, 7.2, 1.1 Hz, H-5’), 7.02 (dd, 1H, J = 8.4, 0.9 Hz, H-3’), 7.49 (ddd, 1H, J = 8.6, 7.2, 1.6 Hz, H-4’), 7.55 (d, 1H, J = 15.1 Hz, H-2), 7.56 (d, 1H, J = 1.4 Hz, H-5”), 7.68 (d, 1H, J = 15.2 Hz, H-3), 7.92 (dd, 1H, J = 8.1, 1.6 Hz, H-6’), 12.89 (s, 1H, -OH).
13C-NMR (151 MHz, CDCl3) δ = 113.03 (C-4”), 117.29 (C-3”), 117.73 (C-2), 118.66 (C-3’), 118.98 (C-5’), 120.17 (C-1’), 129.77 (C-6’), 131.26 (C-3), 136.44 (C-4’), 145.55 (C-5”), 151.65 (C-2”), 163.66 (C-2’), 193.46 (C-1).
The resulting compound (3) was characterized by the following NMR spectral data:
1H-NMR (600 MHz) (CDCl3) δ (ppm): 6.95 (ddd, 1H, J = 8.1, 7.1, 1.1 Hz, H-5’), 7.02 (dd, 1H, J = 8.4, 1.2 Hz, H-3’), 7.12 (ddd, 1H, J = J = 5.0, 3.7, 0,3 Hz, H-4”), 7.41 (d, 1H, J = 3.6, Hz, H-3”), 7.44 (d, 1H, J = 15.2 Hz, H-2), 7.47 (d, 1H, J = 4.9 Hz, H-5”), 7.49 (ddd, 1H, J = 8.4, 7.2, 1.5 Hz, H-4’), 7.89 (dd, 1H, J = 8.1, 1.6 Hz, H-6’), 8.05 (dd, 1H, J = 15.2, 0,5 Hz, H-3), 12.85 (s, 1H, -OH).
13C-NMR (151 MHz, CDCl3) δ (ppm) = 118.74 (C-3’), 118.95 (C-2), 118.99 (C-5’), 120.06 (C-1’), 128.66 (C-4”), 129.65 (C-6’), 129.68 (C-5”), 132.89 (C-3”), 136.49 (C-4’), 138.00 (C-3), 140.30 (C-2”), 163.69 (C-2’), 193.27 (C-1).
3.2. Microorganisms
The studies were carried out on eight strains of yeasts of the species Rhodotorula rubra (KCh 4 and KCh 82), Rhodotorula marina (KCh 77), Rhodotorula glutinis (KCh 242), Yarrowia lipolytica (KCh 71), Candida viswanathii (KCh 120), Saccharomyces cerevisiae (KCh 464), and Candida parapsilosis (KCh 909), which have already been described [,], and come from the collection of the Department of Chemistry of Wrocław University of Environmental and Life Sciences, Poland. All the strains were cultivated on a Sabouraud agar consisting of aminobac (5 g), glucose (40 g) and agar (15 g) dissolved in 1 L of distilled water and pH 5.5 and stored in a fridge at 4 °C.
3.3. Analysis
Initial tests were carried out using TLC plates (SiO2, DC Alufolien Kieselgel 60 F254 (0.2 mm thick), Merck, Darmstadt, Germany). The product separation on a plate in cyclohexane is Ethyl acetate eluent (9:1 v/v). The product was observed (without additional visualization) under the UV lamp for the wavelength of 254 nm.
3.4. Gas Chromatography (GC)
GC analysis were performed using an Agilent 7890A gas chromatograph, equipped with a flame ionization detector (FID) (Agilent, Santa Clara, CA, USA). The capillary column DB-5HT (30 m × 0.25 mm × 0.10 µm) was used to determine the composition of the product mixtures. A temperature program was applied as follows: 80–300 °C, the temperature on the detector: 300 °C, injection 1 µl, flow 1 mL/min, flow H2: 35 mL/min, air flow; 300 mL/min, time of analysis; 18.67 min. The retention time of the substrate 1—9,6 min, product 2 retention time—8,5 min. The retention times for compounds having the thiophene substituent 3 and 4 were recorded as 10,8 min, and 9,7 min, respectively (Supplementary Materials).
3.5. NMR Analysis
NMR analysis was performed using a DRX 600 MHz Bruker spectrometer (Bruker, Billerica, MA, USA). The prepared samples were dissolved in deuterated chloroform CDCL3. The analyses performed, included 1H-NMR, 13C-NMR, HMBC (two-dimensional analysis) HMQC (heteronuclear correlation) and COSY (correlation spectroscopy) (Supplementary Materials).
3.6. Screening
Erlenmeyer flasks with a capacity of 300 mL were used for biotransformation; each contained 100 ml of the culture medium – Sabouraud (3% glucose, 1% peptone). The transplanted microorganisms were incubated for three days at 24 °C on a rotary shaker (144 rpm). After this time, the substrates 1 and 3 were added in an amount of 10 mg, dissolved in 1 mL of DMSO (dimethyl sulfoxide). Samples were collected after 1, 3, 6, 12 hours and 1, 3, and 7 day of incubation. After this time, samples were extracted with ethyl acetate, dried with anhydrous magnesium sulfate (MgSO4), and analyzed by TLC and GC.
3.7. Semi-Preparative Scale
Semi-preparative biotransformations were performed in 2 L Erlenmeyer flasks, each containing 500 mL of culture medium (3% glucose, 1% peptone). The transferred microorganisms were incubated for three days at 24 °C on a rotary shaker. After this time, the substrate was added in 100 mg, dissolved in 2 mL of DMSO. After ten days, the product in the mixture was isolated by triple extraction with ethyl acetate (3 extractions of 300 mL). After drying with anhydrous magnesium sulfate, and concentrating the sample by using a rotary evaporator, the obtained product was analyzed by GC and NMR.
The biotransformation product was separated on preparative TLC plates (1000 µm, silica gel plates (Anatech, Gehrden, Germany) with cyclohexane: Ethyl acetate eluent (9:1 v/v). After separation, the product was scraped from the plate, extracted twice from the gel with ethyl acetate, dried with magnesium sulfate and concentrated by a rotary vacuum evaporator. A one-day transformation of 3-(2”-furyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (1) (100mg) in Y. lipolytica KCh 71 gave 72 mg (colorless oil) of 3-(2”-furyl)-1-(2’-hydroxyphenyl)-propan-1-one (2). One-day transformation of 3-(2”-furyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3) (100mg) in the same strain culture yielded 76 mg (pale yellow oil) of 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-propan-1-one (4). The resulting products were then analyzed by NMR spectroscopy.
4. Conclusions
There is known catalytic activity of ene reductases present in many yeast species, consisting in the hydrogenation of chalcone to dihydrochalcone. In this study, 3-(2”-furyl)- (1) and 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-prop-2-en-1-one (3) were biotransformed using eight yeast strains. The purpose was to test the microorganisms’ ability to selectively hydrogenate the double bond in chalcone containing heteroatom, in one of the rings, and select the most efficiently transforming strain. A significant factor in this type of reaction is not only its duration and the efficiency with which the substrate is transformed, but also the lack of by-products. Out of the available microorganisms, the fastest conversions of substrate 1 to the expected product were observed in Saccharomyces cerevisiae KCh 464 and Y. lipolytica KCh 71 strains. Product 2 was observed following the first hour of biotransformation (constitutive enzyme), and the conversion efficiency >99% was recorded as early as six hours after the start of the biotransformation. High conversion of substrate 1, higher than 90% after 24 hours of biotransformation, was also observed in the R. rubra KCh 4 culture. However, when analyzing the composition of the reaction mixture in twelve hours, the observed substrate conversion did not exceed 10%. This biotransformation pattern indicates that it is probably the result of substrate-induced ene reductases. Comparing these data to the conversion of 2-hydroxychalcone to dihydrochalcone, as previously described in [], where the above-mentioned strains were also tested, we observed that the presence of the heteroatom in the B-ring practically does not affect the transformation efficiency—after 72 hours in the case of KCh 71 and KCh 464 strains; however, it is much more efficient in the case of the strain KCh 4. Substrate 3, which is composed of a sulfur atom in ring B, was analogously converted by the tested biocatalysts in the same way as substrate 1. Substrate 3 was most effectively transformed by Y. lipolytica KCh 71 strain culture. During this biotransformation, 3-(2”-thienyl)-1-(2’-hydroxyphenyl)-propan-1-one (4) was obtained in 99% yield after the third hour of transformation.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
Formal analysis, M.L., M.K. and T.J.; investigation, M.L. and M.K.; methodology, M.L., T.J. and E.K.-S.; project administration, M.L. and T.J.; resources, T.J.; supervision, T.J. and E.K.; visualization, M.L.; writing-original draft, M.L.; writing-review and editing, M.L. and T.J.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winnig, M.; Bufe, B.; A Kratochwil, N.; Slack, J.P.; Meyerhof, W. The binding site for neohesperidin dihydrochalcone at the human sweet taste receptor. BMC Struct. Boil. 2007, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Krammer, G.; Ley, J.; Riess, T.; Haug, M.; Paetz, S.; Kindel, G.; Schmidtmann, R. Use of 4-hydroxydihydrochalcons and their salts for enhancing an impression of sweetness. U.S. Patent 9,445,606, 20 September 2016. [Google Scholar]
- Orlíková, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, Y.; Chen, X.; Wang, Y.; Chen, W.; Xu, Q.; Li, P.; Ma, F. Extraction, identification, and antioxidant and anticancer tests of seven dihydrochalcones from Malus ‘Red Splendor’ fruit. Food Chem. 2017, 231, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radical Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Corrêa, M.J.C.; Nunes, F.M.; Bitencourt, H.R.; Borges, F.C.; Guilhon, G.M.S.P.; Arruda, M.S.P.; Marinho, A.M.R.; Santos, A.S.; Alves, C.N.; Brasil, D.S.B.; et al. Biotransformation of chalcones by the endophytic fungus Aspergillus flavus isolated from Paspalum maritimum trin. J. Braz. Chem. Soc. 2011, 22, 1333–1338. [Google Scholar] [CrossRef]
- Gall, M.; Thomsen, M.; Peters, C.; Pavlidis, I.V.; Jonczyk, P.; Grünert, P.P.; Beutel, S.; Scheper, T.; Gross, E.; Backes, M.; et al. Enzymatic conversion of flavonoids using bacterial chalcone isomerase and enoate reductase. Angew. Chem. Int. Ed. 2014, 53, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Awouafack, M.D.; Kusari, S.; Lamshöft, M.; Ngamga, D.; Tane, P.; Spiteller, M. Semi-synthesis of dihydrochalcone derivatives and their in vitro antimicrobial activities. Planta Med. 2010, 76, 640–643. [Google Scholar] [CrossRef][Green Version]
- Silva, V.D.; Stambuk, B.U.; Nascimento, M.D.G. Efficient chemoselective biohydrogenation of 1,3-diaryl-2-propen-1-ones catalyzed by Saccharomyces cerevisiae yeasts in biphasic system. J. Mol. Catal. B: Enzym. 2010, 63, 157–163. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Yang, X.-W.; Zhang, Y.-B.; Zhai, Y.-Y.; Xu, W.; Zhao, B.; Liu, D.-L.; Yu, H.-J. Biotransformation of phlorizin by human intestinal flora and inhibition of biotransformation products on tyrosinase activity. Food Chem. 2012, 132, 936–942. [Google Scholar] [CrossRef]
- Noe, C.R.; Knollmiiller, M.; Oberhauser, B.; Steinbauer, G. Eine Methode zur Bestimmung der Absolutkonfiguration chiraler a-hydroxysubstituierter Nitrile, Alkine und Aldehyde. Chem. Ber. 1986, 119, 729–743. [Google Scholar] [CrossRef]
- Ecker, G.; Chiba, P.; Hitzler, M.; Schmid, D.; Visser, K.; Cordes, H.P.; Csöllei, J.; Seydel, J.K.; Schaper, K.-J. Structure−Activity Relationship Studies on Benzofuran Analogs of Propafenone-Type Modulators of Tumor Cell Multidrug Resistance. J. Med. Chem. 1996, 39, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Ecker, G.; Noe, C.R.; Fleischhacker, W. Improved synthesis of the enantiomers of propafenone using chiral building blocks. Chem. Monthly 1997, 128, 53–59. [Google Scholar] [CrossRef]
- Shallenberger, R.S. Hydrogen Bonding and the Varying Sweetness of the Sugars. J. Food Sci. 1963, 28, 584–589. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer-Verlag: Berlin, Germany, 2009. [Google Scholar]
- Whitelaw, M.L.; Chung, H.-J.; Daniel, J.R. Synthesis and sensory evaluation of ring-substituted dihydrochalcone sweetener. J. Agric. Food Chem. 1991, 39, 663–667. [Google Scholar] [CrossRef]
- Portmann, M.-O.; Kilcast, D. Psychophysical characterization of new sweeteners of commercial importance for the EC food industry. Food Chem. 1996, 56, 291–302. [Google Scholar] [CrossRef]
- Hunter, J.; Rice, S.; Lowe, R.; Pask, C.M.; Warriner, S.; Sridharan, V. Iridium catalyzed alkylation of 2′-hydroxyacetophenone with alcohols under thermal or microwave conditions. Tetrahedron Lett. 2017, 58, 4400–4402. [Google Scholar] [CrossRef]
- Chen, P.; Li, W.; Wang, Y. Atmospheric hydrogenation of α, β-unsaturated ketones catalyzed by highly efficient and recyclable Pd nanocatalyst. Catal. Commun. 2019, 125, 10–14. [Google Scholar] [CrossRef]
- Stompor, M.; Kałużny, M.; Żarowska, B. Biotechnological methods for chalcone reduction using whole cells of Lactobacillus, Rhodococcus and Rhodotorula strains as a way to produce new derivatives. Appl. Microbiol. Biotechnol. 2016, 100, 8371–8384. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.-F. ChemInform Abstract: Ruthenium-Catalyzed Conjugate Hydrogenation of α,β-Enones by in situ Generated Dihydrogen from Paraformaldehyde and Water. Eur. J. Org. Chem. 2015, 2015, 331–335. [Google Scholar] [CrossRef]
- Chen, S.; Lu, G.; Cai, C. A base-controlled chemoselective transfer hydrogenation of α,β-unsaturated ketones catalyzed by [IrCp*Cl2]2 with 2-propanol. RSC Adv. 2015, 5, 13208–13211. [Google Scholar] [CrossRef]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.D.C.; Pinto, D.C.G.A.; Pinto, D.C.G.A. Chalcone: A Valuable Scaffold Upgrading by Green Methods. ACS Sustain. Chem. Eng. 2017, 5, 7467–7480. [Google Scholar] [CrossRef]
- Winkler, C.K.; Faber, K.; Hall, M. Biocatalytic reduction of activated C C-bonds and beyond: emerging trends. Curr. Opin. Chem. Boil. 2018, 43, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Romano, D.; Amaretti, A.; Molinari, F.; Rossi, M. Enoate reductases from non conventional yeasts: Bioconversion, cloning, and functional expression in Saccharomyces cerevisiae. J. Biotechnol. 2011, 156, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, T.; Dymarska, M.; Siepka, M.; Gniłka, R.; Leśniak, A.; Popłoński, J.; Kostrzewa-Susłow, E. Enantioselective reduction of flavanone and oxidation of cis- and trans-flavan-4-ol by selected yeast cultures. J. Mol. Catal. B: Enzym. 2014, 109, 47–52. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Microbial transformations of 4′-methylchalcones as an efficient method of obtaining novel alcohol and dihydrochalcone derivatives with antimicrobial activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef]
- Żyszka-Haberecht, B.; Poliwoda, A.; Lipok, J. Biocatalytic hydrogenation of the C=C bond in the enone unit of hydroxylated chalcones—process arising from cyanobacterial adaptations. Appl. Microbiol. Biotechnol. 2018, 102, 7097–7111. [Google Scholar] [CrossRef]
- Hall, M.; Stueckler, C.; Hauer, B.; Stuermer, R.; Friedrich, T.; Breuer, M.; Kroutil, W.; Faber, K. Asymmetric Bioreduction of Activated C=C Bonds UsingZymomonas mobilis NCR Enoate Reductase and Old Yellow Enzymes OYE 1–3 from Yeasts. Eur. J. Org. Chem. 2008, 2008, 1511–1516. [Google Scholar] [CrossRef]
- Kohli, R.M.; Massey, V. The oxidative half-reaction of Old Yellow Enzyme. J. Biol. Chem. 1998, 273, 32763–32770. [Google Scholar] [CrossRef]
- Karplus, P.A.; Fox, K.M.; Massey, V. Flavoprotein structure and mechanism. 8. Structure-function relations for old yellow enzyme. FASEB J. 1995, 9, 1518–1526. [Google Scholar] [CrossRef]
- Janeczko, T.; Gładkowski, W.; Kostrzewa-Susłow, E. Microbial transformations of chalcones to produce food sweetener derivatives. J. Mol. Catal. B: Enzym. 2013, 98, 55–61. [Google Scholar] [CrossRef]
- Janeczko, T.; Kostrzewa-Susłow, E. Enantioselective reduction of propiophenone formed from 3-chloropropiophenone and stereoinversion of the resulting alcohols in selected yeast cultures. Tetrahedron Asymmetry 2014, 25, 1264–1269. [Google Scholar] [CrossRef]
- Łużny, M.; Kozłowska, E.; Dymarska, M.; Kostrzewa-Susłow, E.; Janeczko, T. Sposób wytwarzania 1-(2’-hydrokyfenylo)-3-(3”-metoksyfenylo)-propan-1-onu. P.426760. 23 August 2018. [Google Scholar]
- Łużny, M.; Kozłowska, E.; Popłoński, J.; Dymarska, M.; Kostrzewa-Susłow, E.; Janeczko, T. Sposób wytwarzania 1-(2’-hydrokyfenylo)-3-(4”-metoksyfenylo)-propan-1-onu. P.426763. 23 August 2018. [Google Scholar]
- Łużny, M.; Kozłowska, E.; Dymarska, M.; Kostrzewa-Susłow, E.; Janeczko, T. Sposób wytwarzania 1-(2’-hydrokyfenylo)-3-(4”-metoksyfenylo)-propan-1-onu. P.426770. 23 August 2018. [Google Scholar]
- Kostrzewa-Susłow, E.; Dymarska, M.; Guzik, U.; Wojcieszyńska, D.; Janeczko, T. Stenotrophomonas maltophilia: A Gram-Negative Bacterium Useful for Transformations of Flavanone and Chalcone. Molecules 2017, 22, 1830. [Google Scholar] [CrossRef] [PubMed]
- De Matos, I.L.; Nitschke, M.; Porto, A.L.M. Hydrogenation of Halogenated 2′-Hydroxychalcones by Mycelia of Marine-Derived Fungus Penicillium raistrickii. Mar. Biotechnol. 2019, 21, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Żyszka, B.; Anioł, M.; Lipok, J. Highly effective, regiospecific reduction of chalcone by cyanobacteria leads to the formation of dihydrochalcone: two steps towards natural sweetness. Microb. Cell Factories 2017, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, T.; Popłoński, J.; Kozłowska, E.; Dymarska, M.; Huszcza, E.; Kostrzewa-Susłow, E. Application of α- and β-naphthoflavones as monooxygenase inhibitors of Absidia coerulea KCh 93, Syncephalastrum racemosum KCh 105 and Chaetomium sp. KCh 6651 in transformation of 17α-methyltestosterone. Bioorganic Chem. 2018, 78, 178–184. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).