Abstract
This study was designed to explore the in vitro anticancer effects of the bioactive compounds isolated from Ricinodendron heudelotii on selected cancer cell lines. The leaves of the plant were extracted with ethanol and partitioned in sequence with petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate fraction was phytochemically studied using thin layer chromatography (TLC) and column chromatography (CC). Structural elucidation of pure compounds obtained from the ethyl acetate fraction was done using mass spectra, 1H-NMR, and 13C-NMR analysis. The isolated compounds were subsequently screened using five different cancer cell lines: HL-60, SMMC-7721, A-549, MCF-7, SW-480, and normal lung epithelial cell line, BEAS-2B, to assess their cytotoxic effects. Nine compounds were isolated and structurally elucidated as gallic acid, gallic acid ethyl ester, corilagin, quercetin-3-O-rhamnoside, myricetin-3-O-rhamnoside, 1,4,6-tri-O-galloyl glucose, 3,4,6-tri-O-galloyl glucose, 1,2,6-tri-O-galloyl glucose, and 4,6-di-O-galloyl glucose. Corilagin exhibited the most cytotoxic activity with an IC50 value of 33.18 μg/mL against MCF-7 cells, which were comparable to cisplatin with an IC50 value of 27.43 µg/mL. The result suggests that corilagin isolated from R. heudelotii has the potential to be developed as an effective therapeutic agent against the growth of breast cancer cells.
1. Introduction
Cancer is a major cause of death across the globe, affecting all age groups [1]. The World Health Organization has predicted that cancer cases will increase from 14 million in 2012 to 22 million in the next four decades [2]. It has caused a great sense of burden both to individual lives and the society at large [3]. The absolute cure of cancer remains a big challenge despite available anticancer agents, awareness campaigns, and prevention strategies. Breast and prostate cancer have the highest incidence in women and men, respectively [4]. It is therefore pertinent to keep exploring for novel chemotherapeutic agents that can evade drug-resistant cancer cells. Natural or natural based anti-cancer drugs, such as vincristine, paclitaxel, bleomycin, and others, have been clinically used over the years [5]. Ricinodendron heudelotii (family: Euphorbiceae) plant parts have been used in traditional medicine to treat cough, stomach ailments, and pain related to child birth. Decoctions and infusions are mostly prepared from the leaves and stem bark. Traditional practitioners prescribe it for women suffering from miscarriages [6]. Phytochemical screening of the leaf extract revealed the presence of tannins, terpenoids, glycosides, and alkaloids, and it exhibited antimicrobial effects [7]. Two dinorditerpenoids, 1,2-dihydroheudelotinol and heudelotinone, and three other compounds, lupeol, E-ferullic acid octacosylate, and 3-methylmethylorsellinate, have been isolated from the stem bark and roots of R. heudelotii [8]. Seven new tetracyclic triterpenoids ricinodols with potential as 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors has also been reported to be isolated from R. heudelotii [9]. Yakubu et al. [10] also reported the leaf extract to have regulatory effects on artemisinin toxicity when co-administered with artemisinin. This study aims to identify the chemical structure of isolated bioactive compounds in R. heudelotii as well as evaluate the compoundsfor in vitro cytotoxic activity against human leukemia (HL-60), human hepatocellular carcinoma (SMMC-7721), human lung adenocarcinoma (A549), human breast cancer cell (MCF-7), and colon adenocarcinoma (SW480) cell lines.
2. Results
Nine compounds were isolated from the ethyl acetate fraction of R. heudelotii by employing column chromatography, including Sephadex LH-20 and MCI CHP20P gel.
2.1. Structural Elucidation of Isolated Compounds
Structure elucidation of the isolated compounds was carried out using spectroscopic techniques: 1H- (600 MHz) and 13C-NMR (150 MHz) and DEPT along with 2D-NMR for some compounds (Figure 1). Compound 1 was obtained as an off-white amorphous powder, showing a molecular ion peak at m/z 170 [M]− in the negative ESI-MS. The molecular formula, C7H6O5, was deduced from the combination of 1H-, 13C-NMR and DEPT spectroscopic data. Compound 1 was identified as gallic acid when the data was compared with those in the literature [11,12]. Compound 2, an off-white powder, showed a quasi-molecular ion peak at m/z 198 [M]− in the negative ESI-MS. Combining with the 1H-, 13C-NMR and DEPT spectroscopic data, a molecular formula, C9H10O5, was deduced. Comparing the data with those in the literature, compound 2 was identified as gallic acid ethyl ester [13]. Compound 3 was obtained as a yellow amorphous powder, showing a molecular ion peak at m/z 633 [M]− in the negative ESI-MS. From 1H-, 13C-NMR, and DEPT spectroscopic data, the molecular formula of C27H22O18 was deduced. Comparing the data with those in the literature, compound 3 was identified as corilagin [14]. Compound 4 was obtained as a yellow amorphous powder. It showed a molecular ion peak at m/z 447 [M − H]− in the negative ESI-MS while the molecular formula of C21H20O11, was deduced from the 1H-, 13C-NMR, and DEPT spectroscopic data. Likewise, by comparing the data with those in the literature, compound 4 was quercetin-3-O-rhamnoside [15,16]. Compound 5 was obtained as a pale yellow amorphous powder showing a quasi-molecular ion peak at m/z 463 [M − H]− in the negative ESI-MS, and the molecular formula of C21H20O12, was deduced from 1H-, 13C-NMR, and DEPT spectroscopic data. By comparing the data with those in the literature, compound 5 was identified as myricetin-3-O-rhamnoside [12,17]. Compound 6 was obtained as an off white powder, showing a quasi-molecular ion peak at m/z 635 [M − H]− in the negative ESI-MS. Combining with the 1H-, 13C-NMR, and DEPT spectroscopic data, a molecular formula of C27H24O18 was deduced. By comparing the data with those in literature, compound 6 was identified as 1,4,6-tri-O-galloyl-β-d-glucose [18]. Compound 7 was obtained as a white amorphous powder. It showed a molecular ion peak at m/z 636 [M − H]− in the negative ESI-MS. Combining the 1H-, 13C-NMR, and DEPT spectroscopic data, a molecular formula of C27H24O18 was deduced. The data obtained correlates with those in the literature and compound 7 was identified as 3,4,6-tri-O-galloyl-d-glucose [19]. Compound 8, an off-white amorphous powder with a molecular ion peak at m/z 635 [M − H]− in the negative ESI-MS. A molecular formula of C27H24O18, was deduced from the 1H-, 13C-NMR, and DEPT spectroscopic data. Comparing the data with those in the literature, compound 8 was identified as 1,2,6-tri-O-galloyl-β-d-glucose [20,21]. Compound 9 was obtained as a white amorphous powder showing a molecular ion peat at m/z 483 [M − H]− in the negative ESI-MS. A molecular formula was deduced from the 1H-, 13C-NMR, and DEPT spectroscopic data. By comparing the data with those in the literature, compound 9 was identified as 4,6-di-O-galloyl-d-glucose [22,23].
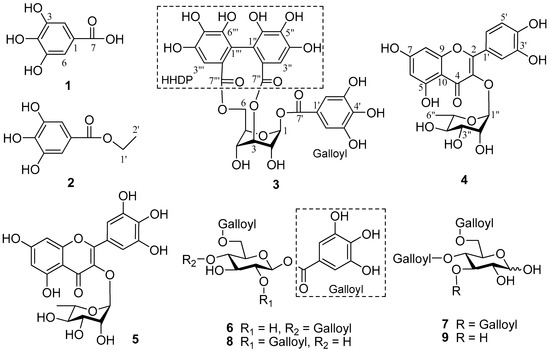
Figure 1.
Structures of compounds (1–9) isolated from Ricinodendron heudelotii.
2.2. Spectroscopic Data of Compounds 1–9
Gallic acid (1): Off-white amorphous powder, C7H6O5, negative ESI-MS m/z: 170 [M]−, 1H-NMR (600 MHz, acetone-d6): δ 7.10 (2H, s, H-2,6), 13C-NMR (150 MHz, acetone-d6): 121.0 (C-1), 109.0 (C-2,6), 145.9 (C-3,5), 138.3 (C-4), 168.0 (C-7).
Gallic acid ethyl ester (2): Off-white amorphous powder, C9H10O5, negative ESI-MS m/z: 198 [M]−, 1H-NMR (600 MHz, acetone-d6): δH 0.84 (3H, t, J = 7.1 Hz, H-2′), 3.82 (2H, q, J = 7.1 Hz, H-1′) 7.05 (2H, s, H-3,5); 13C-NMR (150 MHz, acetone-d6): δC 120.7 (C-1); 109.4 (C-2,6), 138.0 (C-4), 144.9 (C-3,5), 166.8 (C-7), 60.6 (C-1′), 14.1 (C-2′).
Corilagin (3): Off-white amorphous powder, C27H22O18,negative ESI-MS m/z 633 [M − H]−, 1H-NMR (600, CD3OD): δH 7.05 (2H, s, H-2′,6′),6.69, 6.65 (each 1H, s, HHDP-3″,3‴), 6.36 (1H, d, J = 2.4 Hz, H-1), 4.95 (1H, ddd, J = 10.9, 8.0, 1.2 Hz, H-6a), 4.80 (1H, brs, H-3), 4.51 (1H, t, J = 9.6 Hz, H-5), 4.46 (1H, dd, J = 1.7, 1.2 Hz, H-4), 4.16 (1H, dd, J = 8.0, 10.9 Hz, H-6b), 3.98 (1H, brd, J = 1.2 Hz, H-2); 13C-NMR (150 MHz, CD3OD): HHDP δC 167.1 and 167.6 (C=O), 145.8, 145.1 (C-3″,3‴), 144.9, 144.3 (C-5″,5‴), 136.3, 136.2 (C-4″,4‴), 116.2, 116.1 (C-1″,1‴), 124.1, 123.8 (C-2″,2‴), 107.3, 106.7 (C-6″,6‴); galloyl δC 165.2 (C=O), 146.2 (C-3′,C-5′), 139.7 (C-4′), 119.4 (C-1′), 109.7 (C-2′,C-6′); glucose δC 92.9 (C-1), 78.3 (C-3), 76.6 (C-5), 72.4 (C-2), 64.2 (C-6), 62.8 (C-4).
Quercetin-3-O-rhamnoside (4): Yellow amorphous powder, C21H20O11; negative ESI-MS m/z 447 [M − H]−, 1H-NMR (600 MHz, CD3OD): δ 7.33 (1H, d, J = 2.0 Hz, H-2′), 7.31 (1H, dd, J = 2.0, 8.3 Hz, H-6′), 6.91 (1H, d, J = 8.3 Hz, H-5′), 6.19 (1H, s, H-6), 6.36 (1H, s, H-8), 5.34 (1H, s, H-1″), 4.22 (1H, brs, H-2″), 3.76 (1H, dd, J = 3.2, 9.4 Hz, H-3″), 3.34 (1H, dd, J = 7.5, 9.4 Hz, H-4″), 3.41 (1H, m, H-5″), 0.94 (3H, d, J = 6.1 Hz, H-6″); 13C-NMR (150 MHz, CD3OD): δC 18.7 (C-6″), 71.1 (C-5″), 70.6 (C3″), 71.1 (C-2″), 71.2 (C-4″), 94.3 (C-8), 98.7 (C-6), 102.1 (C-1″), 104.1 (C-10), 115.7 (C-2′), 115.8 (C-5′), 120.8 (C-1′), 122.2 (C-6′), 134.3 (C-3), 145.4 (C-3′), 148.8 (C-4′), 156.7 (C-2), 157.3 (C-9), 161.4 (C-5), 165.6 (C-7), 177.8 (C-4).
Myricetin 3-O-rhamnoside (5): Yellow amorphous powder, C21H20O12, negative ESI-MS m/z: 463 [M − H]−, 1H-NMR (600 MHz, CD3OD): δ 6.95 (2H, s, H-2′,6′), 6.36 (1H, d, J = 1.6 Hz, H-6), 6.20 (1H, d, J = 1.6 Hz, H-8), 5.31 (1H, s, H-1″), 3.80 (1H, dd, J = 3.2, 9.5 Hz, H-2″), 3.55 (1H, m, H-3″), 3.17 (1H, m, H-4″), 3.34 (1H, m, H-5″), 0.84 (3H, d, J = 6.1 Hz, H-6″). 13C-NMR (150 MHz, CD3OD): δC 177.6 (C-4), 163.8 (C-7), 161.4 (C-5), 157.1 (C-9), 156.6 (C-2), 145.9 (C-5′, 3′), 135.4 (C-4′), 135.2 (C-3), 119.7 (C-1′), 107.9 (C-2′), 107.9 (C-6′), 104.3 (C-10), 102.4 (C-1″), 98.8 (C-8), 93.7 (C-6), 71.9 (C-4″), 71.3 (C-5″), 70.8 (C-3″), 70.3 (C-2″), 17.8 (C-6″).
1,4,6-Tri-O-galloyl-β-d-glucose (6): Off-white amorphous powder, C27H24O18, negative ESI-MS m/z 635 [M − H]−; 1H-NMR (600 MHz, CD3OD): δH 7.15, 7.10, 7.06 (each 2H, s, galloyl H-2′,6′), 5.78 (1H, d, J = 8.2 Hz, H-1), 5.22 (1H, t, J = 9.6 Hz, H-4), 4.45 (1H, dd, J = 12.4, 2.0 Hz, H-6a), 4.22 (1H, dd, J = 12.4, 4.9 Hz, H-6b), 4.06 (1H, ddd, J = 2.0, 4.9, 9.6 Hz, H-5), 3.84 (1H, dd, J = 9.2, 9.6 Hz, H-3), 3.65 (1H, dd, J = 8.2, 9.2 Hz, H-2).
3,4,6-Tri-O-galloyl-d-glucose (7): Off-white amorphous powder, C27H24O18, negative ESI-MS m/z 635 [M − H]−, 1H-NMR (600 MHz, CD3OD): δH 7.07, 6.99, 6.94 (each 2H, s, galloyl H-2′,6′), 4.42 (1H, m, H-6a), 4.26 (1H, m, H-6b), β-glucose form: δH 4.76 (1/3H, d, J = 7.8 Hz, H-1), 3.56 (1/3H, dd, J = 9.5, 7.8 Hz, H-2), 5.45 (1/3H, t, J = 9.5 Hz, H-3), 5.35 (1/3H, dd, J = 9.5, 9.8 Hz, H-4), 4.07 (1/3H, m, H-5), α-glucose form: δH 5.25 (2/3H, d, J = 3.6 Hz, H-1), 3.81 (2/3H, dd, J = 9.8, 3.6 Hz, H-2), 5.68 (2/3H, t, J = 9.8 Hz, H-3), 5.36 (2/3H, t, J = 9.8 Hz, H-4), 4.47 (2/3H, m, H-5).
1,2,6-Tri-O-galloyl-β-d-glucose (8): Off-white amorphous powder, C27H24O18, negative ESI-MS: m/z 635 [M − H]−, 1H-NMR (600 MHz, CD3OD): δH 7.10, 7.04, 7.00 (each 2H, s, galloyl H-2′,6′), 5.92 (1H, d, J = 8.5 Hz, H-1), 5.23 (1H, dd, J = 8.5, 9.6 Hz, H-2), 4.56 (1H, dd, J = 2.0, 12.2 Hz, H-6a), 4.47 (1H, dd, J = 4.8, 12.2 Hz, H-6b), 3.83 (1H, ddd, J = 2.0, 4.8, 9.6 Hz, H-5), 3.81 (1H, t, J = 9.6 Hz, H-3), 3.66 (1H, t, J = 9.6 Hz, H-4); 13C-NMR (150 MHz, CD3OD): δC 63.1 (C-6), 69.6 (C-4), 72.7 (C-5), 74.4 (C-3), 74.2 (C-2), 93.2 (C-1), 117.0, 119.1, 119.2 (galloyl C-1′), 109.2, 108.8, 108.8 (galloyl C-2′,6′), 145.3, 145.4, 145.8 (galloyl C-3′,5′), 138.6, 138.7, 139.1 (galloyl C-4′), 164.1, 164.9, 165.7 (C=O).
4,6-Di-O-galloyl-β-d-glucose (9): Off-white amorphous powder, C20H20O14, negative ESI-MS: m/z 483 [M − H]−, 1H-NMR (600 MHz, CD3OD): δH 7.09, 7.07, 7.08, 7.06 (each 1H, s, 2× galloyl H-2′,6′), 5.13 (1H, t, J = 9.4 Hz, H-4), 4.39 (1H, m, H-6a), 4.18 (1H, m, H-6b), α-glucose form: 5.17 (1/2H, d, J = 3.7 Hz, H-1), 3.98 (1/2H, t, J = 9.4 Hz, H-3), 4.53 (1/2H, dd, J = 3.7, 9.6 Hz, H-2), β-glucose form: δH 4.60 (1/2H, d, J = 7.8 Hz, H-1), 3.89 (1/2H, m, H-5), 3.70 (1H, t, J = 9.3 Hz, H-3), 3.31 (1/2H, dd, J = 9.3, 7.8 Hz, H-2).
2.3. Bioassay
In an in vitro cytotoxicity assay, compounds 1–5 were applied to HL-60, SMMC-7721, A-549, MCF-7, and SW-480 cancer cells to assess their cytotoxic effects. Compound 3 (Corilagin) exhibited the most cytotoxic activity against MCF-7 cells with IC50 values of 33.18 and 25.81 µg/mL for HL-60 cells. The IC50 value of compound 3 in MCF-7 cells is comparable with the positive control drug (cisplatin), having an IC50 value of 27.43 (Table 1). Corilagin had 29.19, 4.91, and 49.82% inhibition for A-549, SMMC-7721, and SW-480 respectively. The inhibition rate of other compounds (gallic acid, gallic acid ethyl ester, quercetin-3-O-rhamnoside, myricetin-3-O-rhamnoside) wasless than 50% at the concentration of 40 µM (Table 2), suggesting that they showed no significant inhibitory activity against the cancer cell lines; therefore, their IC50 values were not measured.

Table 1.
IC50 values (µg/mL) of corilagin (3) isolated from R. heudelotii against some cancer cell lines.

Table 2.
Percentage of inhibition (I%) of compounds 1–5 (40 µM) isolated from R. heudelotii on different cell lines.
Figure 2, Figure 3 and Figure 4 shows the photomicrograph of the cytotoxic activities of compound 3 on breast, colon, and leukemia cancer cell lines with relatively similar activity when compared with cisplatin (control group).
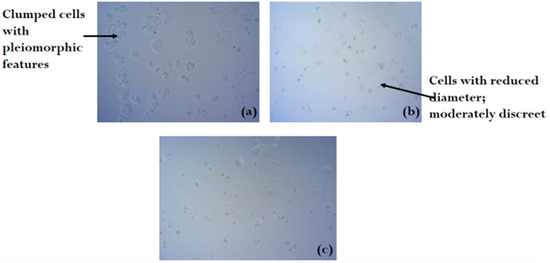
Figure 2.
Photomicrograph of breast cancer cell line (a) untreated, (b) treated with cisplatin, and (c) treated with compound 3 (H&E × 100).
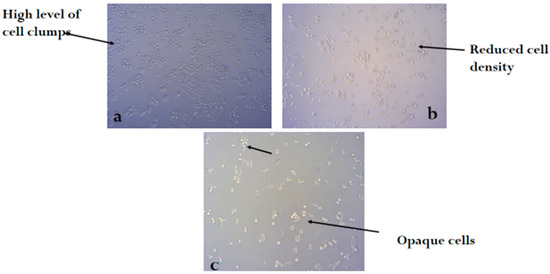
Figure 3.
Photomicrograph of colon cancer cell line (a) untreated, (b) treated with cisplatin, and (c) treated with compound 3 (H&E × 100).
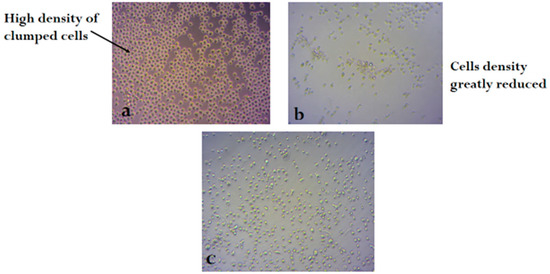
Figure 4.
Photomicrograph of leukemia cancer cell line (a) untreated, (b) treated with cisplatin, and (c) treated with compound 3 (H&E × 200).
3. Discussion
After many years of research on cancer, cancer still remains a destructive disease responsible for over one quarter of the deaths recorded globally. It is one of the major health obstacles, representing the second largest cause of mortality [24]. This is mainly because cancer chemotherapy is often mitigated by a high degree of toxicity to normal cells or failure of treatment due to the development of resistance to anticancer drugs. However, medicinal plants have been increasingly shown to possess anticancer properties, and this has recently become the subject of some scientific studies [25] because they are considered to be less toxic and can be effective for treatment of different diseases. To date, the therapeutic pre-clinical screening of medicinal plants against multiple cancer cells and characterization of the bioactive components in these plants are limited. Our study reports a bioactive compound with cytotoxicity effects in the leaf extracts of R. heudelotii and its potential to be developed for use for cancer treatment.
Corilagin (compound 3) was isolated and structurally identified from the ethyl acetate fraction of the ethanolic extract of R. heudelotii leaves in our study. Only compound 3 showed significant and comparable cytotoxic property relative to cisplatin. Corilagin has been previously identified in several other plants, especially those of the Euphorbiaceae family. It was firstly isolated from Caesalpinia coriaria in 1951, hence the name of the molecule [26]. It was later identified as a weak carbonic anhydrase inhibitor [27]. Studies have reported its antibacterial, hepatoprotective, anti-oxidant [28], anti-inflammatory [29], anti-hypertensive [30], and anti-tumor activities [31]. Yang et al. [32] reported that corilagin can block glioblastoma cells and stem-like cells’ proliferation. In this study, corilagin was found to exhibit an inhibitory effect on HL-60, MCF-7, and SW480 cell growth by 76.23, 57.93, and 49.82%, respectively (Table 2). This could be linked to its ability to activate the Jun N-terminal kinase (JNK) signaling pathway [33]. When these pathways are turned on, it can lead to the activation of the proapoptotic β-cell lymphoma 2 (Bcl-2) protein through phosphorylation and/or upregulation of gene expression and hence mitochondrial cell death [34]. Breast cancer cells treated with corilagin showed reduced cell diameters when compared to the untreated cells, implying that the compound caused cell death of most of the cancerous cells (Plate 1). Corilagin is an ellagitannin, which have been reported to induce pro-apoptotic effects in in both MDA-MB-231 and MCF-7 breast cancer cells [35]. Kuo et al. [36] observed that ellagitannins from Terminalia arjuna bark extract induced apoptosis in A549 cells and in human breast adenocarcinoma MCF-7 cells via blockage of cell-cycle progression in the G0/G1 phase. The cytotoxic properties of tannins are characterised by the release of reactive oxygen species (ROS), which is followed by a reduction in the thioredoxin, superoxide dismutase, and in the level of redox active proteins [37]; this might explain the cytotoxic activity of compound 3. The chemopreventive activity of tannins, especially the ellagitannins and hydrolysable tannins, has been linked to activation of apoptosis [38]. According to the findings of Jia et al. [31], corilagin inhibited SKOv3ip by inducing cell cycle arrest and enhancing apoptosis in the cancer cell lines. Down regulation of cyclin B1 and phosphor-cdc 2 after corilagin treatment on cancer cells was discovered [31]. Corilagin has also been reported to cause the release of TNF-α inhibitor in inflammation [39], decrease the secretion of the transforming growth factor beta 1 (TGF-β1) in a dose dependent manner [31], and inhibit hepatitis C viral replication in vitro [40]. Corilagin enhanced apoptosis by inducing cell cycle arrest at the G2/M stage in ovarian cancer cells. Anti-cancer strategies have focused on possible ways of deactivating the G2/M checkpoint, thereby causing cancer cells to undergo mitosis, and leading to increased DNA damage and then cell death [41,42]. Ming et al. [43] also reported the inhibitory action of corilagin on hepatocellular carcinoma cells (HCC) at the G2/M phase by upregulating p53 and p21, and downregulating cyclin B1, cdc 2, and pAKT. Selectivity index (SI) signifies the differential activity of compounds; the greater the SI value, the more selective the compound [44]. The SI data, as shown in Table 3, indicate that corilagin was non-selective to HL-60, MCF-7, and SW480 cancer cell lines. This suggests its general toxicity to the cell. Adverse effects caused by treatment and increased resistance to tumor cells, leading to treatment failure, has led to a search for therapeutic alternatives. Therefore, it is important to find selective bioactive compounds that could arrest tumor cell proliferation.

Table 3.
Selectivity index of compound 3 (corilagin).
4. Materials and Methods
4.1. General Experimental Procedures
1D- and 2D-NMR spectra were recorded in CD3OD and acetone-d6 with Bruker Avance III600 spectrometer (Bruker, Fällande, Switzerland) operating at 600 MHz for 1H-NMR, and 150 MHz for 13C-NMR, respectively. Coupling constants are expressed in hertz (Hz), and chemical shifts are given on a δ (parts per million, ppm) scale with tetramethylsilane (TMS) as an internal standard. ESI mass spectra were recorded on a VG Auto Spec-300 spectrometer (Waters, Milford, MA, USA). Column chromatography (CC) was performed on Sephadex LH-20 (25–100 μm, GE Healthcare Bio-Science AB, Uppsala, Sweden), MCI-gel CHP20P (75–150 μm, Mitsubishi Chemical Co, Ltd., Tokyo, Japan), and Toyopearl HW-40F (Tosoh Co. Ltd., Tokyo, Japan). Thin-layer chromatography (TLC) was performed on silica gel H-precoated plates, 0.2–0.25 mm thick (Qingdao Haiyang Chemical Co., Qingdao, China), with benzene–ethylformate–formic acid (3:6:1 or 2:7:1, v/v), and compounds were detected by spraying with 2% ethanolic FeCl3 and 10% sulfuric ethanol solution followed by heating.
4.2. Plant Sample Collection
Ricinodendron heudelotii leaves were collected within Covenant University premises Ota, Ogun, Nigeria. The plant was identified by Dr. J.O. Popoola (Botanist, Biological Science Department, Covenant University, Ota, Ogun State, Nigeria) and a voucher specimen was prepared and submitted in the Forest research Institute of Nigeria, Ibadan with voucher number, FHI 110573. Leaves were dried at room temperature (25 °C) and blended using an electric blender into a coarse powder.
4.3. Extraction and Solvent Partitioning ofthe Crude Extract
The powdered leaf samples (10 kg) were extracted using 95% ethanol by macerationat room temperature, the filtrate was further condensed under reduced temperature and pressure, and the yield of the extract obtained was 11.75%. The concentrated crude extract (1 kg) was suspended in 1 L distilled water and partitioned in sequence with petroleum ether (Pet; 8 L), ethyl acetate (EtOAc; 8 L), and n-butanol (8 L). The solvent fractions were concentrated to dryness to afford four (4) fractions as: Pet, EtOAc, n-butanol, and aqueous fractions. The EtOAc fraction was selected for subsequent isolation procedures.
4.4. Isolation of Compounds fromthe Partitioned Fraction
The ethylacetate fraction (246 g) was loaded into a column containing Sephadex LH-20 (800 g). The eluent was a mixture of water and methanol in different ratios starting with 100:0 to 0:100. Fractions with similar TLC patterns were combined to afford 13 fractions. Fraction 11 (36 g) was chromatographed on a Sephadex LH-20 column and MCI-gel CHP20P eluting with water:methanol (4:1) to afford compounds 1 (8.53 mg), 2 (2.1 mg), and 3 (12 mg). Fraction 12 (3.8 g) was subjected to repeated column chromatography also eluting with H2O and methanol (60:1) to afford compound 4 (98 mg) and 5 (13.2 mg). Compounds 6 (9.9 mg), 7 (2.6 mg), 8 (2.7 mg), and 9 (4.4 mg) were obtained from fraction 13 (14.5 g) after repeated purification by chromatography on a Sephadex LH-20, MCI-gel CHP20P and Toyopearl HW-40F columns eluting with H2O/methanol.
4.5. Bioassay
4.5.1. Cell Culture
Cancer cell lines, HL-60, SMMC-7721, A-549, MCF-7, and SW-480, were obtained from the American Type Culture Collection Center (ATCC) (Manassas, VA, USA). They were cultured in RMPI-1640 or DMEM medium supplemented with 10% bovine serum albumin (Gibco, Grand Island, NY, USA). Prior to exposure to drugs, the cancer cell lines were cultured in a CO2 incubator for 48 h and the cell density was adjusted to 5 × 104 cells/well.
4.5.2. Sample Preparation
Compounds (1 mg/mL) were dissolved in either DMSO or H2O depending on their solubility. Samples (2 µL) were suspended in centrifuge tubes containing 48 µL of complete medium to make a final volume of 100 µL.
4.5.3. Assay Procedures
The cytotoxicity assay was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) method by measuring the absorbance of formazan precipitate formed in the cells based on MTS reduction [45]. In this assay, cells were seeded into each of the wells of 96-well cell culture plates, incubated for 12h at 37 °C, and then the test compounds (40 µM) were added and allowed to incubate for 48 h. Each cell line was exposed to different concentrations (0.064, 0.32, 1.6, 8, and 40 µM) of the test compounds in triplicates using cisplatin-an anticancer drug as the positive control. The absorbance of the lysate was measured using a 96-well micro plate reader (Bio-Rad 680, Hercules, CA, USA) at 490 nm. Compounds with a growth inhibition rate of 50% were further evaluated at concentrations of 0.064, 0.32, 1.6, 8, and 40 µM in triplicates with cisplatin as positive control. The concentration that inhibited 50% of the cell growth were expressed as IC50, which was calculated using Reed and Muench’s method [46,47]. The percentage inhibition (I%) was calculated as follows:
where Cc = viable cell count of negative control, Cs = viable cell count of sample, IC50 was calculated by the following equation:
IC50 = 10 Log10 (IC50); IH: I% above 50%; IL: I% below 50%; CH: High drug concentration; and CL: Low drug concentration.
Inhibition (%) = (Cc − Cs)/Cc × 100
Log10 (IC50) = (Log10 (CL) (IH − 50) + log10 (CH) (50 − IL))/(IH − IL)
4.6. Selectivity Index
The selectivity index (SI) for the cytotoxicity of corilagin was calculated using the ratio of the IC50 value of the compound on a normal cell line to the IC50 value of the compound on cancer cell lines [48].
4.7. Statistical Analysis
Samples are expressed as mean ± standard deviation or standard error of mean.
5. Conclusions
In conclusion, we report significant cytotoxic activities of corilagin isolated from leaf extract of R. heudelotii on a breast cancer cell line, and its potential for development as an anticancer agent to reduce the burden of cancer diseases globally.
Author Contributions
O.F.Y. performed the isolation of the compounds, activity assay and the preparation of the manuscript. A.H.A. and E.E.J.I. designed the experiment and the principal investigators of the experiment. Y.-J.Z. participated in structural elucidation of compounds and direction of the experiment. T.M.D. made valuable revision of the manuscript. All authors approved the final version manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31470429) and the OWSD Postgraduate Fellowship. The authors acknowledge the funding support of Covenant University Centre for Research Innovation and Discovery (CUCRID).
Acknowledgments
The authors are grateful to the staffs of the analytical and bioactivity screening groups at the State Key Laboratory of Phytochemistry & Plant Resources in West China, KIB, CAS, for measuring the spectroscopic data and cytotoxic activity.
Conflicts of Interest
The authors declare no conflict of interest
References
- Sylla, B.S.; Wild, C.P. A million Africans a year dying from cancer by 2030: What can cancer research and control offer to the continent? Int. J. Cancer 2012, 130, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. Cancer J. Clin. 2012, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- López-Abente, G.; Aragonés, N.; Pérez-Gómez, B.; Pollan, M.; García-Pérez, J.; Ramis, R.; Fernández-Navarro, P. Time trends in municipal distribution patterns of cancer mortality in Spain. BMC Cancer 2014, 14, 535. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Momeni, J.; Djoulde, R.D.; Akam, M.T.; Kimbu, S.F. Chemical constituents and antibacterial activities of the stem bark extracts of Ricinodendron heudelotii (Euphorbiaceae). Indian J. Pharm. Sci. 2005, 67, 386–389. [Google Scholar]
- Yakubu, O.F.; Adebayo, A.H.; Famakinwa, T.O.; Adegbite, O.S.; Ishola, T.A.; Imonikhe, L.O.; Adeyemi, O.A.; Awotoye, O.A.; Iweala, E.E.J. Antimicrobial and toxicological studies of Ricinodendron heudelotii (Baill.). Asian J. Pharm. Clin. Res. 2018, 11, 299–305. [Google Scholar] [CrossRef]
- Kimbu, S.F.; Keumedjio, F.; Sondengam, L.B.; Connolly, J.D. Two dinorditerpenoids from Ricinodendron heudelotii. Phytochemistry 1991, 30, 619–621. [Google Scholar] [CrossRef]
- Yu, J.H.; Shen, Y.; Wu, Y.; Leng, Y.; Zhang, H.; Yue, J.M. Ricinodols A—G: New tetracyclic triterpenoids as 11β-HSD1 inhibitors from Ricinodendron heudelotii. RSC Adv. 2015, 5, 26777–26784. [Google Scholar] [CrossRef]
- Yakubu, O.F.; Adebayo, A.H.; Okechukwu, E.S.; Adeyemi, O.A.; Iweala, E.J.; Zhang, Y. Co-administration of artemisinin and Ricinodendron heudelotii leaf extract—Effects on selected antioxidants and liver parameters in male Wistar rats. Comp. Clin. Path. 2018, 27, 765–772. [Google Scholar] [CrossRef]
- Eldahshan, O.A. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. J. Biol. Sci. 2011, 3, 52–55. [Google Scholar]
- Soro, Y.; Kassi, A.B.B.; Bamba, F.; Siaka, S.; Toure, S.A.; Coustard, J.M. Flavonons and gallic acid from leaves of Santaloides afzelii (Connaraceae). Rasayan J. Chem. 2012, 5, 332–337. [Google Scholar]
- Mori, T.; Chang, C.; Maurtua, D.; Hammond, G.B. Isolation of the active compound in Mauria heterophylla, a peruvian plant with antibacterial activity. Phytother. Res. 2006, 20, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, L.; Liu, S.; Deng, Y.; Zheng, G.; Gu, Y.; Ming, Y. Bioguided fraction and isolation of the antitumor components from Phyllanthus niruri L. BioMed Res. Int. 2016. [Google Scholar] [CrossRef]
- Bose, S.; Maji, S.; Chakraborty, P. Quercitrin from Ixora coccinea leaves and its anti-oxidant activity. J. Pharmacol. Sci. Technol. 2013, 2, 72–74. [Google Scholar]
- Ibrahim, M.T.; Abu, S.A.; Sleem, A.A. Phytochemical and biological investigation of Thunbergia grandiflora. J. Pharmacol. Phytochem. 2017, 6, 43–51. [Google Scholar]
- Zhong, X.N.; Otsuka, H.; Ide, T.; Hirata, E.; Takushi, A.; Takeda, Y. Three flavonol glycosides from leaves of Myrsine seguinii. Phytochemical 1997, 46, 943–946. [Google Scholar] [CrossRef]
- Hassan, M.; Kubmarawaı, D.; Oladosu, P. Antiallergic polyphenols from Citharexylum spinosum. Trends Phytochem. Res. 2017, 1, 129–134. [Google Scholar]
- Si, C.L.; Xu, J.; Lu, Y.Y.; Su, Z.; Su, Y.F.; Bae, Y.S. Hydrolysable tannins from Juglans sigillata stem barks. Biochem. Syst. Ecol. 2011, 39, 225–227. [Google Scholar] [CrossRef]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. Isolation and identification of a gallotannin 1,2,6-tri-O-galloyl-β-d-glucopyranose from hydroalcoholic extract of Terminaliachebula fruits effective against multidrug-resistant uropathogens. J. Appl. Microbiol. 2013, 115, 390–397. [Google Scholar] [CrossRef]
- Nawwar, M.A.M.; Hussein, S.A.M.; Merfort, I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemical 1994, 36, 793–798. [Google Scholar] [CrossRef]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Saijo, R.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LXXXIV. Isolation and characterization of five new hydrolyzable tannins from the bark of Mallotus japonicus. Chem. Pharm. Bull. 1989, 37, 2063–2070. [Google Scholar] [PubMed]
- Rang, H.P. Pharmacology; Churchill Livingstone: London, UK, 2003; ISBN 0-4430-7145-4. [Google Scholar]
- Abd-Rabou, A.A.; Zoheir, K.M.A.; Ahmed, H.H. Potential impact of curcumin and taurine on human hepatoma cells using Huh-7 cell line. Clin. Biochem. 2012, 45, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.T.; Lademann, R. Corilagin, ein weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung über natürliche Gerbstoffe. Justus Liebigs Annalen Der. Chemie 1951, 571, 232–237. [Google Scholar] [CrossRef]
- Satomi, H.; Umemura, K.; Ueno, A.; Hatano, T.; Okuda, T.; Noro, T. Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol. Pharm. Bull. 1993, 16, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Inoue, Y.; Nakama, S.; Ichiba, T.; Aniya, Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 2007, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Liu, J.; Zhang, S.L.; Luo, T.; Wu, F.; Dong, J.H.; Guo, Y.J.; Zhao, L. Corilagin ameliorates the extreme inflammatory status in sepsis through TLR4 signaling pathways. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Lin, T.C.; Hsu, F.L. Antihypertensive effect of corilagin in the rat. Can. J. Physiol. Pharmacol. 1995, 73, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jin, H.; Zhou, J.; Chen, L.; Lu, Y.; Ming, Y.; Yu, Y. A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement. Altern. Med. 2013, 13, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Li, G.H.; Li, Z.Y.; Feng, S.; Liu, X.Q.; Han, G.K.; Zhang, H.; Qin, X.; Zhang, R.; Nie, Q.; et al. Effect of corilagin on the proliferation and NF-κB in U251 glioblastoma cells and U251 glioblastoma stem-like cells. Evid. Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, K.M.; Kim, N.; Yeom, Y.L. Doxorubicin prevents endoplasmic reticulum stress-induced apoptosis. Biochem. Biophys. Res. Commun. 2006, 339, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; MicKimm-Breschkin, J.; Motoyama, N.; et al. ER stress triggers apoptosis by activating BH3-only protein bim. Cell 2007, 129, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Hsu, Y.L.; Lin, T.C.; Lin, L.T.; Chang, J.K.; Lin, C.C. Casuarinin from the bark of Terminalia arjuna induces apoptosis and cell cycle arrest in human breast adenocarcinoma MCF-7 cells. Planta Med. 2005, 71, 237–743. [Google Scholar] [CrossRef] [PubMed]
- Naus, P.J.; Henson, R.; Bleeker, G.; Wehbe, H.; Meng, F.; Patel, T. Tannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathways. J. Hepatol. 2007, 46, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Esposito, D.; Grace, M.H.; Ha, D.; Pham, A.; Bortolazzo, A.; Bevens, Z.; Kim, J.; Komarnytsky, S.; Lila, M.A.; et al. Cytotoxic effects of ellagitannins isolated from walnuts in human cancer cells. Nutr. Cancer 2014, 66, 1304–1314. [Google Scholar] [CrossRef]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.W.; Kan, C.W.; Hau, D.K.; et al. Corilagin is a potent inhibitor of NF-κB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sudha, G.; Srinivasan, N.; Das, S. Small molecule inhibitors of HCV replication from Pomegranate. Sci. Rep. 2014, 4, 5411. [Google Scholar] [CrossRef]
- Singh, R.P.; Dhanalakshmi, S.; Agarwal, R. Phytochemicals as cell cycle modulators a less toxic approach in halting human cancers. Cell Cycle 2002, 1, 156–161. [Google Scholar] [CrossRef]
- Thakur, V.S.; Deb, G.; Babcook, M.A.; Gupta, S. Plant phytochemicals as epigenetic modulators: Role in cancer chemoprevention. AAPS J. 2014, 16, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Zheng, Z.; Chen, L.; Zheng, G.; Liu, S.; Yu, Y.; Tong, Q. Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell Biol. Int. 2013, 37, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on Breast cancer cell lines. Molecules 2016, 21, 1013–1028. [Google Scholar]
- Iweala, E.E.J.; Liu, F.F.; Cheng, R.R.; Li, Y.; Omonhinmin, C.A.; Zhang, Y.J. Anti-cancer and free radical scavenging activity of some Nigerian food plants in vitro. Int. J. Cancer Res. 2015, 11, 41–51. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Adebayo, A.H.; Tan, N.H.; Akindahunsi, A.A.; Zeng, G.Z.; Zhang, Y.M. Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae). Pharmacogn. Mag. 2010, 21, 62–66. [Google Scholar]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-Caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).