Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Factor Experiment
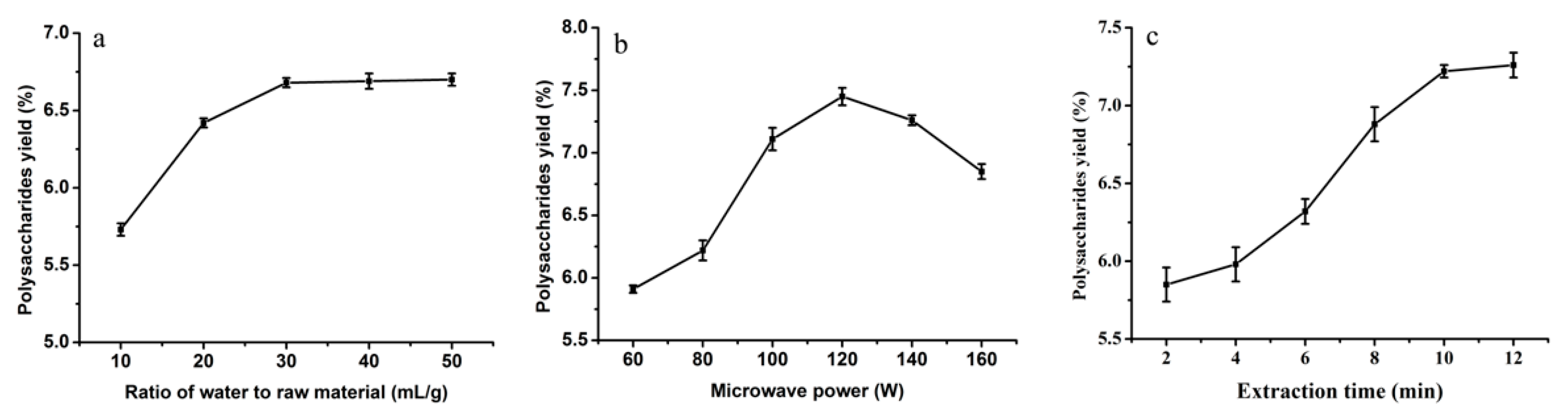
2.1.1. Influence of Ratio of Water to Raw Material on the Yield of Polysaccharides
2.1.2. Influence of Microwave Power on the Yield of Polysaccharides
2.1.3. Influence of Extraction Time on the Yield of Polysaccharides
2.2. Optimization of Extraction of T. orientalis Polysaccharides
2.2.1. Statistical Analysis and Model Fitting
0.718 X12 − 0.633 X22 − 0.448 X32
2.2.2. Optimization of Polysaccharides Extraction Parameters
2.2.3. Verification of Predictive Model
2.3. Comparison with Other Extraction Methods
2.4. Hepatoprotective Activities of TOP-2
2.4.1. Influence of TOP-2 on Body Weight and Organ Index
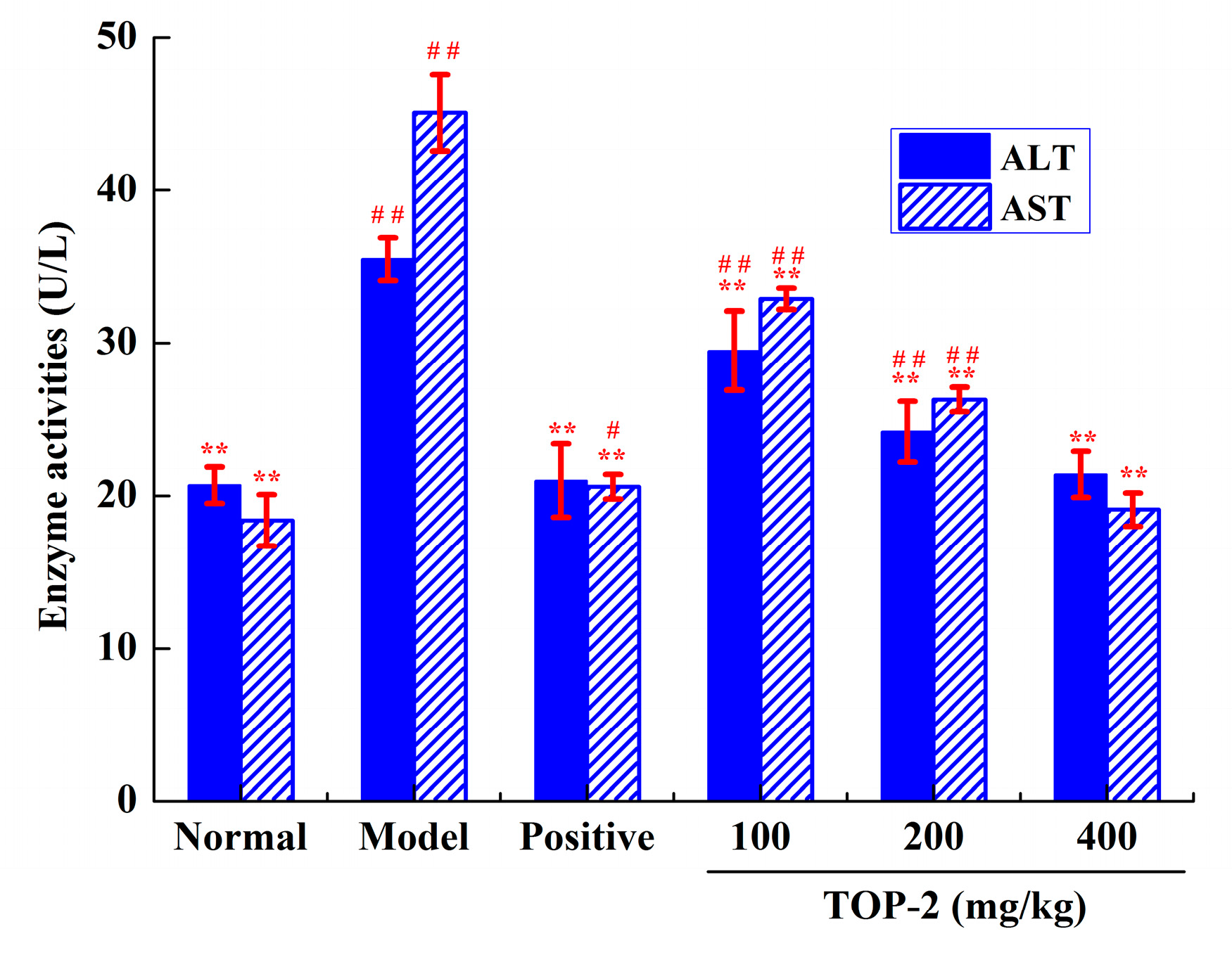
2.4.2. Influence of TOP-2 on Serum Aminotransferase Activities
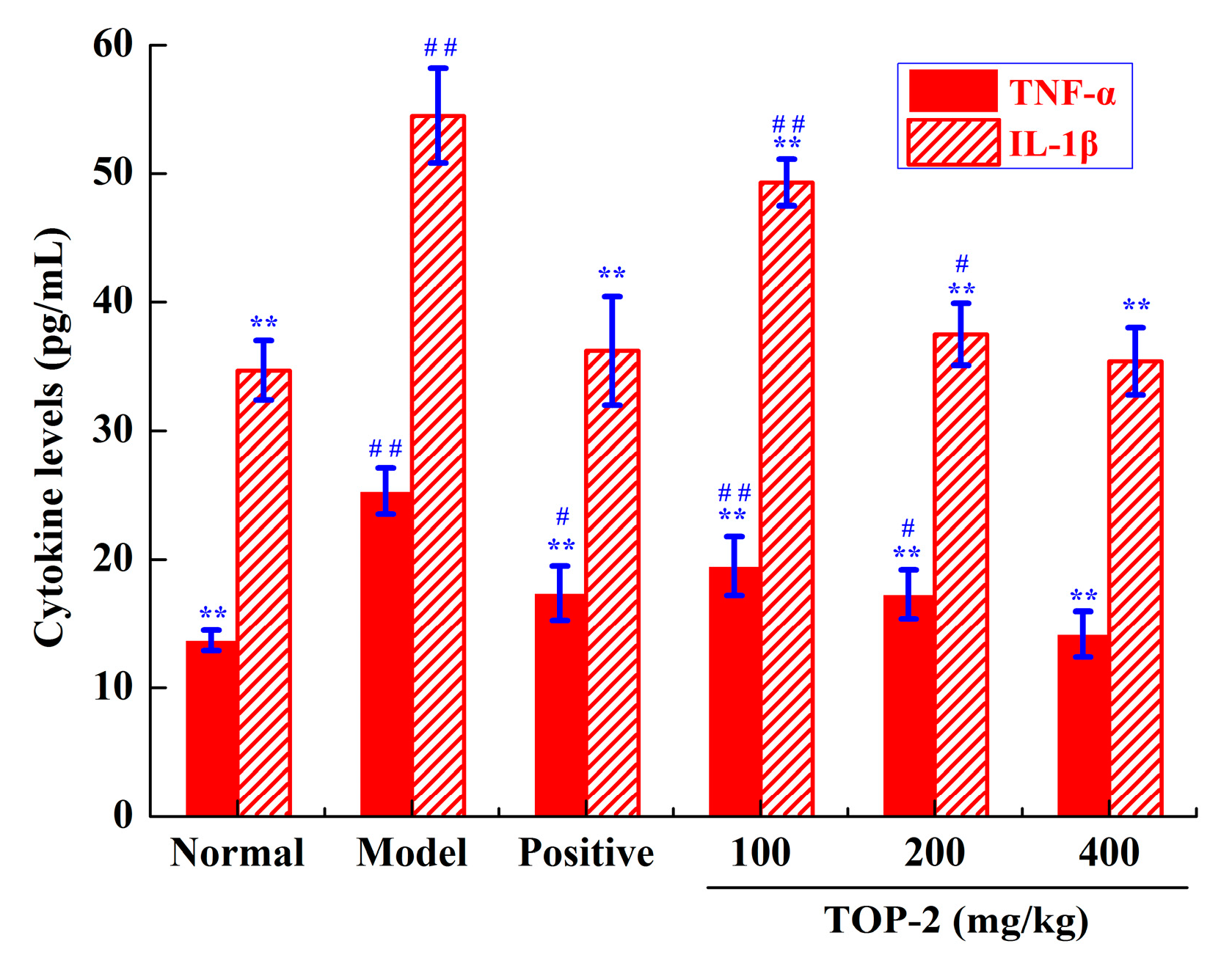
2.4.3. Influence of TOP-2 on Liver Cytokine Levels
2.4.4. Influence of TOP-2 on Liver Antioxidant Enzyme Activities and Malondialdehyde (MDA) Levels
3. Materials and Methods
3.1. Materials and Chemicals
3.2. UMAE of T. Orientalis Polysaccharides
3.2.1. UMAE Process
3.2.2. Single Factor Experiment
3.2.3. Box-Behnken Design
3.3. Comparison with Other Extraction Methods
3.4. Purification of T. orientalis Polysaccharides
3.5. Hepatoprotective Activities of TOP-2
3.5.1. Animal Treatment
3.5.2. Biochemical Assays
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mandayam, S.; Jamal, M.M.; Morgan, T.R. Epidemiology of alcoholic liver disease. Semin. Liver Dis. 2004, 24, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG clinical guideline: Alcoholic liver, disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Xu, M.-J.; Bertola, A.; Wang, H.; Zhou, Z.; Liangpunsakul, S. Animal models of alcoholic liver disease: Pathogenesis and clinical relevance. Gene Expr. 2017, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Mendez, A.; Lugo-Baruqui, A.; Armendariz-Borunda, J. Molecular basis and current treatment for alcoholic liver disease. Int. J. Environ. Res. Public Health 2010, 7, 1872–1888. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Kim, M.Y.; Baik, S.K. Alcoholic liver disease: Treatment. World J. Gastroentero. 2014, 20, 12934. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Li, J.; Xiong, L.; Chen, H.; Liu, X.; Wang, N.; Ouyang, K.; Wang, W. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr. Polym. 2018, 183, 11–20. [Google Scholar] [CrossRef]
- Song, X.; Shen, Q.; Liu, M.; Zhang, C.; Zhang, L.; Ren, Z.; Wang, W.; Dong, Y.; Wang, X.; Zhang, J.; et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases. Int. J. Biol. Macromol. 2018, 114, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ren, D.; Luo, Y.; Hu, Y.; Yang, X. Chemical characteristics of an Ilex Kuding tea polysaccharide and its protective effects against high fructose-induced liver injury and vascular endothelial dysfunction in mice. Food Funct. 2017, 8, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhu, C.; Zhang, Y.; Sun, J.; Alim, A.; Yang, X. Chemical characteristics, antioxidant capacities and hepatoprotection of polysaccharides from pomegranate peel. Carbohydr. Polym. 2018, 202, 461–469. [Google Scholar] [CrossRef]
- Chen, H.L.; Chiang, H.C. Constituents of fruit bodies of Tramete orientalis. J. Chin. Chem. Soc-Taip. 1995, 42, 97–100. [Google Scholar] [CrossRef]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Yu, C.J.; Zhou, L.W. Species diversity and utilization of medicinal mushrooms and fungi in China. Int. J. Med. Mushrooms 2009, 11, 287–302. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W.D. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef]
- Zheng, Y.; Zong, Z.M.; Chen, S.L.; Chen, A.H.; Wei, X.Y. Ameliorative effect of Trametes orientalis polysaccharide against immunosuppression and oxidative stress in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 2017, 95, 1216–1222. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Jiang, Z.; Zhou, X. Optimization for ultrasonic-microwave synergistic extraction of polysaccharides from Cornus officinalis and characterization of polysaccharides. Int. J. Biol. Macromol. 2016, 83, 226–232. [Google Scholar] [CrossRef]
- Huang, S.Q.; Ning, Z.X. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int. J. Biol. Macromol. 2010, 47, 336–341. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhang, S.; Jiang, Z. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr. Polym. 2014, 99, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Wang, W. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Jiang, C.; Ma, L.; Zhang, Z.; Zeng, X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem. Toxicol. 2012, 50, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Lin, Y.H.; Chu, C.C.; Tsai, Y.H.; Chao, J.C.J. Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J. Funct. Foods 2008, 11, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.; Zhang, J.; Hu, C.; Che, G.; Zhou, M.; Jia, L. Antioxidant and hepatoprotective activities of intracellular polysaccharide from Pleurotus eryngii SI-04. Int. J. Biol. Macromol. 2016, 91, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, Y.; Jiao, Y.; Yu, L.; Yang, S.; Yang, X. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 2012, 88, 1453–1459. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, B.; Tang, C.; Gou, Y.; Chen, H.; Wang, Y.; Jin, C.; Liu, J.; Niu, F.; Kan, J.; et al. Characterization, antioxidant activity and hepatoprotective effect of purple sweetpotato polysaccharides. Int. J. Biol. Macromol. 2018, 115, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; French, S.W.; Casey, C.A.; Kharbanda, K.K.; Nanau, R.M.; Rasineni, K.; Mcvicker, B.L.; Kong, V.; Donohue, T.M.; Pathology, M. Changes in the pathogenesis of alcohol-induced liver disease—Preclinical studies. Exp. Mol. Pathol. 2013, 95, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet 2000, 355, 591–592. [Google Scholar] [CrossRef]
- Cai, L.; Zou, S.; Liang, D.; Luan, L. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohydr. Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Zhang, J.; Zhang, C.; Dong, Y.; Ren, Z.; Gao, Z.; Liu, M.; Zhao, H.; Jia, L. Antioxidative and hepatoprotective effects of enzymatic and acidic-hydrolysis of Pleurotus geesteranus mycelium polysaccharides on alcoholic liver diseases. Carbohydr. Polym. 2018, 201, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Pastorino, J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002, 27, 63–68. [Google Scholar] [CrossRef]
- An, L.; Wang, X.; Cederbaum, A.I. Cytokines in alcoholic liver disease. Arch. Toxicol. 2012, 86, 1337–1348. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, Y.; Zhong, M.; Xu, Y.; Li, J.; Chen, Y.; Duan, X.; Zhu, H. Hepatoprotective effects of Dicliptera chinensis polysaccharides on dimethylnitrosamine-induced hepatic fibrosis rats and its underlying mechanism. J. Ethnopharmacol. 2016, 179, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Su, M.; Chen, Q.; Chang, Q.; Wang, W.; Li, H. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride-induced acute liver injury in mice. J. Ethnopharmacol. 2017, 200, 124–135. [Google Scholar] [CrossRef]
- Kandimalla, R.; Kalita, S.; Saikia, B.; Choudhury, B.; Singh, Y.P.; Kalita, K.; Dash, S.; Kotoky, J. Antioxidant and hepatoprotective potentiality of Randia dumetorum Lam. Leaf and bark via inhibition of oxidative stress and inflammatory cytokines. Front. Pharmacol. 2016, 7, 205. [Google Scholar] [CrossRef]
- Masalkar, P.D.; Abhang, S.A. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin. Chim. Acta 2005, 355, 61–65. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2010; pp. 1–151. [Google Scholar]
Sample Availability: Samples of the compounds Trametes orientalis polysaccharides and purified T. orientalis polysaccharide (TOP-2) are available from the authors. |

| Run | X1 (Ratio of Water to Raw Material, mL/g) | X2 (Microwave Power, W) | X3 (Extraction Time, min) | Yield (%) |
|---|---|---|---|---|
| 1 | 0 (30) | 0 (120) | 0 (10) | 7.25 |
| 2 | −1 (20) | 0 (120) | −1 (8) | 5.97 |
| 3 | 1 (40) | 0 (120) | −1 (8) | 5.44 |
| 4 | −1 (20) | −1 (100) | 0 (10) | 6.63 |
| 5 | 0 (30) | 0 (120) | 0 (10) | 7.38 |
| 6 | 1 (40) | 0 (120) | 1 (12) | 6.39 |
| 7 | 1 (40) | 1 (140) | 0 (10) | 6.14 |
| 8 | 0 (30) | 0 (120) | 0 (10) | 7.36 |
| 9 | 0 (30) | −1 (100) | −1 (8) | 5.78 |
| 10 | 1 (40) | −1 (100) | 0 (10) | 5.51 |
| 11 | 0 (30) | 0 (120) | 0 (10) | 7.19 |
| 12 | 0 (30) | 1 (140) | −1 (8) | 5.89 |
| 13 | 0 (30) | 1 (140) | 1 (12) | 6.13 |
| 14 | −1 (20) | 1 (140) | 0 (10) | 5.54 |
| 15 | −1 (20) | 0 (120) | 1 (12) | 6.76 |
| 16 | 0 (30) | 0 (120) | 0 (10) | 7.35 |
| 17 | 0 (30) | −1 (100) | 1 (12) | 7.10 |
| Source | Sum of Squares | df | Mean Square | F Value | Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 8.0979 | 9 | 0.8998 | 91.2539 | <0.0001 | *** |
| X1 | 0.2520 | 1 | 0.2520 | 25.5629 | 0.0015 | ** |
| X2 | 0.2178 | 1 | 0.2178 | 22.0892 | 0.0022 | ** |
| X3 | 1.3612 | 1 | 1.3612 | 138.0578 | <0.0001 | *** |
| X1X2 | 0.7396 | 1 | 0.7396 | 75.0101 | <0.0001 | *** |
| X1X3 | 0.0064 | 1 | 0.0064 | 0.6491 | 0.4469 | ns |
| X2X3 | 0.2916 | 1 | 0.2916 | 29.5740 | 0.0010 | *** |
| X12 | 2.1706 | 1 | 2.1706 | 220.1448 | <0.0001 | *** |
| X22 | 1.6871 | 1 | 1.6871 | 171.1067 | <0.0001 | *** |
| X32 | 0.8451 | 1 | 0.8451 | 85.7068 | <0.0001 | *** |
| Residual | 0.0690 | 7 | 0.0097 | |||
| Lack of fit | 0.0421 | 3 | 0.0140 | 2.0852 | 0.2449 | ns |
| Pure error | 0.0269 | 4 | 0.0067 | |||
| Cor total | 8.1667 | 16 | ||||
| R2 | 0.9915 | |||||
| R2Adj | 0.9807 | |||||
| Adeq precision | 23.58 |
| Group | Dose (mg/kg) | Increase of Body Weight (g) | Liver Index (mg/g) | Spleen Index (mg/g) |
|---|---|---|---|---|
| Normal | 7.57 ± 0.54 * | 42.13 ± 2.25 * | 4.22 ± 0.34 * | |
| Model | 5.32 ± 0.63 | 57.46 ± 2.74 # | 5.05 ± 0.56 # | |
| Positive | 200 | 7.18 ± 0.72 * | 44.68 ± 3.21 * | 4.28 ± 0.31 * |
| TOP-2 | 100 | 6.33 ± 0.57 *,# | 50.85 ± 3.37 *,# | 4.36 ± 0.47 * |
| TOP-2 | 200 | 7.37 ± 0.68 * | 43.29 ± 4.06 * | 4.27 ± 0.18 * |
| TOP-2 | 400 | 7.48 ± 0.55 * | 42.23 ± 3.40 * | 4.31 ± 0.36 * |
| Group | Dose (mg/kg) | SOD (U/mg pro) | CAT (U/mg pro) | GSH-Px (U/mg pro) | MDA (nmol/mg pro) |
|---|---|---|---|---|---|
| Normal | 289.56 ± 5.8 ** | 60.13 ± 2.8 ** | 566.09 ± 34 ** | 0.73 ± 0.04 ** | |
| Model | 196.43 ± 11 ## | 50.72 ± 6.9 ## | 392.32 ± 28 ## | 1.26 ± 0.07 ## | |
| Positive | 200 | 226.73 ± 23 **,## | 55.52 ± 1.3 **,## | 471.37 ± 30 **,## | 0.83 ± 0.06 **,## |
| TOP-2 | 100 | 224.34 ± 15 **,## | 54.64 ± 1.7 **,## | 432.21 ± 14 **,## | 0.97 ± 0.08 **,## |
| TOP-2 | 200 | 253.61 ± 12 **,## | 57.34 ± 3.8 **,## | 487.32 ± 32 **,## | 0.83 ± 0.16 **,## |
| TOP-2 | 400 | 280.45 ± 12 ** | 60.22 ± 5.8 ** | 512.53 ± 24 **,## | 0.78 ± 0.14 ** |
| Independent Variables | Levels | Extraction Parameters |
|---|---|---|
| Ratio of water to raw material (mL/g) | 10, 20, 30, 40, 50 | Microwave power 100 W and extraction time 20 min |
| Microwave power (W) | 60, 80, 100, 120, 140, 160 | Ratio of water to raw material 20 mL/g and extraction time 10 min |
| Extraction time (min) | 2, 4, 6, 8, 10, 12 | Ratio of water to raw material 20 mL/g and microwave power 100 W |
| Independent Variables | Symbol | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ratio of water to raw material (mL/g) | X1 | 20 | 30 | 40 |
| Microwave power (W) | X2 | 100 | 120 | 140 |
| Extraction time (min) | X3 | 8 | 10 | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Cui, J.; Chen, A.-H.; Zong, Z.-M.; Wei, X.-Y. Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis. Molecules 2019, 24, 147. https://doi.org/10.3390/molecules24010147
Zheng Y, Cui J, Chen A-H, Zong Z-M, Wei X-Y. Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis. Molecules. 2019; 24(1):147. https://doi.org/10.3390/molecules24010147
Chicago/Turabian StyleZheng, Yi, Jue Cui, An-Hui Chen, Zhi-Min Zong, and Xian-Yong Wei. 2019. "Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis" Molecules 24, no. 1: 147. https://doi.org/10.3390/molecules24010147
APA StyleZheng, Y., Cui, J., Chen, A.-H., Zong, Z.-M., & Wei, X.-Y. (2019). Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis. Molecules, 24(1), 147. https://doi.org/10.3390/molecules24010147





