Abstract
Oxidative reactions can result in the formation of electronically excited species that undergo radiative decay depending on electronic transition from the excited state to the ground state with subsequent ultra-weak photon emission (UPE). We investigated the UPE from the Fe2+-EGTA (ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid)–H2O2 system with a multitube luminometer (Peltier-cooled photon counter, spectral range 380 to 630 nm). The UPE of 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 reached 4319 ± 755 relative light units during 2 min measurement and was about seven times higher (p < 0.001) than the UPE of incomplete systems (Fe2+-H2O2, EGTA-H2O2) and medium alone. Substitution of Fe2+ with Cr2+, Co2+, Mn2+ or Cu2+ as well as of EGTA with EDTA (ethylenediaminetetraacetic acid) or citrate completely abolished UPE. Experiments with ROS scavengers revealed the dependence of UPE on hydroxyl radicals suggesting occurrence of oxidative attack and cleavage of the ether bond in EGTA backbone structure and formation of triplet excited carbonyl groups with subsequent light emission. Plant phenolics (ferulic, chlorogenic and caffec acids) at concentration 87 µmol/L and ascorbate at 0.46 mmol/L inhibited UPE by 90 ± 4%, 90 ± 5%, 97 ± 2% and 92 ± 1%, respectively. Quenching of UPE from Fe2+-EGTA-H2O2 system can be used for evaluation of antioxidant activity of phytochemicals.
1. Introduction
Oxidative metabolic reactions in living cells can result in the formation of electronically excited species. They undergo radiative decay depending on electronic transition from the singlet or triplet excited state to the singlet ground state what is accompanied by ultra-weak photon emission (UPE) [1]. In vitro oxidation of pure lipids or cell cultures and organ homogenates containing lipids (especially brain homogenates) resulted in UPE along with accumulation of various products of lipid peroxidation [2,3,4,5,6]. Free radical scavengers (mannitol, butylated hydroxytoluene, D-α-tocopherol) quenched UPE related to lipid peroxidation in cell culture and organ homogenates [3,4]. Moreover, rats fed a tocopherol-free diet for seven months revealed higher ex vivo UPE from brain, liver and heart homogenates than animals on normal feed [4]. Fenton’s reagent (solution of H2O2 with Fe2+) and chelate-modified Fenton’s reagent are used to study the hydroxyl radical (•OH)-induced peroxidative damage to various organic compounds and biomolecules and in some cases this can be accompanied by light emission including UPE [7,8,9]. Because, the Fenton reaction involves the creation of reactive oxygen species (ROS) by chemicals that are present in vivo these experimental models have importance in studies on free radicals related pathology in humans [10,11]. Moreover, measurement of UPE as well as other forms of chemiluminescence related to peroxidative damage to biomolecules can be used for monitoring the effectiveness of various compounds as potential ROS scavengers and pharmacological interventions leading to suppression of oxidative stress in vivo [12,13]. In addition, chemiluminescence can reflect the intensity of oxidative processes and current balance between generated ROS and antioxidant capacity in various cells and tissues under normal conditions [1,14].
A recent study showed that chelating agents frequently used to modify Fenton’s reagent such as EDTA and EGTA can react with various oxidants including hypochlorite, peroxyl radicals and peroxynitrite [15]. It cannot be excluded that these reactions lead to generation of electronically excited chemical groups in chelating compounds with subsequent light emission. Although, Fenton’s reagent alone was reported to generate UPE [7,8] no data exist (to the best of our knowledge) on photon emission from Fe2+-EGTA-H2O2 system. Therefore, in this study we investigated the chemilumiescence of Fe2+-EGTA-H2O2 system with special attention to elucidate what ROS are involved in this phenomenon and its possible application as a tool for evaluation of antioxidant activity of selected phenolic acids.
2. Results
2.1. Ultra Weak Photon Emission from Fe2+-EGTA-H2O2 System
The complete system 9.3 µmol/L Fe2+—18.5 µmol/L EGTA—0.26 mmol/L H2O2 emitted 1351 ± 178 relative light units (RLU) during 2 min of measurement. This was about 2.2-times higher (p < 0.001) than UPE from incomplete systems (Fe2+-H2O2 or EGTA-H2O2) and 2.5-times higher (p < 0.001) than that of medium alone (Table 1).

Table 1.
Light emission from Fe2+-EGTA-H2O2 system. Effect of increasing concentrations of EGTA-modified Fenton system under stable ratio of Fe2+ to EGTA to H2O2 molar concentration conditions.
Analysis of 5-, 10- and 20-times higher concentrations of Fe2+-EGTA-H2O2 system (under conditions of the same ratio of molar concentrations of constituents) revealed gradual increase in UPE from 1533 ± 76 RLU up to 6278 ± 502 RLU while light emission from control systems (incomplete systems, H2O2 alone, Fe2+-EGTA-H2O) did not change significantly and ranged from 489 ± 9 RLU to 645 ± 100 RLU (Table 1). This increase was not linear and the difference between mean UPE of Fe2+-EGTA-H2O2 and mean background photon emission of medium alone (H2O injected to PBS) was 821 RLU, 1061 RLU, 3718 RLU and 5781 RLU for the baseline and 5-, 10- and 20-times higher concentrations of the modified Fenton system, respectively. It should be pointed out that light emission from Fe2+-H2O2 (incomplete system I, Fenton reagent) in the case of two highest concentrations (experiment C and D, Table 1) was higher (p < 0.05) than those from corresponding control systems. The UPE of 92.6 µmol/L Fe2+-185.2 µmol/L EGTA-2.6 mmol/L H2O2 reached 4319 ± 755 RLU and was about seven times higher than UPE of incomplete systems and medium alone (Table 1). This concentration of Fenton system was used for further experiments.
2.2. Effect of Iron and EGTA Replacement by other Divalent Cations and Metal Chelators on Light Emission from Fe2+-EGTA-H2O2 System
Replacement of Fe2+ with other divalent cations (Cr2+, Co2+, Mn2+, Cu2+) almost completely abolished any light emission from the Fenton system (Figure 1). Mean UPE ranged from 513 ± 41 RLU for Cu2+-EGTA-H2O2 to 625 ± 62 RLU for Cr2+-EGTA-H2O2 and was comparable to that observed for medium alone (508 ± 28 RLU). Similar results were observed for Fe2+-EDTA-H2O2 and Fe2+-citric acid-H2O2, both systems emitted 6.5- and 6-times less photons (Figure 1) than Fe2+-EGTA-H2O2 over 2 min of counting, respectively. Mean photon emission from all corresponding control systems did not exceed 690 RLU (data not shown).
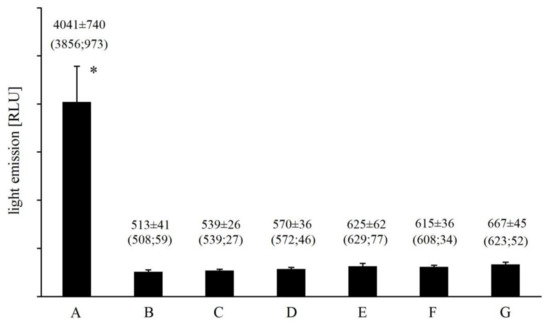
Figure 1.
Effect of iron and EGTA replacement with other divalent cations (Cu2+, Mn2+, Co2+, Cr2+) and metal chelators (EDTA, citric acid) on the light emission from Fe2+-EGTA-H2O2 system. Results obtained from four series of experiments expressed as mean and standard deviation and (median; interquartile range). (A) Fe2+-EGTA-H2O2; (B) Cu2+-EGTA-H2O2; (C) Mn2+-EGTA-H2O2; (D) Co2+-EGTA-H2O2; (E) Cr2+-EGTA-H2O2; (F) Fe2+-EDTA-H2O2; (G) Fe2+-citric acid-H2O2. * vs. value of B, C, D, E, F and G, p < 0.05.
2.3. Effect of Reactive Oxygen Species Scavengers on Light Emission from Fe2+-EGTA-H2O2 System
Catalase and superoxide dismutase (SOD) added to Fe2+-EGTA (final activity of 0.185 U/µL) prior to a H2O2 injection inhibited UPE by 85 ± 4% and 65 ± 14%, respectively (n = 8, p < 0.001). NaN3 (an singlet oxygen scavenger) at the concentration of 0.37 mmol/L had no significant effect on UPE of Fe2+-EGTA-H2O2 (4521 ± 441 RLU for Fenton system without NaN3 vs. 3784 ± 1027 RLU for system with NaN3, p > 0.05, n = 6) while hydroxyl radical (•OH) scavengers, mannitol and dimethyl sulfoxide (DMSO) at the same concentration decreased UPE by 32 ± 8% and 51 ± 10% (p < 0.01), respectively, with the stronger effect of the latter one (p < 0.05) (Figure 2).
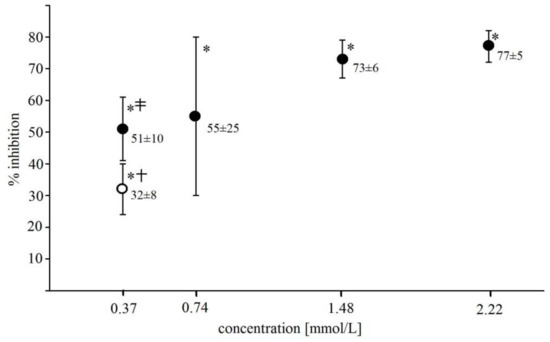
Figure 2.
Inhibitory effect of DMSO (closed circles) and mannitol (open circle) on light emission from 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system. DMSO and mannitol were added to PBS containing Fe2+ and EGTA before automatic H2O2 injection. Results expressed as mean and standard deviation of % inhibition were obtained from 6 separate experiments. * significant inhibition, p < 0.001; † vs. corresponding concentration of DMSO, p < 0.05; ‡ vs. DMSO concentrations of 1.48 mmol/L and 2.22 mmol/L, p < 0.01.
2.4. Effect of Selected Phenolics and Ascorbic Acid on Light Emission from Fe2+-EGTA-H2O2 System
All tested phenolic acids (ferulic, chlorogenic and caffec acids) strongly suppressed the light emission from Fe2+-EGTA-H2O2 system. Addition of ferulic acid, chlorogenic acid or caffeic acid to the final concentration of 87 µmol/L decreased the mean UPE from Fe2+-EGTA-H2O2 by 4.7-, 4.5- and 7.7-times (p < 0.01), respectively. The mean % inhibition of light emission ranged between 90 ± 3% and 98 ± 1% for these compounds at the concentration range of 87 µmol/L to 870 µmol/L and the strongest inhibition was observed in the case of caffeic acid (Table 2). Ascorbic acid at the concentration of 0.46 mmol/L almost completely quenched UPE from Fe2+-EGTA-H2O2 (92 ± 1% inhibition, n = 4, p < 0.001). However, the light emission from Fe2+-EGTA-ascorbic acid-H2O2 was still higher than that of medium alone (963 ± 44 RLU vs. 585 ± 8 RLU, p < 0.05).

Table 2.
Inhibition of light emission from Fe2+-EGTA-H2O2 by selected phenolic acids.
3. Discussion
3.1. Light Emission from Fe2+-EGTA-H2O2 System
We found that Fe2+-EGTA-H2O2 system emitted light in a concentration dependent (but not linear) manner under conditions of a stable ratio of molar concentrations of its constituents. Injection of H2O2 to Fe2+ or EGTA alone did not result in the significant increase in UPE. In these cases the light emission was similar or slightly higher than that observed for medium alone. Substitution of Fe2+ with other divalent cations (Cu2+, Mn2+, Co2+ and Cr2+) almost completely abolished UPE. The same effect was observed for substitution of EGTA with EDTA or citric acid. These indicate that UPE is specific for Fe2+-EGTA-H2O2 system and could not be obtained from the combination of other divalent cations and chelating agents with H2O2.
3.2. Plausible Mechanism of Light Generation from Fe2+-EGTA-H2O2 System
Light generation from Fe2+-EGTA-H2O2 system was inhibited by •OH scavengers (mannitol and DMSO), SOD an effective scavenger of superoxide radical (O2−) and catalase an enzyme decomposing H2O2. Inhibitory effect of catalase on UPE is obvious and clearly indicates the necessity of H2O2 for light emission and is in line with the lack of UPE from incomplete system Fe2+-EGTA-H2O. Numerous reactions can simultaneously take place in our modified Fenton system. Some of them leading to generation of •OH, O2− and singlet oxygen (O2(1Δg)) [7,8,16] are shown below:
Fe2+-EGTA + H2O2 → Fe3+-EGTA + OH− + •OH (hydroxyl radicals generation)
Fe3+-EGTA + H2O2 → Fe3+OOH−-EGTA + H+
Fe3+OOH−-EGTA + H2O2 → FeO2+-EGTA + HO•2 + H2O
FeO2+-EGTA + H2O2 → Fe3+-EGTA + HO•2 + OH−
HO•2 → H+ + O2− (formation of superoxide radicals)
O2− + Fe3+-EGTA → Fe2+-EGTA + O2 (reduced iron can enter reaction 1 to yield •OH)
O2− + •OH + H+ → H2O2 + O2(1Δg) (formation of singlet oxygen)
2 O2− + 2 H+ → H2O2 + O2(1Δg) (formation of singlet oxygen)
It is well known that Fenton reagent (Fe2+-H2O2) generates UPE via O2(1Δg) formation and its decay [7,8] with a three characteristic bands emission at 1270 nm (monomolecular decay from its first excited state to ground state), and at 634 nm and 703 nm (bimolecular transition) [17]. Two of these bands (1270 nm and 703 nm) were far away and one (634 nm) was at the border of the spectral range (from 380 nm to 630 nm) of detection of our luminometer. The width of the 634 nm band is about 35 nm, therefore this may be at least in part responsible for UPE of Fe2+-EGTA-H2O2 under conditions of our experiments. However, NaN3 a scavenger of O2(1Δg) did not significantly decrease UPE which suggests that O2(1Δg) was not involved in UPE of Fe2+-EGTA-H2O2 system. There are two possible explanations of this observation: firstly, the intensity of photons emission related to bimolecular decay of O2(1Δg) was too low to significantly contribute to UPE of Fe2+-EGTA-H2O2 system and was not detected; secondly, almost all O2·− (generated in reaction 5) was consumed for reduction of Fe3+-EGTA complex (reaction 6) and thus formation of O2(1Δg) and subsequent photons emission was inhibited. Another source of UPE are triplet excited carbonyl groups (3(R=C)*) [2,18,19] emitting photons with spectral range of 350 nm to 550 nm [12].
3(R=C)* can be formed by •OH—induced oxidation of various low molecular weight compounds (e.g., uric acid, vitamin B12, tryptophan) as well as lipids and DNA [12]. •OH can attack the ether group in the backbone chain of various molecules resulting in the cleavage of the ether bond [20,21,22] with its further degradation and formation of another radical and carbonyl group [20,23]. EGTA contains two ether bonds in the middle of the backbone chain (Figure 3). It is possible that •OH can react with these bonds and form 3(R=C)* with subsequent photons emission. Figure 4 shows the proposed mechanism of these reactions. This suggested elucidation of light emission from Fe2+-EGTA-H2O2 system is supported by three observations: (A)—substitution of EGTA with EDTA which has no ether bonds in the backbone structure (Figure 3) resulted in the elimination of UPE; (B)—•OH scavengers inhibited UPE probably by protection of EGTA ether bonds from •OH attack and consequent formation of 3(R=C)*; (C)—SOD decreased UPE by scavenging O2− and suppression of •OH formation via inhibition of reaction (6). This reaction in the presence of SOD is as follows:
O2− + 2H+ + Fe3+-EGTA → Fe3+-EGTA + H2O2 (no reduction of Fe3+ and subsequent •OH formation)
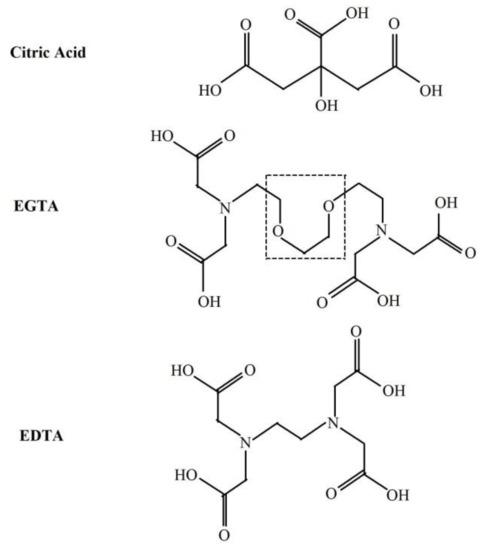
Figure 3.
Chemical structures of citric acid, EGTA and EDTA. The dashed line frame shows the two ether bonds of EGTA most probably involved in the light emission from the Fe2+-EGTA-H2O2 system.
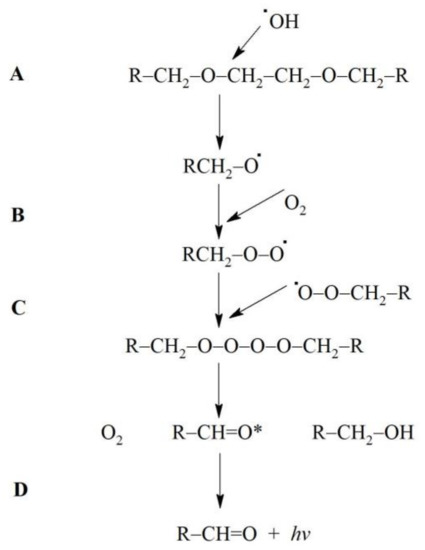
Figure 4.
The proposed mechanism for formation of triplet excited carbonyl groups (3(R=C)*) and subsequent light emission from Fe2+-EGTA-H2O2 system. (A) Hydroxyl radicals (•OH) generated in the Fenton reaction attack one of ether bond in the backbone structure of EGTA (R-CH2-O-CH2-CH2-O-CH2-R) leading to its cleavage and radicals formation (R-CH2-O•). (B) This radical react with molecular oxygen (O2) dissolved in reaction environment to generate peroxyl radical(R-CH2-O-O•). (C) Two peroxyl radicals react with each other (Russel-type mechanism) with subsequent formation of O2, and two products one with hydroxyl group (R-CH2-OH) and the second one with triplet excited carbonyl group (R-CH=O*). (D) Electronic transitions from the triplet excited state to the ground state is accompanied by the photon emission (λν).
Moreover, substitution of EGTA with citric acid (other chelating agent without ether bond) also abolished UPE.
3.3. Inhibitory Effect of Phenolic Acids on Light Emission from Fe2+-EGTA-H2O2 System
According to the proposed mechanism of UPE from Fe2+-EGTA-H2O2 any given compound would inhibit light emission if: (A) it effectively scavenges at least one of the following ROS: H2O2, •OH and O2−; (B) is a stronger Fe2+ chelating agent than EGTA. The first action would result in the decreased activity of •OH and protection of EGTA ether bonds from oxidative attack. The second one would involve abstraction of Fe2+ ions from Fe2+-EGTA complex and formation of another complex to be less effective in reaction with H2O2 leading to •OH formation. Moreover, part of •OH radicals formed in this complex can oxidize other molecules (e.g., added compound with chelating properties or bicarbonate ions derived from dissolved in water atmospheric CO2 [24]) before their reaction with ether bonds of EGTA and subsequent light emission.
All three tested phenolic acids inhibited UPE from the Fe2+-EGTA-H2O2 system. They have hydroxyl substituents in the backbone aromatic ring: ferulic acid one, caffeic and chlorogenic acids have two. It is possible that •OH can grab a hydrogen atom from one of the hydroxyl groups at the phenolic ring to form water and a less reactive and more stable radical. Thus less •OH was available for photon emitting reactions with ether bonds of EGTA. The observation that ferulic acid was a weaker inhibitor of UPE than caffeic acid is in line with this explanation. Moreover, this plausible mechanism of UPE inhibition from Fe2+-EGTA-H2O2 system by phenolic acids is in agreement with previous reports demonstrating an intensification of the •OH scavenging activity of flavonoids with an increased number of –OH substituents in an aromatic ring [25]. Furthermore, hydroxyl groups and catechol group, at position 3, 5, 7 and 40 are critical for the effective scavenging of peroxynitrite by flavonoids [26] as well as the inhibition of total ROS generation in kidney homogenates by flavonoids intensifies as the number of total –OH groups in their structure increases [27]. Moreover, the protective effect of polyphenols against •OH—induced degradation of deoxyribose correlated with the number of –OH substitutions in the backbone structure [28]. Our results correspond well with a studies showing distinct •OH and O2·− scavenging activity of chlorogenic and caffeic acids in vitro [29] and the inhibitory effect of ferulic acid on •OH—induced damage to synaptosomes and neuronal cells [30,31]. In another study ferulic acid scavenged O2·− as proved by using electron spin resonance spectroscopy [32]. Therefore, decomposition of O2·− apart from direct scavenging of •OH may additionally be responsible for inhibitory effect of studied phenolic acids on UPE from Fe2+-EGTA-H2O2 system. These phenolic acids can also from complexes with divalent cations including Fe2+ [33,34,35] which can decrease Fe2+ reactivity with H2O2 [33,34]. However, two-fold molar excess of EGTA compared to Fe2+ ions in the reaction mixture seems to prevent formation of Fe2+-phenolic acid complexes. It is in line with previous studies showing negligible binding of Fe2+ and Fe3+ to polyphenols in the presence of excess of EDTA another strong chelating agent [34,36,37]. Therefore, formation of phenolic acid–iron complexes had insignificant contribution to phenolic acid-induced suppression of UPE from Fe2+-EGTA-H2O2. Caffeic, chlorogenic and ferulic acids were able to reduce Fe3+ to Fe2+ [38]. Thus they can replace O2− as an Fe3+ reducing agent (reaction 6) and enhance •OH generation in the Fe2+-EGTA-H2O2 system. Therefore although all phenolics revealed about 90% inhibition of UPE at concentration of 87 µmol/L we tested 2- and 10-times higher concentrations to exclude any possible pro-oxidant action of these compounds. Ascorbic acid is also a powerful Fe3+ reducing agent [38] and is frequently used as a component of modified Fenton systems (e.g., Fe3+-H2O2-ascorbate and Fe3+-EDTA-H2O2-ascorbate) to enhance •OH generation in in vitro studies on antioxidant properties of various phytochemicals [28]. However, ascorbic acid itself can scavenge various ROS including •OH [39,40] and is recognized as an efficient antioxidant vitamin in vivo [40,41]. Ascorbic acid almost entirely inhibited UPE from Fe2+-EGTA-H2O2 system which shows that scavenging of •OH by this vitamin definitely prevailed over potential pro-oxidant action under conditions of our experiments.
3.4. Strengths and Weaknesses of the Study
The photon counter of luminometer used for measurement of light emission had narrow spectral range from 380 nm to 630 nm and was cooled with Peltier module only to 8 °C. This precluded the measurement of photons derived from decay of O2(1Δg) formed in Fenton system (reactions 7 and 8). Application of photomultiplier device with much wider spectral range sensitivity and lower working temperature of photomultiplier (e.g., 300 nm to 900 nm and −40 °C, 160 nm to 710 nm and −30 °C) provides the opportunity to measure signals from all possible photon emitters (3(R=C)* and O2(1Δg)) and ensures low background and high signal-to-noise ratio [2,3]. Therefore, it cannot be excluded that the real UPE from Fe2+-EGTA-H2O2 system is higher than we observed under conditions of our experiments. On the other hand, the AutoLumat Plus is a commercially available instrument for chemiluminescent determination of various compounds and enzymes in one batch in a quasi-parallel mode and our experiments could be easily repeated and extended to various Fenton systems by other researches. Moreover, we proposed the quenching of UPE from Fe2+-EGTA-H2O2 system as a simple tool for evaluation of antioxidant activity of various phytochemicals. Because the system simplicity and short time of UPE recording the assay is inexpensive, suitable for automation and the obtained results could be easy for interpretation. Therefore, a proven possibility to execute such tests with AutoLumat Plus seems to be the advantage of our study. We did not determine spectrum of light emitted from Fe2+-EGTA-H2O2 system and it could also be recognized as the second weakness of our study. However, with the use of various ROS scavengers and by substitution of EGTA with EDTA we were able to conclude that the photons emitters are 3(R=C)*. Moreover, by substitution of Fe2+ with other divalent cations we proved that UPE emission within the range from 380 nm to 630 nm is specific for Fe2+-EGTA-H2O2 combination. The ratio of molar concentrations of FeSO4 to EGTA to H2O2 in Fenton reaction system was 1:2:28.1. In our previous studies this reaction mixture generated large amounts of •OH as reflected by damage to deoxyribose [28,42] and the cytotoxicity against cell suspensions in vitro [43]. Therefore, we analyzed the relationship between UPE and increasing concentrations of Fenton system under conditions of stable ratio of molar concentrations of its components. The maximal photon emission from Fenton system occurred during the first several dozen seconds after addition of H2O2 to Fe2+ solution and then was terminated [8]. Therefore, we measured UPE from Fe2+-EGTA-H2O2 system for 120 s and did not analyze the kinetics of this phenomenon.
4. Materials and Methods
4.1. Reagents
All chemicals were of analytical grade. DMSO, d-mannitol, sodium azide (NaN3), iron (II) sulfate heptahydrate (FeSO4·7H2O), cupric sulfate pentahydrate (CuSO4·5H2O), cobalt (II) sulfate hydrate (CoSO4·H2O), manganese (II) sulfate monohydrate (MnSO4·H2O), chromium (II) chloride (CrCl2), EDTA, EGTA, sodium citrate, ferulic, chlorogenic and caffeic acids (see Appendix A), sodium L-ascorbate, catalase from bovine liver (2440 units/mg solid), SOD from bovine liver (1500 units/mg protein) were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). H2O2 30% solution (w/w) was from Chempur (Piekary Slaskie, Poland). Sterile phosphate buffered saline (PBS, pH 7.4, without Ca2+ and Mg2+) was obtained from Biomed (Lublin, Poland). Sterile deionized pyrogen-free water (freshly prepared, resistance > 18 MW/cm, HPLC H2O Purification System, USF Elga, Buckinghamshire, UK) was used throughout the study. Working aqueous solutions of FeSO4 (concentrations of 0.5, 2.5, 5, and 10 mmol/L) and 5 mmol/L solutions of CuSO4, MnSO4, CoSO4 were prepared before the assay. To minimize oxidation of Cr2+ ions, aqueous solution (5 mmol/L) of CrCl2 was prepared with deaerated water within 1 min before addition to a luminescent reaction mixture.
Working solutions of H2O2 (2.8, 14, 28 and 56 mmol/L) were prepared by dilution of 30% H2O2 solution and the concentration was confirmed by the measurement of absorbance at 240 nm using a molar extinction coefficient of 43.6/mol cm [44]. Stock solution of EGTA (100 mmol/L) was prepared in PBS with pH adjusted to 8.0 with 5 mol/L NaOH and stored at room temperature in the dark for no longer than 3 months. EGTA working solutions (concentrations of 1, 5, 10 and 20 mmol/L) were obtained by appropriate dilution of EGTA stock solution with water. Catalase and SOD were dissolved in PBS to an activity of 10 U/µL. Phenolic acids (ferulic, chlorogenic and caffeic acids) were dissolved in PBS to concentrations of 0.1, 0.2 and 1 mmol/L. Solutions of DMSO (concentrations of 20, 40, 80 and 120 mmol/L), NaN3 (20 mmol/L), mannitol (20 mmol/L), EDTA (10 mmol/L), citric acid (10 mmol/L) and sodium ascorbate (25 mmol/L) were prepared in PBS freshly before the assay.
4.2. Light Emission from Fe2+-EGTA-H2O2 System
The chemiluminescence was measured with a multitube luminometer (AutoLumat Plus LB 953, Berthold, Germany) equipped with a Peltier-cooled photon counter (spectral range from 380 to 630 nm) to ensure high sensitivity and low and stable background noise signal. Twenty µL of 10 mmol/L EGTA solution was added to the tube (Lumi Vial Tube, 5 mL, 12 × 75 mm, Berthold Technologies, Bad Wildbad, Germany) containing 940 µL of PBS. Then 20 µL of 5 mmol/L solution of FeSO4 was added and after gentle mixing the tube was placed in the luminometer chain and incubated for 10 min in the dark at 37 °C. Then 100 µL of 28 mmol H2O2 solution was added by an automatic dispenser and the total light emission (expressed in RLU) was measured for 120 s. The final concentrations of FeSO4, EGTA and H2O2 in the reaction mixture were 92.6, 185.2 and 2.6 mmol/L respectively. Control systems included: incomplete system I (Fe2+-H2O2 in PBS); incomplete system II (EGTA-H2O2 in PBS); H2O2 in PBS; Fe2+ and EGTA without H2O2 (Fe2+-EGTA-H2O in PBS); and medium alone (H2O in PBS) (Table 3). These experiments were also performed with 2- and 10-times lower and 2-times higher concentrations of FeSO4, EGTA and H2O2 (the ratio of molar concentrations of compounds was always the same).

Table 3.
Design of experiments on light emission from Fe2+-EGTA-H2O2 system.
4.3. Effect of Iron and EGTA Replacement by other Divalent Cations and Metal Chelators on Light Emission from Fe2+-EGTA-H2O2 System
In these experiments we checked whether replacement of Fe2+ and EGTA with other divalent cations (Cr2+, Co2+, Mn2+, Cu2+) and metal chelators (EDTA and sodium citrate) can change the light emission from the Fe2+-EGTA-H2O2 system. The Fenton system was 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 and Fe2+ was replaced by the same concentrations of the aforementioned cations. In another series of experiments EGTA was replaced by the same concentrations of EDTA or sodium citrate and the total light emission was measured as described. The design of these experiments together with appropriate controls is shown in Table 3.
4.4. Determining the Effect of Reactive Oxygen Species Scavengers and Selected Phenolic Acids on Light Emission from Fe2+-EGTA-H2O2 System
To determine what ROS are involved in the UPE of 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system, 20 µL solution of ROS scavenger was added to the luminometer tube containing FeSO4 and EGTA in PBS and incubated for 10 min at 37 °C in the dark and then 100 µL of H2O2 solution was injected and the total light emission was measured for 2 min. Controls included: full system without ROS scavenger (Fe2+-EGTA-H2O2 in PBS); incomplete system I (Fe2+-H2O2 in PBS); incomplete system I with ROS scavenger (Fe2+-ROS scavenger-H2O2 in PBS); Fe2+ and EGTA without H2O2 (Fe2+-EGTA-H2O in PBS); Fe2+ and EGTA and ROS scavenger without H2O2 (Fe2+-EGTA-ROS scavenger-H2O in PBS) (Table 4).

Table 4.
Design of experiments on the effect of reactive oxygen scavengers and selected phenolic acids on light emission from Fe2+-EGTA-H2O2 system.
The following ROS scavengers were used SOD—an O2·− scavenger (final activity of 0.185 U/µL), catalase—an H2O2 scavenger (final activity of 0.185 U/µL), DMSO—a potent •OH scavenger [45] (final concentrations of 0.37 mmol/L to 2.22 mmol/L), mannitol—an •OH scavenger [46] (final concentration of 0.37 mmol/L), NaN3—a O2(1Δg) scavenger [47] (final concentration of 0.37 mmol/L) and sodium ascorbate (final concentration of 0.46 mmol/L). In another series of experiments the effects of three phenolic acids (ferulic, chlorogenic and caffeic acids, final concentrations in the reaction mixture from 0.09 mmol/L to 0.87 mmol/L) on total light emission from Fe2+-EGTA-H2O2 system were studied. The design of these tests and control samples were the same as in the case of ROS scavengers (Table 4). In each series of experiments (repeated at least four times) one ROS scavenger or one phenolic acid was tested. The inhibitory effect of ROS scavengers or phenolic acids on light emission was expressed as percent inhibition (%I) calculated according to the formula: %I = [(A − B)/(A − C)] × 100% where A, B and C are the total light emission from Fe2+-EGTA-H2O2, Fe2+-EGTA-studied compound-H2O2, and medium (H2O in PBS), respectively.
4.5. Statistical Analysis
Results (total light emission or % inhibition of light emission) were expressed as mean (standard deviation) and median and interquartile range (IQR). The comparisons between total light emission from Fe2+-EGTA-H2O2 system and light emission from corresponding samples of modified system (e.g., incomplete system, system with addition of ROS scavengers or phenolic acids, system based on other divalent cations or chelating agents, medium alone) were analyzed with independent-samples (unpaired) t-test or Mann–Whitney U test depending on data distribution which was tested with Kolmogorov-Smirnov-Liliefors test. The Brown-Forsythe test for analysis of the equality of the group variances was used prior to the application of the unpaired t-test and if variances were unequal, the Welch’s t-test was used instead of the standard t-test. The comparisons of % inhibition of light emission caused by ROS scavengers and phenolic acids were performed in the same way. A p value < 0.05 was considered significant.
5. Conclusions
We found that Fenton system composed of Fe2+-EGTA-H2O2 emits light within the range from 380 nm to 630 nm. The UPE of Fe2+-EGTA-H2O2 depends upon •OH and O2− and could be attributed to •OH—induced cleavage of ether bond in the backbone structure of EGTA with consequent formation of 3(R=C)* and photons emission. Plant phenolic acids with known antioxidant properties (caffeic, chlorogenic and ferulic acids) and ascorbic acid significantly quenched UPE what suggests possible application of this phenomenon as the assay for evaluation of antioxidant activities of various phytochemicals. However, further studies involving optimization of the Fenton system parameters, time of UPE measurement, control tests with other types of antioxidants and validation are necessary before the successful development of this assay.
Acknowledgments
This study was supported by a research grant from the Medical University of Lodz No: 503/1-079-01/503-11-001.
Author Contributions
Michal Nowak, Wieslaw Tryniszewski and Dariusz Nowak conceived and designed the experiments. Michal Nowak, Agata Sarniak and Anna Wlodarczyk performed the experiments. Michal Nowak and Piotr J. Nowak analyzed the data. Michal Nowak, Agata Sarniak and Anna Wlodarczyk and Dariusz Nowak contributed reagents, materials and analysis tools. Michal Nowak, Wieslaw Tryniszewski and Dariusz Nowak wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
| UPE | ultra-weak photon emission |
| •OH | hydroxyl radical |
| ROS | reactive oxygen species |
| EDTA | ethylenediaminetetraacetic acid, disodium salt |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| RLU | relative light units |
| SOD | superoxide dismutase |
| DMSO | dimethyl sulfoxide |
| O2− | superoxide radical |
| O2(1Δg) | singlet oxygen |
| 3(R=C)* | triplet excited carbonyl groups |
Appendix A. Chemical Compounds Studied in This Article
Caffeic acid (PubChem CID: 689043); Chlorogenic acid (PubChem CID: 1794427); EDTA disodium salt (PubChem CID: 8758); EGTA (PubChem CID: 6207); Ferulic acid (PubChem CID: 445858); Sodium citrate (PubChem CID: 6224).
References
- Cifra, M.; Pospíšil, P. Ultra-weak photon emission from biological samples: Definition, mechanisms, properties, detection and applications. J. Photochem. Photobiol. B 2014, 139, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Varsavsky, A.I.; Boveris, A.; Chance, B. Oxygen- or organic hydroperoxide-induced chemiluminescence of brain and liver homogenates. Biochem. J. 1981, 198, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Pospíšil, P. Linoleic acid-induced ultra-weak photon emission from Chlamydomonas reinhardtii as a tool for monitoring of lipid peroxidation in the cell membranes. PLoS ONE 2011, 6, e22345. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Kaneda, T. Extra-week chemiluminescence of organ homogenate and blood in tocopherol-deficient rats. J. Nutr. Sci. Vitaminol. 1981, 27, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Kaneda, T.; Takyu, C.; Inaba, H. Characteristics of tissue ultraweak chemiluminescence in rats fed with autoxidized linseed oil. J. Nutr. Sci. Vitaminol. 1983, 29, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Boveris, A.; Chance, B. Chemiluminescence of lipid vesicles supplemented with cytochrome c and hydroperoxide. Biochem. J. 1980, 188, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, H.; Zhou, Y.; Ogawa, N.; Lin, J.M. Self-catalytic degradation of ortho-chlorophenol with Fenton’s reagent studied by chemiluminescence. J. Environ. Sci. 2012, 24, 550–557. [Google Scholar] [CrossRef]
- Ivanova, I.P.; Trofimova, S.V.; Piskarev, I.M.; Aristova, N.A.; Burhina, O.E.; Soshnikova, O.O. Mechanism of chemiluminescence in Fenton reaction. J. Biophys. Chem. 2012, 3, 88–100. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Lu, C.; Lin, J.M. Improved chemiluminescence in fenton-like reaction via dodecylbenzene-sulfonate-intercalated layered double hydroxides. J. Phys. Chem. C 2012, 116, 14711–14716. [Google Scholar] [CrossRef]
- Friedman, A.; Arosio, P.; Finazzi, D.; Koziorowski, D.; Galazka-Friedman, J. Ferritin as an important player in neurodegeneration. Parkinsonism Relat. Disord. 2011, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron and carcinogenesis: From Fenton reaction to target genes. Redox Rep. 2002, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photochem. Photobiol. B 2014, 139, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Pospišil, P. Two-dimensional imaging of spontaneous ultra-weak photon emission from the human skin: Role of reactive oxygen species. J. Biophotonics 2011, 4, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Highly sensitive imaging for ultra-weak photon emission from living organisms. J. Photochem. Photobiol. B 2014, 139, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Sowa, K.; Bartosz, G. Metal chelators react also with reactive oxygen and nitrogen species. Biochem. Biophys. Res. Commun. 2007, 352, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.; Maidt, L.; Poyer, L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem. J. 1990, 269, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B 2014, 139, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wijk, E.P.; Wijk, R.V. Multi-site recording and spectral analysis of spontaneous photon emission from human body. Forsch. Komplementarmed. Klass. Naturheilkd. 2005, 12, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rác, M.; Sedlářová, M.; Pospíšil, P. The formation of electronically excited species in the human multiple myeloma cell suspension. Sci. Rep. 2015, 5, 8882. [Google Scholar] [CrossRef] [PubMed]
- Antiñolo, M.; Ocaña, A.J.; Aranguren, J.P.; Lane, S.I.; Albaladejo, J.; Jiménez, E. Atmospheric degradation of 2-chloroethyl vinyl ether, allyl ether and allyl ethyl ether: Kinetics with OH radicals and UV photochemistry. Chemosphere 2017, 181, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann-Ingrand, S.; Favreliere, S.; Fauconneau, B.; Mauco, G.; Tallineau, C. Plasmalogen degradation by oxidative stress: Production and disappearance of specific fatty aldehydes and fatty alpha-hydroxyaldehydes. Free Radic. Biol. Med. 2001, 31, 1263–1271. [Google Scholar] [CrossRef]
- Castro, G.; Casado, J.; Rodríguez, I.; Ramil, M.; Ferradás, A.; Cela, R. Time-of-flight mass spectrometry assessment of fluconazole and climbazole UV and UV/H2O2 degradability: Kinetics study and transformation products elucidation. Water Res. 2016, 88, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Jellison, J.; Goodell, B. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 2012, 94, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Elliot, A.J. Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Radiat. Phys. Chem. 1986, 27, 241–243. [Google Scholar] [CrossRef]
- Hussain, R.S.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory activity of flavonoids from Prunus davidiana and other flavonoids on total ROS and hydroxyl radical generation. Arch. Pharm. Res. 2003, 26, 809–815. [Google Scholar] [CrossRef] [PubMed]
- De Graft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Totsuka, M.; Shimizu, M. Catechol groups enable reactive oxygen species scavenging-mediated suppression of PKD-NFkappaB-IL-8 signaling pathway by chlorogenic and caffeic acids in human intestinal cells. Nutrients 2017, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Perluigi, M.; Sultana, R.; Agrippino, R.; Calabrese, V.; Butterfield, D.A. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): Insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem. Int. 2006, 48, 318–327. [Google Scholar] [PubMed]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002, 22, 2711–2717. [Google Scholar] [PubMed]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Granieri, L.; Del Pino, A.M.; Mazzoni, M.; Mancinelli, L.; Proietti, P.; Perretti, G.; Palmerini, C.A. Chelating properties of beer: Implications on calcium homeostasis in PE/CA-PJ15 cells. J. Nutr. Intermed. Metab. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Minakata, K.; Fukushima, K.; Nakamura, M.; Iwahashi, H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe and NADPH. J. Clin. Biochem. Nutr. 2011, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- DeGraft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric-reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.C.; Bode, A.M. Biology of free radical scavengers: An evaluation of ascorbate. FASEB J. 1993, 7, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54 (Suppl. 6), 1119S–1124S. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Piasecka, G.; Antczak, A.; Pietras, T. Effect of ascorbic acid on hydroxyl radical generation by chemical, enzymatic and cellular systems. Importance for antioxidant prevention of pulmonary emphysema. Biomed. Biochim. Acta 1991, 50, 265–272. [Google Scholar] [PubMed]
- Nowak, D.; Piasecka, G.; Pietras, T.; Antczak, A. Effect of ascorbic acid on killing of lymphocytes and macrophages by hydrogen peroxide. Biomed. Biochim. Acta 1991, 50, 1079–1086. [Google Scholar] [PubMed]
- Noble, R.W.; Gibson, Q.H. The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J. Biol. Chem. 1970, 245, 2409–2413. [Google Scholar] [PubMed]
- Rosenblum, W.I.; El-Sabban, F. Dimethyl sulfoxide (DMSO) and glycerol, hydroxyl radical scavengers, impair platelet aggregation within and eliminate the accompanying vasodilation of injured mouse pial arterioles. Stroke 1982, 13, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Bancirova, M. Sodium azide as a specific quencher of singlet oxygen during chemiluminescent detection by luminol and Cypridina luciferin analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the ferulic acid, chlorogenic acid and caffeic acid are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).