Abstract
Herein, we report an environmentally friendly, rapid, and convenient ionic liquid ([Et3NH][HSO4])-promoted facile synthesis of ethyl 4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivatives 4(a–f) and 4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5- carbohydrazide derivatives 6(a–f). All the synthesized derivatives 4(a–f) and 6(a–f) were evaluated for their in vitro antifungal and antibacterial activity, by method recommended by National Committee for Clinical Laboratory Standards (NCCLS). The compound 6c bearing a fluoro group on the chromone ring and oxygen and a hydrazino group (–NHNH2) on the pyrimidine ring, was found to be the most potent antibacterial compound amongst the synthesized derivatives. The compound 6f bearing a methoxy group (–OCH3) on the chromone ring and sulphur group on the pyrimidine ring, was found to exhibit equipotent antifungal activity when compared with the standard drug miconazole. A d-alanine-d-alanine ligase (DdlB) enzyme assay study and an ergosterol extraction and quantitation assay study were performed to predict the mode of action of the synthesized compounds. A molecular docking study was performed to predict the binding interactions with receptors and mode of action of the synthesized derivatives. Further, analysis of the ADMET parameters for the synthesized compounds has shown that these compounds have good oral drug-like properties and can be developed as oral drug candidates. To establish the antimicrobial selectivity and safety, the most active compounds 6c and 6f were further tested for cytotoxicity against the human cancer cell line HeLa and were found to be non-cytotoxic in nature. An in vivo acute oral toxicity study was also performed for the most active compounds 6c and 6f and the results indicated that the compounds are non-toxic in nature.
1. Introduction
In the past few decades many drug-resistant human pathogenic microbes have been observed [1] and this is a serious public health dilemma in a wide range of infectious disease [2,3]. Failure in the antimicrobial treatment is mostly observed due to the resistance developed in the microbes, which leads to increased risks to mortality and sometimes contributes to complications. To prevail over this challenge the best way is the design and development of novel bioactive compounds which will be effective against strains which have developed resistance. In spite of the large number of antibiotics and chemotherapeutics available for medical use, antimicrobial resistance has created a substantial medical need for new classes of antimicrobial agents. The design and synthesis of novel antimicrobials agents will forever remain an area of gigantic connotation [4,5]. Novel and potent antimicrobial agents can be obtained by revamping the structure of the available antimicrobial agents or combining together two or more different active pharmacophores present in antimicrobial agents into one molecule.
Coumarins are an elite class of naturally occurring compounds with promising therapeutic perspectives [6,7]. This compound has become obligatory structural unit that is utilitarian in medicinal chemistry, with a wide variety of activities such as anticancer [8], antioxidant [9], antiplasmodial [10], antimalarial [11], antirhinovirus [12], antifungal [13] and antibacterial properties [14]. 4-oxo-4H-Chromen-3-carbaldehyde (3-formylchromone) is a most significant starting point for the synthesis of quite a lot of biologically active compounds due to the existence of an unsaturated keto functional group, a conjugated second carbonyl group at the C-3 position, and an electrophilic centre at the C-2 position. Much research has been focused on the inhibition of bacterial growth using naturally occurring coumarins such as xanthoxin, herniarin, umbelliferone, and scopoletin and on the antifungal activity of umbelliferone, scopoletin, and coumarin itself [15]. The coumarin scaffold is present in many antimicrobial agents such as semigrabrin, novobiocin, quercetin, apigenin, etc.
Nitrogen-containing heterocycles such as pyrimidine are a subject of continuous interest because of their diverse biological activities and occurrence in natural medicinal plants. Pyrimidine and its derivatives are used as pesticides, herbicides and insecticides [16,17]. Marketed antifungal drugs such as flucytosine, voriconazole and albaconazole also contain pyrimidine nuclei [18].
Molecular hybridization is a rational drug design approach whereby new chemical entities are obtained by combining two or more pharmacophoric units from different bioactive compounds into a single molecule. Through this approach, medicinal chemists hope that the new hybrid derivatives will present better affinity and efficacy when compared to the individual parent drugs, a modified selectivity profile with improved pharmacokinetic and pharmacodynamic properties, dual or multiple modes of action, reduction of undesirable side effects, decreases in drug-drug interactions, reduced emergence or spread of drug resistance in microorganisms and protozoans and lower cost [19]. Pharmacophore hybridization is believed to be analogous to conventional combination therapy, with the exception that the two pharmacophores are covalently linked and available as a single entity. The selection of the two principles in the dual molecule is usually based on their observed (or anticipated) synergistic or additive pharmacological activities to enable the identification of highly active novel chemical entities [20,21,22,23]. In view of this and in continuation to our search for more effective antifungal and antibacterial agents [24,25,26,27,28], we planned the synthesis of coupled systems containing coumarin and pyrimidine rings with appropriate pharmacophores. The design protocol for the synthesized derivatives is as shown in Scheme 1. Considering the importance of both these scaffolds the molecular hybridization technique was used to couple them together, in the hope to getting newer derivatives with the higher antimicrobial activity.
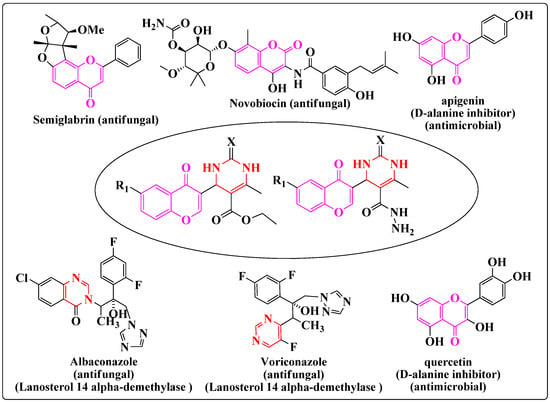
Scheme 1.
The design protocol for the synthesis of the target molecules.
Considering the focus on green synthesis in recent years, ionic liquids have attracted the attention of many researchers. Ionic liquids have been referred to as “designer solvents/green solvents” because of their physical and chemical properties which can be adjusted by varying the cation and anion. Taking into consideration the abovementioned points, we have carried out the synthesis of coumarin-pyrimidine coupled hybrid derivatives 4(a–f) and 6(a–f) using triethylammonium hydrogen sulphate [Et3NH][HSO4] as an solvent and easily recoverable green catalyst [26].
All the synthesized ethyl 4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivatives 4(a–f) and 4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide derivatives 6(a–f) were screened for their in vitro antifungal and antibacterial activity, by the method recommended by National Committee for Clinical Laboratory Standards (NCCLS), using miconazole and d-cycloserine as standard drugs, respectively. Minimum inhibitory concentration (MIC100) values were determined using the standard agar method as per CLSI guidelines [26,29,30,31].
The drug target selection was based on different biological roles such as: (i) cell wall synthesis; (ii) protein synthesis; (iii) nucleic acid synthesis; and (iv) cell metabolism [26]. ATP-dependent d-alanine-d-alanine ligase (Ddl) is one of the parts of biochemical machinery which is involved in biosynthesis of peptidoglycan, as it catalyzes the formation of the terminal d-ala-d-ala dipeptide of the peptidoglycan precursor UDP MurNAc pentapeptide. Bacterial growth is inhibited if the enzyme d-alanine ligase (Ddl) is been inhibited by the designed molecule. d-Alanine ligase (Ddl) enzyme inhibition is an attractive and tenable target in the search for novel and effective antimicrobial drugs [32]. Recently, coumarin derivatives have been reported to inhibit E. coli DdlB enzyme [33]. Hence, the designed and synthesized compounds were appraised for their inhibiton effect using d-alanine-d-alanine ligase (DdlB) enzyme assay study.
The main function of most antifungal agents is to prevent the synthesis of ergosterol, which is a key element of the fungal plasma membrane. Lanosterol 14α-demethylase enzyme plays an important role in ergosterol synthesis in fungi [18]. Exposure of fungi to lanosterol 14α-demethylase enzyme inhibitors causes reduction of ergosterol. This restricts and disturbs the structure of the membrane and its nutrient transport and chitin synthesis functions. The net result is to inhibit fungal growth. Most fungi cannot survive without ergosterol, and the enzymes that participate in its formation have become important targets for drug discovery. Recently, coumarin derivatives have been reported to inhibit cytochrome P450 lanosterol 14α-demethylase (CYP51) [34]. Based on literature reports [35] and the antifungal activity exhibited by the synthesized compounds, an ergosterol extraction and quantitation assay method was used to study the mode of action of the most potent antifungal compound 6f [26].
Molecular docking is a well-known computational method for drug discovery which can be used to mock-up the interaction between a ligand and a protein at the atomic level and to predict, and characterize the behavior of ligands in the binding site of target proteins [36]. The synthesized compounds were thus docked into a homology model of cytochrome P450 lanosterol 14α-demethylase of C. albicans [37] to predict their antifungal activity mode of action. The synthesized compounds were also docked into the E. coli DdlB enzyme (PDB entry: 1IOV [38]) to predict its antibacterial activity mode of action [26].
To evaluate the drug-like properties of the synthesized compounds, the physicochemical parameters based on the Lipinski RO5 (Rule of Five) [39] and Jogersons rule of three were determined using Qikprop v. 3.5 (Schrodinger LLC). This pharmacological investigation of the compounds can support pharmaceutical scientist in selecting the most effective compounds for the development of better drugs [26]. Cytotoxicity studies and in vivo acute oral toxicity examinations of the most active synthesized compounds 6c and 6f were also performed.
2. Result and Discussion
2.1. Chemistry
The utility of triethylammonium hydrogen sulphate [Et3NH][HSO4] in molten state, which is a low cost, mild, non-volatile and non-corrosive acidic ionic liquid, as an efficient Bronsted acid catalyst under solvent-free conditions for the Biginelli reaction is described, as shown in Scheme 2.
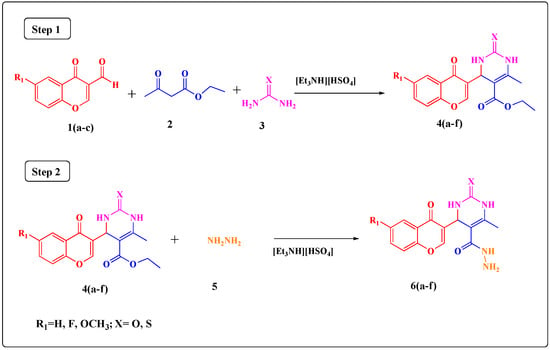
Scheme 2.
Scheme for green synthesis of the target compounds 4(a–f) and 6(a–f).
The model reaction of 4-oxo-4H-chromene-3-carbaldehydes, ethyl acetoacetate and urea in [Et3NH][HSO4] was optimized by investigating various parameters such as the mol percent of catalyst at various temperatures. The triethylammonium hydrogen sulphate [Et3NH][HSO4] ionic liquid was screened as catalyst at various loads such as 5, 10, 15, 20 mol % and at various temperatures such as 80, 90, 100, 110 °C as shown in Table 1. When 5 mol % of the catalyst was used at 100 °C the product 4a was obtained in 72% yield in 150 min. Furthermore, the effect of the amount of catalyst was examined by examining the effect of various catalyst loads on the yield of product and the time required for completion of reaction for compound 4a. When 10 mol % of catalyst was used at 100 °C, compound 4a was obtained in 76% yield in 105 min. When 15 mol % of catalyst was used at 100 °C, 95% of 4a was obtained in 60 min. The same yield resulted when 20 mol % of catalyst was used at 100 °C. Therefore, considering 15 mol % as an efficient amount the reaction was carried out at various temperatures like 80 °C, 90 °C, 100 °C and 110 °C. Temperatures both above and below 100 °C gave lower yields, therefore 15 mol % of the [Et3NH][HSO4] ionic liquid as catalyst and solvent at 100 °C was considered to give the best yield (95%) in the shortest reaction time (60 min) (Entry 3 of Table 1).

Table 1.
Effect of different reaction conditions on [Et3NH][HSO4] catalyzed synthesis of 4a.
These observations make the process under study more expeditious, economic, safe and eco-friendly. The recovery and reusability of the catalyst was investigated for the synthesis of compound 4a. The results are given in Table 2; the recovered catalyst can be reused for at least four additional times in subsequent reactions without a considerable decrease in its catalytic activity.

Table 2.
Reusability of [Et3NH][HSO4] catalyst for model reaction 4a.
A total of six novel ethyl 4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivatives 4(a–f) were synthesized according to the abovementioned green synthetic protocol. The time required for the completion of reactions was around 60–85 min time (monitored by TLC). The yields of the synthesized novel compounds were in the 86–95% range. The structures of the synthesized compounds were confirmed by IR, 1H-NMR, 13C-NMR, mass spectral analysis and elemental analysis.
The synthesized derivatives 4(a–f) (1 mmol) were next allowed to react with hydrazine hydrate (1.2 mmol) under solvent free condition using [Et3NH][HSO4] as a catalyst and also as a solvent to furnish compounds 6(a–f). The catalyst load and the temperature required for the synthesis of 6(a–f) were also studied. The effect of different [Et3NH][HSO4] ionic liquid catalyst loads on the yield of product and time required for completion of reaction were studied at various catalyst loads such as 5, 10, 15 mol % and at various temperatures such as 70, 80, 90 and 100 °C, as shown in Table 3. When 5 mol % of the catalyst was used at 90 °C the product 6a was obtained in 88% yield in 30 min. When 10 mol % catalyst was used at the same temperature, compound 6a was obtained in 87% yield in 30 min. When 15 mol % catalyst was used at 90 °C, compound 6a was obtained in 85% yield in 30 min. Therefore, considering 5 mol % as an efficient amount, the reaction was carried out at various temperatures like 70 °C, 80 °C, 90 °C and 100 °C. At 5 mol % catalyst loading the use of the higher (100 °C) or lower (70 °C, 80 °C) temperatures than 90 °C gave the compound 6a in reduced yields after longer times, therefore, 5 mol % of the [Et3NH][HSO4] ionic liquid as catalyst and solvent was shown to give the best yield (88%) in a short reaction time (30 min) at 90 °C (Entry 3 of Table 3). The recovery and reusability of the catalyst in the synthesis of compound 6a was also investigated. The findings are listed in Table 4; the recovered catalyst can be reused for at least four additional times in subsequent reactions without any considerable decrease in its catalytic activity.

Table 3.
Effect of different reaction conditions on [Et3NH][HSO4] catalyzed synthesis of 6a.

Table 4.
Reusability of [Et3NH][HSO4] catalyst for model reaction 6a.
A total of six 4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetra-hydropyrimidine-5-carbohydrazides 6(a–f) were synthesized according to the abovementioned green synthetic protocol. The time required for the completion of reactions was around 20–35 min (monitored by TLC), and the yields of the synthesized novel compounds were in the 80–95% range The structures of the synthesized compounds were confirmed by IR, 1H-NMR, 13C-NMR, mass spectral analysis and elemental analysis.
2.2. Biological Activity
2.2.1. In Vitro Antibacterial Activity
All the synthesized compounds 4(a–f) and 6(a–f) were screened for their in vitro antibacterial activity against Staphylococcus aureus (NCIM-2901), Escherichia coli 1411 (standard laboratory strains) and Escherichia coli SM1411 (an acrAB deficient derivative of 1411). Minimum inhibitory concentration (MIC100) values were determined using methods recommended by National Committee for Clinical Laboratory Standards (NCCLS) [26,29,30,31]. Dimethyl sulfoxide (DMSO) was used as solvent control. The results of the in vitro antibacterial activity are presented in Table 5. The compound 6c bearing a fluoro group on the chromone ring and oxygen and –NHNH2 groups on the pyrimidine ring was found to be the most potent antibacterial compound against E. coli 1411, E. coli SM1411 and S. aureus, with MIC100 values of 14 µg/mL, 14 µg/mL and 32 µg/mL, respectively. The compound 4c was found to be the most potent antibacterial compound among the synthesized derivatives against S. aureus, with the MIC100 value 30 µg/mL.

Table 5.
In vitro antibacterial activity of the synthesized compounds 4(a–f) and 6(a–f).
The compound 6d bearing a fluoro group on the chromone ring and –NHNH2 and sulphur groups on the pyrimidine ring was found to be equipotent to the standard drug d-cycloserine against S. aureus, with a MIC100 value of 32 µg/mL. The compounds 4d, 4e, 6e and 6f were found to be less active against E. coli 1411, E. coli SM1411 and S. aureus.
2.2.2. In Vitro Antifungal Activity
The newly synthesized derivatives 4(a–f) and 6(a–f) were screened for their in vitro antifungal activity against different yeast and filamentous fungal pathogens. Minimum inhibitory concentration (MIC100) values for in vitro antifungal activity were determined by the methods recommended by National Committee for Clinical Laboratory Standards (NCCLS) [26,29,30,31]. Miconazole was used as standard drug. Dimethyl sulfoxide (DMSO) was used as solvent control. The MIC100 (µg/mL) of all the tested compounds and that of the reference drug miconazole are listed in Table 6. Results from Table 6 indicated that all the synthesized compounds showed good to moderate in vitro antifungal activity against the tested fungal strains.

Table 6.
In vitro antifungal activity of the synthesized compounds 4(a–f) and 6(a–f).
The compounds 6f, 6e and 4e were found to be the most potent antifungal agents among the synthesized derivatives. The compound 6f bearing a –OCH3 group on the chromone ring and a sulphur group on the pyrimidine ring, was found to exhibit equipotent antifungal activity when compared with the standard drug miconazole. Compound 6f had MIC100 values of 24 µg/mL for C. albicans, 25 µg/mL for C. glabrata, 28 µg/mL for F. oxysporum, 33 µg/mL for A. fumigates, 15 µg/mL for A. flavus, 16 µg/mL for A. niger and 15 µg/mL for C. neoformans. Compound 6e was found to be the second most potent antifungal agent among the synthesized compounds, with MIC100 values 28 µg/mL for C. albicans, 30 µg/mL for C. glabrata, 30 µg/mL for F. oxysporum, 32 µg/mL for A. fumigates, 16 µg/mL for A. flavus, 18 µg/mL for A. niger and 16 µg/mL for C. neoformans. Compound 6f exhibited more potent in vitro antifungal activity than the compounds 6b and 6d, which may be due to the presence of the electron donating –OCH3 substituent on the chromone ring. The compounds 4a, 4b and 4c were found to be the least active antifungal agents among the synthesized series.
From the structure activity relationship (SAR) it could be manifested that the nuclei such as chromone and pyrimidine pharmacophores along with X = O/S are responsible for the antimicrobial activity. It could be deduced from the structure activity relationships (SARs), in general that the moderation on the chromone ring and the pyrimidine ring appreciably influenced the antimicrobial activity. The compounds 6c with R1 = F group on the chromone ring and X = O group and –NHNH2 group on the pyrimidine ring was found to be more potent antibacterial agents than the standard drug d-cycloserine against all the tested bacterial strains. The compound 4c with R1 = F group on the chromone ring and X = O group and –OC2H5 group on the pyrimidine ring was found to be a more potent antibacterial agent than the standard drug d-cycloserine against the S. aureus bacterial strain. The compound 6d with R1 = F group on the chromone ring and X = S group and –NHNH2 group on the pyrimidine ring was found to be equipotent to the standard drug d-cycloserine against the E. coli bacterial strain. The compounds 4b, 4e, 4f, 6b, 6d and 6f with the sulphur group on the pyrimidine ring were found to be more moderately active antibacterial agents than those with oxygen groups on the pyrimidine ring 4a, 4c, 4d, 6a, 6c and 6e. Replacement of the –OC2H5 group with a –NHNH2 group on the pyrimidine ring significantly increased the in vitro antifungal activity. The compound 6f with the –OCH3 group on the chromone ring and NHNH2 and S group on the pyrimidine ring was found to be the most active antifungal agent among the synthesized series against all the tested fungal strains. The compound 4e (–OCH3 on the chromone ring and S group and –OC2H5 group on the pyrimidine ring) and compound 6e (–OCH3 on the chromone ring and S group and on the pyrimidine ring) showed excellent in vitro antifungal activity against all the selected fungal strains. The compounds 4b, 4e, 4f, 6b, 6d and 6f with the sulphur group on the pyrimidine ring were found to be more active antifungal agents than those with oxygen group on the pyrimidine ring 4a, 4c, 4d, 6a, 6c and 6e. The chromone ring when substituted with an electron donating group such as R1 = OCH3 (4d, 4e, 6e and 6f) was found to show better antifungal activity than when substituted with an electron withdrawing group like R1 = F (4c, 4f, 6c and 6d).
2.2.3. Ergosterol Extraction and Quantitation Assay
Considering that ergosterol is an important fungal cell membrane lipid, changes in its biosynthetic pathway may also cause damage to the fungal cell, preventing growth in a way similar as azole compounds such as miconazole, fluconazole, etc. do [36]. To reveal the antifungal mechanism of the most potent synthesized compound 6f, its influence on the sterol composition on the C. albicans membrane was monitored by analyzing the changes in the sterol composition in the cells of C. albicans by U.V. analysis. The assay was performed at various concentrations of the most potent synthesized compound 6f such as MIC/16, MIC/8, MIC/4, MIC/2 and MIC value to quantify the content of sterol produced by C. albicans, as shown in Figure 1.
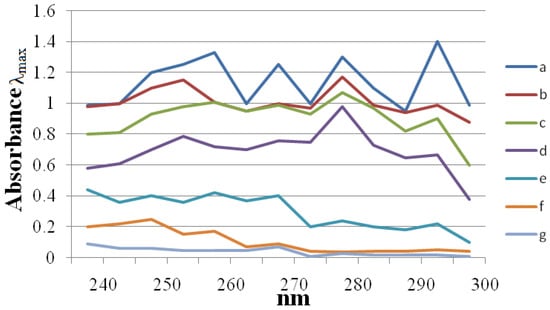
Figure 1.
UV spectrophotometric sterol profile of C. albicans (NCIM3471) treated with, 0 (curve a), 1.5 (curve b), 3 (curve c), 6 (curve d), 12 (curve e) and 24 (curve f) µg/mL of synthesized compound 6f and 25 (curve g) µg/mL of miconazole. Sterols were extracted from cells and spectral profiles between 240 and 300 nm were determined.
Curve “a” in Figure 1 represents the negative control (no compound). The absorption of sterols extracted from fungal culture at the wavelengths of 230 nm, and 281.5 nm was analyzed and the results obtained are as shown in Figure 1 for compound 6f. Ergosterol and an intermediate of the metabolic pathway, ergosterol-24(28) dehydroergosterol (DHE), absorb energy at 281.5 nm, but only DHE shows an intense absorption at 230 nm. Changes in this absorption pattern are therefore indicative of interference in the synthetic pathway of ergosterol [26,40]. There was a change in the absorption pattern for the synthesized compound 6f as shown in Figure 1, which proves that the synthesized compound 6f inhibits ergosterol biosynthesis by inhibiting the enzyme cytochrome P450 lanosterol 14 α-demethylase of C. albicans.
Curve “a” shows an intense ergosterol peak at 281.5 nm signifying the presence of this compound. Curve “g” in Figure 1 indicates the inhibition of ergosterol by miconazole (standard drug) at its MIC value. As the concentration of the synthesized compound 6f increases the intensity of the ergosterol peak at 281.5 nm decreases, which indicates a decrease in the concentration of ergosterol in the culture medium. In Figure 2 there is almost a flat peak of compound 6f at its MIC100 value, which is almost similar to that of standard drug miconazole at its MIC100 value. These results suggest that the compound 6f might inhibit the important enzyme in fungus, i.e., lanosterol 14α-demethylase, in a similar manner to that accepted mechanism of the standard drug miconazole.
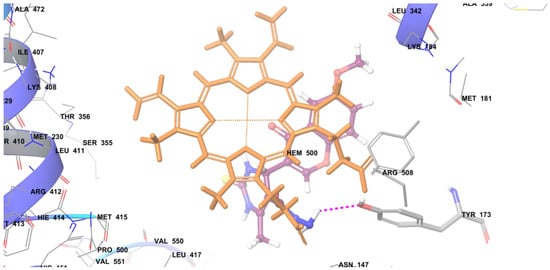
Figure 2.
The docking pose of compound 6f in the active pocket of cytochrome P450 lanosterol 14α-demethylase of C. albicans. (Light orange colored structure represents the heme co-factor, Pink bond represents the hydrogen bonding between ligand and receptor, Purple colored structure represents the molecule).
2.2.4. d-Alanine-d-alanine ligase (Ddl) Enzyme Assay
The key enzyme involved in the peptidoglycan biosynthesis is d-alanine-d-alanine ligase (Ddl), making it an important target for drug discovery. Inhibition of DdlB as an essential enzyme in either Gram-positive or Gram-negative organisms leads to the loss of cell shape and integrity followed by bacterial death. The synthesized derivatives 4(a–f) and 6(a–f) were tested for their inhibitory activity on DdlB from E.coli. The results are presented as IC50 values. The compounds 4a, 4c, 6a, 6b, 6c and 6d were found to be better DdlB enzyme inhibitors than the standard drug d-cycloserine. The compound 6c bearing R1 = fluoro on the chromone ring and X = O and –NHNH2 groups on the pyrimidine ring, was found to be the most potent DdlB enzyme inhibitor among the synthesized series. The compounds 4a, 4c, 4d, 6a, 6c and 6e with an oxygen group on the pyrimidine ring were found to be good inhibitors of DdlB enzyme.
2.3. Computational Study
2.3.1. Molecular Docking
Docking is an effective and reliable approach to simulate the probable binding mode of ligands and proteins. Recently, coumarin derivatives have been reported to inhibit cytochrome P450 lanosterol 14α-demethylase (CYP51) [35]. Based on literature reports [36] and the antifungal activity exhibited by the synthesized compounds, molecular docking studies were performed in the active site of cytochrome P450 lanosterol 14α-demethylase enzyme of C. albicans [38] to understand its mechanism of action. In silico approaches like molecular docking have become very useful to identify potential targets for different ligands and they are associated with the thermodynamic interactions with the target enzyme governing the inhibition of the target microorganism [26]. The docking scores of the synthesized compounds 4(a–f) and 6(a–f) are presented in Table 7.

Table 7.
The docking scores of the synthesized compounds 4(a–f) and 6(a–f) for cytochrome P450 lanosterol 14α-demethylase enzyme of C. albicans.
The docking data results revealed that the synthesized compounds were held in the active pocket by forming various interactions with the cytochrome P450 lanosterol 14α-demethylase of C. albicans such as hydrogen and hydrophobic interactions. The docking score of compound 6f i.e −7.24 was found to be almost similar to that of standard drug miconazole i.e., −7.33. Figure 2 represents the docking pose of compound 6f in the active pocket of cytochrome P450 lanosterol 14α-demethylase of C. albicans and it can be seen that the NH2 group of compound 6f forms a hydrogen bond interaction with the HEM 500 and TYR 173. The phenyl ring containing a methoxy group fits well into the hydrophobic pocket of the enzyme.
On the basis of the in vitro antifungal activity, ergosterol extraction and quantitation assay and molecular docking study result, it was established that the synthesized compounds could inhibit the enzyme cytochrome P450 lanosterol 14α-demethylase of C. albicans. From the docking score data and the in vitro antifungal activity results it can be concluded that the compounds 4e, 6e and 6f have good potential to inhibit the enzyme cytochrome P450 lanosterol 14α-demethylase of C. albicans.
Inhibition of Ddl in bacteria by drugs hinders the growth of bacteria, which makes this enzyme an irresistible and important target for the discovery of novel antimicrobial drug. Hence, molecular docking was performed against E. coli DdlB (PDB entry: 1IOV). The docking scores of the synthesized compounds 4(a–f) and 6(a–f) are presented in Table 8. The docking results indicated that the compounds were held in the active pocket by combination of various hydrogen and hydrophobic interactions with DdlB enzyme. The docking results revealed that, the highest binding compound to DdlB enzyme was compound 6c with a G-Score of −6.71.

Table 8.
Docking score of the synthesized compounds 4(a–f) and 6(a–f) for DdlB enzyme.
From the in vitro antibacterial data, d-alanine-d-alanine enzyme assay study and molecular docking study it was found that the synthesized compounds 6a, 6b, 6c and 6d are capable of inhibiting the DdlB enzyme. Figure 3 represents the docking pose of compound 6c into the active pocket of DdlB enzyme. The compound shows a hydrogen bond interaction with amino acid residue LYS159.
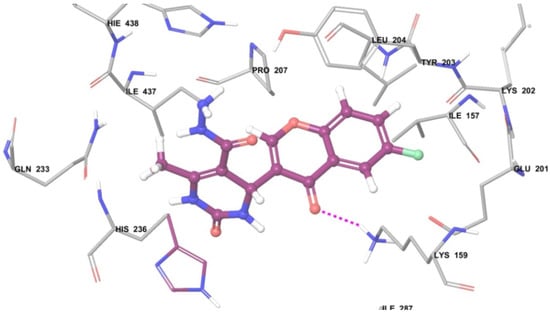
Figure 3.
The docking pose of compound 6c into the active pocket of DdlB enzyme. (Pink bond represents the hydrogen bonding between ligand and receptor, Purple colored structure represents the molecule).
2.3.2. In Silico Absorption, Distribution, Metabolism, Elimination and Toxicity (ADMET) Prediction
2D structures of all the synthesized ethyl 4-(6-substituted-4-oxo-4H-chromen-3-yl)- 6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate and 4-(6-substituted-4-oxo-4H- chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide derivatives 4(a–f) and 6(a–f) were subjected to in silico pharmacokinetic screening using Qikprop v. 3.5 (Schrodinger LLC) to study compliance with the Lipinski rule of five and Jorgensen’s rule of three. Understanding of in silico physicochemical pharmacokinetic parameters is imperative to predict the oral bioavailability of the synthesized compounds 4(a–f) and 6(a–f). It was contemplated that none of the synthesized compounds 4(a–f) and 6(a–f) violated Lipinski rule of 5 and Jorgensen’s rule of three, as shown in Table 9 and Table 10, respectively. The synthesized compounds 4(a–f) and 6(a–f) showed polar surface area (PSA) value ranges between 96.6 to 160.3 Å, as shown in Table 9, which suggests the good cell permeability properties of the synthesized compounds [41].

Table 9.
In silico physicochemical pharmacokinetic parameters important for good bioavailability according to Lipinski rule of 5 for the synthesized compounds 4(a–f) and 6(a–f).

Table 10.
In silico physicochemical pharmacokinetic parameters important for good oral bioavailability according to Jorgensen’s rule of 3 for the synthesized compounds 4(a–f) and 6(a–f).
All the synthesized compounds 4(a–f) and 6(a–f) have Log S values within the desired range (−5.11–4.04) as shown in Table 10. The synthesized compounds showed BIPCaco-2 value ranges between 48.37 to 1137.55, which indicates good in silico intestinal absorption. The synthesized compounds exhibited MDCK values which range from 18.73 to 2820.05, indicating good in silico oral absorption [42]. The synthesized compounds showed Log Khsa values ranging between −0.01 to 0.24, as shown in Table 10, which is an indication that a significant proportion of the compounds are likely to circulate freely in the blood stream and hence reach the target sites. The most active antimicrobial compounds 6c, 6e and 6f showed 69.97%, 64.09% and 79.39% absorption, respectively. The HERG K+ channel, which is best known for its contribution to the electrical activity of the heart that coordinates the heart’s beating, appears to be the molecular target responsible for the cardiac toxicity of a wide range of therapeutic drugs [43]. Thus, HERG K+ channel blockers are potentially toxic and the predicted IC50 values often provide reasonable predictions for cardiac toxicity of drugs in the early stages of drug discovery [26,44]. From the Log HERG values in Table 10 it is observed that all the synthesized compounds 4(a–f) and 6(a–f) are non-toxic in nature.
2.4. In Vitro Cytotoxicity Study
Toxicity scrutiny of synthesized compounds at the early phase of research paves the way to clinical trials and reduces the failure of potential therapeutics at later stages of clinical trials [24]. To scrutinize the safety and selectivity of antimicrobial activity of the synthesized compounds, the compounds 6c and 6f were appraised for their toxicity against HeLa (a human cervical cancer cell line) and PC-3 (human prostate cancer cell line) by the Sulforhodamine B (SRB) assay using adriamycin as a standard drug [26,45]. The cytotoxic effect of the synthesized compounds 6c and 6f was checked on cancer cell lines using the concentration range between 80 and 0.55 μg/mL, to determine the 50% growth inhibition (GI50). The results obtained are summarized in Table 11. The results indicate that in cytotoxicity studies, the most active synthesized compounds 6c and 6f can be considered as antimicrobial agent leads due to their lack of cell toxicity against HeLa at the maximum concentration evaluated. Figure 4 represents that cell inhibition did not occur even up to 80 µg/mL concentration of the synthesized compounds 6c and 6f and hence they can be considered as non-toxic in nature and selective in their antimicrobial activity.

Table 11.
In vitro cytotoxicity study of the synthesized compounds 6c and 6f.
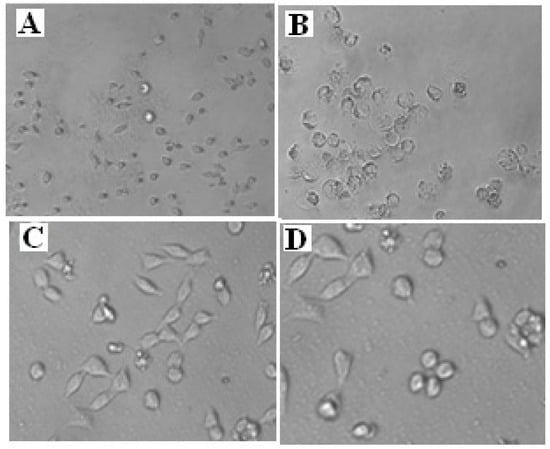
Figure 4.
Images of in vitro cytotoxicity screening against HeLa (Human cervical cancer cell line) for Control (A), Positive control (B), Compound 6c (C) at 80 µg/mL concentration, Compound 6f (D) at 80 µg/mL concentration.
2.5. In Vivo Acute Oral Toxicity Study and Behavioral Study
Animals treated with the newly synthesized compounds 6c and 6f were free of any toxicity as per acceptable range given by the OECD guideline No. 425 and no mortality was found up to 2000 mg/kg, which indicated that the lethal dose of the compounds is above 2000 mg/kg body weight in mice and that the compounds 6c and 6f can be considered to be safe and could be developed in the future as good antimicrobial agents. The in vivo acute oral toxicity and gross behavioral study results of the newly synthesized compounds 6c and 6f are as listed in Table 12 [26].

Table 12.
In vivo acute oral toxicity study and gross behavioral studies of the synthesized compounds 6c and 6f.
3. Materials and Methods
3.1. General Information
All the reactions were performed in oven-dried glassware. All reagents and solvents were used as obtained from the supplier or recrystallized/redistilled unless otherwise noted. The purity of the synthesized compounds was monitored by ascending thin layer chromatography (TLC) on silica gel-G (Merck, Darmstadt, Germany) coated aluminum plates, visualized by iodine vapor. Melting points were determined in open capillary tubes and are uncorrected. Infrared (IR) spectra were recorded on a PS 4000 FTIR instrument (JASCO, Tokyo, Japan) using KBr pellets. Elemental analyses (C, H, and N) were done with a FLASHEA 112 analyzer (Shimadzu, Mumbai, Maharashtra, India) and all analyses were consistent (within 0.4%) with theoretical values. The 1H-NMR and 13C-NMR spectra of synthesized compounds were recorded on an Avance II 400 NMR spectrometer (Bruker, Billerica, MA, USA) at 400/100 MHz frequency in DMSO-d6 or CDCl3 and using TMS as internal standard (chemical shift δ values are expressed in ppm). Mass spectra were scanned on a Micromass Q-Tof system (Waters, Manchester, UK) [26].
3.2. Synthesis of Ethyl 4-(6-Substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydro- pyrimidine-5-carboxylate Derivatives 4(a–f)
A mixture of substituted 4-oxo-4H-chromene-3-carbaldehydes 1(a–c) (1 mmol), ethyl acetoacetate (2) (1 mmol), urea/thiourea (3) (1 mmol) and [Et3NH][HSO4] (15 mol %) was refluxed under solvent-free conditions at 100 °C for the required time while the reactions were monitored by TLC. After completion of the reaction, the reaction mixture was poured into crushed ice and stirred for 5 min. The solid obtained was filtered, washed with cold water and then recrystallized from ethanol to afford the pure product. The ionic liquid present in the filtrate was recovered and was used for other reactions.
Ethyl 6-methyl-2-oxo-4-(4-oxo-4H-chromen-3-yl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate 4a. Yield 95%; M.P.: 270–272 °C; IR (KBr vmax in cm−1): 3238 (N–H stretching), 3005 (C–H stretching), 2900 (–CH3 stretching), 2815 (alkyl CH stretching), 1746 (C=O stretching), 1601 (C=C stretching), 1454 (CH bending of CH2), 1356 (C–N stretching), 1002 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 1.14 (t, J = 7.10 Hz, 3 H, CH3), 2.28 (s, 3 H, CH3), 4.05 (q, J = 7.04 Hz, 2 H, CH2), 4.72 (d, J = 1.49 Hz, 1H, CH), 7.41–7.59 (m, 3H, aromatic), 7.75 (s, 1H, NH), 8.05 (s, 1H, aromatic), 8.10 (d, J = 1.44 Hz, 1H, aromatic), 9.12 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 14.45, 18.51, 45.91, 61.37, 101.94, 118.07, 121.61, 123.87, 126.27, 126.68, 133.85, 149.33, 150.72, 155.65, 155.77, 167.37, 172.17; MS: m/z: 329.21 [M + 1]+; Anal. Calcd. for C17H16N2O5: C, 62.19; H, 4.91; N, 8.53. Found: C, 62.20; H, 4.93; N, 8.50.
Ethyl 6-methyl-4-(4-oxo-4H-chromen-3-yl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 4b. Yield 87%; M.P.: 210–212 °C; IR (KBr vmax in cm−1): 3235 (N–H stretching), 3005 (C–H stretching), 2905 (–CH3 stretching), 2815 (CH alkyl stretching), 1600 (C=C stretching), 1454 (CH bending of CH2), 1360 (C–N stretching), 1140 (C=S stretching), 1002 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 1.17 (t, J = 7.10 Hz, 3 H, CH3), 2.29 (s, 3 H, CH3), 4.74 (d, J = 1.49 Hz, 1H, CH), 7.47–7.74 (m, 3H, aromatic), 8.03 (s, 1H, NH), 8.06 (s, 1H, aromatic), 8.12 (d, J = 1.44 Hz, 1H, aromatic), 8.89 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 14.50, 18.44, 50.62, 61.77, 106.24, 118.71, 121.32, 123.99, 126.12, 133.91, 150.11, 155.60, 160.39, 167.33, 171.94, 177.89; MS: m/z: 345.01 [M + 1]+; Anal. Calcd. for C17H16N2O4S: C, 59.29; H, 4.68; N, 8.13. Found: C, 59.31; H, 4.69; N, 8.10.
Ethyl 4-(6-fluoro-4-oxo-4H-chromen-3-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 4c. Yield 92%; M.P.: 200–202 °C; IR (KBr vmax in cm−1): 3230 (N–H stretching), 3000 (C–H stretching), 2900 (–CH3 stretching), 2815 (CH alkyl stretching), 1745 (C=O stretching), 1600 (C=C stretching), 1454 (CH bending of CH2), 1364 (C–N stretching), 1002 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 1.15 (t, J = 7.10 Hz, 3 H, CH3), 2.29 (s, J = 2.11 Hz, 3 H, CH3), 4.09 (q, J = 7.04 Hz, 2 H, CH2), 4.74 (d, J = 1.51 Hz, 1H, CH), 6.91–7.11 (m, 2 H, aromatic), 7.43 (s, 1 H, NH), 7.74 (d, J = 2.93 Hz, 1 H, aromatic) 8.06 (s, 1 H, aromatic), 9.13 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 14.22, 18.11, 45.51, 61.70, 101.99, 111.52, 118.71, 119.23, 122.00, 126.88, 149.14, 150.79, 155.13, 156.11, 164.19, 167.19, 172.10; MS: m/z: 347.11 [M + 1]+; Anal. Calcd. for C17H15FN2O5: C, 58.96; H, 4.37; N, 8.09. Found: C, 58.99; H, 4.39; N, 8.04.
Ethyl 4-(6-methoxy-4-oxo-4H-chromen-3-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 4d. Yield 88%; M.P.: 230–232 °C; IR (KBr vmax in cm−1): 3238 (N–H stretching), 3005 (C–H stretching), 2900 (–CH3 stretching), 2845 (–OCH3 stretching), 2815 (alkyl CH stretching), 1742 (C=O stretching), 1600 (C=C stretching), 1454 (CH bending of CH2), 1362 (C–N stretching), 1002 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 1.17 (t, J = 7.10 Hz, 3 H, CH3), 2.29 (s, J = 2.11 Hz, 3 H, CH3), 3.80 (s, 3 H, O CH3), 4.10 (q, J = 7.04 Hz, 2 H, CH2) 4.74 (d, J = 1.51 Hz, 1H, CH), 7.37 (d, J = 8.90 Hz, 1H), 7.42 (s, 1H, NH), 7.48–7.52 (m, 2 H), 8.06 (s, 1H, aromatic), 9.13 (s, 1 H, NH); 13C-NMR (DMSO-d6, δC ppm): 14.45, 18.51, 45.91, 55.08, 61.37, 101.94, 107.44, 117.98, 119.35, 122.42, 149.33, 150.72, 152.63, 155.65, 156.18, 167.37, 171.94; MS: m/z: 359.53 [M + 1]+; Anal. Calcd. for C18H18N2O6: C, 60.33; H, 5.06; N, 7.82. Found: C, 60.35; H, 5.09; N, 7.80.
Ethyl 4-(6-methoxy-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 4e. Yield 86%; M.P.: 222–224 °C; IR (KBr vmax in cm−1): 3235 (N–H stretching), 3008 (C–H stretching), 2908 (–CH3 stretching), 2845 (–OCH3 stretching), 2813 (alkyl CH stretching), 1740 (C=O stretching), 1600 (C=C stretching), 1454 (CH bending of CH2), 1360 (C–N stretching), 1148 (C=S stretching), 1002 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 1.17 (t, J = 7.10 Hz, 3 H, CH3), 2.29 (s, 3 H, CH3), 3.80 (s, 3 H, O CH3), 4.11 (q, J = 7.04 Hz, 2 H, CH2), 4.73 (d, J = 1.49 Hz, 1 H), 7.37 (d, J = 8.90 Hz, 1 H), 7.36 (s, 1 H, NH), 7.48–8.08 (m, 3 H), 8.89 (s, 1 H, NH); 13C-NMR (DMSO-d6, δC ppm): 14.45, 18.51, 49.08, 55.80, 61.37, 104.43, 107.44, 117.98, 119.35, 122.42, 126.68, 149.33, 152.63, 154.52, 156.18, 167.37, 171.94, 177.89; MS: m/z: 375.17 [M + 1]+; Anal. Calcd. for C18H18N2O5S: C, 57.74; H, 4.85; N, 7.48. Found: C, 57.77; H, 4.86; N, 7.46.
Ethyl 4-(6-fluoro-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 4f. Yield 92%; M.P.: 228–230 °C; IR (KBr vmax in cm−1): 3230 (N–H stretching), 3000 (C–H stretching), 2910 (–CH3 stretching), 2810 (alkyl CH stretching), 1742 (C=O stretching), 1605 (C=C stretching), 1450 (CH bending of CH2), 1360 (C–N stretching), 1140 (C=S stretching), 1005 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 1.15 (t, J = 7.10 Hz, 3 H, CH3), 2.29 (s, 3 H, CH3), 4.11 (q, J = 7.04 Hz, 2 H, CH2), 4.73 (d, J = 1.49 Hz, 1 H, CH), 6.91–7.74 (m, 3 H, aromatic), 7.76 (s, 1 H, NH), 8.08 (s, 1 H, aromatic), 8.89 (s, 1 H, NH); 13C-NMR (DMSO-d6, δC ppm): 14.44, 18.46, 50.66, 61.79, 106.33, 111.50, 118.73, 119.31, 122.18, 126.89, 149.15, 152.36, 160.19, 162.58, 167.79, 171.96, 177.88; MS: m/z: 363.15 [M + 1]+; Anal. Calcd. for C17H15FN2O4S: C, 56.35; H, 4.17; N, 7.73. Found: C, 56.37; H, 4.19; N, 7.70.
3.3. Synthesis of 4-(6-Substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydro- pyrimidine-5-carbohydrazide Derivatives 6(a–f)
A mixture of ethyl4-(6-substituted-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo/oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivative 4(a–f) (1 mmol), hydrazine hydrate (5) (1.2 mmol) and [Et3NH][HSO4] (5 mol %) was refluxed under solvent-free conditions at 90 °C for the required time, while reaction completion was monitored by TLC. After completion of the reaction, the reaction mixture was poured into crushed ice and stirred for 5 min. The solid obtained was filtered, washed with cold water and then recrystallized from ethanol to afford the pure product. The ionic liquid present in the filtrate was recovered and was used for other reactions.
6-Methyl-2-oxo-4-(4-oxo-4H-chromen-3-yl)-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide 6a. Yield 88%; M.P.: 256–258 °C; IR (KBr vmax in cm−1): 3520 (NH2 stretching), 3450 (N–H stretching), 3000 (C–H stretching), 2900 (–CH3 stretching), 1742 (C=O stretching), 1605 (C=C stretching), 1368 (C–N stretching), 1005 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 2.03 (s, 3H, CH3), 3.13 (s, 2 H, NH2), 4.74 (d, J = 1.51 Hz, 1H, CH), 7.08 (s, 1 H, NH), 7.42 (s, 1H, NH), 7.47–8.13 (m, 5H, aromatic), 9.39 (s, 1 H, NH); 13C-NMR (DMSO-d6, δC ppm): 18.49, 46.11, 102.31, 118.71, 121.61, 123.44, 126.23, 126.85, 133.88, 149.45, 150.71, 155.36, 159.17, 168.62, 172.49; MS: m/z: 315.25 [M + 1]+; Anal. Calcd. for C15H14N4O4: C, 57.32; H, 4.49; N, 17.83. Found: C, 57.32; H, 4.49; N, 17.83.
6-Methyl-4-(4-oxo-4H-chromen-3-yl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide 6b. Yield 88%; M.P.: 198–200 °C; IR (KBr vmax in cm−1): 3515 (–NH2 stretching), 3450 (N–H stretching), 3000 (C–H stretching), 2908 (–CH3 stretching), 1742 (C=O stretching), 1600 (C=C stretching), 1360 (C–N stretching), 1145 (C=S stretching), 1002 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 2.26 (s, 3 H, CH3), 3.13 (s, 2H, NH2), 4.74 (d, J = 1.49 Hz, 1 H, CH), 7.08 (s, 1 H, NH), 7.47 (s, 1 H, NH), 7.59–8.13 (m, 5 H, aromatic), 8.89 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 18.97, 51.16, 106.11, 118.23, 121.14, 123.43, 126.28, 126.81, 133.69, 150.11, 155.49, 160.19, 168.66, 172.32, 177.80; MS: m/z: 331.29 [M + 1]+; Anal. Calcd. for C15H14N4O3S: C, 54.53; H, 4.27; N, 16.96. Found: C, 54.55; H, 4.29; N, 16.93.
4-(6-Fluoro-4-oxo-4H-chromen-3-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide 6c. Yield 95%; M.P.: 210–212 °C; IR (KBr vmax in cm−1): 3510 (NH2 stretching), 3455 (N–H stretching), 3000 (C–H stretching), 2910 (–CH3 stretching), 1742 (C=O stretching), 1605 (C=C stretching), 1365 (C–N stretching), 1005 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 2.03 (s, 3 H, CH3), 3.13 (s, 2H, NH2), 4.74 (d, J = 1.51 Hz, 1 H, CH), 6.91 (d, J = 8.93 Hz, 1H), 7.08 (s, 1H, NH), 7.10–8.06 (m, 4 H, aromatic), 9.39 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 18.26, 46.83, 106.12, 109.11, 118.36, 121.33, 123.39, 126.10, 147.77, 150.18, 155.60, 155.99, 162.45, 168.20, 172.45; MS: m/z: 333.55 [M + 1]+; Anal. Calcd. for C15H13FN4O4: C, 54.22; H, 3.94; N, 16.86. Found: C, 54.25; H, 3.96; N, 16.83.
4-(6-Fluoro-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide 6d. Yield 90%; M.P.: 146–148 °C; IR (KBr vmax in cm−1): 3512 (NH2 stretching), 3458 (N–H stretching), 3000 (C–H stretching), 2912 (–CH3 stretching), 1742 (C=O stretching), 1605 (C=C stretching), 1360 (C–N stretching), 1140 (C=S stretching), 1005 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 2.27 (s, 3H, CH3), 3.14 (s, 2H, NH2), 4.74 (d, J = 1.49 Hz, 1H, CH), 6.92 (d, J = 8.93 Hz, 1H), 7.09 (s, 1H, NH), 7.11–8.09 (m, 4H, aromatic), 8.90 (s, 1 H, NH); 13C-NMR (DMSO-d6, δC ppm): 18.10, 52.61, 106.21, 109.14, 118.24, 121.39, 123.41, 126.52, 150.19, 155.62, 160.41, 162.56, 168.21, 171.96, 177.81; MS: m/z: 349.72 [M + 1]+; Anal. Calcd. for C15H13FN4O3S: C, 54.22; H, 3.94; N, 16.86. Found: C, 54.27; H, 3.99; N, 16.80.
4-(6-Methoxy-4-oxo-4H-chromen-3-yl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide 6e. Yield 82%; M.P.: 208–210 °C; IR (KBr vmax in cm−1): 3520 (NH2 stretching), 3452 (N–H stretching), 3000 (C–H stretching), 2905 (–CH3 stretching), 2845 (–OCH3 stretching), 1742 (C=O stretching), 1605 (C=C stretching), 1362 (C–N stretching), 1005 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 2.03 (s, 3H, CH3), 3.13 (s, 2H, NH2), 3.80 (s, 3 H, OCH3), 4.74 (d, J = 1.51 Hz, 1 H), 7.08 (s, 1H, NH), 7.37–8.06 (m, 5 H, aromatic), 9.39 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 18.92, 46.48, 56.82, 106.66, 109.11, 118.24, 121.48, 123.49, 125.76, 147.41, 150.62, 155.12, 156.30, 168.61, 172.27; MS: m/z: 345.02 [M + 1]+; Anal. Calcd. for C16H16N4O5: C, 55.81; H, 4.68; N, 16.27. Found: C, 55.84; H, 4.69; N, 16.25.
4-(6-Methoxy-4-oxo-4H-chromen-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbohydrazide 6f. Yield 80%; M.P.: 158–160 °C; IR (KBr vmax in cm−1): 3522 (NH2 stretching), 3455 (N–H stretching), 3000 (C–H stretching), 2910 (–CH3 stretching), 2845 (–OCH3 stretching), 1742 (C=O stretching), 1605 (C=C stretching), 1360 (C–N stretching), 1146 (C=S stretching), 1005 (–O– stretching); 1H-NMR (DMSO-d6, δH ppm): 2.26 (s, 3H, CH3), 3.13 (s, 2H, NH2), 3.80 (s, 3 H, OCH3), 4.74 (d, J = 1.49 Hz, 1 H), 7.08 (s, 1H, NH), 7.37–8.08 (m, 5H, aromatic), 8.89 (s, 1H, NH); 13C-NMR (DMSO-d6, δC ppm): 18.90, 51.50, 105.66, 106.41, 118.90, 121.71, 123.44, 126.47, 150.53, 150.66, 156.30, 160.31, 168.22, 171.96, 177.98; MS: m/z: 361.10 [M + 1]+; Anal. Calcd. for C16H16N4O4S: C, 53.32; H, 4.47; N, 15.55. Found: C, 53.35; H, 4.49; N, 15.51.
3.4. Biological Activity Assays
3.4.1. In Vitro Antibacterial Activity
All the synthesized compounds 4(a–f) and 6(a–f) were screened for their in vitro antibacterial activity against Staphylococcus aureus (NCIM-2901) and Escherichia coli 1411 (standard laboratory strains) and Escherichia coli SM1411 (an acrAB deficient derivative of 1411). Minimum inhibitory concentration (MIC100) values were determined using the method recommended by National Committee for Clinical Laboratory Standards (NCCLS). In vitro antibacterial activities of the synthesized compounds 4(a–f) and 6(a–f) were tested in Nutrient Broth (NB) for Bacteria by the two fold serial dilution method. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media) at 37 ± 1 °C. The bacterial suspension was adjusted with sterile saline to a concentration of 1 × 10−4–10−5 C.F.U. The synthesized compounds and standard drug d-cycloserine were prepared by two fold serial dilutions to obtain the required concentrations. The tubes were incubated in BOD incubators at 37 ± 1 °C for bacteria. The MICs were recorded by visual observations after 24 h (for bacteria) of incubation [26,29,30,31].
3.4.2. d-Alanine-d-alanine ligase (DdlB) Enzyme Inhibition Study
The d-Ala-adding activity of DdlB ligase was monitored by the detection of orthophosphate generated during the reaction based on the colorimetric malachite green method described by Walsh et al. [46]. Assays were performed at 37 °C in a mixture (final volume: 50 µL) containing 38.5 mM Hepes, pH 8.0, 3.25 mMMgCl2, 6.5 mM (NH4)2SO4, 700 µM d-Ala, 500 µM ATP, purified DdlB (diluted in 20 mM HEPES, pH 7.2, and 1 mM dithiothreitol) and the test compound (IC50 values were determined for a range of inhibitor concentrations). All the compounds were soluble in the assay mixture containing 5% DMSO. After 30 min of incubation, 100 µL Biomol reagent was added. After 5 min, absorbance was read at 650 nm. Residual activity was calculated with respect to a similar assay without inhibitor. To exclude possible non-specific (promiscuous) inhibitors, representative compounds 4(a–f) and 6(a–f) were also tested in the presence of Tween (0.003%), Triton X-114 (0.005%), and SDS (420 µM), as described by McGovern et al. [47,48]. No significant differences were found when compared to measurements without detergents [49].
3.4.3. In Vitro Antifungal Activity
In vitro antifungal activity was determined as per CLSI (formerly, NCCLS) guidelines [29,30,31]. The synthesized compounds 4(a–f), 6(a–f) and the standard drug miconazole were dissolved in DMSO solvent. The medium yeast nitrogen base was dissolved in phosphate buffer pH 7 and it was autoclaved at 110 °C for 10 min. With each set a growth control without the antifungal agent and solvent control DMSO were included. The fungal strains were freshly sub cultured on to Sabouraud dextrose agar (SDA) and incubated at 25 °C for 72 h. The fungal cells were suspended in sterile distilled water and diluted to get 105 cells/mL. Further, ten microlitres of the standardized suspension was inoculated onto the control plates and the media incorporated with the antifungal agents. The inoculated plates were incubated at 25 °C for 48 h. The readings were taken at the end of 48 h and 72 h. The MIC was the lowest concentration of drug preventing growth of macroscopically visible colonies on drug containing plates when there was visible growth on the drug free control plates [26].
3.4.4. Ergosterol Extraction and Quantitation Assay
A single Candida albicans (NCIM-3471) colony from an overnight Sabouraud dextrose agar plate culture was used to inoculate 50 mL of Sabouraud dextrose broth for control and for various concentrations of the molecules. The cultures were incubated for 16 hours and harvested by centrifugation at 2700 rpm (856× g) for five min. The net weight of the cell pellet was determined. Three mL of 25% alcoholic potassium hydroxide solution was added to each pellet and vortex mixed for one min. Cell suspensions were transferred to sterile borosilicate glass screw-cap tubes and were incubated in an 85 °C water bath for one hour. Following incubation, the tubes were allowed to cool. Sterols were then extracted by addition of a mixture of 1 mL of sterile distilled water and 3 mL of n-heptane followed by vigorous vortex mixing for 3 min. The heptane layer was transferred to a clean borosilicate glass screw-cap tube and stored at −20 °C. Prior to analysis, 0.6 mL aliquot of sterol extract was diluted five fold in 100% ethanol and scanned spectrophotometrically between 240 nm and 300 nm with a spectrophotometer (UV-Visible Spectrophotometer 2100, Thermo Fischer Scientific, Waltham, MA, USA). The presence of ergosterol and the late sterol intermediate 24(28)-dehydroergosterol (DHE) in the extracted sample results in a characteristic four-peaked curve. The absence of detectable ergosterol in extracts was indicated by a flat line. A dose-dependent decrease in the height of the absorbance peaks was evident and corresponded to decreased ergosterol concentration [26,50].
3.5. Molecular Docking
The 3D model structure of cytochrome P450 lanosterol 14-α demethylase of C. albicans was built using homology modeling [37]. Molecular docking studies into a homology model of cytochrome P450 lanosterol 14 α-demethylase of C. albicans and into the E. coli DdlB enzyme (PDB entry: 1IOV [38]) were performed using Glide v. 6.8 (Schrodinger, LLC, New York, NY, USA, 2015) to predict its antifungal and antibacterial activity mode of action, respectively.
3.5.1. Homology Modeling Procedure for Cytochrome P450 Lanosterol 14α-Demethylase of C. albicans
The 3D model structure of cytochrome P450 lanosterol 14α-demethylase of C. albicans was built using homology modeling. The amino acid sequence of the enzyme was obtained from the Universal Protein Resource (http://www.uniprot.org/) (Accession Code: P10613), and sequence homologous was obtained from Protein Data Bank (PDB) using Blast search. In literature, the structure of cytochrome P450 lanosterol 14α-demethylase was developed homologically using crystal structure of lanosterol 14α-demethylase from C. albicans as template. Based on the result of blast search, we used the crystal structure of human lanosterol 14-α DM (CYP51) with miconazole as a template for homology modeling (PDB ID. 3I3K). A hidden Markov model (HMM) was generated from a sequence alignment for the identification of sequence motifs and query family, which provides the information about which residues are conserved in the consensus sequence. This information was used as constraints in the generation of the protein sequence alignment. In addition, secondary structure prediction algorithms SSpro [51] were used for alignment of C. albicans lanosterol 14α-demethylase to human lanosterol 14α-demethylase (DM) enzyme. The combination of sequence motifs and secondary structure provides accurate picture of each helix. Alignment of C. albicans lanosterol 14α-demethylase with human lanosterol 14α-demethylase enzyme was carried out using the automated alignment program in PRIME v. 2.1 (Schrodinger LLC, New York, NY, USA). Following automated alignment, manual inspection was made to ensure the conserved motifs, and loops were correctly aligned. The final model of C. albicans lanosterol 14α-DM enzyme was developed using PRIME v2.1. The binding sites were generated using SITEMAP v2.3 [52] (Schrodinger LLC, New York, NY, USA), and side chain and loops around active binding site (site with highest sitemap score) were refined using Prime refinement tool. The quality of generated C. albicans lanosterol 14 α-demethylase model was assessed by using the well-validated programs PROCHECK [53] and WHATIF [37,54].
3.5.2. Molecular Docking Study into E. coli DdlB Enzyme
Molecular docking study was performed in Maestro 9.1 using Glide v. 6.8 (Schrodinger LLC). All compounds were built using Maestro build panel and optimized to lower energy conformers using Ligprep v3.5 (Schrodinger, Inc., New York, NY, USA). The coordinates for E. coli DdlB enzyme were taken from RCSB Protein Data Bank and prepared for docking using ‘protein preparation wizard’ in Maestro v10.3. The bond orders and formal charges were added for hetero-groups and hydrogen’s were added to all atoms in the structure. Side chains that are not close to the binding cavity and do not participate in salt bridges were neutralized and termini were capped by adding ACE and NMA residue. After preparation, the structure was refined to optimize the hydrogen bond network using OPLS_2005 force field. The minimization was terminated when the energy converged or the RMSD reached a maximum cutoff of 0.30 Å. The extra precision (XP) docking mode for all compounds was performed on generated grid of protein structure [55]. The final evaluation of ligand-protein binding was done with Glide score [17].
3.6. In Silico ADMET Prediction
A computational study of the synthesized compounds 4(a–f) and 6(a–f) was performed for prediction of ADMET properties. The absorption, distribution, metabolism, elimination and toxicity (ADMET) properties of all the synthesized compounds were predicted using Qikprop v. 3.5 (Schrodinger LLC). In the present study, we have calculated the molecular volume (M.V.), molecular weight (M.W.), predicted octanol–water partition coefficient (log P o/w), number of hydrogen bond acceptors (n-ON), number of hydrogen bonds donors (n-OHNH), percentage human oral absorption (% ABS), Van der waals surface area of polar nitrogen and oxygen atoms (Polar Surface Area), Log S (water solubility), BIPCaco-2 (apparent Caco-2 cell permeability), Log Khsa (binding to human serum albumin) and Log HERG (toxicity study) [24].
3.7. In Vitro Cytotoxicity Studies
To explore the selective antifungal activity of the synthesized compounds in vitro cytotoxicity study of the most active compounds 6c and 6f was performed against HeLa (human cervical cancer cell line) and PC-3 (human prostate cancer cell line) using Sulforhodamine B (SRB) assay using Adriamycin as positive control [45]. The images were captured using an Eclipse Ti-S Inverted Research Microscope (Nikon, Tokyo, Japan). Image processing software used was NIS-Elements.
3.8. In Vivo Acute Oral Toxicity Study and Gross Behavioral Studies
Two of the newly synthesized compounds 6c and 6f, which are most potent among the synthesized compounds, were further subjected to an in vivo acute oral toxicity study and gross behavioral study as per the OECD guideline No. 425. Each group, consisting of six Swiss albino mice (18–22 gm weight, overnight fasted), were kept in colony cage at 25 ± 2 °C with 55% relative humidity and 12 h of light and dark cycle. A specified dose of 100, 250, 500, 750, 1000, 1500 and 2000 mg/kg body weight of mice was administered orally as a single dose. The acute toxic symptoms and the behavioral changes produced by the test compounds were observed continuously for 4 h periods at 8th, 12th and 24th h on set of toxic symptoms and the gross behavioral changes were also recorded. These animals were maintained for further 10 days with observation made daily. The in vivo acute oral toxicity study was carried out at animal house at the Y.B. Chavan College of Pharmacy, Aurangabad IAEC approval number CPCSEA/IAEC/P’col-52/2015-16/115 [26].
4. Conclusions
In this study, we have synthesized a suite of novel compounds 4(a–f) and 6(a–f) containing chromone-pyrimidine coupled hybrid derivatives as antifungal and antibacterial agents using a green synthetic protocol. The use of a green method, i.e., use of ionic liquid helped us in the synthesis of the target derivatives in good yield. The eco-friendly reaction conditions, good to excellent yields in a short reaction time and avoidance of cumbersome work-up procedures make this process economically worthwhile for industrial application, with the additional advantage of reusability of the catalyst. The in vitro antibacterial activity results suggest that compounds 4c, 6c and 6d possess potent in vitro antibacterial activity. The in vitro antifungal activity results suggest that compounds 4e, 6e and 6f possess potent in vitro antifungal activity. A d-alanine-d-alanine ligase (DdlB) enzyme assay study indicates that the synthesized derivatives have the ability to inhibit the DdlB enzyme. The synthesized derivative 6f was found to be the most active antifungal agent; its mode of action was studied using an ergosterol extraction and quantitation assay method. The results of this test indicate that the synthesized compound 6f acts by inhibiting ergosterol biosynthesis by inhibiting lanosterol 14α-demethylase enzyme. A molecular docking study was performed to predict the mechanism of action of the synthesized derivatives. Further, analysis of the ADMET parameters for the synthesized compounds performed using Qikprop v3.5 has revealed that these compounds have good oral drug-like properties and which makes them suitable for development as future oral drug candidates. Moreover, the cytotoxicity study revealed that the compounds 6c and 6f did not show any significant cytotoxicity against HeLa and PC-3 cell lines at the maximum evaluated concentration, thus indicating the selectivity of their antimicrobial action. The newly synthesized compounds 6c and 6f were also tested for their in vivo acute oral toxicity study and there was no mortality and no significant behavioral changes observed in Swiss albino mice for the first 24 h. Thus, the synthesized coupled compounds have shown excellent in vitro antimicrobial activity and compounds 4c, 4e, 6c and 6f could be in future further developed as lead antimicrobial molecules.
Acknowledgments
The authors are thankful to Fatima Rafiq Zakaria, Chairman, Maulana Azad Educational Trust and Principal, Y.B. Chavan College of Pharmacy, Rafiq Zakaria Campus, Aurangabad 431001 (MS), India for providing the laboratory facility.
Author Contributions
S.V.T. research student, A.P.G.N. research guide, J.A.S., M.P.V.-T. co-operated for spectral analysis, A.P.S., K.S.K. performed molecular docking study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zomorodian, K.; Moein, N.R.; Rahimi, M.J.; Pakshir, K.; Ghasemi, Y.; Abdi, S.; Sharbatfar, S. Possible Application and Chemical Compositions of Carumcopticum Essential Oils Against Food borne and Nosocomial Pathogens. Middle-East J. Sci. Res. 2011, 9, 239–245. [Google Scholar]
- Mamishi, S.; Zomorodian, K.; Saadat, F.; Gerami-Shoar, M.; Tarazooie, B.; Siadati, S.A. A case of invasive aspergillosis in CGD patient successfully treated with amphotericin B and INF-gamma. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shoar, M.G.; Zomorodian, K.; Saadat, F.; Hashemi, M.J.; Tarazoei, B. Fatal endocarditis due to Aspergillus flavus in Iran. J. Pak. Med. Assoc. 2004, 54, 485–486. [Google Scholar] [PubMed]
- Drakensjo, I.T.; Chryssanthou, E. Epidemiology of dermatophyte infections in Stockholm, Sweden: A retrospective study from 2005–2009. Med. Mycol. 2011, 49, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Alborzi, A. Invasive fungal infections in renal transplant recipients. Exp. Clin. Transplant. 2011, 9, 355–362. [Google Scholar] [PubMed]
- Ji, Q.; Ge, Z.; Chen, K.; Wu, H.; Liu, X.; Huang, Y.; Yuan, L.; Yang, X.; Liao, F. Synthesis and biological evaluation of novel phosphoramidate derivatives of coumarin as chitin synthase inhibitors and antifungal agents. Eur. J. Med. Chem. 2016, 108, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti-cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.S.; Divya, B.; Swain, M.; Rao, T.P.; Yadav, J.S.; Vishnuvardhan, M.V.P.S. A domino Knoevenagel hetero-Diels–Alder reaction for the synthesis of polycyclic chromene derivatives and evaluation of their cytotoxicity. Bioorg. Med. Chem. Lett. 2012, 22, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Raghunathan, R.; Almansour, A.I.; Karama, U. An efficient synthesis of highly functionalized novel chromeno [4, 3-b] pyrroles and indolizino [6, 7-b] indoles as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Devakaram, R.; Black, D.S.; Choomuenwai, V.; Davis, R.A.; Kumar, N. Synthesis and antiplasmodial evaluation of novel chromeno[2,3-b]chromene derivatives. Bioorg. Med. Chem. 2012, 20, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Devakaram, R.; Black, D.S.; Andrews, K.T.; Fisher, G.M.; Davis, R.A.; Kumar, N. Synthesis and antimalarial evaluation of novel benzopyrano[4,3-b]benzopyran derivatives. Bioorg. Med. Chem. 2011, 19, 5199–5206. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Proietti Monaco, L.; Desideri, N. Design, synthesis and in vitro evaluation of novel chroman-4-one, chroman, and 2H-chromene derivatives as human rhinovirus capsid-binding inhibitors. Bioorg. Med. Chem. 2011, 19, 7357–7364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Z.; Yin, W.; Liu, P.; Zhang, W. Microwave-Assisted Synthesis and Antifungal Activities of Poly substituted Furo[3,2-c]chromen-4-ones and 7,8,9,10-Tetrahydro-6H-benzofuro[3,2-c]chromen-6-ones. Synth. Commun. 2014, 44, 3257–3263. [Google Scholar] [CrossRef]
- Hosseinnia, R.; Mamaghani, M.; Tabatabaeian, K.; Shirini, F.; Rassa, M. An expeditious regioselective synthesis of novel bioactive indole-substituted chromene derivatives via one-pot three-component reaction. Bioorg. Med. Chem. Lett. 2012, 22, 5956–5960. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, N.; Puerta, M.C.; Valerga, P. Synthesis, structure, antimicrobial and antioxidant investigations of dicoumarol and related compounds. Eur. J. Med. Chem. 2008, 43, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, K.M.; Youssef, R. Synthesis and evalution of some 2,4 and 2,4-disubstituted6 methylpyrimidine derivatives for antimicrobial activity. J. Indian Chem. Soc. 1986, 53, 914–917. [Google Scholar]
- Tiwari, S.V.; Seijas, J.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.G. Ultrasound Mediated One-Pot, Three Component Synthesis, Docking and ADME prediction of Novel 5-amino-2-(4-chlorophenyl)-7-substitutedphenyl-8,8α-dihydro-7H-[1,3,4]thiadiazolo[3,2-α]pyrimidine-6-carbonitrile Derivatives as Anticancer Agents. Molecules 2016, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and Emerging Azole Antifungal Agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar] [PubMed]
- Viegas-Junior, C.; Danuello, A.; Silva, B.V.; Barreiro, E.J.; Fraga, C.A. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Hulsman, N.; Medema, J.P.; Bos, C.; Jongejan, A.; Leurs, R.; Smit, M.J.; de Esch, I.J.; Richel, D.; Wijtmans, M. Chemical insights in the concept of hybrid drugs: The antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J. Med. Chem. 2007, 50, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Contelles, J.M.; Soriano, E. The Medicinal Chemistry of Hybrid-Based Drugs Targeting Multiple Sites of Action. Curr. Top. Med. Chem. 2011, 11, 2714–2715. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific and Technical: Harlow, UK, 1989; Volume 5, p. 1193. [Google Scholar]
- Nikalje, A.G.; Tiwari, S.V.; Sarkate, A.P.; Karnik, K.S. Imidazole-thiazole coupled derivatives as novel lanosterol 14-α demethylase inhibitors: Ionic liquid mediated synthesis, biological evaluation and molecular docking study. Med. Chem. Res. 2018, 27, 592–606. [Google Scholar] [CrossRef]
- Nimbalkar, U.D.; Tupe, S.G.; Seijas, J.A.; Khan, F.A.K.; Sangshetti, J.N.; Nikalje, A.P.G. Ultrasound and molecular sieves-assisted synthesis, molecular docking and antifungal evaluation of 5-(4-(benzyloxy)-substituted phenyl)-3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones. Molecules 2016, 21, 484. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.V.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Karnik, K.S.; Nikalje, A.P.G. Facile Synthesis of Novel Coumarin Derivatives, Antimicrobial Analysis, Enzyme Assay, Docking Study, ADMET Prediction and Toxicity Study. Molecules 2017, 22, 1172. [Google Scholar] [CrossRef] [PubMed]
- Piotto, S.; Concilio, S.; Sessa, L.; Diana, R.; Torrens, G.; Juan, C.; Caruso, U.; Iannelli, P. Synthesis and antimicrobial studies of new antibacterial azo-compounds active against staphylococcus aureus and listeria monocytogenes. Molecules 2017, 22, 1372. [Google Scholar] [CrossRef] [PubMed]
- Concilio, S.; Sessa, L.; Petrone, A.M.; Porta, A.; Diana, R.; Iannelli, P.; Piotto, S. Structure modification of an active azo-compound as a route to new antimicrobial compounds. Molecules 2017, 22, 875. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, R.; Duguid, J.P.; Marmion, B.P.; Swain, R.H.A. Medicinal Microbiology, 2nd ed.; Churchill Livingstone: London, UK, 1975; Volume 2. [Google Scholar]
- Collins, A.H. Microbiological Methods, 2nd ed.; Butterworth: London, UK, 1976. [Google Scholar]
- Khan, Z.K. In vitro and in vivo screening techniques for bioactivity screening and evaluation. In Proceedings of the International Workshop UNIDO-CDRI, Lucknow, India, 2–5 December 1997; pp. 210–211. [Google Scholar]
- Tytgat, I.; Colacino, E.; Tulkens, P.M.; Poupaert, J.H.; Prevost, M.; Van Bambeke, F. DD-Ligases as a Potential Target for Antibiotics: Past, Present and Future. Curr. Med. Chem. 2009, 16, 2566–2580. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. d-Alanine: d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ayine-Tora, D.M.; Kingsford-Adaboh, R.; Asomaning, W.A.; Harrison, J.J.; Mills-Robertson, F.C.; Bukari, Y.; Sakyi, P.O.; Kaminta, S.; Reynisson, J. Coumarin Antifungal Lead Compounds from Millettiathonningii and Their Predicted Mechanism of Action. Molecules 2016, 21, 1369. [Google Scholar] [CrossRef] [PubMed]
- Granados, T.V.; Garcia, S.; Vazquez, M.A.; Robles, J. Molecular docking-based screening of newly designed coumarin derivatives with potential antifungal activity against lanosterol 14α-demethylase. Theor. Chem. Acc. 2016, 135, 210–216. [Google Scholar] [CrossRef]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Lokwani, D.K.; Sarkate, A.P.; Shinde, D.B. Synthesis, Antifungal Activity, and Docking Study of Some New 1,2,4-triazole Analogs. Chem. Biol. Drug Des. 2011, 78, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Park, I.S.; Walsh, C.T.; Knox, J.R. d-alanine: d-alanine ligase: Phosphonate and phosphinate intermediates with wild type and the Y216F mutant. Biochemistry 1997, 36, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 2, 3–26. [Google Scholar] [CrossRef]
- Lima, I.O.; de Medeiros Nobrega, F.; de Oliveira, W.A.; Lima, E.O.; Menezes, E.A.; Cunha, F.; MeloDiniz, A.M.F.F. Anti-Candida albicans effectiveness of citral and investigation of mode of action. Pharm. Biol. 2012, 50, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.V.; Suresh, L.; Chandramouli, G.V.P. Ionic liquid catalysed multicomponent synthesis, antifungal activity, docking studies and in silico ADMET properties of novel fused Chromeno-Pyrazolo-Phthalazine derivatives. J. Saudi Chem. Soc. 2015, 21, 306–314. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Walker, B.D.; Campbell, T.J. HERG K+ channels: Friend and foe. Trends Pharmacol. Sci. 2001, 22, 240–246. [Google Scholar] [CrossRef]
- Aronov, A.M. Predictive in silico modeling for hERG channel blockers. Drug Discov. Today 2005, 10, 149–155. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.W.; Falk, P.J.; Thanassi, J.; Discotto, L.; Pucci, M.J.; Ho, H.T. Comparison of the d-glutamate-adding enzymes from selected gram-positive and gram-negative bacteria. J. Bacteriol. 1999, 181, 5395–5401. [Google Scholar] [PubMed]
- McGovern, S.L.; Helfand, B.T.; Feng, B.; Shoichet, B.K. A Specific Mechanism of Nonspecific Inhibition. J. Med. Chem. 2003, 46, 4265–4272. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Gray, N.M.; Lowe, P.N.; Chung, C.W. Effect of Detergent on “Promiscuous” Inhibitors. J. Med. Chem. 2003, 46, 3448–3451. [Google Scholar] [CrossRef] [PubMed]
- Kovac, A.; Majce, V.; Lenarsic, R.; Bombek, S.; Bostock, J.M.; Chopra, I.; Polanc, S.; Gobec, S. Diazenedicarboxamides as inhibitors of d-alanine-d-alanine ligase (Ddl). Bioorg. Med. Chem. Lett. 2007, 17, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Arthington-Skaggs, B.A.; Warnock, D.W.; Morrison, C.J. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, G.; Przybylski, D.; Rost, B.; Baldi, P. Improving the prediction of protein secondary structure in three and eight classes using recurrent neural networks and profiles. Proteins 2002, 47, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Hooft, R.W.; Vriend, G.; Sander, C.; Abola, E.E. Errors in protein structures. Nature 1996, 381, 272. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).