Abstract
The transforming growth factor-β (TGF-β), in which overexpression has been associated with various diseases, has become an attractive molecular target for the treatment of cancers. Thirty-two quinoxaline-derivatives of 3-substituted-4-(quinoxalin-6-yl) pyrazoles 14a–d, 15a–d, 16a–d, 17a–d, 18a–d, 19a–d, 25a, 25b, 25d, 26a, 26b, 26d, 27b, and 27d were synthesized and evaluated for their activin TGF-β type I receptor kinase and p38α mitogen activated protein (MAP) kinase inhibitory activity in enzymatic assays. Among these compounds, the most active compound 19b inhibited TGF-β type I receptor kinase phosphorylation with an IC50 value of 0.28 µM, with 98% inhibition at 10 µM. Compound 19b also had good selectivity index of >35 against p38α MAP kinase, with 9.0-fold more selective than clinical candidate, compound 3 (LY-2157299). A molecular docking study was performed to identify the mechanism of action of the synthesized compounds and their good binding interactions were observed. ADMET prediction of good active compounds showed that these ones possess good pharmacokinetics and drug-likeness behavior.
1. Introduction
Transforming growth factor-β (TGF-β) superfamily members have a wide range of cellular functions, including cell proliferation, differentiation, adhesion, migration, and apoptosis [1]. Moreover, TGF-β superfamily members are proteins with similar structures, including TGF-βs, activins, bone morphogeneticproteins (BMPs), growth and differentiation factors. TGF-β plays a crucial role in initiation and progression of fibrosis in various tissues such as the heart [2], lung [3], liver [4], and kidney [5]. TGF-βs are composed of five homogeneous isomers with highly homologous amino acid sequences, TGF-β1, TGF-β2, TGF-β3, TGF-β4, and TGF-β5, though only the first three exist in humans. Among these isoforms, TGF-β1 is the prototype and major isoform of this family. TGF-β conducts signaling through two distinct serine and threonine kinase receptors as TGF-β type I (activin receptor-like kinase 5, ALK5) and type II receptors [6]. ALK5 is activated by the combination of TGF-β and the type II receptor in the juxtamembrane GS domain, stimulating its kinase activity. The activated ALK5 spread the signals through phosphorylation of Smad2 and Smad3, and followed by binding with Smad4 to form complexes. These Smad complexes will translocate into the nuclei, where they regulate target gene transcription such as cell differentiation, proliferation, apoptosis, migration, and extracellular matrix production [1]. Nevertheless, overexpression of TGF-β signaling was shown to result in various human diseases such as hematological malignancy [7], cancer [8], and pancreatic diseases [9].
For this reason, many small molecule ALK5 inhibitors, such as compounds 1 (SB-505124) [10], 2 (SD-208) [11], 3 (LY-2157299) [12], and 4 (EW-7197) [13] were synthesized at major research institutions (Figure 1). These compounds inhibited ALK5 autophosphorylation and TGF-β-induced transcription of extracellular matrix genes at sub-micromolar concentrations in reporter assays, as shown in Figure 1. Among them, clinical candidates, compounds 3 and 4 have progressed to Phase II and Phase I trials as antitumor agents, respectively.
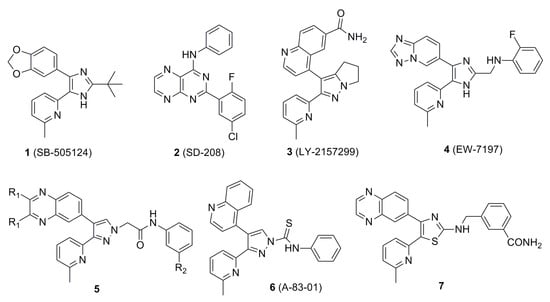
Figure 1.
ALK5 inhibitors under development.
We previously showed that a series of compounds, denoted as 5, containing the quinoxaline moiety, except for the 2,3-dimethyl substituted analogs, showed significant ALK5 inhibition in enzymatic assay [14]. P38α MAP kinase domain is among the most homologous to that of ALK5, so we selected p38α MAP kinase to evaluate the selectivity profile of these series of compounds. In addition, previous studies have found that compounds with high p38α MAP kinase selectivity are also highly selective to other kinases, such as compound 4, which showed high selectivity to 320 kinases in human body [13]. These series of compounds were selective for ALK5, compared with p38α MAP kinase. The most active compound inhibited ALK5 phosphorylation with an IC50 value of 0.013 µM and a selectivity index of >77 against p38α MAP kinase. We reported the effect of quinoxaline groups and length of side chains attached to pyrazole ring, and positional isomers on the activity of compounds containing pyrazole rings, but the effect of the pyridine portion attached to pyrazole ring on the activity of the compounds was not discussed.
Tojo et al. described a novel class of ALK5 inhibitors possessing a thioamide linkage between the phenyl and pyrazole rings [15]. Among these, compound 6 (A-83-01) inhibited ALK5 with an IC50 value of 0.012 µM. Although including a thioamide linkage between the phenyl and pyrazole ring distinctly increased ALK5 inhibitory activity, as previously shown [16], the thioamide linkage was rather unstable and was slowly cleaved, to release a pyrazole ring, during long-term storage.
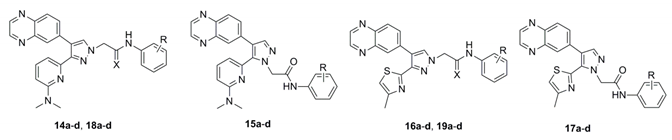
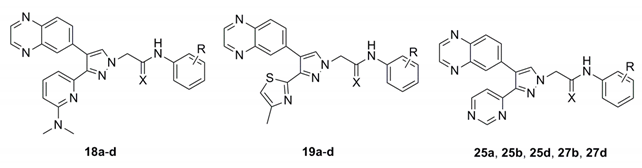
Previously, we showed that the methyl group of 6-methylpyridine in compound 4 formed hydrophobic interactions with the aromatic ring of Tyr249 and the nitrogen atom of the same moiety formed a water-mediated hydrogen bonding network with the side chains of Tyr249 and Glu245 and the backbone of Asp351 [13]. In previous studies, we found that the introduction of electron-donating groups into quinoxaline or quinoline rings failed to increase ALK5 inhibition activity [14]. This is due to the steric hindrance of their rigid structures. Therefore, we assumed that the ALK5 inhibition activity will be enhanced by introducing the electron-donating groups or the structure with many binding sites into the non-rigid pyridine ring on the premise of maintaining the non-substituent quinoxaline structure. To examine whether the capability of the nitrogen atom of the 6-methylpyridine moiety as an H-bond acceptor would be increased by other substitutions, we introduced 6-(dimethylamino)pyridin-2-yl, 4-methylthiazol-2-yl [17,18,19], and pyrimidin-4-yl groups [20], instead of the 6-methylpyridine moiety in 5 series compound. Based on this finding and previous research, we tried to replace the thioamide linkage with a chemically stable thioamidomethylene linkage and, designed compounds 18a–d, 19a–d, 27b, and 27d (Figure 2). To compare the effects of the thioamidomethylene linkage in 18a–d, 19a–d, 27b, and 27d on ALK5 inhibitory activity, their counterpart derivatives 14a–d, 15a–d, 16a–d, 17a–d, 25a, 25b, 25d, 26a, 26b, and 26d possessing an amidomethylene linkage, were also designed. The target compounds 14b–d, 16b–d, 15b–d, 17b–d, 18b–d, 19b–d, 25b, 25b, 25d, 26b, 26b, 26d, 27b, and 27d each possess a substituent, either o-F, m-F or m-CN, in the phenyl ring because these were previously found to be most beneficial for ALK5 inhibitory activity and selectivity [13].
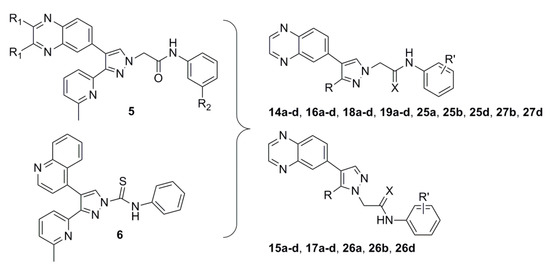
Figure 2.
Design strategy based on compound 5 and 6.
2. Results and Discussion
2.1. Chemistry
The 3-(6-(dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles 14a–d and 3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)pyrazoles 16a–d were synthesized as shown in Scheme 1. The 6-(dimethylamino)picolinaldehyde (8) [21] and 4-methylthiazole-2-carbaldehyde (9) were treated with aniline and diphenyl phosphite in i-PrOH at room temperature to give the (phenylamino)methylphosphonates 10a and 10b in 90% and 70% yields, respectively. Coupling of the 10a and 10b with quinoxaline-6-carbaldehyde [22] in a mixture of THF and i-PrOH (4:1) at room temperature in the presence of Cs2CO3, followed by hydrolysis with 1 N HCl, produced the corresponding monoketones 11a and 11b in 71% and 52% yields, respectively [14]. Treatment of 11a and 11b with N,N-dimethylformamide dimethyl acetal (DMF•DMA) in N,N-dimethylformamide (DMF) at 80 °C, followed by cyclization with hydrazine monohydrate in absolute EtOH, produced the pyrazoles 12a and 12b in 68% and 71% yields, respectively [23]. The pyrazoles 12a and 12b were alkylated with 2-chloro-N-phenylacetamide (13a) [24], 2-chloro-N-(2-fluorophenyl)acetamide (13b), 2-chloro-N-(3-fluorophenyl)acetamide (13c) or 2-chloro-N-(3-cyanophenyl)acetamide (13d) [25] in the presence of NaH in anhydrous DMF to yield the target compounds 14a–d, 16a–d and their positional isomers 15a–d and 17a–d in 40–81% and 7–15% yields, respectively. The positional isomers were separated by column chromatography and their structures were confirmed by nuclear Overhauser enhancement (NOE) experiments. In NOE experiments, irradiation of the methylene protons of compound 14a at δ 5.06 gave an enhancement of the proton H-5 in the pyrazole ring at δ 7.80, while irradiation of the methylene protons of compound 15a at δ 5.17 gave no enhancement of the proton H-5 in the pyrazole ring at δ 8.02, confirming the respective alkylation positions.
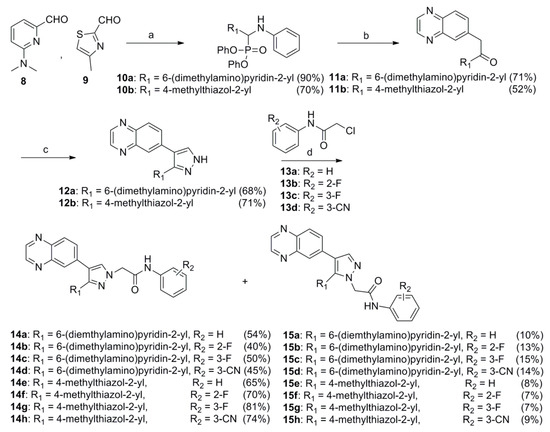
Scheme 1.
Reagents and conditions: (a) aniline, (PhO)2P(O)H, rt, 4 h; (b) (i) quinoxaline-6-carbaldehyde, Cs2CO3, rt, 16 h; (ii) 1 N HCl, 1 h; (c) (i) DMF•DMA, 80 °C, 2 h; (ii) N2H4•H2O, EtOH, reflux, 4 h; (d) NaI (cat.), NaH, rt, 2 h.
Thionation of compounds 14a–d and 16a–d with Lawesson’s reagent in anhydrous 1,2-dimethoxyethane (DME) at 85 °C produced the thioamides 18a–d and 19a–d in 37–89% yields as shown in Scheme 2.
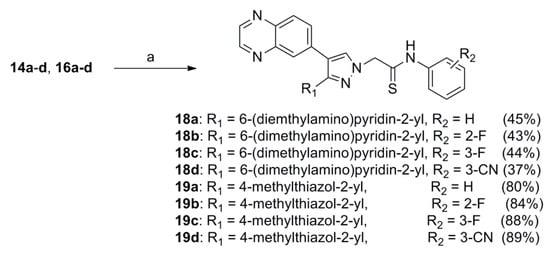
Scheme 2.
Reagents and conditions: (a) Lawesson’s reagent, 85 °C, 12 h.
To increase binding sites with key proteins, the 3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)pyrazoles 25a, 25b, and 25d were synthesized as shown in Scheme 3. Pyrimidine-4-carbaldehyde (20) [26] was synthesized from commercially available 1,1-dimethoxyacetone and N,N-dimethylformamide dimethyl acetal via 3 steps. Compound 23 was synthesized from compound 20 via 3 steps in the same reaction condition as described in Scheme 1. The pyrazole 23 was further alkylated with substituted phenylacetamides 24a, 24b, or 24d in the presence of NaH in anhydrous DMF to yield the target compounds 25a, 25b, 25d and their positional isomers 26a, 26b, 26d in 58–65% and 7–13% yields, respectively. These positional isomers were also separated by column chromatography and their structures were confirmed by NOE experiments (Scheme 3). Similarly, the thioamide compounds 27b and 27d were synthesized from 25b and 25d in the same reaction condition as described in Scheme 2 (Scheme 4). As expected, all synthesized target compounds were quite stable during long-term storage at room temperature.
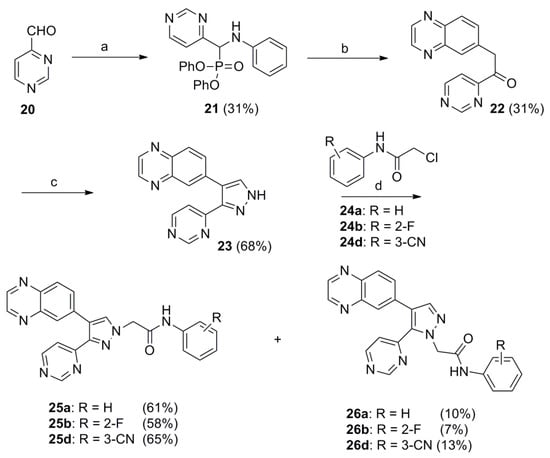
Scheme 3.
Reagents and conditions: (a) aniline, (PhO)2P(O)H, rt, 4 h; (b) (i) quinoxaline-6-carbaldehyde, Cs2CO3, rt, 16 h; (ii) 1 N HCl, 1 h; (c) (i) DMF•DMA, 80 °C, 2 h; (ii) N2H4•H2O, EtOH, reflux, 4 h; (d) NaI (cat.), NaH, rt, 2 h.
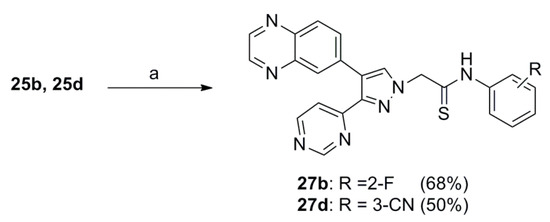
Scheme 4.
Reagents and conditions: (a) Lawesson’s reagent, 85 °C, 12 h.
2.2. Residual Activity in an Enzymatic Assay
To investigate whether compounds 14a–d, 15a–d, 16a–d, 17a–d, 18a–d, and 19a–d would inhibit ALK5, a kinase assay for preliminary screening was performed using the purified human ALK5 kinase domain produced in Sf9 insect cells and compounds at 10 μM. To examine the activity and potency of the synthesized compounds, compound 3 (LY-2157299) was used as a positive control. All compounds with a 4-methylthiazol-2-yl moiety (16a–d, 17a–d, and 19a–d) showed potent ALK5 inhibitory activity (27–98%), whereas those with a 6-(dimethylamino)pyiridin-2-yl moiety (14a–d, 15a–d, and 18a–d) showed moderate ALK5 inhibition activity (5–71%) (Table 1).

Table 1.
Residual ALK5 and p38α MAP kinase activities in the presence of 3-substituted-4-(quinoxalin-6-yl) pyrazoles 14a–d, 15a–d, 16a–d, 17a–d, 18a–d, and 19a–d.
The amides 14a–d (5–63%) and 16a–d (95–97%) showed more potent ALK5 inhibitory activity than their respective positional isomers, 15a–d (5–13%) and 17a–d (27–54%), respectively. Among compounds containing a 6-(dimethylamino)pyridin-2yl moiety, the thioamides 18a–d (30–71%) showed more potent ALK5 inhibition than the corresponding amides 14a–d at 10 μM. Among compounds containing a 4-methylthiazol-2-yl moiety, the thioamides 19a–d (87–98%) also showed similar ALK5 inhibition with the corresponding amides 16a–d at 10 μM. We speculated that insertion of electron-donating groups at the 6-position of the pyridine moiety in 5 series compound would increase the capacity of the nitrogen atom in that moiety as an H-bond acceptor, thus, potentiating its ALK5 inhibitory activity. Instead, insertion of the 6-(dimethylamino)pyridin-2-yl moiety does not seem to fit ATP binding pocket of ALK5 compared to its structural counterparts bearing 6-methylpyrine. Fortunately, introduction of 4-methylthiazol-2-yl moiety effectively improved ALK5 inhibitory activity.
2.3. p38a MAP Kinase Assay
We selected p38α MAP kinase to survey the selectivity profile of this series of compounds because its kinase domain is among the most homologous to that of ALK5 [27]. All target compounds except 17a–d (3–46%) did not inhibit p38α MAP kinase, even at their maximum concentration of 10 μM (Table 1).
Figure 3 intuitively illustrates the inhibitory activity of 3-substituted-4-(quinoxalin-6-yl)pyrazoles against ALK5 and p38α MAP kinase. All compounds with a 4-methylthiazol-2yl moiety (16a–d, 17a–d, and 19a–d) showed more potent ALK5 inhibition than those with a 6-(dimethylamino)pyridin-2-yl moiety (14a–d, 15a–d, and 18a–d).
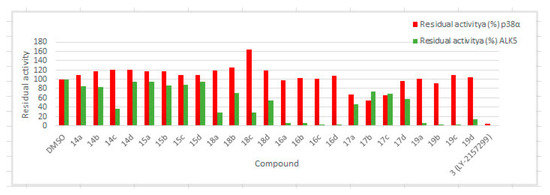
Figure 3.
Residual activities of ALK5 and p38α MAP kinase in the presence of 3-substituted-4-(quinoxalin-6-yl)pyrazoles 14a–d, 15a–d, 16a–d, 17a–d, 18a–d, and 19a–d.
2.4. ALK5 Inhibitory Activity in an Enzymatic Assay
In previous studies, we found that the activity of thioamide compounds was superior to that of the corresponding amide ones [14]. To evaluate ALK5 inhibitory activity and selectivity of the compounds possessing 6-(dimethylamino)pyridin-2-yl or 4-methylthiazol-2-yl moieties as electron donating group, the thioamides 18a–d and 19a–d was selected and their half maximal inhibitory concentration (IC50) values were measured. All compounds with a 4-methylthiazol-2-yl moiety (19a–d) showed potent ALK5 inhibition (IC50 = 0.28–0.57 μM), whereas those with a 6-(dimethylamino)pyridin-2-yl moiety (18a–d) showed no significant ALK5 inhibitory activity at up to 5.0 μM (Table 2).

Table 2.
Inhibitory activity of 3-substituted-4-(quinoxalin-6-yl) pyrazoles 18a–d, 19a–d, 25a, 25b, 25d, 27b, and 27d against ALK5 and p38α MAP kinase.
To evaluate ALK5 inhibitory activity and selectivity of the compounds possessing pyrimidin-4-yl moiety as multiple binding site, the amides 25a, 25b, 25d and thioamides 27b and 27d were also selected and evaluated. However, all compounds with a pyrimidin-4-yl moiety (25a, 25b, 25d, 27b, 27d) also showed no significant ALK5 inhibition activity at up to 2.26 μM (Table 2).
Compound 19b showed the most potent ALK5 inhibitory activity with an IC50 value of 0.28 μM in these three series of compounds. It was slightly less potent than compounds 3 (0.12 μM). Furthermore, all thioamides 18a–d, 19a–d, 27b, and 27d failed to inhibit p38α MAP kinase up to 10.0 μM. Compound 19b was the most selective in these three series, showing a selectivity index of >35, higher than that of positive control compound 3 (4). In this series of compounds (19a–d), the activity of compounds with substituents is superior to that of unsubstituted one. Notably, 2-fluorine substituted compound 19b, which is two-fold more potent than unsubstituted compound 19a (IC50 = 0.57 μM).
2.5. Docking Study of 18b and 19b in the Alk5 Active Site
To rationalize the SAR shown in Table 1 and Table 2, we examined the binding modes of two representative ligands (18b and 19b) using the semi-flexible molecular docking program DS CDOCKER [28]. Docking analyses were performed using the recently reported X-ray structure of ALK5 complexed to a pyrazole ALK5 inhibitor (PDB: 1RW8) [13] (Figure 4).
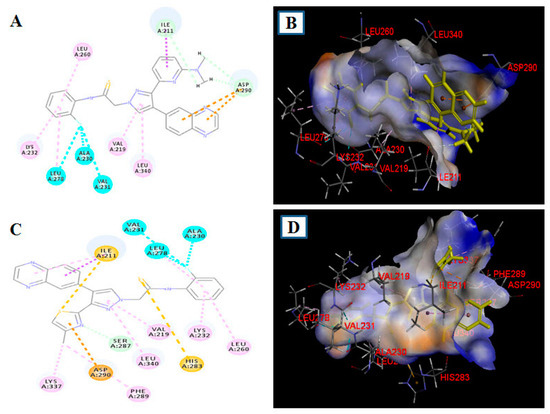
Figure 4.
Docking pose of compounds 18b and 19b in the active site of ALK5 (PDB: 1RW8). (A) 2D binding model of 18b; (B) Proposed pose of 18b in the binding pocket of ALK5; (C) 2D binding model of 19b; (D) Proposed pose of 19b in the binding pocket of ALK5. The ligands are shown in yellow.
The sulfur atom of the thioamide in 19b contacted the hinge of ALK5, forming hydrogen bonds with the imidazole ring of His283, a residue previously reported to be important for inhibitory activity (C) [14]. The phenyl ring of 19b interacted with Lys232 via Pi-alkyl bond. The central pyrazole ring of 19b formed Pi-alkyl bond with the side chains of Leu340 and Val219. The thiazole N atom of 19b formed carbon–hydrogen bond with the backbone of Ser287 and the methyl group of 19b formed alkyl bond with the backbone of Lys337 and Pi-alkyl bond with the backbone of Phe289. Not only the calculated binding energy scores (CDOCKER_INTERATION_ENERGY) of these two compounds indicated that 19b (−56.18 kcal/mol) formed more stable complexes with ALK5 than did 18b (−54.81 kcal/mol), but also compound 19b (Lys232, His283, Ser287, Leu340, and Lys337) showed more bonding with previously reported key amino acids than did compound 18b (Lys232 and Leu340) (A) [29]. In particular, compound 18b did not form bond with the most important amino acid, His283. The 2-fluorophenyl ring of 18b and 19b was stretched to the backside hydrophobic pocket consisting of Lys232, Leu260, Leu278, Val231, and Ala230. Furthermore, compound 19b seemed to be more favorably accommodated in the binding pocket of ALK5 than compound 18b (B and D). Our docking results indicated that the most active compound, 19b, showed the more favorable intermolecular interactions in the ALK5 active site than compound 18b. This supported the conclusion that the substitution group size of the pyridine moiety and selection of a heterocycle in compound 5 may have been important for improving ALK5 inhibition.
2.6. ADMET Analysis
ADMET pharmacokinetics is a very important method in drug design and drug screening, which is responsible for drug failure [30,31]. The ADMET properties of the drug molecules are greatly influenced by the optimum value of the intestinal absorption, water solubility, blood–brain barrier (BBB) penetration, human cytochrome P450 2D6 (CYP2D6) inhibition, and plasma protein binding (PPB) level. The ADMET parameters of these good targeted compounds 19a–d was measured using Discovery Studio software as a drug reference was reported in Table 3.

Table 3.
Prediction of ADMET properties of compounds 19a–d.
The preferred and most widely used route of drug is the oral route, and the mechanism of absorption from the gastrointestinal tract is passive diffusion through the intestinal epithelial cells. Hence, the absorption and solubility of the drug are two major factors for oral administration. All of the 3-substituted-4-(quinoxalin-6-yl) pyrazoles 19a–d showed good intestinal absorption. All compounds showed low or very low aqueous solubility at room temperature. However, the structure of these compounds contain thiazole and quinoxaline moiety, so it is easy to make a salt in stomach acid and dissolve in water. The BBB is an important organizational structure to maintain the stability of the central nervous system, which maintains the relative stability of the environment in the nervous system by restricting the entry of compounds into the central nervous system, and protects nerve cells from being invaded by harmful substances. All compounds, except compound 19b, showed BBB penetration in permissible level (1). These compounds are suitable for the treatment of systemic diseases. Compound 19b showed low BBB permeability and is suitable for non-brain diseases, which is characterized by cyano group at 3-position on the phenyl ring. In addition, the PPB binding ability of all compounds was good. CYP2D6 is an important drug metabolism enzyme in the family of cytochrome P450, and its catalysis is widely used. Over the years, the genes encoding CYP2D6 enzyme have been closely related to the genetic polymorphism, drug metabolism, production of adverse drug reactions, and activation of carcinogens. Also, all compounds did not inhibit CYP2D6, so they will be shown no or low side effects such as drug-drug interaction and wide metabolism. All of the parameters were within the acceptable range defined for human use and these good targeted compounds may exhibit significant pharmacokinetic and drug-likeliness properties.
3. Experimental
3.1. Chemistry
All solvents and chemicals (Aladdin, Shanghai, China) were commercially available without further purification. In general, all reactions were performed under normal atmosphere and at room temperature unless otherwise noted. Melting points were measured in open glass capillaries tube in an electrical melting point and are uncorrected. Spots were detected by viewing under UV lamps (254 nm). 1H and 13C NMR spectra were recorded on Bruker NMR spectrometers (Bruker, Billerica, MA, USA) at 300 MHz and 500 MHz, respectively, tetramethylsilane (TMS) was used as internal standard. High resolution mass spectra electrospray ionization (HRMS-ESI) was obtained on a Thermo Scientific LTQ Orbitrap XL spectrometer (Thermo Scientific, Markham, Ontario, Canada). The purity of the tested compounds was determined using an Agilent 1260 series HPLC system (Agilent Technologies, Waldbronn, Germany) using a C18 column (packing ODS HG 5 µM, 4.6 × 250 mm), and that for all the compounds was found to be >95%.
3.1.1. General procedure for the preparation of diphenyl ((6-(dimethylamino)pyridin-2-yl)(phenylamino)methyl)phosphonate (10a), Diphenyl ((4-methylthiazol-2-yl)(phenylamino)methyl)phosphonate (10b) and Diphenyl ((phenylamino)(pyrimidin-4-yl)methyl)phosphonate (21)
To a stirred solution of 8, 9, or 20 (12.90 mmol) in i-PrOH (40 mL), aniline (15.48 mmol) and diphenyl phosphite (20.64 mmol) were added. The mixture was stirred at room temperature for 4 h. The reaction mixture evaporated to dryness under reduced pressure. The residue was purified by silica gel column chromatography (Petroleum ether/Ethyl acetate, 6:1) to give the titled compound 10a, 10b, or 21 as a white solid.
Diphenyl ((6-(dimethylamino)pyridin-2-yl)(phenylamino)methyl)phosphonate (10a): Yield 90%; 1H NMR (300 MHz, CDCl3) δ 7.42 (t, J = 9.0 Hz, 1H), 7.36–7.21 (m, 6H), 7.18–7.08 (m, 6H), 6.86–6.77 (m, 4H), 6.42 (dd, J = 9.0, 3.0 Hz, 1H), 5.56 (br s, 1H), 5.27 (d, J = 18.0 Hz, 1H), 3.09 (s, 6H).
Diphenyl ((4-methylthiazol-2-yl)(phenylamino)methyl)phosphonate (10b): Yield 70%; 1H NMR (300 MHz, CDCl3) δ 7.36–7.14 (m, 10H), 7.04 (d, J = 9.0 Hz, 2H), 6.85–6.80 (m, 2H), 6.73 (d, J = 9.0 Hz, 1H), 6.21 (br s, 1H), 5.58 (d, J = 24.0 Hz, 1H), 2.41 (s, 3H).
Diphenyl ((phenylamino)(pyrimidin-4-yl)methyl)phosphonate (21): Yield 31%; 1H NMR (300 MHz, CDCl3) δ 9.23 (s, 1H), 8.68 (d, J = 3.0 Hz, 1H), 7.62 (s, 1H), 7.27–7.01 (m, 13H), 6.79 (t, J = 9.0 Hz, 1H), 6.70 (d, J = 9.0 Hz, 1H), 6.09 (br s, 1H), 5.30 (d, J = 24.0 Hz, 1H).
3.1.2. General Procedure for the Preparation of 1-(6-(Dimethylamino)pyridin-2-yl)-2-(quinoxalin-6-yl)ethanone (11a) 1-(4-Methylthiazol-2-yl)-2-(quinoxalin-6-yl)ethanone (11b) and 1-(Pyrimidin-4-yl)-2-(quinoxalin-6-yl)ethan-1-one (22)
To a stirred solution of 10a, 10b, or 21 (10.9 mmol) in a mixture of THF (23.2 mL) and i-PrOH (5.8 mL), Cs2CO3 (1.41 mmol) and quinoxaline-6-carbaldehyde (10.9 mmol) were added. The mixture was stirred at room temperature for 16 h, and to it, 1 N HCl (43.4 mL) was added dropwise over a period of 5 min. The reaction mixture was diluted with tert-butyl methyl ether (MTBE) (17.4 mL). The aqueous layer was separated, and the organic layer was extracted with 1 N HCl (3 × 50 mL). The combined aqueous layer was neutralized with saturated NaHCO3 solution (pH 7–8) and extracted with EtOAc (3 × 100 mL). The EtOAc solution was dried over anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. The residue was purified by silica gel column chromatography (Petroleum ether/Ethyl acetate, 4:1) to give the titled compound 11a, 11b, or 22 as a yellow solid.
1-(6-(Dimethylamino)pyridin-2-yl)-2-(quinoxalin-6-yl)ethanone (11a): Yield 71%; 1H NMR (300 MHz, DMSO-d6) δ 8.92 (d, J = 3.0 Hz, 2H), 8.06 (d, J = 9.0 Hz, 1H), 8.01 (s, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.69 (t, J = 7.5 Hz, 1H), 7.22 (d, J = 9.0 Hz, 1H), 6.94 (d, J = 6.0 Hz, 1H), 4.76 (s, 2H), 3.15 (s, 6H).
1-(4-Methylthiazol-2-yl)-2-(quinoxalin-6-yl)ethanone (11b): Yield 52%; 1H NMR (300 MHz, DMSO-d6) δ 8.95 (br s, 2H), 8.08 (d, J = 9.0 Hz, 2H), 7.88 (s, 1H), 7.82 (d, J = 9.0 Hz, 1H), 4.81 (s, 2H), 2.54 (s, 3H).
1-(Pyrimidin-4-yl)-2-(quinoxalin-6-yl)ethan-1-one (22): Yield 62%; 1H NMR (300 MHz, CDCl3) δ 9.45 (s, 1H), 9.01 (d, J = 6.0 Hz, 1H), 8.83 (s, 2H), 8.11–8.06 (m, 2H), 7.94 (d, J = 3.0 Hz, 1H), 7.75 (d, J =9.0 Hz, 1H), 4.79 (s, 2H).
3.1.3. General Procedure for the Preparation of N,N-Dimethyl-6-(4-(quinoxalin-6-yl)-1H-pyrazol-3-yl)pyridin-2-amine (12a) 4-Methyl-2-(4-(quinoxalin-6-yl)-1H-pyrazol-3-yl)thiazole (12b) and 6-(3-(Pyrimidin-4-yl)-1H-pyrazol-4-yl)quinoxaline (23)
To a stirred solution of 11a, 11b, or 22 (1.71 mmol) in anhydrous DMF (4.5 mL), N,N-dimethylformamide dimethyl acetal (5.12 mmol) were added. The mixture was heated at 80 °C for 4 h. After cooled to room temperature, the reaction mixture was evaporated to dryness under reduced pressure. The residue was dissolved in EtOH (6.43 mL), and to it, hydrazine monohydrate (35.36 mmol) was added. The mixture was heated at reflux temperature for 4 h, then cooled to room temperature, and evaporated to dryness under reduced pressure. The residue was diluted with CH2Cl2 (60 mL) and washed with water (20 mL) and brine (20 mL). The CH2Cl2 solution was dried over anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure. The residue was purified by silica gel column chromatography (Petroleum ether/Ethyl acetate, 1:1) to give the titled compound 12a, 12b, or 23 as a yellow solid.
N,N-Dimethyl-6-(4-(quinoxalin-6-yl)-1H-pyrazol-3-yl)pyridin-2-amine (12a): Yield 68%; 1H NMR (300 MHz, CDCl3) δ 8.84 (d, J = 6.0 Hz, 2H), 8.21 (d, J = 3.0 Hz, 1H), 8.10 (d, J = 9.0 Hz, 1H), 7.88 (dd, J = 9.0, 3.0 Hz, 1H), 7.77 (s, 1H), 7.32 (t, J = 9.0 Hz, 1H), 6.68 (d, J = 9.0 Hz, 1H), 6.48 (d, J = 9.0 Hz, 1H), 3.10 (s, 6H).
4-Methyl-2-(4-(quinoxalin-6-yl)-1H-pyrazol-3-yl)thiazole (12b): Yield 71%; 1H NMR (300 MHz, CDCl3) δ 8.87 (d, J = 3.0 Hz, 2H), 8.29 (d, J = 3.0 Hz, 1H), 8.15 (d, J = 9.0 Hz, 1H), 7.95 (dd, J = 9.0, 3.0 Hz, 1H), 7.87 (s, 1H), 6.87 (s, 1H), 2.49 (s, 3H).
6-(3-(Pyrimidin-4-yl)-1H-pyrazol-4-yl)quinoxaline (23): Yield 58%; 1H NMR (300 MHz, DMSO-d6) δ 13.72 (s, 1H), 9.02 (s, 1H), 8.93 (br s, 2H), 8.85 (br s, 1H), 8.36 (br s, 1H), 8.14 (s, 1H), 8.03 (br s, 1H), 7.91 (d, J = 9.0 Hz, 2H).
3.1.4. General Procedure for the Preparation of 2-(3-(6-(Dimethylamino)pyridin-2-yl)-, 2-(3-(4-Methylthiazol-2-yl)- or 2-(3-(Pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (14a–d, 16a–d, 25a, 25b, 25d) and 2-(5-(6-(Dimethylamino)pyridin-2-yl)-, 2-(5-(4-Methylthiazol-2-yl)- or 2-(5-(Pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylacetamide (15a–d, 17a–d, 26a, 26b, 26d)
To a solution of pyrazole 12a, 12b, or 23 (0.63 mmol) in anhydrous DMF (8.3 mL), a catalytic amount of sodium iodide, NaH (0.75 mmol), and 2-chloro-N-phenylacetamide 13a, 13b, 13c, 13d, 24a, 24b, or 24d (0.79 mmol) were added. The mixture was stirred at room temperature for 2 h and then evaporated to dryness under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane/methanol, 50:1) to give the two positional isomers 14a–d, 16a–d, 25a, 25b, 25d and 15a–d, 17a–d, 26a, 26b, 26d as white solids.
2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylacetamide (14a): Yield 54%; HPLC purity: 98.68% (acetonitrile: 40%); mp 212.5–214.0 °C; 1H NMR (300 MHz, CDCl3) δ 8.87 (br s, 1H, NH), 8.84 (d, J = 6.0 Hz, 2H), 8.18 (s, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.84 (dd, J = 9.0, 3.0 Hz, 1H), 7.80 (s, 1H), 7.58–7.52 (m, 3H), 7.34 (t, J = 9.0 Hz, 2H), 7.15 (d, J = 9.0 Hz, 2H), 6.49 (d, J = 6.0 Hz, 1H), 5.06 (s, 2H), 2.72 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 164.60, 158.64, 151.02, 149.38, 145.10, 144.50, 142.95, 142.02, 137.87, 137.07, 135.58, 132.76, 132.50, 129.02 (2C), 128.50, 128.14, 124.86, 121.69, 120.19 (2C), 109.86, 105.36, 55.91, 37.58 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C26H24N7O 450.20368, found 450.20370.
2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-(2-fluorophenyl)acetamide (14b): Yield 40%; mp 190.5–193.0 °C; HPLC purity: 99.39% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 9.30 (s, 1H), 8.81 (d, J = 6.0 Hz, 2H), 8.31 (t, J = 7.5 Hz, 1H), 8.16 (d, J = 3.0 Hz, 1H), 8.01 (d, J = 9.0 Hz, 1H), 7.83 (dd, J = 9.0, 3.0 Hz, 1H), 7.76 (s, 1H), 7.54 (t, J = 7.5 Hz, 1H), 7.25 (d, J = 6.0 Hz, 1H), 7.17–7.06 (m, 3H), 6.45 (d, J = 9.0 Hz, 1H), 5.06 (s, 2H), 2.64 (s, 6H); 13C NMR (125 MHz, DMSO-d6) δ 166.30, 158.69, 153.95 (d, J = 243.75. Hz), 150.70, 149.00, 146.16, 145.38, 142.74, 141.50, 138.21, 136.43, 133.76, 132.70, 128.58, 127.50, 126.10 (d, J = 16.25 Hz), 126.09, 124.97 (d, J = 3.75 Hz), 124.24, 120.25, 116.07 (d, J = 18.75 Hz), 110.08, 105.21. 55.01, 30.48 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C26H23FN7O 468.19426, found 468.19427.
2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-(3-fluorophenyl)acetamide (14c): Yield 50%; mp 176.5–178.5 °C; HPLC purity: 98.18% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 9.16 (s, 1H), 8.87–8.82 (m, 2H), 8.15 (s, 1H), 8.03 (d, J = 9.0 Hz, 1H), 7.83–7.78 (m, 2H), 7.56–7.48 (m, 2H), 7.32–7.22 (m, 2H), 7.16 (d, J = 9.0 Hz, 1H), 7.11 (d, J = 9.0 Hz, 1H), 6.83 (t, J = 8.0 Hz, 1H), 6.48 (d, J = 9.0 Hz, 1H), 5.03 (s, 2H), 2.71 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 164.71, 161.23, 154.77 (d, J = 252.0 Hz), 153.97, 153.51, 145.10, 144.52, 142.90, 141.98, 138.28, 135.79, 135.36, 132.52 (d, J = 7.5 Hz), 130.03 (d, J = 9.0 Hz), 128.62, 128.04, 121.69, 115.44, 111.41 (d, J = 20.7 Hz), 110.07, 107.71, 107.40 (d, J = 6.75 Hz), 105.83, 55.82, 30.93 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C26H23FN7O 468.19426, found 468.19431.
N-(3-Cyanophenyl)-2-(3-(6-(dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (14d): Yield 45%; mp 218.5–221.5 °C; HPLC purity: 99.52% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 9.39 (s, 1H), 8.83 (d, J = 3.0 Hz, 2H), 8.14 (d, J = 3.0 Hz, 1H), 8.02 (d, J = 9.0 Hz, 1H), 7.94 (s, 1H), 7.81–7.78 (m, 2H), 7.71–7.68 (m, 1H), 7.53 (t, J = 9.0 Hz, 1H), 7.41 (d, J = 6.0 Hz, 2H), 7.07 (d, J = 9.0 Hz, 1H), 6.49 (d, J = 9.0 Hz, 1H), 5.05 (s, 2H), 2.73 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δ 166.48, 158.67, 150.65, 149.04, 146.17, 145.38, 142.72, 141.49, 139.80, 138.21, 136.41, 133.77, 132.69, 130.92, 128.59, 127.79, 127.50, 124.32, 122.42, 120.30, 119.07, 112.20, 110.08, 105.21, 55.28, 37.61 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C27H23N8O 475.19893, found 475.19891.
2-(3-(4-Methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylacetamide (16a): Yield 65%; mp 190.2–192.5 °C; HPLC purity: 99.29% (acetonitrile: 45%); 1H NMR (300 MHz, CDCl3/DMSO-d6) δ 9.28 (s, 1H), 8.86 (br s, 2H), 8.27 (s, 1H), 8.10 (d, J = 9.0 Hz, 1H), 7.94 (d, J = 9.0 Hz, 1H), 7.88 (s, 1H), 7.58 (d, J = 9.0 Hz, 2H), 7.30 (d, J = 9.0 Hz, 2H), 7.10 (t, J = 6.0 Hz, 1H), 6.89 (s, 1H), 5.19 (s, 2H), 2.44 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 164.16, 159.08, 153.48, 145.30, 144.90, 144.50 (2C), 142.95, 142.47, 137.05, 133.28, 132.86, 132.05, 129.06, 129.03 (2C), 124.92, 121.60, 120.14 (2C), 114.49, 55.99, 17.05; HRMS-ESI (m/z): [M + H]+ calcd for C23H19N6OS 427.13356, found 427.13354.
N-(2-Fluorophenyl)-2-(3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (16b): Yield 70%; mp 197.5–200.0 °C; HPLC purity: 96.75% (acetonitrile: 45%); 1H NMR (300 MHz, CDCl3) δ 8.89 (s,1H) 8.85 (d, J = 6.0 Hz, 2H), 8.32 (s, 1H), 8.28 (d, J = 9.0 Hz, 1H), 8.11 (d, J = 9.0 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.85 (s, 1H), 7.15–7.06 (m, 3H), 6.92 (s, 1H), 5.09 (s, 2H), 2.43 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 164.07, 158.99, 153.61, 152.71 (d, J = 243.75 Hz), 145.17, 145.07, 144.79, 142.91, 142.45, 133.39, 132.77, 132.22, 128.95, 128.89, 128.76, 125.55 (d, J = 9.8 Hz), 125.21 (d, J = 7.5 Hz), 124.56 (d, J = 3.8 Hz), 121.80, 121.67, 115.02 (d, J = 19.5 Hz), 55.97, 17.12; HRMS-ESI (m/z): [M + H]+ calcd for C23H18FN6OS 445.12413, found 445.12405.
N-(3-Fluorophenyl)-2-(3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (16c): Yield 81%; mp 212.1–213.5 °C; HPLC purity: 100.00% (acetonitrile: 45%); 1H NMR (300 MHz, CDCl3) δ 9.41 (s, 1H), 8.85 (br s, 2H), 8.27 (s, 1H), 8.10 (d, J = 9.0 Hz, 1H), 8.02 (s, 1H), 7.95 (d, J = 9.0 Hz, 1H), 7.87 (s, 1H), 7.52 (d, J = 9.0 Hz, 1H), 7.23 (d, J = 9.0 Hz, 1H), 6.90 (s, 1H), 6.81 (d, J = 6.0 Hz, 1H), 5.16 (s, 2H), 2.43 (s, 3H); 13C NMR (75 MHz, CDCl3/CD3OD) δ 168.83, 167.13, 166.78 (d, J = 243.0 Hz), 163.54, 156.91, 149.04, 148.52, 147.19, 146.58, 145.91, 143.17 (d, J = 9.7 Hz), 138.13, 136.26, 133.97 (d, J = 9.5 Hz), 132.51, 132.16, 125.03, 119.20, 118.66, 115.09 (d, J = 21.3 Hz), 111.24 (d, J = 27.0 Hz), 59.22, 20.44; HRMS-ESI (m/z): [M + H]+ calcd for C23H18FN6OS 445.12413, found 445.12415.
N-(3-Cyanophenyl)-2-(3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (16d): Yield 74%; mp 222.3–223.0 °C; HPLC purity: 95.14% (acetonitrile: 45%); 1H NMR (300 MHz, CDCl3/DMSO-d6) δ 10.48 (s, 1H), 8.76 (d, J = 6.0 Hz, 2H), 8.28 (s, 1H), 8.04–7.95 (m, 4H), 7.75 (d, J = 9.0 Hz, 1H), 7.38 (d, J = 6.0 Hz, 1H), 7.32 (t, J = 6.0 Hz, 1H), 6.84 (s, 1H), 5.08 (s, 2H), 2.32 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 166.11, 160.40, 152.90, 146.42, 145.74, 142.95, 142.79, 141.84, 139.73, 134.79, 134.25, 132.08, 130.93, 128.87, 128.11, 127.82, 124.32, 122.44, 119.87, 119.04, 115.33, 112.21, 55.37, 17.36; HRMS-ESI (m/z): [M + H]+ calcd for C24H18N7OS 452.12881, found 452.12878.
N-Phenyl-2-(3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (25a): Yield 61%; mp 170.3–172.5 °C; HPLC purity: 99.97% (acetonitrile: 30%); 1H NMR (300 MHz, CDCl3) δ 9.14 (s, 1H), 8.85 (s, 2H), 8.71 (d, J = 6.0 Hz, 1H), 8.65 (s, 1H), 8.13 (d, J = 3.0 Hz, 1H), 8.08 (d, J = 6.0 Hz, 1H), 7.86 (s, 1H), 7.78 (dd, J = 9.0, 3.0 Hz, 1H), 7.67 (d, J = 6.0 Hz, 1H),7.48(d, J = 9.0 Hz, 2H), 7.30 (d, J = 6.0 Hz, 1H), 7.11 (t, J = 9.0 Hz, 1H), 5.12 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 163.83, 159.00, 158.62, 157.47, 147.42, 145.43, 145.05, 143.00, 142.46, 136.79, 133.78, 133.19, 131.97, 129.31, 129.14 (2C), 128.86, 125.19, 123.29, 120.22 (2C), 119.02, 56.24. HRMS-ESI (m/z): [M + H]+ calcd for C23H18N7O 408.15673, found 408.15622.
N-(2-Fluorophenyl)-2-(3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (25b): Yield 58%; mp 192.3–194.5 °C; HPLC purity: 99.97% (acetonitrile: 30%); 1H NMR (300 MHz, CDCl3) δ 9.10 (s, 1H), 9.02 (br s, 1H, NH), 8.84 (s, 2H), 8.77 (d, J = 6.0 Hz, 1H), 8.29 (t, J = 7.5 Hz, 1H), 8.15 (d, J = 3.0 Hz, 1H), 8.10 (d, J = 6.0 Hz, 1H), 7.85–7.77 (m, 3H), 7.16–7.05 (m, 3H), 5.13 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 163.72, 158.86, 158.57, 157.19, 152.56 (d, J = 244.0 Hz), 147.57, 145.27, 144.95, 142.90, 142.46, 133.88, 133.31, 132.24, 129.07, 128.83, 125.55 (d, J = 10.2 Hz), 125.22 (d, J = 7.7 Hz), 124.73 (d, J = 3.6 Hz), 123.45, 121.60, 118.80, 114.99 (d, J = 19.0 Hz), 56.13; HRMS (ESI) m/z calcd for C23H17FN7O 426.14731, found 426.14719.
N-(3-Cyanophenyl)-2-(3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (25d): Yield 65%; mp 178.3–180.5 °C; HPLC purity: 99.27% (acetonitrile: 30%); 1H NMR (300 MHz, DMSO-d6) δ 10.86 (s, 1H), 9.03 (s, 1H), 8.95–8.93 (m, 2H), 8.85 (d, J = 6.0 Hz, 1H), 8.39 (s, 1H), 8.11 (s, 2H), 8.06 (d, J = 9.0 Hz, 1H), 7.91–7.88 (m, 3H), 7.59–7.58 (m, 2H), 5.28 (s, 2H); 13C NMR (125 MHz, DMSO-d6/CD3OD) δ 165.93, 159.82, 157.96, 157.08, 145.76, 145.46, 144.96, 142.54, 141.84, 139.25, 135.22, 134.16, 132.45, 130.03, 128.20, 127.82, 127.49, 123.96, 122.55, 122.17, 119.09, 118.18, 112.55, 54.91; HRMS-ESI (m/z): [M + H]+ calcd for C24H17N8O 433.15198, found 433.15179.
2-(5-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylacetamide (15a): Yield 10%; 145.4–147.5 °C; 1H NMR (300 MHz, CDCl3) δ 8.81 (d, J = 6.0 Hz, 2H), 8.22 (s, 1H), 8.09 (s, 1H), 8.02–7.99 (m, 2H), 7.69 (dd, J = 9.0, 3.0 Hz, 1H), 7.49 (d, J = 9.0 Hz, 2H), 7.34–7.28 (m, 3H), 7.08 (t, J = 9.0 Hz, 1H), 6.52 (d, J = 9.0 Hz, 1H), 6.45 (d, J = 6.0 Hz, 1H), 5.17 (s, 2H), 3.07 (s, 6H); HRMS-ESI (m/z): [M + H]+ calcd for C26H24N7O 450.20368, found 450.20364.
2-(5-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-(2-fluorophenyl)acetamide (15b): Yield 13%; mp 130.2–133.0 °C; 1H NMR (300 MHz, CDCl3) δ 8.81 (d, J = 6.0 Hz, 2H), 8.45 (s, 1H), 8.30 (t, J = 7.5 Hz, 1H), 8.09 (s, 1H), 8.01–7.98 (m, 2H), 7.67 (dd, J = 9.0, 3.0 Hz, 1H), 7.33 (t, J = 9.0 Hz, 1H), 7.14–6.95 (m, 3H), 6.51 (d, J = 6.0 Hz, 1H), 5.24 (s, 2H), 3.07 (s, 6H); HRMS-ESI (m/z): [M + H]+ calcd for C26H23FN7O 468.19426, found 468.19406.
2-(5-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-(3-fluorophenyl)acetamide (15c): Yield 15%; mp 146.5–147.6 °C; 1H NMR (300 MHz, CDCl3) δ 8.81 (d, J = 3.0 Hz, 2H), 8.41 (s, 1H), 8.09 (s, 1H), 8.00 (d, J = 9.0 Hz, 2H), 7.68 (d, J = 9.0, 3.0 Hz, 1H), 7.50 (d, J = 9.0 Hz, 1H), 7.35 (t, J = 7.5 Hz, 1H), 7.23 (t, J = 9.0 Hz, 1H), 7.11 (d, J = 9.0 Hz, 1H), 6.81 (t, J = 9.0 Hz, 1H), 6.53 (d, J = 9.0 Hz, 1H), 6.45 (d, J = 6.0 Hz, 1H), 5.16 (s, 2H), 3.07 (s, 6H); HRMS-ESI (m/z): [M + H]+ calcd for C26H23FN7O 468.19426, found 468.19427.
N-(3-Cyanophenyl)-2-(5-(6-(dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (15d): Yield 14%; mp 145.5–148.0 °C; 1H NMR (300 MHz, CDCl3) δ 8.81 (d, J = 3.0 Hz, 2H), 8.63 (s, 1H), 8.08 (d, J = 3.0 Hz, 1H), 8.02–7.96 (m, 3H), 7.67 (d, J = 9.0 Hz, 2H), 7.43–7.33 (m, 3H), 6.54 (d, J = 9.0 Hz, 1H), 6.43 (d, J = 9.0 Hz, 1H), 5.18 (s, 2H), 3.07 (s, 6H); HRMS-ESI (m/z): [M + H]+ calcd for C27H23N8O 475.19893, found 475.19891.
2-(5-(4-Methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylacetamide (17a): Yield 8%; 1H NMR (300 MHz, CDCl3) δ 9.64 (s, 1H), 8.90 (s, 2H), 8.14 (d, J = 6.0 Hz, 1H), 8.04 (s, 4H), 7.83–7.65 (m, 1H), 7.60 (d, J = 6.0 Hz, 1H), 7.39–7.27 (m, 2H), 7.14 (d, J = 6.0 Hz, 1H), 5.29 (s, 2H), 2.60 (s, 3H); HRMS-ESI (m/z): [M + H]+ calcd for C23H19N6OS 427.13356, found 427.13361.
N-(2-Fluorophenyl)-2-(5-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (17b): Yield 7%; 1H NMR (300 MHz, CDCl3) δ 9.66 (s, 1H), 8.86 (br s, 2H), 8.37 (t, J = 9.0 Hz, 1H), 8.13–8.09 (m, 2H), 7.87 (s, 1H), 7.73 (dd, J = 9.0, 3.0Hz, 1H), 7.16–7.03 (m, 3H), 6.98 (s, 1H), 5.33 (s, 2H), 2.57 (s, 3H); HRMS-ESI (m/z): [M + H]+ calcd for C23H18FN6OS 445.12413, found 445.12418.
N-(3-Fluorophenyl)-2-(5-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (17c): Yield 7%; 1H NMR (300 MHz, CDCl3) δ 9.98 (s, 1H), 8.86 (br s, 2H), 8.14 (d, J = 9.0 Hz, 1H), 8.01 (br s, 2H), 7.87 (s, 1H), 7.72 (d, J = 9.0 Hz, 1H), 7.55 (d, J = 9.0 Hz, 1H), 7.25 (br s, 1H), 7.03 (br s, 1H), 6.81 (t, J = 9.0 Hz, 1H), 5.22 (s, 2H), 2.59 (s, 3H); HRMS-ESI (m/z): [M + H]+ calcd for C23H18FN6OS 445.12413, found 445.12408.
N-(3-Cyanophenyl)-2-(5-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)acetamide (17d): Yield 9%; 1H NMR (300 MHz, CDCl3) δ 10.43 (s, 1H), 8.75 (br s, 2H), 8.36 (d, J = 3.0 Hz, 1H), 8.26 (d, J = 9.0, 1H), 8.06–7.91 (m, 3H), 7.74–7.6 (m, 2H), 7.37–7.26 (m, 2H), 5.42 (s, 2H), 2.34 (s, 3H); HRMS-ESI (m/z): [M + H]+ calcd for C24H18N7OS 452.12881, found 452.12881.
N-Phenyl-2-(5-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-2,3-dihydro-1H-pyrazol-1-yl)acetamide (26a): Yield 10%; HPLC purity: 96.43% (acetonitrile: 30%); 1H NMR (300 MHz, CDCl3) δ 9.43 (s, 1H), 8.87 (s, 2H), 8.80 (s, 1H), 8.66 (d, J = 6.0 Hz, 1H), 8.11 (d, J = 9.0 Hz, 1H), 8.05 (s, 1H), 7.95 (s, 1H), 7.62 (dd, J = 9.0, 3.0 Hz, 1H), 7.53 (d, J = 6.0 Hz, 2H), 7.33 (t, J = 7.5 Hz, 2H), 7.20 (dd, J = 6.0, 3.0 Hz, 1H), 7.13 (t, J = 7.5 Hz, 1H), 5.29 (s, 2H); HRMS-ESI (m/z): [M + H]+ calcd for C23H18N7O 408.15673, found 408.15698.
N-(2-Fluorophenyl)-2-(5-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-2,3-dihydro-1H-pyrazol-1-yl)acetamide (26b): Yield 7%; HPLC purity: 99.29% (acetonitrile: 30%); 1H NMR (300 MHz, CDCl3) δ 9.55 (s, 1H), 9.45 (s, 1H), 8.86 (s, 2H), 8.65 (d, J = 6.0 Hz, 1H), 8.34 (t, J = 9.0 Hz, 1H), 8.11 (d, J = 9.0 Hz, 1H), 8.06 (s, 1H), 7.94 (s, 1H), 7.62 (dd, J = 9.0, 3.0 Hz, 1H), 7.19 (d, J = 6.0 Hz, 1H), 7.15–7.05 (m, 3H), 5.31 (s, 2H); HRMS-ESI (m/z): [M + H]+ calcd for C23H17FN7O 426.14731, found 426.14783.
N-(3-Cyanophenyl)-2-(5-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-2,3-dihydro-1H-pyrazol-1-yl)acetamide (26d): Yield 13%; HPLC purity: 97.98% (acetonitrile: 45%); 1H NMR (300 MHz, CDCl3) δ 10.04 (s, 1H), 9.25 (s, 1H), 8.78 (br s, 2H), 8.54(s, 1H), 7.96 (br s, 3H), 7.89–7.81 (m, 2H), 7.69 (br s, 1H), 7.58 (br s, 1H), 7.26 (s, 1H), 7.19 (s, 1H), 5.44 (s, 2H); HRMS (ESI) m/z calcd for C24H17N8O 433.15198, found 433.15216.
3.1.5. General Procedure for the Preparation of 2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylethanethioamide 18a–d, 2-(3-(4-Methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylethanethioamide 19a–d or N-Phenyl-2-(3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (27b and 27d)
A stirred mixture of 14a–d, 16a–d, 25b, or 25d (0.34 mmol), Lawesson’s reagent (0.34 mmol), and anhydrous DME (5 mL) in a dry sealed tube was heated at 85 °C for 12 h. After cooled to room temperature, the solvent was evaporated to dryness under reduced pressure, and the residue was purified by silica gel column chromatography (dichloromethane/methanol, 100:1) to give the titled compounds 18a–d, 19a–d, 27b, or 27d as a light yellow solid.
2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylethanethioamide (18a): Yield 45%; mp 182.0–184.0 °C; HPLC purity: 96.95% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 10.79 (s, 1H), 8.82 (d, J = 6.0 Hz, 2H), 8.15 (s, 1H), 8.03 (d, J = 9.0 Hz, 1H), 7.82–7.70 (m, 3H), 7.52 (t, J = 7.5 Hz, 2H), 7.39 (t, J = 7.5 Hz, 2H), 7.26 (d, J = 9.0 Hz, 1H), 7.09 (d, J = 9.0 Hz, 1H), 6.49 (d, J = 9.0 Hz, 1H), 5.44 (s, 2H), 2.74 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 192.76, 167.73, 145.14, 144.56, 142.96, 142.06, 138.24, 138.00, 132.68, 132.39, 132.31, 130.93 (2C), 128.90, 128.85 (2C), 128.60, 128.25, 127.00, 123.04, 121.71, 109.87, 105.56, 65.58, 37.67 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C26H24N7S 466.18084, found 466.18082.
2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-(2-fluorophenyl)ethanethioamide (18b): Yield 43%; mp 88.3–91.2 °C; HPLC purity: 99.31% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 10.79 (s, 1H), 8.82 (d, J = 6.0 Hz, 2H), 8.70 (t, J = 7.5 Hz, 1H), 8.16 (d, J = 3.0 Hz, 1H), 8.01 (d, J = 9.0 Hz, 1H), 7.82 (dd, J = 9.0, 3.0 Hz, 1H), 7.79 (s, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.24–7.17 (m, 4H), 6.46 (d, J = 9.0 Hz, 1H), 5.44 (s, 2H), 2.64 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 193.51, 158.67, 154.33 (d, J = 248.6 Hz), 151.59, 149.39, 145.06, 144.48, 142.98, 142.07, 137.80, 135.72, 132.94, 132.23, 128.36 (d, J = 18.0 Hz), 127.63 (d, J = 7.8 Hz), 126.72 (d, J = 10.0 Hz), 124.02 (d, J = 3.8 Hz), 123.79, 121.91, 115.48 (d, J = 19.1 Hz), 109.76, 105.30, 63.66, 37.48 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C26H23FN7S 484.17142, found 484.17133.
2-(3-(6-(Dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-(3-fluorophenyl)ethanethioamide (18c): Yield 44%; mp 68.5–70.2 °C; HPLC purity: 98.45% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 10.94 (s, 1H), 8.82 (d, J = 6.0 Hz, 2H), 8.14 (d, J = 3.0 Hz, 1H), 8.02 (d, J = 9.0 Hz, 1H), 7.88 (d, J = 9.0 Hz, 1H), 7.81–7.78 (m, 2H), 7.52 (t, J = 7.5 Hz, 1H), 7.42 (d, J = 9.0 Hz, 1H), 7.34 (t, J = 9.0 Hz, 1H), 7.09 (d, J = 9.0 Hz, 1H), 6.96 (t, J = 9.0 Hz, 1H), 6.47 (d, J = 9.0 Hz, 1H), 5.40 (s, 2H), 2.71 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 192.94, 164.11, 159.68 (d, J = 175.7 Hz), 148.85, 145.17, 144.61, 142.96, 142.08, 139.63 (d, J = 10.5 Hz), 138.03, 135.26, 133.85, 132.50 (d, J = 15.0 Hz), 130.06 (d, J = 9.2 Hz), 128.67, 128.27, 121.74, 118.38 (d, J = 3.2 Hz), 113.74 (d, J = 21.2 Hz), 110.24, 109.89, 105.66, 63.58, 37.69 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C26H23FN7S 484.17142, found 484.17133.
N-(3-Cyanophenyl)-2-(3-(6-(dimethylamino)pyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (18d): Yield 37%; mp 108.5–110.0 °C; HPLC purity: 96.46% (acetonitrile: 40%); 1H NMR (300 MHz, CDCl3) δ 11.13 (s, 1H), 8.83 (d, J = 6.0 Hz, 2H), 8.26 (s, 1H), 8.14 (s, 1H), 8.01 (t, J = 9.0 Hz, 2H), 7.81–7.78 (m, 2H), 7.54–7.49 (m, 3H), 7.05 (d, J = 9.0 Hz, 1H), 6.49 (d, J = 9.0 Hz, 1H), 5.41 (s, 2H), 2.73 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δ 197.16, 158.56, 146.18, 145.39, 142.71, 141.48, 140.23, 138.33, 137.50, 136.35, 134.03, 132.69, 130.75, 130.50, 128.59, 127.49, 126.65, 120.36, 120.15, 118.77, 111.89, 110.23, 105.33, 62.82, 39.12 (2C); HRMS-ESI (m/z): [M + H]+ calcd for C27H23N8S 491.17609, found 491.17609.
2-(3-(4-Methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)-N-phenylethanethioamide (19a): Semi-solid; Yield 80%; HPLC purity: 96.56% (acetonitrile: 45%); 1H NMR (300 MHz, CDCl3) δ 11.02 (s, 1H), 8.91 (br s, 2H), 8.29 (s, 1H), 8.17 (t, J = 9.0 Hz, 1H), 7.96–7.88 (m, 3H), 7.38 (t, J = 9.0 Hz, 2H), 7.26 (b r s, 2H), 6.96 (s, 1H), 5.54 (s, 2H), 2.57 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 192.07, 158.81, 153.81, 145.31, 145.10, 144.93, 142.98, 142.53, 138.17, 133.19, 132.62, 132.07, 129.16, 129.07, 128.99 (2C), 127.12, 122.94 (2C), 121.70, 114.54, 63.83, 17.13; HRMS-ESI (m/z): [M + H]+ calcd for C23H19N6S2 443.11071, found 443.11072.
N-(2-Fluorophenyl)-2-(3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (19b): Semi-solid; Yield 84%; HPLC purity: 96.40% (acetonitrile: 45%); 1H NMR (300 MHz, DMSO-d6) δ 11.79 (s, 1H), 8.94 (d, J = 9.0 Hz, 2H), 8.50 (d, J = 9.0 Hz, 2H), 8.16 (d, J = 9.0 Hz, 1H), 8.08 (d, J = 9.0 Hz, 1H), 7.73–7.61 (m, 4H), 7.39–7.25 (m, 2H), 5.48 (s, 2H), 2.35 (s, 3H); 13C NMR (75 MHz, CDCl3/DMSO-d6) δ 194.18, 167.96, 159.08, 154.96 (d, J = 248.25 Hz), 153.40, 145.09, 144.63, 142.66, 142.09, 133.79, 132.61, 132.27, 131.08 (d, J = 14.4 Hz), 130.99, 130.43, 128.77 (d, J = 3.0 Hz), 128.60 (d, J = 8.3 Hz), 128.21 (d, J = 8.3 Hz), 125.28, 115.68 (d, J = 19.4 Hz), 114.68, 65.63, 19.07; HRMS-ESI (m/z): [M + H]+ calcd for C23H18FN6S2 461.10129, found 461.10120.
N-(3-Fluorophenyl)-2-(3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (19c): Semi-solid; Yield 88%; HPLC purity: 96.37% (acetonitrile: 45%); 1H NMR (300 MHz, DMSO-d6) δ 12.21 (s, 1H), 8.93 (d, J = 9.0 Hz, 2H), 8.52 (s, 1H), 8.48 (s, 1H), 8.16 (d, J = 9.0 Hz, 1H), 8.06 (t, J = 9.0 Hz, 1H), 7.73–7.68 (m, 2H), 7.50 (d, J = 9.0 Hz, 1H), 7.31 (s, 1H), 7.14 (s, 1H), 5.43 (s, 2H), 2.34 (s, 3H); 13C NMR (75 MHz, CDCl3/DMSO-d6) δ 197.82, 166.35 (d, J = 243.0 Hz), 163.66, 157.17, 149.06, 148.50, 147.52, 146.54, 145.81, 138.34, 136.12 (d, J = 17.5 Hz), 135.66, 135.10 (d, J = 11.4 Hz), 133.83 (d, J = 9.0 Hz), 132.68, 132.34, 124.62, 122.40 (d, J = 2.3 Hz), 118.65, 117.20 (d, J = 21.0 Hz), 114.12, 69.57, 17.24; HRMS-ESI (m/z): [M + H]+ calcd for C23H18FN6S2 461.10129, found 461.10132.
N-(3-Cyanophenyl)-2-(3-(4-methylthiazol-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (19d): Semi-solid; Yield 89%; HPLC purity: 96.31% (acetonitrile: 45%); 1H NMR (300 MHz, DMSO-d6) δ 12.33 (s, 1H), 8.93 (d, J = 9.0 Hz, 2H), 8.50 (d, J = 6.0 Hz, 2H), 8.44 (s, 1H), 8.14–8.06 (m, 2H), 7.77 (d, J = 6.0 Hz, 1H), 7.69 (t, J = 7.5 Hz, 1H), 7.31 (s, 1H), 6.91 (s, 1H), 5.45 (s, 2H), 2.34 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 196.70, 160.48, 152.89, 146.42, 145.73, 143.12, 142.80, 141.82, 140.22, 135.10, 134.31, 132.08, 130.75, 130.48, 128.87, 128.53, 128.08, 126.60, 119.71, 118.76, 115.34, 111.90, 61.84, 17.36; HRMS-ESI (m/z): [M + H]+ calcd for C24H18N7S2 468.10596, found 468.10599.
N-(2-Fluorophenyl)-2-(3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (27b): Yield 68%; HPLC purity: 98.75% (acetonitrile: 35%); 1H NMR (300 MHz, CDCl3) δ 10.57 (s, 1H, NH), 9.12 (s, 1H), 8.84 (s, 2H), 8.78 (s, 1H), 8.64 (t, J = 7.5 Hz, 1H), 8.16 (s, 1H), 8.08 (d, J = 9.0 Hz, 1H), 7.92 (s, 1H), 7.82 (d, J = 9.0 Hz, 2H), 7.23–7.10 (m, 3H), 5.48 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 192.31, 167.73, 158.80, 157.49, 154.12 (d, J = 247.5 Hz), 147.81, 145.36, 145.01, 142.94, 142.47, 133.77, 133.11, 132.15, 130.92, 128.89, 128.85, 127.72 (d, J = 7.5 Hz), 126.62 (d, J = 10.0 Hz), 124.19 (d, J = 3.75 Hz), 123.47, 118.84, 115.44 (d, J = 18.75 Hz), 65.58; HRMS-ESI (m/z): [M + H]+ calcd for C23H17FN7S 442.12447, found 442.12447.
N-(3-Cyanophenyl)-2-(3-(pyrimidin-4-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl)ethanethioamide (27d): Yield 50%; HPLC purity: 99.68% (acetonitrile: 35%); 1H NMR (300 MHz, CDCl3) δ 10.57 (s, 1H, NH), 9.21 (s, 1H), 8.88 (s, 2H), 8.76 (d, J = 6.0 Hz, 1H), 8.27 (s, 1H), 8.16–8.11 (m, 2H), 7.91 (br s, 2H), 7.79 (d, J = 9.0 Hz, 1H), 7.64 (s, 1H), 7.54–7.49 (m, 2H), 5.50 (s, 2H); 13C NMR (125 MHz, CDCl3) δ 194.60, 159.15, 158.61, 157.07, 146.27, 145.21, 144.73, 142.88, 142.19, 139.77, 134.36, 133.27, 132.07, 129.72, 129.61, 128.89, 128.47, 127.38, 126.19, 122.38, 119.06, 118.16, 112.49, 63.61; HRMS-ESI (m/z): [M + H]+ calcd for C24H17N8S 449.12914, found 449.1285.
3.2. Kinase Assay
All kinase experiments were completed by the ProQinase (Freiburg, Germany). All protein kinases were expressed in Sf9 insect cells or in E. coli as recombinant GST-fusion proteins or His-tagged proteins, either as full-length or enzymatically active fragments. All kinases were obtained from human cDNAs and purified by either GSH-affinity chromatography or immobilized metal. The purity of the protein kinases was examined by SDS-PAGE/Coomassie staining. The identity was checked by mass spectroscopy.
A radiometric protein kinase assay (33PanQinase® activity assay) was used for measuring the kinase activity of the two protein kinases. All kinase assays were performed in 96-well FlashPlatesTM from PerkinElmer (Boston, MA, USA) in 50 μL reaction volumes. The reaction cocktail was pipetted in four steps in the following order: 20 μL of assay buffer (standard buffer), 5 μL of ATP solution (in H2O), 5 μL of test compound (in 10% DMSO), 20 μL enzyme/substrate mix.
The assay for all protein kinases contained 70 mM HEPES-NaOH pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 50 μg/mL PEG20000, ATP, [γ-33P]-ATP, protein kinase, and substrate.
The reaction cocktail was incubated at 30 °C for 60 min. The reaction was halted with 50 μL of 2% (v/v) H3PO4, plates were aspirated and washed two times with 200 μL 0.9% (w/v) NaCl. Incorporation of 33Pi was established with a microplate scintillation counter (PerkinElmer, Boston, MA, USA). All assays were performed with a BeckmanCoulter/SAGIAN™ Core System.
3.3. Docking Assay
All molecular computation studies were carried out using Discovery Studio 2017 (Accelrys, San Diego, CA, USA). The X-ray crystal structure of ALK5 complexed with 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitor was obtained from protein data bank (PDB: 1RW8). The water molecules and heavy atom in protein were removed and the protein was prepared by adding hydrogen and correcting incomplete residues using Clean Protein tool of DS, then the protein was refined with CHARMm. The structures of compounds 18b and 19b were sketched in 2D and converted into 3D using the DS molecule editor. Automated docking studies were carried out to investigate the binding mode of compounds 18b and 19b in the crystal structure of 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole utilizing DS-CDOCKER protocol. The pose with the top CDOCKER_INTERACTION_ENERGY was chosen for analyzing the binding features of compounds 18b and 19b with ALK5.
3.4. Prediction of ADMET Properties
ADMET properties of good targeted compounds 19a–d as drug lead compound were predicted using ADMET descriptors in Discovery Studio 2017 (Accelrys, San Diego, CA, USA). It is a quick, easy and accurate method for prediction of absorption, distribution, metabolism, elimination and toxicity (ADMET) properties. In this work, for the aforementioned compounds, human intestinal absorption level, aqueous solubility (log(SW)), blood–brain barrier (BBB) penetration level (AlogP98), human cytochrome P450 2D6 (CYP2D6) inhibitory ability, hepatotoxicity possibility, and plasma protein binding (PPB) levels were measured.
4. Conclusions
In our study, 32 quinoxaline-derivatives of 3-substituted-4-(quinoxalin-6-yl) pyrazoles 14a–d, 15a–d, 16a–d, 17a–d, 18a–d, 19a–d, 25a, 25b, 25d, 26a, 26b, 26d, 27b, and 27d were synthesized and evaluated for ALK5 and p38α MAP kinase inhibitory activities in enzymatic assays. We found that insertion of a 4-methylthiazol-2-yl moiety at the 3-position of the pyrazole ring showed more potent ALK5 inhibitory activity and selectivity than introduction of electron-donating groups into quinoxaline or quinoline ring. The most potent compound, 19b, inhibited ALK5 phosphorylation with an IC50 value of 0.28 µM and showed 98% inhibition at 10 µM in the enzymatic assay. The selectivity index of 19b against p38α MAP kinase was >35, much higher than that of positive control compound 3 (4). Although compound 19b has slightly lower activity than the positive control compound 3, its selectivity is much higher than that of the positive control compound 3, so its side effects may be lower. The docking study described that compounds possessing 4-methylthazol-2-yl moiety was found to show better docking interaction than compounds possessing 6-(dimethylamino)pyridine-2-yl moiety on its active site. All good targeted compounds were subjected to ADMET prediction and the predicted ADMET parameters were within the acceptable range defined for human use. This result provides good data for the design of substituents for the 3-position introduction of the pyrazole ring, which may be a good choice if the lipophilic compounds are needed. In particular, compound 19b was the most promising and it could be considered worthwhile lead compound worthy of further investigation.
Author Contributions
L.-M.Z. and Z.G. are co-authors; they contributed equally to this work. All authors (L.-M.Z., Z.G., Y.-J.X., J.Z.M., W.-J.Z., X.-Y.L., H.-R.P., and C.H.J.) have read and approved the final manuscript.
Funding
This work was supported by the National Science Foundation of China (No. 81560557) and the Education Department of Jilin Province Scientific Research Fund Project (No. 2016-283).
Acknowledgments
We thank Susan R. Doctrow, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Zhu, Y.Z. Role of transforming growth factor-β in the progression of heart failure. Cell. Mol. Life Sci. 2006, 63, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhu, Y.J.; Yang, X.; Guo, Z.J.; Xu, W.B.; Tian, X.L. Effect of TGF-β/Smad signaling pathway on lung myofibroblast differentiation. Acta. Pharmacol. Sin. 2007, 28, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hao, X.; Xu, L.; Cui, J.; Xue, L.; Tian, Z. Intestinal flora imbalance promotes alcohol-induced liver fibrosis by the TGF-β/Smad signaling pathway in mice. Oncol Lett. 2017, 14, 4511–4516. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.Y.; Park, S.R.; Cho, S.; Yu, S.L.; Lee, H.Y.; Park, C.G.; Kang, J.; Jung, D.Y.; Park, M.H.; Hwang, W.M.; et al. TGF-β-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induce acute kidney injury. J. Antimicrob. Chemoth. 2018, 73, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Miyazono, K.; Ten Dijke, P. TGF-beta signaling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Blobe, G.C. Role of transforming growth factor-β in hematologic malignancies. Blood 2006, 107, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Hendrik, U.; Frank, G.; Roland, K.; Utz, S.; Hendrik, L.; Bernhard, H.R. Signaling Crosstalk of TGF-β/ALK5 and PAR2/PAR1: A Complex Regulatory Network Controlling Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1568. [Google Scholar] [CrossRef]
- Tobias, B.; Benjamin, R.; Dirk, R.; Roland, K.; Harald, B.; Hendrik, L.; Frank, G.; Hendrik, U. Dasatinib blocks transcriptional and promigratory responses to transforming growth factor-beta in pancreatic adenocarcinoma cells through inhibition of Smad signaling: Implications for in vivo mode of action. Mol. Cancer 2015, 14, 199/1–199/12. [Google Scholar] [CrossRef]
- Byfield, S.D.; Major, C.; Laping, N.J.; Roberts, A.B. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004, 65, 744–752. [Google Scholar] [CrossRef]
- Uhl, M.; Aulwurm, S.; Wischhusen, J.; Weiler, M.; Ma, J.Y.; Almirez, R.; Mangadu, R.; Liu, Y.W.; Platten, M.; Herrlinger, U.; et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004, 64, 7954–7961. [Google Scholar] [CrossRef]
- Bueno, L.; De Alwis, D.P.; Pitou, C.; Yingling, J.; Lahn, M.; Glatt, S. Semi-mechanistic modeling of the tumor growth inhibitory effects of LY2157299, a new type I receptor TGF-β kinase antagonist, in mice. Eur. J. Cancer 2008, 44, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Krishnaiah, M.; Sreenu, D.; Subrahmanyam, V.B.; Rao, K.S.; Lee, H.J.; Park, S.J.; Park, H.J.; Lee, K.; Sheen, Y.Y.; et al. Discovery of N-((4-([1,2,4]triazolo- [1,5-a]pyridin-6-yl)-5-(6-methylpyridin- 2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): A highly potent, selective, and orally bioavailable inhibitor of TGF-β type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J. Med. Chem. 2014, 57, 4213–4238. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Sreenu, D.; Krishnaiah, M.; Subrahmanyam, V.B.; Rao, K.S.; Mohan, A.V.N.; Park, C.V.; Son, J.Y.; Son, D.H.; Park, H.J.; et al. Synthesis and biological evaluation of 1-substituted-3(5)- (6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrzoles as transforming growth factor-β type I receptor kinase inhibitors. Eur. J. Med. Chem. 2011, 46, 3917–3925. [Google Scholar] [CrossRef]
- Tojo, M.; Hamashima, Y.; Hanyu, A.; Kajimoto, T.; Saitoh, M.; Miyazono, K.; Node, M.; Imamura, T. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-β. Cancer Sci. 2005, 96, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Dewang, P.M.; Kim, D.K. Synthesis and biological evaluation of 2-pyridyl-substituted pyrazoles and imidazoles as transforming growth factor-β type I receptor kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4228–4232. [Google Scholar] [CrossRef]
- Belveren, S.; Ali Dondas, H.; Ulger, M.; Poyraz, S.; Garcia, M.E.; Saperas, M.F.; Sansano, J.M. Synthesis of highly functionalized 2-(pyrrolidin-1-yl)thiazole frameworks with interesting antibacterial and antimycobacterial activity. Tetrahedron 2017, 73, 6718–6727. [Google Scholar] [CrossRef]
- Vale, N.; Correia-Branco, A.; Patricio, B.; Duarte, D.; Martel, F. In vitro studies on the inhibition of colon cancer by amino acid derivatives of bromothiazole. Bioorg. Med. Chem. Lett. 2017, 27, 3507–3510. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.M.; Carda, M.; Crespo, B.; Cunat, A.C.; Cozar, C.; Leon, M.L.; Marco, J.A.; Roda, N.; Cervera, J.F.S. Design, synthesis and antimalarial evaluation of novel thiazole derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Jin, J.; Chen, H.; Cao, R.; Chen, X.; Xu, B. Synthesis and biological evaluation of pyrimidine derivatives as novel human Pin1 inhibitors. Bioorg. Med. Chem. 2018, 26, 2186–2197. [Google Scholar] [CrossRef]
- Tsukamoto, I.; Koshio, H.; Kuramochi, T.; Saitoh, C.; Inamura, H.Y.; Nozawa, C.K.; Yamamoto, E.; Yatsu, T.; Shimada, Y.; Sakamoto, S.; et al. Synthesis and structure-activity relationships of amide derivatives of (4,4-difluoro-1,2,3,4-tetrahydro-5H- benzazepin-5- ylidene)acetic as selective arginine vasopressin V2 receptor agonists. Bioorg. Med. Chem. 2009, 17, 3130–3141. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, P.M.; Ana, B.S.M.; Benedicto del, R.; Pelaez, R.; Caballero, E.; Medarde, M. A new family of quinolone and quinoxaline analogues of combretastatins. Bioorg. Med. Chem. Lett. 2004, 14, 3771–3774. [Google Scholar] [CrossRef]
- Jin, C.H.; Krishnaiah, M.; Sreenu, D.; Rao, K.S.; Subrahmanyam, V.B.; Park, C.Y.; Son, J.Y.; Sheen, Y.Y.; Kim, D.K. Synthesis and biological evaluation of 1-substituted-3(5)-(6-methylpyridin- 2-yl)-4-(quinolin-6-yl)pyrazoles as transforming growth factor-β type I receptor kinase inhibitors. Bioorg. Med. Chem. 2011, 19, 2633–2640. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Preti, D.; Tabrizi, M.A.; Fruttarolo, F.; Saponaro, G.; Baraldi, S.; Romagnoli, R.; Moorman, A.R.; Gessi, S.; Varani, K.; et al. N6-[(Hetero)aryl/(cyclo)alkyl-carbamoyl- methoxy-phenyl]-(2-chloro)-5’-N-ethylcarboxamido-adenosines: The first example of adenosine-related structures with potent agonist activity at the human A2B adenosine receptor. Bioorg. Med. Chem. 2007, 15, 2514–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Zhu, Q.; Liu, J.; Chen, L.; Leng, Y.; Jiang, H.; Liu, H. Benzamide derivatives as dual-action hypoglycemic agents that inhibit glycogen phosphorylase and activate glucokinase. Bioorg. Med. Chem. 2009, 17, 7301–7312. [Google Scholar] [CrossRef]
- Subramanyam, C.; Wager, T.T. Novel Compounds as Casein Kinase Inhibitors. U.S. Patent US2011,0098,272,A1, 28 April 2011. [Google Scholar]
- Eyers, P.A.; Craxton, M.; Morrice, N.; Cohen, P.; Goedert, M. Conversion of SB 203580-insensitive MAP kinase family members to drug-senstive forms by a single amino-acid substitution. Chem. Biol. 1998, 5, 321–328. [Google Scholar] [CrossRef]
- Wang, Z.C.; Qin, Y.J.; Wang, P.F.; Yang, Y.A.; Wen, Q.; Zhang, X.; Qiu, H.Y.; Duan, Y.T.; Wang, Y.T.; Sang, Y.L.; et al. Sulfonamides containing coumarin moieties selectively and potently inhibit carbonic anhydrases II and IX: Design, synthesis, inhibitory activity and 3D-QSAR analysis. Eur. J. Med. Chem. 2013, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gellibert, F.; Fouchet, M.H.; Nguyen, V.L.; Wang, R.; Krysa, G.; Gouvile, A.C.; Huet, S.; Dodic, N. Design of novel quinazoline deirivatives and related analogues as potent and selective ALK5 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Feng, Y.D.; Lu, X.; Nie, J.B.; Li, W.; Wang, L.N.; Tian, L.J.; Liu, Q.H. Isosteroidal alkaloids as potent dual-binding site inhibitors of both acetylcholinesterase and butyrylcholinesterase from the bulbs of Fritillaria walujewii. Eur. J. Med. Chem. 2017, 137, 280–291. [Google Scholar] [CrossRef]
- Patel, T.S.; Vanparia, S.F.; Patel, U.H.; Dixit, R.B.; Chudasama, C.J.; Patel, B.D.; Dixit, B.C. Novel 2,3-disubstituted quinazoline-4(3)-one derived from amino acid linked sulphonamide as a potent malarial antifolates for DHFR inhibition. Eur. J. Med. Chem. 2017, 129, 251–265. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 18a–d, 19a–d, 25a, 25b, 25d, 27b, and 27d are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).