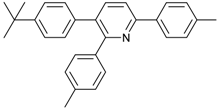
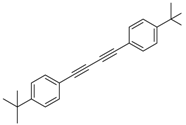
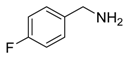
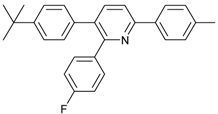
Abstract
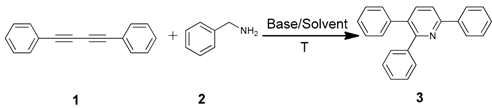
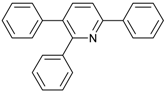
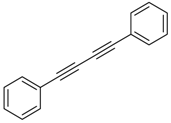
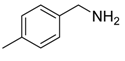
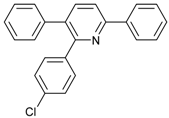
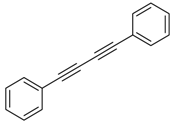
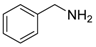
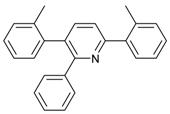
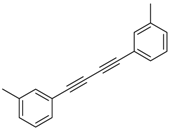
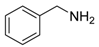
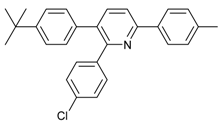
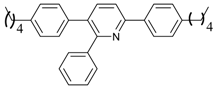
An efficient base-catalyzed synthesis of arylated pyridines has been disclosed. This reaction involving conjugated acetylenes and substituted benzylamines proceeded smoothly, giving rise to tri-aryl substituted pyridines which are biologically relevant compounds in good to excellent yields in N,N-dimethylformamide (DMF) under air at 140 °C with K2CO3 as catalyst.
1. Introduction
The importance of pyridine motif comes from its unique biological activity in natural products [1,2,3], pharmaceutical compounds [4,5,6,7,8] and agrochemicals [9]. In addition, pyridine derivatives are widely applied in organometallic chemistry [10,11], catalysis [12], material science [13,14,15] and supramolecular chemistry [16,17,18]. Therefore, the more efficient synthesis of pyridine derivatives is still an important topic [19,20]. However, there are only very few examples reported on this topic: in 1974, Chalk [21] reported a new pyridine synthesis from conjugated acetylenes and substituted methylamines, leading to 51% of 2-p-tolyl-3,6-diphenylpyridine and 38% of 2-p-tolyl-3,6-diphenylpyridine N-oxide at 145 °C under nitrogen with dimethylsulfoxide as solvent. In 2013, Shaand coworkers [22] disclosed a facile synthetic method for the preparation of trisubstituted pyridines with high regioselectivity through a three-component assembly strategy of arynes, isocyanides, and 3-bromo- or 3-acetoxypropynes, leading to 65% of 2-(4-fluorophenyl)-3,6-diphenylpyridine. In recent years, transition-metal-catalyzed C-C cross-coupling reaction has been applied to a diverse array of fields. Peter [3] recently reported the site-selective arylation of commercially available 2,3,5,6-tetrachloropyridine using the Suzuki–Miyaura reaction, allowing the selective synthesis of mono-, di-, tri- and tetraarylated pyridines in good to quantitative yields. In this context, based on the advantages of conjugated acetylenes, which are readily prepared by the catalytic oxidative coupling of terminal alkynes [23], studying more efficient synthesis of pyridine derivatives between conjugated acetylenes and substituted methylamines is still highly desirable and challenging.
2. Resultsand Discussion
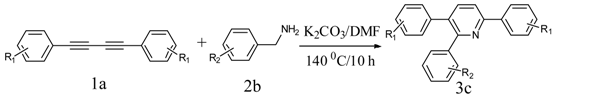
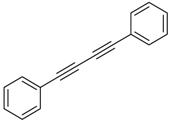
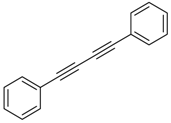
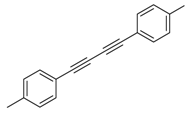
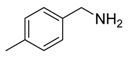
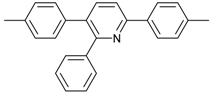
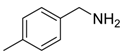
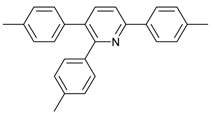
Our interest in increasing the synthetic yield of arylated pyridines from conjugated acetylenes and substituted benzylamines under optimum conditions stemmed from the fact that Chalk’s [24] work gave only a 70% yield of 2,3,6-triphenylpyridine fromsolutions of 1,4-diphenylbutadiyne in benzylamine (1:6.13 mmol) after two to three hours at 180 °C under nitrogen. Initially, we tested the reaction of 1,4-diphenylbutadiyne 1 (1 mmol) and benzylamine 2 (6 mmol) in DMSO at 140 °C in the presence of K2CO3 (0.5 mmol) under air. To our delight, 2,3,6-triphenylpyridine 3c was obtained in 85% isolated yield (Table 1, entry 3). Then, the effects of the ratio of starting materials 1:2 were examined (Table 1, entries 1–5). The yield of 3 improved to 96% with a 1:2 ratio of 1:8 or 1:10 (Table 1, entries 1–2). This result really encouraged us and extensive exploration of the conditions was further carried out. When the reaction temperature was dropped from 120 °C to 80 °C, 70% and 30% of the desired product 3 were obtained respectively (Table 1, entries 6–7). Subsequent solvent screening suggested that N,N-dimethylformamide (DMF) was the optimal one with 1,4-diphenylbutadiyne 1 (1 mmol) and benzylamine 2 (8 mmol) catalyzed by K2CO3 (0.5 mmol), and the desired product 3 was obtained in 99% isolated yield without any byproducts at 140 °C under air. It is worth noting that the reaction could proceed without a base, also as a catalyst, rendering the desired product in 38% isolated yield (Table 1, entry 11), which demonstrated that the yield of desired product 3 depends on the catalytic activity of the base. To demonstrate the catalytic value of a variety of bases, the synthetic reactions of 2,3,6-triphenylpyridine between 1,4-diphenylbutadiyne 1 (1 mmol) and benzylamine 2 (8 mmol) were carried out in DMF using different bases at 140 °C for 10 h with 0.5 mmol catalyst loading under air (Table 1, entries 12–20). The almost quantitative yield (99%) was obtained by using K2CO3 as the catalyst (Table 1, entry 8). Use of other bases, such as Na2CO3, NaOH, KOH and KHCO3 also gave good yields (Table 1, entries 13–15, 17). Under similar reaction conditions, Cs2CO3, NaF, NaH2PO4, KH2PO4 and CH3COONa afforded only moderate yield (Table 1, entries 12, 16, 18–20). These resultsindicate that K2CO3 is very effective in promoting the synthesis of arylated pyridines from conjugated acetylenes and substituted benzylamines under facile conditions.

Table 1.
Optimization of the reaction conditions a.
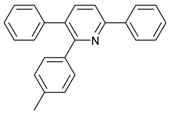
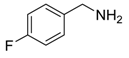
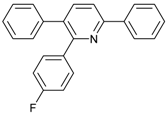
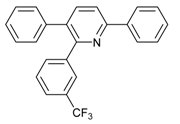
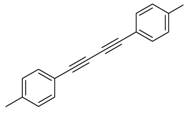
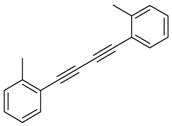
Under the optimized reaction conditions, the scope of this synthetic protocol was evaluated to test the compatibility of varying symmetrical 1,4-diarylbuta-1,3-diynes as starting materials (Table 2). The 1,4-diarylbuta-1,3-diyne bearing two methyl groups at the 1- and 4-position was easily converted to give the desired products with excellent yield (90%) in the synthesis of arylated pyridines using benzylamine (3cbb). However, 1,4-bis(4-butylphenyl)buta-1,3-diyne was slightly less reactive, giving the desired product with 60% yield under the same conditions, and this result clearly demonstrated that steric hindrance has an effect on the yield of desired product (3cfb). The reaction using sterically hindered 1,4-di-o-tolylbuta-1,3-diyne and 1,4-di-m-tolylbuta-1,3-diyne led to 77% and 78% yields, respectively (3ccb, 3cdb).Investigations of substituted benzylamines in the synthesis of arylated pyridines using 1,4-diphenylbutadiyne were also conducted. The reaction with substituted benzylamine having an electron-donating group was carried out efficiently, affording almost quantitative yield (99%) (3cac).Various substituted benzylamines bearing electron-withdrawing groups, such as -F, -Cl, and -CF3, provided the corresponding products in moderate to good yields (3cad, 3cae, 3caf). The steric and electronic effects of the substrate bearing electron-withdrawing substituent in the 3-position of benzylamine remarkably affected the reaction yield: upon using [3-(trifluoromethyl)phenyl]methanamine, product 3,6-diphenyl-2-[3-(trifluoromethyl)phenyl]pyridine was obtained in 50% yield (3caf).

Table 2.
Synthesis of arylated pyridines from conjugated acetylenes and substituted benzylamines under optimized conditions. a
3. Materials and Methods
3.1. General Conditions
All manipulations were performed under air. All reagents employed in the synthesis were analytical grade, purchased from J&K Scientific Ltd. (Shanghai, China) and used as received without any prior purification. The products were isolated by thin layer chromatography on silica gel using petroleum ether as the eluent. 1H-NMR, 13C-NMR spectra were recorded on a Bruker Avance III (400 MHz, Bruker Corporation, Billerica, MA, USA) spectrometer using tetramethylsilane as the internal standard and CDCl3 as the solvent. Chemical shift values are expressed in ppm relative to external TMS (see Supplementary).
3.2. General Procedure for the Preparation of Arylated Pyridines
1,4-Disubstituted-1,3-diacetylene (0.25 mmol) and K2CO3 (0.5 mmol) were added, under air, to a solution of appropriate benzylamine (2.0 mmol) in DMSO (0.5 mL) previously heated at 140 °C. The resulting solution was stirred for 10 h at this temperature and washed with saturated aqNaCl, extracted with ethyl acetate (3 × 15 mL). The combined organic phase was dried with anhydrous Na2SO4, filtrated and concentrated under vacuum to yield the crude product. The crude product was purified by thin layer chromatography on silica gel with petroleum ether as eluent.
3.3. Analytical Data of Representative Products
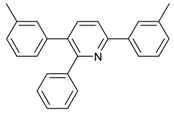
2,3,6-Triphenylpyridine: White crystals (m.p. = 110–111 °C, lit [24] 110.5–112 °C, lit [25] 111–112 °C). 1H-NMR (400 MHz, CDCl3) δ 8.20 (d, 2H), 7.98–7.75 (m, 2H), 7.50 (dq, 5H), 7.30 (ddd, 8H).13C-NMR (101 MHz, CDCl3) δ 156.64, 155.68, 140.43, 140.01, 139.43, 139.10, 134.43, 130.23, 129.59, 129.01, 128.75, 128.37, 127.84, 127.18, 127.02, 118.59. lit [25]: 1H-NMR (400MHz, CDCl3) δ 8.16–8.14 (m, 2H), 7.78–7.77 (m, 2H), 7.51–7.42 (m, 5H), 7.30–7.21 (m, 9H); 13C-NMR (100 MHz, CDCl3) δ 156.6, 155.6, 140.4, 140.0, 139.4, 139.1, 134.4, 130.2, 129.5, 129.0, 128.7, 128.3, 127.8, 127.1, 127.0, 118.5. HRMS (EI) calcd. for C23H17N: 307.1361, found: 307.2.
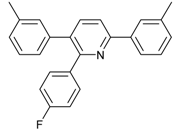
2-(4-Fluorophenyl)-3,6-diphenylpyridine: White solid (m.p. = 115–117 °C, lit [22] 115–116 °C). 1H-NMR (400 MHz, CDCl3) δ 8.19 (d, 2H), 7.82 (d, 2H), 7.52 (dd, 5H), 7.32 (d, 3H), 7.27 (d, 2H), 7.01 (d, 2H). 13C-NMR (101 MHz, CDCl3) δ 162.53 (JC−F = 245.6 Hz), 155.73, 155.53, 139.81, 139.56, 138.95, 134.31 (JC−F = 4.3 Hz), 132.06, 131.97 (JC−F = 8.2 Hz), 129.55, 129.13, 128.82, 128.53, 127.34, 126.99, 118.70, 114.74(JC−F = 21.5 Hz). lit [22]: 1H-NMR (400 MHz, CDCl3): δ 8.13 (d, 2H), 7.77 (s, 2H), 7.51–7.42 (m, 5H), 7.31–7.29 (m, 3H), 7.22–7.20 (m, 2H), 6.94 (t, 2H); 13C-NMR (100 MHz, CDCl3): 162.5 (JC−F = 245.6 Hz), 155.7, 155.5, 139.8, 139.5, 138.9, 136.4 (JC−F = 4.3 Hz), 134.2, 131.9 (JC−F = 8.2 Hz), 129.5, 129.0, 128.7, 128.4, 127.2, 126.9, 118.6, 114.7 (JC−F = 21.5Hz). HRMS (EI) calcd. for C23H16FN: 325.1267, found: 325.2.
4. Conclusions
In summary, an efficient protocol for arylated pyridines from conjugated acetylenes and substituted benzylamines catalyzed by base was developed, which gives a much more convenient approach to obtain arylated pyridines with good to excellent yields. Compared to the approachreported by Chalk [21], the advantages of this protocol are inthe absence ofbyproduct detected by GC-MS even if the reaction was carried out in the air. Efforts to understand this reaction mechanism are in progress in our laboratory.
Supplementary Materials
Supplementary materials are available online.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 21363026), the Scientific and Technological Landing Project of Higher Education of Jiangxi Province (No. KJLD13091).
Author Contributions
M.G. and Q.Z. conceived and designed the experiments; B.C. performed the experiments; M.G., H.J., Q.P. and Y.K. analyzed the data and contributed with different analysis tools; finally, M.G. and B.C. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2005, 22, 627–646. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.W.N.; Druker, B.J. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol. Rev. 2003, 55, 401–423. [Google Scholar] [PubMed]
- Reimann, S.; Ehlers, P.; Parpart, S.; Surkus, A.; Spannenberg, A.; Langer, P. Site-selective synthesis of arylated pyridines by Suzuki-Miyaura reactions of 2,3,5,6-tetrachloropyridine. Tetrahedron 2015, 71, 5371–5384. [Google Scholar]
- O’Hagen, D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep. 2000, 17, 435–446. [Google Scholar]
- Cui, J.-J.; Tran-Dube, M.; Shen, H.; Nambu, M.; Kung, P.P.; Pairish, M.; Jia, L.; Meng, J.; Funk, L.; Botrous, I.; et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and Anaplastic Lymphoma Kinase (ALK). J. Med. Chem. 2011, 54, 6342–6363. [Google Scholar] [CrossRef] [PubMed]
- Nagamitsu, T.; Sunazuka, T.; Obata, R.; Tomoda, H.; Tanaka, H.; Harigaya, Y.; Omura, S. Total synthesis of (+)-pyripyropene A. A potent, orally bioavailable inhibitor of Acyl-CoA: Cholesterol acyltransferase. J. Org. Chem. 1995, 60, 8126–8127. [Google Scholar] [CrossRef]
- Trecourt, F.; Gervais, B.; Mallet, M.; Quéguiner, G. First synthesis of caerulomycin C. J. Org. Chem. 1996, 61, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Sammakia, T.; Stangeland, E.L.; Whitcomb, M.C. Total synthesis of caerulomycin C via the halogen dance reaction. Org. Lett. 2002, 4, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Matolcsy, G. Pesticide Chemistry; Elsevier: Amsterdam, The Netherlands, 1988; p. 427. [Google Scholar]
- Sweetman, B.A.; Muller-Bunz, H.; Guiry, P.J. Synthesis, resolution and racemisation studies of new tridentate ligands for asymmetric catalysis. Tetrahedron Lett. 2005, 46, 4643–4646. [Google Scholar] [CrossRef]
- Durola, F.; Sauvage, J.P.; Wenger, O.S. Sterically non-hindering endocyclic ligands of the bi-isoquinoline family. Chem. Commun. 2006, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Jha, R.R.; Chaudhary, R.; Tiwari, R.K.; Danodia, A.K. 2-(1-Benzotriazolyl)pyridine: A robust bidentate ligand for the palladium-catalyzed CC (Suzuki, Heck, Fujiwara Moritani, Sonogashira), CN and CS coupling reactions. Adv. Synth. Catal. 2013, 355, 421–438. [Google Scholar] [CrossRef]
- Zhou, G.; Wong, W.-Y.; Yang, X. New design tactics in OLEDs using functionalized 2-phenylpyridine-type cyclometalates of iridium (III) and platinum (II). Chemistry 2011, 6, 1706–1719. [Google Scholar]
- Cowley, M.J.; Adams, R.W.; Atkinson, K.D.; Cockett, M.C.R.; Duckett, S.B.; Green, G.G.R.; Lohamn, J.A.B.; Kerssebaum, R.; Kilgour, D.; Mewis, R.E. Iridium N-Heterocyclic carbene complexes as efficient catalysts for magnetization transfer from para-hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.D.; Ruggi, A.; Pascu, M.; Scopelliti, R.; Severin, K. Clathrochelate-based Bipyridyl Ligands of Nanoscale Dimensions: Easy-to-access building blocks for supramolecular chemistry. Chem. Sci. 2013, 4, 1658–1662. [Google Scholar] [CrossRef]
- Wu, D.; Zhi, L.; Bodwell, G.J.; Cui, G.; Tsao, N.; Müllen, K. Self-assembly of positively charged discotic PAHs: From nanofibers to nanotubes. Angew. Chem. Int. Ed. 2007, 46, 5417–5420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Li, X.-P.; Lu, X.-C.; Hsieh, I.-F.; Cao, Y.; Moorefield, C.N.; Wesdemiotis, C.; Cheng, S.Z.D.; Newkome, G.R. Stoichiometric self-assembly of shape-persistent 2D complexes: A facile route to a symmetric supramacromolecular spoked wheel. J. Am. Chem. Soc. 2011, 133, 11450–11453. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Naohiko, Y. Modular pyridine synthesis from oximes and enals through synergistic copper/iminium catalysis. J. Am. Chem. Soc. 2013, 135, 3756–3759. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Matthew, D.-H.; Omar, K.-A. Direct synthesis of pyridine derivatives. J. Am. Chem. Soc. 2007, 129, 10096–10097. [Google Scholar]
- Chalk, A.J. A new pyridine synthesis from conjugated acetylenes and substituted methylamines. Tetrahedron 1974, 30, 1387–1391. [Google Scholar] [CrossRef]
- Sha, F.; Shen, H.; Wu, X.Y. Highly regioselective assembly of Di- or trisubstituted pyridines fromarynes, isocyanides, and 3-bromo- or 3-acetoxypropynes. Eur. J. Org. Chem. 2013, 2013, 2537–2540. [Google Scholar] [CrossRef]
- Chen, B.; Guo, M.-P.; Wen, Y.-J.; Shen, X.-L.; Zhou, X.-L.; Lv, M.-Y. Efficient P, O chelate palladium (II)/AgNO3 cocatalyzed homocoupling of aromatic terminal alkynes in aqueous media under ambient atmosphere. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 259–263. [Google Scholar] [CrossRef]
- Chalk, A.J. A new pyridine synthesis and its redirection to a pyrrole synthesis with cuprous chloride. Tetrahedron Lett. 1972, 33, 3487–3490. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Park, C.M.; Loh, T.P. Transition-metal-free synthesis of substituted pyridines via ring expansion of 2-Allyl-2H-azirines. Organ. Lett. 2014, 16, 3432–3435. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).