Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of New Pyrazoline Derivatives
2.2. MTT Assay
2.3. MTS Assay
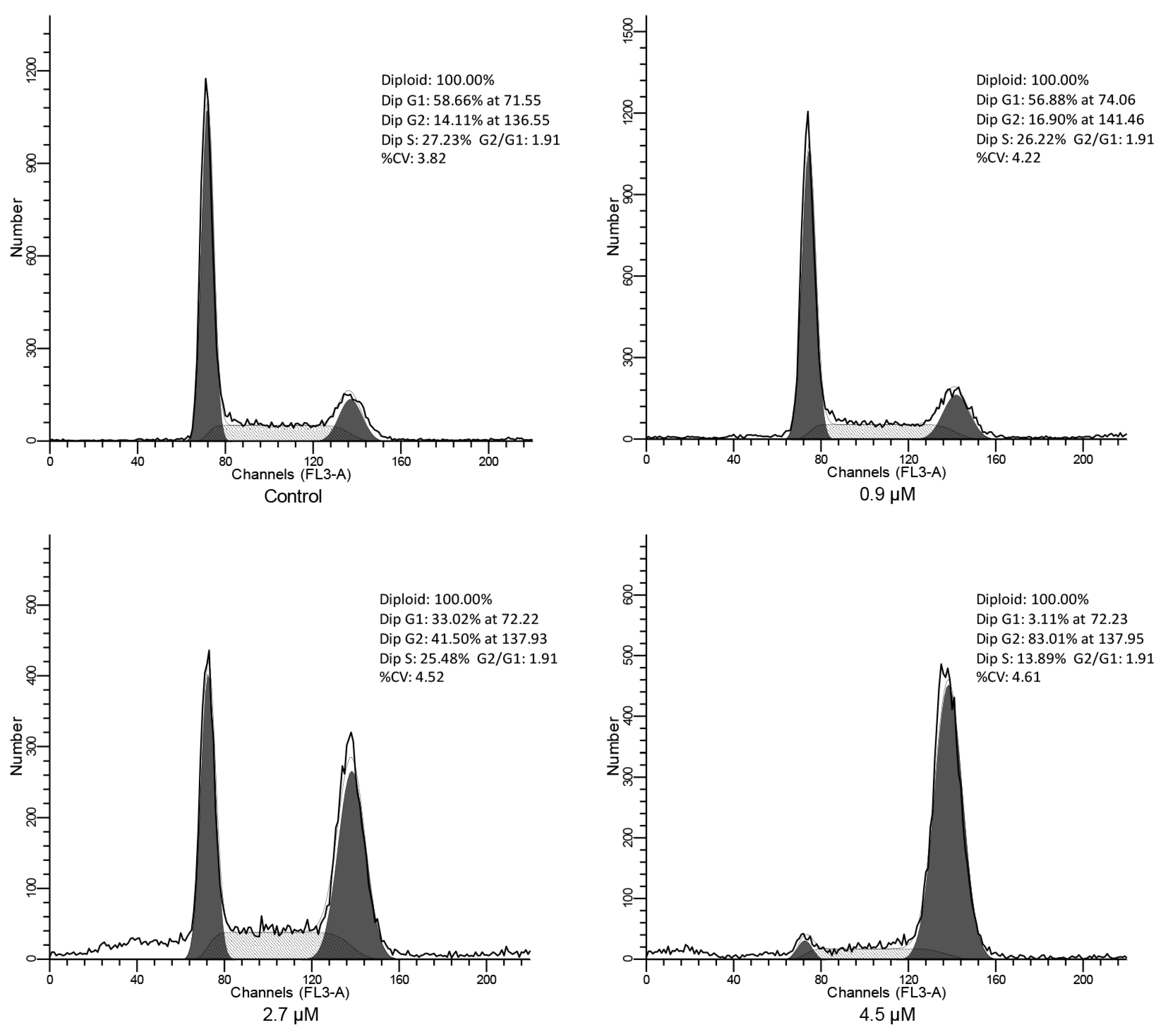
2.4. Cell Cycle Analysis
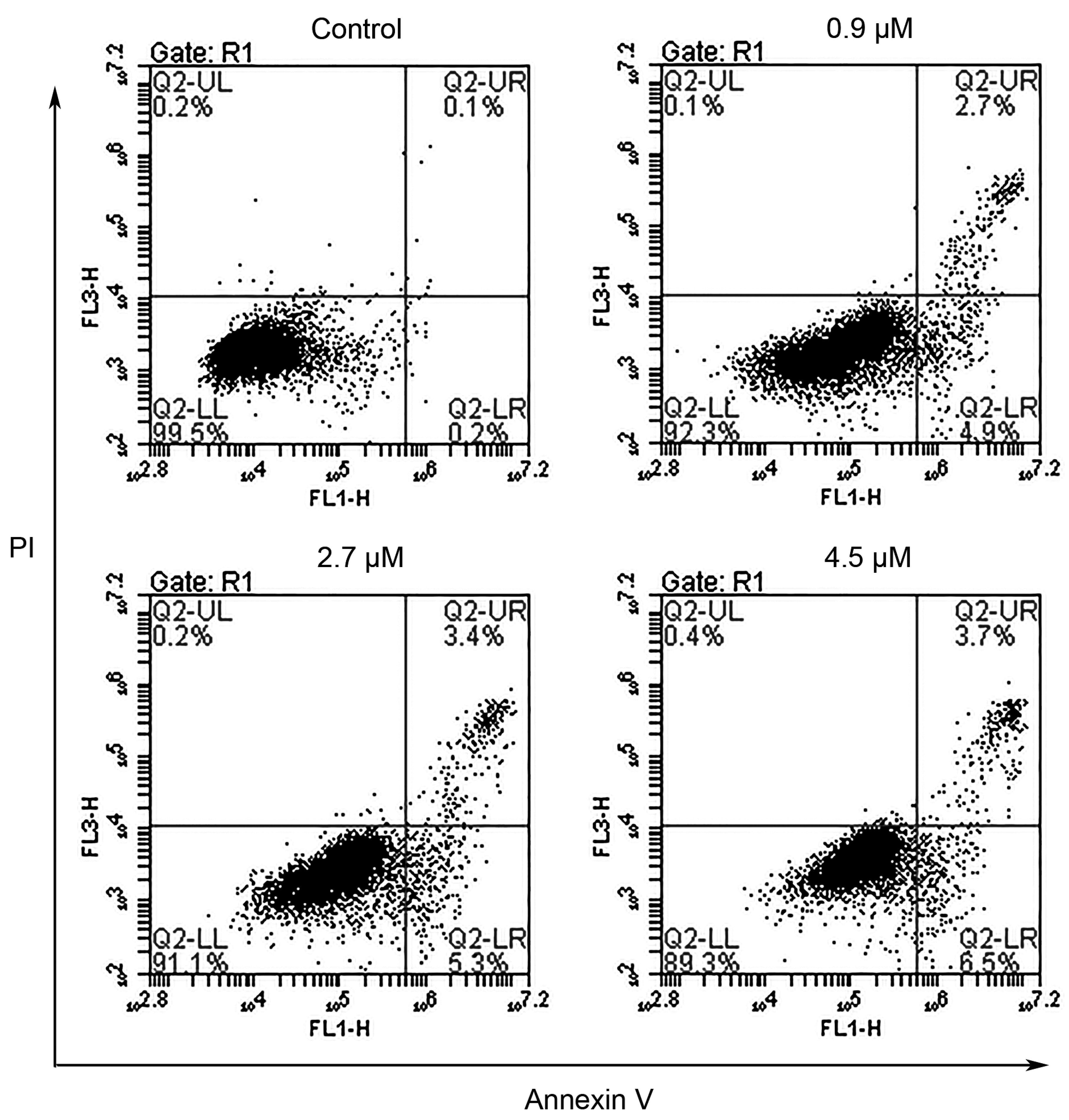
2.5. Annexin-V Assay
3. Experimental Section
3.1. Chemical Reagents and Equipment
3.2. General Procedures for the Synthesis of Compounds a1–5
3.3. General Procedures for the Synthesis of Compounds b1–19
3.4. Pharmacology
3.4.1. Cell Culture and Treatment
3.4.2. MTT Assay
3.4.3. MTS Assay
3.4.4. Cell Cycle Analysis
3.4.5. Annexin-V Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poustchi, H.; Sepanlou, S.; Esmaili, S.; Mehrabi, N.; Ansarymoghadam, A. Hepatocellular carcinoma in the world and the Middle East. Middle East J. Dig. Dis. 2010, 2, 31–41. [Google Scholar] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef] [PubMed]
- Rebucci, M.; Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013, 85, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Molecular and physiologic mechanisms of drug resistance in cancer: An overview. Cancer Metastasis Rev. 2001, 20, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Medina, P.P.; Slack, F.J. microRNAs and cancer: An overview. Cell Cycle 2008, 7, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, M.; Altıntop, M.D.; İbrahim Çiftçi, H.; Koga, R.; Otsuka, M.; Fujita, M.; Özdemir, A. Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents. Molecules 2015, 20, 19066–19084. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J.; Insuasty, B. Synthesis and in vitro antitumor activity of a novel series of 2-pyrazoline derivatives bearing the 4-aryloxy-7-chloroquinoline fragment. Molecules 2014, 19, 18656–18675. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.J.; Li, Y.J.; Jiang, A.Q.; Yang, M.R.; Zhu, Q.Z.; Dong, H.; Zhu, H.L. Design, synthesis and biological evaluation of novel pyrazoline-containing derivatives as potential tubulin assembling inhibitors. Eur. J. Med. Chem. 2015, 94, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Dees, E.C.; Rowinsky, E.K.; Noe, D.A.; O’Reilly, S.; Adjei, A.A.; Elza-Brown, K.; Donehower, R.C. A phase I and pharmacologic study of pyrazoloacridine and cisplatin in patients with advanced cancer. Investig. New Drugs 2003, 21, 75–84. [Google Scholar] [CrossRef]
- Berg, S.L.; Blaney, S.M.; Sullivan, J.; Bernstein, M.; Dubowy, R.; Harris, M.B. Phase II trial of pyrazoloacridine in children with solid tumors: A Pediatric Oncology Group phase II study. J. Pediatr. Hematol. Oncol. 2000, 22, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, B.; Mrozek, E.; Kuebler, J.P.; Bekaii-Saab, T.; Kraut, E.H. Phase II trial of pyrazoloacridine (NSC#366140) in patients with metastatic breast cancer. Investig. New Drugs 2011, 29, 347–351. [Google Scholar]
- Jin, X.; Mo, Q.; Zhang, Y.; Gao, Y.; Wu, Y.; Li, J.; Hao, X.; Ma, D.; Gao, Q.; Chen, P. The p38 MAPK inhibitor BIRB796 enhances the antitumor effects of VX680 in cervical cancer. Cancer Biol. Ther. 2016, 17, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Kuma, Y.; Sabio, G.; Bain, J.; Shpiro, N.; Márquez, R.; Cuenda, A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J. Biol. Chem. 2005, 280, 19472–19479. [Google Scholar] [CrossRef] [PubMed]
- George, D.J. Phase 2 studies of sunitinib and AG013736 in patients with cytokine-refractory renal cell carcinoma. Clin. Cancer Res. 2007, 13, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.; Wilding, G.; Hudes, G.; Stadler, W.M.; Kim, S.; Tarazi, J.; Bycott, P.; Liau, K.; Dutcher, J. Axitinib (AG-013736; AG) in patients (pts) with metastatic clear cell renal cell cancer (RCC) refractory to sorafenib. EJC Suppl. 2007, 5, 300–304. [Google Scholar] [CrossRef]
- Kumar, R.; Knick, V.B.; Rudolph, S.K.; Johnson, J.H.; Crosby, R.M.; Crouthamel, M.C.; Hopper, T.M.; Miller, C.G.; Harrington, L.E.; Onori, J.A.; et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol. Cancer Ther. 2007, 6, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Mammi, M.; di Paola, E.D.; Russo, E.; Gallelli, L.; Citraro, R.; Gadaleta, C.D.; Marech, I.; Ammendola, M.; de Sarro, G. Pazopanib a tyrosine kinase inhibitor with strong anti-angiogenetic activity: A new treatment for metastatic soft tissue sarcoma. Crit. Rev. Oncol. Hematol. 2014, 89, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Rubin, B.; Bertazza, L.; Redaelli, M.; Barollo, S.; Monticelli, H.; Baldini, E.; Mian, C.; Mucignat, C.; Scaroni, C.; et al. The aurora kinase inhibitor VX-680 shows anti-cancer effects in primary metastatic cells and the SW13 cell line. Investig. New Drugs 2016, 34, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J.; Swords, R.T.; Nagler, A.; Hochhaus, A.; Ottmann, O.G.; Rizzieri, D.A.; Talpaz, M.; Clark, J.; Watson, P.; Xiao, A.; et al. MK-0457, an Aurora kinase and BCR-ABL inhibitor, is active in patients with BCR-ABL T315I leukemia. Leukemia 2013, 27, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Na, J.I.; Na, J.Y.; Choi, W.Y.; Lee, M.C.; Park, M.S.; Choi, K.H.; Lee, J.K.; Kim, K.T.; Park, J.T.; Kim, H.S. The HIF-1 inhibitor YC-1 decreases reactive astrocyte formation in a rodent ischemia model. Am. J. Transl. Res. 2015, 7, 751–760. [Google Scholar] [PubMed]
- Chang, L.C.; Lin, H.Y.; Tsai, M.T.; Chou, R.H.; Lee, F.Y.; Teng, C.M.; Hsieh, M.T.; Hung, H.Y.; Huang, L.J.; Yu, Y.L.; et al. YC-1 inhibits proliferation of breast cancer cells by down-regulating EZH2 expression via activation of c-Cbl and ERK. Br. J. Pharmacol. 2014, 171, 4010–4025. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C.T. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Ovais, S.; Yaseen, S.; Hamid, H.; Alam, M.S.; Samim, M.; Singh, S.; Javed, K. Synthesis of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2011, 21, 4301–43055. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, L.T. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr. Cancer Drug Targets 2002, 2, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Novel 1,4-naphthoquinone-based sulfonamides: Synthesis, QSAR, anticancer and antimalarial studies. Eur. J. Med. Chem. 2015, 20, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Ying, P.; Chen, Y.; Yu, X.; Wang, J.; Zhang, L.; He, Y.; Zheng, J. The synthesis of a novel chalcone and evaluation for anti-free radical activity and antagonizing the learning impairments in Alzheimer’s model. Cell Physiol. Biochem. 2012, 29, 949–958. [Google Scholar]
- De Vasconcelos, A.; Campos, V.F.; Nedel, F.; Seixas, F.K.; Dellagostin, O.A.; Smith, K.R.; de Pereira, C.M.; Stefanello, F.M.; Collares, T.; Barschak, A.G. Cytotoxic and apoptotic effects of chalcone derivatives of 2-acetyl thiophene on human colon adenocarcinoma cells. Cell Biochem. Funct. 2013, 31, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Roman, O.; Lesyk, R. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline-thiazolidine-based hybrids. Eur. J. Med. Chem. 2016, 113, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of compounds b1–19 are available from the authors.
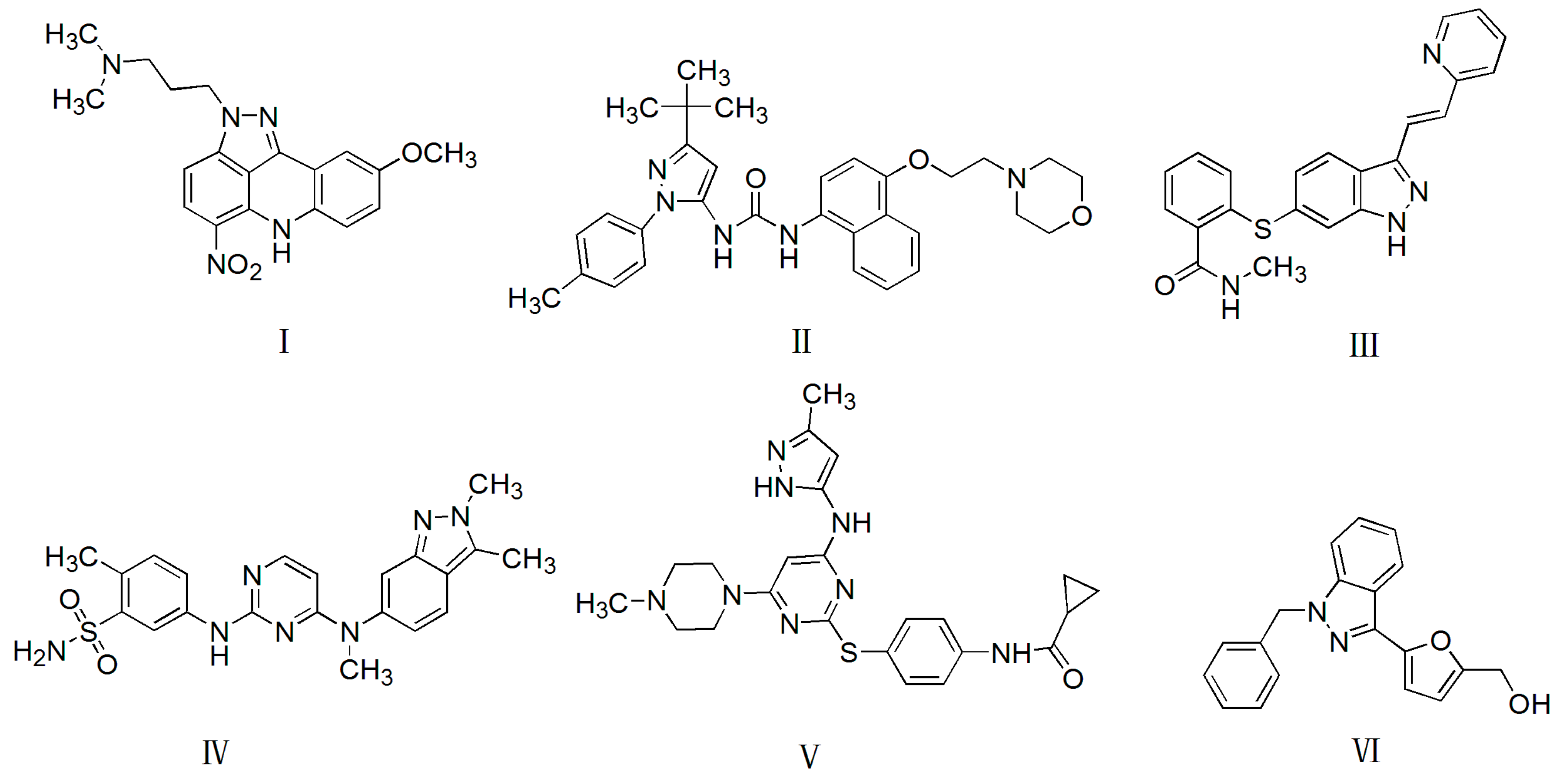


| Compounds | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| a1 | –OCH3 | –OH | –OCH3 | –OH | H |
| a2 | –OCH3 | –OH | –OCH3 | H | –NO2 |
| a3 | –Br | –OH | –OCH3 | –OH | H |
| a4 | –Br | –OH | –OCH3 | H | –NO2 |
| a5 | –OCH3 | –OCH3 | –OCH3 | H | –CH3 |
| Compounds | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| b1 | –OCH3 | –OH | –OCH3 | –OH | H | H |
| b2 | –OCH3 | –OH | –OCH3 | H | –NO2 | H |
| b3 | –Br | –OH | –OCH3 | –OH | H | H |
| b4 | –Br | –OH | –OCH3 | H | –NO2 | H |
| b5 | –OCH3 | –OH | –OCH3 | –OH | H | 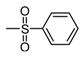 |
| b6 | –OCH3 | –OH | –OCH3 | –OH | H | 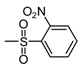 |
| b7 | –OCH3 | –OH | –OCH3 | –OH | H |  |
| b8 | –OCH3 | –OH | –OCH3 | –OH | H | 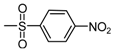 |
| b9 | –OCH3 | –OH | –OCH3 | –OH | H | 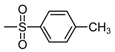 |
| b10 | –OCH3 | –OH | –OCH3 | –OH | H | 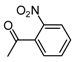 |
| b11 | –OCH3 | –OH | –OCH3 | –OH | H | 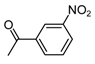 |
| b12 | –OCH3 | –OH | –OCH3 | –OH | H | 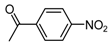 |
| b13 | –OCH3 | –OH | –OCH3 | –OH | H | 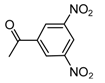 |
| b14 | –OCH3 | –OH | –OCH3 | –OH | H | 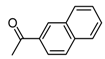 |
| b15 | –OCH3 | –OH | –OCH3 | –OH | H | 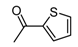 |
| b16 | –OCH3 | –OH | –OCH3 | –OH | H |  |
| b17 | –OCH3 | –OH | –OCH3 | –OH | H |  |
| b18 | –OCH3 | –OH | –OCH3 | –OH | H | 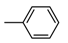 |
| b19 | –OCH3 | –OCH3 | –OCH3 | –H | –CH3 | 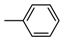 |
| Compounds | IC50/μmol·L−1 | Compounds | IC50/μmol·L−1 | ||
|---|---|---|---|---|---|
| HepG-2 | Primary Hepatocytes | HepG-2 | Primary Hepatocytes | ||
| b1 | >50 | — | b12 | >50 | — |
| b2 | >50 | — | b13 | >50 | — |
| b3 | >50 | — | b14 | 17.99 ± 1.37 | 22.65 ± 1.21 |
| b4 | >50 | — | b15 | 4.51 ± 1.49 | 19.24 ± 0.08 |
| b5 | 28.76 ± 1.32 | 35.13 ± 2.21 | b16 | 4.61 ± 1.27 | 20.73 ± 1.72 |
| b6 | >50 | — | b17 | 3.57 ± 1.39 | 33.47 ± 2.33 |
| b7 | >50 | — | b18 | 28.47 ± 1.34 | 50.71 ± 3.21 |
| b8 | >50 | — | b19 | >50 | — |
| b9 | 12.01 ± 1.83 | 29.66 ± 2.43 | Cisplatin | 8.45 ± 1.05 | — |
| b10 | >50 | — | |||
| b11 | >50 | — | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Pan, Y.; Wang, H.; Li, H.; Peng, Q.; Wei, D.; Chen, C.; Zheng, J. Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line. Molecules 2017, 22, 467. https://doi.org/10.3390/molecules22030467
Xu W, Pan Y, Wang H, Li H, Peng Q, Wei D, Chen C, Zheng J. Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line. Molecules. 2017; 22(3):467. https://doi.org/10.3390/molecules22030467
Chicago/Turabian StyleXu, Weijie, Ying Pan, Hong Wang, Haiyan Li, Qing Peng, Duncan Wei, Cheng Chen, and Jinhong Zheng. 2017. "Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line" Molecules 22, no. 3: 467. https://doi.org/10.3390/molecules22030467
APA StyleXu, W., Pan, Y., Wang, H., Li, H., Peng, Q., Wei, D., Chen, C., & Zheng, J. (2017). Synthesis and Evaluation of New Pyrazoline Derivatives as Potential Anticancer Agents in HepG-2 Cell Line. Molecules, 22(3), 467. https://doi.org/10.3390/molecules22030467






