Abstract
This paper presents the possibility of producing phosphorus fertilizers through Acidithiobacillus ferrooxidans utilization in secondary raw materials solubilization. Phosphorus was obtained from the bones of poultry and fish as well as from Morocco phosphorite. Four doses of poultry bones and fish bones were used in the experiment (2, 4, 10 and 20 g/L) and two doses (2 and 4 g/L) of phosphorite were also used. The experimenters measured the final pH, which increased in proportion to the increase in the number of poultry bone doses, whereas in the case of fish bones it decreased in proportion to the increase in the number of fish bone doses. Only in the case of phosphorite, where 10 g/L were used, there was a slight increase in pH during solubilization observed. The highest phosphorus concentration of 1.9% (expressed as P2O5) was found for the solubilization performed on fish bones with the highest dose (20 g/L). The formulation obtained in this study meets the necessary requirements for use as a bio-fertilizer because of the relatively low content of P2O5 and the low content of toxic elements. The results confirm the utilization of Acidithiobacillus ferrooxidans in the biosolubilization of phosphorus renewable raw materials that can alleviate the problem of the world’s depleting phosphorite deposits.
1. Introduction
The manufacturing costs of mineral fertilizers largely depend on the price of raw materials, which could be reduced if renewable by-products could be used [1]. Limited resources of natural phosphates and the unstable geopolitical situations in the regions where the largest deposits of phosphorites are located have made the recovery of phosphorus from wastes rich in fertilizers of utmost importance in recent years [2].
A wider application of biotechnologies to activate phosphate raw materials may help reduce the adverse phenomena attendant on the manufacturing of conventional fertilizers [3]. These processes can involve bacteria and fungi that solubilize phosphate (Phosphate Solubilizing Bacteria—PSB, Phosphate Solubilizing Fungi—PSF) [4].
PSB and PSF are integral parts of the phosphorus cycle in soil [5]. PSB are chiefly responsible for phosphorus release from organic and inorganic pools by solubilization and mineralization and are more efficient than PSF [6]. Pseudomonas, Bacillus, Rhizobium, Burkholderia, Achromobacter, Agrobacterium, Microccocus, Aerobacter, Flavobacterium and Erwinia are among the bacteria strains that change such forms of inorganic phosphorus as tricalcium phosphate, dicalcium phosphate, hydroxyl apatite and rock phosphate into forms available to plants [4]. PSB activity is driven by mechanisms producing organic (such as citric, gluconic, lactic, and propionic) as well as inorganic (such as sulfuric) acids [7]. Thiobacillus ferrooxidans and Acidithiobacillus thiooxidans raise the greatest expectations because they produce sulfuric acid [8,9].
In order to make phosphorus from soil available for plants, the organic compounds must be mineralized, which in most cases requires enzymes such as phosphatase (phosphohydrolases), phytase, phosphonoacetate hydrolase, and d-α-glycerophosphatase [4].
The use of microorganisms for phosphorus solubilization was first patented in 1910 [10]. It proposed the utilization of bacteria from disintegrated, weathered rock for activating phosphorus from phosphate. Fertilizers containing PSB can be manufactured in liquid and solid form. The former contains phosphorus compounds activated by bacteria at the manufacturing stage. In this type of fertilizer, phosphorus can be obtained from different, mostly poor raw materials, such as wastes from the agro-food industry, sludge, etc. [3,11]. In the case of the latter, the solubilization of phosphorus compounds occurs after the application of the fertilizer to the soil environment. Also, phosphate rock is used as an addition to poor products [1,12,13,14]. Such phosphorus biofertilizers are enriched with sulfur, which is transformed into sulfuric acid by Thiobacillus.
Microorganisms may be considered a source of solubilizing agents for insoluble phosphates present in the form of hydroxyapatite (poultry bones and fish bones as well as phosphorite). Acidithiobacillus ferrooxidans (A. ferrooxidans) is one of the most common mesophilic and acidophilic ferrous iron and sulphur oxidizing bacterial strains [15], and one of the most important autotrophic bacterium involved in bioleaching of low-grade sulfide minerals. It has been used to dissolve non-metallic elements, for example, to recover arsenic from medicinal realgar [16,17,18,19], but also to solubilize phosphorus from rock phosphates. A. ferrooxidans is of special interest as a phosphate-solubilizing microorganism because of its natural ability to produce sulfuric acid, which can be used in the solubilization of hydroxyapatite [20]. Pyrites (FeS2) can be oxidized by A. ferrooxidans, producing H2SO4 and FeSO4. Rock phosphate is dissolved by H2SO4, forming soluble phosphorus. Fe2+ in FeSO4 is oxidized to Fe3+, producing energy to sustain the growth of A. ferrooxidans [21]. Yet its ability to oxidize ferrous ion and/or sulfur and at the same time to release phosphates has been found to be repressed to different degrees by the addition of naturally-occurring organic compounds in growth media [22].
Although rock phosphate can be successfully solubilized by these bacteria [23], the low release of soluble phosphate is a major challenge, since rock phosphate is poor in organic matter that enhances bacteria growth and at the same time induces a higher efficiency of solubilization. As far as we know, the release of soluble phosphate from phosphorus-bearing materials such as poultry bones and fish bones by A. ferrooxidans has never been studied, which is precisely why we cultivated A. ferrooxidans on the medium containing renewable materials in order to find out how to make its use in agriculture more effective and economical. To this purpose, we used poultry bones and fish bones as a raw material, which are a source of phosphorus and organic compounds that could increase the effectiveness of A. ferrooxidans-induced solubilization. Our objective was to investigate the possibility of phosphorus solubilization from poultry bones and fish bones, as well as phosphate rock apatite, using sulfuric acid generated by A. ferrooxidans.
2. Results and Discussion
Autotrophic acidophilic bacteria have a key role in the production of sulfuric acid, which critically affects the solubilization of phosphates. Sulfuric acid creates an acidic environment, and thus benefits the solubilization of the hydroxyapatite structure present in the poultry bones, fish bones and phosphate rock (phosphorite). Equation (1) presents the solubilization of hydroxyapatite:
whereas Equation (2) presents the solubilization of fluoroapatite present in phosphorite:
Ca5(OH)(PO4)3 + 5H2SO4 + 10H2O → 5CaSO4·2H2O + 3H3PO4 + H2O
Ca5F(PO4)3 + 5H2SO4 + 10H2O → 5CaSO4·2H2O + 3H3PO4 + HF
2.1. Changes in pH
The pH drops significantly during solubilization in most studied microorganisms, since most of them solubilize by the production of organic acids [24]. For Bacillus megaterium, the initial pH is close to neutral, so even though the pH drops to ca. 3.5, the ΔpH is much higher than that recorded for the solubilization performed by A. ferrooxidans, despite the fact that the final pH is much lower (since the starting point is lower).
An increase in pH was observed only in the case of phosphorite (10 g/L), as a result of the consumption of acid by the proton attack on rock phosphate, which was also reported by Xiao et al. [18]. Table 1 shows the comparison of initial and final as well as ΔpH, recorded for different doses of raw materials. The highest change in pH in the cultures was observed for the highest concentration of fish bones 20 g/L ΔpH = 0.861, while the lower changes in the pH were found in the cultures with higher doses of poultry bones (20 g/L). The value of the final pH increases as the poultry bone doses increase, while in the case of fish bones the value of the final pH decreases as the fish bone doses increase. The addition of fish bones could improve the pH reducing ability of autotrophic acidophilic bacterium A. ferrooxidans. This relationship between higher doses of fish bones and higher changes of pH may be explained by the fact that the metabolites of bacterial cells could interact and thus release various compounds from the fish bones, which could enhance the production of bacterial acid [25,26]. The relationship between the dose of poultry bones and the effect of the corresponding pH is consistent with observations presented previously [27]. Another explanation for the different pH effect for poultry bones and fish bones might be the chemical activity and stability that, in the case of fish bones, is lower because it represents the “younger” form of hydroxyapatite, when compared with poultry bones.

Table 1.
The comparison of initial and final as well as ΔpH recorded for different doses of raw materials.
The utilization of hydroxyapatite of biological origin instead of phosphate rock had a significant influence on bacterial performance. The pH of the broth inoculated with the culture of A. ferrooxidans and with the addition of a renewable source of phosphorus was lower than that measured for solubilization with the use of rock phosphate. The addition of renewable resources can, on top of delivering phosphorus, deliver other compounds that potentially enhance the growth of bacterial cells and at the same time improve the pH-reducing ability of A. ferrooxidans by the production of higher amounts of acid or the synthesis of other organic molecules, which can affect the phosphates solubilization in other ways due to acid attacks on the fluorapatite structure [28].
2.2. Concentration of Phosphorus (Expressed as P2O5)
The changes in phosphorus concentrations during the solubilization experiments (expressed as P2O5) are described by the model presented below (Equation (3)).
where the , mg/L is the maximum concentration of phosphorus (expressed as P2O5) and K, 1/day is the constant that reflects the variable slope, which is called the Hill slope. When K is greater, the curve changes more sharply, and it means that the solubilization is performed faster.
There was no influence of the doses of applied raw materials on the K (1/day). Only in the case of phosphorite higher doses of substrate raised the value of K, but in the case of the other parameters of the proposed model, there was a close relation between the maximum concentrations of phosphorus (expressed as P2O5) and the doses. The evaluated maximum concentration of P2O5 from the proposed model ranged between 454 and 1980 mg/L, with variations among different sources of phosphate and their doses (Table 2). The highest maximum concentration of 1.98% phosphorus (expressed as P2O5) was found for the solubilization performed on the highest dose of fish bones (20 g/L). The solubilization of apatite from poultry bones also resulted in high levels of 1.6% phosphorus (expressed as P2O5) obtained for the 10 g/L. An increase in the dose of poultry bones above 10 g/L results in lowering the (Figure 1 and Table 2). This is probably because too high doses of poultry bones, or to be more precise, some organic or inorganic compounds originating from poultry bones, act as an inhibitor of solubilization [19].

Table 2.
Parameters of the model describing P2O5 concentration changes during solubilization (left side), and those describing the Solubilization Factor change during solubilization (right side). SE—standard error; RM—raw material; p—p-value.
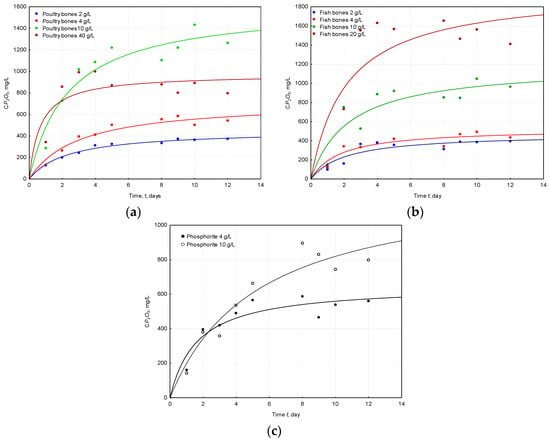
Figure 1.
Changes of phosphorus (expressed as P2O5) concentrations during the solubilization performed by A. ferrooxidans with the utilization of three different phosphorus raw materials (a) poultry bones; (b) fish bones; and (c) phosphorite.
The differences in the obtained concentrations of phosphorus (expressed as P2O5) between the same doses for different raw materials were found to be statistically significant: for a dose of 4 g/L the differences were: fish bones vs. phosphorite (p = 0.0288, N = 9); for 10 g/L: fish bones vs. phosphorite (p = 0.00828), poultry bones vs. phosphorite (p = 0.000246, N = 9), poultry bones vs. fish bones (p = 0.0865, N = 9); in the case of 20 g/L: poultry bones vs. fish bones (p = 0.0209). The observed differences might be a result of the components that were delivered with phosphorus by the different raw materials used in the experiment. In the case of bones (poultry and fish), many organic compounds such as amino acids, fatty acids, and lipids as well as minerals are delivered [29]. Lower concentrations of phosphorus (expressed as P2O5) were obtained for Morocco phosphorite when compared with the results obtained for bones. This can be explained by the origin of the phosphate rock. The immediate source of sedimentary phosphate is considered to be released from organic matter, which is degraded by microbially-mediated redox reactions [30]. Phosphate rock may occur with small amounts of organic matter that did not undergo phosphogenesis. These findings are consistent with the results described in the literature [24], where the growth rate of B. megaterium was monitored with the utilization of four different components of growth medium: poultry bones, fish bones, Morocco phosphorite and ash originating from the incineration of sludge from a waste water treatment plant. It was reported that phosphorite, when compared with ashes that are deprived of organic matter during incineration, served as a better source of nutrients since the plant growth rate was higher with the growth medium of phosphorite when compared with ashes which deliver only nutrients in an inorganic form. This, in turn, might have an effect on the affectivity of the solubilization of A. ferrooxidans. Additionally, some elements that may be released during organism growth in different compositions of the medium could affect the composition of the produced metabolites and, consequently, the microbial solubilization [29,31,32].
Another issue that may be the reason for the differences between the amounts of released phosphorus from bones and phosphorite by solubilizing bacteria is the fact that the chemical activity of the materials that have been used in the experiments varies significantly—a shorter formation time and a lower mineralization degree—bones—results in a higher solubilization efficiency; longer formation time and a higher mineralization degree—phosphorite—results in a lower solubilization efficiency [25].
2.3. Solubilization of Apatite
The solubilization effect or the Solubilization Factor (SF, %) is defined as the ratio (in percentage terms) of soluble P2O5 present in the solution and phosphorus (expressed as P2O5) introduced to the solubilization medium in solid form.
Attempts to describe the changes of the Solubilization Factor in time were undertaken with the use of the model (Equation (4)):
where SFmax, % is the maximum solubilization factor, L is the time at which the SF is equal to ½ of SFmax and k, 1/day constant is the variable slope which is called the Hill slope. When k is greater, the curve changes more sharply, and it means that solubilization occurs faster. Figure 2 shows the changes of the phosphates solubilization factor in the process performed with the utilization of phosphorus resources. Table 2 collects all parameters of the proposed model together with the p-value and errors that express the fit of the model to the experimental data.
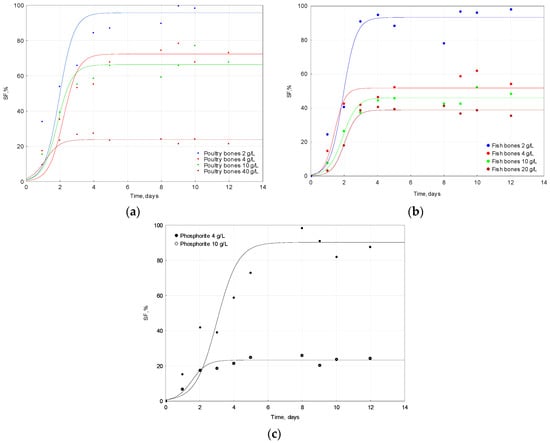
Figure 2.
Solubilization Factor changes of phosphates in the process performed by A. ferrooxidans with the utilization of three different sources of phosphorus: (a) poultry bones; (b) fish bones; and (c) phosphorite.
The evaluated parameter SFmax for bones was found to be statistically significant and correlated with the raw materials doses applied in the experiment, when the relationship was described as a linear regression: f(dose) = SF: y = 96.8 − 3.58x, R2 = 0.937; p = 0.032. Another empirical model was used to describe the experimental points in the case of fish bones solubilization, since the linear regression did not describe the experimental data well. The following model was used: SF % = (A + (B* Dose of raw material))/Dose of raw material [27]. It yielded the following parameters: A = 118 mg/L (p = 0.0292), a constant describing the reaching plateau, and B = 30.9% (p = 0.0346) that can be interpreted as the minimal value of SF %. The proposed model gave a better fit (R2 = 0.975, χ2 = 1.79) to the experimental points than the linear regression (R2 = 0.544; p = 0.262).
Parameter k, which reflects the solubilization rate, has been found to increase when the dose of the substrate increases. For poultry bones, this relation was statistically significant: f(dose) = k: y = 0.129 − 0.0749x, R2 = 0.917; p = 0.042 (Table 2). For fish bones, the linear regression did not result in a high correlation coefficient (R2 = 0.675; p = 0.178).
The doses of phosphorus substrates did not strongly affect parameter L in all discussed cases, but small differences occurred between the average of parameter L and used materials; 1.78 ± 0.468; 1.83 ± 0.272 and 2.24 ± 1.02 for poultry bones, fish bones and phosphate rock, respectively.
As it was discussed above, two empirical models were used to describe the changes in SF and phosphorus concentrations (expressed as P2O5) over time. Table 3 summarizes the relationship between the parameters of both models. It was found that K, 1/day (()) and k, 1/day (f(t) = SF) were statistically correlated (r = 0.794, p < 0.05), and since both describe the performance of solubilization, this correlation is consistent with the model assumptions. A strong correlation was also discovered between K 1/day (()) and L, day (f(t) = SF) (r = 0.7978, p < 0.05).

Table 3.
The table of correlation factors between the parameters of considered kinetic describing models (* p < 0.1; ** p < 0.05), N = 10.
The high dose of the substrate had a negative influence on the biosolubilization of phosphates. This phenomenon was observed in the case of poultry bones and fish bones, as well as phosphorite. These findings are consistent with the results reported in the literature [24,25,27]. The highest solubilization factor was reached for the lowest doses of substrates: 95.7% for 2 g/L of poultry bones, 93.0% for 2 g/L of fish bones and 90.8% for 4 g/L of phosphorite. Similar results were reported in the literature [8].
The solubilization factor has previously been reported to be much higher when compared with the solubilization performed by B. megaterium [25,27], probably because acids produced by B. megaterium are much weaker than the sulfuric acid produced by A ferrooxidans. The drop in pH is lower for solubilization performed by A. ferrooxidans than that by B. megaterium. When the dose of the substrate used in the experiments was doubled, the SF decreased by 24%, 45% and 74% for poultry bones, fish bones and phosphorite, respectively.
The fraction of phosphorus solubilized from rock phosphate by biosolubilization performed by A. ferrooxidans was reported by Chi et al. to be up to 11.8% [33]. The reason for such significantly higher solubilization effectiveness in the case of phosphorite (up to 91%) presented in this paper, might be the growth medium composition, where FeSO4 was used instead of pyrite FeS2, as reported by Chi et al. [33]. Table 3 presents a matrix correlation of the concentrations of elements that occur in the microbial broth after the solubilization in all discussed cases. A statistically significant correlation was found between P and Ca (r = 0.209, p = 0.048): it is two times lower than the molar ratio in the hydroxyapatite (0.465). Many statistically significant correlations were observed between sulfur and others elements, such as Fe (r = 0.818), K (r = −0.587), Mg (r = −0.587), Mn (r = −0.566), Na (r = −0.0531) and Ni (r = 0.516), which is consistent with the solubilization mechanism performed by A. ferrooxidans, since sulfuric acid is produced and affects the release of many compounds present in the raw materials used as a phosphorus source.
The phosphate rock, as well as other materials used in this study, meet the necessary requirements for biofertilizers, primarily because of the relatively low content of P2O5 (0.08% of P2O5 in the case of the fertilizer obtained from poultry bones—20 g/L; 0.14% of P2O5 in the case of the fertilizer obtained from fish bones—20 g/L), but also because of the low content of toxic elements (Cd, Cu, Cr, Ni, Pb, Zn). Biogenic apatite (bone) has the lowest total concentration of all elements. The low concentration of impurities in biogenic apatite is the result of a short accumulation time (bone formation takes a few weeks) [34]. Another advantage of the application of bones is the lack of fluorine, which plays a major role in sedimentary apatite formation and contributes to its preservation in sediments [35,36]. Multi-elemental analysis (ICP-OES) (Table 4) of the content of toxic elements and heavy metals in the formulations based on renewable raw materials was lower as compared to their presence in the formulation obtained with the utilization of phosphorite, with one exception of Ni that, in the case of fish bones, was 50% higher when compared with phosphorite. All findings are below the acceptable levels of toxic elements, which is essential for potential biofertilizers. Literature data demonstrated that fish bones show a higher quality than poultry bones in terms of chemical impurities (i.e., magnesium and alkali metals), which is an added advantage of fish bones utilization over poultry bones utilization.

Table 4.
The content of toxic elements present in the obtained formations (mg/kg) (N = 10), mean ± SD, and acceptable limits of their content in the fertilizers pursuant to the Regulation of the Minister of Agriculture and Rural Development of Poland on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization (Journal of Laws of 2004 No. 236, item 2369). a–j indicate statistically significant differences at p < 0.05.
3. Materials and Methods
3.1. Microorganisms
Solubilizing bacteria A. ferrooxidans used in the experiments were obtained from Professor Zygmunt Sadowski from Wroclaw University of Technology. A. ferrooxidans is an autochthonous strain of bacteria (F7-01) isolated from the tailings impoundment “Iron Bridge,” Poland.
The strain was cultivated in 9 K medium of Silverman and Lundgren [37] composed of 3 g (NH4)2SO4, 0.5 g MgSO4·7H2O, 0.5 g K2HPO4, 0.1 g KCl, 0.01 g Ca(NO3)2 and 44.2 g FeSO4·7 H2O (per liter of distilled water), containing elemental sulfur (0.8 g/L). The pH of the medium was adjusted to 2.5 with H2SO4 [32] prepared with technical grade reagents (from POCh S.A., Gliwice, Poland). The inoculation was undertaken by transferring 10% (v/v) of 72 h-old well-grown culture of A. ferrooxidans (cell density—1.0 × 108 CFU·mL−1) to fresh medium (Figure 3). During the experiment’s first seven days, pre-cultures were conducted at 30 °C without the addition of phosphorus-bearing substrates to enable the bacteria to utilize acids present in the solution and ensure sufficient time for bacterial cells to produce their own sulfuric acid. The color of the suspension changed from green to purple since, at the growth stage, bacteria oxidize iron ions (from Fe2+ to Fe3+). After the initial cultivation of bacteria, the given amounts (mentioned below in Section 3.2) of phosphorus-bearing materials were added to Erlenmeyer’s flasks where the initial phase was undertaken and the main solubilization experiment was conducted.
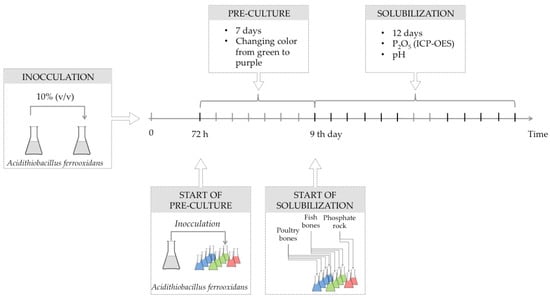
Figure 3.
The scheme sampling timing of the experiment.
3.2. Solubilization Experiments
Solubilization experiments were conducted for three different types of phosphorus raw material: crushed boiled poultry bones (18.6% P2O5) [25], crushed fish bone (20.0% P2O5), and ground Morocco phosphorite (22.7% P2O5) [25]. All samples were pulverized and sieved to pass through 1 mm particle size fractions for chemical and solubilization studies.
Four doses of poultry bones and fish bones were used in the experiment (2, 4, 10 and 20 g/L), and two doses (2 and 4 g/L) were used in the case of phosphorite. Ten Erlenmeyer’s flasks, containing 250 mL of medium for A. ferrooxidans (four with medium containing poultry bones and four with medium containing fish bones as well as two with phosphorite as a phosphorus source) were used in the experiment. All experimental samples were carried out in triplicate. All presented values are an arithmetic mean.
The solutions were sterilized and then inoculated with A. ferrooxidans. During 12 days of cultivation/solubilization (Figure 3), culture media were shaken and incubated at 34 °C (Thermoshake Gerhardt, Bonn, Germany). The process was performed in the batch culture mode. Samples of microorganism suspension from both culture groups were collected at the same time. The permeates were used for the evaluation of pH and P2O5 concentrations that were measured by the ICP-OES method [38]. The pH measurements were conducted with the Mettler-Toledo (Seven Multi, Greifensee, Switzerland) pH-meter equipped with an electrode InLab413 compensating for temperature.
3.3. Analytical Methods
One half milliliter of permeates were digested with 2.5 mL concentrated—65% m/m HNO3 suprapur grade from Merck (Darmstadt, Germany) in Teflon vessels (microwave oven Milestone MLS-1200). After mineralization, all samples were diluted to 50 mL. The concentrations of elements in all digested and diluted samples were determined by means of the Inductively Coupled Plasma-Optical Emission Spectrometer (Varian VISTA-MPX ICP-OES, Victoria, Australia) fitted with an ultrasonic nebulizer (U5000AT+, CETAC, Omaha, UNO, USA) in the Chemical Laboratory of the Multielemental Analyses at Wroclaw University of Technology, which is accredited by ILAC-MRA and the Polish Centre for Accreditation according to PN-EN ISO/IEC 17025 (nr AB 696) [38].
3.4. Calculations
The arithmetic mean values, standard deviations (SD) and t tests as well as the model parameters of equations describing the experimental data were determined with nonlinear estimation and multiple regression modules of Statistica software ver. 9.0 (StatSoft, Krakow, Poland). Correlation was considered statistically significant at p < 0.05.
Chi-square test (χ2 test) was also used, which was calculated from Equation (5), which more accurately described the fit of the model to the experimental data compared to the determination coefficient R2.
4. Conclusions
To become available for plants, phosphorus needs to be converted into a soluble form; this solubilization occurs in nature in the presence of a large number of acidogenic autotrophs and heterotrophs (bacteria, fungi and yeasts), which produce inorganic or organic acids with which they are capable of dissolving insoluble phosphates.
The results presented here demonstrate the usefulness of A. ferrooxidans in the biosolubilization of phosphorus occurring in poultry bones, fish bones and phosphorite. The solubilization factor was the highest for the lower dose of poultry bones (96%) and fish bones (94%), as well as for phosphorite (91%). Fertilizers obtained from the solubilization performed with the highest doses of phosphorus substrates contained 1.6% and 1.9% of phosphorus (expressed as P2O5) for poultry bones and fish bones, respectively. The formulations can be classified as organic phosphorus fertilizers in liquid form, pursuant to the Regulation of the Minister of Agriculture and Rural Development of Poland on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization (Journal of Laws of 2004, no. 236, item. 2369 &14), which specifies that the minimum quality requirements for the phosphorus content (expressed as P2O5) in phosphorus fertilizers is 0.05% (m/m).
Acknowledgments
This project is financed within the framework of grant PBS 2/A1/11/2013 entitled: “Phosphorus renewable raw materials—a resource base for new generation of fertilizers,” attributed by the National Center for Research and Development.
Author Contributions
A.S. conceived and designed the experiments; M.W. and P.M. performed the experiments; A.S. and K.C. analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Besharati, H.; Atashnama, K.; Hatami, S. Biosuper as a phosphate fertilizer in a calcareous soil with low available phosphorus. Afr. J. Biotechnol. 2007, 6, 1325–1329. [Google Scholar]
- Malinowski, P.; Olech, M.; Sas, J.; Wantuch, W.; Biskupski, A.; Urbańczyk, L.; Borowik, M.; Kotowicz, J. Production of compound mineral fertilizers as a method of utilization of waste products in chemical company Alwernia S.A. Pol. J. Chem. 2010, 12, 6–9. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K. In situ solubilization of phosphorus bearing raw materials by Bacillus megaterium. Eng. Life Sci. 2017. [Google Scholar] [CrossRef]
- Hayat, R.; Safdar, A.; Ummay, A.; Rabia, K.; Iftikhar, A. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.-A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Santos, N.P.; Moura, P.R.; Freire, A.M.M. Biofertilizers with natural phosphate, sulphur and Acidithiobacillus in a soil with low available-P. Sci. Agricola 2003, 60, 767–773. [Google Scholar]
- Wyciszkiewicz, M.; Saeid, A.; Samoraj, M.; Chojnacka, K. Solid-state solubilization of bones by B. megaterium in spent mushroom substrate as a medium for a phosphate enriched substrate. J. Chem. Technol. Biotechnol. 2016. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Górecki, H.; Chojnacka, K. New generation of phosphate fertilizer from bones, produced by bacteria. Open Chem. 2015, 13, 951–958. [Google Scholar] [CrossRef]
- Coates, L.R. Method of Producing Fertilizers. Patent US947798, 1 February 1910. [Google Scholar]
- Du, S.Q.; DU, S.W.; Qin, Z.W. Process for Preparing biologic Organic and Mineral Compound Fertilizer. Patent Apl. CN1262260, 9 August 2000. [Google Scholar]
- Wang, D.Z.; Wang, L. Three microbe Granular Fertilizer and Producing Process Thereof. Patent Apl. CN1175565, 11 March 1998. [Google Scholar]
- Liu, W. Method for Producing Organic and Inorganic Mixed Microbe Fertilizer. Patent CN1054362, 12 July 2000. [Google Scholar]
- Rajan, S.S.S. Use of low grade phosphate rocks as biosuper fertilizer. Fertil. Res. 1981, 2, 199–209. [Google Scholar] [CrossRef]
- Kucera, J.; Pakostova, E.; Lochman, J.; Janiczek, O.; Mandl, M. Are there multiple mechanisms of anaerobic sulfur oxidation with ferric iron in Acidithiobacillus ferrooxidans? Res. Microbiol. 2016, 167, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Guo, C.; Zhang, T.; Lu, G.; Wan, J.; Liao, C.; Dang, Z. Investigation of intermediate sulfur species during pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Hydrometallurgy 2017, 167, 58–65. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Tao, J.; Feng, X.; Liu, X.; Qin, W. Differential fluoride tolerance between sulfur- and ferrous iron-grown Acidithiobacillus ferrooxidans and its mechanism analysis. Biochem. Eng. J. 2017, 119, 59–66. [Google Scholar] [CrossRef]
- Martínez-Bussenius, C.; Navarro, C.A.; Orellana, L.; Paradela, A.; Jerez, C.A. Global response of Acidithiobacillus ferrooxidans ATCC 53993 to high concentrations of copper: A quantitative proteomics approach. J. Proteom. 2016, 145, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Nguyen, T.A.H.; Taran, E.; Mahler, S.M.; Nguyen, A.V. Effect of energy source, salt concentration and loading force on colloidal interactions between Acidithiobacillus ferrooxidans cells and mineral surfaces. Colloids Surf. B. Biointerfaces 2015, 132, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Avdalović, J.; Beškoski, V.; Gojgić-Cvijovića, G.; Mattinenc, M.-L.; Stojanovićd, M.; Zildžovićd, S.; Vrvić, M.M. Microbial solubilization of phosphorus from phosphate rock by iron-oxidizing Acidithiobacillus sp. B2. Miner. Eng. 2015, 72, 17–22. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil-phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the. Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Xiao, C.X.; Chi, R.-A.; Fang, Y.-J. Effects of Acidiphilium cryptum on biosolubilization of rock phosphate in the presence of Acidithiobacillus ferrooxidans. Trans. Nonferrous Meter. Soc. China. 2013, 23, 2153–2159. [Google Scholar] [CrossRef]
- Wyciszkiewicz, M.; Saeid, A.; Dobrowolska-Iwanek, J.; Chojnacka, K. Utilization of microorganisms in the solubilization of low-quality phosphorus raw material. Ecol. Eng. 2016, 89, 109–113. [Google Scholar] [CrossRef]
- Saeid, A.; Labuda, M.; Chojnacka, K.; Górecki, H. Valorization of Bones to Liquid Phosphorus Fertilizer by Microbial Solubilization. Waste Biomass Valori. 2014, 5, 265–272. [Google Scholar] [CrossRef]
- Phiraphinyo, P.; Taepakpurenat, S.; Lakkanatinaporn, P.; Suntornsuk, W.; Suntornsuk, L. Physical and chemical properties of fish and chicken bones as calcium source for mineral supplements. J. Sci. Technol. 2006, 28, 327–335. [Google Scholar]
- Wyciszkiewicz, M.; Saeid, A.; Chojnacka, K.; Górecki, H. Production of phosphate biofertilizers from bones by phosphate-solubilizing bacteria Bacillus megaterium. Open Chem. 2015, 13, 1063–1070. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Yawar, W. Bacterial solubilization of phosphorus from phosphate rock containing sulfur-mud. Hydrometallurgy. 2010, 103, 54–59. [Google Scholar] [CrossRef]
- Mendes, G.O.; Vassilev, N.B.; Bonduki, V.H.A.; Silva, I.R.; Ribeiro, J.I.; Costa, D. Inhibition of Aspergillus niger Phosphate Solubilization by Fluoride Released from Rock Phosphate. Appl Environ Microbiol. 2013, 16, 4906–4913. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Strother, P.; McMahon, G.; Nittler, L.R.; Wang, J.; Zhang, J.; Sang, L.; Ma, Ch.; Papineau, D. Terminal Proterozoic cyanobacterial blooms and phosphogenesis documented by the Doushantuo granular phosphorites I: In situ micro-analysis of textures and composition. Precambrian Res. 2013, 235, 20–35. [Google Scholar] [CrossRef]
- Mendes, G.O.; Zafra, D.L.; Vassilev, N.B.; Silva, I.R.; Ribeiro, J.I.; Costa, M.D. Biochar Enhances Aspergillus niger Rock Phosphate Solubilization by Increasing Organic Acid Production and Alleviating Fluoride Toxicity. Appl. Environ. Microbiol. 2014, 80, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.; Xiao, C.; Gao, H. Bioleaching of phosphorus from rock phosphate containing pyrites by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 979–981. [Google Scholar] [CrossRef]
- Tutken, T.; Vennemann, T.W.; Pfretzschner, H.-U. Early diagenesis of bone and tooth apatite in fluvial and marine settings: Constraints from combined oxygen isotope, nitrogen and REE analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 254–268. [Google Scholar] [CrossRef]
- Soudry, D.; Nathan, Y. Microbial infestation: A pathway of fluorine enrichment in bone apatite fragments (Negev phosphorites, Israel). Sediment. Geol. 2000, 132, 171–176. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; AbdelKareem, H.N. Sustainable approach for recycling waste lamb and chicken bones for fluoride removal from water followed by reusing fluoride-bearing waste in concrete. Waste Manag. 2015, 45, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yield. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [PubMed]
- Górecka, H.; Chojnacka, K.; Górecki, H. The application of ICP-MS and ICP-OES in determination of micronutrients in wood ashes used as soil conditioners. Talanta 2006, 70, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).