Abstract
With the aim of developing novel antitumor scaffolds, a novel series of polysubstituted pyrazole derivatives linked to different nitrogenous heterocyclic ring systems at the C-4 position were synthesized through different chemical reactions and characterized by means of spectral and elemental analyses and their antiproliferative activity against 60 different human tumor cell lines was validated by the U.S. National Cancer Institute using a two stage process. The in vitro anticancer evaluation revealed that compound 9 showed increased potency toward most human tumor cell lines with GI50MG-MID = 3.59 µM, as compared to the standard drug sorafenib (GI50 MG-MID = 1.90 µM). At the same time, compounds 6a and 7 were selective against the HOP-92 cell line of non-small cell lung cancer with GI50 1.65 and 1.61 µM, respectively.
1. Introduction
The development of new antitumor agents is an important field of scientific activity, due to the toxic side effect problems of recent drugs. Many of the obtainable anticancer agents display unwanted side effects such as reduced bioavailability, toxicity and drug-resistance [1,2,3,4,5]. Incorporation of a pyrazole ring into different heteroaryl ring systems results in significant anticancer activities [6,7,8,9]. Different substituted pyrazole compounds have also been examined for their antiproliferative activities in vitro and antitumor activity in vivo, resulting in promising target products [10,11,12]. On the other hand, compounds containing pyrazole derivatives represent an advantageous choice for the synthesis of compounds with a broad spectrum of pharmacological activities, including anti-inflammatory [13], antibacterial, antifungal [14], inhibition of cyclooxygenase-2 [15], antiangiogenic [16], antipyretic [17], antihypertensive [18], antiplatelet [19], nitric oxide synthase (NOS) inhibitors [20] and anticancer activities [21]. Based on these observations and in continuation of our research on biologically active heterocycles [22,23,24,25], it was of interest to incorporate the 1,2,4-polysubstituted pyrazole ring system into different heteroaryl ring systems in one molecule in an attempt to obtain a new target anticancer agents.
2. Results and Discussion
2.1. Chemistry
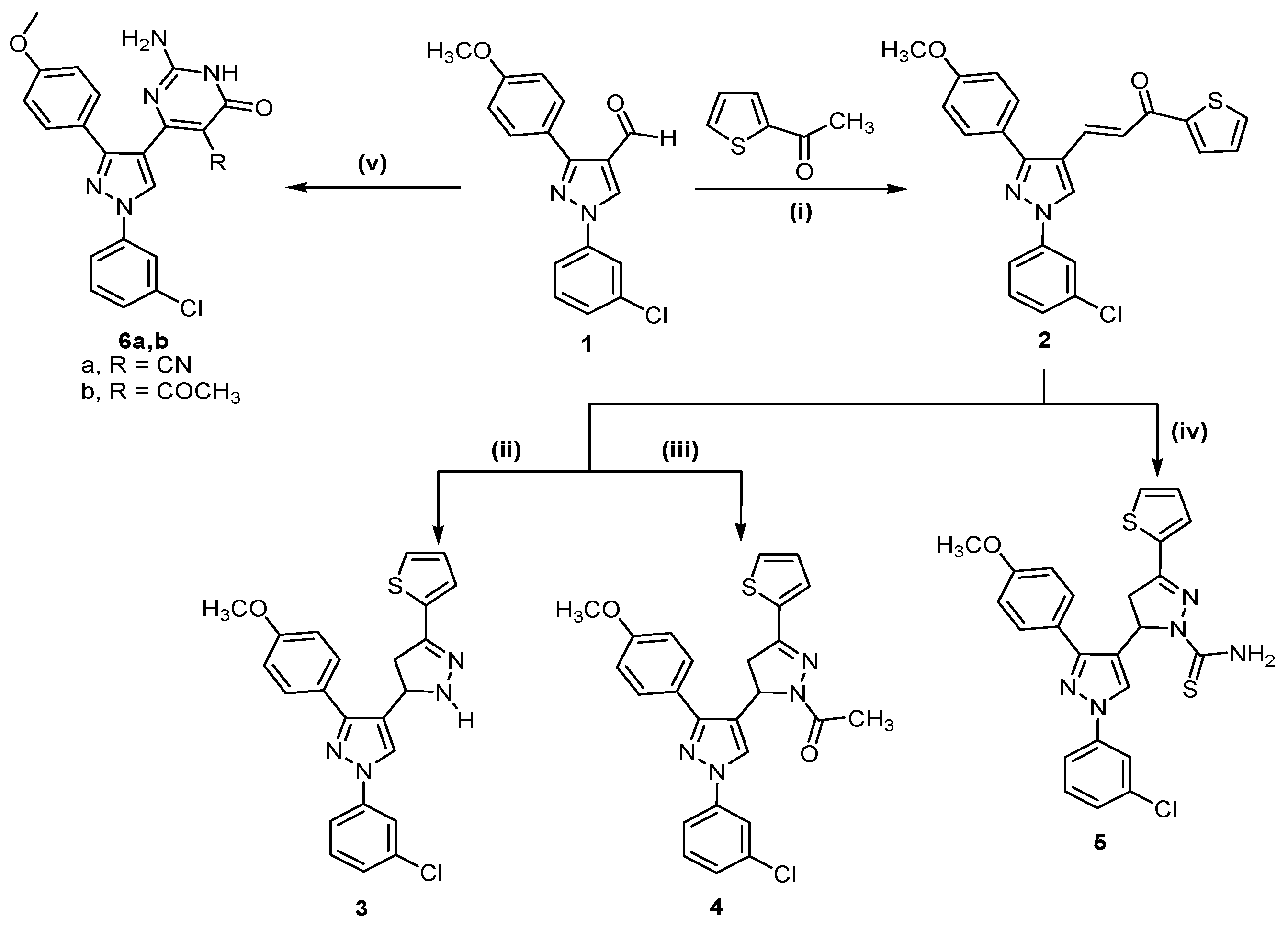
The reaction sequences outlined in Scheme 1 and Scheme 2 were used for the synthesis of the target compounds. Application of the Claisen Schmidt condensation on 2-acetylthiophene and 1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazole-4-carboxaldehyde (1) in ethanolic sodium hydroxide solution according to literature methods [26,27] afforded (E)-3-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (2), which was used as starting material. Cyclocondensation of the α,β-unsaturated ketone 2 with hydrazine hydrate in absolute ethanol or glacial acetic acid yielded the corresponding pyrazoline derivative 3 and N-acetyl-pyrazoline derivative 4, respectively. On the other hand, heating of 2 with thiosemicarbazide in ethanolic NaOH gave 1-thiocarbamoyl pyrazole derivative 5. In addition, condensation of compound 1 with ethyl cyanoacetate, or ethyl acetoacetate in the presence of guanidine hydrochloride gave 2-amino-5-cyano/acetyl-6-hydroxy-4-aryl pyrimidines 6a,b, respectively (Scheme 1).
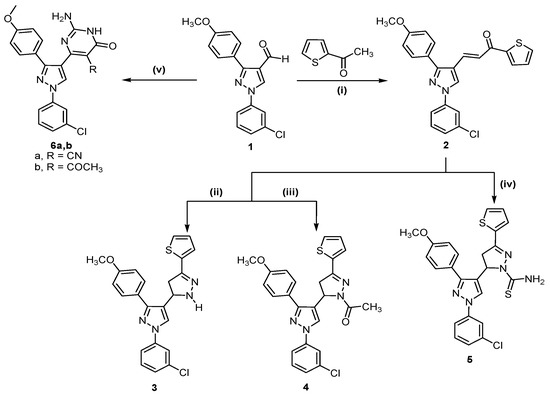
Scheme 1.
Synthetic route for trisubstituted pyrazole compounds 2–6.
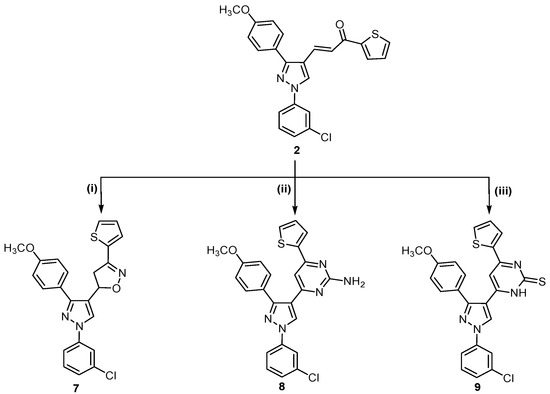
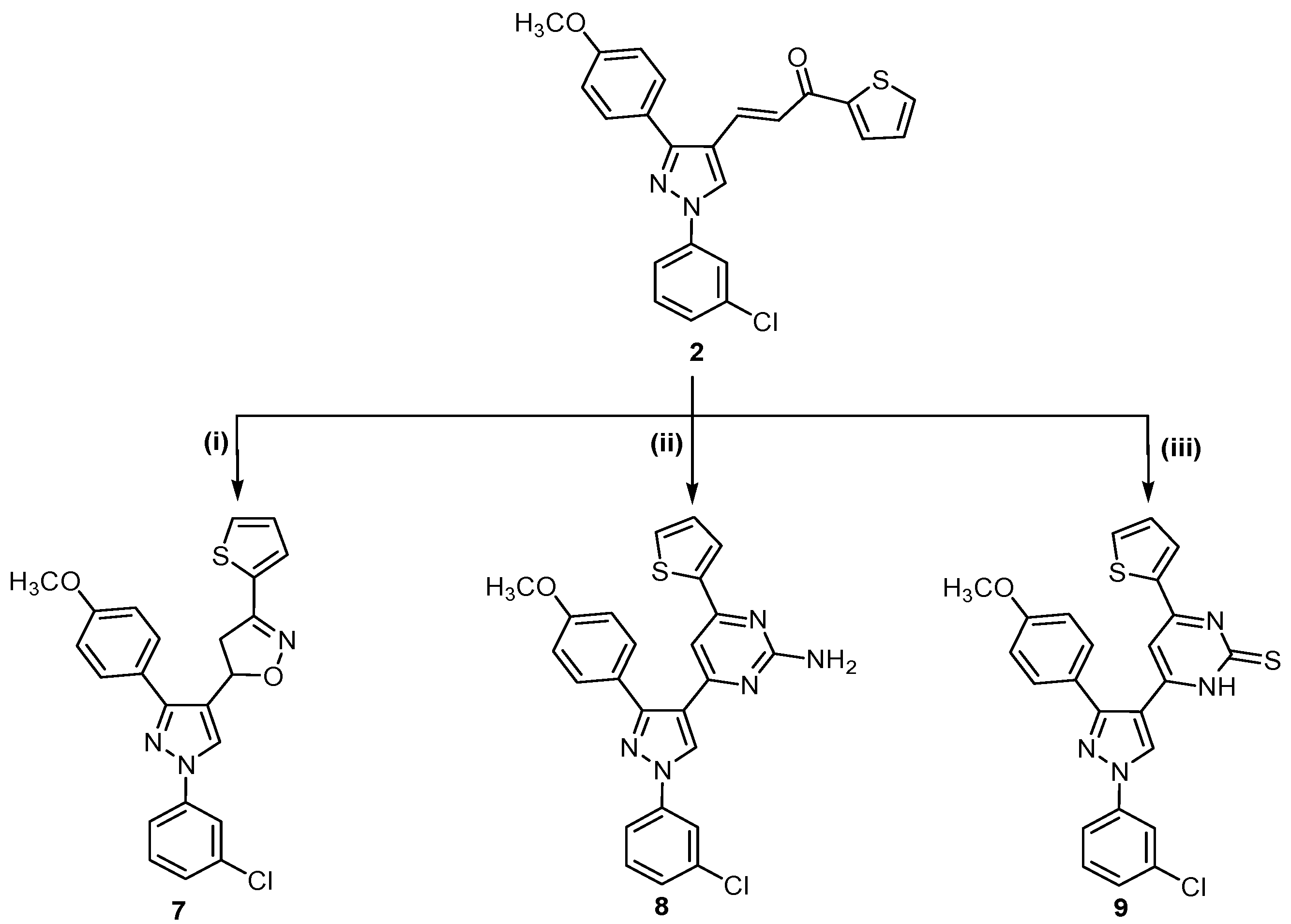
Scheme 2.
Synthetic route for trisubstituted pyrazole compounds 7–9.
Finally, α,β-unsaturated ketone 2 was reacted with hydroxylamine hydrochloride in refluxing ethanol in the presence of sodium hydroxide as alkaline medium to afford the corresponding isoxazoline 7. Treatment of 2 with guanidine sulfate in ethanolic sodium hydroxide gave 2-aminopyrimidine derivative 8, which was reacted with thiourea in the presence of sodium hydroxide to give the corresponding pyrimidine-2-thione derivative 9 (Scheme 2).
2.2. In Vitro Anticancer Screening
The target compounds were selected by the U.S. National Cancer Institute (NCI), for anticancer activity screening. The screening is a two-stage process, beginning with the evaluation at a single dose (10 µM) and the compounds which display significant growth inhibition are then evaluated at five concentration levels. The first screening, where the selected compounds are evaluated at a single dose (10 µM) and the culture is incubated for 48 h, utilizes 60 different human tumor cell lines, representing leukemia, melanoma and cancers of lung, colon, central nervous system (CNS), ovary, kidney, prostate as well as breast.
The percentages of growth of the tested compounds against the full 60-cell line panel are listed in Table 1. The one dose mean graphs of the selected compounds revealed that compounds 6a, 7 and 9 showed increased potency against most human cancer cell lines, so these compounds were selected for further evaluation at five dose concentration levels (0.01–100 µM).Regarding sensitivity against individual cell lines, compound 9 showed potent anticancer activity against all human cancer cell lines with GI50 1.9–5.50 µM. It had the highest selectivity against the non-small cell lung cancer cell line EKVX, with GI50 1.9 µM. At the same time, compounds 6a and 7 showed the highest activity against the cell line HOP-92 belonging to the non-small cell lung cancer class with GI50 1.7 and 1.6 µM, respectively, and against the renal cancer cell line A498 with GI50 1.47 and 1.81 µM, respectively.

Table 1.
The mean growth percent of compounds 2, 3, 4, 6a, 6b, 7, 8 and 9.
Examination of all the ligand structural modifications performed at the 4-position of 1,3,4-trisubstituted pyrazole scaffold to establish the structure activity relationships with anticancer acitivity shows the following results: first, introduction of the pyrimidine-2(1H)-thione moiety in the 4-position of the pyrazole moiety in compound 9 enhanced the potency towards most cancer cell lines. It has GI50 MG-MID = 3.6 µM against all subpanel tumor cell lines, comparable to that of sorafenib (GI50 MG-MID = 1.90 µM. Concerning the pyrazolyl derivative 6a, it was observed that the activity was enhanced in 2-amino-6-oxopyrimidine-5-carbonitrile, which suggests that the polar 6-aminopyrimidine moiety has a role in enhancing anticancer activity (GI50 MG-MID = 5.70 µM), but less so than the 2-mercaptopyrimidine group. Finally, introduction of a 1-carbothioamide to a pyrazole or isoxazoline group substituted on the pyrazole greatly reduces the activity and these compounds showed the least potent activity. The results are presented in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6.

Table 2.
GI50 (μM) of five-dose screening results of compounds 6a, 7 and 9.

Table 3.
TGI (μM) of five-dose screening results of compounds 6a, 7 and 9.

Table 4.
LC50 (μM) of five-dose screening results of compounds 6a, 7 and 9.

Table 5.
Median growth inhibitory concentrations (GI50, μM) of in vitro subpanel tumor cell lines and GI50 (μM) full panel mean-graph mid-points (MG-MID) of compounds 6a, 7 and 9 in comparison with sorafenib.

Table 6.
Selectivity ratios for compounds 6a, 7 and 9 towards the nine tumor cell lines.
3. Experimental Section
3.1. General Information
Melting points were measured in open capillary tubes using a Griffin apparatus and are uncorrected. Structures of compounds were confirmed by routine spectrometric analysis. Elemental analyses were carried results were within ±0.4% of the theoretical values. Infrared spectra were recorded on a 435 IR spectrophotometer (Shimadzu Bruker, Tokyo, Japan) using KBr discs. 1H-NMR and 13C-NMR spectra were obtained on a Gemini 500 MHz spectrophotometer (Varian, Polo Alto, Ca, USA) or on a Bruker 500 MHz spectrophotometer, and measured in δ scale using TMS as an internal standard. Mass Spectra were recorded on a 5988 spectrometer (Hewlett Packard, California, USA). Analytical thin layer chromatography (TLC) was performed using silica gel aluminum sheets, 60 F254 (E. Merck, Darmstadt, Germany) for the progress of reactions and visualization with ultraviolet light (UV) at 365 and 254 nm.
3.2. 3-(1-(3-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (2)
A mixture of carbaldehyde derivative 1 (0.01 mol) and 2-acetylthiophene (0.01 mol) in of 30% ethanolic solution of NaOH (40 mL) was stirred for 12 h at room temperature. The progress of reaction was monitored by TLC. After completion, the reaction mixture was poured into acidified ice cold water of pH~2. The precipitated solid was filtered, washed with water and recrystallized to afford compound 2 in 87% yield; m.p. 144–146 °C (EtOH); IR (KBr) ν: 3079 (CH-Ar), 1641 (C=O), 1600 (C=C) cm−1; 1H-NMR (DMSO-d6): δ 3.81 (s, 3H, OCH3); 7.00–7.98 (m, 12H, ArH + CH=CH), 8.73 (s,1H, CH of pyrazole) ppm; 13C-NMR (DMSO-d6): δ 55.60, 114.34, 116.62, 117.75, 125.11, 125.95, 127.67, 129.08, 129.81, 131.74, 133.85, 134.47, 135.44, 141.08, 144.32, 151.54, 159.58, 191.96 ppm; MS (EI, 70 eV): m/z (%): 420 (11) [M]+; Anal. Calcd for C23H17ClN2O2S (420.91): C, 65.63; H, 4.07; N, 6.66; Found: C, 65.59; H, 4.16; N, 6.72.
3.3. 1-(3-Chlorophenyl)-4-(4,5-dihydro-3-(thiophen-2-yl)-1H-pyrazol-5-yl)-3-(4-methoxyphenyl)-1H-pyrazole (3)
To a solution of compound 2 (0.01 mol) in ethanol (30 mL) containing a catalytic amount of glacial acetic acid, a solution of hydrazine hydrate (98%, 0.5 mL) was added and the mixture was refluxed for 6 h. The reaction mixture was cooled to room temperature and the precipitated solid was filtered, dried and recrystallization provided compound 3 in 57% yield; m.p. 175–178 °C (EtOH); IR (KBr) ν: 3177 (NH), 1590 (C=C) cm−1; 1H-NMR (DMSO-d6): δ 2.99 (dd, 1H, CH), 3.70 (s, 3H, OCH3), 3.84 (dd, 1H, CH), 5.42 (dd, 1H, CH), 6.39–7.77 (m, 11H, Ar-H), 8.91 (s, 1H, CH of pyrazole), 11.52 (s, 1H, NH D2O exchangeable) ppm; 13C-NMR (DMSO-d6): δ 42.10, 55.61, 60.74, 114.22, 116.78, 117.76, 125.18, 125.90, 126.17, 127.38, 128.67, 129.65, 130.87, 131.26, 134.53, 137.55, 140.98, 151.24, 159.73, 158.76, 161.48 ppm; MS (EI, 70 eV): m/z (%): 434 (18) [M]+; Anal. Calcd for C23H19ClN4OS (434.94): C, 63.51; H, 4.40; N, 12.88; Found: C, 63.59; H, 4.53; N, 12.92.
3.4. 1-(5-(1-(3-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydro-3-(thiophen-2yl)pyrazol-1-yl)-ethanone (4)
To a solution of compound 2 (0.01 mol) in glacial acetic acid (20 mL), hydrazine hydrate (0.01 mol) was added and the mixture was refluxed for 3 h. The reaction mixture was cooled to room temperature and the solid formed was filtered off, dried and recrystallized to get compound 4 in 65% yield; m.p. 150–154 °C (EtOH); IR (KBr) ν: 3080 (CH-Ar), 1677 (C=O), 1619 (C=N), 1588 (C=C) cm−1; 1H-NMR (DMSO-d6): 2.43 (s, 3H, COCH3), 3.30 (dd, 1H, CH), 3.81 (s, 3H, OCH3), 3.88 (dd, 1H, CH), 5.57 (dd, 1H, CH), 6.70–7.65 (m, 11H, Ar-H), 9.18 (s, 1H, CH of pyrazole) ppm; 13C-NMR (DMSO-d6): δ 24.01, 43.81, 55.60, 63.87, 114.26, 116.84, 117.62, 125.35, 125.86, 126.10, 127.56, 129.14, 129.78, 130.69, 131.08, 134.41, 137.82, 140.84, 150.39, 155.19, 159.12, 160.92, 168.19 ppm; MS (EI, 70 eV): m/z (%): 476 (43) [M]+; Anal. Calcd for C25H21ClN4O2S (476.98): C, 62.95; H, 4.44; N, 11.75; Found: C, 63.02; H, 4.35; N, 11.81.
3.5. 5-(1-(3-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4,5-dihydro-3-(thiophen-2-yl)-pyrazole-1-carbothioamide (5)
To a mixture of chalcone 2 (0.01 mol) in absolute ethanol (30 mL), sodium hydroxide (1 g, 0.025 mol) was added. The reaction mixture was heated under reflux for 5 h. The contents were reduced, cooled and poured onto crushed ice. The resulting precipitate was collected by filtration and recrystallized to give in 61% yield; m.p. >300 °C (MeOH); IR (KBr) ν: 3407 (NH2), 1658 (C=N), 1523 (C=C), 1078 (C=S) cm−1; 1H-NMR (DMSO-d6): 3.13 (dd, 1H, CH), 3.84 (s, 3H, OCH3), 4.14 (dd, 1H, CH), 5.51 (dd, 1H, CH), 7.14–8.02 (m, 11H, Ar-H), 9.12 (s, 1H, CH of pyrazole), 9.93 (s, 2H, NH2 D2O exchangeable) ppm; 13C-NMR (DMSO-d6): δ 43.14, 55.64, 62.93, 114.33, 116.72, 117.58, 125.37, 125.80, 126.08, 127.54, 128.79, 129.22, 130.65, 131.16, 138.12, 137.82, 140.85, 151.23, 156.19, 159.30, 161.02, 176.55 ppm; MS (EI, 70 eV): m/z (%): 494 (6) [M]+; Anal. Calcd for C24H20ClN5OS2 (494.03): C, 58.35; H, 4.08; N, 14.18; Found: C, 58.27; H, 4.12; N, 14.23.
3.6. 2-Amino-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,6-dihydro-6-oxopyrimidine-5-carbonitrile (6a) and 5-acetyl-2-amino-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-pyrimidin-4(3H)-one (6b)
A mixture of compound 1 (0.01 mol), ethyl cyanoacetate or ethyl acetoacetate (0.01 mol) and (5 mL) of 40% ethanolic sodium hydroxide was stirred for 10 min, followed by addition of guanidine hydrochloride (0.01 mol) and the heating continued under refluxed for 3 h. The reaction mixture was diluted with ice-water and the formed precipitate was collected by filtration, washed several times with water, dried and recrystallized to afford the title compounds 6a,b.
2-Amino-4-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-1,6-dihydro-6-oxopyrimidine-5-carbonitrile (6a). Yield 64%; m.p. 144–148 °C (EtOH); IR (KBr) ν: 3341, 3145 (NH2, NH), 2219 (C≡N), 1663 (C=O), 1599 (C=C) cm−1; 1H-NMR (DMSO-d6): 2.85 (s, 2H, NH2 D2O exchangeable), 3.88 (s, 3H, OCH3), 7.03–8.75 (m, 9H, Ar-H and NH D2O exchangeable), 9.20 (s, 1H, CH of pyrazole) ppm; 13C-NMR (DMSO-d6): δ 55.63, 113.75, 115.31, 116.79, 117.77, 123.48, 125.21, 126.4, 127.94, 128.30, 129.82, 130.80, 134.57, 140.89, 155.04, 159.88, 160.60, 164.33, 171.88 ppm; MS (EI, 70 eV): m/z (%): 418 (14) [M]+; Anal. Calcd for C21H15ClN6O2 (418.84): C, 60.22; H, 3.61; N, 20.07; Found: C, 60.26; H, 3.58; N, 20.15.
5-Acetyl-2-amino-6-(1-(3-chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)pyrimidin-4-(3H)-one (6b). Yield 68%, m.p. 135–137 °C (EtOH); IR (KBr) ν: 3410, 3152 (NH2,NH), 1675 (C=O), 1589 (C=C) cm−1; 1H-NMR (DMSO-d6): 2.30 (s, 2H, NH2 D2O exchangeable), 2.39 (s, 3H, CH3), 3.86 (s, 3H, OCH3), 6.90–7.96 (m, 9H, Ar-H and NH exchangeable with D2O), 9.14 (s, 1H, CH of pyrazole) ppm; 13C-NMR (DMSO-d6): δ 24.38, 55.60, 113.72, 115.94, 116.85, 117.73, 125.10, 126.01, 128.36, 130.76, 132.95, 134.47, 141.05, 151.14, 154.78, 159.60, 160.82, 164.21, 165.38, 182.70 ppm; MS (EI, 70 eV): m/z (%): 435 (7) [M]+; Anal. Calcd for C22H18ClN5O3 (435.86): C, 60.62; H, 4.16; N, 16.07; Found: C, 60.59; H, 4.11; N, 16.12.
3.7. 1-(3-Chlorophenyl)-3-(4-methoxyphenyl)-4-(3-(thiophen-2-yl)isoxazol-5-yl)-1H-pyrazole (7)
A mixture of compounds 2 (0.01 mol) and hydroxylamine hydrochloride (0.01 mol) in ethanol (30 mL) containing sodium hydroxide solution (0.5 g NaOH in 0.5 mL water) was refluxed for 3 h. The reaction mixture was poured onto ice-water, neutralized with drops of conc. Hydrochloric acid and the solid precipitate formed filtered off, washed with water and recrystallized to yield the desired compound 7 in 73% yield; m.p. >300 °C (EtOH); IR (KBr) ν: 3064 (CH-Ar), 1601 (C=C) cm−1; 1H-NMR (DMSO-d6): 3.99 (s, 3H, OCH3), 7.30 (d, 2H, H J = 20), 7.60 (d, 1H, CH); 7.75–8.45 (m, 10H, Ar-H), 9.15 (s, 1H, CH of pyrazole) ppm; 13C-NMR (DMSO-d6): δ 43.19, 55.61, 73.10, 114.38, 116.72, 117.68, 125.68, 125.84, 126.10, 127.43, 129.07, 129.57, 130.81, 131.29, 134.47, 138.21, 140.85, 151.20, 154.63, 160.92, 162.89 ppm; MS (EI, 70 eV): m/z (%): 433 (12) [M]+; Anal. Calcd for C23H16ClN3O2S (433.91): C, 63.66; H, 3.72; N, 9.68; Found: C, 63.74; H, 3.79; N, 9.71.
3.8. 4-(1-(3-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-6-(thiophen-2-yl)pyrimidin-2-amine (8)
An aqueous solution 5 mL of 40% sodium hydroxide was added gradually during a period of 3 h to a mixture of chalcone 2 (0.01 mol) and guanidine sulfate (0.01 mol) in ethanol (25 mL). The reaction mixture was refluxed for 5 h and the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured onto ice-cold water and the solid product formed was collected by filtration, washed with water then recrystallized to get compound 8 in 67% yield; m.p. >300 °C (MeOH); IR (KBr) ν: 3347 (NH2); 1632 (C=N); 1589 (C=C) cm−1; 1H-NMR (DMSO-d6): 3.80 (s, 3H, OCH3), 6.68-8.08 (t, 12H, Ar-H), 8.93 (s,1H, CH of pyrazole), 10.18 (s, 2H, NH2 D2O exchangeable) ppm; 13C-NMR (DMSO-d6): δ 55.64, 82.10, 114.31, 116.80, 117.59, 125.30, 125.78, 126.09, 127.41, 128.67, 129.48, 130.79, 131.16, 134.49, 139.86, 141.05, 150.88, 152.10, 160.37, 164.21, 166.58, 164.25 ppm; Anal. Calcd for C24H18ClN5OS (459.95): C, 62.67; H, 3.94; N, 15.23; Found: C, 62.63; H, 3.88; N, 15.29.
3.9. 6-(1-(3-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-4-yl)-4-(thiophen-2-yl)pyrimidine-2-(1H)-thione (9)
A solution of the chalcone 2 (0.01 mol), thiourea (0.01 mol) and sodium hydroxide (0.1 g) in absolute ethanol (30 mL) was refluxed for 6 h. The reaction mixture was concentrated under vacuum, cooled and neutralized with dilute HCl. The formed product was filtered off, washed with water and recrystallized to get compound 9 in 76% yield; m.p. >300 °C (MeOH); IR (KBr) ν: 3422 (NH), 1595 (C=C), 1176 (C=S) cm−1; 1H-NMR (DMSO-d6): 3.82 (s, 3H, OCH3), 6.90–8.60 (m, 12H, Ar-H), 9.20 (s, 1H, CH of pyrazole), 9.95 (s, 1H, NH D2O exchangeable) ppm; 13C-NMR (DMSO-d6): δ 55.63, 104.56, 113.82, 115.89, 117.66, 125.38, 125.70, 126.03, 127.38, 128.34, 129.45, 130.77, 131.09, 134.42, 138.17, 140.95, 150.76, 157.21, 160.85, 161.80, 162.46, 184.33 ppm; MS (EI, 70 eV): 477 (8) [M]+; Anal. Calcd for C24H17ClN4OS2 (477): C, 60.43; H, 3.59; N, 11.75; Found: C, 60.38; H, 3.66; N, 11.82.
3.10. Measurement of Anticancer Activity
The experimental method used in anticancer screening has been adopted by U.S. National Cancer Institute according to reported standard procedure [28,29,30].
4. Conclusions
In summary, we have synthesized a series of novel pyrazole derivatives incorporated different heteroaryl ring systems in one molecule and evaluated these compounds for their anticancer activities against different 60 human cancer cell lines representing leukemia, melanoma and cancers of lung, colon, brain, ovary, breast, prostate and kidney cancer using a two-stage process. The pyrimidine-2(1H)-thione derivative 9 showed good anticancer activity with (GI50 MG-MID = 3.59 µM) compared to the standard drug sorafenib. The structures of the new compounds were elucidated using spectroscopic and elemental analysis.
Acknowledgments
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
Author Contributions
The listed authors contributed to this work as described in the following. Hoda H. Fahmy gave the concepts of the work, interpreted the results and prepared the manuscript, Eman S. Nossier, carried out the synthetic work, interpreted the results and prepared the manuscript and Nagy M. Khalifa, Magda M. F. Ismail and Hend M. El-Sahrawy interpreted the results and cooperated in the preparation of the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S. Recent progress in the development of anticancer agents. Curr. Med. Chem. Anticancer Agents 2002, 2, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Altmann, K.H. Microtubule-stabilizing agents: A growing class of important anticancer drugs. Curr. Opin. Chem. Biol. 2001, 5, 424–431. [Google Scholar] [CrossRef]
- Wartmann, M.; Altmann, K.H. The biology and medicinal chemistry of epothilones. Curr. Med. Chem. Anticancer Agents 2002, 2, 1231–1248. [Google Scholar] [CrossRef]
- O’Dwyer, M.E.; Druker, B.J. The role of the tyrosine kinase inhibitor STI571 in the treatment of cancer. Curr. Cancer Drug Targets 2001, 1, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Dong, Z.W.; Zhao, B.X.; Ge, X.; Meng, N.; Shin, D.S.; Miao, J.Y. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg. Med. Chem. 2007, 15, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, A.R. Synthesis of some new indole derivatives containing pyrazoles with potential antitumor activity. ARKIVOC 2010, 11, 177–187. [Google Scholar]
- El-Zahar, M.I.; EL-Karim, S.A.; Haiba, M.E.; Khedr, M.A. Synthesis, antitumor activity and molecular docking study of novel benzofuran-2-yl pyrazole pyrimidine derivatives. Acta Pol. Pharm. Drug. Res. 2011, 68, 357–373. [Google Scholar]
- Kalirajan, R.; Rathore, L.; Jubie, S.; Gowramma, B.; Gomathy, S.; Sankar, S. Microwave assisted synthesis of some novel pyrazole substituted benzimidazoles and evaluation of their biological activities. Indian J. Chem. 2011, 50B, 1794–1799. [Google Scholar] [CrossRef]
- Perchellet, E.M.; Ward, M.M.; Skaltsounis, A.L.; Kostakis, I.K.; Pouli, N.; Marakos, P.; Perchellet, J.H. Antiproliferative and proapoptotic activities of pyranoxanthenones, pyranothioxanthenones and their pyrazole-fused derivatives in HL-60 cells. Anticancer Res. 2006, 26, 2791–2804. [Google Scholar] [PubMed]
- Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonia, R.; Nogueras, M.; Sanchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar] [CrossRef] [PubMed]
- Labbozzetta, M.; Baruchello, R.; Marchetti, P.; Gueli, M.C.; Poma, P.; Notarbartolo, M.; Simoni, D.; D’Alessandro, N. Lack of nucleophilic addition in the isoxazole and pyrazole diketone modified analogs of curcumin; implications for their antitumor and chemosensitizing activities. Chem. Biol. Interact. 2009, 181, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Fischer, J.; Kis-Varga, A.; Gyires, K. New celecoxib derivatives as anti-inflammatory agents. J. Med. Chem. 2008, 51, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Tanitame, A.; Oyamada, Y.; Ofuji, K.; Terauchi, H.; Kawasaki, M.; Wachi, M.; Yamagishi, J. Synthesis and antibacterial activity of a novel series of DNA gyrase inhibitors: 5-[(E)-2-arylvinyl]pyrazoles. Bioorg. Med. Chem. Lett. 2005, 15, 4299–4303. [Google Scholar] [CrossRef] [PubMed]
- Rida, S.M.; Saudi, M.N.S.; Youssef, A.M.; Halim, M.A. Synthesis and biological evaluation of the pyrazole class of cyclooxygenase-2-inhibitors. Lett. Org. Chem. 2009, 6, 282–288. [Google Scholar] [CrossRef]
- Abadi, A.H.; Abdel Haleem, A.; Hassan, G.S. Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bul. 2003, 51, 838–844. [Google Scholar] [CrossRef]
- Sridhar, S.; Parsad, R.Y. Synthesis and Analgesic Studies of Some New 2-pyrazolines. Eur. J. Chem. 2012, 9, 1810–1815. [Google Scholar] [CrossRef]
- Turan-Zitouni, G.; Chevallet, P.; Kilic, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- Zhou, S.; Ren, J.; Liu, M.; Ren, L.; Liu, Y.; Gong, P. Design, synthesis and pharmacological evaluation of 6,7-disubstituted-4-phenoxyquinoline derivatives as potential antitumor agents. Bioorg. Chem. 2014, 57, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Gupta, H. Biological activities of pyrazoline derivatives—A recent development. Drug Discov. 2009, 4, 154–163. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Gzella, A.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.M.; Al-Omar, M.A.; Amr, A.E.; Baiuomy, A.R.; Abdel Rahman, R.F. Synthesis and biological evaluation of some novel fused thiazolo[3,2-a]pyrimidines as potential analgesic and antiinflammatory agents. Russ. J. Bioorg. Chem. 2015, 41, 192–201. [Google Scholar] [CrossRef]
- Eweas, A.F.; Khalifa, N.M.; Ismail, N.S.; Al-Omar, M.A.; Soliman, A.M. Synthesis, molecular docking of novel 1,8-naphthyridine derivatives and their cytotoxic activity against HepG2 cell lines. Med Chem Res. 2014, 23, 76–86. [Google Scholar] [CrossRef]
- Almutairi, M.S.; Hegazy, G.H.; Haiba, M.E.; Ali, H.I.; Khalifa, N.M.; Soliman, A.M. Synthesis, Docking and Biological Activities of Novel Hybrids Celecoxib and Anthraquinone Analogs as Potent Cytotoxic Agents. Int. J. Mol. Sci. 2014, 15, 22580–22603. [Google Scholar] [CrossRef] [PubMed]
- Haiba, M.E.; Al-Abdullah, E.S.; Edrees, M.M.; Khalifa, N.M. Synthesis and characterization of some substituted 3,4-dihydronaphthalene derivatives through different enaminones as potent cytotoxic agents. Drug Res. 2015, 65, 9–17. [Google Scholar]
- Babasaheb, P.B.; Shrikant, S.G.; Ragini, G.B.; Jalinder, V.T.; Chandrahas, N.K. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, P.A.; Hursey, M.L.; Fine, M.J.; Czerwinski, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar] [PubMed]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).