Abstract
Seven metabolites of 2′,3′,5′-tri-O-acetyl-N6-(3-hydroxyphenyl) adenosine (WS070117) were synthesized by deacetylation, hydrolysis, cyclization, sulfonylation and glycosylation reactions, respectively. All these compounds, which could be useful as material standards for metabolic research, were characterized by NMR and HPLC-MS (ESI) analyses.
1. Introduction
2′,3′,5′-Tri-O-acetyl-N6-(3-hydroxyphenyl) adenosine (also known as WS070117) is a new adenosine analog anti-hyperlipidemic drug candidate currently in many preclinical studies [1,2,3,4,5]. Guo and coworkers have investigated and elucidated the in vivo metabolites of WS070117 in rat urine after oral administration of WS070117 by HPLC-DAD, ESI-MS and Off-Line Microprobe NMR [6]. In the study, seven metabolites of WS070117 were observed in the HPLC trace (the metabolites are ranked M2–M8 according to the retention time; Figure 1). The structure elucidation results unambiguously revealed that there are two phase I metabolites, including a deacetylation product of WS070117 (M8) and an adenine derivative formed by the loss of ribofuranose (M7). In addition, there are five phase II metabolites: M6 is the oxidation product of M7 at C-8; M2 and M4 are the glycosylation products of M7 and M8 on the phenolic hydroxyl groups, respectively; M3 and M5 are the sulfonylation products of M6 and M8, respectively. Herein, the synthesis of the above metabolites was carried out to provide metabolites material standards for preclinical pharmacokinetic studies.
2. Results and Discussion
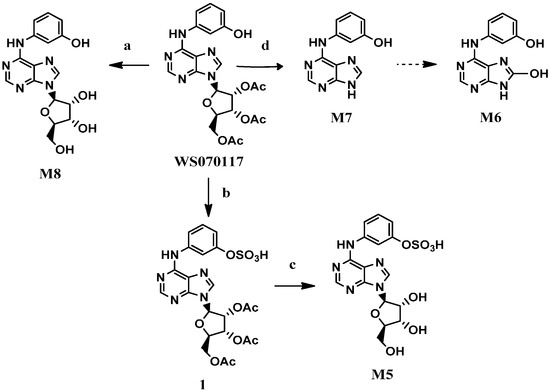
The metabolite M8 was prepared in quantitative yield by the hydrolysis of the acetyl groups in WS070117 with NaOH [7] (Scheme 1). Treatment of WS070117 with chlorosulfonic acid [8] afforded the corresponding sulphonate 1, which was converted to the desired product M5 by subsequent deacetylation with Na2CO3. Direct hydrolysis of the glycosidic bond in WS070117 with concentrated hydrochloric acid and 95% ethanol solution at reflux smoothly afforded M7.
We initially attempted to synthesize M6 by bromination and hydrolysis reactions at C-8 of M7 [9], but liquid bromine and NaH did not afford the needed C8-brominated adenine. Then 4,5-diamino-6-chloropyrimidine 2 and N,N′-carbonyldiimidazole (CDI) were employed to synthesize 8-hydroxy-6-chloroadenine (3) [10], which could be ammoniated at C-6 to prepare M6 (Scheme 2). The amination was catalyzed by hydrochloric acid to afford the desired product in 70% yield at 120 °C in a microwave reactor. Subsequent sulfonylation of M6 with sulfur trioxide-pyridine complex gave the target metabolite M3.
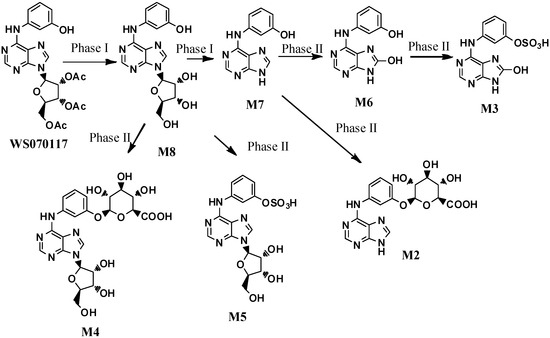
Figure 1.
The structures of the WS070117 metabolites in rat urine.
Figure 1.
The structures of the WS070117 metabolites in rat urine.
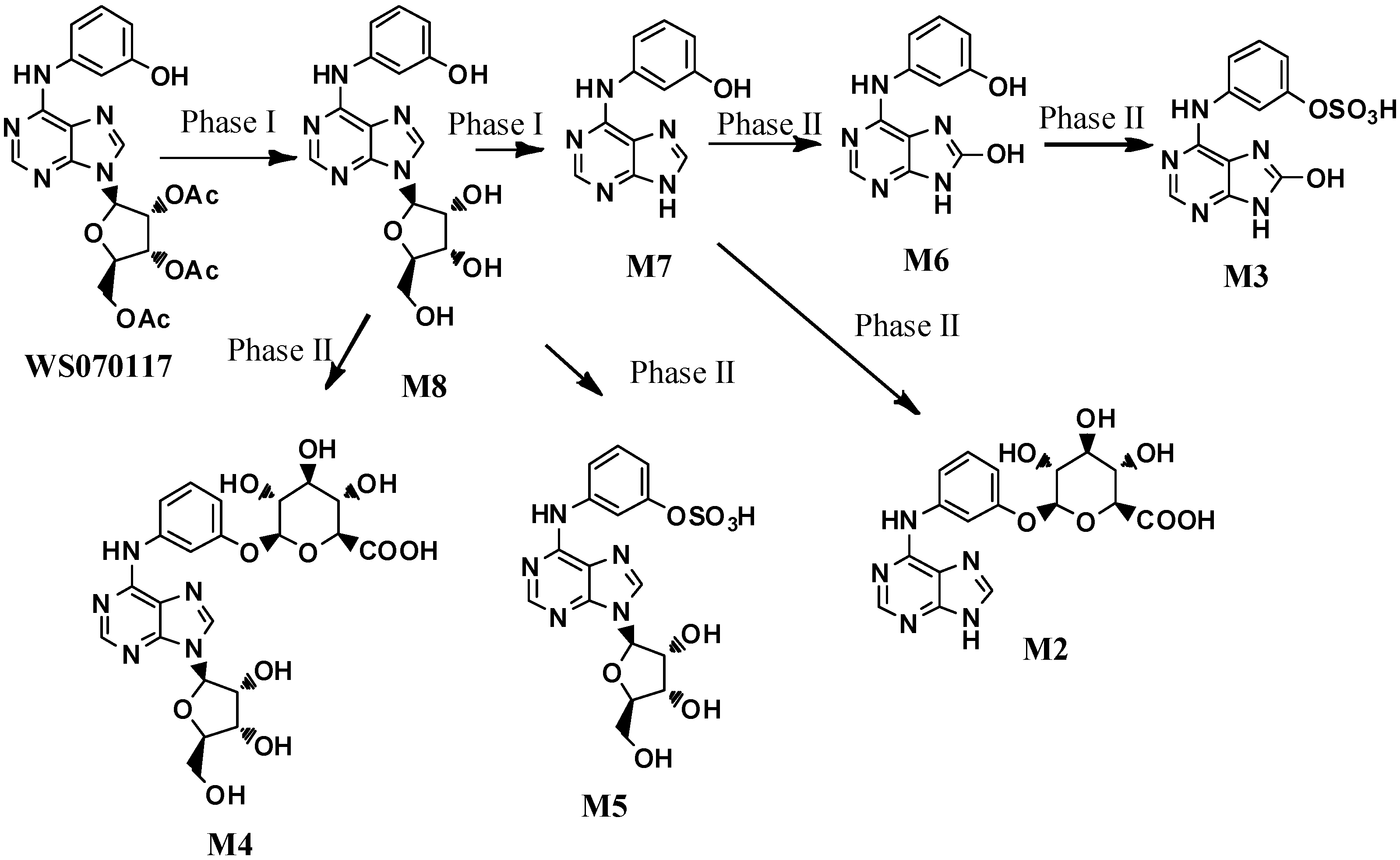
Scheme 1.
The synthesis of M5, M7 and M8.
Scheme 1.
The synthesis of M5, M7 and M8.
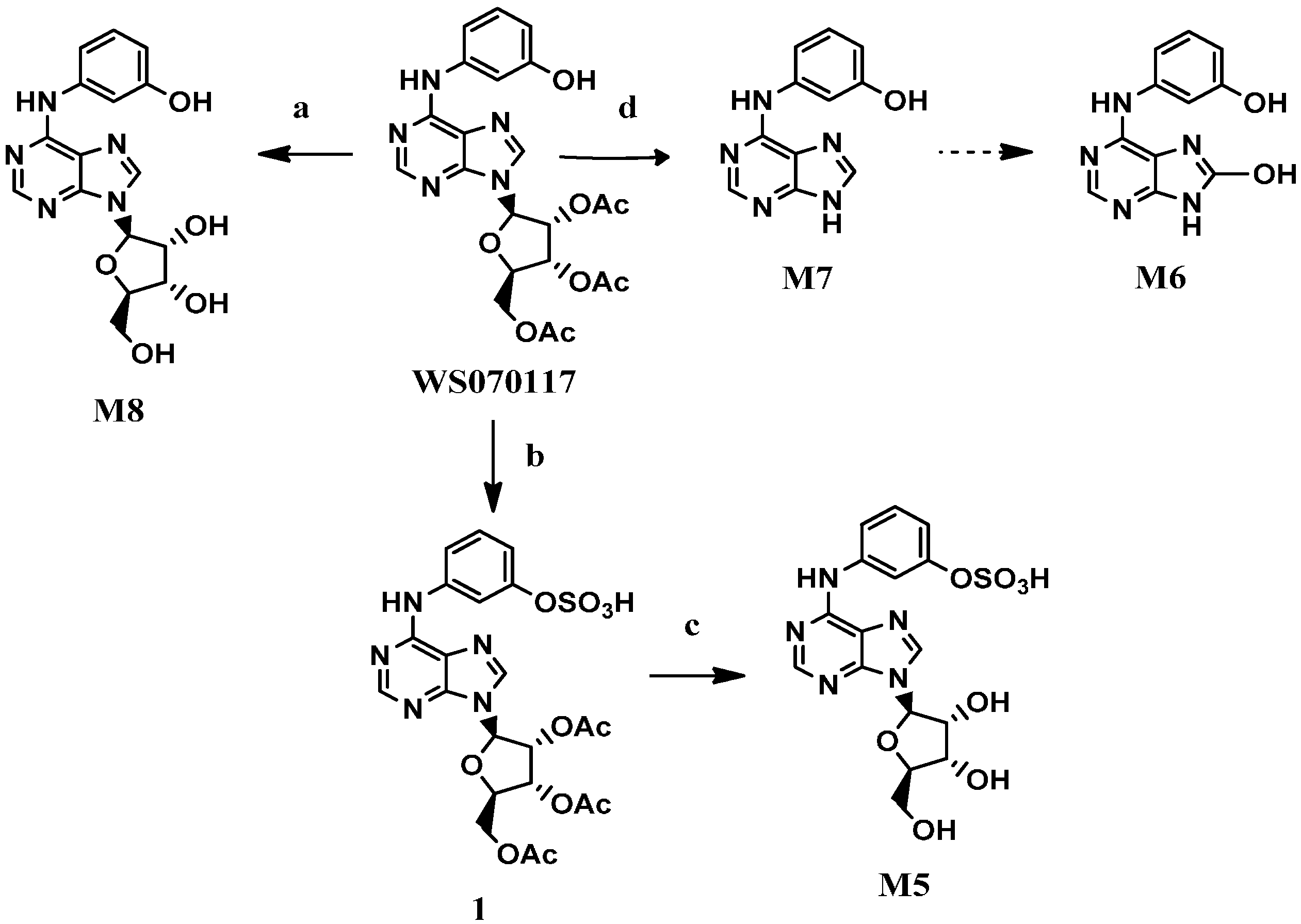
Reagents and conditions: (a) NaOH, H2O, 2 h, 99%; (b) HSO3Cl, pyridine, r.t., 12 h; (c) Na2CO3, H2O, 12 h, 66.3% for two steps; (d) HCl/95%EtOH, reflux,1 h, 92.5%.
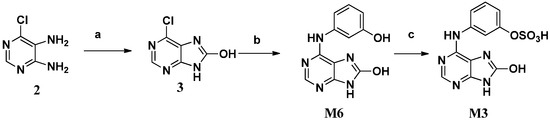
Scheme 2.
The synthesis of M3 and M6.
Scheme 2.
The synthesis of M3 and M6.
Reagents and conditions: (a) CDI, dioxane, reflux, 6 h; (b) 3-aminophenol, HCl(aq), ethanol, MW 120 °C, 4 h, 70%; (c) SO3·pyr., pyridine, DMF, 24 h, 67.2%
Initially, the glycosylation reaction with tetra-O-acetyl-β-d-glucopyranuronic acid methyl ester as the donor and WS070117 as the receptor was catalyzed by SnCl4 to synthesize M4, but the yield of the desired product was poor, with the formation of a complex mixture of by-products. The Koenigs–Knorr glycosylation reaction with acetobromo-α-d-glucuronic acid methyl ester as the donor was then employed, but Ag2O failed to catalyze the reaction even at two equivalents, which may be due to its complexation with the nitrogen-atoms. Afterwards the Schmitt glycosylation reaction was tried with trichloroacetimidate as donor (Scheme 3). A series of conditions for selective anomeric deacetylation of 4 were investigated, including benzylamine/DMF [11], FeCl3·6H2O/CH3CN [12] and Nd(OTf)3/CH3OH [13]. The results showed that Nd(OTf)3/CH3OH gave a cleaner reaction in quantitative yield and the product could be used directly in the next step without purification. Under the catalysis of BF3·Et2O, trichloroacetimidate 6 reacted smoothly with WS070117 to give the desired β-glucoside 7 due the neighboring group participation effect. After deacetylation with Cs2CO3, M4 was obtained in 83.3% yield. However, the glycosylation reaction of M7 under the same conditions only afforded the desired product M2 with a 25% conversion because of the poor solubility of the raw material in dichloromethane. An alternate route such as selective hydrolysis of the N-glycoside bond of M4 with aqueous hydrochloric acid solution afforded M2 in moderate yield.
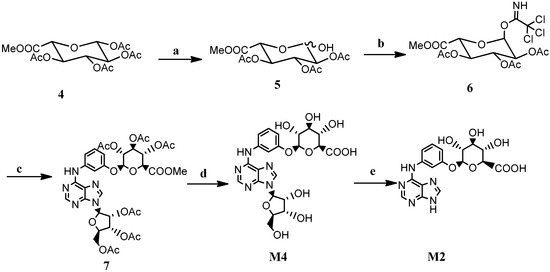
Scheme 3.
The synthesis of M4 and M2.
Scheme 3.
The synthesis of M4 and M2.
Reagents and conditions: (a) Nd(OTf)3/CH3OH, reflux, 6 h; (b) Cl3CCN/DBU/CH2Cl2, 0 °C, 2 h; (c) WS070117, BF3·Et2O, CH2Cl2, 0 °C,12 h, 86.7%; (d) Cs2CO3/MeOH/H2O, 40°C, 0.5 h, 83.3%; (e) HCl/H2O, reflux, 74.1%.
3. Materials and Methods
3.1. General Information
WS070117 was prepared in our laboratory in 98% purity. Unless otherwise indicated, all commercial reagents and solvents were used without additional purification. 1H-NMR and 13C-NMR spectra were recorded on a Mercury-300M (Varian, Salt Lake City, UT, USA,) or Bruker 400M (Varian) spectrometer. Chemical shifts (in ppm) were referenced to tetramethylsilane (δ = 0) in deuterated solvent as internal standard. HPLC-mass spectra (ESI) were recorded on an Acquity UPLC-MS system (Waters, Milford, MA, UK). Optical rotations were recorded on p-2000 polarimeter (Jasco, Tokyo, Japan) at 20 °C in 589 nm.
3.2. Synthesis of N6-(3-Hydroxyphenyl) Adenosine (M8)
NaOH (2.1 g, 52.5 mmol) was added to a stirred suspension of WS070117 (8.0 g, 16.5 mmol) in H2O (50 mL), and the mixture was stirred at room temperature for 2 h until the disappearance of the solid. The solution was cooled to 5 °C and the precipitated solid was filtered and washed with water to give M8 (5.90 g, 99%) as a white solid, = −40.7 (c = 0.4, H2O), ESI/MS: 360.15 [M + H]+, 1H-NMR (300 MHz, methanol-d4, see Supplementary Materials) δ 8.34 (d, J = 5.0 Hz, 2H), 7.27 (t, J = 2.1 Hz, 1H), 7.15–6.99 (m, 2H), 6.52 (dt, J = 7.5, 1.8 Hz, 1H), 6.00 (d, J = 6.3 Hz, 1H), 4.77 (dd, J = 6.3, 5.1 Hz, 1H), 4.34 (dd, J = 5.1, 2.6 Hz, 1H), 4.19 (q, J = 2.6 Hz, 1H), 3.90 (dd, J = 12.6, 2.6 Hz, 1H), 3.76 (dd, J = 12.5, 2.8 Hz, 1H). 13C-NMR (100 MHz, methanol-d4) δ 161.6, 156.5, 155.8, 152.5, 151.9, 144.8, 143.9, 133.1, 115.6, 114.4, 111.6, 93.9, 90.7, 78.1, 75.2, 66.0.
3.3. Synthesis of N6-(3-O-Sulfophenyl) Adenosine (M5)
Chlorosulfonic acid (300 μL, 4.6 mmol) was added dropwise (carefully!) into a stirred solution of WS070117 (130 mg, 0.27 mmol) in dry pyridine (3 mL) at 0 °C under N2. The solution was warmed to room temperature and stirred for 12 h. Na2CO3 (aq, 0.5 M, 20 mL) was then added to adjust the pH to 10. The reaction mixture was stirred for another 12 h at room temperature, and the solvent was removed in vacuo and the remaining solid was purified by Sephadex LH-20 chromatography eluting with water to give M5 (78 mg, 66.3%) as a white solid, = −33.5 (c = 0.15, H2O), ESI/MS: 440.10 [M + H]+, 1H-NMR (300 MHz, D2O, see Supplementary Materials) δ 8.28 (s, 1H), 8.21 (s, 1H), 7.50 (t, J = 3.8 Hz, 1H), 7.40 (t, J = 5.7 Hz, 2H), 7.10 (p, J = 7.7 Hz, 1H), 6.03 (dd, J = 5.9, 2.6 Hz, 1H), 4.76–4.67 (m, 1H), 4.41 (dd, J = 5.4, 3.7 Hz, 1H), 4.28 (dq, J = 6.2, 3.4 Hz, 1H), 3.93 (dd, J = 12.9, 2.94 Hz, 1H), 3.83 (dd, J = 12.9, 3.7 Hz, 1H). 13C-NMR (100 MHz, D2O) δ180.2, 152.0, 151.5, 148.4, 140.9, 138.6, 130.1, 120.1, 117.7, 115.4, 88.5, 85.8, 73.9, 70.7, 68.0, 61.5.
3.4. Synthesis of N6-(3-Hydroxyphenyl) Adenine (M7)
Concentrated hydrochloric acid (5 mL) was added into a stirred solution of WS070117 (4.85 g, 10 mmol) in 95% ethanol (100 mL). The reaction was refluxed for 1 h and then cooled to room temperature. The precipitated solid was filtered and washed with water to give M7 (2.1 g, 92.5%) as a white solid, ESI/MS: 228.05 [M + H]+, 1H-NMR (300 MHz, DMSO-d6, see Supplementary Materials) δ11.22 (s, 1H), 9.61 (brs, 1H), 8.74 (s, 1H), 8.70 (s, 1H), 7.42 (s, 1H), 7.31 (d, J = 10.4 Hz, 1H), 7.20 (t, J = 8.0 Hz, 1H), 6.62 (d, J = 8.0 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 158.3, 150.0, 148.9, 148.8, 143.8, 139.1, 130.1, 113.3, 112.6, 112.6, 109.0.
3.5. Synthesis of 8-Hydroxy-N6-(3-hydroxy-phenyl) Adenine (M6)
N,N′-Carbonyldiimidazole (CDI, 1.6 g, 10 mmol) was added into a stirred solution of 4,5-diamino-6-chloropyrimidine (2, 0.76 g, 5.23 mmol) in 1,4-dioxane. The reaction mixture was refluxed for 6 h and then cooled to room temperature. The precipitated solid was filtered and washed with water to give 8-hydroxy-6-chloroadenine (3, 700 mg). Concentrated hydrochloric acid (1 mL) was then added into a stirred solution of intermediate 3 (0.7 g, 4.12 mmol) and 3-aminophenol (0.7 g, 6.42 mmol) in ethanol (20 mL) in a sealed tube, which was irradiated in a microwave reactor at 120 °C for 4 h and then cooled to room temperature. The precipitating solid was filtered and washed with water to give M6 (0.9g, 70%) as a white solid, ESI/MS: 244.07 [M + H]+, 1H-NMR (400 MHz, DMSO-d6, see Supplementary Materials) δ 11.69 (s, 1H), 10.80 (s, 1H), 9.43 (s, 1H), 8.25 (s, 1H), 7.33 (s, 1H), 7.22–7.10 (m, 2H), 6.48 (d, J = 4.0 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 158.2, 152.9, 149.5, 148.1, 142.5, 140.9, 129.9, 110.8, 110.2, 107.1, 106.3.
3.6. Synthesis of 8-Hydroxy-N6-(3-O-sulfophenyl) Adenine (M3)
SO3·Pyr complex (9.54 g, 60 mmol) was added into a stirred solution of M6 (2.27 g, 9.3 mmol) in pyridine (50 mL) and DMF (50 mL) at room temperature. After 24 h, acetone (200 mL) was added and the precipitated solid was filtered and washed with water to give M3 (2.01 g, 67.2%) as a white solid, 1H-NMR (300 MHz, DMSO-d6) δ 8.84 (s), 8.20 (s, 1H), 7.61 (dd, J = 8.2, 2.3 Hz, 1H), 7.51 (dd, J = 2.3, 2.2 Hz, 1H), 7.18 (dd, J = 8.2 Hz, 1H), 6.77 (dd, J = 8.2, 2.2 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 154.3, 153.5, 150.7, 149.4, 142.4, 141.2, 129.3, 114.5, 114.7, 111.4, 106.3.
3.7. Synthesis of Intermediate 7
Nd(OTf)3 (0.95 g, 1.6 mmol) was added into a stirred solution of tetra-O-acetyl-β-d-glucopyranuronic acid methyl ester (4, 6.0 g, 16 mmol) in methanol (200 mL). The solution was refluxed for 6 h and cooled to room temperature. The solution was then concentrated in vacuo and the residue was dissolved with CH2Cl2 (30 mL). The organic layer was washed with water, dried with Na2SO4 and concentrated in vacuo after filtration to give an orange oil of intermediate 5. Next DBU (0.75 mL, 5 mmol) was added dropwise into a stirred solution of the above product and Cl3CCN (8 mL) in CH2Cl2 at 0 °C. The mixture was stirred for 2 h and concentrated. The residue was purified by column chromatography (petroleum ether–ethyl acetate = 3:1) to give intermediate 6 (3.45 g).
BF3·Et2O (1.5 mL, 15.3 mmol) was added dropwise into a stirred mixture of WS070117 (3.64 g, 7.50 mmol), intermediate 6 (5.4 g, 11.3 mmol) and 4 Å molecular sieves in CH2Cl2 (200 mL) at 0 °C. The mixture was stirred for 12 h and neutralized with 2 mL Et3N. The solution was concentrated in vacuo after filtration and the residue was purified by column chromatography (CH2Cl2–MeOH = 100:1) to give intermediate 7 (5.2 g, 86.7%). ESI/MS:802.53 [M + 1]+, 1H-NMR (300 MHz, DMSO-d6, see Supplementary Materials) δ: 10.07 (s, 1H), 8.56 (s, 1H), 8.44 (s, 1H), 7.80 (s, 1H), 7.65 (d, J = 8.2 Hz, 1H), 7.28 (t, J = 8.2 Hz, 1H), 6.71 (d, J = 9.8 Hz, 1H), 6.29 (d, J = 5.2 Hz, 1H), 6.06 (t, J = 5.6 Hz, 1H), 5.66 (dd, J = 10.7 Hz, 6.7 Hz, 2H), 5.52 (t, J = 9.6 Hz, 1H), 5.09 (m, 2H), 4.71 (d, J = 9.9 Hz, 1H), 4.43 (m, 2H), 4.28 (m, 1H), 3.64 (s, 3H), 2.13 (s, 3H), 2.03 (m, 15H).
3.8. Synthesis of N6-(3-O-β-d-Glucuronyphenyl) Adenosine (M4)
Cs2CO3 (2.5 g, 7.7 mmol) was added into a stirred solution of intermediate 7 (4.5 g, 5.6 mmol) in methanol (20 mL). The mixture was stirred at 40 °C for 0.5 h and then cooled to room temperature. The precipitated solid was filtered, redissolved with water (2 mL), and was then cation exchange resin was added to adjust the pH to 6–7. The solution was concentrated in vacuo after filtration to give M4 (2.5 g, 83.3%) as a white solid, = −51.2 (c = 0.1, H2O), ESI/MS:536.40 [M + H]+, 1H-NMR (400 MHz, methanol-d4, see Supplementary Materials) δ 8.42 (s, 1H), 8.38 (s, 1H), 7.95 (s, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.28 (t, J = 8.3 Hz, 1H), 6.84 (d, J = 8.2 Hz, 1H), 6.01 (d, J = 6.0 Hz, 1H), 5.01 (d, J = 6.3 Hz, 1H), 4.77 (t, J = 5.7 Hz, 1H), 4.34 (s, 1H), 4.18 (s, 1H), 4.02 (d, J = 10.0 Hz, 1H), 3.90 (d, J = 12.6 Hz, 1H), 3.76 (d, J = 12.5 Hz, 1H), 3.62 (d, J = 9.5 Hz, 1H), 3.53 (m, 2H). 13C-NMR (100 MHz, methanol-d4) δ 170.9, 158.0, 152.4, 151.8, 148.6, 140.9, 140.1, 129.1, 120.7, 114.2, 111.5, 108.8, 101.1, 89.8, 86.7, 76.0, 75.3, 74.1, 73.1, 71.7, 71.2, 62.0.
3.9. Synthesis of N6-(3-O-β-d-Glucuronyphenyl) Adenine (M2)
Concentrated hydrochloric acid (1 mL) was added into a stirred solution of M4 (1.6 g, 3 mmol) in water (10 mL). The reaction mixture was refluxed for 1 h and then cooled to room temperature. The precipitated solid was filtered and washed with water to give M2 (0.89 g, 74.1%) as a white solid, = −139.8 (c = 0.08, H2O), ESI/MS: 404.33 [M + H]+, 1H-NMR (400 MHz, DMSO-d6, see Supplementary Materials) δ 9.95 (brs, 1H), 8.42 (s, 1H), 8.27 (s, 1H), 7.89 (s, 1H), 7.65 (d, J = 7.2 Hz, 1H), 7.23 (t, J = 8.1 Hz, 1H), 6.70 (d, J = 7.9 Hz, 1H), 5.00 (d, J = 7.1 Hz, 1H), 3.88 (d, J = 9.0 Hz, 1H), 3.34 (m, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 170.6, 157.7, 152.0, 151.5, 141.4, 141.1, 129.6, 114.4, 110.1, 108.7, 100.5, 76.4, 76.1, 73.4, 71.9.
4. Conclusions
Seven metabolites of 2′,3′,5′-tri-O-acetyl-N6-(3-hydroxyphenyl) adenosine (WS070117) were successfully synthesized by deacetylation, hydrolysis, cyclization, sulfonylation and glycosylation reactions. All these compounds were characterized by NMR and HPLC-MS (MS) analyses, and the purities (HPLC) are all higher than 98%, so they could be used as material standards for metabolic research on WS070117.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/1/8/s1.
Acknowledgments
We thank National Major Scientific and Technological Special Project for “Significant New Drugs Innovation” (No. 2012ZX09301002-002).
Author Contributions
Study design, experimental work and writing of first draft were performed by Wen-Xuan Zhang. Hong-Na Wu, Bo Li and Hong-Lin Wu participated in experimental work and revision of the first draft of paper. DongMei Wang and Song Wu participated in the revision of the manuscript and gave good suggestions. All authors reviewed and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hao, L.H.; Qu, K.; Xiong, L.; Wei, J.Z.; Zhu, H.B.; Wu, S. Synthesis and hypolipidemic activity of O5,N6-disubstituted adenosine derivative. Chin. J. Med. Chem. 2011, 21, 7–11. [Google Scholar]
- Huang, L.Z.; Fan, B.Y.; Ma, A.; Shaul, P.W.; Zhu, H.B. Inhibition of ATP-Binding Cassette Transporter A1 Protein Degradation Promotes HDL Cholesterol Efflux Capacity and Reverse Cholesterol Transport and Reduces Atherosclerosis in Mice. J. Lipid Res. 2015. [Google Scholar] [CrossRef]
- Guo, P.; Lian, Z.Q.; Sheng, L.H.; Wu, C.M.; Gao, J.; Li, J.; Wang, Y.; Guo, Y.S.; Zhu, H.B. The adenosine derivative 2′,3′,5′-tri-O-acetyl-N6-(3-hydroxylaniline) adenosine activates AMPK and regulates lipid metabolism in vitro and in vivo. Life Sci. 2012, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.Q.; Li, Y.; Gao, J.; Qu, K.; Li, J.; Hao, L.; Wu, S.; Zhu, H.B. A novel AMPK activator, WS070117, improves lipid metabolism discords in hamsters and HepG2 cells. Lipids Health Dis. 2011, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.M.; Abliz, Z.; Zhu, H.B. In vitro stability and metabolism of O2′,O3′,O5′-tri-acetyl-N6-(3-hydroxylaniline) adenosine in rat, dog and human plasma: Chemical hydrolysis and role of plasma esterases. Xenobiotica 2011, 41, 549–560. [Google Scholar] [PubMed]
- Guo, W.; Jin, M.; Miao, Z.; Qu, K.; Liu, X.; Zhang, P.; Qin, H.; Zhu, H.; Wang, Y. Structure Elucidation of the Metabolites of 2′,3′,5′-Tri-O-Acetyl-N6-(3-Hydroxyphenyl) Adenosine in Rat Urine by HPLC-DAD, ESI-MS and Off-Line Microprobe NMR. PLoS ONE 2015, 10, e0127583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Li, B.; Wu, H.N.; Wang, D.M.; Wu, S. Synthesis of impurities from novel cholesterol-lowering drug candidate WS070117. Chin. J. New Drugs 2015, 24, 1166–1170. [Google Scholar]
- Soidinsalo, O.; Wähälä, K. Synthesis of daidzein 7-O-β-d-glucuronide-4-O-sulfate. Steroids 2007, 72, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Brill, W.K.D.; Riva-Toniolo, C. The bromination of purines with a charge transfer complex between bromine and lutidine. Tetrahedron Lett. 2001, 42, 6279–6282. [Google Scholar] [CrossRef]
- Shepherd, T.A.; Dally, R.D.; Joseph, S. Preparation of Pyrrolopyrimidinone Derivatives as AKT and p70S6 Kinase Inhibitors. U.S. Patent 2010012080, 2009. [Google Scholar]
- Chen, Y.; Li, Y.; Yu, H.; Sugiarto, G.; Thon, V.; Hwang, J.; Ding, L.; Hie, L.; Chen, X. Tailored Design and Synthesis of Heparan Sulfate Oligosaccharide Analogues Using Sequential One-Pot Multienzyme Systems. Angew. Chem. 2013, 125, 12068–12072. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, G.D.; Gong, S.S.; Sun, Q. Microwave-Assisted FeCl3·6H2O-Catalyzed Regioselective Deprotection of Pyranose Anomeric O-Acetyl Group. Adv. Mater. Res. 2014, 830, 155–158. [Google Scholar] [CrossRef]
- Tran, A.T.; Deydier, S.; Bonnaffé, D.; Le Narvor, C. Regioselective green anomeric deacetylation catalyzed by lanthanide triflates. Tetrahedron Lett. 2008, 49, 2163–2165. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
