Abstract
Three types of ent-kaurane diterpenoids were isolated from the aerial parts of Isodon excisoides, including three new diterpenoids, 1α,7α,14β-trihydroxy-20-acetoxy-ent-kaur-15-one (1); 1α,7α,14β,18-tetrahydroxy-20-acetoxy-ent-kaur-15-one (2); and 1α-acetoxy-14β-hydroxy-7α,20-epoxy-ent-kaur-16-en-15-one (3); together with six known diterpenes henryin (4); kamebanin (5); reniformin C (6); kamebacetal A (7); kamebacetal B (8); and oridonin (9). The structures of the isolated compounds were elucidated by means of nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry in conjunction with published data for their analogs, as well as their fragmentation patterns. Compounds 5 and 9 were isolated from Isodon excisoides for the first time. To explore the structure-activity relationships of the isolated compounds, they were tested for their cytotoxic effects against five human cancer cell lines: HCT-116, HepG2, A2780, NCI-H1650, and BGC-823. Most of the isolated compounds showed certain cytotoxic activity against the five cancer cell lines with IC50 values ranging from 1.09–8.53 µM. Among the tested compounds, compound 4 exhibited the strongest cytotoxic activity in the tested cell lines, with IC50 values ranging from 1.31–2.07 µM. Compounds 1, 6, and 7 exhibited selective cytotoxic activity.
1. Introduction
The genus Isodon (Lamiaceae) includes approximately 100 species of wild plants, of which 80 species and 25 varieties are found in China. About 30 species are used to treat esophageal cancer, rheumatism and chronic pharyngitis in Chinese folk medicine [1,2]. Diterpenoids are the primary bioactive constituents of Isodon plants. Due to their complex, fast-changing carbon skeleton and multiple pharmacological activities, diterpenoids as anti-cancer drug candidates have gained attention [3,4,5,6,7,8,9,10,11]. To date, approximately 600 diterpenoids are known to exhibit significant cytotoxicity. Cyclopentanone conjugated with an exomethylene moiety has been shown to be necessary for diterpenoid cytotoxicity [12,13]. Currently, a large number of diterpenoids have been developed into anti-cancer drugs such as oridonin, eriocalyxin A, and rabdophyllin G. Furthermore, the extracts and effective fractions enriched in diterpenoids are also approved into the market as drugs, such as Dong-ling-cao tablets (Isodon rubescens), Xiao-yan-lidan tablets (containing Isodon lophanthoides var. graciliflorus, as one of three ingredients), and Weifuchun tablets (containing Isodon amethystoides, as the main ingredient) [12,13]. Isodon excisoides (Lamiaceae) is a perennial medicinal herb widely distributed in the western region of Henan and Yunnan Provinces in China [11,14]. The aerial parts of I. excisoides are used to treat esophageal cancer by local people [14]. The air-dried leaves are collected and boiled in water to make tea that is consumed several times per day as a treatment for esophageal cancer. Although the chemical constituents of Isodon plants have been extensively studied, only approximately 20 compounds have been isolated from I. excisoides and its active anti-cancer constituents remain unidentified [3,15,16]. In order to search for new anti-tumor compounds and clarify the therapeutic basis of the anti-cancer effects of I. excisoides, we carried out a systematic phytochemical investigation on I. excisoides. Three new diterpenoids 1α,7α,14β-trihydroxy-20-acetoxy-ent-kaur-15-one (1); 1α,7α,14β,18-tetrahydroxy-20-acetoxy-ent-kaur-15-one (2); and 1α-acetoxy-14β-hydroxy-7α,20-epoxy-ent-kaur-16-en-15-one (3); together with six known diterpenoids, namely henryin (4); kamebanin (5); reniformin C (6); kamebacetal A (7); kamebacetal B (8); and oridonin (9), were isolated from the water extract of the aerial part of I. excisoides.(Figure 1). Furthermore, to explore the structure-activity relationships of the isolated compounds, the cytotoxic activities of the isolated diterpenoids were tested against five human cancer cell lines: HCT-116, A2780, NCI-H1650, BGC-823, and HepG2. Although some of the compounds isolated in our study are not new entities, to the best of our knowledge they were subjected to a consistent cytotoxic screening for the first time. In this experiment, we found that the 7,20-epoxy moiety may be associated with reduced cytotoxic activity.
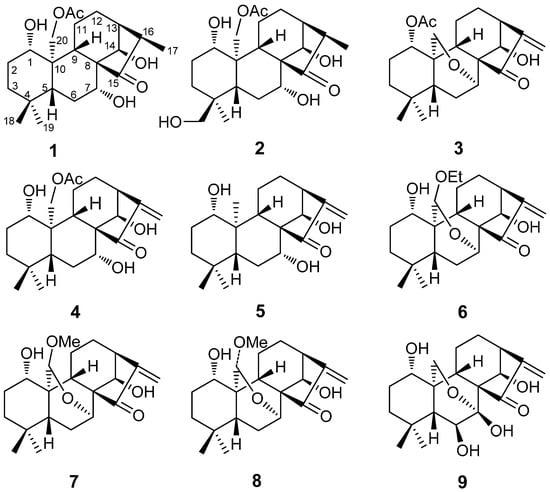
Figure 1.
Structures of compounds 1–9.
2. Results and Discussion
2.1. Structure Elucidation
Compound 1 was obtained as white, needle-like crystals. The molecular formula of 1 was determined to be C22H34O6 on the basis of positive HR-ESI-MS at m/z 417.22367 [M + Na]+ (calcd for C22H34O6Na+, 417.22467). Its IR spectrum displayed the absorption bands of hydroxyl group (3422 cm−1) and free carbonyl group (1731 cm−1). The NMR spectra revealed three methyls (δC 9.2, 33.3, 21.5(each q); δH 1.12 (3H, d, 7.1 Hz), 0.91 (3H, s), 0.85 (3H, s)), one acetoxyl group (δC 170.8(s), 21.5(q); δH 2.13(3H, s)), one ketone carbonyl group (δC 222.8), three oxy-methines (δC 81.6, 76.1, 75.4) and one oxy-methylene (δC 63.7). Considering the diterpenoids previously isolated from the plant, 1 was tentatively presumed to be a 7,20-non-epoxy-ent-kaurane skeleton, substituted with three hydroxyl groups and one acetoxyl group.
The 1H- and 13C-NMR data of 1 were nearly identical with that of a known diterpene henryin (4) (Table 1) [17], and their only difference was in the moiety at C-16. For 1, the methyl signal at δH 1.12 (3H, d, J = 7.2 Hz) has correlations with C-13 (δC 42.4) and C-15 (δC 222.8) in the HMBC spectrum (Figure 2), revealing that the exo-methylene at C-16 in 4 had been replaced by a methyl at C-16 in 2.

Table 1.
1H- and 13C-NMR data of compounds 1–3 (500 and 125 MHz δ in ppm).
| No. | 1 (In CDCl3) | 2 (In DMSO-d6 and D2O) | 3 (In CDCl3) | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | β 3.31, dd, (11.0, 4.6) | 81.6 | β 3.09, dd, (10.8, 4.5) | 80.5 | β 4.57, dd, (11.2, 5.3) | 76.6 |
| 2 | α 1.82, m | 30.5 | α 1.63, overlapped | 29.2 | α 1.69, overlapped | 25.1 |
| β 1.65, overlapped | β 1.53, overlapped | β 1.47, m | ||||
| 3 | α 1.47, dt, (13.8, 3.7) | 39.4 | α 1.53, overlapped | 32.9 | α 1.44, m | 37.9 |
| β 1.29, dt, (13.8, 4.4) | β 1.05, m | β 1.24, m | ||||
| 4 | – | 32.9 | – | 36.6 | – | 33.6 |
| 5 | β 0.98, dd, (11.6, 3.5) | 52.5 | β 1.21, d, (12.2) | 42.0 | β 1.33, ddd, (11.8, 7.4, 1.5) | 47.1 |
| 6 | 1.93, m | 28.6 | β 1.80, d, (12.2) | 28.6 | β 2.85, ddd, (14.0, 11.8, 1.9) | 25.1 |
| α 1.63, overlapped | α 1.84, overlapped | |||||
| 7 | β 4.20, dd (11.4, 6.0) | 75.4 | β 3.77, dd, (11.8, 4.3) | 73.8 | β 3.95, dd, (3.8, 1.9) | 64.5 |
| 8 | – | 61.0 | – | 59.8 | – | 58.7 |
| 9 | β 1.55, d, (8.8) | 55.5 | β 1.37, d, (8.6) | 55.7 | β 1.69, overlapped | 50.8 |
| 10 | – | 46.0 | – | 44.1 | – | 39.2 |
| 11 | α 2.77, q, (6.1) | 19.9 | α 2.82, dd, (15.9, 5.6) | 19.3 | α 1.84, overlapped | 17.8 |
| β 1.20, m | β 0.98, m | β 1.15, q, (6.5) | ||||
| 12 | α 1.88, m | 24.2 | α 1.63, overlapped | 24.3 | α 2.42, dt, (14.1, 9.0) | 30.9 |
| β 1.65, overlapped | β 1.53, overlapped | β 1.54, m | ||||
| 13 | α 2.42, m | 42.4 | α 2.24, m | 42.5 | α 2.99, br d, (9.9) | 42.0 |
| 14 | α 4.82, d, (1.1) | 76.1 | α 4.72, br. s | 75.2 | α 4.61, br s | 71.5 |
| 15 | – | 222.8 | – | 221.2 | – | 204.5 |
| 16 | 2.88, m | 43.2 | 2.67, m | 44.6 | – | 151.1 |
| 17 | 1.12, d, (7.1) | 9.2 | 1.00, d, (7.1) | 9.1 | 6.00, br s; 5.38, br.s | 117.5 |
| 18 | 0.91, s | 33.3 | 3.19, d, (10.6) | 69.5 | 0.88, s | 31.4 |
| 2.84, d, (10.6) | ||||||
| 19 | 0.85, s | 21.5 | 0.62, s | 17.3 | 1.08, s | 20.3 |
| 20 | α 4.74, d, (13.2) | 63.7 | α 4.35, d, (13.3) | 64.1 | α 4.09, dd, (10.3, 1.5) | 61.0 |
| β 4.37, d, (13.2) | β 4.28, d, (13.3) | β 4.03, dd, (10.3, 1.6) | ||||
| OAc | – | 170.8 | – | 170.2 | – | 170.1 |
| OAc | 2.13, s | 21.5 | 2.07, s | 21.2 | 1.95, s | 21.5 |
In the HMBC spectrum, correlations between H-1 (δ 3.31) with C-5 (δ 52.5), C-9 (δ 55.5) and C-20 (δ 63.7), H-7 (δ 4.20) with C-14 (δC 76.1), H-14 (δ 4.82) with C-12 (δC 24.2), and C-15 (δC 222.8), indicated that the hydroxy groups were at C-1, C-7, and C-14, respectively. Moreover, the acetoxyl group was assigned at C-20 based on the correlation of H-20 (δH 4.74 and 4.37) with the carbonyl at δC 170.8 (–OCOCH3) in the HMBC spectrum (Figure 2).
The relative configuration of the substituents was revealed by the ROESY spectrum, in which the correlations of H-1 with H-5 and H-9, Me-18 with H-5, H-7 with H-5 and H-9, and H-13 with H-14 and H-16, indicated that they were positioned on the same side, and that H-14, H-13, and H-16 were on the other side (Figure 3).
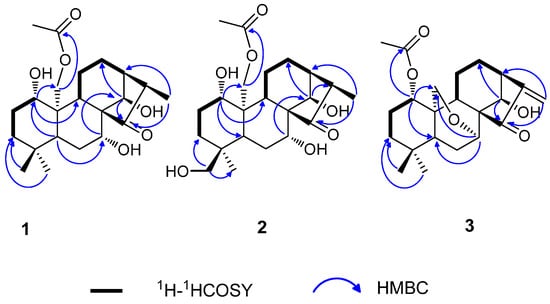
Figure 2.
Key HMBC and 1H-1HCOSY correlations for compounds 1–3.
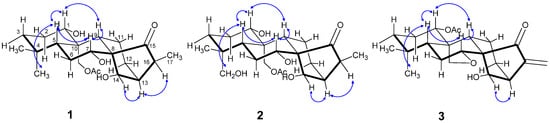
Figure 3.
Key ROESY correlations for compounds 1–3.
The absolute configuration of 1 was confirmed using the CD spectrum. According to the octant rule for saturated cyclopentanone [9], the negative Cotton effect at 305 nm (Δε-0.196), based on the n-π* transition of the saturated cyclopentanone moiety, indicated that the D ring was in a β-orientation (Figure 4). Finally, the structure of compound 1 was elucidated as 1α,7α,14β-trihydroxy-20-acetoxy-ent-kaur-15-one.
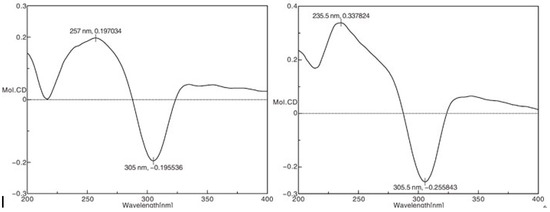
Figure 4.
Experimental CD spectra of compounds 1 and 2.
Compound 2 was obtained as a white crystal. The molecular formula was established as C22H34O7 by HR-ESI-MS at m/z 433.22012 [M + Na]+ (calcd for C22H34O7Na+, 433.21967),which indicated that 2 had an additional oxygen atom compared to 1. The molecular formulas, NMR, and IR data suggested that 2 was an oxygenated analog of 1. Comparison of the NMR spectral data of 2 with those of 1 indicated that one angular methyl (δC 33.3, δH 0.91 (3H, s)) at C-4 in 1 had been replaced by one hydroxymethyl (δC 69.5, δH 3.19 (1H, d, J = 10.6) and 2.82 (1H, d, J = 10.6)) in 2. Furthermore, the downfield shift of C-4 and the upfield shift of C-3, C-5 and C-19 suggested the presence of one hydroxyl group at C-18 in 2. The planar structure of 2 was indicated by the HMBC data (Figure 2). In the HMBC spectrum, correlations were observed for δH 3.19 (H-18) with δC 32.9 (C-3) and 17.3 (C-19) also confirmed that a hydroxymethyl group was linked to C-4.
The same relative stereo-structure for 1 and 2 was deduced from their similar ROESY correlations (Figure 3) and almost identical 1H- and 13C-NMR data. In addition, compound 2 exhibited almost the same CD absorption as that of 1 (Figure 4). Thus, the structure of 2 was determined to be 1α,7α,14β,18-tetrahydroxy-20-acetoxy-ent-kaur-15-one(Figure 1).
Compound 3 was obtained as a white crystalline powder. The molecular formula of 3 was deduced to be C22H30O5 by positive HR-ESI-MS at m/z 397.1992 [M + Na]+ (calcd for C22H30O5Na+, 397.19855). The UV spectrum of 3 showed an absorption maximum at 230 nm. The IR spectrum of 3 showed the presence of hydroxyl (3418 cm−1), carbonyl (1732 cm−1), and double bond (1647 cm−1) groups. The 1H- and 13C-NMR spectra of 3, together with the results from a HSQC experiment showed the presence of one exocyclic double bond (δH 6.00, 5.38 (each 1H, brs); δC 117.5, 151.1), one acetoxyl group (δH 1.95 (3H, s); δC 170.1 (s), 21.5 (q)), one ketone carbonyl (δC 204.6), and two angular methyl groups (δH 0.88 and 1.08 (each 3H, s); δC 31.4 (q) and 21.3 (q)). In addition, the other carbon signals were assigned to six methenes (including one oxygenated signal), six methine carbons (including three oxygenated signals), and three quaternary carbons. These carbon signals were the characteristic signals of the structures of the diterpenoids isolated from the Isodon genus. The 1H- and 13C-NMR spectra of 3 were very similar to those of kamebacetal A (7) [18], except for the absence of a dioxygenated methine (δH 5.51 (1H, d, J = 1.1 Hz, H-20); δC 101.9) and a methoxyl group (δH 3.38 (3H, s); δC 54.9) as well as the presence of an acetoxyl group (δH 1.95 (s, 3H); δC170.1 (s), 21.5 (q)) and an oxygenated methylene (δH 4.09 (1H, dd, J = 10.3, 1.5 Hz, H-20) and 4.03 (1H, dd, J = 10.3, 1.6 Hz, H-20); δC 61.0) in 3. Meanwhile, the following cross-peaks were observed in the HMBC spectrum: δH 3.95 (H-7β) with δC 61.0 (C-20), δH 4.57 (H-1) with δC 170.1 (OAc), and δH 4.61 (H-14) with δC 30.9 (C-12), 204.5 (C-15) and 151.1 (C-16). Thus, the basic skeleton of 3 was assumed to be 1-acetoxy-14-hydroxy-7,20-epoxy-ent-kaur-16-en-15-one.
The relative configuration of 3 was revealed by ROESY experiments (Figure 3). In the ROESY spectrum, the correlations of H-1/H-5 and H-9, Me-18/H-5, H-7/H-5 and H-9, H-14/H-13 were observed. These results indicated that H-1, H-5, H-9, and Me-18 should be on the same side of the molecule, and H-14 and H-13 should be on the other side.
The negative Cotton effect at 356 nm (Δε-0.02), based on the n-π* transition of the unsaturated cyclopentanone moiety, indicated that the D ring was in a β-orientation [11]. According to the analysis described above, the structure of 3 was determined to be 1α-acetoxy-14β-hydroxy-7α,20-epoxy-ent-kaur-16-en-15-one.The six known compounds isolated from I.excisoides were identified as henryin (4) [17], kamebanin (5) [19], reniformin C (6) [20], kamebacetal A (7) [18], kamebacetal B (8) [21], and oridonin (9) [22], by comparison of their spectral data to the reported in the literature.
2.2. Cytotoxicity Assay
Using the MTT method [23], all compounds were evaluated for their cytotoxic effects against five human cancer cell lines HCT-116, A2780, NCI-H1650, BGC-823, and HepG2. Most of the tested compounds exhibited potent cytotoxicity. The results are presented in Table 2.

Table 2.
Cytotoxic activities of all tested compounds on five human cancer cell lines.
| Sample | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HCT-116 | HepG2 | BGC-823 | NCI-H1650 | A2780 | |
| 1 | 2.94 ± 0.06 | 3.07 ± 0.02 | 5.59 ± 0.19 | >10 | 6.33 ± 0.34 |
| 2 | 2.45 ± 0.12 | 3.21 ± 0.09 | 4.17 ± 0.25 | >10 | 5.61 ± 0.19 |
| 3 | 2.13 ± 0.81 | 2.20 ± 1.12 | >10 | 5.68 ± 0.73 | 1.09 ± 0.13 |
| 4 | 1.77 ± 0.22 | 1.54 ± 0.32 | 1.31 ± 0.76 | 2.07 ± 0.36 | 1.42 ± 0.20 |
| 5 | 4.85 ± 0.33 | 7.88 ± 1.02 | 2.99 ± 0.76 | >10 | 1.56 ± 0.34 |
| 6 | 4.81 ± 0.01 | 7.45 ± 0.22 | 5.17 ± 0.61 | >10 | 1.98 ± 0.13 |
| 7 | 4.87 ± 1.12 | 1.09 ± 0.06 | 5.31 ± 0.14 | 2.58 ± 0.23 | 1.44 ± 0.07 |
| 8 | 4.55 ± 0.02 | 7.35 ± 0.48 | 4.97 ± 0.84 | 2.38 ± 0.31 | 4.88 ± 0.22 |
| 9 | 4.85 ± 0.33 | 7.88 ± 1.02 | 2.99 ± 0.76 | >10 | 1.56 ± 0.34 |
| DDP | 7.81 ± 0.14 | >10 | 8.56 ± 1.05 | >10 | 8.65 ± 0.59 |
| Taxol | (3.07 ± 0.12) × 10−2 | (1.31 ± 0.44) × 10−2 | (4.06 ± 0.35) × 10−3 | (2.61 ± 1.02) × 10−2 | (7.13 ± 0.51) × 10−3 |
DDPH (cisplatin) and taxol were used as positive controls.
2.3. Analysis of Structure-Activity Relationships
We assessed the structure-activity relationships of the isolated compounds, based on the results of cytotoxic activity test. Compounds 1, 2, 4, and 5 were 7, 20-non-epoxy kaurane diterpenoids and were present in large amounts in I. excisoides. Compounds 4 and 5 contained α,β-unsaturated pentone and exocyclic methylene. In addition, compound 4 also contained 20-OAc. No exocyclic double bond was found in compounds 1 or 2; however, 20-OAc was present in these compounds. Previous reports have suggested that α,β-unsaturated pentones and exocyclic methylene are essential structural requirements for the cytotoxic activity of diterpenoids [24,25,26]. The results of the cytotoxic activity tests indicated that all 4 compounds had cytotoxic activity. Although the NCI-H1650 cell line was resistant to compounds 1 and 2, these compounds exhibited significant cytotoxic activities (IC50: 2.94–6.47 μM) against the other four tumor cell lines, while compound 4 displayed the highest cytotoxic activity. This suggests that α,β-unsaturated pentone and exocyclic methylene are not the only moieties required for the cytotoxic activity of diterpenoids. Furthermore, 7,20-non-epoxy kaurane diterpenoid, and 20-OAc may also be responsible for the cytotoxic activity of diterpenoids. this possibility should be further investigated.
Compounds 3, 6, 7, 8, and 9 are 7,20-epoxy kaurane diterpenoids composed of α,β-unsaturated pentone and exocyclic methylene. A large number of studies have confirmed that compound 9 has definite cytotoxic activity [25,26,27]. Pre-clinical studies are being conducted on the use of compound 9 as a potential new drug. Compounds 3 and 6–9 exhibited expected levels of cytotoxic activity consistent with previous reports suggesting that α,β-unsaturated pentones and exocyclic methylene are responsible for the cytotoxic activity of diterpenoids.
Compounds 6–8 are variants of diterpenoids comprising an oxygen-containing substituent on the C-20 chiral carbon, but have different configurations (compounds 6 and 7 have an α-configuration, while compound 8 has a β-configuration). A desirable result is that compounds of different configurations display selective activity against NCI-H1650 and HepG2 cell lines. Compound 8 exhibited significant cytotoxic effects on both NCI-H1650 and HepG2 cells (IC50: 1.09–2.58 μM), whereas compounds 6 and 7 had an insignificant effect on the NCI-H1650 cell line (IC50 > 10 μM), suggesting that the relative configuration of the C-20 chiral carbon affected the cytotoxic effect of the tested diterpenoids on some of the tumor cell lines (NCI-H1650 and HepG2), whereas the β-configuration enhanced their cytotoxic activity.
Among the tested compounds, compound 4 exhibited the highest cytotoxic activity against the five tested tumor cell lines (IC50: 1.31–2.07 μM). The cytotoxic activity of compound 4 was almost 5-fold higher than that of compound 9. These results indicate that 7,20-non-epoxy, α,β-unsaturated pentones and exocyclic methylene, as well as the 20-OAc group, had a positive effect on the cytotoxic activity of diterpenoids. α,β-unsaturated pentones (compounds 1 and 2), exocyclic methylene, and configurations of C-20 chiral carbon (compounds 6–8) significantly affected the cytotoxic activity of the tested diterpenoids in some of the cell lines, such as NCI-H1650 and HepG2.
3. Experimental Section
3.1. General Information
Melting points were measured on a Boetius micro melting point apparatus and were uncorrected. IR spectra were recorded on a Spectrum 100 FT-IR Spectrometer (PerkinElmer, Waltham, MA, USA). UV spectra were recorded on a Shimadzu double-beam 210A spectrophotometer (Shimadzu, Kyoto, Japan). Optical rotation was measured using a SEPA-300 polarimeter (Horiba, Tokyo, Japan). NMR spectra were recorded on a Bruker Avance III spectrometer (Bruker, Billerica, Germany) with TMS as the internal standard. Chemical shifts (δ) are expressed in ppm with reference to the solvent signals. HRESIMS data was acquired using an LTQ orbitrap (Thermo Fisher Scientific, Inc., Bremen, Germany). Semi-preparative HPLC was performed on a Waters 600/Waters 2487 (Waters, Milford, MA, USA) with a YMC (250 mm × 10 mm I.D. 5 μm) column. Column chromatography was performed either on silica gel (100–200 mesh and 200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) or on MCI gel CHP 20P (75–150 μm, Mitsubishi Chemical Corp., Tokyo, Japan), ODS (50 μm, YMC, Kyoto, Japan), Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). Solvents were distilled prior to use. Spectroscopic grade solvents were used. TLC was carried out on pre-coated silica gel HF254 plates. Spots were visualized by heating silica gel plates sprayed with 20% H2SO4 in ethanol (v/v).
3.2. Plant Material
The aerial parts of I. excisoides were collected from Luanchuan County in Henan Province, China, in September 2011 and authenticated by professor Xiao-Zheng Luo of the Henan College of Traditional Chinese Medicine. A voucher specimen (No. 2011-0905) was deposited in the Key Laboratory of Traditional Chinese Medicine Chemistry and Resource of Henan Province.
3.3. Extraction and Isolation
The air-dried and powdered aerial parts of I. excisoides (10 kg) were decocted three times with water (320 L × 1.5 h) at 100 °C. The decoction was then evaporated to obtain a concentrate with a concentration equivalent to 0.1 g/mL of crude drug. The concentrate was subjected to chromatography on D-101 macroporous resin and successively eluted with different concentrations of EtOH (0, 30%, 70% and 95% EtOH), to obtain four fractions (Fr. A–D).
Fr. B (20 g, 30% EtOH elute) was subjected to column chromatography over MCI gel (50 cm× 4 cm) and eluted with MeOH–H2O (30:70, 1.5 L; 50:50, 2.5 L; 100:0, 1 L) to yield three subfractions (Fr. B1–B3). Compound 4 (40 mg) was crystallized from Fr. B2 using MeOH. Fr. B1 (5 g) was further subjected to column chromatography on a Sephadex LH-20 column and eluted with MeOH–H2O (30:70) to afford a mixture of three diterpenoids, which was further purified by semi-preparative HPLC (MeOH–H2O, 33:67, 2.7 mL/min). Detection was monitored at 190, 190, and 230 nm. Compounds 1 (8 mg), 2 (7 mg) and 9 (10 mg) were obtained at 28.4, 26.9, and 21.0 min, respectively. Fr. C (36 g, 70% EtOH elute) was further chromatographed on MCI gel (50 cm× 4 cm), eluted with MeOH–H2O (30:70 1.5 L; 50:50 3.5 L; 100:0 1 L) to yield three subfractions (Fr. C1–C3). Compound 7 (70 mg) was crystallized from Fr. C2 using CHCl3. The Fr. C2 residue was subjected to column chromatography over silica gel (100–200 mesh), eluted with petroleum ether–Me2CO (10:1, 8:1, 5:1, 3:1, 0:1) to afford compounds 5 (15 mg) and 8 (15 mg). Compounds 3 (8 mg) and 6 (4 mg) were purified after repeated chromatography over silica gel and a RP-18 column (30%–100% MeOH) from the petroleum ether–Me2CO (5:1) fraction of Fr. C2.
1α,7α,14β-Trihydroxy-20-acetoxy-ent-kaur-15-one (1): white crystals (MeOH); mp: 235 °C; −27.02 (c 0.18, MeOH), UV (MeOH) λmax (log ε): no absorption; IR (KBr) λmax (cm−1): 3544, 3422, 2931, 2875, 1731, 1465, 1381, 1253, 1093, 1062, 1043 cm−1; HRESIMS m/z: 417.22367 [M + Na]+ (calcd for C22H34O6Na+, 417.22467); CD (MeOH) λmax (Δε) 257 (+0.20), 305 (−0.20) nm; See Table 1 for 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz) spectral data.
1α,7α,14β,18-Tetrahydroxy-20-acetoxy-ent-kaur-15-one (2): white crystals (MeOH); mp: 231 °C; −19.48 (c 0.77, MeOH); UV (MeOH) λmax (log ε):no absorption; IR (KBr) λmax (cm−1): 3473, 2918, 2866, 1737, 1725, 1485, 1377, 1269, 1082, 1056, 957 cm−1; HR-ESI-MS m/z: 433.22012 [M + Na]+ (calcd for C22H34O7Na+, 433.21967); CD (MeOH) λmax (Δε) 236 ( +0.34), 306 (−0.26) nm; See Table 1 for 1H-NMR (DMSO-d6 + D2O, 500 MHz) and 13C-NMR(DMSO-d6 + D2O, 125 MHz) spectral data.
1α-Acetoxy-14β-hydroxy-7α,20-epoxy-ent-kaur-16-en-15-one (3): white crystalline powder; mp:165 °C; −50 (c 0.06, MeOH); UV (MeOH) λmax (log ε): 230 (3.52) nm; IR (KBr) λmax (cm−1): 3418, 2959, 2871, 1732, 1647, 1496, 1372, 1242, 1078, 1030, 984 cm−1; HRESIMS m/z: 397.19923 [M + Na]+ (calcd for C22H30O5Na+, 397.19855); CD (MeOH) λmax (Δε) 223 (+3.43), 284 (+1.19), 356 (−0.02) nm; See Table 1 for 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz) spectral data.
3.4. Cytotoxicity Assay
Five human cancer cell lines (colon carcinoma cell line HCT-116, hepatic cancer cell line HepG2, ovarian cancer cell line A2780, lung cancer cell line NCI-H1650, and gastric cancer cell line BGC-823) were used. All cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. The cytotoxicity assay was performed according to the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method using 96-well microplates [28]. Briefly, the cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. Next, 100 μL of adherent cells at a density of 5 × 104 cell/mL were seeded into each well of the 96-well cell culture plates and incubated in 5% CO2 at 37 °C for 24 h to form a monolayer on the flat bottom. Next, the supernatant of each well was removed, after which 100 μL of fresh medium and 100 μL of medium containing a test sample were added to the well. The plate was then incubated in 5% CO2 at 37 °C for 72 h. Next, 20 μL of 5 mg/mL MTT in DMSO was added to each well and further incubated for 4 h. The supernatant was carefully removed from each well and 150 μL of DMSO were added. The plate was then vortex-shaken for 15 min to dissolve the blue formazan crystals. The optical density (OD) of each well was measured on a Genois microplate reader (Tecan GENios, Männedorf, Switzerland) at a wavelength of 570 nm.
In each experiment, each tumor cell line was exposed to the test compound at concentrations of 1 × 10−5, 1 × 10−6, and 1 × 10−7 mol/L. The inhibitory rate of the cell growth was calculated according to the following formula: Inhibition rate (%) = (ODcontrol − ODtreated)/ODcontrol × 100. Finally, IC50 values were calculated using SPSS 16.0 statistical software.
4. Conclusions
Phytochemical investigations on the water extract of the aerial parts of I. excisoides resulted in the isolation of three new compounds 1–3, along with six known compounds 4–9. The isolated compounds were identified as 1α,7α,14β-trihydroxy-20-acetoxy-ent-kaur-15-one (1); 1α,7α,14β,18-tetrahydroxy-20-acetoxy-ent-kaur-15-one (2); 1α-acetoxy-14β-hydroxy-7,20-epoxy-ent-kaur-16-en-15-one (3); henryin (4); kamebanin (5); reniformin C (6); kamebacetal-A (7); kamebacetal B (8); and oridonin (9). Compounds 5 and 9 were isolated from I. excisoides for the first time.
All the compounds were evaluated for their cytotoxic effects against five human tumor cell lines (HCT-116, HepG2, A2780, NCI-H1650, and BGC-823) and all showed certain cytotoxic activity against all five types of human tumor cells. Furthermore, we studied the cytotoxic activity of kaurane diterpenoids with different structural features for the first time. It was observed that diterpenoids with a 7,20-non-epoxy group, α,β-unsaturated pentones and an exocyclic methylene group, the 20-OAc group can improve cytotoxic activity. These results suggest that the cyclopentanone conjugated with an exomethylene group is mainly responsible, although not the sole factor, for the cytotoxic activity of 7,20-non-epoxy diterpenoids. Diterpenoids containing the 20-OAc group also displayed significant cytotoxic activity (compounds 1 and 2). The presence of the 20-OAc group has a positive effect on the cytotoxic activity (compounds 1, 2, and 4), and the mechanism underlying their cytotoxic activity requires further investigation. There have been many reports on 7, 20-epoxy kaurane diterpenoids [23,24,25,26,27]. Our study revealed that the configuration of the C-20 chiral carbon had a positive effect on the cytotoxic activity. In addition, β-configuration of C-20 (compound 8) showed marked selective cytotoxic activity against the NCI-H1650 and HepG2 cell lines. More similar compounds should be used to further study this structure-activity relationship in the future.
Supplementary Materials
The IR, HR-ESI-MS, NMR (1D and 2D) and CD spectra of compounds 1–3 are available as supporting information. Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/09/17544/s1.
Acknowledgments
The investigation was financially supported by the National Natural Science Foundation of China (no. 81173486), Young Backbone Teachers of Colleges and Universities in Henan Province Fund (no. 2012GGJS-095), Medical Science and Technology Project of Henan Province (no. 2013ZY02063), the Fundamental Scientific Research Funds of Provincial Universities (no. 2014KYYWF-QN02) and the Collaborative Innovation Center of Diagnosis, Treatment and Drug Research for Respiratory Disease, Henan Province, China. We are thankful to the Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College for testing the cytotoxic activities. We are also grateful to the Department of Instrumental Analysis, Henan University of Traditional Chinese Medicine for measuring the IR, UV, NMR and MS spectra.
Author Contributions
Li-Ping Dai and Yan-Qing Lu performed the isolation and structure elucidation of the constituents, and participated in the bioassay experiments. Chun Li, Hui-Min Gao partially contributed the structure elucidation and together with Li-Ping Dai prepared the manuscript. Han-Ze Yang conducted the cytotoxic assay. Hong-Yan Yu collected the plant material and carried out the extraction and isolation. Zhi-Min Wang planned, designed and organized the whole research of this study. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, H.D.; Xu, Y.L.; Jiang, B. Diterpenoids from Isodon Species; Science Press: Beijing, China, 2001; p. 2. [Google Scholar]
- Li, H.; PU, J.X.; Li, J. Diterpenoids chemodiversity of the genus Isodon spach from Lamiaceae. Plant Divers. Resour. 2013, 35, 81–88. [Google Scholar]
- Jiao, K.; Li, H.Y.; Zhang, P.; Pi, H.F.; Ruan, H.L.; Wu, J.Z. Two new ent-kaurane diterpenoids from the aerial parts of Isodon excisoides. Chin. Chem. Lett. 2014, 25, 131–133. [Google Scholar] [CrossRef]
- Wang, T.; Tang, F.M.; Zhang, Y.H.; Chen, Z. A natural diterpenoid kamebacetal with anti-tumor activity. J. Mol. Struct. 2010, 97, 317–322. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kim, J.Y.; Dong, S.P.; Wang, K.Y. Study on the immunomodulation effect of Isodon japonicus extract via splenocyte function and NK anti-tumor activity. Int. J. Mol. Sci. 2012, 13, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Zhang, W.; Li, J.C.; Liu, N. Chemical constituents of flowers and fruits of Rabdosia excisa. Chin. J. Nat. Med. 2012, 10, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, T.S.; Yamashiro, A.; Dohzono, I.M.; Maki, M. Development of microsatellite markers for Isodon Longitubus (lamiaceae). Appl. Plant Sci. 2013, 1, 128–130. [Google Scholar]
- Liao, Y.J.; Bai, H.Y.; Li, Z.H.; Zou, J. Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells. Cell. Death Dis. 2014, 5, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Cheng, P.Y. Structure elucidation of a bis-ent-kauranoid isolated from Isodon pharicus. Acta Pharm. Sin. 1994, 29, 532–538. [Google Scholar]
- Wang, Z.M.; Cheng, P.Y. Structure elucidation of a new bis-ent-kaurane compound, isodopharicin E, isolated from Isodon pharicus (Prain) Murata. Chin. Chem Lett. 1991, 2B, 847. [Google Scholar]
- Wang, Z.M.; Feng, H.; Zhang, Q.; Liu, F.S.; Jin, W.S.; Mu, M.; Fang, Q.H.; Kong, M.; He, W.Y. The structures elucidation of isodopharicin D and F. Acta Pharm. Sin. 1998, 33, 207–211. [Google Scholar]
- Jiang, H.L.; Zhu, M.; Chen, Y.; Li, W.T. Simultaneous determination of glaucocalyxin A, glaucocalyxin B and glaucocalyxin D in weifuchun tablets by HPLC. Chin. Tradit. Patent Med. 2013, 35, 86–89. [Google Scholar]
- Wu, M.J.; Fu, J.G.; Dong, J.J.; Li, J.C.; Zhao, T.Z. Advances on the plants of Isodon of the chemical constituents and pharmacological effects. World Latest Med. Inf. 2004, 3, 1214–1218. [Google Scholar]
- Ding, B.Z.; Wang, S.Y.; Gao, Z.Y. Flora of Henan; Henan People Publishing Press: Zhengzhou, China, 1981; Volume 3, p. 76. [Google Scholar]
- Li, H.Y.; Jiao, K.; Zhang, P.; Gong, S.Z.; Sun, Y.; Wu, J.Z. Chemical constituents from Isodon excisoides. Chin. Tradit. Herb. Drug 2014, 45, 154–160. [Google Scholar]
- Wang, Y.X.; Zhu, L.L.; He, Z.A.; Zhang, J.X. New diterpenoids from Isodon excisoides. Chin. Chem. Lett. 2010, 21, 610–612. [Google Scholar] [CrossRef]
- Li, J.C.; Liu, C.J.; An, X.Z.; Sun, H.D.; Lin, Z.W. The structure of Henryin. Acta Bot. Yunnan 1984, 6, 453–456. [Google Scholar]
- Takeda, Y.; Ichihara, T.; Takaishi, Y. Structure elucidation of new diterpenoids Rabdosia umbrosa var. leucantha f. Kameba. J. Chem. Soc. Perkin Trans. 1987, 1, 2403–2409. [Google Scholar] [CrossRef]
- Lan, D.; Liu, G.A.; Yang, D.J.; Wang, H.; Wang, L.; Sun, K. Cytotoxic ent-kaurane diterpenoids from Isodon weisiensis. Pharmazie 2005, 60, 458–460. [Google Scholar]
- Wang, X.R.; Wang, Z.Q.; Dong, J.G. Chemical structures of Reniformin A, B and C. Acta Bot. Sin. 1986, 28, 292–298. [Google Scholar]
- Sun, H.D.; Li, J.C. Excisanin A and B, new diterpenoids from Rabdosia excisa. Chem. Lett. 1981, 753–756. [Google Scholar]
- Zhang, H.B.; PU, J.X.; Wang, Y.Y.; He, F.; Zhao, Y.; Li, X.N.; Luo, X.; Xiao, W.L.; Li, Y.; Sun, H.D. Four New ent-Kauranoids from Isodon rubescens var. lushanensis and Data Reassignment of Dayecrystal B. Chem. Pharm. Bull. 2010, 58, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.M.; Kuo, C.Y.; Lin, C.Y.; Hung, M.F.; Shen, J.J.; Wang, T.L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014, 19, 3327–3344. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Li, X.N.; Du, X.; Wang, W.G.; Dong, K.; Su, J.; Li, Y.; Pu, J.X.; Sun, H.D. ent-Atisane and ent-kaurane diterpenoids from Isodon rosthornii. Fitoterapia 2013, 88, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Zhan, R.; Wang, W.G.; Jiang, H.Y.; Du, X.; Li, X.N.; Li, Y.; Pu, J.X.; Sun, H.D. Cytotoxic ent-kaurane diterpenoids from Isodon wikstroemioides. J. Nat. Prod. 2014, 77, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Li, S.; Liu, X.; Ji, A.; Xu, B.; Xu, J. Oridonin alters the expression profiles of microRNAs in BxPC-3 human pancreatic cancer cells. BMC Complement. Altern. Med. 2015, 115, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, S.; Gao, Y.; Zhang, X.; Lu, C. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med. Oncol. 2015, 32, 365. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Xie, H.H.; Hao, J.; Jiang, Y.M.; Wei, X.Y. Eudesmane sesquiterpene glucosides from lycheeseed and their cytotoxic activity. Food Chem. 2010, 123, 1123–1126. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).