Abstract
Reactions of hydrazonoyl halides and each of methyl 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazine-1-carbodithioate and 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazine-1-carbothioamide afforded 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-3-phenyl-5-substituted-2,3-dihydro-1,3,4-thiadiazoles and 5-(4-substituted)diazenyl)-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-4-arylthiazoles, respectively. Analogously, the reactions of hydrazonoyl halides with 7-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-phenyl-2-thioxo-2,3-dihydropyrido[2,3-d]pyrimidin-4(1H)-one gave 3-(4-substituted)-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-6-phenyl-1-arylpyrido[2,3-d]-[1,2,4]-triazolo-[4,3-a]pyrimidin-5(1H)-ones in a good yield. The structures of the newly synthesized were elucidated via elemental analysis, spectral data and alternative synthesis routes whenever possible. Twelve of the newly synthesized compounds have been evaluated for their antitumor activity against human breast carcinoma (MCF-7) and human hepatocellular carcinoma (HepG2) cell lines. Their structure activity relationships (SAR) were also studied. The 1,3,4-thiadiazole derivative 9b (IC50 = 2.94 µM) has promising antitumor activity against the human hepatocellular carcinoma cell line and the thiazole derivative 12a has promising inhibitory activity against both the human hepatocellular carcinoma cell line and the breast carcinoma cell line (IC50 = 1.19, and 3.4 µM, respectively).
1. Introduction
1,2,3-Triazoles are an important class of heterocycles due to their wide range of applications as synthetic intermediates and pharmaceuticals [1,2,3,4]. Several therapeutically interesting 1,2,3-triazoles have been reported, including anti-HIV agents [5,6,7,8], antimicrobial compounds [9], β3-selective adrenergic receptor agonists [10], kinase inhibitors [11,12], other enzyme inhibitors [13,14], the β-lactam antibiotic tazobactam [15] and the cephalosporin cefatrizine [16].
1,3,4-Thiadiazole derivatives have attracted considerable interest owing to their wide spectra of biological activities such as antibacterial, antifungal, antituberculosis, antihepatitis B viral, antileishmanial, anti-inflammatory, analgesic, CNS depressant, anticancer, antioxidant, antidiabetic, molluscicidal, antihypertensive, diuretic, analgesic, antimicrobial, antitubercular, and anticonvulsant activities [17,18,19,20,21,22,23,24,25,26].
Thiazoles can found in drug development for the treatment of allergies [27], hypertension [28], inflammation [29], schizophrenia [30], bacterial [31], HIV infections [32], hypnotics [33] and more recently for the treatment of pain [34], as fibrinogen receptor antagonists with antithrombotic activity [35] and as new inhibitors of bacterial DNA gyrase B [36].
The 1,2,4-triazolopyrimidines have also attracted growing interest due to their important pharmacological activities, such as antitumor potency, antimalarial, antimicrobial, anti-inflammatory, antifungal and macrophage activation [37,38,39,40,41,42]. In continuation of our ongoing work [43,44,45,46,47,48], we report herein the synthesis of some new thiadiazole, thiazole and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives containing 1,2,3-triazole moieties.
2. Results and Discussion
2.1. Chemistry
Treatment of 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1) [49] with methyl hydrazino-carbodithioate (2) in 2-propanol afforded methyl 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinecarbodithioate (3) (Scheme 1). Structure 3 was elucidated by elemental analysis, spectral analysis, and chemical transformation. Compound 3 when reacted with ethyl 2-chloro-2-(2-phenylhydrazono)acetate (4b) in ethanolic triethylamine at room temperature gave one isolated product formulated as ethyl 5-((1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-ethylidene)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (9b). Structure 9b was confirmed by elemental analysis, spectral data, and an alternative synthesis route. Thus, 2,3-dihydro-1,3,4-thiadiazole 8b [50] was reacted with 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1) in ethanol afforded a product identical to 9b in all aspects (m.p., mixed m.p., and spectra). In the light of the these results, the mechanism outlined in Scheme 1 seems to be the most plausible pathway for the formation of 9b from the reaction of the 3 with 4b. The reaction involves initial formation of thiohydrazonate 6, which undergoes intermolecular cyclization as soon as it is formed to yield the intermediate 7 or via 1,3-dipolar cycloaddition of nitrileimine 5b [prepared in situ from 4b with triethylamine] to the C=S double bond of 3. The formation of 6 and 7 are similar to the reactions of hydrazonoyl chloride with 1-phenyl-1,4-dihydrotetrazole-5-thione [51] and 5-phenyl-1,3,4-thiadiazole-2(3H)-thione [52]. Compound 7 was converted to 9 by elimination of methanthiol. Analogously, treatment of the appropriate 3 with each of 4a, 4c–g gave 2,3-dihydro-1,3,4-thiadiazoles 9b–g, respectively, in good yield (Scheme 1).
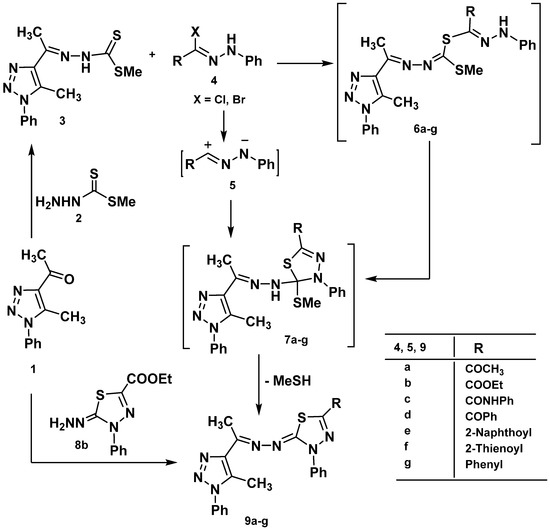
Scheme 1.
Synthesis of thiadiazoles 9a–g.
Reaction of 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1) with thiosemicarbazide (10) in ethanol afforded 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinecarbothioamide (11) in a good yield. The structure of 11 was elucidated via elemental analysis, spectral data and chemical transformation. Its 1H-NMR showed signals at δ 2.47 (s, 3H, CH3), 2.68 (s, 3H, CH3), 6.67 (s, br., 2H, NH2), 7.32–7.58 (m, 5H, ArH’s), 8.73 (s, br., 1H, NH). Compound 11 was reacted hydrazonoyl chloride 4a in ethanol under refluxed gave the corresponding 4-methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(phenyldiazenyl)thiazole (12a) in quantitative yield (Scheme 2). Structure 12a was confirmed by elemental analysis, spectral data and alternative synthesis. Thus, 2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-4-phenyl-thiazole (14), which was prepared from reaction of 1 with 2-hydrazinyl-4-phenylthiazole (13) [53], was coupled with benzenediazonium chloride in ethanolic sodium acetate at 0–5 °C to afford a product identical in all respects (mp, mixed mp, and spectra) to 12a. Analogously, treatment of 11 with the appropriate 4 gave thiazole derivatives12b–i, respectively, in good yield (Scheme 2).
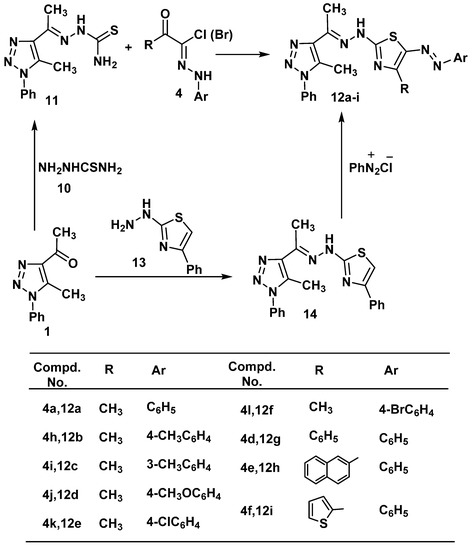
Scheme 2.
Synthesis of thiazolederivatives 12a–i.
Next, 1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-3-phenylprop-2-en-1-one (15) [54] was reacted with 6-amino-2-thioxo-2,3-dihydropyrimidin-4(1H)-one (16) in ethanol to afford 7-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-phenyl-2-thioxo-2,3-dihydropyrido[2,3-d]pyrimidin-4(1H)-one (17) in a good yield. Structure 17 was elucidated by elemental analysis, spectral data and chemical transformation. Thus, when compound 17 was reacted with 4a in chloroform under reflux it afforded one isolable product, as evidenced by tlc, formulated as 3-acetyl-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1,6-diphenylpyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one (22a, Scheme 3). The mechanism outlined in Scheme 3 seems to be the most plausible pathway for the formation of 22 from the reaction of thione 17 with 4 via two pathways: (1) 1,3-addition of the thiol tautomer 18 to the nitrilium imide 5 to give the thiohydrazonate ester 19 which undergoes nucleophilic cyclization to yield spiro compounds 20. The latter ring opened and cyclized to yield 22 by loss of hydrogen sulfide; and (2) 1,3-cycloaddition of nitrilium imide 5 to the C=S double bond of 17 to give 20 directly (Scheme 3). Attempts to isolate the thiohydrazonate ester 19, spiro intermediate 20 and thiohydrazide 21 did not succeed, even under mild conditions as they readily undergo in situ cyclization followed by elimination of hydrogen sulfide to give the final product 22 in Scheme 3.
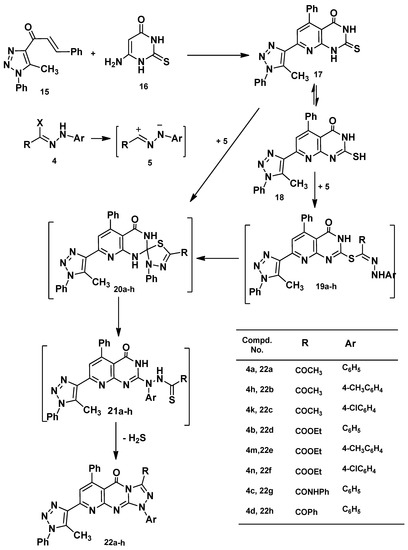
Scheme 3.
Synthesis of pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones 22a–h.
2.2. Cytotoxic Activity
Our literature survey showed that many thiazole and 1,3,4-thiadiazole derivatives have antitumor activity with excellent IG50 and IC50 values, as depicted in Figure 1 [55,56,57,58]. In view of these facts, we examined the antitumor activity of a new series of substituted thiadiazoles and thiazoles against the human breast carcinoma cell line (MCF-7) and against the human hepatocellular carcinoma (HepG2).
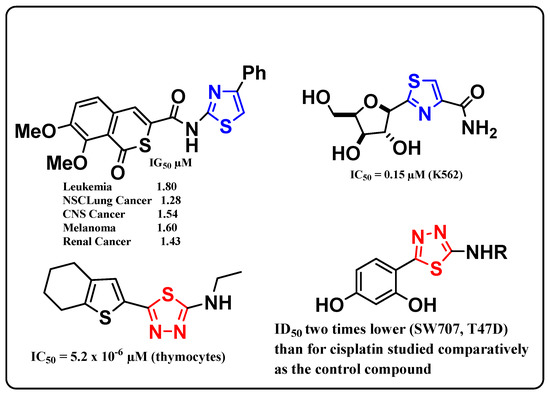
Figure 1.
Antitumor activity of thiazoles and 1,3,4-thiadiazoles.

Table 1.
The in vitro inhibitory activity of tested compounds against tumor cell lines expressed as IC50 values (μM) ± standard deviation from six replicates.
| Tested Compounds | Tumor Cell Lines | |
|---|---|---|
| MCF-7 | HepG2 | |
| 9a | 29.11 ± 0.21 | 25.42 ± 0.21 |
| 9b | 8.67 ± 0.30 | 2.94 ± 0.12 |
| 9c | 7.72 ± 0.18 | 17.60 ± 0.23 |
| 9d | 22.40 ± 0.20 | 16.13 ± 0.21 |
| 9e | 22.94 ± 0.18 | 21.72 ± 0.14 |
| 9f | 38.21 ± 0.16 | 43.43 ± 0.19 |
| 9g | 19.72 ± 0.20 | 10.71 ± 0.27 |
| 12a | 3.4 ± 0.23 | 1.19 ± 0.07 |
| 12b | 22.5 ± 0.24 | 27.90 ± 0.24 |
| 12c | 20.1 ± 0.12 | 29.41± 0.07 |
| 22a | 37.7 ± 0.11 | 27.94 ± 0.13 |
| 22d | 39.9 ± 0.07 | 43.62 ± 0.14 |
| Doxorubicin | 0.46 ± 0.21 | 0.42 ± 0.22 |
The in vitro growth inhibitory activity of the synthesized compounds was investigated in comparison with the well-known anticancer standard drug doxorubicin using a crystal violet colorimetric viability assay. Data generated were used to plot a dose response curve of which the concentration of test compounds required to kill 50% of the cell population (IC50) was determined. The cytotoxic activity was expressed as the mean IC50 of three independent experiments (Table 1) and the results revealed that all the tested compounds showed inhibitory activity to the tumor cell lines in a concentration dependent manner. The small values of IC50 for the selected compounds indicate that, for more anticancer effect higher concentrations can be used. The results are represented in Table 1, Figure 2 and Figure 3 show that:
- -
- The in vitro inhibitory activities of tested compounds against the human breast carcinoma (MCF-7) have the following descending order: 12a > 9c > 9b > 9g > 12e > 9d > 12b > 9e > 9a > 22d > 9f > 22a.
- -
- The in vitro inhibitory activities of tested compounds against the human hepatocellular carcinoma (HepG2) cell line have the following descending order: 12a > 9b > 9g > 9d > 9c > 9e > 9a > 12e > 22d > 12b > 9f > 22a.
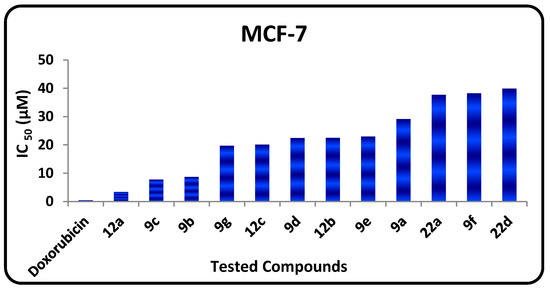
Figure 2.
IC50 values of tested compounds against MCF-7.
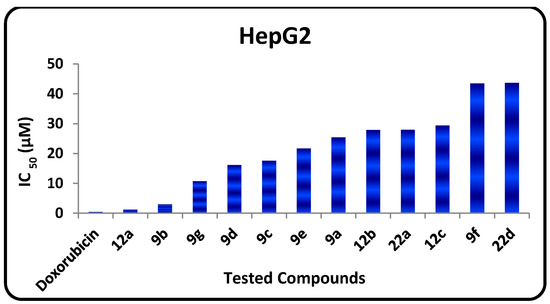
Figure 3.
IC50 values of tested compounds against HepG2.
Examination of the SAR leads to the following conclusions:
- -
- The 1,3,4-thiadiazole 9b (IC50 = 2.94 µM) has promising antitumor activity against the human hepatocellular carcinoma cell line while the other 1,3,4-thiadiazole derivatives 9a, 9c–f have moderate activities (IC50 = 7.72‒43.43 µM).
- -
- Thiazole 12a has promising inhibitory activity against both the human hepatocellular carcinoma cell line and the breast carcinoma cell line (IC50 = 1.19, and 3.4 µM, respectively) while the other thiazole derivatives 12b and 12e have moderate activity.
- -
- Pyridotriazolopyrimidinone derivatives 22a,d have moderate activity.
- -
- For substituents at position 2 of the 1,3,4-thiadiazole ring, the in vitro inhibitory activity of tested compounds against the human breast carcinoma cell line have the following descending order: CONHC6H5 > COOC2H5 > C6H5 > C6H5CO > C10H7CO > CH3CO > C4H3SCO group.
- -
- For substituents at position 2 of the 1,3,4-thiadiazole ring, the in vitro inhibitory activity of tested compounds against the human hepatocellular carcinoma cell line have the following descending order: COOC2H5 > C6H5 > C6H5CO > CONHC6H5 > C10H7CO > CH3CO > C4H3SCO group.
3. Experimental Section
3.1. Chemistry
3.1.1. General
Melting points were measured on an Electrothermal IA 9000 series digital melting point apparatus. IR spectra were recorded in potassium bromide discs on PyeUnicam SP 3300 and Shimadzu FTIR 8101 PC infrared spectrophotometers. NMR spectra were recorded on a Varian Mercury VX-300 NMR spectrometer operating at 300 MHz (1H-NMR) and run in deuterated dimethylsulfoxide (DMSO-d6). Chemical shifts were related to that of the solvent. 13C-NMR was recorded on a Bruker spectrometer at 75 MHz. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer at 70 eV. Elemental analyses were measured by using an ElementarVario LIII CHNS analyzer. Antitumor activity of the productswas carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt. Hydrazonoyl halides 4 [59,60,61,62,63,64,65] were prepared as reported in the respective literature.
3.1.2. Synthesis of Methyl 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazine-1-carbodithioate (3)
To a solution of 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1, 2.01 g, l0 mmol) in 2-propanol (20 mL), methyl hydrazinecarbodithioate 2 (1.22 g, 10 mmol) was added. The mixture was stirred at room temperature for 2 h. The solid product was filtered off, recrystallized from ethanol to afford 3 as a yellow solid in 85% yield; mp: 182–184 °C; IR: ν = 3198 (NH), 2993, 2918 (CH), 1601 (C=N) cm−1; 1H-NMR: δ = 2.30 (3H, s, CH3), 2.46 (3H, s, CH3), 2.67 (3H, s, SCH3), 7.56–7.69 (5H, m, Ar-H), 8.38 (1H, s, NH); 13C-NMR: δ = 14.9 (CH3), 17.9 (CH3), 21.0 (CH3), 116.4, 125.8, 129.6, 129.8, 132.4, 133.1, 134.7, 164.6 (Ar-C), 191.3 (C=S); MS m/z (%): 305 (M+, 14), 258 (100), 200 (43), 119 (75), 91 (24). Anal. Calcd for C13H15N5S2(305.42): C, 51.12; H, 4.95; N, 22.93. Found C, 51.03; H, 4.73; N, 22.74%.
3.1.3. General Procedure for Synthesis of 2-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)-hydrazono)-3-phenyl-5-subsitituted-2,3-dihydro-1,3,4-thiadiazoles 9a–g
To a mixture of alkyl carbodithioate 3 (0.305 g, 1 mmol) and the appropriate hydrazonoyl halide 4a–g (1 mmol) in ethanol (20 mL), triethylamine (0.5 mL) was added, the mixture was stirred at room temperature for 2 h. The resulting solid was collected and recrystallized from dimethylformamide to give the corresponding 1,3,4-thiadiazolines 9a–g. The products 9a–g together with their physical constants are listed below.
1-(5-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethanone (9a). Yellow solid, (77% yield); mp: 271–273 °C; IR: ν = 3062, 2921 (CH), 1676 (C=O), 1607 (C=N) cm−1; 1H-NMR: δ = 2.49 (3H, s, CH3), 2.50 (3H, s, CH3), 2.58 (3H, s, CH3), 7.39–8.09 (10H, m, Ar-H); MS, m/z (%) 417 (M+, 52), 346 (14), 259 (23), 143 (77), 78 (100). Anal. calcd for C21H19N7OS (417.49): C, 60.42; H, 4.59; N, 23.49. Found: C, 60.26; H, 4.51; N, 23.28%.
Ethyl 5-((1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (9b). Yellow solid, (70% yield); mp: 184–186 °C; IR: ν = 3064, 2983 (CH), 1704 (C=O), 1606 (C=N) cm−1; 1H-NMR: δ = 1.32 (3H, t, J = 7.2, CH2CH3), 2.49 (3H, s, CH3), 2.65 (3H, s, CH3), 4.38 (2H, q, J = 7.2, CH2CH3), 7.38–8.01 (10H, m, Ar-H); 13C-NMR: δ = 9.6, 11.1, 19.2 CH3), 61.2 (CH2), 115.2, 116.3, 116.4, 117.2, 118.3, 119.2, 120.6, 120.8, 122.3, 124.4, 126.3, 137.4, 151.2 (Ar-C), 166.3 (CO); MS, m/z (%) 447 (M+, 17), 346 (6), 289 (11), 170 (49), 143 (69), 118 (27), 78 (100). Anal. calcd for C22H21N7O2S (447.51): C, 59.05; H, 4.73; N, 21.91. Found: C, 59.02; H, 4.70; N, 21.79%.
5-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-N,4-diphenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide (9c). Yellow solid, (79% yield); mp: 260–262 °C; IR: ν = 3398 (NH), 3059, 2918 (CH), 1672 (C=O), 1602 (C=N) cm−1; 1H-NMR: δ = 2.47 (3H, s, CH3), 2.65 (3H, s, CH3), 7.18–8.08 (15H, m, Ar-H), 11.38 (1H, s, NH); MS, m/z (%) 494 (M+, 47), 336 (14), 170 (60), 142 (76), 119 (59), 78 (100). Anal. calcd for C26H22N8OS (494.57): C, 63.14; H, 4.48; N, 22.66. Found: C, 63.05; H, 4.40; N, 22.44%.
5-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)(phenyl)methanone (9d). Yellow solid, (73 yield); mp: 250–252 °C; IR: ν = 3061, 2918 (CH), 1620 (C=O), 1605(C=N) cm−1; 1H-NMR: δ = 2.43 (3H, s, CH3), 2.60 (3H, s, CH3), 7.12–7.98 (15H, m, Ar-H);MS, m/z (%) 479 (M+, 15), 346 (8), 135 (42), 106 (100), 78 (63), 65 (56). Anal. calcd for C26H21N7OS (479.56): C, 65.12; H, 4.41; N, 20.45. Found: C, 65.05; H, 4.37; N, 20.37%.
(5-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)(naphthalen-2-yl)methanone (9e). Yellow solid, (70% yield); mp: 244–246 °C; IR: ν = 3059, 2928 (CH), 1629 (C=O), 1603 (C=N) cm−1; 1H-NMR: δ = 2.43 (3H, s, CH3), 2.62 (3H, s, CH3), 7.13–7.95 (16H, m, Ar-H), 8.22 (s, 1H, naphthalene-H1); MS, m/z (%) 529 (M+, 31), 512 (100), 324 (64), 155 (57), 135 (58), 78 (89). Anal. calcd for C30H23N7OS (529.61): C, 68.03; H, 4.38; N, 18.51; found: C, 67.89; H, 4.31; N, 18.42%.
(5-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)(thien-2-yl)methanone (9f). Orange solid, (79% yield); mp: 264–266 °C; IR: ν = 3071, 2920 (CH), 1648 (C=O), 1606 (C=N) cm−1;1H-NMR: δ = 2.48 (3H, s, CH3), 2.62 (3H, s, CH3), 7.12–7.97 (13H, m, Ar-H); MS, m/z (%) 485 (M+, 29), 346 (11), 170 (36), 112 (95), 78 (100). Anal. calcd for C24H19N7OS2(485.58): C, 59.36; H, 3.94; N, 20.19. Found: C, 59.28; H, 3.79; N, 20.12%.
2-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazono)-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole (9g). Yellow solid, (72% yield); mp: 205–207 °C; IR: ν = 3058, 2916 (CH), 1603 (C=N) cm−1; 1H-NMR: δ = 2.49 (3H, s, CH3), 2.62 (3H, s, CH3), 7.10–7.91 (15H, m, Ar-H); 13C-NMR: δ = 11.4 (CH3), 17.3 (CH3), 116.0, 116.2, 117.3, 118.2, 118.6, 119.3, 119.6, 120.0, 120.2, 120.6, 122.3, 127.0, 128.5, 128.8, 139.0, 147.7, 151.4 (Ar-C); MS, m/z (%) 451 (M+, 49), 293 (12), 194 (73), 136 (88), 92(56), 78 (100). Anal. calcd for C25H21N7S (451.55): C, 66.50; H, 4.69; N, 21.71. Found: C, 66.53; H, 4.58; N, 21.64%.
3.1.4. Alternate synthesis of 9b
To a solution of 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1, 0.201 g, l mmol) in 2-propanol (10 mL), ethyl 5-hydrazono-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (8b, 0.264 g, 1 mmol) was added. The mixture was refluxed for 2 h then cooled to room temperature. The solid precipitated was filtered off, washed with water, dried and recrystallized from dimethylformamide to give in 69% yield a product which was identical in all aspects (m.p., mixed m.p. and IR spectra) to that obtained from reaction of 3 with 4b.
3.1.5. Synthesis of 2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinecarbothioamide (11)
A mixture of 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1, 2.01 g, 10 mmol) and thio-semicarbazide 10 (0.91 g, 10 mmol) in ethanol (50 mL) containing a catalytic amount of hydrochloric acid was refluxed for 6 h. The desired thiosemicarbazone precipitated from reaction mixture was filtered, washed with ethanol and recrystallized from acetic acid to give pure product of compound 11 as white solid (82%); mp = 221–223 °C; IR: ν =3420, 3262, 3191 (NH2, NH), 1596 (C=N) cm−1; 1H-NMR: δ = 2.47 (s, 3H, CH3), 2.49 (s, 3H, CH3), 3.46 (s, br, 2H, NH2), 7.57–7.65 (m, 5H, Ar-H),10.22 (s, br, 1H, NH); MS m/z (%): 274 (M+, 30), 158 (37), 118 (34), 77 (100). Anal. Calcd: for C12H14N6S (274.34): C, 52.54; H, 5.14; N, 30.63. Found: C, 52.48; H, 5.10; N, 30.48%.
3.1.6. Synthesis of 2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(aryl-diazenyl)-4-substitutedthiazoles 12a–i
A mixture of thiosemicarbazone 11 (0.274 g, 1 mmol) and the appropriate hydrazonoyl halide 4 (1 mmol) in dioxane (20 mL) containing TEA (0.07 mL) was refluxed for 6 h, allowed to cool and the solid formed was filtered off, washed with EtOH, dried and recrystallized from DMF to give the corresponding 1,3,4-thiadiazolines 12a–i. The products 12a–i together with their physical constants are listed below.
4-Methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(phenyldiazenyl)-thiazole (12a). Red solid, (72% yield); mp 187–189 °C; IR: ν = 3414 (NH), 1600 (C=N) cm−1; 1H-NMR: δ = 2.41 (s, 3H, CH3), 2.49 (s, 3H, CH3), 2.64 (s, 3H, CH3), 7.18–7.92 (m, 11H, Ar-H and NH); 13C-NMR: δ =10.0, 13.4, 16.5 (CH3), 111.6, 117.5, 118.0, 116.2, 122.1, 122.6, 129.0,133.7, 139.3, 144.1, 144.5, 153.6, 154.0, 163.4 (Ar-C); MS, m/z (%) 416 (M+, 15), 283 (66), 118 (28), 77 (100), 65 (15). Anal. calcd for C21H20N8S (416.50): C, 60.56; H, 4.84; N, 26.90. Found: C, 60.63; H, 4.81; N, 26.76%.
4-Methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(p-tolyldiazenyl)-thiazole (12b). Red solid, (76% yield); mp 193–195 °C; IR: ν = 3426 (NH), 1601 (C=N) cm−1; 1H-NMR: δ = 2.21 (s, 3H, CH3), 2.50 (s, 3H, CH3), 2.52 (s, 3H, CH3), 2.59 (s, 3H, CH3), 7.08–7.64 (m, 10H, Ar-H and NH); MS, m/z (%)430 (M+, 25), 185 (9), 118 (19), 77 (100). Anal. calcd for C22H22N8S (430.53): C, 61.37; H, 5.15; N, 26.03. Found: C, 61.29; H, 5.08; N, 25.84%.
4-Methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(m-tolyldiazenyl)-thiazole (12c). Red solid, (68% yield); mp 156–158 °C; IR: ν = 3435 (NH), 1600 (C=N) cm−1; 1H-NMR: δ 2.20 (s, 3H, CH3), 2.46 (s, 3H, CH3), 2.52 (s, 3H, CH3), 2.57 (s, 3H, CH3), 7.10–7.62 (m, 10H, Ar-H and NH); MS, m/z (%)430 (M+, 22), 158 (37), 118 (13), 77 (100). Anal. calcd for C22H22N8S (430.53): C, 61.37; H, 5.15; N, 26.03. Found: C, 61.42; H, 5.11; N, 25.87%.
5-((4-Methoxyphenyl)diazenyl)-4-methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)-hydrazinyl)thiazole (12d). Dark red solid, (72% yield); mp 178–180 °C; IR: ν = 3431 (NH), 1600 (C=N) cm−1; 1H-NMR: δ = 2.20 (s, 3H, CH3), 2.48 (s, 3H, CH3), 2.59 (s, 3H, CH3), 3.58 (s, 3H, OCH3), 7.13–7.74 (m, 10H, ArH’s and NH); MS, m/z (%)446 (M+, 35), 158 (54), 118 (39), 107 (35), 77 (100). Anal. calcd for C22H22N8OS (446.53): C, 59.18; H, 4.97; N, 25.09. Found: C, 59.11; H, 4.92; N, 25.02%.
5-((4-Chlorophenyl)diazenyl)-4-methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)-hydrazinyl)thiazole (12e). Orange solid, (76% yield); mp 202–204 °C; IR: ν = 3425 (NH), 1597 (C=N) cm−1; 1H-NMR: δ = 2.23 (s, 3H, CH3), 2.47 (s, 3H, CH3), 2.60 (s, 3H, CH3), 7.19–7.79 (m, 10H, ArH’s and NH); MS, m/z (%) 450 (M+, 2), 388 (12), 171 (2), 64 (100). Anal. calcd for C21H19ClN8S (450.95): C, 55.93; H, 4.25; N, 24.85. Found: C, 55.92; H, 4.13; N, 24.76%.
5-((4-Bromophenyl)diazenyl)-4-methyl-2-(2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)-hydrazinyl)thiazole (12f). Orange solid, (78% yield); mp 214–216 °C; IR: ν = 3422 (NH), 1596 (C=N) cm−1; 1H-NMR: δ = 2.22 (s, 3H, CH3), 2.47 (s, 3H, CH3), 2.60 (s, 3H, CH3), 7.17–7.82 (m, 10H, ArH’s and NH); MS, m/z (%) 494 (M+, 14), 414 (31), 171 (16), 158 (56), 142 (63), 77 (100). Anal. calcd for: C21H19BrN8S (495.40): C, 50.91; H, 3.87; N, 22.62. Found: C, 50.79; H, 3.77; N, 22.49%.
2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-4-phenyl-5-(phenyldiazenyl)-thiazole (12g). Orange solid, (73% yield); mp 198–200 °C; IR: ν = 3429(NH), 1596 (C=N) cm−1; 1H-NMR: δ = 2.49 (s, 3H, CH3), 2.64 (s, 3H, CH3), 7.32–8.23 (m, 16H, ArH’s and NH); MS, m/z (%) 478 (M+, 12), 171 (31), 158 (34), 130 (10), 118 (29), 77 (100). Anal. calcd for C26H22N8S (478.57): C, 65.25; H, 4.63; N, 23.41.Found: C, 65.18; H, 4.60; N, 23.27%.
2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-4-(naphthalen-2-yl)-5-(phenyldiazenyl)thiazole (12h). Red solid, (67% yield); mp 187–189 °C; IR: ν = 3439 (NH), 1595 (C=N) cm−1; 1H-NMR: δ = 2.24 (s, 3H, CH3), 2.62 (s, 3H, CH3), 7.17–7.83 (m, 17H, ArH’s and NH), 8.12 (s, 1H, naphthalene-H1); MS, m/z (%) 528 (M+, 2), 484 (6), 286 (8), 244 (4), 127 (39), 77 (100). Anal. calcd for C30H24N8S (528.63): C, 68.16; H, 4.58; N, 21.20.Found: C, 68.11; H, 4.46; N, 21.03%.
2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(phenyldiazenyl)-4-(thien-2-yl)thiazole (12i). Dark red solid, (70% yield); mp 176–178 °C; IR: υ ν = 3424 (NH), 1599 (C=N) cm−1; 1H-NMR: δ = 2.23 (s, 3H, CH3), 2.60 (s, 3H, CH3), 7.10–7.75 (m, 14H, ArH’s and NH); MS, m/z (%) 484 (M+, 11), 158 (31), 142 (15), 118 (26), 77 (100). Anal. calcd for C24H20N8S2(484.60): C, 59.48; H, 4.16; N, 23.12.Found: C, 59.49; H, 4.11; N, 23.03%.
3.1.7. Synthesis of 2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-4-phenylthiazole (14)
To a solution of 4-acetyl-5-methyl-1-phenyl-1H-1,2,3-triazole (1, 0.201 g, l mmol) in 2-propanol (10 mL), 2-hydrazinyl-4-phenylthiazole (13, 0.191 g, 1 mmol) was added. The mixture was refluxed for 2 h then cooled to room temperature. The solid product was filtered off, washed with ethanol and recrystalized from ethanol to afford the thiazole derivative 14 as a white solid, (73% yield); mp 182–184 °C; IR: υ = 3198 (NH), 1603 (C=N) cm−1; 1H-NMR: δ = 2.47 (s, 3H, CH3), 2.60 (s, 3H, CH3), 7.24–7.77 (m, 12H, ArH’s, thiazole H-5 and NH); MS, m/z (%) 374 (M+, 12), 230 (73), 158 (36), 104 (63), 77 (100). Anal. calcd for C20H18N6S (374.46): C, 64.15; H, 4.85; N, 22.44.Found: C, 64.10; H, 4.69; N, 22.31%.
3.1.8. Alternate Synthesis of 12g
To a solution of 14 (0.374 g, 1 mmol) in ethanol (20 mL) was added sodium acetate trihydrate (0.138 g, 1 mmol), and the mixture was cooled to 0–5 °C in an ice bath. To the resulting cold solution was added portionwise a cold solution of benzenediazonium chloride [prepared by diazotizing aniline (1 mmol) dissolved in hydrochloric acid (6 M, 1 mL) with a solution of sodium nitrite (0.07 g, 1 mmol) in water (2 mL). After complete addition of the diazonium salt, the reaction mixture was stirred for a further 30 min in an ice bath. The solid that separated was filtered off, washed with water and finally recrystallized from DMF to give a 76% of a product which was identical in all aspects (m.p., mixed m.p. and IR spectra) with those obtained from reaction of 11 with 4d.
3.1.9. Synthesis of 7-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-phenyl-2-thioxo-2,3-dihydropyrido-[2,3-d]pyrimidin-4(1H)-one (17)
A mixture of 1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-3-phenylprop-2-en-1-one (15, 2.89 g, 10 mmol) and 6-amino-2-thioxo-2,3,4-trihydro-1H-pyrimidin-4-one (16, 1.43 g, 10 mmol) in glacial acetic acid (30 mL) was heated under reflux for 5 h. After cooling, the reaction mixture was poured into ice/HCl mixture and the formed solid was collected and recrystallized from DMF to give thione 17 as yellow crystals, 79%, mp 253–255 °C; IR: ν = 3425, 3205 (2NH), 1668 (C=O), 1598 (C=N) cm−1; 1H-NMR: δ = 2.41(s, 3H, CH3), 7.15–8.24 (m, 11H, ArH’s and pyridine-H), 11.41 (br.s, 1H, NH), 11.93 (s, br., 1H, NH); MS, m/z (%) 412 (M+, 51), 294 (63), 209 (71), 149 (19), 66 (16); Anal. Calcd. For C22H16N6OS (412.47): C, 64.06; H, 3.91; N, 20.38. Found: C, 64.06; H, 3.91; N, 20.38%.
3.1.10. General Procedure for Synthesis of Pyrido[2,3-d][1,2,4]triazolo-[4,3-a] pyrimidin-5(1H)-ones 22a–h
To a solution of 17 (0.412 g, 1 mmol) and the appropriate hydrazonoyl halides 4 (1 mmol) in dioxane (20 mL) was added triethylamine (0.14 mL, 1 mmol). The reaction mixture was refluxed till all of the starting materials had disappeared (20–24 h, monitored by TLC). The solvent was evaporated and the residue was triturated with methanol. The solid formed was collected and recrystallized from the appropriate solvent to give products 22a–h. The products 22a–h together with their physical constants are listed below.
3-Acetyl-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1,6-diphenylpyrido[2,3-d][1,2,4]triazolo-[4,3-a] pyrimidin-5(1H)-one (22a). Yellow solid, (82% yield), mp 262–264 °C; IR: ν = 1670, 1651 (2C=O), 1599 (C=N) cm−1; 1H-NMR: δ = 2.42 (s, 3H, CH3), 2.68 (s, 3H, CH3),7.30–8.38 (m, 16H, ArH’s and pyridine-H); MS, m/z (%) 538 (M+, 17), 373 (18), 260 (23), 156 (21), 80 (100), 56 (23). Anal. Calcd. forC31H22N8O2 (538.56): C, 69.13; H, 4.12; N, 20.81. Found: C, 69.08; H, 4.02; N, 20.68%.
3-Acetyl-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-6-phenyl-1-(p-tolyl)pyrido[2,3-d][1,2,4]triazolo [4,3-a]pyrimidin-5(1H)-one (22b). Yellow solid, (76% yield), mp 262–264 °C; IR: ν = 1721, 1670 (2C=O), 1601(C=N) cm−1; 1H-NMR: δ = 2.26 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.64 (s, 3H, CH3), 7.14–7.99 (m, 15H, ArH’s and pyridine-H); MS, m/z (%) 552 (M+, 23), 515 (23), 370 (25), 217 (28), 106 (79), 52 (100). Anal. Calcd. For C32H24N8O2 (552.59): C, 69.55; H, 4.38; N, 20.28. Found: C, 69.38; H, 4.19; N, 20.21%.
3-Acetyl-1-(4-chlorophenyl)-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-6-phenylpyrido[2,3-d] [1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one (22c). Yellow solid, (79% yield), mp 278–280 °C; IR: ν = 1721, 1670 (2C=O), 1600 (C=N) cm−1; 1H-NMR: δ = 2.42 (s, 3H, CH3), 2.64 (s, 3H, CH3), 7.19–7.97 (m, 15H, ArH’s and pyridine-H); MS, m/z (%) 573 (M+, 5), 213 (25), 129 (32), 98 (100), 57 (94). Anal. Calcd. For C31H21ClN8O2 (573.00): C, 64.98; H, 3.69; N, 19.56. Found: C, 64.75; H, 3.61; N, 19.44%.
Ethyl 8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-oxo-1,6-diphenyl-1,5-dihydropyrido[2,3-d] [1,2,4]triazolo[4,3-a]pyrimidine-3-carboxylate (22d). Yellow solid, (73% yield), mp 246–248 °C; IR: ν = 1749, 1670 (2C=O), 1601 (C=N) cm−1; 1H-NMR: δ = 1.29 (t, J = 7.2, 3H, CH3), 2.40 (s, 3H, CH3), 4.13 (q, J = 7.2, 2H, CH2), 7.17–7.64 (m, 16H, Ar-H and pyridine-H) ppm; MS, m/z (%) 568 (M+, 14), 481 (19), 236 (17), 111 (32), 69 (10), 55 (100). Anal. Calcd. For C32H24N8O3 (568.58): C, 67.60; H, 4.25; N, 19.71. Found: C, 67.43; H, 4.20; N, 19.58%.
Ethyl 8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-oxo-6-phenyl-1-(p-tolyl)-1,5-dihydropyrido[2,3-d] [1,2,4]triazolo[4,3-a]pyrimidine-3-carboxylate (22e). Yellow solid, (71% yield), mp 225–227 °C; IR: ν = 1750, 1653 (2C=O), 1601 (C=N) cm−1; 1H-NMR: δ = 1.32 (t, J = 7.2, 3H, CH3), 2.24 (s, 3H, CH3), 2.41 (s, 3H, CH3), 4.16 (q, J = 7.2, 2H, CH2), 7.12–7.83 (m, 15H, Ar-H and pyridine-H); MS, m/z (%) 582 (M+, 22), 431 (20), 222 (33), 131 (26), 76 (100). Anal. Calcd. For C33H26N8O3 (582.61): C, 68.03; H, 4.50; N, 19.23. Found: C, 68.17; H, 4.37; N, 19.07%.
Ethyl 1-(4-chlorophenyl)-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-oxo-6-phenyl-1,5-dihydro-pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine-3-carboxylate (22f). Yellow solid, (77% yield), mp 270–272 °C; IR: ν = 1750, 1652 (2C=O), 1601 (C=N) cm−1; 1H-NMR: δ = 1.34 (t, J = 7.2, 3H, CH3), 2.43 (s, 3H, CH3), 4.21 (q, J = 7.2, 2H, CH2), 7.18–7.89 (m, 15H, Ar-H and pyridine-H); MS, m/z (%) 603 (M+, 64), 504 (67), 314 (85), 279 (73), 176 (95), 66 (100). Anal. Calcd. For C32H23ClN8O3 (603.03): C, 63.74; H, 3.84; N, 18.58. Found: C, 63.70; H, 3.75; N, 18.47%.
8-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-oxo-N,1,6-triphenyl-1,5-dihydropyrido[2,3-d][1,2,4]-triazolo[4,3-a]pyrimidine-3-carboxamide (22g). Yellow solid, (75% yield), mp 270–272 °C; IR: ν = 3325 (NH), 1668, 1651 (2C=O), 1601 (C=N) cm−1; 1H-NMR: δ = 2.24 (s, 3H, CH3), 7.13–8.06 (m, 21H, Ar-H and pyridine-H), 11.27 (br.s, 1H, NH); 13C-NMR: δ = 10.3 (CH3), 120.1, 121.2, 121.3, 121.4, 121.8, 121.9, 125.2, 125.5, 125.8, 126.8, 127.8, 128.2, 128.8, 129.2, 129.6, 129.7, 130.2, 134.1, 135.7, 136.6, 138.0,138.4, 146.6, 148.8, 153.3 (Ar-C), 165.4, 173.6 (C=O); MS, m/z (%) 538 (M+, 17), 373 (18), 260 (23), 156 (21), 80 (100), 56 (23). Anal. Calcd. For C36H25N9O2 (615.64): C, 70.23; H, 4.09; N, 20.48. Found: C, 70.28; H, 4.02; N, 20.27%.
3-Benzoyl-8-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-1,6-diphenylpyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one (22h). Yellow solid, (78% yield), mp 255–257 °C; IR: ν = 1669, 1652 (2C=O), 1600 (C=N) cm−1; 1H-NMR: δ = 2.28 (s, 3H, CH3), 7.11–8.06 (m, 21H, Ar-H and pyridine-H); MS, m/z (%) 600 (M+, 27), 504 (32), 300 (40), 148 (87), 95 (59), 67 (100). Anal. Calcd. For C36H24N8O2 (600.63): C, 71.99; H, 4.03; N, 18.66. Found: C, 71.69; H, 4.01; N, 18.54%.
3.2. Evaluation of the Antitumor Activity Using Viability Assay
Human breast carcinoma (MCF-7) and human hepatocellular carcinoma (HepG2) cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were grown on RPMI-1640 medium supplemented with 10% inactivated fetal calf serum and 50 µg/mL gentamycin. The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and were subcultured two to three times a week.
Potential cytotoxicity of the compounds was evaluated on tumor cells using the method of Gangadevi and Muthumary [66]. The cells were grown as monolayers in growth RPMI-1640. The monolayers of 104 cells adhered at the bottom of the wells in a 96-well microtiter plate incubated for 24 h at 37 °C in a humidified incubator with 5% CO2. The mono layers were then washed with sterile phosphate buffered saline (0.01 M, pH 7.2) and simultaneously the cells were treated with 100 µL from different dilutions of tested sample in fresh maintenance medium and incubated at 37 °C. A control of untreated cells was made in the absence of tested sample. Positive control containing doxroubcin drug was also tested as reference drug for comparison. Six wells were used for each concentration of the test sample. Every 24 h the observation under the inverted microscope was made. The number of the surviving cells was determined by staining the cells with crystal violet [66,67] followed by cell lysing using 33% glacial acetic acid and reading the absorbance at 590 nm using a microplate reader (SunRise, TECAN, Inc, Männedorf, Switzerland) after mixing well. The absorbance values from untreated cells were considered as 100% proliferation. The number of viable cells was determined using the microplate reader as previously mentioned and the percentage of viability was calculated as [1 − (ODt/ODc)] × 100%, where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells. The relation between surviving cells and drug concentration is plotted to get the survival curve of each tumor cell line after treatment with the specified compound. The 50% inhibitory concentration (IC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from the graphic plots.
4. Conclusions
Some newly synthesized compounds were evaluated for their anti-cancer activity against the human breast carcinoma (MCF-7) and human hepatocellular carcinoma (HepG2) cell lines. Also, their structure activity (SAR) was studied. The results revealed that the thiazole derivative 12a has promising antitumor activities (IC50 = 3.41 and 1.12 µM, respectively) and most of the tested compounds showed moderate anti-cancer activities.
Author Contributions
AOA, SMG designed research; AOA, SMG and SAA performed research, analyzed the data, wrote the paper. AOA, SMG read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, N.N.; Li, Y.; Yu, S.J.; Zhang, X.; Liu, X.H.; Zhao, W.G. Microwave-assisted synthesis of some novel 1,2,3-triazoles by click chemistry, and their biological activity. Res. Chem. Intermed. 2013, 39, 759–766. [Google Scholar] [CrossRef]
- Su, N.N.; Xiong, L.X.; Yu, S.J.; Zhang, X.; Cui, C.; Li, Z.M.; Zhao, W.G. Larvicidal activity and click synthesis of 2-alkoxyl-2-(1,2,3-triazole-1-yl)acetamide library. Comb. Chem. High Throughput Screen. 2013, 16, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.-Q.; Katritzky, A.R. 1,2,3-Triazoles. In Comprehensive Heterocycle Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 1–126. [Google Scholar]
- Katritzky, A.R.; Zhang, Y.; Singh, S.K. 1,2,3-Triazole formation under mild conditions via 1,3-dipolar cycloaddition of acetylenes with azides. Heterocycles 2003, 60, 1225–1239. [Google Scholar] [CrossRef]
- Christian, W.T.; Caspar, C.; Morten, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper (i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Biorn, A.C.; Cocklin, S.; Madani, N.; Si, Z.; Ivanovic, T.; Samanen, J.; Ryk, D.I.V.; Pantophlet, R.; Burton, D.R.; Freire, E.; et al. Mode of action for linear peptide inhibitors of HIV-1 gp120 interactions. Biochemistry 2004, 43, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.; Muldoon, J.; Lin, Y.C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.G.; Sharpless, B.K.; Elder, J.H.; et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 2006, 45, 1435–1439. [Google Scholar] [CrossRef]
- Brik, A.; Muldoon, J.; Lin, Y.C.; Elder, J.C.; Goodsell, D.S.; Olson, A.J.; Fokin, V.V.; Sharpless, B.K.; Wong, C.H. Rapid diversity-oriented synthesis in microtiter plates for in situ screening of HIV protease inhibitors. ChemBioChem. 2003, 4, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Gao, Y.; Hou, Y.L.; Zhang, C.; Yu, S.J.; Bian, Q.; Li, Z.M.; Zhao, W.G. Design, synthesis, and fungicidal evaluation of a series of novel 5-methyl-1H-1,2,3-trizole-4-carboxyl amide and ester analogues. Eur. J. Med. Chem. 2014, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Brockunier, L.L.; Parmee, E.R.; Ok, H.O.; Candelore, M.R.; Cascieri, M.A.; Colwell, L.F.; Eng, L.; Feeney, W.P.; Forrest, M.J.; Hom, G.J.; et al. Human beta3-adrenergic receptor agonists containing 1,2,3-triazole substituted benzenesulfonamides. Bioorg. Med. Chem. Lett. 2000, 10, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Ramos, M.J. Structural basis for the GSK-3beta binding affinity and selectivity against CDK-2 of 1-(4-aminofurazan-3yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Olesen, P.H.; Sørensen, A.R.; Ursö, B.; Kurtzhals, P.; Bowler, A.N.; Ehrbar, U.; Hansen, B.F. Synthesis and in vitro characterization of 1-(4-Aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J. Med. Chem. 2003, 46, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Krasinski, A.; Radic, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, B.K.; Kolb, H.C. In situ selection of lead compounds by click chemistry: Target-guided optimization of aceylcholinesterase inhibitors. J. Am. Chem. Soc. 2005, 127, 6686–6692. [Google Scholar] [CrossRef] [PubMed]
- Mocharla, V.P.; Colasson, B.; Lee, L.V.; Roeper, S.; Sharpless, B.K.; Wong, C.H.; Kolb, H.C. In situ click chemistry: Enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. Int. Ed. 2005, 44, 116–120. [Google Scholar] [CrossRef]
- Caballé, C.; Urdaneta, E.; Marzo, F.; Larralde, J.; Santidrián, S. Inhibition of in vitro intestinal absorption of d-galactose by cefroxadine, cefatrizine and cefaloglycin. Indian J. Pharm. 2003, 35, 163–167. [Google Scholar]
- Syed, M.A.; Ramappa, A.K.; Alegaon, S. Synthesis and evaluation of antitubercular and anti fungal activity of some novel 6-(4-substituted aryl)-2-(3,5-dimethyl-1H-pyrazol-1-yl)imidazo[2,1-b][1,3,4] thiadiazole derivatives. Asian J. Pharm. Clin. Res. 2013, 6, 47–51. [Google Scholar]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Tong, J.Y.; Sun, N.B.; Wu, H.K.; Liu, X.H. Synthesis, crystal structure and biological activity of N-(5-(O-tolyl)-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamide. J. Chem. Soc. Pak. 2013, 35, 1349–1353. [Google Scholar]
- Yang, M.Y.; Zhao, W.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Liu, X.H. Synthesis and biological activity of acylthiourea derivatives contain 1,2,3-thiadiazole and 1,3,4-thiadiazole. Lett. Drug Des. Discov. 2015. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Da, Y. Synthesis of 2-(5-(2-chlorophenyl)-2-furoylamino)-5-aryloxymethyl-1,3,4-thiadiazoles under microwave irradiation. Synth. Commun. 2001, 31, 1829–1836. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Ma, Y.; Zhang, C.; Dong, W.; Pan, L.; Wang, B.; Li, Z. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropane carboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Singh, A.K.; Jaiswal, N.; Singh, D. Synthesis and pharmacological activity of 1,3,4-thiadiazole derivatives: A review. Int. Res. J. Pharm. 2012, 3, 70–82. [Google Scholar]
- Gomha, S.M.; Khalil, K.D.; El-Zanate, A.M.; Riyadh, S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 2013, 87, 1109–1120. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis under microwave irradiation of [1,2,4]triazolo[3,4-b] [1,3,4]thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011, 16, 8244–8256. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new heterocycles derived from 3-(3-methyl-1H-indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull. Korean Chem. Soc. 2012, 33, 2985–2990. [Google Scholar] [CrossRef]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G., Jr.; Connolly, C.J.C.; Doherty, A.M.; Klutchko, S.R.; Sircar, I.; Steinbaugh, B.A.; et al. Structure-activity relationships of a series of 2-amino-4-thiazole containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.N.; Xavier, F.P.; Vasu, K.K.; Chaturvedi, S.C.; Pancholi, S.S. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzym. Inhib. Med. Chem. 2009, 24, 890–897. [Google Scholar] [CrossRef]
- Jaen, J.C.; Wise, L.D.; Caprathe, B.W.; Tecle, H.; Bergmeier, S.; Humblet, C.C.; Heffner, T.G.; Meltzner, L.T.; Pugsley, T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990, 33, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Ishikawa, H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg. Med. Chem. Lett. 1994, 4, 1601–1606. [Google Scholar] [CrossRef]
- Bell, F.W.; Cantrell, A.S.; Hogberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordon, C.L.; Kinnick, M.D.; Lind, P.; Morin, J.M., Jr.; Noreen, R.; et al. Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Ergenc, N.; Capan, G.; Gunay, N.S.; Ozkirimli, S.; Gungor, M.; Ozbey, S.; Kendi, E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 343–347. [Google Scholar] [CrossRef]
- Carter, J.S.; Kramer, S.; Talley, J.J.; Penning, T.; Collins, P.; Graneto, M.J.; Seibert, K.; Koboldt, C.; Masferrer, J.; Zweifel, B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Badorc, A.; Bordes, M.F.; de Cointet, P.; Savi, P.; Bernat, A.; Lale, A.; Petitou, M.; Maffrand, J.P.; Herbert, J.M. New orally active non-peptide fibrinogen receptor (GpIIb-IIIa) antagonists: Identification of ethyl 3-[N-[4-[4-amino[(ethoxycarbonyl)imino]methyl]phenyl]-1,3-thiazol-2-yl]-N-[1-(ethoxycarbonyl)methyl]piperid-4-yl]amino]propionate (SR 121787) as a potent and long-acting antithrombotic agent. J. Med. Chem. 1997, 40, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Theis, H.; Hanke, R.; Endermann, R.; Johannsen, L.; Geschke, F.U. seco-Cyclothialidines: New concise synthesis, inhibitory activity toward bacterial and human DNA topoisomerases, and antibacterial properties. J. Med. Chem. 2001, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abbas, S.E.S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d] [1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G(1) cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, A.V.; Chernyshev, V.M. Molecular structure of 3-amino[1,2,4]triazolo-[4,3-a] pyrimidin-5-one in various tautomeric forms: Investigation by DFT and QTAIM methods. Chem. Heterocycl. Compd. 2014, 50, 319–326. [Google Scholar] [CrossRef]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Hassaneen, H.M.E. Synthesis of pyrido[2,3-d][1,2,4] triazolo[4,3-a]pyrimidin-5-ones as potential antimicrobial agents. Arch. Pharm. Res. 2013, 36, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Gomha, S.M. Badrey, M.G. Ecofriendly regioselective one-pot synthesis of chromeno[4,3-d] [1,2,4]triazolo[4,3-a]pyrimidine. Eur. J. Chem. 2013, 4, 180–184. [Google Scholar] [CrossRef]
- Mohmed, A.M.; Abdelall, E.K.A.; Zaki, Y.H.; Abdelhamid, A.O. Synthesis of some new of thieno[2,3-b]pyridines, pyrazolo[1,5-a]pyrimidine, [1,2,4]triazolo[1,5-a]pyrimidine and pyrimido[1,2-a]benzimidazole derivatives containing pyridine moiety. Eur. J. Chem. 2011, 2, 509–513. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Shokry, A.S.; Tawfiek, S.M. A new approachforthe synthesis of some pyrazolo[5,1-c]triazines and pyrazolo[1,5-a]pyrimidines containing naphtofuran moiety. J. Heterocycl. Chem. 2012, 49, 116–124. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Fahmi, A.A.; Halim, K.N.M. Design and synthesis of some new pyrazolo[1,5-a]pyrimidines, pyrazolo[5,1-c]triazines, pyrazolo[3,4-d]pyridazines, oxazolo[3,4-d]pyridazines containing pyrazole moiety. Synth. Commun. 2013, 43, 1101–1126. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; Abdelhamid, A.O. One pot synthesis of pyridine, thiazolidine, pyrazole and 2,3-dihydro-1,3,4-thiadiazole derivatives under solvent-free condition. Int. J. Adv. Res. 2013, 1, 717–728. [Google Scholar]
- Abdelhamid, A.O.; Gomha, S.M. Synthesis of new pyrazolo[1,5-a]pyrimidine, triazolo[4,3-a] pyrimidine derivatives and thieno[2,3-b]pyridine derivatives from sodium 3-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-3-oxoprop-1-en-1-olate. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Gomha, S.M.; Shawali, S.A.; Abdelhamid, A.O. Convenient methods for synthesis of various fused heterocycles via utility of 4-acetyl-5-methyl-1-phenyl-pyrazole as precursor. Turk. J. Chem. 2014, 38, 865–879. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Savka, R.D.; Matiichuk, V.S.; Obushak, N.D. Synthesis and selected transformations of 1-(5-methyl-1-aryl-1H-1,2,3-triazol-4-yl)ethanones and 1-[4-(4-R-5-methyl-1Н-1,2,3-triazol-1-yl)phenyl]ethanones. Zh. Obshch. Khim. 2009, 79, 320–325. [Google Scholar]
- Abdelhamid, A.O.; Zohdi, H.F.; Rateb, N.M. Reactions with hydrazonoyl halides XXI: Reinvestigation of the reactions of hydrazonoyl bromides with 1,1-dicyanothioacetanilide. J. Chem. Res. 1999, 184, 920. [Google Scholar]
- Butler, R.N. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 621–678. [Google Scholar]
- Huisgen, R.; Grashey, R.; Seidel, M.; Knupfer, H.; Schmidt, R. 1.3-Dipolare additionen, III. Umsetzungen des diphenylnitrilimins mit carbonyl und thiocarbonyl-verbindungen. Justus Liebigs Annalen der Chemie 1962, 658, 169–180. [Google Scholar] [CrossRef]
- Yadav, R.C.; Sharma, P.K.; Singh, J. Synthesis and biological activity of 4''-substituted-2-(4'-formyl-3'-phenylpyrazole)-4-phenyl thiazole. J. Chem. Pharm. Res. 2013, 5, 78–84. [Google Scholar]
- Abdelhamid, A.O.; Abdel‐Riheem, N.A.; El‐Idreesy, T.T.; Rashdan, H.R.M. Synthesis of 5‐arylazothiazoles, pyridines and thieno[2,3‐b]pyridines derivatives containing 1,2,3‐triazole moiety. Eur. J. Chem. 2012, 3, 322–331. [Google Scholar] [CrossRef]
- Popsavin, M.; Spaić, S.; Svirčev, M.; Kojić, V.; Bogdanović, G.; Popsavin, V. Synthesis and in vitro antitumour screening of 2-(β-d-xylofuranosyl)thiazole-4-carboxamide and two novel tiazofurin analogues with substituted tetrahydrofurodioxol moiety as a sugar mimic. Bioorg. Med. Chem. Lett. 2012, 22, 6700–6704. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Nektegayev, I.; Vasylenko, O.; Grellier, P.; Lesyk, R. Isothiocoumarin-3-carboxylic acid derivatives: Synthesis, anticancer and antitrypanosomal activity evaluation. Eur. J. Med. Chem. 2014, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Opolski, A. Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorg. Med. Chem. 2006, 14, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxicity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Zayed, M.E.M.; Ng, S.W. Ethyl (Z)-2-chloro-2-(2-phenylhydrazin-1-ylidene)acetate. Acta Cryst. 2011, 67, o1962. [Google Scholar]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles-2. New routes to acetylthiadiazolines and arylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–324. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Synthesis and reactions of phenyl carbamoyl-aryl hydrazidic chlorides. Tetrahedron 1971, 27, 2517–2528. [Google Scholar]
- Abdelhamid, A.O.; El-Shiatey, F.H.H. Reactions with hydrazonoyl halides II. Synthesis and reactions of 2-bromothienyl-2-phenylhydrazone. Phosphorus Sulfur Silicon Relat. Elem. 1988, 39, 45–49. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Shawali, A.S.; Elwan, N.M.; Abounada, N.M. Reaction of 1-(2-naphthoyl) methyl-2-dimethylsulfonium bromide with N-nitroso-N-arylacetamides and reactions of the products with some nucleophiles. Sulfur Lett. 1992, 13, 273–285. [Google Scholar]
- Wolkoff, P. A new method of preparing hydrazonyl halides. Can. J. Chem. 1975, 53, 1333–1335. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr. J. Biotechnol. 2007, 6, 1382–1386. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the synthesized compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).