Abstract
To supply the increasing demand of natural high potency sweeteners to reduce the calories in food and beverages, we have looked to steviol glycosides. In this work we report the bioconversion of rebaudioside A to rebaudioside I using a glucosyltransferase enzyme. This bioconversion reaction adds one sugar unit with a 1→3 linkage. We utilized 1D and 2D NMR spectroscopy (1H, 13C, COSY, HSQC-DEPT, HMBC, 1D TOCSY and NOESY) and mass spectral data to fully characterize rebaudioside I.
1. Introduction
In the past decade the food industry has dedicated concerted efforts to develop natural non-caloric high potency sweeteners. The demand for alternative sweeteners comes from the overwhelming consumer preference for taste of sugar, but without calories. The food industry has heavily invested in the discovery of novel natural steviol glycosides that have the ability to sweeten products and provide no caloric content. Steviol glycosides are most commonly isolated from Stevia rebaudiana Bertoni, a perennial shrub of the Asteraceae (Compositae) family native to Paraguay and Brazil [1]. Isolation of steviol glycosides from S. rebaudiana leaves over the past thirty years have uncovered the wide variety of their molecular complexity [2,3,4,5,6,7,8,9,10,11]. Most recently, Ibrahim et al. isolated two novel diterpene glycosides and five known glycosides from a commercial extract of S. rebaudiana [12]. Steviolbioside, stevioside, rebaudioside A–F, dulcoside A and rubusoside are the most abundant steviol glycosides found in the leaves of S. rebaudiana [13].
Recently we reported the isolation, full characterization, and sensory properties of rebaudioside M (1) from S. rebaudiana Bertoni (Figure 1) [14,15]. Rebaudioside M is approximately 200–350 times as potent as sucrose [15]. Sensory evaluations determined rebaudioside M’s taste profile to be a clean, sweet taste, with a slightly bitter and licorice-like aftertaste. This work illustrated that the configuration of glycosylation (number of sugar units and the sugar linkages) influences the sweet taste profile of steviol glycoside molecules.
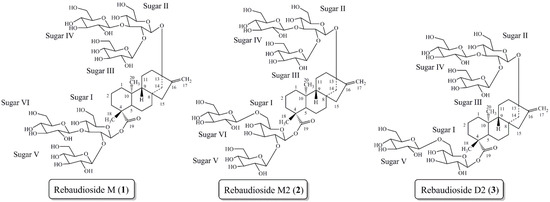
Figure 1.
Steviol glycoside rebaudioside M (1) isolated from S. rebaudiana Bertoni. Steviol glycosides rebaudioside M2 (2) and rebaudioside D2 (3) isolated from the bioconversion reaction of rebaudioside A to rebaudioside D.
Figure 1.
Steviol glycoside rebaudioside M (1) isolated from S. rebaudiana Bertoni. Steviol glycosides rebaudioside M2 (2) and rebaudioside D2 (3) isolated from the bioconversion reaction of rebaudioside A to rebaudioside D.
Due to the relative low abundance of certain sweet steviol glycosides in plant material, numerous organizations have invested in biotechnology to produce larger quantities of desired steviol glycosides [16,17,18,19,20,21,22,23]. Most recently we have pursued the bioconversion reaction of rebaudioside A to rebaudioside D with UGT (UDP-glycosyltransferase) enzymes. This bioconversion reaction allowed for the isolation and characterization of two novel steviol glycosides, rebaudioside M2 (2) and rebaudioside D2 (3) (Figure 1) [24,25]. Both molecules contain a 1→6 sugar linkage, which is rarely found in the steviol glycoside family.
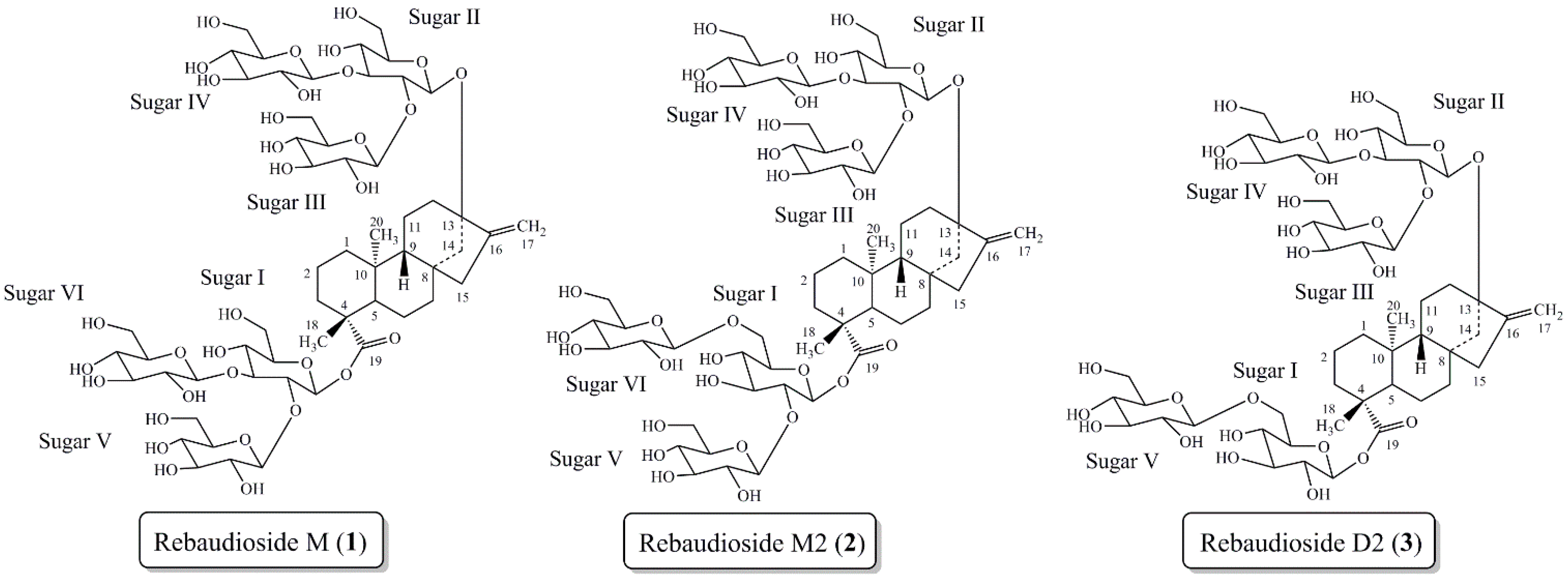
Based on previous experiences with the bioconversion reactions we investigated the bioconversion of rebaudioside A (4) to rebaudioside I (5) (Figure 2). The bioconversion reaction was analyzed by HPLC with reference standards. Rebaudioside I, a natural non-claoric sweetener, was produced with a 22.5% yield (135 mg) (based on total area percent of HPLC-MS SIM chromatography) (Figure 3). We report herein the isolation and full characterization of rebaudioside I, determined by 1D and 2D NMR experiments together with mass spectral data. Ohta et al. first reported the isolation of rebaudioside I in S. rebaudiana Morita, although full spectral assignment was not reported [9,26].
Figure 2.
Bioconversion of rebaudioside A (4) to rebaudioside I (5).
Figure 2.
Bioconversion of rebaudioside A (4) to rebaudioside I (5).
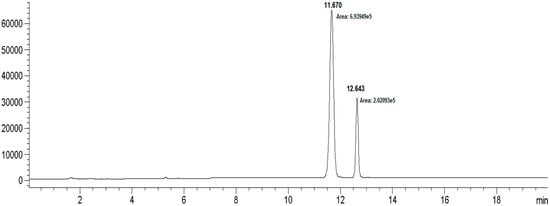
Figure 3.
HPLC chromatogram for the bioconversion of rebaudioside I from rebaudioside A.
Figure 3.
HPLC chromatogram for the bioconversion of rebaudioside I from rebaudioside A.
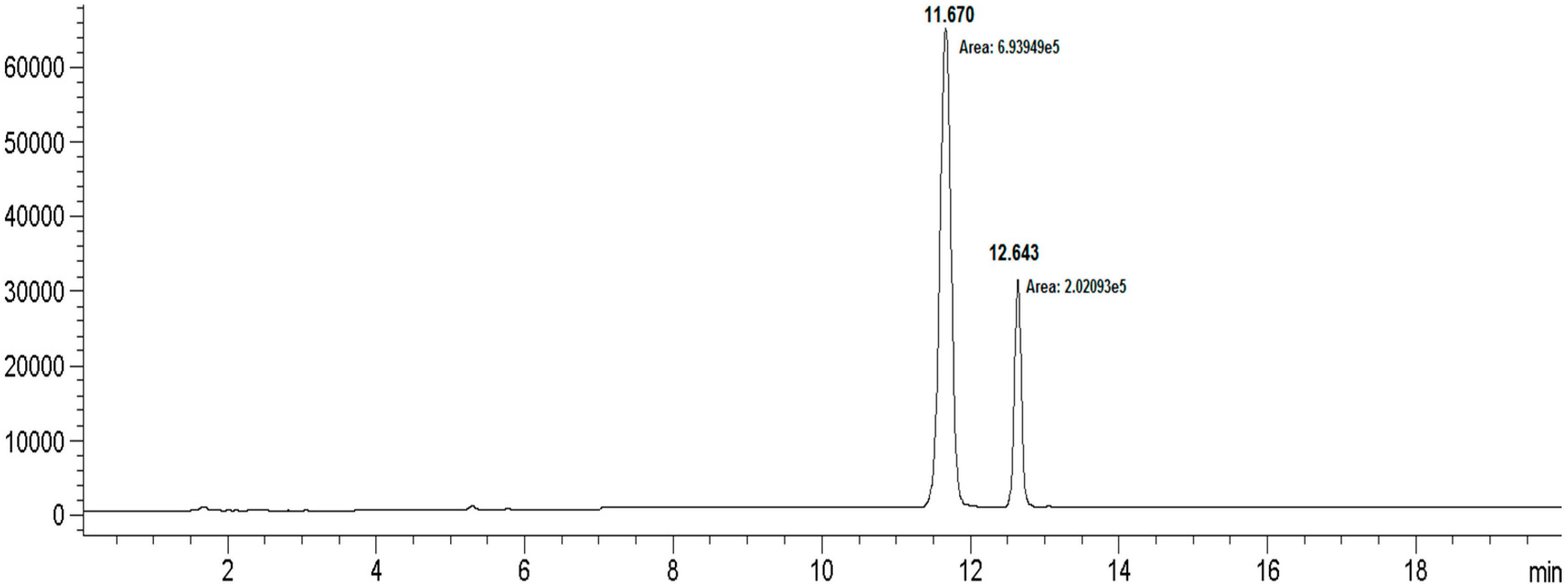
| Compound | Retention Time | MW | Peak Area |
|---|---|---|---|
| Rebaudioside I (5) | 11.670 | 1129.16 | 693,949 |
| Rebaudioside A (4) | 12.643 | 967.02 | 202,093 |
2. Results and Discussion
Rebaudioside I (5) was isolated as a white solid and accurate mass measurement using High Resolution Mass Spectrometry (HRMS) provided an exact mass m/z of 1127.4741, [M−H]− in its negative ESI-TOF mass spectrum, corresponding to the molecular formula C50H79O28 (see Supplementary Information). The MS/MS spectrum of 5, selecting the [M−H]− ion at m/z 1127.4 for fragmentation, indicated loss of two sugar units at m/z 803.5301, however it did not show additional fragmentations with a collision energy of 30 V. When a higher collision energy was applied (60 V), the parent ion was not observed, but sequential loss of three sugar units at m/z 641.4488, 479.3897, and 317.3023 were observed from m/z 803.5301.
Having confirmed the molecular weight of compound 5, a series of 1D and 2D NMR experiments were performed to allow for the full assignment of rebaudioside I. Our initial 1H-NMR spectrum was acquired at 300 K, where one of the anomeric protons was completely obscured by the water resonance. Therefore, 1H-NMR spectrum of the sample was acquired at lower temperature (292 K), to shift out the water resonance, and at this temperature all anomeric protons were sufficiently resolved (Supplementary Information). Thus, all other NMR data of 5 was acquired at 292 K.
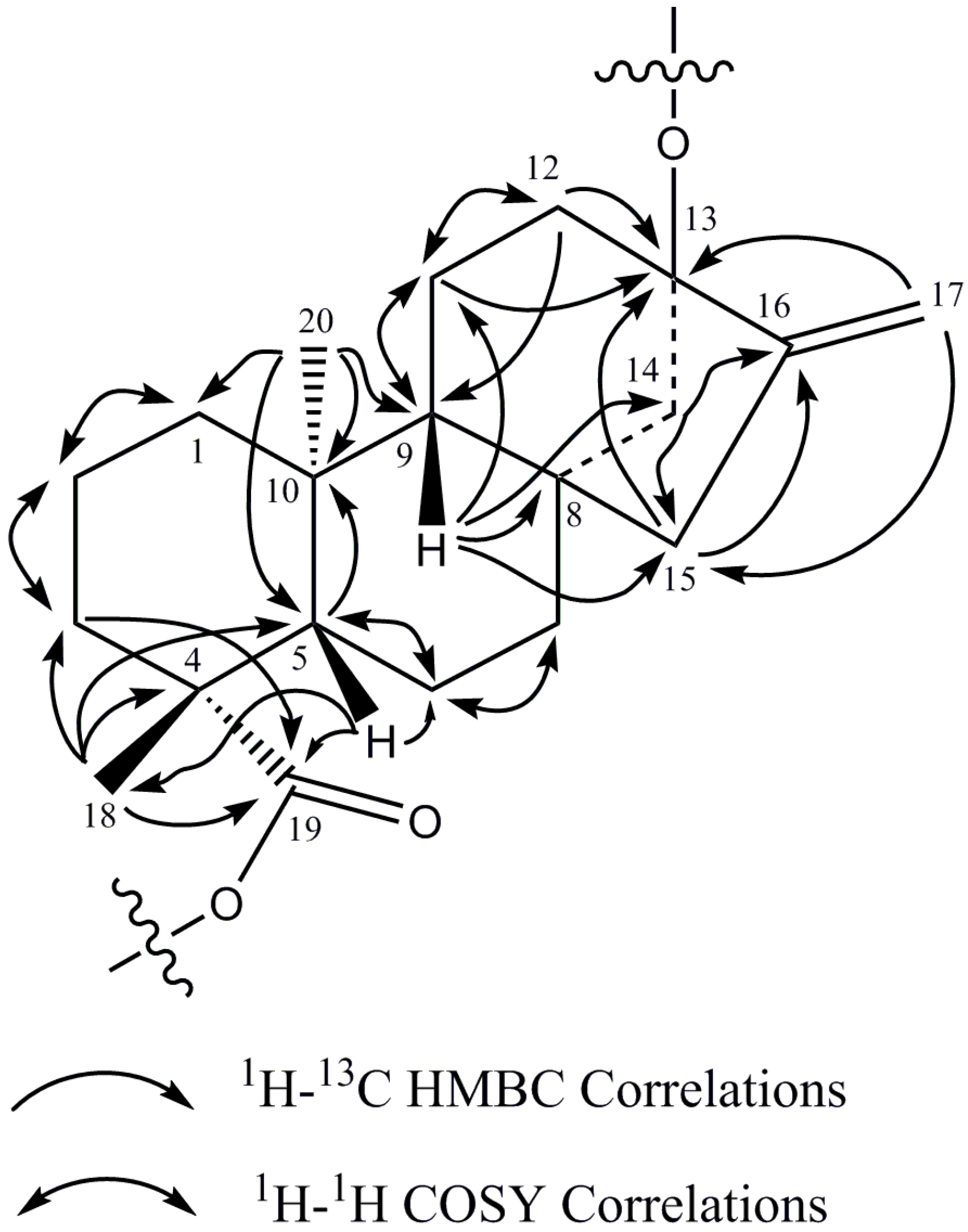
The 1H-NMR spectrum and the HSQC-DEPT data of rebaudioside I (5) indicated the existence of two methyl singlets at δ 1.22 (C-18) and 1.26 (C-20), two olefinic proton singlets corresponding to an exocyclic double bond at δH 5.02 and at 5.67 (C-17), nine methylene and two methine protons between δH 0.74–2.59 characteristic for the ent-kaurane diterpenoid isolated from other Stevia extracts [27,28]. The ent-kaurane diterpenoid aglycone central core was supported by 1H-1H COSY and 1H-13C HMBC correlations shown in Figure 4. The complete 1H and 13C assignments of the central diterpene core are provided in Table 1 (positions 1–20).
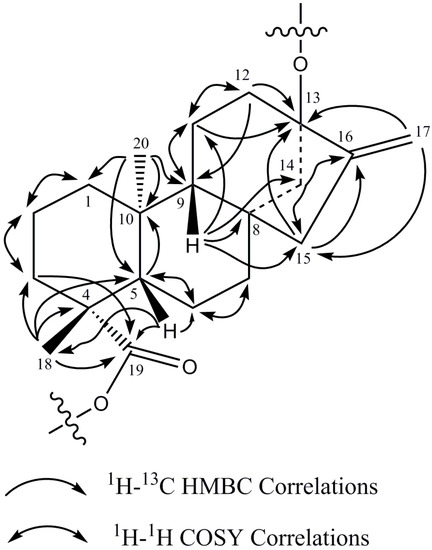
Figure 4.
1H-1H COSY and 1H-13C HMBC correlations of the diterpene core of rebaudioside I (5).
Figure 4.
1H-1H COSY and 1H-13C HMBC correlations of the diterpene core of rebaudioside I (5).
Correlations observed in the NOESY spectrum were used to assign the relative stereochemistry of the central diterpene core. In the NOESY spectrum, NOE correlations were observed between H-14 and H-20 indicating that H-14 and H-20 are on the same face of the rings. Similarly, NOE correlations were observed between H-9 and H-5 as well as H-5 and H-18. NOE correlations between H-9 and H-14 were not observed. The NOESY data thus indicate that H-5, H-9 and H-18 were on the opposite face of the rings compared to H-14 and H-20. These data thus indicate that the relative stereochemistry in the central core was retained during the glycosylation step.

Table 1.
1H- and 13C-NMR (500 and 150 MHz, pyridine-d5 + TMS), assignments of the rebaudioside I (5).
| Sugar | Position | 1H-NMR | 13C-NMR |
|---|---|---|---|
| 1 | 0.74 t (11.6), 1.75 m | 40.7 | |
| 2 | 1.44 m, 2.20 m | 19.4 | |
| 3 | 1.02 m, 2.35 m | 38.5 | |
| 4 | --- | 44.0 | |
| 5 | 1.03 m | 57.2 | |
| 6 | 1.90 m, 2.33 m | 22.2 | |
| 7 | 1.29 m, 1.31 m | 41.7 | |
| 8 | --- | 42.3 | |
| 9 | 0.88 d (6.3) | 54.1 | |
| 10 | --- | 39.8 | |
| 11 | 1.67 m, 1.70 m | 20.5 | |
| 12 | 1.98 m, 2.28 m | 37.3 | |
| 13 | --- | 86.7 | |
| 14 | 1.78 m, 2.59 d (11.9) | 44.3 | |
| 15 | 2.04 brs | 47.6 | |
| 16 | --- | 154.0 | |
| 17 | 5.02 s, 5.67 s | 104.8 | |
| 18 | 1.22 s | 28.4 | |
| 19 | --- | 176.9 | |
| 20 | 1.26 s | 15.7 | |
| I | 1' | 6.14 d (8.2) | 95.3 |
| 2' | 4.18 m | 72.5 | |
| 3' | 4.27 m | 89.4 | |
| 4' | 4.25 m | 69.2 | |
| 5' | 3.93 m | 78.2–78.8 † | |
| 6' | 4.27 m, 4.37 m | 61.7 | |
| II | 1'' | 5.06 d (7.9) | 98.0 |
| 2'' | 4.35 m | 80.6 | |
| 3'' | 4.20 m | 87.5 | |
| 4'' | 3.97 m | 70.1 | |
| 5'' | 3.80 m | 77.6 | |
| 6'' | 4.18 m, 4.49 m | 62.5 | |
| III | 1''' | 5.57 d (7.7) | 104.6 |
| 2''' | 4.21 m | 76.3 | |
| 3''' | 4.27 m | 78.2–78.6 † | |
| 4''' | 4.25 m | 72.1 | |
| 5''' | 3.94 m | 78.2–78.8 † | |
| 6''' | 4.41 m, 4.53 m | 63.1 | |
| IV | 1'''' | 5.38 d (7.9) | 104.7 |
| 2'''' | 4.01 m | 75.3 or 75.5 | |
| 3'''' | 4.28 m | 78.2–78.6 † | |
| 4'''' | 4.11 m | 72.1 | |
| 5'''' | 4.13 m | 78.2–78.6 † | |
| 6'''' | 4.25 m, 4.58 m | 62.3 or 62.4 | |
| V | 1''''' | 5.29 d (7.9) | 105.0 |
| 2''''' | 4.04 m | 75.3 or 75.5 | |
| 3''''' | 4.27 m | 78.2–78.6 † | |
| 4''''' | 4.12 m | 71.5 or 71.6 | |
| 5''''' | 4.05 m | 78.5 or 78.6 † | |
| 6''''' | 4.26 m, 4.56 m | 62.3 or 62.4 |
† Five carbon resonances in the range of 78.2–78.8 (78.16, 78.47, 78.50, 78.55, and 78.77), hence chemical shift could not be unequivocally assigned.
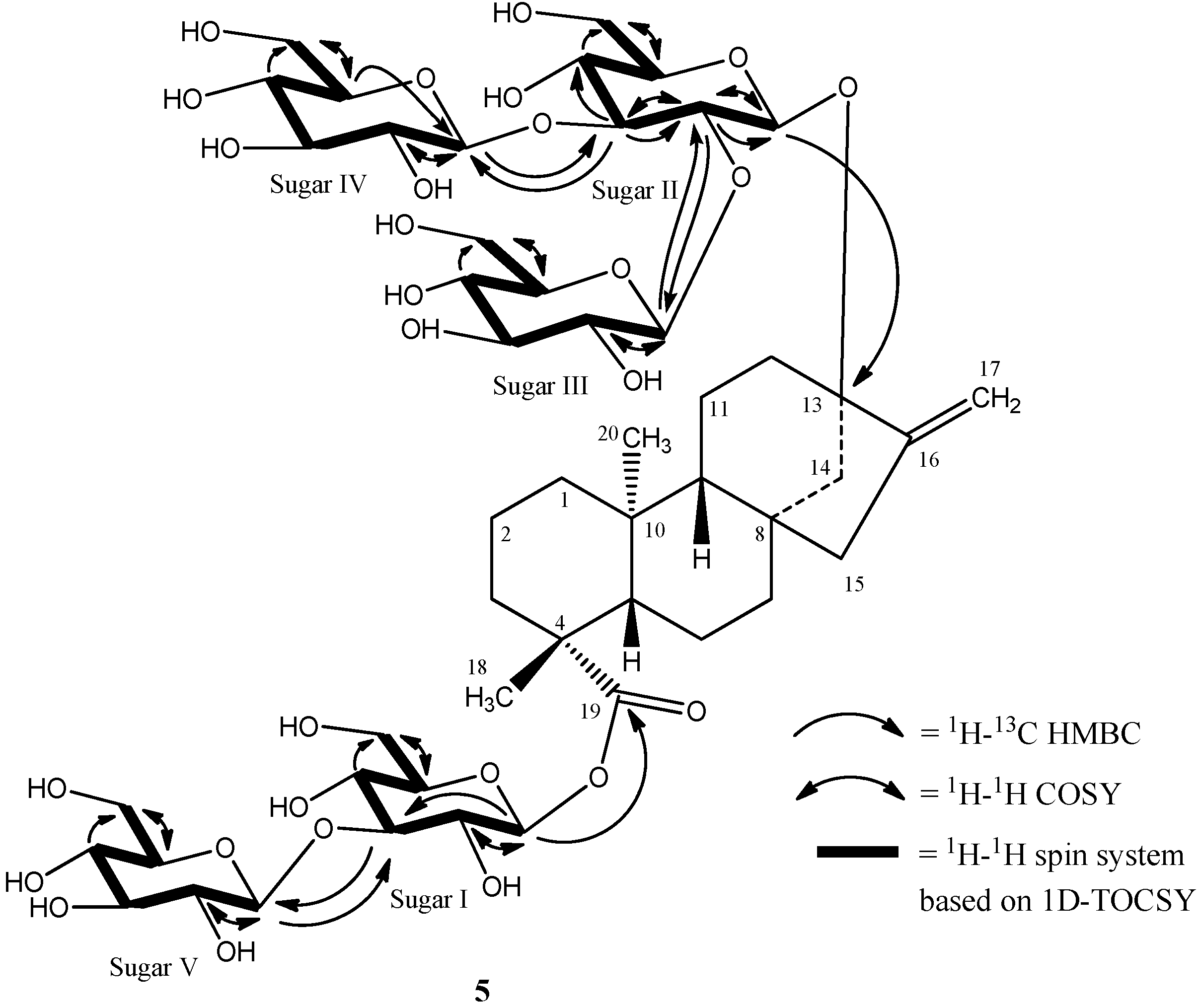
The breakdown of the 1H-13C HSQC-DEPT data for 5 confirmed the presence of five anomeric protons. All five anomeric protons were resolved in the spectra acquired at 292 K at δH 6.14 (δC 95.3), 5.57 (δC 104.6), 5.38 (δC 104.7), 5.29 (δC 105.0), and 5.06 (δC 98.0) (Table 1). Additionally, all five anomeric protons had large couplings (7.7–8.2 Hz) indicating that they had β-configurations. The anomeric proton observed at δH 6.14 showed an HMBC correlation to C-19 (δC 176.9) which indicated that it corresponds to the anomeric proton of GlcI. Similarly, the anomeric proton observed at δH 5.06 showed an HMBC correlation to C-13 (δC 86.7) allowing it to be assigned as the anomeric proton of GlcII (Figure 4).
Additional analysis of the 1D and 2D NMR data allowed the assignment of the remaining three sugars in 5. The relatively downfield chemical shift of C-3' (δC 89.4) in sugar I suggested the presence of a sugar substituent at C-3' of sugar I. Long range 1H-13C correlations observed in the HMBC experiment from the anomeric proton observed at δH 5.29 (H-1''''') to the carbon at δC 89.4 (C-3') and from H-3' at δH 4.27 to an anomeric carbon at δC 105.0 (C-1''''') confirmed the substitution at C-3' in sugar I.
The remaining two glucose moieties were assigned in a similar manner. The relatively downfield chemical shift of C-2'' (δC 80.6) and C-3'' (δC 87.5) in sugar II suggested a 2,3-branched-d-glucotriosyl substituent at C-13. Long range 1H-13C correlations observed in the HMBC experiment from the anomeric proton observed at δH 5.57 (H-1''') to the carbon at δC 80.6 (C-2'') and from H-2'' at δH 4.35 to an anomeric carbon at δC 104.6 (C-1''') confirmed the presence of a 1→2 sugar linkage between sugar III and sugar II. Similarly, the sugar substituent at C-3'' in sugar II was also corroborated by HMBC correlations observed from the anomeric proton at δH 5.38 (H-1'''') to the carbon at δC 87.5 (C-3'') and from H-3'' at δH 4.20 to the anomeric carbon (δC 104.7) of sugar IV confirmed the presence of a 1→3 sugar linkage between sugar IV and sugar II.
The complete 1H and 13C assignments for the glycoside at C-13 and C-19 were made on the basis of COSY, HSQC-DEPT, HMBC, and 1D-TOCSY data and are provided in Table 1. A summary of the key HMBC, COSY, and 1D-TOCSY correlations used to assign the glycoside are provided in Figure 5.Thus the structure of rebaudioside I (5), was confirmed as (13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl)-β-d-glucopyranosyl)oxy] ent-kaur-16-en-19-oic acid-(3-O-β-d-glucopyranosyl)-β-d-glucopyranosyl) ester].
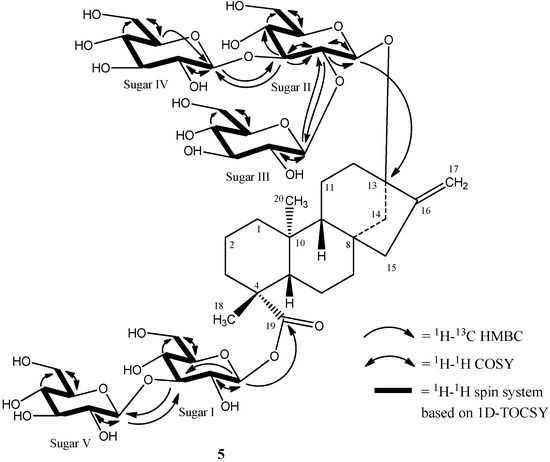
Figure 5.
Key COSY, HMBC, and 1D-TOCSY correlations of rebaudioside I (5).
Figure 5.
Key COSY, HMBC, and 1D-TOCSY correlations of rebaudioside I (5).
3. Experimental Section
3.1. General Experimental Procedures for Rebaudioside I (5)
3.1.1. Isolation of Rebaudioside I
Preliminary HPLC analyses of samples were performed using a Waters 2695 Alliance System (Waters Corp., Milford, MA, USA) equipped with a Waters 2996 Photodiode Array (PDA, Waters Corp.) and Dionex Corona Charged Aerosol (CAD Plus, Dionex, Sunnyvale, CA, USA) detectors by the following method: Phenomenex Synergi Hydro-RP, 4.6 × 250 mm, 4 µm (p/n 00G-4375-E0); Column Temp: 55 °C; Mobile Phase A: 0.00284% NH4OAc and 0.0116% HOAc in water; Mobile Phase B: Acetonitrile (MeCN); Flow Rate: 1.0 mL/min; Injection volume: 10 µL. Detection was by UV (210 nm) and CAD. Gradient: 0–8.5 min (75A:25B), 10 min (71A:29B), 16.5 min (70A:30B), 18.5–24.5 min (66A:34B), 26.5–29.0 min (48A:52B), 31–37 min (30A:70B), 38 min (75A:25B).
HPLC Isolation: The purification was performed in two chromatographic steps. The first method used a Waters Symmetry Shield RP18 (30 × 150 mm, 7 µm, p/n WAT248000) column with isocratic mobile phase conditions of 80:20 water/methanol (MeOH). Flow rate was maintained at 45 mL/min and injection load was 180 mg. Detector wavelength was set at 210 nm. The second chromatographic method used a Waters Atlantis dC18 (30 × 100 mm, 5 µm, p/n 186001375) column with isocratic mobile phase conditions of 80:20 water/MeCN. Flow rate was maintained at 45 mL/min and detector wavelength was set at 210 nm. The analyses of fractions were performed using a Waters Atlantis dC18 (4.6 × 150 mm, 5 µm, p/n 186001342) column; Mobile Phase A: water; Mobile Phase B: MeCN; Flow Rate: 1 mL/min; Isocratic mobile phase conditions: 75:25 A/B for 30 min. The peak for 5 was observed at a retention time (tR) of approximately 17 min. The isolated compound was recovered from the eluent via rotary evaporation (Buchi R-114 Rotovapor) and lyophilization (FTS System benchtop lyophilizer). Purity of the final product was 91% as confirmed by LC-CAD. Approximately 1 mg of 5 was provided for spectroscopic and spectrometric analyses.
3.1.2. Nuclear Magnetic Resonance
The sample was prepared by dissolving ~1.0 mg in 180 µL of pyridine-d5 + TMS, and NMR data were acquired on a Bruker Avance 500 MHz instrument with either a 2.5 mm inverse probe or a 5 mm broad band probe. The 13C- and HMBC-NMR data were acquired at Rensselaer Polytechnic Institute using their Bruker Avance 600 MHz and 800 MHz instruments with 5 mm cryo-probe, respectively. The 1H- and 13C-NMR spectra were referenced to the TMS resonance (δH 0.00 ppm and δC 0.0 ppm).
3.1.3. Mass Spectrometry
MS and MS/MS data were generated with a Waters Quadrupole Time-of-Flight Micro mass spectrometer equipped with an electrospray ionization source. The sample was analyzed by negative ESI. The sample was diluted to a concentration of 0.25 mg/mL with H2O:MeCN (1:1) and introduced via flow injection for MS data acquisition. The sample was diluted further to 0.01 mg/mL to yield good s/n to tune for MS/MS and acquired by direct infusion. The collision energy was set to 60 V in order to acquire MS/MS data with increased fragment ion peaks due to the nature of the molecule.
3.2. Bioconversion Reaction
Rebaudioside I (5) was isolated from bioconversion reaction of rebaudioside A (4) by a proprietary glucosyltransferase from PureCircle Ltd. In vivo production of glycosylation enzymes were expressed in E. coli. Rebaudioside A to rebaudioside I conversion with glucosyltransferase UGT76G1-R11-F12 experimental condition are as follows: the reaction mixture (40 mL) contained 0.5 mM rebaudioside A, 3 mM MgCl2, 50 mM sodium phosphate buffer at pH 7.5, 2.5 mM UDP-glucose, and 4.0 mL of UGT76G1-R11-F12 (2.5 U/mL). The reaction was run at 30 °C on an orbitary shaker at 135 rpm. For sampling 125 μL of the reaction mixture was quenched with 10 μL of 2N H2SO4 and 115 μL of methanol/water (7/3). The samples were immediately centrifuged and kept at 10 °C before analysis by by LC-MS. An Agilent 1200 series HPLC system, equipped with binary pump (G1312B), autosampler (G1367D), thermostatted column compartment (G1316B), DAD detector (G1315C), connected with Agilent 6110A MSD, and interfaced with “LC/MSD Chemstation” software, was used. After 42 h. of reaction, 20 mL of the reaction mixture was quenched with 20 mL of ethanol and used for isolation and structure elucidation.
HPLC analyses of samples were performed using an Agilent 1200 series HPLC system equipped with a binary pump (G1312B), autosampler (G1367D), thermostatted compartment (G13136B) and DAD detector (G1315C), connected with Agilent 6110 A MSD, and interfaced with “LC/MSD Chemstation” software. The conditions used were Phenomenex Kinetex, 2.6 μm C18 100A, 4.6 mm × 150 mm, 2.6 μm; Column Temp: 55 °C; Mobile Phase A: 0.1% formic acid in water; Mobile Phase B: Acetonitrile (MeCN); Flow Rate: 0.8 mL/min; Injection volume: 2 µL. Detection was by DAD (210 nm) and MSD (Scan and SIM mode, ES-API, negative polarity). Gradient: 0–8.5 min (76A:24B), 10 min (71A:29B), 16.5 min (70A:30B). SIM parameters 0–4.0 min (SIM ion 1289–1291), 4–11 min (SIM ion 1127–1290), 11–22 min (SIM ion 965–967).
4. Conclusions
To our best knowledge this is the first report of full isolation and spectral characterization of (13-[(2-O-β-d-glucopyranosyl-3-O-β-d-glucopyranosyl)-β-d-glucopyranosyl)oxy]-ent-kaur-16-en-19-oic acid-(3-O-β-d-glucopyranosyl)-β-d-glucopyranosyl), ester] (rebaudioside I, 5), from a bioconversion reaction of rebaudioside A. By employing 1D and 2D NMR spectroscopy (1H, 13C, COSY, HSQC-DEPT, HMBC, 1D TOCSY and NOESY) and mass spectral data, we have completed the full structure elucidation of rebaudioside I. The conversion of rebaudioside A to rebaudioside I demonstrated the power of biotechnology to synthesize a novel steviol glycoside. With continued progress of isolation and characterization of novel steviol glycosides, further relationships between the amount of sugar units connected at C-13 and C-19 of the steviol aglycone core and degree of sweetness will be uncovered.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/11/17345/s1.
Supplementary Files
Supplementary File 1Acknowledgments
We thank PureCircle and Libragen for the bioconversion reaction material. We also wish to thank all our partners at AMRI.
Author Contributions
Indra Prakash, Cynthia Bunders and Romila D. Charan wrote the manuscript and did interpretation of NMR data. Krishna P. Devkota ran NMR experiments and analyzed NMR data. Catherine Ramirez, Tara Snyder and Christopher Priedemann isolated rebaudioside I. Avetik Markosyan, Cyrille Jarrin and Robert Ter Halle performed bioconversion reactions of rebaudioside I.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lewis, W.H. Early uses of Stevia rebaudiana (Asteraceae) leaves as a sweetener in Paraguay. Econ. Bot. 1992, 46, 336–337. [Google Scholar]
- Ceunen, S; Geuns, J.M.C. Steviol Glycosides: Chemical Diversity, Metabolism, and Function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar]
- Chaturvedula, V.S.P.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpenoid glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar]
- Kohda, H.; Kasai, R.; Yamsaki, K.; Murakami, K.; Tanaka, O. New sweet diterpene glucosides from Stevia rebaudiana. Phytochemistry 1976, 15, 981–983. [Google Scholar] [CrossRef]
- Kobayashi, M.; Horikawa, S.; Degrandi, I.H.; Ueno, J.; Mitsuhashi, H. Dulcosides A and B, new diterpene glycosides from Stevia rebaudiana. Phytochemistry 1977, 16, 1405–1408. [Google Scholar] [CrossRef]
- Starratt, A.N.; Kirby, C.W.; Pocs, R.; Brandle, J.E. Rebaudioside F, a diterepene glycoside from Stevia rebaudiana. Phytochemistry 2002, 59, 367–370. [Google Scholar] [CrossRef]
- Sakamoto, I.; Yamasaki, K.; Tanaka, O. Rebaudioside-C, a new sweet diterpene glycosides of Stevia rebaudiana. Chem. Pharm. Bull. 1977, 25, 844–846. [Google Scholar] [CrossRef]
- Sakamoto, I.; Yamasaki, K.; Tanaka, O. Application of 13C-NMR spectroscopy to chemistry of plant glycosides: Rebaudioside-D and -E, new sweet diterpene-glucosides of Stevia rebaudiana Bertoni. Chem. Pharm. Bull. 1977, 25, 3437–3439. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Mosettig, E.; Beglinger, U.; Dolder, F.; Lichti, H.; Quitt, P.; Waters, J.A. The absolute configuration of steviol and isosteviol. J. Am. Chem. Soc. 1963, 85, 2305–2309. [Google Scholar] [CrossRef]
- Kinghorn, A.A.D.; Soejarto, D.D.; Nanayakkara, N.P.D.; Compadre, C.M.; Makapugay, H.C.; Hovanec-Brown, J.M.; Medon, P.J.; Kamath, S.K. A phytochemical screening procedure for sweet ent-Kaurene glycosides in the genus Stevia. J. Nat. Prod. 1984, 47, 439–444. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Rodenburg, D.L.; Alves, K.; Fronczek, F.R.; McChesney, J.D.; Wu, C.; Nettles, B.J.; Venkataraman, S.V.; Jaksch, F. Minor Diterpene Glycosides from the Leaves of Stevia rebaudiana. J. Nat. Prod. 2014, 77, 1231–1235. [Google Scholar] [CrossRef]
- JEFCA. Steviol glycosides. In Combined Compendium of Food Additive Specifications [FAO JECFA Monographs 10], Proceedings of the 73rd Joint FAO/WHO Expert Committee on Food Additives (JECFA), Geneva, Switzerland, 8–17 June 2010; Food and Agriculture Organization of the United nations (FAO), Joint FAO/WHO Expert Committee on Food Additives (JECFA): Rome, Italy, 2010. [Google Scholar]
- Prakash, I.; Chaturvedula, V.S.P.; Markosyan, A. Isolation, characterization and sensory evaluationof a Hexa-d-glucopyranosyl diterpene from Stevia rebaudiana. Nat. Prod. Commun. 2013, 8, 1523–1526. [Google Scholar]
- Prakash, I.; Markosyan, A.; Bunders, C. Development of Next Generation Stevia Sweeteners: Rebaudioside M. Foods 2014, 3, 162–175. [Google Scholar]
- Richman, A.; Swanson, A.; Humphrey, T.; Chapman, R.; McGarvey, B.; Pocs, R.; Brandle, J.E. Functional genomics uncovers three glucosyltransferases involved in the synthesis of the major sweet glucosides of Stevia rebaudiana. Plant J. 2005, 41, 55–67. [Google Scholar]
- Philippe, R.; de Mey, M.; Anderson, J.; Kumaran Ajikumar, P. Biotechnological production of natural zero-calorie sweeteners. Curr. Opin. Biotechnol. 2014, 26, 155–161. [Google Scholar] [CrossRef]
- Kumar, H.; Kaul, K.; Bajpai-Gupta, S.; Kaul, V.; Kumar, S. A comprehensive analysis of fifteen genes of steviol glycosides biosynthesis pathway in Stevia rebaudiana (Bertoni). Gene 2012, 492, 276–284. [Google Scholar] [CrossRef]
- Humphrey, T.; Richman, A.; Menassa, R.; Brandle, J.E. Spatial organization of four enzymes from Stevia rebaudiana that are involved in steviol glycoside synthesis. Plant Mol. Biol. 2006, 61, 47–62. [Google Scholar] [CrossRef]
- Brandle, J.E.; Telmer, P.G. Steviol glycoside biosynthesis. Phytochemistry 2007, 68, 1855–1863. [Google Scholar] [CrossRef]
- Mohahed, A.; Ceunen, S.; Geuns, J.M.C.; van den Ende, W.; de Ley, M. UDP-dependent glycosyltransferases involved in the biosynthesis of steviol glycosides. J. Plant Physiol. 2011, 168, 1136–1141. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Hansen, J.; Simon, E.; Brianza, F.; Semmler, A.; Olsson, K.; Carlsen, S.; During, L.; Ouspenski, A.; Hicks, P. Methods for Improved Production of Redaudioside D and Rebaudioside M. WO2014/122227 14 August 2014. [Google Scholar]
- Markosyan, A.; Prakash, I.; Prakash Chaturvedula, V.S. High-purity Steviol Glycosides. WO2013/176738, 28 November 2013. [Google Scholar]
- Prakash, I.; Bunders, C.; Devkota, K.P.; Charan, R.D.; Ramirez, C.; Priedemann, C.; Markosyan, A. Isolation and Characterization of a Novel Rebaudioside M Isomer from a Bioconversion Reaction of Rebaudioside A and NMR Comparison Studies of Rebaudioside M Isolated from Stevia rebaudiana Bertoni and Stevia rebaudiana Morita. Biomolecules 2014, 4, 374–389. [Google Scholar] [CrossRef]
- Prakash, I.; Bunders, C.; Devkota, K.P.; Charan, R.D.; Ramirez, C.; Priedemann, C.; Markosyan, A. Isolation and Structure Elucidation of Rebaudioside D2 from Bioconversion Reaction of Rebaudioside A to Rebaudioside D. Nat. Prod. Commun. 2014, 9, 1135–1138. [Google Scholar]
- Morita, T.; Isao, F.; Fumito, M.; Masaya, O. New Steviol Glycoside. U.S. Patent 2011/0183056, 28 July 2011. [Google Scholar]
- Chaturvedula, V.S.P.; Clos, J.F.; Rhea, J.; Milanowski, D.; Mocek, U.; DuBois, G.E.; Prakash, I. Minor diterpenoid glycosides from the leaves of Stevia rebaudiana. Phytochem. Lett. 2011, 4, 209–212. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Structures of the novel α-glucosyl linked diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 2034–2038. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
