Abstract
A series of chalcones a1–20 bearing a 4-OMe groups on the A-ring were initially synthesized and their anticancer activities towards HepG2 cells evaluated. Subsequently, a series of chalcones b1–42 bearing methoxy groups at the 2' and 6'-positions of the B-ring were synthesized and their anticancer activities towards five human cancer cell lines (HepG2, HeLa, MCF-7, A549 and SW1990) and two non-tumoral human cell lines evaluated. The results showed that six compounds (b6, b8, b11, b16, b18, b22, b23 and b29) displayed promising activities, with compounds b22 and b29 in particular showing higher levels of activity than etoposide against all five cancer cell lines. Compound b29 showed a promising SI value compared with both HMLE and L02 (2.1–6.5 fold in HMLE and > 33 > 103.1 fold in L02, respectively).
1. Introduction
Chalcones (1,3-diaryl-2-propen-1-ones, Figure 1) are naturally occurring compounds that are widely distributed in a variety of plant species. Compounds belonging to this structural class have been identified as both intermediates and end products in the biosynthesis of flavonoids, and make significant contributions to the medicinal value of herbs. The ease of preparation of these compounds, as well as their potential for oral administration and safety, make them good candidates for use as therapeutic agents in drug discovery [1]. For these reasons, chalcones have attracted considerable attention from chemists and pharmacologists, and a large number of results have been reported in the literature pertaining to the biological activities of both naturally occurring and synthetic chalcone compounds, including their anti-inflammatory [2,3], antimicrobial [4,5,6], antifungal [7,8], antioxidant [9] and anticancer activities [10,11,12,13,14,15].
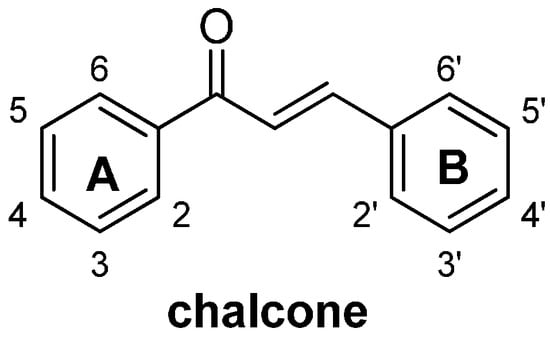
Figure 1.
General structure of chalcones.
The antitumor properties of chalcones have received considerable attention during the last few years, because they operate via a similar mode of action to the structurally related combretastatin [16]. The frequent occurrence of polymethoxyphenyl-type moieties in naturally occurring anticancer agents, such as colchicine and combretastatin, has inspired scientists to investigate the synthesis and biological evaluation of methoxylated chalcones, and the results of these studies have shown that methoxy groups generally make a significant contribution to the cytotoxicity of these compounds [17,18,19].
To develop potential anticancer drugs based on chalcones, as well as improve our understanding of the structure-activity relationships (SARs) of these compounds, we report herein the synthesis of a series of alkoxylated chalcones. The cytotoxic activities of these 62 compounds have also been evaluated against a panel of five cancer cell lines and two normal human cell lines, including HepG2, HeLa, MCF-7, A549, SW1990, HMLE and L02 cells.
2. Results and Discussion
Compounds a1–20 bearing a 4-OMe on their A-ring (i.e., their 1-phenyl moiety) were initially synthesized and evaluated in terms of their activity towards human liver carcinoma HepG2 cells using a SRB assay. This evaluation was conducted to develop a better understanding of the structural-activity relationship (SAR) of the B-ring of these compounds and the activity results (percentage rates at concentration of 10 μM) are shown in Table 1.

Table 1.
Inhibitory activities of the 4-methoxyl chalcones a1–20 against HepG2 cells (10 μM). 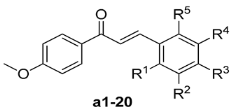

| Compound | R1 | R2 | R3 | R4 | R5 | Inhibition (%) ** |
|---|---|---|---|---|---|---|
| a1 | H | H | H | H | H | 42.4 ± 2.0 |
| a2 | H | H | Br | H | H | 37.6 ± 4.4 |
| a3 | F | H | Br | H | H | 41.8 ± 5.9 |
| a4 * | Br | H | F | H | H | 41.2 ± 4.6 |
| a5 * | Cl | H | Br | H | H | 9.0 ± 5.6 |
| a6 | H | H | NO2 | H | H | 9.8 ± 5.0 |
| a7 | H | H | COOH | H | H | 32.7 ± 3.0 |
| a8 | OCH3 | H | H | H | H | 61.4 ± 3.4 |
| a9 | H | OCH3 | H | H | H | 59.0 ± 2.9 |
| a10 | H | H | OCH3 | H | H | 27.7 ± 17.7 |
| a11 | H | H | OCH2CH3 | H | H | 13.1 ± 8.4 |
| a12 | OCH3 | OCH3 | H | H | H | 66.3 ± 4.2 |
| a13 | OCH3 | H | H | OCH3 | H | 77.0 ± 2.2 |
| a14 * | OCH3 | H | H | H | OCH3 | 78.1 ± 4.7 |
| a15 | H | OCH3 | OCH3 | H | H | 65.0 ± 17.6 |
| a16 | H | OCH3 | H | OCH3 | H | 70.6 ± 2.0 |
| a17 | H | OCH2Ph | OCH3 | H | H | 44.1 ± 6.2 |
| a18 | OCH3 | OCH3 | OCH3 | H | H | 31.8 ± 7.3 |
| a19 | OCH3 | H | OCH3 | OCH3 | H | 38.1 ± 10.3 |
| a20 | H | OCH3 | OCH3 | OCH3 | H | 71.1 ± 1.3 |
| Etoposide | 63.9 ± 0.1 |
*: Novel compound; **: The results are shown as the average percentage of the individual mean (±SD, standard deviation) for three independent experiments.
The introduction of halogen atoms (i.e., compounds a2–5), a NO2 substituent (a6) and a COOH group (a7) on the B-ring led to a decrease in the activity of these compounds compared with the parent compound a1.Contrary to a2–7, compounds a8–20 which were substituted with electron donating groups (i.e., OMe, OEt and OBn), generally exhibited moderate to high levels of inhibition. A single OMe group substituted at the 2'-position (a8) or 3'-position (a9) led to much better inhibition than that of the 4'-position (a10). A comparison of a10 vs. a11 and a15 vs. a17 revealed that bulky substituents seemed to be poorly tolerated at the 3' and 4'-position. Interestingly, the substitution of the B ring with two OMe groups (compounds a12–16) led to a general increase in the activity (inhibition 65.0%–78.1%), with bis-substitution at the 2' and 6' positions (a14) providing the best results. The trimethoxy- substituted chalcones synthesized in the current study (i.e., compound a18–20) showed weak anti-proliferative activity towards HepG2 cells, except for the 3',4',5'-trimethoxy compound a20.
It is possible to draw some conclusions from the results in Table 1, including: (1) the introduction of electron donating groups to the B-ring generally made a positive contribution to the cytotoxicity, whereas electron withdrawing groups tended to have an adverse impact on the cytotoxicity; (2) the position and size of the substituents might have a specific impact on the activity of these compounds; and (3) the presence of two OMe moiety on the B-ring led to an remarkable increase in the cytotoxic activity.
With a aim of identifying potential anticancer agents with enhanced levels of activity, as well as verifying the conclusions provided above, we took a14 as a starting compound, and then designed and synthesized a series of chalcones b1–42 bearing methoxy groups at the 2' and 6'-positions of the B-ring, whilst varying the substituents on the A-ring. The cytotoxicities of the resulting chalcones were evaluated in vitro against five human cancer cell lines and other two non-tumoral cell lines, including HepG2, HeLa, MCF-7, A549, SW1990, HMLE and L02, and the results are shown in Table 2.
Compounds bearing electron donating groups (i.e., Me, OMe, OEt, OBn, OCH2O, CH2CH2O, Ph, NH2 and OH) generally exhibited higher levels of anticancer activity than those with electron withdrawing groups (i.e., F, Cl, Br, NO2, CN and CF3). Furthermore, chalcones substituted with alkoxyl groups exhibited greater cytotoxic activities than those substituted with methyl, amino or hydroxyl groups (e.g., the IC50 values of b6–11 were lower than those of b2–5 and b23–27). A comparison of b2–4, b6–13 and b24–26 revealed that both the position and the size of the substituents had a significant impact on the cytotoxic activities of the compounds, with the position effect appearing to be of the order o > m > p (confirmed by b30–33), and the size contribution following the order OMe > OEt > OBn (b11 is an exception to this order).
A review of compounds b30 to b42 revealed that those with electron withdrawing groups exhibited weak cytotoxic activity, and that the cytotoxic activities of these compounds were related to the electrophilic nature of their substituents. For example, nearly all of the IC50 values for compounds b30 and b39–42, which had a CN, NO2 or CF3 group on their A-ring, were greater than 30 µM.
It’s noteworthy that compound b6, b8, b11, b16, b18, b22, b23 and b29 exhibited the most outstanding activities among all the compounds tested in this study, with most of their in vitro IC50 values against the five human cancer lines being less than 10 µM. Compound b22 was 20.4- and 23.6-fold as potent than etoposide against HepG2 and SW1990 cells, respectively, and compound b29 was 11.5-fold more potent than etoposide against A549 cells. These two compounds displayed the best activity and therefore are deserving of further investigation.
All compounds were also evaluated for their cytotoxic activity against two non-tumoral human cell lines named human mammary epithelial cell (HMLE) and L02 (a human liver cell line) in Table 2 and SI values between the CC50 in non-tumoral cell lines and IC50 in tumoral cells calculated. The data indicate that about one third of the compounds (b8, b11–13, b16, b18–24, b28–29, b31–32, b34, b36–38) exhibit more than 2-fold better SI between the CC50 in L02 and IC50 in tumoral cells, which is similar to the value of etoposide. Among them, compounds b11, b16, b22, b23 exhibited over 5-fold better SI toward L02. However, most compounds’ SI values between the CC50 in HMLE and IC50 in tumoral cells are unsatisfactory, except for b11, b27, b29 and b31 (2–7 fold), which is also similar to etoposide. Fortunately, compound b11, and especially b29, showed promising SI values compared with both HMLE and L02 (2.1–6.5 fold in HMLE and >33–>103.1 fold, respectively).

Table 2.
Cytotoxicities of the 2',6'-dimethoxyl chalcones b1–b42 against five human cancer cell lines and two non-tumoral cell lines. 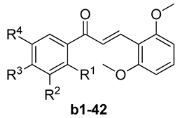

| Compound | R1 | R2 | R3 | R4 | Human Cancer Cell Lines, IC50 (µM) & SI | Normal Human Cell Lines, CC50 (µM)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HepG2 | HeLa | MCF7 | A549 | SW1990 | HMLE | L02 | ||||||||||
| IC50 a | IC50 a | IC50 a | IC50 a | IC50 a | ||||||||||||
| SI c | SI d | SI e | SI f | SI g | SI h | SI i | SI j | SI k | SI l | |||||||
| b1 # | H | H | H | H | 20.98 ± 1.75 | >30 | 28.18 ± 0.04 | >30 | >30 | 9.47 ± 0.39 | 33.340.39e | |||||
| 0.5 | 1.6 | <0.3 | <1.1 | 0.3 | 1.2 | <0.3 | <1.1 | <0.3 | <1.1 | |||||||
| b2 | CH3 | H | H | H | 14.07 ± 1.84 | 18.40 ± 0.75 | 19.08 ± 2.11 | 25.47 ± 4.14 | >30 | 14.98 ± 0.78 | 35.87 ± 3.40 | |||||
| 1.1 | 2.5 | 0.8 | 1.9 | 0.8 | 1.9 | 0.6 | 1.4 | <0.5 | <1.2 | |||||||
| b3 | H | CH3 | H | H | 12.81 ± 0.50 | 13.28 ± 2.14 | 26.78 ± 4.26 | 20.69 ± 0.59 | 25.23 ± 2.00 | 16.20 ± 0.19 | 33.26 ± 1.61 | |||||
| 1.3 | 2.6 | 1.2 | 2.5 | 0.6 | 1.2 | 0.8 | 1.6 | 0.6 | 1.3 | |||||||
| b4 | H | H | CH3 | H | 19.51 ± 1.28 | 18.60 ± 0.36 | 25.44 ± 0.15 | 22.07 ± 0.60 | >30 | 13.77 ± 1.69 | 59.59 ± 5.56 | |||||
| 0.7 | 3.1 | 0.7 | 3.2 | 0.5 | 2.3 | 0.6 | 2.7 | <0.5 | <2.0 | |||||||
| b5 | H | CH3 | CH3 | H | 26.79 ± 0.30 | 20.33 ± 1.01 | 30.31 ± 0.29 | >30 | >30 | 15.66 ± 0.76 | 25.15 ± 2.71 | |||||
| 0.6 | 0.9 | 0.8 | 1.2 | 0.5 | 0.8 | <0.5 | <0.8 | <0.5 | <0.8 | |||||||
| b6 # | OCH3 | H | H | H | 2.05 ± 0.22 | 5.00 ± 0.03 | 5.60 ± 0.17 | 6.35 ± 0.32 | 10.02 ± 0.14 | 4.01 ± 0.05 | 8.78 ± 1.21 | |||||
| 2.0 | 4.3 | 0.8 | 1.8 | 0.7 | 1.6 | 0.6 | 1.4 | 0.4 | 0.9 | |||||||
| b7 | H | OCH3 | H | H | 6.97 ± 0.55 | 9.98 ± 0.37 | 6.37 ± 0.05 | 14.02 ± 0.19 | 15.62 | 8.14 ± 0.53 | 18.08 ± 5.82 | |||||
| 1.2 | 2.6 | 0.8 | 1.8 | 1.3 | 2.8 | 0.6 | 1.3 | 0.5 | 1.2 | |||||||
| a16 | OCH3 | 9.20 ± 0.31 | 12.42 ± 0.34 | 23.10 ± 0.79 | 21.18 ± 0.55 | 17.80 ± 0.07 | 3.28 ± 0.31 | 8.87 ± 1.83 | ||||||||
| 0.4 | 1.0 | 0.3 | 0.7 | 0.1 | 0.4 | 0.2 | 0.4 | 0.2 | 0.5 | |||||||
| b8 | OCH2CH3 | H | H | H | 4.23 ± 0.09 | 8.72 ± 0.07 | 8.56 ± 0.17 | 7.36 ± 0.37 | 8.20 ± 0.12 | 0.83 ± 0.21 | 39.55 ± 7.53 | |||||
| 0.2 | 9.3 | 0.1 | 4.5 | 0.1 | 4.6 | 0.1 | 5.4 | 0.1 | 4.8 | |||||||
| b9 | H | OCH2CH3 | H | H | 7.71 ± 0.21 | 12.16 ± 0.25 | 11.59 ± 0.07 | 13.22 ± 0.43 | 10.68 ± 0.08 | 9.58 ± 0.44 | 17.42 ± 5.96 | |||||
| 1.2 | 2.3 | 0.8 | 1.4 | 0.8 | 1.5 | 0.7 | 1.3 | 0.9 | 1.6 | |||||||
| b10 | H | H | OCH2CH3 | H | 10.92 ± 0.46 | 14.36 ± 0.15 | 19.76 ± 0.69 | 22.72 ± 0.57 | 19.47 ± 0.23 | 13.81 ± 3.77 | 36.66 ± 3.41 | |||||
| 1.3 | 3.4 | 1.0 | 2.6 | 0.7 | 1.9 | 0.6 | 1.6 | 0.7 | 1.9 | |||||||
| b11 | OCH2Ph | H | H | H | 2.76 ± 0.04 | 3.62 ± 0.07 | 4.15 ± 0.2 | 3.63 ± 0.13 | 7.43 ± 0.56 | 20.99 ± 1.26 | 38.28 ± 5.63 | |||||
| 7.6 | 13.9 | 5.8 | 10.6 | 5.1 | 9.2 | 5.8 | 10.5 | 2.8 | 5.2 | |||||||
| b12 | H | OCH2Ph | H | H | 23.59 ± 0.84 | 25.59 ± 1.16 | 29.03 ± 0.09 | 13.25 ± 0.02 | 22.30 ± 0.07 | 31.40 ± 1.12 | >100 | |||||
| 1.3 | >4.2 | 1.2 | >3.9 | 1.1 | >3.4 | 2.4 | >7.5 | 1.4 | >7.5 | |||||||
| b13 # | H | H | OCH2Ph | H | 21.75 ± 0.62 | >30 | >30 | >30 | >30 | >100 | >100 | |||||
| >4.6 | >4.6 | - | - | - | - | - | - | - | - | |||||||
| b14 | OCH2Ph | H | OCH3 | H | >30 | >30 | >30 | >30 | >30 | >100 | >100 | |||||
| - | - | - | - | - | - | - | - | - | - | |||||||
| b15 | H | OCH3 | OCH2Ph | H | >30 | >30 | >30 | >30 | >30 | >100 | >100 | |||||
| - | - | - | - | - | - | - | - | - | - | |||||||
| b16 | H | OCH3 | OCH2CH3 | H | 4.14 ± 0.11 | 5.02 ± 0.09 | 4.79 ± 0.13 | 5.78 ± 0.11 | 7.67 ± 0.04 | 9.15 ± 1.33 | 44.70 ± 8.15 | |||||
| 2.2 | 10.8 | 1.8 | 8.9 | 1.9 | 9.3 | 1.6 | 7.7 | 1.2 | 5.8 | |||||||
| b17 | OCH2CH3 | H | OCH3 | H | 11.97 ± 0.23 | 8.54 ± 0.14 | 7.74 ± 0.10 | 9.33 ± 0.30 | 10. 80 ± 0.50 | 9.03 ± 0.80 | 10.92 ± 0.42 | |||||
| 0.8 | 0.9 | 1.1 | 1.3 | 1.2 | 1.4 | 1.0 | 1.2 | 0.8 | 1.0 | |||||||
| b18 | OCH3 | H | H | OCH3 | 1.59 ± 0.15 | 4.56 ± 0.22 | 5.60 ± 0.06 | 4.16 ± 0.72 | 9.64 ± 0.18 | 2.48 ± 0.34 | 16.97 ± 2.73 | |||||
| 1.6 | 10.7 | 0.5 | 3.7 | 0.4 | 3.0 | 0.6 | 4.1 | 0.3 | 1.8 | |||||||
| b19 # | H | OCH3 | OCH3 | H | 7.12 ± 0.67 | 9.26 ± 0.04 | 19.67 ± 0.20 | 13.62 ± 0.85 | 13.84 ± 0.06 | 10.53 ± 0.41 | 39.75 ± 6.32 | |||||
| 1.5 | 5.6 | 1.1 | 4.3 | 0.5 | 2.0 | 0.8 | 2.9 | 0.8 | 2.9 | |||||||
| b20 # | H | OCH2O | H | 17.46 ± 0.54 | 16.23 ± 0.95 | 22.54 ± 0.37 | 19.50 ± 0.90 | 25.85 ± 0.65 | 14.21 ± 1.06 | 60.90 ± 5.61 | ||||||
| 0.8 | 3.5 | 0.9 | 3.8 | 0.6 | 2.7 | 0.7 | 3.1 | 0.5 | 2.4 | |||||||
| b21 | H | CH2CH2O | H | 17.34 ± 0.23 | 21.57 ± 0.43 | 22.62 ± 2.10 | 22.26 ± 0.99 | 24.37 ± 0.60 | 14.58 ± 0.81 | 57.48 ± 13.57 | ||||||
| 0.8 | 3.3 | 0.7 | 2.7 | 0.6 | 2.5 | 0.7 | 2.6 | 0.6 | 2.4 | |||||||
| b22 | H | H | Ph | H | 0.25 ± 0.03 | 0.87 ± 0.25 | 2.40 ± 0.08 | 1.75 ± 0.15 | 1.24 ± 0.08 | 2.21 ± 0.09 | 14.54 ± 6.58 | |||||
| 8.8 | 58.2 | 2.5 | 16.7 | 0.9 | 6.1 | 1.3 | 8.3 | 1.8 | 11.7 | |||||||
| b23 # | H | NH2 | H | H | 5.70 ± 0.09 | 7.23 ± 0.07 | 8.56 ± 0.75 | 7.32 ± 0.29 | 10.07 ± 0.03 | 11.41 ± 0.76 | 43.18 ± 11.74 | |||||
| 2.0 | 7.6 | 1.6 | 6.0 | 1.3 | 5.0 | 1.6 | 5.9 | 1.1 | 4.3 | |||||||
| b24 # | OH | H | H | H | 7.77 ± 0.08 | 13.38 ± 0.74 | 10.68 ± 0.38 | 8.64 ± 0.31 | 16.11 ± 0.32 | 8.96 ± 0.44 | 24.22 ± 2.71 | |||||
| 1.2 | 3.1 | 0.7 | 1.8 | 0.8 | 2.3 | 1.0 | 2.8 | 0.6 | 1.5 | |||||||
| b25 | H | OH | H | H | 7.27 ± 0.13 | 9.56 ± 0.33 | 8.92 ± 0.25 | 12.73 ± 0.28 | 21.03 ± 0.43 | 6.31 ± 0.13 | 19.64 ± 1.29 | |||||
| 0.9 | 2.7 | 0.7 | 2.1 | 0.7 | 2.2 | 0.5 | 1.5 | 0.3 | 0.9 | |||||||
| b26 # | H | H | OH | H | 14.38 ± 0.81 | 12.73 ± 0.81 | 20.24 ± 0.30 | 23.75 ± 1.56 | 20.76 ± 0.54 | 12.55 ± 2.82 | 28.16 ± 1.69 | |||||
| 0.9 | 2.0 | 1.0 | 2.2 | 0.6 | 1.4 | 0.5 | 1.2 | 0.6 | 1.4 | |||||||
| b27 | OH | H | OH | H | 17.39 ± 1.01 | 24.84 ± 0.17 | 23.08 ± 0.82 | 17.27 ± 0.76 | >30 | >100 | 35.46 ± 5.72 | |||||
| >5.8 | 2.0 | >4.0 | 1.4 | >4.3 | 1.5 | >5.8 | 2.1 | - | <1.2 | |||||||
| b28 # | OH | H | OCH3 | H | 19.52 ± 0.79 | 17.02 ± 0.65 | 28.37 ± 1.25 | 17.37 ± 0.97 | 28.30 ± 0.26 | 16.25 ± 2.48 | >100 | |||||
| 0.8 | >5.1 | 1.0 | >5.9 | 0.6 | >3.5 | 0.9 | >5.8 | 0.6 | >3.5 | |||||||
| b29 | H | OCH3 | OH | H | 0.97 ± 0.04 | 1.83 ± 0.08 | 1.79 ± 0.20 | 1.50 ± 0.07 | 3.03 ± 0.05 | 6.31 ± 0.13 | >100 | |||||
| 6.5 | >103.1 | 3.4 | >54.6 | 3.5 | >55.9 | 4.2 | >66.7 | 2.1 | >33.0 | |||||||
| b30 | H | H | CN | H | 20.45 ± 0.21 | >30 | >30 | >30 | >30 | 12.55 ± 2.82 | >100 | |||||
| 0.6 | >4.9 | <0.4 | - | <0.4 | - | <0.4 | - | <0.4 | - | |||||||
| b31 | F | H | H | H | 17.98 ± 0.88 | 23.33 ± 0.42 | 23.51 ± 0.98 | 27.44 ± 0.67 | >30 | >100 | >100 | |||||
| >5.6 | >5.6 | >4.3 | >4.3 | >4.3 | >4.3 | >3.6 | >3.6 | - | - | |||||||
| b32 | H | F | H | H | 21.56 ± 0.97 | 20.67 ± 0.78 | 25.52 ± 0.78 | 27.15 ± 1.39 | 28.38 ± 0.27 | 11.91 ± 1.28 | 72.01 ± 12.10 | |||||
| 0.6 | 3.3 | 0.6 | 3.5 | 0.5 | 2.8 | 0.4 | 2.7 | 0.4 | 2.5 | |||||||
| b33 | H | H | F | H | 24.55 ± 0.36 | 17.41 ± 0.39 | 32.22 ± 1.34 | >30 | >30 | 12.73 ± 2.35 | 63.86 ± 13.78 | |||||
| 0.5 | 2.6 | 0.7 | 3.7 | 0.4 | 2.0 | <0.4 | <2.1 | <0.4 | <2.1 | |||||||
| b34 | H | Cl | H | H | 13.45 | 16.55 | 21.97 | 16.08 | 21.44 | 9.42 ± 1.21 | 46.87 ± 13.91 | |||||
| 0.7 | 3.5 | 0.6 | 2.8 | 0.4 | 2.1 | 0.6 | 2.9 | 0.4 | 2.2 | |||||||
| b35 # | H | H | Cl | H | 27.09 ± 0.88 | 18.40 ± 0.06 | 29.76 ± 0.80 | >30 | >30 | 17.71 ± 1.35 | 85.43 ± 6.86 | |||||
| 0.7 | 3.2 | 1.0 | 4.6 | 0.6 | 2.9 | <0.6 | <2.8 | <0.6 | <2.8 | |||||||
| b36 | H | Br | H | H | 11.30 ± 1.45 | 13.38 ± 0.44 | 23.33 ± 0.79 | 25.03 ± 3.17 | 17.13 ± 0.57 | 13.07 ± 1.21 | 61.24 ± 12.11 | |||||
| 1.2 | 5.4 | 1.0 | 4.6 | 0.6 | 2.6 | 0.5 | 2.4 | 0.8 | 3.6 | |||||||
| b37 | Cl | H | Cl | H | 11.73 ± 0.18 | 15.69 ± 1.09 | 30.35 ± 0.71 | >30 | 15.36 ± 0.83 | 19.82 ± 1.44 | >100 | |||||
| 1.7 | >8.5 | 1.3 | >6.4 | 0.7 | >3.3 | <0.7 | - | 1.3 | >6.5 | |||||||
| b38 | H | Cl | Cl | H | 18.58 ± 1.24 | 17.55 ± 2.01 | 29.58 ± 2.13 | 27.63 ± 0.58 | 26.16 ± 0.08 | 28.34 ± 3.74 | >100 | |||||
| 1.5 | >5.4 | 1.6 | >5.7 | 1.0 | >3.4 | 1.0 | >3.6 | 1.1 | >3.8 | |||||||
| b39 | H | NO2 | H | H | >30 | >30 | >30 | >30 | >30 | >100 | >100 | |||||
| - | - | - | - | - | - | - | - | - | - | |||||||
| b40 | H | H | NO2 | H | >30 | >30 | >30 | >30 | >30 | >100 | >100 | |||||
| - | - | - | - | - | - | - | - | - | - | |||||||
| b41 | H | CF3 | H | H | 24.37 ± 5.87 | 29.07 ± 3.06 | >30 | >30 | >30 | >100 | >100 | |||||
| >4.1 | >4.1 | >3.4 | >3.4 | - | - | - | - | - | - | |||||||
| b42 | H | CF3 | H | CF3 | >30 | >30 | >30 | >30 | >30 | >100 | >100 | |||||
| - | - | - | - | - | - | - | - | - | - | |||||||
| Etoposide | 5.11 ± 0.45 | 3.96 ± 0.17 | 20.57 ± 0.34 | 3.23 ± 0.28 | 29.32 ± 1.73 | 6.42 ± 0.39 | >100 | |||||||||
| 1.3 | >19.6 | 1.6 | >25.3 | 0.3 | >4.9 | 2.0 | >31 | 0.2 | >3.4 | |||||||
# Known compound; a IC50 values were calculated by SigmaPlot 10.0 as the compounds concentrations producing half maximal inhibition after incubating the cells with increasing concentrations up to 30 μM. Data are the mean ± SD of at least three independent experiments; b CC50 values were calculated by SigmaPlot 10.0 as the compounds concentrations producing half maximal inhibition after incubating the cells with increasing concentrations up to 100 μM. Data are the mean ± SD of at least three independent experiments. SI = selectivity index, given by c (CC50HMLE/IC50HepG2); d (CC50L02/IC50HepG2); e (CC50HMLE/IC50Hela); f (CC50L02/IC50Hela); g (CC50HMLE/IC50MCF7); h (CC50L02/IC50MCF7); i (CC50HMLE/IC50A549); j (CC50L02/IC50A549); k (CC50HMLE/IC50SW1990) or l (CC50L02/IC50SW1990); “-” means SI value can’t be calculated.
3. Experimental
3.1. Chemistry
3.1.1. General Methods
All of the solvents and reagents used in the current study were purchased from commercial suppliers and used without further purification. Melting points were determined in open capillaries and are uncorrected. The reaction products were purified by crystallization or flash column chromatography using a mixture of petroleum ether and ethyl acetate as the eluent. 1H-NMR spectra were recorded on Varian Inova-400 MHz, Varian Inova-500 MHz and SYS-600 MHz instruments (Varian, Palo Alto, CA, USA). 13C-NMR spectra were recorded on a 600 MHz Bruker ARX-600 spectrometer (Bruker Bioscience, Billerica, MA, USA). The chemical shifts (δ) are reported in ppm relative to the internal reference standard tetramethylsilane (TMS) and the coupling constants (J values) have been reported in Hertz (Hz). MS data were obtained using time-of-flight mass spectrometer (TOF-MS) or Bruker microTOF-Q instrument (Bruker, Billerica, MA, USA). High resolution mass spectra (HRMS) were obtained on a Q-TOF Ultima ESI instrument (micrOTOF-Q II, Bruker Daltonics, Leipzig, Germany). Analysis by thin layer chromatography (TLC) was performed on silica gel plates (Merck, Billerica, MA, USA). Automated column chromatography was conducted over silica gel using a Companion Rf 200 automated chromatography system (Teledyne ISCO, Lincoln, NE, USA). The chalcones were prepared according to methods previously described in the literature 6, 13–15 [20,21,22].
The chalcones evaluated in the current study were synthesized by the base catalyzed Claisen-Schmidt condensation reaction of the appropriately substituted acetophenones and aldehydes (Scheme 1). The chalcones prepared according to this method were formed predominantly with the (E)-configuration (JH2-H3 = 15–16 Hz).
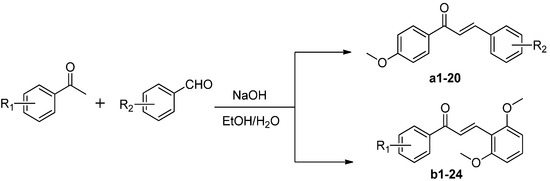
Scheme 1.
Synthesis of compounds a1–20 and b1–42.
3.1.2. General Procedure for Synthesis of Chalcones
A 50 mL flask was charged with substituted acetophenone (5 mmol) and a solution of sodium hydroxide (10 mmol) in a 4:1 (v/v) mixture of ethanol/H2O (25 mL), and the resulting mixture was stirred at room temperature for 5 min. A substituted benzaldehyde (5 mmol) was then added to the reaction, and the resulting mixture was stirred at room temperature. The reaction was then monitored by TLC using ethyl acetate/petroleum ether (1:4 or 1:2 v/v) as the solvent system. Upon completion of the reaction, the crude product was filtered off and recrystallized from a mixture of dichloromethane and ethanol or purified by column chromatography over silica gel eluting with a mixture of petroleum ether and ethyl acetate to give the pure product.
3.1.3. Characterization Data
(E)-1-(4-Methoxyphenyl)-3-phenylprop-2-en-1-one (a1). White solid (76%), mp: 104.1–105.3 °C ([23] 103–105 °C). 1H-NMR (400 MHz, DMSO-d6) δ 8.19–8.17 (m, 2H), 7.95 (d, J = 15.6 Hz, 1H), 7.91–7.86 (m, 2H), 7.72 (d, J = 15.6 Hz, 1H), 7.47–7.45 (m, 3H), 7.11–7.09 (m, 2H), 3.88 (s, 3H); MS (ESI) m/z = 261 (M+Na)+; HRMS (ESI) m/z calcd. for C16H14O2 [M+Na] 261.0896, found 261.0875 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(4-bromophenyl)prop-2-en-1-one (a2). Yellow solid (84%), mp: 153.7–155.1 °C ([24] 157 °C). 1H-NMR (400 MHz, DMSO-d6) δ 8.18 (d, J = 8.8 Hz, 2H), 7.99 (d, J = 15.6 Hz, 1H), 7.86 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 15.6 Hz, 1H), 7.66 (d, J = 8.4 Hz, 2H), 7.09 (d, J = 8.8 Hz, 2H), 3.88 (s, 3H); MS (ESI) m/z = 339 (M+Na)+; HRMS (ESI) m/z calcd. for C16H13BrO2 [M+Na] 338.9991, found 338.9980 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(4-bromo-2-fluorophenyl)prop-2-en-1-one (a3). White solid (99%), mp: 122.2–123.6 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.16 (d, J = 9.0 Hz, 2H), 8.11 (m, 1H), 8.03 (d, J = 15.5 Hz, 1H), 7.73 (d, J = 15.5 Hz, 1H), 7.71–7.68 (m, 1H), 7.56–7.54 (m, 1H), 7.10 (d, J = 9.0 Hz, 2H), 3.88 (s, 3H); MS (ESI) m/z = 357 (M+Na)+.
(E)-1-(4-Methoxyphenyl)-3-(2-bromo-4-fluorophenyl)prop-2-en-1-one (a4). White solid (63%), mp: 98.7–101.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.31–8.27 (m, 1H), 8.19 (d, J = 9.2 Hz, 2H), 7.96 (d, J = 15.6 Hz, 1H), 7.92 (d, J = 15.6 Hz, 1H), 7.75–7.72 (m, 1H), 7.44–7.39 (m, 1H), 7.10 (d, J = 9.2 Hz, 2H), 3.88 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 187.5, 163.9, 139.8, 131.6, 131.4, 130.9, 130.6, 126.2, 125.3, 120.9, 116.2, 114.6, 56.1; MS (ESI) m/z = 357 (M+Na)+; HRMS (ESI) m/z calcd. for C16H12BrFO2 [M+Na] 356.9897, found 356.9889 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(4-bromo-2-chlorophenyl)prop-2-en-1-one (a5). White solid (86%), mp: 145.3–146.4 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.20–8.18 (m, 3H), 8.05 (d, J = 15.5 Hz, 1H), 7.92 (d, J = 15.5 Hz, 1H), 7.87 (s, 1H), 7.68–7.67 (m, 1H), 7.10 (m, 2H), 3.88 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): δ 187.4, 164.0, 137.1, 135.6, 132.7, 132.3, 131.6, 131.2, 130.5, 130.4, 125.8, 124.4, 114.6, 56.1; MS (ESI) m/z = 351/353 (M+H)+.
(E)-1-(4-Methoxyphenyl)-3-(4-nitrophenyl)prop-2-en-1-one (a6).Yellow solid (63%), mp: 170.2–171.5 °C ([25] 168–169 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.30–8.28 (m, 2H), 8.21 (d, J = 9.0 Hz, 2H), 8.16–8.13 (m, 3H), 7.79 (d, J = 16.0 Hz, 1H), 7.11 (d, J = 9.0 Hz, 2H), 3.89 (s, 3H); MS (ESI) m/z = 284 (M+H)+; HRMS (ESI) m/z calcd. for C16H13NO4 [M+H] 284.0917, found 284.0916 [M+H].
(E)-1-(4-Methoxyphenyl)-3-(4-carbonylphenyl)prop-2-en-1-one (a7) [26]. Yellow solid (40%), mp: >350 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.18 (d, J = 8.8 Hz, 2H), 7.92 (d, J = 15.6 Hz, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.77 (d, J = 8.0 Hz, 2H), 7.71 (d, J = 15.6 Hz, 1H), 7.09 (d, J = 8.8 Hz, 2H), 3.88 (s, 3H); MS (ESI) m/z = 321 (M+K)+.
(E)-1-(4-Methoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one (a8). Yellow solid (82%). mp: 43.2–45.5 °C ([27] 44–45 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.16–8.14 (m, 2H), 8.05 (d, J = 15.5 Hz, 1H), 7.99–7.97 (m, 1H), 7.89 (d, J = 15.5 Hz, 1H), 7.47–7.44 (m, 1H), 7.13–7.09 (m, 3H), 7.06–7.03 (m, 1H), 3.91 (s, 3H), 3.88 (s, 3H); MS (ESI) m/z = 269 (M+H)+; HRMS (ESI) m/z calcd. for C17H16O3 [M+H] 269.1172, found 269.1177 [M+H].
(E)-1-(4-Methoxyphenyl)-3-(3-methoxyphenyl)prop-2-en-1-one (a9). Yellow solid (78%), mp: 95.7–97.0 °C ([28] 103–104 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.18 (d, J = 9.0 Hz, 2H), 7.95 (d, J = 15.5 Hz, 1H), 7.68 (d, J = 15.5 Hz, 1H), 7.48 (s, 1H), 7.44–7.43 (m, 1H), 7.38–7.35 (m, 1H), 7.10 (d, J = 9.0 Hz, 2H), 7.03–7.01 (m, 1H), 3.88 (s, 3H), 3.84 (s, 3H); MS (ESI) m/z = 269 (M+H)+; HRMS (ESI) m/z calcd. for C17H16O3 [M+H] 269.1172, found 269.1179 [M+H].
(E)-1,3-bis(4-Methoxyphenyl)prop-2-en-1-one (a10). Yellow solid (72%), mp: 97.8–100.5 °C ([28] 103–104 °C). 1H-NMR (400 MHz, DMSO-d6) δ 8.16 (d, J = 9.2 Hz, 2H), 7.85 (d, J = 8.8 Hz, 2H), 7.81 (d, J = 15.6 Hz, 1H), 7.69 (d, J = 15.6 Hz, 1H), 7.08 (d, J = 9.2 Hz, 2H), 7.02 (d, J = 8.8 Hz, 2H), 3.87 (s, 3H), 3.83 (s, 3H); MS (ESI) m/z = 291 (M+Na)+; HRMS (ESI) m/z calcd. for C17H16O3 [M+Na] 291.0992, found 291.0998 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one (a11). White solid (83%), mp: 106.3–107.9 °C ([29] 109 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.15 (d, J = 8.5 Hz, 2H), 7.84–7.78 (m, 3H), 7.68 (d, J = 15.5 Hz, 1H), 7.08 (d, J = 9.0 Hz, 2H), 7.00 (d, J = 8.5 Hz, 2H), 4.10 (q, J = 7.0 Hz, 2H), 3.87 (s, 3H), 1.35 (t, J = 7.0 Hz, 3H); MS (ESI) m/z = 283 (M+H)+; HRMS (ESI) m/z calcd. for C18H18O3 [M+H] 283.1329, found 283.1347 [M+H].
(E)-1-(4-Methoxyphenyl)-3-(2,3-dimethoxyphenyl)prop-2-en-1-one (a12).White solid (91%), mp: 99.8–102.0 °C ([30] 99.0–99.5 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.15 (d, J = 9.0 Hz, 2H), 7.96 (d, J = 15.5 Hz, 1H), 7.89 (d, J = 15.5 Hz, 1H), 7.63–7.61 (m, 1H), 7.16–7.15 (m, 2H), 7.09 (d, J = 9.0 Hz, 2H), 3.88 (s, 3H), 3.85 (s, 3H), 3.80 (s, 3H); MS (ESI) m/z = 299 (M+H)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+H] 299.1278, found 299.1300 [M+H].
(E)-1-(4-methoxyphenyl)-3-(2,5-dimethoxyphenyl)prop-2-en-1-one (a13) [31]. Yellow solid (87%), mp: 54.7–56.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.16 (d, J = 8.8 Hz, 2H), 8.01 (d, J = 15.6 Hz, 1H), 7.91 (d, J = 15.6 Hz, 1H), 7.56–7.55 (m, 1H), 7.09 (d, J = 8.8 Hz, 2H), 7.07–7.00 (m, 2H), 3.87 (s, 3H), 3.85 (s, 3H), 3.80 (s, 3H); MS (ESI) m/z = 321 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 321.1097, found 321.1098 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (a14). Yellow solid (70%), mp: 123.7–125.7 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 15.6 Hz, 1H), 8.04–7.99 (m, 3H), 7.42–7.38 (m, 1H), 7.10–7.08 (m, 2H), 6.77–6.75 (m, 2H), 3.92 (s, 6H), 3.87 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): δ188.9, 163.4, 160.4, 134.5, 132.6, 131.4, 130.9, 124.1, 114.5, 112.1, 104.6, 56.5, 56.0; MS (ESI) m/z = 321 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 321.1097, found 321.1104 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one (a15). Yellow solid (72%), mp: 104.9–106.4 °C ([32] 93–98 °C). 1H-NMR (400 MHz, DMSO-d6) δ 8.17 (d, J = 8.8 Hz, 2H), 7.83 (d, J = 15.6 Hz, 1H), 7.68 (d, J = 15.6 Hz, 1H), 7.54–7.53 (m, 1H), 7.40–7.37 (m, 1H), 7.09 (d, J = 8.8 Hz, 2H), 7.03–7.01 (m, 1H), 3.87 (s, 6H), 3.82 (s, 3H); MS (ESI) m/z = 321 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 321.1097, found 321.1083 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(3,5-dimethoxyphenyl)prop-2-en-1-one (a16) [33]. Yellow solid (85%), mp: 87.6–89.1 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.19 (d, J = 9.0 Hz, 2H), 7.94 (d, J = 15.5 Hz, 1H), 7.64 (d, J = 15.5 Hz, 1H), 7.09 (d, J = 9.0 Hz, 2H), 7.07–7.06 (m, 2H), 6.59–6.58 (m, 1H), 3.88 (s, 3H), 3.82 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 187.9, 163.7, 161.2, 143.8, 137.2, 131.5, 130.9, 123., 114.5, 107.1, 103.1, 56.0, 55.9; MS (ESI) m/z = 299 (M+H)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+H]+ 299.1278, found 299.1298 [M+H].
(E)-1-(4-Methoxyphenyl)-3-(3-(benzyloxy)-4-methoxyphenyl)prop-2-en-1-one (a17). Yellow solid (97%), mp: 115.0–118.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.17 (d, J = 8.8 Hz, 2H), 7.82 (d, J = 15.2 Hz, 1H), 7.68–7.64 (m, 2H), 7.52–7.50 (m, 2H), 7.44–7.34 (m, 4H), 7.10 (d, J = 8.8 Hz, 2H), 7.06–7.04 (m, 1H), 5.19 (s, 2H), 3.87 (s, 3H), 3.83 (s, 3H); MS (ESI) m/z = 397 (M+Na)+; HRMS (ESI) m/z calcd. for C22H24O4 [M+Na]+ 397.1410, found 397.1407 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one (a18). Yellow solid (99%), mp: 94.4–96.5 °C ([34] 94 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.13 (d, J = 9.0 Hz, 2H), 7.88 (d, J = 15.5 Hz, 1H), 7.81 (d, J = 15.5 Hz, 1H), 7.79–7.77 (m, 1H), 7.09 (d, J = 9.0 Hz, 2H), 6.94–6.92 (m, 1H), 3.88 (s, 3H), 3.87 (s, 3H), 3.87 (s, 3H), 3.78 (s, 3H); MS (ESI) m/z = 351 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O5 [M+Na]+ 351.1203, found 351.1216 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one (a19). Yellow solid (94%), mp: 122.1–123.7 °C ([35] 107–110 °C). 1H-NMR (400 MHz, DMSO-d6) δ 8.14 (d, J = 8.8 Hz, 2H), 8.03 (d, J = 15.6 Hz, 1H), 7.76 (d, J = 15.6 Hz, 1H), 7.52 (s, 1H), 7.08 (d, J = 8.8 Hz, 2H), 6.75 (s, 1H), 3.90 (s, 3H), 3.87 (s, 3H), 3.87 (s, 3H), 3.83 (s, 3H); MS (ESI) m/z = 351 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O5 [M+Na]+ 351.1203, found 351.1194 [M+Na].
(E)-1-(4-Methoxyphenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (a20). Yellow solid (84%), mp: 109.7–111.5 °C ([36] 125 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.18 (d, J = 9.0 Hz, 2H), 7.88 (d, J = 15.5 Hz, 1H), 7.66 (d, J = 15.5 Hz, 1H), 7.22 (m, 2H), 7.10 (d, J = 9.0 Hz, 2H), 3.88 (s, 3H), 3.87 (s, 6H), 3.72 (s, 3H); MS (ESI) m/z = 351 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O5 [M+Na]+ 351.1203, found 351.1183 [M+Na].
(E)-1-Phenyl-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b1). Yellow solid (84%), mp: 52.6–54.7 °C ([37] 80 °C). 1H-NMR (500 MHz, DMSO-d6) δ 8.13 (d, J = 16.0 Hz, 1H), 8.03–7.98 (m, 3H), 7.67–8.65 (m, 1H), 7.59–7.56 (m, 2H), 7.43–7.39 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); MS (ESI) m/z = 291 (M+Na)+; HRMS (ESI) m/z calcd. for C17H16O3 [M+Na] 291.0992, found 291.1007 [M+Na].
(E)-1-(2-Methylphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b2). Yellow solid (91%), mp: 79.8–81.4 °C. 1H-NMR (500 MHz, DMSO-d6) δ 7.86 (d, J = 16.0 Hz, 1H), 7.51 (d, J = 16.0 Hz, 1H), 7.49–7.47 (m, 1H), 7.44–7.38 (m, 2H), 7.34–7.31 (m, 2H), 6.74–6.73 (m, 2H), 3.85 (s, 6H), 2.36 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 196.8, 160.3, 140.0, 136.4, 136.3, 133.0, 131.6, 130.8, 128.7, 128.3, 126.2, 111.6, 104.7, 56.5, 20.34; MS (ESI) m/z = 305 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 305.1148, found 305.1134 [M+Na].
(E)-1-(3-Methylphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b3). Yellow solid (94%), mp: 102.5–103.8 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.12 (d, J = 16.0 Hz, 1H), 7.98 (d, J = 16.0 Hz, 1H), 7.81–7.77 (m, 2H), 7.48–7.44 (m, 2H), 7.42–7.39 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H), 2.42 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 190.9, 160.5, 138.7, 138.6, 135.3, 133.9, 132.8, 129.2, 128.9, 125.9, 124.3, 112.0, 104.6, 56.5, 21.41; MS (ESI) m/z = 305 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 305.1148, found 305.1155 [M+Na].
(E)-1-(4-Methylphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b4). Yellow solid (74%), mp: 82.7–84.5 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.10 (d, J = 16.0 Hz, 1H), 7.99 (d, J = 16.0 Hz, 1H), 7.91–7.89 (m, 2H), 7.42–7.37 (m, 3H), 6.77–6.75 (m, 2H), 3.92 (s, 6H), 2.40 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 190.2, 160.4, 143.6, 136.1, 135.0, 132.8, 129.9, 128.7, 124.1, 112.0, 104.7, 56.5, 21.64; MS (ESI) m/z = 305 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 305.1148, found 305.1135 [M+Na].
(E)-1-(3,4-Dimethylphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b5). Yellow solid (90%), mp: 98.9–101.1 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.08 (d, J = 16.0 Hz, 1H), 7.98 (d, J = 16.0 Hz, 1H), 7.75–7.72 (m, 2H), 7.41–7.38 (m, 1H), 7.33–7.32 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H), 2.32 (s, 3H), 2.31 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 190.3, 160.4, 142.5, 137.4, 136.5, 134.9, 132.7, 130.3, 129.5, 126.4, 124.3, 112.0, 104.7, 56.6, 20.1, 19.9; MS (ESI) m/z = 319 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O3 [M+Na] 319.1305, found 319.1302 [M+Na].
(E)-1-(2-Methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b6) [38]. Yellow solid (84%), mp: 109.0–111.4 °C. 1H-NMR (500 MHz, DMSO-d6) δ 7.91 (d, J = 16.5 Hz, 1H), 7.71 (d, J = 16.5 Hz, 1H), 7.56–7.46 (m, 2H), 7.39–7.18 (m, 2H), 7.07–7.04 (m, 1H), 6.74–6.72 (m, 2H), 3.88 (s, 3H), 3.86 (s, 6H); MS (ESI) m/z = 321 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 321.1097, found 321.1080 [M+Na].
(E)-1-(3-Methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b7). Yellow solid (93%), mp: 93.5–95.6 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.12 (d, J = 16.0 Hz, 1H), 7.97 (d, J = 16.0 Hz, 1H), 7.58–7.51 (m, 1H), 7.49–7.45 (m, 1H), 7.44–7.41 (m, 2H), 7.25–7.22 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H), 3.85 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 190.5, 160.5, 160.0, 140.1, 135.5, 132.9, 130.5, 124.2, 121.1, 119.3, 113.0, 111.9, 104.7, 56.6, 55.7; MS (ESI) m/z = 321 (M+Na)+. HRMS (ESI) m/z calcd. for C18H18O4 [M+Na] 321.1097, found 321.1098 [M+Na].
(E)-1-(2-Ethoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b8). Yellow solid (94%). mp: 48.8–50.4 °C. 1H-NMR (400 MHz, DMSO-d6) δ 7.93 (d, J = 16.0 Hz, 1H), 7.74 (d, J = 16.0 Hz, 1H), 7.53–7.44 (m, 2H), 7.39–7.35 (m, 1H), 7.17–7.15 (m, 1H), 7.05–7.02 (m, 1H), 6.74–6.72 (m, 2H), 4.16 (q, J = 7.2 Hz, 2H), 3.86 (s, 6H), 1.31 (t, J = 7.2 Hz, 3H); 13C-NMR (150 MHz, DMSO-d6): 193.8, 160.3, 157.4, 133.5, 133., 132.5, 130.1, 130.0, 129.5, 121.0, 113.7, 111.9, 104.7, 64.4, 56.4, 14.9; MS (ESI) m/z = 335 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O4 [M+Na] 335.1254, found 335.1248 [M+Na].
(E)-1-(3-Ethoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b9). Yellow solid (42%), mp: 63.1–64.8 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.11 (d, J = 16.0 Hz, 1H), 7.95 (d, J = 16.0 Hz, 1H), 7.57–7.55 (m, 1H), 7.50–7.46 (m, 1H), 7.44–7.38 (m, 2H), 7.24–7.18 (m, 1H), 6.77–6.75 (m, 2H), 4.12 (q, J = 7.2 Hz, 2H), 3.92 (s, 6H), 1.36 (t, J = 7.2 Hz, 3H); 13C-NMR (150 MHz, DMSO-d6): 190.5, 160.5, 159.2, 140.1, 135.5, 132.8, 130.4, 124.3, 120.9, 119.7, 113.5, 112.0, 104.6, 63.7, 56.5, 15.0; MS (ESI) m/z = 335 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O4 [M+Na] 335.1254, found 335.1255 [M+Na].
(E)-1-(4-Ethoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b10). White solid (82%), mp: 99.0–101.2 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.09 (d, J = 15.5 Hz, 1H), 8.03–7.98 (m, 3H), 7.41–7.38 (m, 1H), 7.09–7.07 (m, 2H), 6.77–6.75 (m, 2H), 4.14 (q, J = 7.0 Hz, 2H), 3.92 (s, 6H), 1.37 (t, J = 7.0 Hz, 3H); 13C-NMR (150 MHz, DMSO-d6): 188.9, 162.7, 160.4, 134.5, 132.6, 131.2, 130.9, 124.0, 114.9, 112.1, 104.6, 64.0, 56.5, 15.0; MS (ESI) m/z = 335 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O4 [M+Na] 335.1254, found 335.1256 [M+Na].
(E)-1-(2-(Benzyloxy>)phenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b11). Yellow solid (20%), mp: 94.6–97.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 7.92 (d, J = 16.0 Hz, 1H), 7.76 (d, J = 16.0 Hz, 1H), 7.53–7.47 (m, 2H), 7.45–7.35 (m, 3H), 7.25–7.21(m, 4H), 7.09–7.05 (m, 1H), 6.73–6.71 (m, 2H), 5.24 (s, 2H), 3.78 (s, 6H); 13C-NMR(150 MHz, DMSO-d6): 193.1, 157.6, 157.4, 137.4, 133.4, 131.7, 130.6, 129.9, 129.8, 128.9, 128.8, 128.1, 127.7, 120.7, 114.0, 113.7, 104.0, 70.11, 55.7; MS (ESI) m/z = 397 (M+Na)+; HRMS (ESI) m/z calcd. for C24H22O4 [M+Na] 397.1410, found 397.1395 [M+Na].
(E)-1-(3-(Benzyloxy)phenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b12). Yellow solid (81%), mp: 108.8–110.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.11 (d, J = 16.0 Hz, 1H), 7.95 (d, J = 16.0 Hz, 1H), 7.60–7.58 (m, 1H), 7.54–7.47 (m, 4H), 7.43–7.38 (m, 3H), 7.37–7.33 (m, 1H), 7.32–7.29 (m, 1H), 6.77–7.75 (m, 2H), 5.21 (s, 2H), 3.91 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 190.5, 160.5, 159.1, 140.1, 137.3, 135.5, 132.9, 130.5, 128.9, 128.4, 128.2, 124.2, 121.3, 120.2, 114.0, 111.9, 104.7, 69.8, 56.6; MS (ESI) m/z = 397 (M+Na)+; HRMS (ESI) m/z calcd. for C24H22O4 [M+Na] 397.1410, found 397.1394 [M+Na].
(E)-1-(4-(Benzyloxy)phenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b13) [39]. White solid (97%), mp: 96.6–97.8 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.10 (d, J = 16.0 Hz, 1H), 8.04–7.98 (m, 3H), 7.49–7.47 (m, 2H), 7.44–7.33 (m, 4H), 7.18–7.16 (m, 2H), 6.77–6.75 (m, 2H), 5.23 (s, 2H), 3.92 (s, 6H); MS (ESI) m/z = 375 (M+H)+; HRMS (ESI) m/z calcd. for C24H22O4 [M+H] 375.1591, found 375.1591 [M+H].
(E)-1-(2-(Benzyloxy)-4-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b14). Yellow solid (75%), mp: 82.2–83.6 °C. 1H-NMR (400 MHz, DMSO-d6) δ 7.94 (d, J = 16.0, 1H), 7.90 (d, J = 16.0, 1H), 7.59–7.57 (m, 1H), 7.45–7.43 (m, 2H), 7.38–7.34 (m, 1H), 7.24–7.22 (m, 3H), 6.76–6.75 (m, 1H), 6.72–6.70 (m, 2H), 6.67–6.64 (m, 1H), 5.27 (s, 2H), 3.83 (s, 3H), 3.76 (s, 6H); MS (ESI) m/z = 427 (M+Na)+; HRMS (ESI) m/z calcd. for C25H24O5 [M+Na] 427.1516, found 427.1512 [M+Na].
(E)-1-(4-(Benzyloxy)-3-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b15). Yellow solid (80%), mp: 112.8–114.1 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.09 (d, J = 16.0 Hz, 1H), 8.02 (d, J = 16.0 Hz, 1H), 7.67–7.65 (m, 1H), 7.55–7.54 (m, 1H), 7.51–7.45 (m, 2H), 7.45–7.32 (m, 4H), 7.21–7.19 (m, 1H), 6.77–6.75 (m, 2H), 5.21 (s, 2H), 3.92 (s, 6H), 3.87 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 188.9, 160.4, 152.4, 149.5, 137.0, 134.5, 132.6, 131.7, 129.0, 128.5, 128.4, 124.1, 123.0, 112.9, 112.1, 111.2, 104.7, 70.4, 56.5, 56.0; MS (ESI) m/z = 405 (M+H)+; HRMS (ESI) m/z calcd. for C25H24O5 [M+H] 405.1697, found 405.1693 [M+H].
(E)-1-(4-Ethoxy-3-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b16). Yellow solid (91%), mp: 105.3–107.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.08 (d, J = 16.0 Hz, 1H), 8.02 (d, J = 16.0 Hz, 1H), 7.68–7.65 (m, 1H), 7.52–7.51 (m, 1H), 7.41–7.37 (m, 1H), 7.11–7.09 (m, 1H), 6.77–6.75 (m, 2H), 4.14 (q, J = 7.2 Hz, 2H), 3.92 (s, 6H), 3.86 (s, 3H), 1.38 (t, J = 7.2 Hz, 3H); 13C-NMR (150 MHz, DMSO-d6): 188.9, 160.4, 152.7, 149.3, 134.3, 132.5, 131.3, 124.1, 123.1, 112.1, 112.0, 111.0, 104.7, 64.4, 56.5, 55.8, 15.0; MS (ESI) m/z = 365 (M+Na)+; HRMS (ESI) m/z calcd. for C20H22O5 [M+Na] 365.1359, found 365.1350 [M+Na].
(E)-1-(2-Ethoxy-4-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b17). Yellow solid (65%), mp: 98.7–100.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 16.0 Hz, 1H), 7.89 (d, J = 16.0 Hz, 1H), 7.57–7.55 (m, 1H), 7.38–7.33 (m, 1H), 6.74–6.72 (m, 2H), 6.68–6.59 (m, 2H), 4.18 (q, J = 7.2 Hz, 2H), 3.87 (s, 6H), 3.84 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H); 13C-NMR (150 MHz, DMSO-d6): 191.3, 164.1, 160.3, 159.7, 132.4, 132.3, 132.1, 129.8, 122.7, 112.3, 106.5, 104.6, 99.8, 64.5, 56.4, 56.0, 14.8; MS (ESI) m/z = 365 (M+Na)+; HRMS (ESI) m/z calcd. for C20H22O5 [M+Na] 365.1359, found 365.1357 [M+Na].
(E)-1-(2,5-Dimethoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b18). White solid (92%), mp: 121.0–122.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ 7.91 (d, J = 16.0 Hz, 1H), 7.72 (d, J = 16.0 Hz, 1H), 7.40–7.36 (m, 1H), 7.16–7.08 (m, 2H), 7.02–7.01 (m, 1H), 6.73–6.71 (m, 2H), 3.87 (s, 6H), 3.82 (s, 3H), 3.75 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 193.1, 160.3, 153.6, 152.4, 134.1, 132.6, 130.3, 129.3, 118.8, 114.5, 114.4, 112.0, 104.7, 56.8, 56.5, 56.0; MS (ESI) m/z = 351 (M+Na)+; HRMS (ESI) m/z calcd. for C19H20O5Na [M+Na] 351.1203, found 351.1189 [M+Na].
(E)-1-(3,4-Dimethoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b19) [15]. Yellow solid (80%), mp: 120.5–122.7 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.08 (d, J = 16.0 Hz, 1H), 8.03 (d, J = 16.0 Hz, 1H), 7.69–7.67 (m, 1H), 7.52–7.51 (m, 1H), 7.40–7.38 (m, 1H), 7.13–7.11 (m, 1H), 6.77–7.75 (m, 2H), 3.92 (s, 6H), 3.87 (s, 3H), 3.85 (s, 3H); MS (ESI) m/z = 329 (M+H)+.
(E)-1-(Benzo[d][1,3]dioxol-5-yl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b20) [40]. White solid (95%), mp: 138.6–140.4 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.08 (d, J = 16.0 Hz, 1H), 7.96 (d, J = 16.0 Hz, 1H), 7.66–7.64 (m, 1H), 7.47–7.46 (m, 1H), 7.40–7.38 (m, 1H), 7.08–7.06 (m, 1H), 6.76–7.75 (m, 2H), 6.16 (s, 2H), 3.92 (s, 6H); MS (ESI) m/z = 313 (M+H)+; HRMS (ESI) m/z calcd. for C18H16O5 [M+H] 313.1071, found 313.1068 [M+H].
(E)-1-(2,3-Dihydrobenzofuran-5-yl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b21). Yellow solid (99%), mp: 118.8–120.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.07 (d, J = 15.6 Hz, 1H), 7.98 (d, J = 15.6 Hz, 1H), 7.91–7.90 (m, 1H), 7.87–7.84 (m, 1H), 7.41–7.37 (m, 1H), 6.91–6.89 (m, 1H), 6.77–6.75 (m, 2H), 4.65 (t, J = 8.8 Hz, 2H), 3.92 (s, 6H), 3.27 (t, J = 8.8 Hz, 2H); 13C-NMR (150 MHz, DMSO-d6): 188.7, 164.3, 160.3, 134.3, 132.5, 131.7, 130.4, 128.9, 126.0, 124.2, 112.1, 109.4, 104.7, 72.6, 56.5, 28.9; MS (ESI) m/z = 333 (M+Na)+; HRMS (ESI) m/z calcd. for C19H18O4 [M+Na] 333.1097, found 333.1098 [M+Na].
(E)-1-([1,1'-Biphenyl]-4-yl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b22). Yellow solid (70%), mp: 121.7–123.5 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.17 (d, J = 16.0 Hz, 1H), 8.10–8.08 (m, 2H), 8.06 (d, J = 16.0 Hz, 1H), 7.88–7.86 (m, 2H), 7.78–7.76 (m, 2H), 7.54–7.51 (m, 2H), 7.46–7.40 (m, 2H), 6.78–6.76 (m, 2H), 3.94 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 190.1, 160.5, 144.7, 139.5, 137.4, 135.4, 132.9, 129.6, 129.3, 128.8, 127.5, 127.4, 124.1, 112.0, 104.7, 56.6; MS (ESI) m/z = 345 (M+H)+; HRMS (ESI) m/z calcd. for C23H20O3 [M+H] 345.1485, found 345.1478 [M+H].
(E)-1-(3-Aminophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b23) [41]. Yellow solid (67%), mp: 148.6–150.3 °C. 1H-NMR (600 MHz, DMSO-d6) δ 8.06 (d, J = 16.2 Hz, 1H), 7.92 (d, J = 16.2 Hz, 1H), 7.41–7.38 (m, 1H), 7.21–7.17 (m, 2H), 7.13–7.12 (m, 1H), 6.84–6.80 (m, 1H), 6.77–6.75 (m, 2H), 5.39 (s, 2H), 3.92 (s, 6H); MS (ESI) m/z = 306 (M+Na)+; HRMS (ESI) m/z calcd. for C17H17NO3 [M+Na] 306.1101, found 306.1097 [M+Na].
(E)-1-(2-Hydroxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b24) [42]. Yellow solid (83%), mp: 114.2–115.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.55 (s, 1H), 8.25 (d, J = 15.6 Hz, 1H), 8.13 (d, J = 15.6 Hz, 1H), 7.96–7.94 (m, 1H), 7.59–7.52 (m, 1H), 7.45–7.41 (m, 1H), 7.03–6.99 (m, 2H), 6.78–6.76 (m, 2H), 3.94 (s, 6H); MS (ESI) m/z = 285 (M+H)+; HRMS (ESI) m/z calcd. for C17H16O4 [M+H] 285.1121, found 285.1133 [M+H].
(E)-1-(3-Hydroxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b25). Yellow solid (70%), mp: 138.1–139.7 °C. 1H-NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H), 8.09 (d, J = 16.0 Hz, 1H), 7.94 (d, J = 16.0 Hz, 1H), 7.46–7.33 (m, 4H), 7.05–7.02 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 190.5, 160.5, 158.2, 140.0, 135.2, 132.8, 130.4, 124.2, 120.4, 119.5, 114.8, 112.0, 104.7, 56.6; MS (ESI) m/z = 307 (M+Na)+; HRMS (ESI) m/z calcd. for C17H16O4 [M+Na] 307.0941, found 307.0939 [M+Na].
(E)-1-(4-Hydroxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b26) [43]. Yellow solid (82%), mp: 189.6–191.4 °C. 1H-NMR (500 MHz, DMSO-d6) δ 10.35 (s, 1H), 8.06 (d, J = 16.0 Hz, 1H), 7.99 (d, J = 16.0 Hz, 1H), 7.92–7.90 (m, 2H), 7.40–7.36 (m, 1H), 6.91–6.89 (m, 2H), 6.76–6.74 (m, 2H), 3.92 (s, 6H); MS (ESI) m/z = 307 (M+Na)+; HRMS (ESI) m/z calcd. for C17H16O4 [M+Na] 307.0941, found 307.0938 [M+Na].
(E)-1-(2,4-Dihydroxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b27). Yellow solid (85%), mp: 218.7–220.2 °C. 1H-NMR (600 MHz, DMSO-d6) δ 13.51 (s, 1H), 10.70 (s, 1H), 8.21 (d, J = 15.6 Hz, 1H), 8.06 (d, J = 15.6 Hz, 1H), 7.88–7.86 (m, 1H), 7.43–7.40 (m, 1H), 6.77–6.76 (m, 2H), 6.46–6.44 (m, 1H), 6.30–6.29 (m, 1H), 3.93 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 192.8, 166.2, 165.4, 160.5, 135.1, 133.1, 132.7, 122.7, 113.6, 111.9, 108.9, 104.7, 103.2, 56.6; MS (ESI) m/z = 323 (M+Na)+; HRMS (ESI) m/z calcd. for C17H16O5 [M+Na] 323.0890, found 323.0876 [M+Na].
(E)-1-(2-Hydroxy-4-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b28) Yellow solid (74%), mp: 143.5–144.7 °Cs ([13] 140 °C). 1H-NMR (500 MHz, DMSO-d6) δ 13.55 (s, 1H), 8.21 (d, J = 15.5 Hz, 1H), 8.10 (d, J = 15.5 Hz, 1H), 7.93–7.91 (m, 1H), 7.42–7.40 (m, 1H), 6.77–6.75 (m, 2H), 6.57–6.47 (m, 2H), 3.93 (s, 6H), 3.83 (s, 3H); MS (ESI) m/z = 315 (M+H)+; HRMS (ESI) m/z calcd. for C18H18O5 [M+H] 315.1227, found 315.1243 [M+H].
(E)-1-(4-Hydroxy-3-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b29). Yellow solid (40%), mp: 128.7–131.2 °C. 1H-NMR (500 MHz, DMSO-d6) δ 10.02 (s, 1H), 8.06 (d, J = 16.0 Hz, 1H), 8.01 (d, J = 16.0 Hz, 1H), 7.59–7.57 (m, 1H), 7.53–7.52 (m, 1H), 7.40–7.36 (m, 1H), 6.94–6.92 (m, 1H), 6–6.74 (m, 2H), 3.92 (s, 6H), 3.86 (s, 3H); 13C-NMR (150 MHz, DMSO-d6): 188.6, 160.3, 152.1, 148.3, 134.0, 132.4, 130.4, 124.2, 123.5, 115.5, 112.2, 111.7, 104.6, 56.5, 56.0; MS (ESI) m/z = 337 (M+Na)+; HRMS (ESI) m/z calcd. for C18H18O5 [M+Na] 337.1046, found 337.1042 [M+Na].
(E)-1-(4-Cyanophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b30). Yellow solid (95%), mp: 144.3–145.9 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.16 (d, J = 16.0 Hz, 1H), 8.13–8.11 (m, 2H), 8.04–8.02 (m, 2H), 7.96 (d, J = 16.0 Hz, 1H), 7.45–7.42 (m, 1H), 6.78–6.76 (m, 2H), 3.92 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 190.1, 160.7, 142.0, 136.8, 133.4, 133.3, 129.2, 123.6, 118.7, 115.2, 111.7, 104.7, 56.6; MS (ESI) m/z = 294 (M+H)+; HRMS (ESI) m/z calcd. for C18H15NO3 [M+H] 294.1125, found 294.1130 [M+H].
(E)-1-(2-Fluorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b31). Yellow solid (93%), mp: 66.6–68.1 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.06–8.02 (m, 1H), 7.76–7.70 (m, 2H), 7.66–7.64 (m, 1H), 7.44–7.34 (m, 3H), 6.76–6.74 (m, 2H), 3.88 (s, 6H); 190.2, 160.52, 136.0, 134.5, 133.2, 130.9, 127.9, 125.3, 117.1, 111.6, 104.7, 56.5; MS (ESI) m/z = 287 (M+H)+; HRMS (ESI) m/z calcd. for C17H15FO3 [M+H] 287.1078, found 287.1097 [M+H].
(E)-1-(3-Fluorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b32). Yellow solid (93%), mp: 105.4–107.3 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.15 (d, J = 16.0 Hz, 1H), 7.96 (d, J = 16.0 Hz, 1H), 7.86–7.84 (m, 1H), 7.72–7.70 (m, 1H), 7.64–7.62 (m, 1H), 7.53–7.51 (m, 1H), 7.43–7.41 (m, 1H), 6.78–7.76 (m, 2H), 3.93 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.6, 160.6, 140.9, 136.2, 133.2, 131.5, 124.8, 123.7, 120.2, 115.0, 111.8, 104.6, 56.6; MS (ESI) m/z = 287 (M+H)+; HRMS (ESI) m/z calcd. for C17H15FO3 [M+H] 287.1078, found 287.1086 [M+H].
(E)-1-(4-Fluorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b33). Yellow solid (89%), mp: 108.2–109.6 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.13 (d, J = 16.0 Hz, 1H), 8.10–8.06 (m, 2H), 7.99 (d, J = 16.0 Hz, 1H), 7.44–7.36 (m, 3H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.2, 160.5, 135.6, 135.2, 133.0, 131.6, 123.7, 116.4, 111.9, 104.7, 56.5. MS (ESI) m/z = 309 (M+Na)+; HRMS (ESI) m/z calcd. for C17H15FO3 [M+Na] 309.0897, found 309.0890 [M+Na].
(E)-1-(3-Chlorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b34). Yellow solid (96%), mp: 116.5–117.2 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.14 (d, J = 16.0 Hz, 1H), 7.96–7.92 (m, 3H), 7.75–7.71 (m, 1H), 7.63–7.60 (m, 1H), 7.44–7.41 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.6, 160.6, 140.5, 136.3, 134.3, 133.2, 132.9, 131.3, 128.1, 127.3, 123.6, 111.8, 104.7, 56.6; MS (ESI) m/z = 303 (M+H)+; HRMS (ESI) m/z calcd. for C17H15ClO3 [M+H] 303.0782, found 303.0782 [M+H].
(E)-1-(4-Chlorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b35) [44]. Yellow solid (92%), mp: 107.9–109.7 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.14 (d, J = 16.0 Hz, 1H), 8.02–7.99 (m, 2H), 7.97 (d, J = 16.0 Hz, 1H), 7.65–7.61 (m, 2H), 7.44–7.40 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); MS (ESI) m/z = 325 (M+Na)+; HRMS (ESI) m/z calcd. for C17H15ClO3 [M+Na] 325.0602, found 325.0597 [M+Na].
(E)-1-(3-Bromophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b36). Yellow solid (98%), mp: 135.7–138.2 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.14 (d, J = 16.0 Hz, 1H), 8.06–8.05 (m, 1H), 8.01–7.97 (m, 1H), 7.93 (d, J = 16.0 Hz, 1H), 7.87–7.85 (m, 1H), 7.56–7.53 (m, 1H), 7.44–7.40 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.5, 160.6, 140.7, 136.3, 135.8, 133.2, 131.6, 131.0, 127.7, 123.6, 122.8, 111.8, 104.7, 56.6; MS (ESI) m/z = 347 (M+H)+; HRMS (ESI) m/z calcd. for C17H15BrO3 [M+H] 347.0277, found 347.0257 [M+H].
(E)-1-(2,4-Dichlorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b37). Yellow solid (99%), mp: 111.1–112.7 °C. 1H-NMR (500 MHz, DMSO-d6) δ 7.82 (d, J = 16.5 Hz, 1H), 7.78 (s, 1H), 7.59–7.54 (m, 2H), 7.45 (d, J = 16.5 Hz, 1H), 7.42–7.40 (m, 1H), 6.75–6.73 (m, 2H), 3.85 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 193.6, 160.5, 138.5, 137.7, 135.9, 133.7, 131.6, 131.1, 130.1, 128.1, 128.0, 111.3, 104.7, 56.6; MS (ESI) m/z = 359 (M+Na)+; HRMS (ESI) m/z calcd. for C17H14Cl2O3 [M+Na] 359.0212, found 359.0215 [M+Na].
(E)-1-(3,4-Dichlorophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b38). Yellow solid (98%), mp: 142.1–143.4 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.15 (d, J = 16.0 Hz, 1H), 8.12–8.10 (m, 1H), 7.97–7.95 (m, 1H), 7.93 (d, J = 16.0 Hz, 1H), 7.84–7.82 (m, 1H), 7.44–7.41 (m, 1H), 6.77–6.75 (m, 2H), 3.92 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 188.7, 160.6, 138.8, 136.6, 136.0, 133.3, 132.4, 131.7, 130.3, 128.7, 123.3, 111.8, 104.7, 56.6; MS (ESI) m/z = 359 (M+Na)+; HRMS (ESI) m/z calcd. for C17H14Cl2O3 [M+Na] 359.0212, found 359.0189 [M+Na].
(E)-1-(3-Nitrophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b39). Yellow solid (92%), mp: 172.3–173.7 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.66–8.63 (m, 1H), 8.49–8.47 (m, 1H), 8.44–8.42 (m, 1H), 8.20 (d, J = 16.0 Hz, 1H), 8.02 (d, J = 16.0 Hz, 1H), 7.89–7.86 (m, 1H), 7.46–7.42 (m, 1H), 6.77–6.75 (m, 2H), 3.93 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.0, 160.7, 148.6, 139.7, 136.9, 134.7, 133.5, 131.2, 127.5, 123.2, 122.9, 111.7, 104.7, 56.6; MS (ESI) m/z = 336 (M+Na)+; HRMS (ESI) m/z calcd. for C17H15NO5 [M+Na] 336.0842, found 336.0825 [M+Na].
(E)-1-(4-Nitrophenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b40). Yellow solid (97%), mp: 147.3–148.6 °C. 1H-NMR (400 MHz, DMSO-d6) δ 8.39–8.36 (m, 2H), 8.21–8.16 (m, 3H), 7.98 (d, J = 15.6 Hz, 1H), 7.46–7.42 (m, 1H), 6.78–6.76 (m, 2H), 3.93 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.9, 160.7, 150.1, 143.5, 136.9, 133.5, 130.0, 124.4, 123.7, 111.7, 104.7, 56.6; MS (ESI) m/z = 314 (M+H)+; HRMS (ESI) m/z calcd. for C17H15NO5 [M+H] 314.1023, found 314.1022 [M+H].
(E)-1-(3-(Trifluoromethyl)phenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b41). Yellow solid (97%), mp: 145.3–146.7 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.31–8.29 (m, 1H), 8.19 (s, 1H), 8.17 (d, J = 16.0 Hz, 1H), 8.04–8.02 (m, 1H), 7.99 (d, J = 16.0 Hz, 1H), 7.85–7.82 (m, 1H), 7.45–7.41 (m, 1H), 6.77–6.75 (m, 2H), 3.93 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 189.7, 160.6, 139.3, 136.6, 133.3, 132.6, 130.8, 130.2, 129.9, 129.6, 124.8, 123.6, 111.8, 104.7, 56.6; MS (ESI) m/z = 337 (M+H)+; HRMS (ESI) m/z calcd. for C18H15F3O3 [M+H] 337.1046, found 337.1039 [M+H].
(E)-1-(3,5-bis(Trifluoromethyl)phenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one (b42). Yellow solid (99%), mp: 126.5–127.8 °C. 1H-NMR (500 MHz, DMSO-d6) δ 8.47 (s, 2H), 8.43 (s, 1H), 8.16 (d, J = 16.0 Hz, 1H), 7.95 (d, J = 16.0 Hz, 1H), 7.46–7.43 (m, 1H), 6.77–6.75 (m, 2H), 3.91 (s, 6H); 13C-NMR (150 MHz, DMSO-d6): 188.9, 160.6, 140.6, 137.5, 133.6, 131.4, 131.2, 129.0, 126.4, 124.4, 123.6, 122.6, 111.7, 104.7, 56.6; MS (ESI) m/z = 405 (M+H)+; HRMS (ESI) m/z calcd. for C19H14F6O3 [M+H] 405.0920, found 405.0917 [M+H].
3.2. Biology
3.2.1. Cell Culture
HepG2 and MCF7 cells were grown in the Eagle’s minimum essential medium with Earle’s balanced salts (MEM-EBSS) medium, HeLa, A549 and L02 cells were grown in Dulbecco’s Modified Eagle Media (DMEM), SW1990 cells were grown in RPMI-1640 medium, and HMLE cell were grown in DMEM/F-12 medium. The media were supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin and 10% fetal bovine serum (FBS), and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
3.2.2. In Vitro Anti-Proliferative Assay
Cells were seeded into 96-well plates (4000 cells/well) and incubated at 37 °C in a humidified 5% CO2 atmosphere. After 24 h, the cells were treated with different concentrations of the compounds for 48 h in triplicate to generate dose-response curves. Cell viability was evaluated using the sulforhodamine B (SRB) assay as previous described [45]. The IC50 values of the compounds were calculated using SigmaPlot10.0 software, which defined the IC50 value as the concentration required to inhibit cell growth by 50%. Etoposide was used as the positive control.
4. Conclusions
In summary, we have developed a class of alkoxylated chalcones and evaluated their cytotoxicities in vitro against a panel of five different cancer cell lines and two non-tumoral cell lines. Most of the chalcones displayed potent cytotoxic activities. The results of this study also revealed that chalcones bearing electron donating alkoxy groups on their A- or B-ring generally exhibited good levels of cytotoxicity, and that the position and size of the substituents had a significant impact on the cytotoxic activity, with ortho and meta substituents being beneficial to the activity and large substituents having the opposite effect. The most potent compounds were b22 and b29, which had a 4-phenyl moiety and a 3-OMe-4-OH moiety, respectively. The SI values indicate that about one third of the compounds exhibit more than a 2-fold SI between the CC50 in L02 and IC50 in tumoral cells, which is similar with etoposide. Compounds b11, especially b29 showed promising SI values compared with both HMLE and L02 (2.1–6.5 fold in HMLE and over 33 fold in L02, respectively). These compounds are therefore worthy of further investigation.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/11/17256/s1.
Supplementary Files
Supplementary File 1Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 81102376, 81302644), and the National S&T Major Special Project on Major New Drug Innovation (No. 2012ZX09301002-001-023-03).
Author Contributions
Yu-Cheng Wang and Ju-Xian Wang designed the research; Xiao-Guang Bai, Chang-Liang Xu, Shuang-Shuang Zhao and Hong-Wei He performed the experimental work; Xiao-Guang Bai and Chang-Liang Xu wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure-Activity Relationship Studies of Chalcone Leading to 3-Hydroxy-4,3',4',5'-tetramethoxychalcone and Its Analogues as Potent Nuclear Factor κB Inhibitors and Their Anticancer Activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar]
- Lee, S.H.; Seo, G.S.; Kim, J.Y.; Jin, X.Y.; Kim, H.D.; Sohn, D.H. Heme oxygenase 1 mediates anti-inflammatory effects of 2',4',6'-tris(methoxymethoxy) chalcone. Eur. J. Pharmacol. 2006, 532, 178–186. [Google Scholar]
- Yang, H.M.; Shin, H.R.; Cho, S.H.; Bang, S.C.; Song, G.Y.; Ju, J.H.; Kim, M.K.; Lee, S.H.; Ryu, J.C.; Kim, Y.; et al. Structural requirement of chalcones for the inhibitory activity of interleukin-5. Bioorg. Med. Chem. 2007, 15, 104–111. [Google Scholar]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia 2002, 73, 536–539. [Google Scholar]
- Chiaradia, L.D.; Martins, P.G.; Cordeiro, M.N.; Guido, R.V.; Ecco, G.; Andricopulo, A.D.; Yunes, R.A.; Vernal, J.; Nunes, R.J.; Terenzi, H. Synthesis, biological evaluation, and molecular modeling of chalcone derivatives as potent inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatases (PtpA and PtpB). J. Med. Chem. 2012, 55, 390–402. [Google Scholar]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar]
- Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008, 43, 2220–2228. [Google Scholar]
- Cheng, J.H.; Hung, C.F.; Yang, S.C.; Wang, J.P.; Won, S.J.; Lin, C.N. Synthesis and cytotoxic, anti-inflammatory, and anti-oxidant activities of 2',5'-dialkoxylchalcones as cancer chemopreventive agents. Bioorg. Med. Chem. 2008, 16, 7270–7276. [Google Scholar]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar]
- Kachadourian, R.; Day, B.J.; Pugazhenti, S.; Franklin, C.C.; GenouxBastide, E.; Mahaffey, G.; Gauthier, C.; di Pietro, A.; Boumendjel, A. A synthetic chalcone as a potent inducer of glutathione biosynthesis. J. Med. Chem. 2012, 55, 1382–1388. [Google Scholar]
- Achanta, G.; Modzelewska, A.; Feng, L.; Khan, S.R.; Huang, P. A boronic-chalcone derivative exhibits potent anticancer activity through inhibition of the proteasome. Mol. Pharmacol. 2006, 70, 426–433. [Google Scholar]
- Valdameri, G.; Gauthier, C.; Terreux, R.; Kachadourian, R.; Day, B.J.; Winnischofer, S.M.; Rocha, M.E.; Frachet, V.; Ronot, X.; di Pietro, A.; et al. Investigation of Chalcones as Selective Inhibitors of the Breast Cancer Resistance Protein: Critical Role of Methoxylation in both Inhibition Potency and Cytotoxicity. J. Med. Chem. 2012, 55, 3193–3200. [Google Scholar]
- Rangel, L.P.; Winter, E.; Gauthier, C.; Terreux, R.; Chiaradia-Delatorre, L.D.; Mascarello, A.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B.; Macalou, S.; et al. New structure—Activity relationships of chalcone inhibitors of breast cancer resistance protein: Polyspecificity toward inhibition and critical substitutions against cytotoxicity. Drug Des. Dev. Ther. 2013, 7, 1043–1052. [Google Scholar]
- Winter, E.; Gozzi, G.J.; Chiaradia-Delatorre, L.D.; Daflon-Yunes, N.; Terreux, R.; Gauthier, C.; Mascarello, A.; Leal, P.C.; Cadena, S.M.; Yunes, R.A.; et al. Quinoxaline-substitutedchalconesasnew inhibitors of breast cancer resistance protein ABCG2: Polyspecificity at B-ring position. Drug Des. Dev. Ther. 2014, 8, 609–619. [Google Scholar]
- Kamal, A.; Shankaraiah, N.; Prabhakar, S.; Reddy, C.R.; Markandeya, N.; Reddy, K.L.; Devaiah, V. Solid-phase synthesis of new pyrrolobenzodiazepine-chalcone conjugates: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett. 2008, 18, 2434–2439. [Google Scholar]
- Jin, C.; Liang, Y.J.; He, H.; Fu, L. Synthesis and antitumor activity of novel chalcone derivatives. Biomed. Pharmacother. 2013, 67, 215–217. [Google Scholar]
- Shenvi, S.; Kumar, K.; Hatti, K.S.; Rijesh, K.; Diwakar, L.; Reddy, G.C. Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: Structure-activity relationship. Eur. J. Med. Chem. 2013, 62, 435–442. [Google Scholar]
- Boumendjel, A.; Boccard, J.; Carrupt, P.-A.; Nicolle, E.; Blanc, M.; Geze, A.; Choisnard, L.; Wouessidjewe, D.; Matera, E.-L.; Dumontet, C. Antimitotic and antiproliferative activities of chalcones: Forward structure-activity relationship. J. Med. Chem. 2008, 51, 2307–2310. [Google Scholar]
- Kumar, V.; Kumar, S.; Hassan, M.; Wu, H.; Thimmulappa, R.K.; Kumar, A.; Sharma, S.K.; Parmar, V.S.; Biswal, S.; Malhotra, S.V. Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J. Med. Chem. 2011, 54, 4147–4159. [Google Scholar]
- Hsieh, C.T.; Hsieh, T.J.; ElShazly, M.; Chuang, D.W.; Tsai, Y.H.; Yen, C.T.; Wu, S.F.; Wu, Y.C.; Chang, F.-R. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar]
- Lin, A.S.; NakagawaGoto, K.; Chang, F.R.; Yu, D.; MorrisNatschke, S.L.; Wu, C.C.; Chen, S.L.; Wu, Y.C.; Lee, K.H. First total synthesis of protoapigenone and its analogues as potent cytotoxic agents. J. Med. Chem. 2007, 50, 3921–3927. [Google Scholar]
- Montes-Avila, J.; Diaz-Camacho, S.P.; Sicairos-Felix, J.; Delgado-Vargas, F.; Rivero, I.A. Solution-phase parallel synthesis of substituted chalcones and their antiparasitary activity against Giardia lamblia. Bioorg. Med. Chem. 2009, 17, 6780–6785. [Google Scholar]
- Ashtekar, K.D.; Staples, R.J.; Borhan, B. Development of a Formal Catalytic Asymmetric [4 + 2] Addition of Ethyl-2,3-butadienoate with Acyclic Enones. Org. Lett. 2011, 13, 5732–5735. [Google Scholar]
- Braun, R.U.; Ansorge, M.; Müller, T.J.J. Coupling–Isomerization Synthesis of Chalcones. Chemistry 2006, 12, 9081–9094. [Google Scholar]
- Dimmock, J.R.; Jha, A.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Nienaber, K.H.; Kowalczyk, E.S.; Allen, T.M.; Santos, C.L.; de Clercq, E.; et al. Cytotoxic N-[4-(3-aryl-3-oxo-1 propenyl)phenylcarbonyl]-3,5-bis(phenylmethylene)-4-piperidones and related compounds. Eur. J. Med. Chem. 2002, 37, 961–972. [Google Scholar]
- Brown, B.R.; Cummings, W. Polymerisation of flavans. Part II. The condensation of 4[prime or minute]-methoxyflavan with phenols. J. Chem. Soc. (Resum.) 1958. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Srivastava, S. Synthesis of chalcones and flavanones using Julia–Kocienski olefination. Tetrahedron 2010, 66, 9445–9449. [Google Scholar]
- Barnes, R.P.; Goodwin, T.C.; Cotten, T.W. A Study of the Direction of Enolization of p-Methoxy-p'-bromodibenzoylmethane and p-Methoxy-p'-ethoxydibenzoylmethane. J. Am. Chem. Soc. 1947, 69, 3135–3138. [Google Scholar]
- Wu, J.; Li, J.; Cai, Y.; Pan, Y.; Ye, F.; Zhang, Y.; Zhao, Y.; Yang, S.; Li, X.; Liang, G. Evaluation and Discovery of Novel Synthetic Chalcone Derivatives as Anti-Inflammatory Agents. J. Med. Chem. 2011, 54, 8110–8123. [Google Scholar]
- D’Orazio, D.; de Saizieu, A.; Raederstorff, D.; Schueler, G.; Wang-Schmidt, Y.; Wehrli, C.; Wertz, K.; Wolfram, S. Compounds for the Treatment of Non-Autoimmune Type 2 Diabetes Mellitus and/or Syndrome X. WO Patent 2,006,136,429, 28 December 2006. [Google Scholar]
- Shettigar, V.; Patil, P.S.; Naveen, S.; Dharmaprakash, S.M.; Sridhar, M.A.; Shashidhara Prasad, J. Crystal growth and characterization of new nonlinear optical chalcone derivative: 1-(4-Methoxyphenyl)-3-(3, 4-dimethoxyphenyl)-2-propen-1-one. J. Cryst. Growth 2006, 295, 44–49. [Google Scholar]
- Hwang, D.; Hyun, J.; Jo, G.; Koh, D.; Lim, Y. Synthesis and complete assignment of NMR data of 20 chalcones. Magn. Reson. Chem. 2011, 49, 41–45. [Google Scholar]
- Russell, A. The constitution of tannins. Part I. Reduction products of chalkones and the synthesis of a typical phlobatannin. J. Chem. Soc. (Resum.) 1934. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, A.; Kumar, R.; Shard, A.; Sinha, A.K. One-Pot Two-Step Oxidative Cleavage of 1,2-Arylalkenes to Aryl Ketones Instead of Arylaldehydes in an Aqueous Medium: A Complementary Approach to Ozonolysis. Eur. J. Org. Chem. 2010, 31, 6025–6032. [Google Scholar]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.D.; McGown, A.T.; Lawrence, N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: Synthesis and biological evaluation of antivascular activity. Bioorg. Med. Chem. 2009, 17, 7698–7710. [Google Scholar]
- Simonis, H.; Danischewski, S. Über die Anwendung der Friedel-Craftsschen Reaktion zum Aufbau der Flavone. Eur. J. Inorg. Chem. 1926, 59, 2914–2919. [Google Scholar]
- Johnson, I.T.; Fenwick, G.R. (Eds.) Royal Society of Chemistry; Johnson, I.T.; Fenwick, G.R. (Eds.) Royal Society of Chemistry: England, UK, 2000; Volume 255, pp. 189–192.
- Yong, K.; Lu, J.; Gu, H.; Chen, X. Process for Preparation of 4'-Hydroxy-2,6-dimethoxydihydrochalcone. CN Patent 1,01,250,098, 27 August 2008. [Google Scholar]
- Cohen, F.E.; McKerrow, J.H.; Kenyon, G.L.; Li, Z.; Chen, X.; Gong, B.; Li, R. Inhibitors of Metazoan Parasite Proteases. U.S. Patent 5,739,170 A, 14 April 1998. [Google Scholar]
- George, F.; Fellague, T. Preparation of Amino Substituted Chalcone Derivatives for Use in Cosmetic Compositions as Sunscreens and Suntanning Agents. F.R. Patent 2,839,717, Al, 21 November 2003. [Google Scholar]
- Song, L.L.; Kosmeder, J.W., II; Lee, S.K.; Gerhauser, C.; Lantvit, D.; Moon, R.C.; Moriarty, R.M.; Pezzuto, J.M. Cancer chemopreventive activity mediated by 4'-bromoflavone, a potent inducer of phase II detoxification enzymes. Cancer Res. 1999, 59, 578–585. [Google Scholar]
- Nielsen, S.F.; Christensen, S.B.; Cruciani, G.; Kharazmi, A.; Liljefors, T. Antileishmanial Chalcones: Statistical Design, Synthesis, and Three-Dimensional Quantitative Structure-Activity Relationship Analysis. J. Med. Chem. 1998, 41, 4819–4832. [Google Scholar]
- Sahu, N.K.; Bari, S.B.; Kohli, D.V. Molecular modeling studies of some substituted chalcone derivatives as cysteine protease inhibitors. Med. Chem. Res. 2012, 21, 3835–3847. [Google Scholar]
- Sun, H.X.; He, H.W.; Zhang, S.H.; Liu, T.G.; Ren, K.H.; He, Q.Y.; Shao, R.G. Suppression of N-Ras by shRNA-expressing plasmid increases sensitivity of HepG2 cells to vincristine-induced growth inhibition. Cancer Gene Ther. 2009, 16, 693–702. [Google Scholar]
- Sample Availability: Samples of the compounds a1–20 and b1–42 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).