Abstract
Two new phenolic glycosides, named gnaphaffine A and B (compounds 1 and 2), were isolated from Gnaphalium affine. together with six known compounds, including caffeic acid (3), everlastoside L (4), isorhamnetin-7-O-β-d-glucopyranoside (5), quercetin-3-O-β-d-glucopyranoside (6), scutellarein-7-O-β-d-glucoside (7) and api-genin-7-O-β-d-glucopyranoside (8). Their structures were elucidated by spectroscopic methods, including ESI-MS and 2D NMR spectroscopy (HMQC and HMBC). All compounds were evaluated for their anti-complementary activity on the classical pathway of the complement system in vitro.
1. Introduction
The genus Gnaphalium belongs to the family Compositae and consists of approximately 200 species found all over the World, among which 19 are distributed in China [1]. Gnaphalium affine D. Don is an annual herbaceous plant, locally named Ching Ming vegetable in China. G. affine has been used as a traditional medicine for the relief of swelling, wounds, lumbago, angina ache in some Latin American countries [2]. Previous phytochemical investigation on Gnaphalium affine have reported the isolation of flavonoids [3,4,5,6,7,8], phenolic constituents [9], polysaccharides [10], essential oil compounds [11], diterpenes [12,13], and other chemical constituents [14]. Many of these components have been demonstrated to possess anti-complementary and antifeedant activities [2]. During our ongoing research to discover novel or bioactive constituents from G. affine, two novel phenolic glycosides were isolated from this plant, and named gnaphalium A (1) and B (2) (Figure 1).
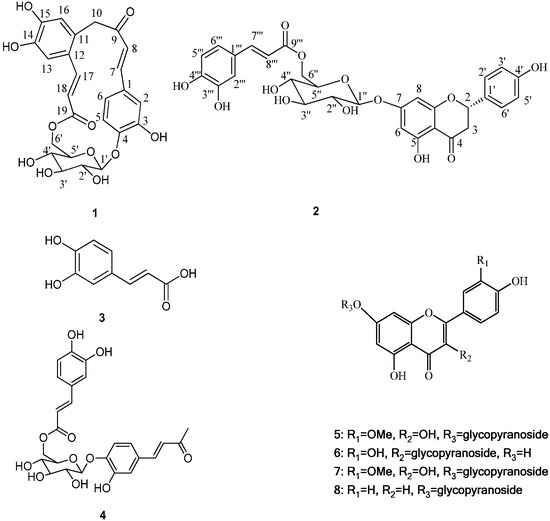
Figure 1.
Structures of compounds 1–8.
Figure 1.
Structures of compounds 1–8.
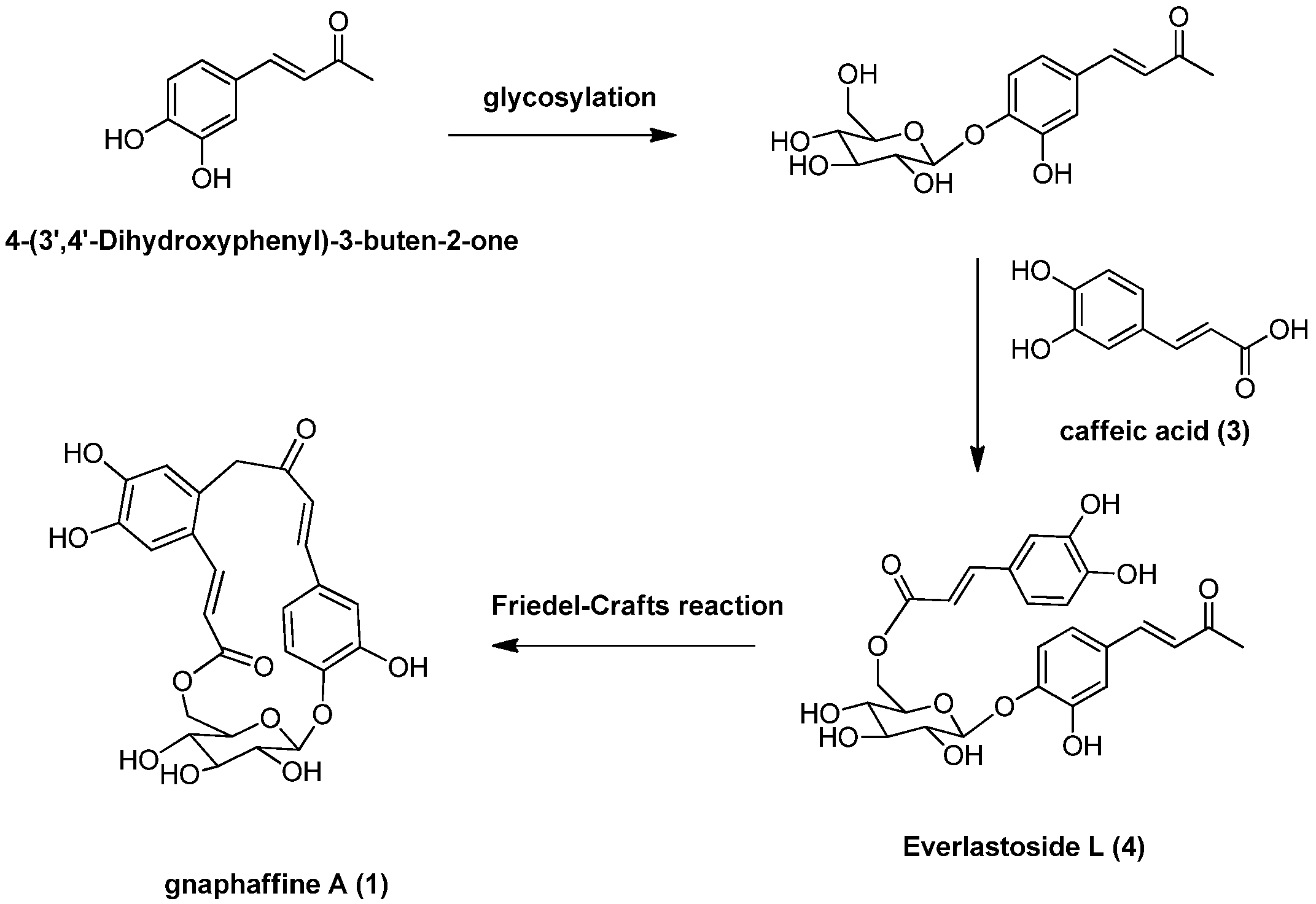
Besides, several known compounds were isolated and identified as caffeic acid (3) [15], everlastoside L (4) [16], isorhamnetin-7-O-β-d-glucopyranoside (5) [17], quercetin-3-O-β-d-gluco-pyranoside (6) [18], scutellarein-7-O-β-d-glucopyranoside (7) [19] and apigenin-7-O-β-d-gluco-pyranoside (8) [20], by comparison of their spectroscopic data with published values. Herein the isolation, characterization, and anti-complementary activity of these compounds were reported. In addition, a plausible biogenetic pathway for compound 1 has been proposed (Scheme 1).
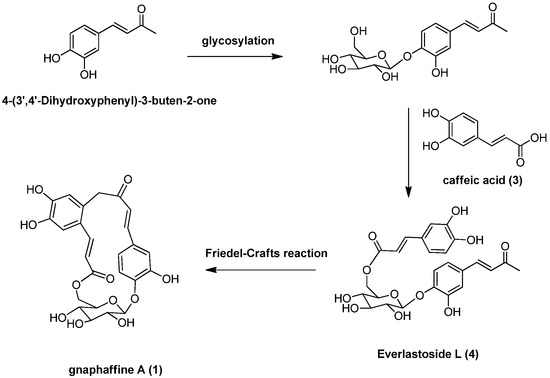
Scheme 1.
Plausible biogenetic pathway for compound 1.
Scheme 1.
Plausible biogenetic pathway for compound 1.
2. Results and Discussion
An 80% ethanolic extract of dried G. affine whole plant was suspended in distilled water and extracted with EtOAc. The EtOAc soluble fraction was concentrated under reduced pressure to produce a residue that was subjected multiple chromatography, two new compounds 1 and 2 and six known compounds 3–8 were isolated and identified.
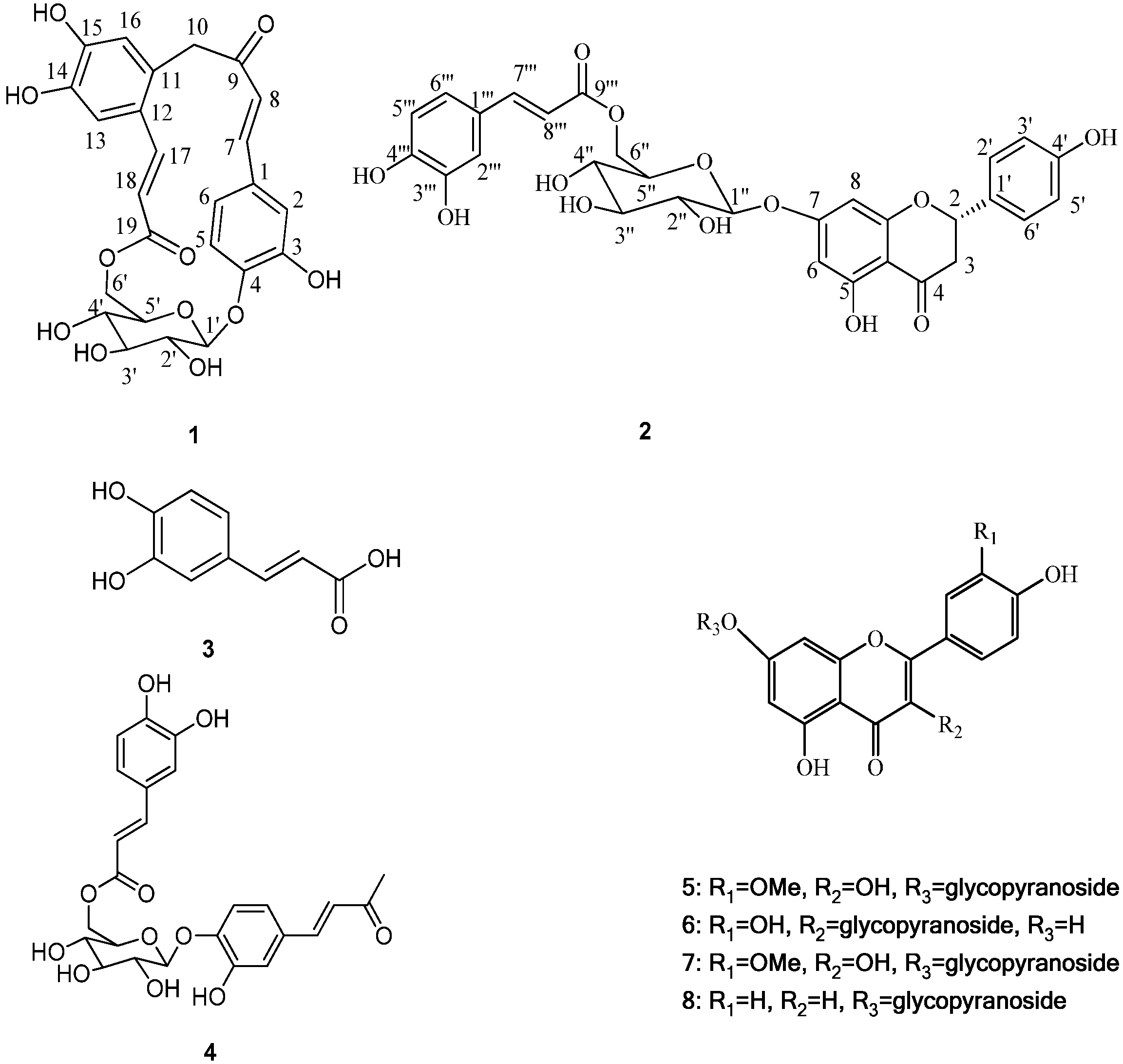
Gnaphaffine A (1) was isolated as a yellow, amorphous powder. On the basis of a HR-ESI-MS peak at m/z 501.1393 [M+H]+ and 13C-NMR data the molecular formula of 1 was determined to be C25H24O11, indicating 14 degrees of unsaturation. The IR spectrum showed the presence of hydroxyl (3535 cm−1), carbonyl (1668 cm−1) and benzene ring (1606, 1506 cm−1) groups. The structure of the compound was established from detailed analysis of its 1H- and 13C-NMR spectra, including 2D NMR. The 13C-NMR spectrum of 1 (Table 1), exhibited 25 signals that together with the information from a DEPT spectrum, indicated two methylene, fourteen methine, and nine quaternary carbons. Among these were one carbonyl group at δ 196.1 (C-9) and one ester function at δ 166.1 (C-19); as well as one anomeric methine at δ 100.7 (C-1') and four methines at δ 72.7 (C-2'), 75.5 (C-3'), 70.6 (C-4') and 73.7 (C-5'), and one methylene at δ 65.2 (C-6'), indicating the presence of a glucopyranose unit. Acid hydrolysis of 1 released D-glucopyranose, with an 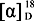 +38.2 (c 0.15, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1' from the large coupling constant of H-1' (J = 7.8 Hz) [2]. The 1H-NMR spectrum (Table 1) displayed signals for an ABX spin system at δ 7.40 (1H, d, J = 1.8 Hz, H-2), 7.08 (1H, dd, J = 8.4, 1.8 Hz, H-6) and 6.80 (1H, d, J = 8.4 Hz, H-5); two trans-configured double bonds at δ 7.33 (1H, d, J = 15.6 Hz, H-7) and δ 6.90 (1H, d, J = 15.6 Hz, H-8), δ 8.02 (1H, d, J = 15.6 Hz, H-17) and δ 6.10 (1H, d, J = 15.6 Hz, H-18) which were further confirmed by the HMQC correlations (H-7/C-7, H-8/C-8, H-17/C-17 and H-18/C-18) and 1H-1H COSY (Figure 2) correlations (H-7/H-8 and H-17/H-18); two singlet signals at δ 7.00 (1H, s, H-13) and δ 6.75 (1H, s, H-16) which suggests the presence of an 1,2,4,5-tetrasubstituted aromatic ring; one methylene group signals at δ 3.38 (1H, m, H-10a) and δ 4.26 (1H, m, H-10b) showed a correlation with δ 44.3 (C-10) in the HMQC. In addition to the above signals, a sugar moiety was identified due to the distinct anomeric signal at δ 4.88 (1H, d, J = 7.8 Hz, H-1'), four oxymethine protons signals between δ 4.10 and δ 3.38 and two oxymethylene protons signals at δ 4.38 and δ 4.29, which were further confirmed by the HMQC correlations and 1H-1H COSY correlations (Figure 2). Since the above-mentioned groups accounted for 13 degrees of unsaturation, the remaining degree suggested the presence of an additional ring system in the structure of 1. The HMBC experiment (Figure 2) showed clear correlations of ABX protons H-2 and H-6 with C-7, H-7 with C-2 and C-6 and of H-8 with C-1, which confirmed the presence of the carbon-carbon double bond attached at C-1 of the ABX system, while correlations of H-7 with C-9 and of H-8 with C-9 and C-10 confirmed the presence of the carbonyl group C-9 attached at C-8. Correlations of H-10a and H-10b with C-9, C-12 (δ 123.4) and C-16 (δ 117.9), and of H-16 with C-10 confirmed the attachment of C-10 at carbonyl group (C-9) and C-11 of the tetrasubstituted aromatic ring at C-10.
+38.2 (c 0.15, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1' from the large coupling constant of H-1' (J = 7.8 Hz) [2]. The 1H-NMR spectrum (Table 1) displayed signals for an ABX spin system at δ 7.40 (1H, d, J = 1.8 Hz, H-2), 7.08 (1H, dd, J = 8.4, 1.8 Hz, H-6) and 6.80 (1H, d, J = 8.4 Hz, H-5); two trans-configured double bonds at δ 7.33 (1H, d, J = 15.6 Hz, H-7) and δ 6.90 (1H, d, J = 15.6 Hz, H-8), δ 8.02 (1H, d, J = 15.6 Hz, H-17) and δ 6.10 (1H, d, J = 15.6 Hz, H-18) which were further confirmed by the HMQC correlations (H-7/C-7, H-8/C-8, H-17/C-17 and H-18/C-18) and 1H-1H COSY (Figure 2) correlations (H-7/H-8 and H-17/H-18); two singlet signals at δ 7.00 (1H, s, H-13) and δ 6.75 (1H, s, H-16) which suggests the presence of an 1,2,4,5-tetrasubstituted aromatic ring; one methylene group signals at δ 3.38 (1H, m, H-10a) and δ 4.26 (1H, m, H-10b) showed a correlation with δ 44.3 (C-10) in the HMQC. In addition to the above signals, a sugar moiety was identified due to the distinct anomeric signal at δ 4.88 (1H, d, J = 7.8 Hz, H-1'), four oxymethine protons signals between δ 4.10 and δ 3.38 and two oxymethylene protons signals at δ 4.38 and δ 4.29, which were further confirmed by the HMQC correlations and 1H-1H COSY correlations (Figure 2). Since the above-mentioned groups accounted for 13 degrees of unsaturation, the remaining degree suggested the presence of an additional ring system in the structure of 1. The HMBC experiment (Figure 2) showed clear correlations of ABX protons H-2 and H-6 with C-7, H-7 with C-2 and C-6 and of H-8 with C-1, which confirmed the presence of the carbon-carbon double bond attached at C-1 of the ABX system, while correlations of H-7 with C-9 and of H-8 with C-9 and C-10 confirmed the presence of the carbonyl group C-9 attached at C-8. Correlations of H-10a and H-10b with C-9, C-12 (δ 123.4) and C-16 (δ 117.9), and of H-16 with C-10 confirmed the attachment of C-10 at carbonyl group (C-9) and C-11 of the tetrasubstituted aromatic ring at C-10.
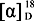 +38.2 (c 0.15, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1' from the large coupling constant of H-1' (J = 7.8 Hz) [2]. The 1H-NMR spectrum (Table 1) displayed signals for an ABX spin system at δ 7.40 (1H, d, J = 1.8 Hz, H-2), 7.08 (1H, dd, J = 8.4, 1.8 Hz, H-6) and 6.80 (1H, d, J = 8.4 Hz, H-5); two trans-configured double bonds at δ 7.33 (1H, d, J = 15.6 Hz, H-7) and δ 6.90 (1H, d, J = 15.6 Hz, H-8), δ 8.02 (1H, d, J = 15.6 Hz, H-17) and δ 6.10 (1H, d, J = 15.6 Hz, H-18) which were further confirmed by the HMQC correlations (H-7/C-7, H-8/C-8, H-17/C-17 and H-18/C-18) and 1H-1H COSY (Figure 2) correlations (H-7/H-8 and H-17/H-18); two singlet signals at δ 7.00 (1H, s, H-13) and δ 6.75 (1H, s, H-16) which suggests the presence of an 1,2,4,5-tetrasubstituted aromatic ring; one methylene group signals at δ 3.38 (1H, m, H-10a) and δ 4.26 (1H, m, H-10b) showed a correlation with δ 44.3 (C-10) in the HMQC. In addition to the above signals, a sugar moiety was identified due to the distinct anomeric signal at δ 4.88 (1H, d, J = 7.8 Hz, H-1'), four oxymethine protons signals between δ 4.10 and δ 3.38 and two oxymethylene protons signals at δ 4.38 and δ 4.29, which were further confirmed by the HMQC correlations and 1H-1H COSY correlations (Figure 2). Since the above-mentioned groups accounted for 13 degrees of unsaturation, the remaining degree suggested the presence of an additional ring system in the structure of 1. The HMBC experiment (Figure 2) showed clear correlations of ABX protons H-2 and H-6 with C-7, H-7 with C-2 and C-6 and of H-8 with C-1, which confirmed the presence of the carbon-carbon double bond attached at C-1 of the ABX system, while correlations of H-7 with C-9 and of H-8 with C-9 and C-10 confirmed the presence of the carbonyl group C-9 attached at C-8. Correlations of H-10a and H-10b with C-9, C-12 (δ 123.4) and C-16 (δ 117.9), and of H-16 with C-10 confirmed the attachment of C-10 at carbonyl group (C-9) and C-11 of the tetrasubstituted aromatic ring at C-10.
+38.2 (c 0.15, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1' from the large coupling constant of H-1' (J = 7.8 Hz) [2]. The 1H-NMR spectrum (Table 1) displayed signals for an ABX spin system at δ 7.40 (1H, d, J = 1.8 Hz, H-2), 7.08 (1H, dd, J = 8.4, 1.8 Hz, H-6) and 6.80 (1H, d, J = 8.4 Hz, H-5); two trans-configured double bonds at δ 7.33 (1H, d, J = 15.6 Hz, H-7) and δ 6.90 (1H, d, J = 15.6 Hz, H-8), δ 8.02 (1H, d, J = 15.6 Hz, H-17) and δ 6.10 (1H, d, J = 15.6 Hz, H-18) which were further confirmed by the HMQC correlations (H-7/C-7, H-8/C-8, H-17/C-17 and H-18/C-18) and 1H-1H COSY (Figure 2) correlations (H-7/H-8 and H-17/H-18); two singlet signals at δ 7.00 (1H, s, H-13) and δ 6.75 (1H, s, H-16) which suggests the presence of an 1,2,4,5-tetrasubstituted aromatic ring; one methylene group signals at δ 3.38 (1H, m, H-10a) and δ 4.26 (1H, m, H-10b) showed a correlation with δ 44.3 (C-10) in the HMQC. In addition to the above signals, a sugar moiety was identified due to the distinct anomeric signal at δ 4.88 (1H, d, J = 7.8 Hz, H-1'), four oxymethine protons signals between δ 4.10 and δ 3.38 and two oxymethylene protons signals at δ 4.38 and δ 4.29, which were further confirmed by the HMQC correlations and 1H-1H COSY correlations (Figure 2). Since the above-mentioned groups accounted for 13 degrees of unsaturation, the remaining degree suggested the presence of an additional ring system in the structure of 1. The HMBC experiment (Figure 2) showed clear correlations of ABX protons H-2 and H-6 with C-7, H-7 with C-2 and C-6 and of H-8 with C-1, which confirmed the presence of the carbon-carbon double bond attached at C-1 of the ABX system, while correlations of H-7 with C-9 and of H-8 with C-9 and C-10 confirmed the presence of the carbonyl group C-9 attached at C-8. Correlations of H-10a and H-10b with C-9, C-12 (δ 123.4) and C-16 (δ 117.9), and of H-16 with C-10 confirmed the attachment of C-10 at carbonyl group (C-9) and C-11 of the tetrasubstituted aromatic ring at C-10.
Table 1.
1H-NMR (600 MHz) and 13C-NMR (150 MHz) data for compound 1 (DMSO-d6, δH in ppm, J in Hz).
| Position | δ H | δ C |
|---|---|---|
| 1 | - | 125.7 |
| 2 | 7.40 (d, 1.8) | 112.4 |
| 3 | - | 145.6 |
| 4 | - | 149.4 |
| 5 | 6.80 (d, 8.4) | 116.0 |
| 6 | 7.08 (dd, 8.4, 1.8) | 126.4 |
| 7 | 7.33 (d, 15.6) | 143.2 |
| 8 | 6.90 (d, 15.6) | 122.3 |
| 9 | - | 196.1 |
| 10a | 3.38 (m) | 44.3 |
| 10b | 4.26 (m) | |
| 11 | - | 127.5 |
| 12 | - | 123.4 |
| 13 | 7.00 (s) | 113.2 |
| 14 | - | 144.8 |
| 15 | - | 148.3 |
| 16 | 6.75 (s) | 117.9 |
| 17 | 8.02 (d, 15.6) | 140.9 |
| 18 | 6.10 (d, 15.6) | 116.4 |
| 19 | - | 166.1 |
| 1' | 4.88 (d, 7.8) | 100.7 |
| 2' | 3.38 (m) | 72.7 |
| 3' | 3.38 (m) | 75.5 |
| 4' | 3.15 (m) | 70.6 |
| 5' | 4.10 (t, 9.4) | 73.7 |
| 6'a | 4.38 (d, 11.4) | 65.2 |
| 6'b | 4.29 (m) |
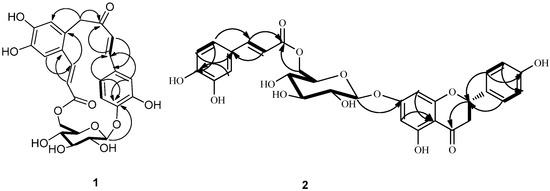
Figure 2.
Key 1H-1H COSY (bold lines) and HMBC (H→C) correlations of compounds 1 and 2.
Figure 2.
Key 1H-1H COSY (bold lines) and HMBC (H→C) correlations of compounds 1 and 2.
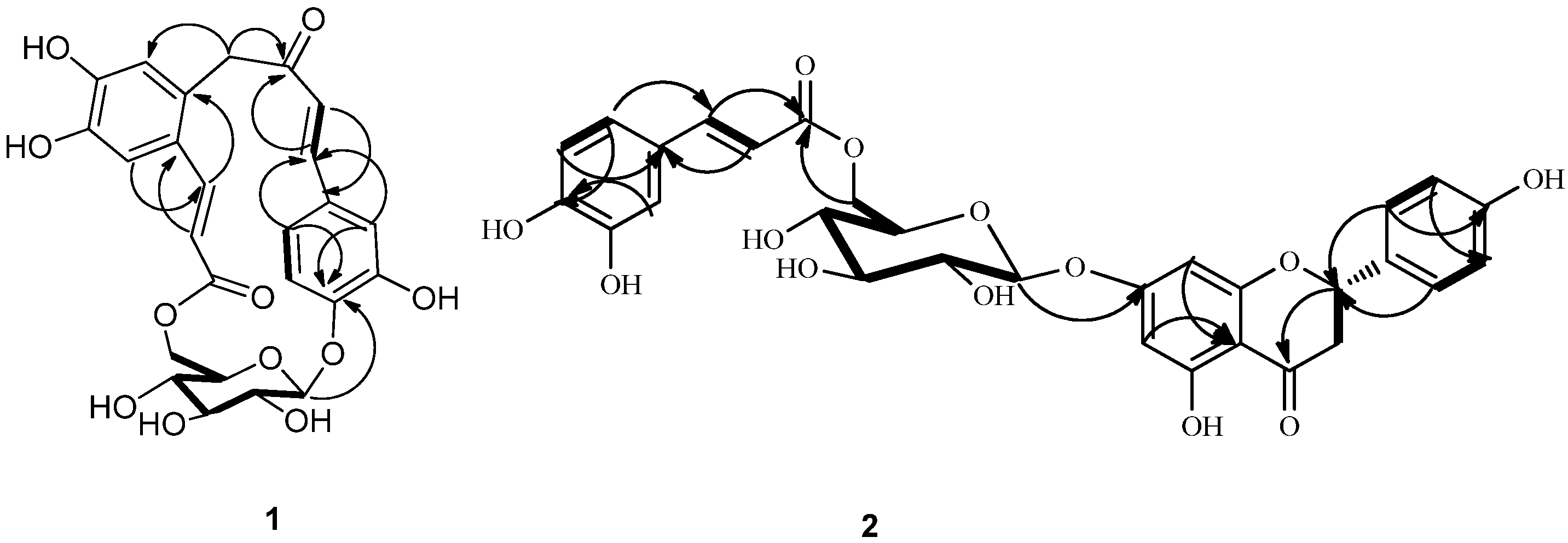
Another carbon-carbon double bond attached at C-12 was deduced from correlations of H-18 with C-12, H-17 with C-11 (δ 127.5) and C-13 (δ 113.2) and of H-13 (δ 7.00) with C-17 (δ 140.9). In addition, correlations of H-17 and H-18 with C-19 suggested the ester function was attached to C-18. The glucopyranose moiety was found to be attached at C-4 as evidenced by the HMBC correlation from H-1' to C-4 (δ 149.4). Besides, the glucopyranose group was linked to C-19 through C-6' as determined by the HMBC correlations from H-6' (δ 4.38 and 4.29) to C-19. Based on this combined evidence, structure of compound 1 was confirmed as that of a novel phenolic glycoside that was trivially named gnaphaffine A. A plausible biogenetic pathway has been discussed for compound 1 herein (Scheme 1). Everlastoside L (4) was proposed as the precursor of compound 1 via Friedel-Crafts reaction. The common biological intermediate (4-(3',4'-Dihydroxyphenyl)-3-buten-2-one) [21] may convert into everlastoside L (4) via saccharification and esterification reactions.
Gnaphaffine B (2) was obtained as a pale yellow solid. Its molecular formula was determined as C30H28O13 by its HR-ESI-MS peak at m/z 597.1597 [M−H]− (calcd for C30H29O13, 597.1524). IR absorptions suggested the presence of hydroxyl (3455 cm−1), carbonyl (1692 cm−1) and phenyl groups (1632, 1502 cm−1). The 1H-NMR spectrum of compound 2 showed three protons at δ 2.70 (dd, J = 17.4, 3.0 Hz), 3.26 (dd, J = 12.0, 17.4 Hz) and 5.45 (dd, J = 12.0, 3.0Hz), typically assigned to H2-3 and H-2 of a flavanol skeleton, an AA'XX' spin system at δ 7.27 (2H, d, J = 8.4 Hz, H-2', 6') and 6.76 (2H, d, J = 8.4 Hz, H-3', 5'), and two meta-coupled protons at δ 6.18 (1H, brs, H-6) and 6.13 (1H, dd, J = 1.8, 4.8 Hz, H-8) and 5.45 (1H, dd, J = 3.0, 12.0 Hz, H-2), 3.26 (1H, dd, J = 12.0, 17.4 Hz, H-3a), 2.70 (1H, dd, J = 17.4, 3.0 Hz, H-3b), which suggested the presence of a (S)-Naringenin skeleton [22,23,24]; a sugar moiety due to the distinct anomeric signal at δ 5.05 (1H, d, 7.8Hz, H-1'') and oxymethine protons in the rang δ 3.21–5.05. The 13C-NMR data (Table 2) showed four methines at δ 69.6 (C-4''), 73.9 (C-2''), 76.4 (C-3'') and 77.1 (C-5''), one methylene at δ 63.2 (C-6'') and an anomeric methine at δ 99.3 (C-1''), indicating the presence of a glucopyranose unit.

Table 2.
1H-NMR (600 MHz) and 13C-NMR (150 MHz) data for compound 2 (DMSO-d6, δH in ppm, J in Hz).
| Position | δ H | δ C |
|---|---|---|
| 2 | 5.45 (1H, dd, 3.0, 12.0) | 78.7 |
| 3 | 3.26 (1H, dd, 12.0, 17.4) | 42.1 |
| 2.70 (1H, dd, 17.4, 3.0) | ||
| 4 | - | 197.1 |
| 5 | - | 162.7 |
| 6 | 6.18 (1H, brs) | 96.3 |
| 7 | - | 163.2 |
| 8 | 6.13 (1H, dd, 1.8, 4.8) | 95.5 |
| 9 | - | 165.0 |
| 10 | - | 103.3 |
| 1' | - | 128.6 |
| 2', 6' | 7.27 (2H, d, 8.4) | 12.4 |
| 3', 5' | 6.76 (2H, d,8.4) | 115.2 |
| 4' | - | 157.8 |
| 1'' | 5.05 (1H, d, 7.8) | 99.3 |
| 2'' | 3.24 (1H, m) | 73.79 |
| 3'' | 3.30 (1H, m) | 76.4 |
| 4'' | 3.15 (1H, m) | 69.6 |
| 5'' | 3.40 (1H, m) | 77.1 |
| 6'' | 4.42 (1H, brd, 12.0) | 63.2 |
| 4.11 (1H, dd, 18.0,6.6) | ||
| 1''' | - | 125.5 |
| 2''' | 7.02 (1H, d, 4.2 ) | 114.9 |
| 3''' | - | 145.6 |
| 4''' | - | 148.4 |
| 5''' | 6.76 (1H, d, 8.4) | 115.7 |
| 6''' | 6.94 (1H, dd, 8.4,2.4) | 121.2 |
| 7''' | 6.24 (1H, d, 15.6) | 113.7 |
| 8''' | 7.45(1H, d, 15.6) | 145.3 |
| 9''' | - | 166.4 |
Acid hydrolysis of 2 yielded a free sugar that was identified as D-glucopyranose by measurement of the corresponding optical rotation 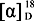 +40.3 (c 0.20, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1'' from the large coupling constant of H-1'' (J = 7.8 Hz) [2]. Furthermore, presence of a caffeoly moiety was confirmed by the detection of ABX spin system signals at 7.02 (1H, d, J = 4.2 Hz, H-2'''), 6.76 (1H, d, J = 8.4Hz, H-5'''), 6.94 (1H, dd, J = 8.4, 2.4 Hz, H-6'''), and the trans-configuration of the double bond was recognized by the large coupling constant (J = 15.6) observed for the olefinic resonances H-7''' and H-8'''. The glucopyranose attachment at C-7 was supported by the HMBC (Figure 2) correlation of H-1'' (5.05) to C-7 (165.0). The glucopyranose group was linked to C-9''' through C-6''', as confirmed by the HMBC correlation from H-6'' (δ 4.42 and 4.11) to C-9'''. These suggested the glucopyranose group was linked to C-9''' through C-6''. All proton and carbon signals were assigned via HMQC, HMBC (Figure 2) and 1H-1H COSY spectra. Therefore, compound 2 was identified as naringenin-7-O-β-d-(6''-E-caffeoyl)-glucopyranoside, and named gnaphaffine B.
+40.3 (c 0.20, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1'' from the large coupling constant of H-1'' (J = 7.8 Hz) [2]. Furthermore, presence of a caffeoly moiety was confirmed by the detection of ABX spin system signals at 7.02 (1H, d, J = 4.2 Hz, H-2'''), 6.76 (1H, d, J = 8.4Hz, H-5'''), 6.94 (1H, dd, J = 8.4, 2.4 Hz, H-6'''), and the trans-configuration of the double bond was recognized by the large coupling constant (J = 15.6) observed for the olefinic resonances H-7''' and H-8'''. The glucopyranose attachment at C-7 was supported by the HMBC (Figure 2) correlation of H-1'' (5.05) to C-7 (165.0). The glucopyranose group was linked to C-9''' through C-6''', as confirmed by the HMBC correlation from H-6'' (δ 4.42 and 4.11) to C-9'''. These suggested the glucopyranose group was linked to C-9''' through C-6''. All proton and carbon signals were assigned via HMQC, HMBC (Figure 2) and 1H-1H COSY spectra. Therefore, compound 2 was identified as naringenin-7-O-β-d-(6''-E-caffeoyl)-glucopyranoside, and named gnaphaffine B.
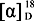 +40.3 (c 0.20, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1'' from the large coupling constant of H-1'' (J = 7.8 Hz) [2]. Furthermore, presence of a caffeoly moiety was confirmed by the detection of ABX spin system signals at 7.02 (1H, d, J = 4.2 Hz, H-2'''), 6.76 (1H, d, J = 8.4Hz, H-5'''), 6.94 (1H, dd, J = 8.4, 2.4 Hz, H-6'''), and the trans-configuration of the double bond was recognized by the large coupling constant (J = 15.6) observed for the olefinic resonances H-7''' and H-8'''. The glucopyranose attachment at C-7 was supported by the HMBC (Figure 2) correlation of H-1'' (5.05) to C-7 (165.0). The glucopyranose group was linked to C-9''' through C-6''', as confirmed by the HMBC correlation from H-6'' (δ 4.42 and 4.11) to C-9'''. These suggested the glucopyranose group was linked to C-9''' through C-6''. All proton and carbon signals were assigned via HMQC, HMBC (Figure 2) and 1H-1H COSY spectra. Therefore, compound 2 was identified as naringenin-7-O-β-d-(6''-E-caffeoyl)-glucopyranoside, and named gnaphaffine B.
+40.3 (c 0.20, H2O). The glucopyranose moiety was determined to have a β-configuration at C-1'' from the large coupling constant of H-1'' (J = 7.8 Hz) [2]. Furthermore, presence of a caffeoly moiety was confirmed by the detection of ABX spin system signals at 7.02 (1H, d, J = 4.2 Hz, H-2'''), 6.76 (1H, d, J = 8.4Hz, H-5'''), 6.94 (1H, dd, J = 8.4, 2.4 Hz, H-6'''), and the trans-configuration of the double bond was recognized by the large coupling constant (J = 15.6) observed for the olefinic resonances H-7''' and H-8'''. The glucopyranose attachment at C-7 was supported by the HMBC (Figure 2) correlation of H-1'' (5.05) to C-7 (165.0). The glucopyranose group was linked to C-9''' through C-6''', as confirmed by the HMBC correlation from H-6'' (δ 4.42 and 4.11) to C-9'''. These suggested the glucopyranose group was linked to C-9''' through C-6''. All proton and carbon signals were assigned via HMQC, HMBC (Figure 2) and 1H-1H COSY spectra. Therefore, compound 2 was identified as naringenin-7-O-β-d-(6''-E-caffeoyl)-glucopyranoside, and named gnaphaffine B.Previous studies have already reported on the anti-complementary activity of components from G. affine [2,16]. Compounds 1–8 were also evaluated in vitro for anti-complementary activity on the classical pathway of the complement. Heparin, with an IC50 value of 0.016 mg/mL, was used as positive control in this study. Compounds 2, 3, 4 and 7 caused moderate inhibtion, showing IC50 values of 0.471, 0.221, 0.577 and 1.041 mg/mL, respectively. IC50 values for the remaing compounds 1, 5, 6 and 8 were 69.63, 81.50, 23.01, and 13.36 mg/mL, respectively.
3. Experimental
3.1. General
Optical rotations were measured with Perkin-Elmer 341 polarimeter. UV and IR spectra were recorded on Shimadzu UV-2550 and Perkin-Elmer 577 (using KBr disks) spectrophotometers, respectively. NMR spectra were acquired on a Bruker Avance III spectrometer (600 MHz for 1H-NMR, 150 MHz for 13C-NMR, data in ppm relative to TMS). ESI-MS spectra were recorded on an Agilent 1200 series HPLC interfaced to an Agilent 6410 triple-quadrupole mass spectrometer equipped with an electrospray ionization source, and HR-ESI-MS spectra were recorded on an Agilent 1290 series HPLC interfaced to an Agilent 6538 UHD Accurate-Mass Q-TOF LC/MS (Agilent Corporation, Santa Clara, MA, USA). Semi-preparative RP-HPLC isolation was performed with an Agilent 1200 instrument with a refractive index detector (RID) using a YMC 5 μm C8 column (250 mm × 10 mm). Methanol for semi-preparative HPLC was of HPLC-grade (Merck, Darmstadt, Germany). Column chromatography: silica gel (200−300 mesh); macroporous adsorbing resin (D-101, ZTC-1, 0.3−1.2 mm, Tianjin Zhentiancheng Science & Technology Co., Ltd., Tianjin, China); Sephadex LH-20 gel (40−70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden); silica gel H (Qingdao Haiyang Chemical Co. Ltd., Qingdao, China). All solvents for column chromatography and acid hydrolysis were of analytical grade (Shanghai Chemical Reagents Company, Ltd., Shanghai, China). Spots of compounds on TLC were developed using 10% H2SO4-EtOH solution
3.2. Plant Material
The whole G. affine plants were purchased at the Bozhou herbal market in Anhui Province, China, in July 2008 and identified by Prof. Wansheng Chen. A voucher specimen was deposited in the Department of Pharmacognosy, Second Military Medical University, Shanghai, China.
3.3. Extraction and Isolation
The dried, whole plant materials of G. affine (2.7 kg) were ground and extracted with 80% EtOH three times (25 L, each for 2 h) under reflux at 70–80 °C. Evaporation of the solvent at 60 °C yielded a crude extract (338 g), which was suspended in distilled water and successively partitioned with petroleum ether, ethyl acetate, and n-butanol. The ethyl acetate extract (60 g) was fractionated by silica gel column chromatography (100–200 mesh, 720 g), using a gradient of CH2Cl2: MeOH (50:1→1:1; each 5 L, v/v) to yield seven fractions (A→G). Fraction C was chromatographed over a Sephadex LH-20 column eluted with a gradient of MeOH: H2O (50:50, 80:20, 0:100, v/v) to give five combined sub-fractions (C1‒C3). Fraction C1 was eluted on RP-18 gel column (MeOH: H2O, 3:7, 5:5, 8:2, v/v, each 500ml) to obtain three parts (C1-1-C1-3). Fraction C1-1 was further purified by Sephadex LH-20 CC with MeOH: H2O (5:5, 8:2, v/v) to afford compounds 1 (20 mg) and 2 (28 mg). Fraction C1-2 was purified on a Sephadex LH-20 column (MeOH: H20, 3:2, v/v) and further subjected to RP-18 gel CC (MeOH:H2O, 2:3, v/v) to yield compound 3 (18 mg). Compounds 4 (25 mg) and 5 (18 mg) were obtained from fraction C1-3 using the same way as described for compound 3. Further purification of subfraction C2 by RP-18 gel CC (MeOH: H2O, 2:3; v/v) and Sephadex LH-20 CC (MeOH: H2O, 1:1; v/v) yielded compounds 6 (17 mg) and 7 (20 mg). Further purification of C3 by RP-18 gel CC (MeOH: H2O, 8:2; v/v) and Sephadex LH-20 CC (CHCl3: MeOH, 1:1; v/v) yielded compound 8 (26 mg).
3.4. Characterization of Compounds 1 and 2
Compound 1. Yellow, amorphous powder; IR (KBr) νmax 3535, 3414, 1668, 1591, 1515, 1288, 1172, 1077 cm−1; 13C- (150 MHz, DMSO-d6) and 1H-NMR (600 MHz, DMSO-d6), see Table 1 and Supplementary Data; HR-ESI-MS: m/z 501.1393 [M+H]+ (calcd for C25H24O11, m/z 501.1391).
Compound 2. Pale yellow solid; IR (KBr) νmax 3856, 3455, 1787, 1692, 1632, 1502, 1274, 1175, 1085 cm−1; 13C- (150 MHz, DMSO-d6) and 1H-NMR (600 MHz, DMSO-d6), see Table 2 and Supplementary Data; HR-ESI-MS: m/z 597.1597 [M−H]− (calcd for C30H29O13, m/z 597.1524).
3.5. Acid Hydrolysis of Compounds 1 and 2
A solution of compound 1 (10 mg) or compound 2 (10 mg) in 2 N aqueous CF3COOH (10 mL) was refluxed at 80 °C for 2 h. The mixture was then diluted in water (10 mL) and extracted with EtOAc (3 × 3 mL). The combined EtOAc layers were washed with H2O and evaporated to dryness to afford the glycoside. The residue was purified over an ODS column to afford D-glucopyranose (1.2 mg), which was identified on the basis of its specific rotation: 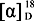 +38.2 (c 0.15, H2O); 0.8 mg,
+38.2 (c 0.15, H2O); 0.8 mg, 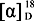 +40.3 (c 0.20, H2O).
+40.3 (c 0.20, H2O).
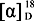 +38.2 (c 0.15, H2O); 0.8 mg,
+38.2 (c 0.15, H2O); 0.8 mg, 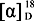 +40.3 (c 0.20, H2O).
+40.3 (c 0.20, H2O).3.6. Anti-Complementary Activity Assay
Based on Mayers’ modified method, nornal human serum (NHS) obtained from healthy male donors (mean age 20 years) was used as the complement source, and it was treated with SRBC to remove the anti-sheep erythrocyte antibody. The NHS was diluted 1:10 with veronal-buffered saline (VBS2+, PH 7.4, containing 0.5 mM Mg2+ and 0.15 mM Ca2+) and selected to give submaximallysis in the absence of complement inhibitors. Each saple was dissolved in VBS2+ with 1% dimethyl sulfoxide (DMSO). Heparin served as the positive control. The cells culture and biological activity assay were very similar to our previous study [25]. Optical density of the suupernatant was measured at 405nm with a spectrophotometer.The inhition rate of haemolysis was calculated by the following formula: [A − (A1 − A0)]/A × 100%, where A is the absorbance of 100% lysis; A1 is the absorbance of the sample; and A0 is the absorbance of control.
4. Conclusions
In conclusion, our phytochemical investigation of the roots extract of G. affine has identified two new phenolic glycosides, gnaphaffine A (1) and B (2), together with six known compounds, caffeic acid (3), everlastoside L (4), isorhamnetin-7-O-β-d-glucopyranoside (5), quercetin-3-O-β-d-glucopyranoside (6), scutellarein-7-O-β-d-glucopyranoside (7) and apigenin-7-O-β-d-glucopyranoside (8). A plausible biogenetic pathway has been proposed for compound 1 herein (Scheme. 1). The key precursor (4-(3',4'-Dihydroxyphenyl)-3-buten-2-one) may convert into key intermediate everlastoside L (4) via saccharification and esterification reactions. Cyclization of the everlastoside L (4) yielded compound 1, which was converted via Friedel-Crafts reaction. Anti-inflammatory activities for compounds 1–8 have been evaluated and compounds 2, 3, 4 and 7 exhibited significant in vitro anti-inflammatory activities through the classic anti-complementary pathway (IC50 value < 10 mg/mL).
Acknowledgments
This project was financially supported by a grant from the Science and Technology Commission of Shanghai Municipality (13ZR1448800). The authors thank Daofeng Chen (Department of Pharmacognosy, School of Pharmacy, Fudan University, China), and Zhihong Cheng (Department of Pharmacognosy, School of Pharmacy, Fudan University, China) for the biological activity evaluation of the isolated compounds. We are also grateful to Genjin Yang and Xin Dong (School of Pharmacy, Second Military Medical University, China) for the measurement of NMR and mass spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qian, C.P.; Chen, H.Y. Flora reipublicae popularis sinicae. In Tomus 75 Anglospermae dicotyledonae Compositae (2); Lin, R., Ed.; Science Press: Beijing, China, 1959; Volume 26, pp. 221–242. [Google Scholar]
- Xi, Z.X.; Chen, W.S.; Wu, Z.J.; Wang, Y.; Zeng, P.Y.; Zhao, G.J.; Li, X.; Sun, L.N. Anti-complementary activity of flavonoids from Gnaphalium affine D. Don. Food Chem. 2012, 130, 165–170. [Google Scholar] [CrossRef]
- Aritomi, M.; Kawasaki, T. Dehydro-para-asebotin, a new chalconeglucoside in the flowers of Gnaphalium affine D. Don. Chem. Pharm. Bull. 1974, 22, 1800–1805. [Google Scholar] [CrossRef]
- Aritomi, M.; Shimojō, M.; Mazaki, T. Chemical Constituents in Flowers of Gnaphalium Affine D. Don. Yakugaku Zasshi 1964, 84, 895–896. [Google Scholar]
- Morimoto, M.; Kumeda, S.; Komai, K. Insect Antifeedant Flavonoids from Gnaphalium affine D. Don. J. Agric. Food Chem. 2000, 48, 1888–1891. [Google Scholar] [CrossRef]
- Cuadra, P.; Harborne, J.B.; Waterman, P.G. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochemistry 1997, 45, 1377–1383. [Google Scholar] [CrossRef]
- Iwashina, T.; Kitajima, J.; Takemura, T. Flavonoids from the leaves of six Corylopsis species (Hamamelidaceae). Bio. System. Eco. 2012, 44, 361–363. [Google Scholar] [CrossRef]
- Torrengra, R.D.; Escarria, S.; Raffelsberger, B.; Achenbach, H. 5,7-dihydroxy-3,6,8-trimethoxyflavone from the flowers of Gnaphalzum elegans. Phytochemistry 1980, 19, 2795–2796. [Google Scholar] [CrossRef]
- Shikov, A.N.; Kundracikova, M.; Palama, T.L.; Pozharitskaya, O.N.; Kosman, V. M.; Makarov, V.G.; Galambosi, B.; Kim, H.J.; Jang, Y.P.; Choi, Y.H.; et al. Phenolic constituents of Gnaphalium uliginosum L. Phytochemistry 2010, 3, 45–47. [Google Scholar]
- Aoshima, Y.; Hasegawa, Y.; Hasegawa, S.; Nagasaka, A.; Kimura, T.; Hashimoto, S.; Torii, Y.; Tsukagoshi, N. Isolation of GnafC, a Polysaccharide Constituent of Gnaphalium affine, and Synergistic Effects of GnafC and Ascorbate on the Phenotypic Expression of Osteoblastic MC3T3-E1 Cells. Bios. Biotechnol. Biochem. 2003, 67, 2068. [Google Scholar] [CrossRef]
- Wei, C.Z.; Rui, X.Z.; Li, R.J.; Hong, G.; Yue, Z.; Qun, S. Chemical composition, antimicrobial and antioxidant activities of essential oil from Gnaphlium affine. Food Chem. Toxicol. 2011, 49, 1322. [Google Scholar] [CrossRef]
- Meragelman, T.L.; Silva, G.L.; Mongelli, E.; Gil, R.R. ent-Pimarane type diterpenes from Gnaphalium gaudichaudianum. Phytochemistry 2003, 62, 569–572. [Google Scholar] [CrossRef]
- Urzúa, A.; Torres, R.; Bueno, C.; Mendoza, L. Flavonoids and diterpenoids in the trichome resinous exudate from Pseudognaphalium cheiranthifolium, P. heterotrichium and P. vira vira. Biochemical Systematics and Ecology 1995, 23, 459. [Google Scholar] [CrossRef]
- Xi, Z.X.; Wan, Y.; Zhao, G.J.; Li, X.; Wu, Z.J.; Sun, L.N. Chemical constituents of petroleum ether fractions of Gnaphalium affine D. Don. Acad. J. Sec. Mil. Med. Univ. 2011, 32, 311–313. [Google Scholar]
- Wu, Z.J.; Ouyang, M.A.; Yang, C.R. Polyphenolic constituents of Salvia sonchifolia. Acta Bot. Yunnan 1999, 21, 393–398. [Google Scholar]
- Morikawa, T.; Wang, L.B.; Ninomiya, K.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Wu, L.J.; Yoshikawa, M. Eight New Glycosides, Everlastosides F-M, from the Flowers of Helichrysum arenarium. Chem. Pharm. Bull. 2009, 57, 853–859. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.B.; Zhang, J.; Li, P.; Yao, Y.Z. Studies on chemical constituents of Salsola collina. Zhongguo Zhong Yao Za Zhi 2007, 32, 409–413. [Google Scholar]
- Zhou, Z.H.; Yang, C.R. Chemical Constituents of Crude Green Tea, the Material of Pu-er Tea in Yunnan. Acta Bot. Yunnanica 2000, 22, 343–350. [Google Scholar]
- Wang, L.B.; Toshio, M.; Gao, H.Y.; Huang, J.; Masayuki, Y.; Wu, L.J. Isolation and identification of chemical constituents of flavones from Flos Helichrysi Arenarii. J. Shengyang Pharm. Univ. 2009, 26, 792–795. [Google Scholar]
- Calzada, F. Additional antiprotozoal constituents from Cuphea pinetorum, a plant used in Mayan traditional medicine to treat diarrhoea. Phytother. Res. 2005, 19, 725–727. [Google Scholar] [CrossRef]
- Zan, L.F.; Qin, J.C.; Zhang, Y.M.; Yao, Y.H.; Bao, H.Y.; Li, X. Antioxidan hipidin derivatives from medicinal mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011, 59, 770–772. [Google Scholar] [CrossRef]
- Helle, T.O.; Gary, I.S.; Johannes, V.S.; Søren, B.C.; Anna, K.J. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef]
- Li, X.C.; Joshi, A.S.; Tan, B.; Elsohly, H.N.; Walker, L.A.; Zjawiony, J.K.; Ferreira, D. Absolute configuration, conformation, and chiral properties of flavanone-(3–8′′)-flavone biflavonoids from Rheedia acuminate. Tetrahedron 2002, 58, 8709–8717. [Google Scholar] [CrossRef]
- Liu, Y.M.; Yang, J.S.; Liu, Q.H. Chemical constituents research from Carthamus tinctorius L. J. Chin. Med. Mater. 2005, 28, 289. [Google Scholar]
- Zhu, H.W.; Di, H.Y.; Zhang, Y.Y.; Zhang, J.W.; Chen, D.F. A protein-bound polysaccharide from the stem bark of Eucommia ulmoides and its anti-complementary effect. Carbohydr. Res. 2009, 344, 1319–1324. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1−8 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
