Abstract
Microwave-promoted efficient synthesis of a (Z)-3-methyleneisoindolin-1-one library from 2-bromobenzamides and terminal alkynes using Cu(OAc)2•H2O/DBU is described. Various benzamide substituents, ring substitutions, including heteroaryl, aryl acetylenes and aliphatic alkynes, could be applied to afford the desired products in good to moderate yield with high stereoselectivity. It is noteworthy that DBU maybe play a dual role as not only the base, but also as a ligand for copper. The reaction is catalyzed by the complex of Cu(OAc)2•H2O and DBU without other additives.
1. Introduction
3-Methyleneisoindolin-1-one is an important scaffold that is present in a number of natural products and biologically active compounds (Figure 1). For example, the alkaloid fumaridine is a secophthalide-isoquinoline ene-lactam [1]. DN-2574 is an isoindoloquinoline derivative used as a cognition enhancing agent [2]. Additionally, a 3-methyleneisoindolin-1-one is a key intermediate in the total synthesis of lennoxamine [3].
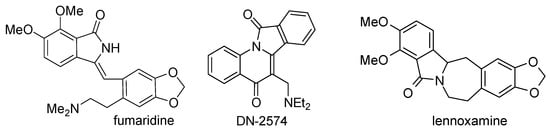
Figure 1.
Structures of some natural products and biologically active 3-methyleneisoindolin-1-one compounds.
There are several methods available for the synthesis of 3-methyleneisoindolin-1-ones. General methods rely on the Wittig reaction [4], nucleophilic additions of organometallic reagents to phthalimides followed by dehydration [5], Horner-Wadsworth-Emmons condensation of 3-(diphenyl-phosphinoyl)isoindolin-1-ones with aldehydes [6,7], base promoted nucleophilic additions to benzonitrile derivatives [8,9], dimethyl isoindolin-1-one-3-yl-phosphonates followed by a Horner reaction [10] or photodecarboxylative addition of carboxylates to phthalimides [11]. In recent years, efficient approaches based on palladium-mediated cyclization reactions have been reported [12,13,14,15,16,17,18,19,20]. Ma et al. [21] reported a CuI/L-proline-catalyzed domino reaction to form 3-methyleneisoindolin-1-ones. The desired products were formed at 85–110 °C for 24–48 h, L-proline had to be used as a ligand and K2CO3 was used as base. However, most of these methods are not suitable for parallel synthesis to obtain large numbers of (Z)-3-methyleneisoindolin-1-ones analogues for drug discovery research. As part of our ongoing drug discovery research, we required a facile route to prepare a library of (Z)-3-methyleneisoindolin-1-ones.
Therefore, we investigated an efficient parallel synthesis of (Z)-3-methyleneisoindolin-1-ones from 2-bromobenzamides and terminal alkynes in a stereoselective manner through a Cu(OAc)2·H2O/DBU catalyzed domino reaction under microwave irradiation. As microwave-assisted synthesis has become a powerful tool with the potential to improve the yields and dramatically shorten reaction times [22,23], there are several advantages to this method: (i) simple use of inexpensive complex of Cu(OAc)2•H2O with DBU, without other additives, (ii) the reaction is not sensitive to water, (iii) environmentally-friendly EtOH as the solvent, (iv) remarkably short reaction time, only 20 min being needed, (v) moderate to high yields with high stereoselectivity, only Z-isomers were obtained as product, and (vi), a broader substrate scope accommodating various benzamide substituents, aromatic rings including heteroaryl substitutions, aryl acetylenes and aliphatic alkynes were used. Therefore, this method are suitable to synthesize (Z)-3-methyleneisoindolin-1-one libraries efficiently and conveniently.
2. Results and Discussion
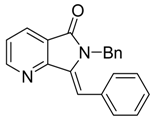
In order to develop an efficient methodology for the synthesis of a (Z)-3-methyleneisoindolin-1-one library, N-benzyl-2-bromobenzamide 1{1} and phenylacetylene 2{1} were used as representative reactants for optimization of the reaction conditions (Scheme 1). Various catalysts, bases, solvents, temperatures and reaction times were investigated (Table 1). Firstly, we compared the system of CuI and Cu(OAc)2•H2O as catalyst, the result showed that the Cu(OAc)2•H2O under microwave irradiation was better than the CuI system (Table 1, entries 1–2). Next, Cu(OAc)2•H2O was used as catalyst, and we compared different solvent effects under microwave irradiation, whereby CH3CN and EtOH showed similar results and better yields than DMSO, DMF or dioxane (Table 1, entries 3–7). Due to its lower toxicity and price, EtOH was chosen as the solvent for subsequent optimization. Further investigation showed that the nature of the base played an important role in the reaction process, with DBU exhibiting the best results (Table 1, entry 7). Only traces of product were detected when Cs2CO3 was used as base (Table 1, entry 10) and no product was detected in the presence of other organic or inorganic bases, such as DABCO, NEt3, NaOH and K3PO4 (Table 1, entries 8–9 and 11–12). DBU maybe play the dual role as not only the base, but also the ligand for copper. We also found that increasing the reaction temperature to 130 °C afforded improved yields (Table 1, entry 13). Finally, the ratio of catalyst to base was optimized. The best result was obtained when the reaction was carried out with 0.3 equiv. Cu(OAc)2•H2O and 4.0 equiv. DBU at 130 °C for 20 min under microwave irradiation (Table 1, entry 14). When the reaction time was increased to 30 min, the yield was found to decrease slightly (Table 1, entry 15).
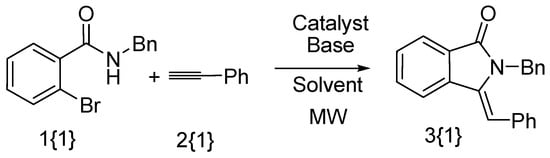
Scheme 1.
Synthesis of (Z)-2-benzyl-3-benzylideneisoindolin-1-one.

Table 1.
Optimization for the synthesis of (Z)-2-benzyl-3-benzylideneisoindolin-1-one a.
Utilizing the above optimized conditions, a library of (Z)-3-methyleneisoindolin-1-ones was designed and synthesized. Firstly, electronic and steric effects of various N-substituents on the 2-bromobenzamides were explored (Scheme 2). Both aromatic and aliphatic substituents were tolerated in the reaction (Table 2, entry 2–10). N-benzyl-substituted-2-bromobenzamides gave better yields than N-aryl-substituted-2-bromobenzamides (Table 2, entries 2–8). For the primary amide (R1 = H), the desired product was also obtained in moderate yield (Table 2, entry 1). (Z)-N-ethylheteroaryl 3-benzylideneisoindolin-1-ones could also be obtained with good yields (Table 2, entries 9–10).

Scheme 2.
Synthesis of N-substituted-3-benzylideneisoindolin-1-one.

Table 2.
Synthesis of N-substituted-3-benzylideneisoindolin-1-one a.
Next, we explored the scope of substituents tolerated on the aromatic ring of the aryl bromides (Scheme 3). Good yields could be obtained regardless of the nature of the substituent (Table 3, entries 1–4). Heteroaryl bromide was also found to provide desired product in moderate yield (Table 3, entry 5).
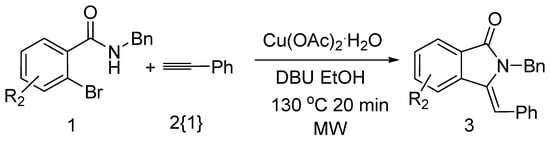
Scheme 3.
Synthesis of substituted (Z)-2-benzyl-3-benzylideneisoindolin-1-one.

Table 3.
Synthesis of substituted (Z)-2-benzyl-3-benzylideneisoindolin-1-one a.
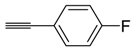
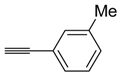
In addition, different arylacetylenes and aliphatic alkynes were chosen to investigate the reaction scope (Scheme 4). When the triple bond was substituted with an aromatic ring, the reaction afforded better yields than that substituted with an aliphatic group. Specially, 3-methylphenyl substitution of the acetylene provided excellent result (Table 4, entry 2). 4-Fluorophenylacetylene also provided the desired product in good yield (Table 4, entry 1). Aliphatic alkynes with steric hindrance afforded the desired product in lesser yield than arylacetylenes (Table 4, entries 4).

Scheme 4.
Synthesis of 3{17}-3{20}.

Table 4.
Substrate Scope of alkyne in the Reaction with N-benzyl-2-bromobenzamide a.
All products were isolated in high purity and the process was highly stereoselective. All compounds were confirmed to be Z-isomers. The geometry of unpublished compounds was established via NOESY studies.
The possible reaction mechanism was shown in Scheme 5. The first step is likely to be the Sonogashira coupling reaction [24]. The second step involves copper coordination to the triple bond, followed by intramolecular hydroamination of the triple bond, and protodemetalation to form the desired substituted 3-methyleneisoindolin-1-one. DBU might act as ligand and base in the reaction.
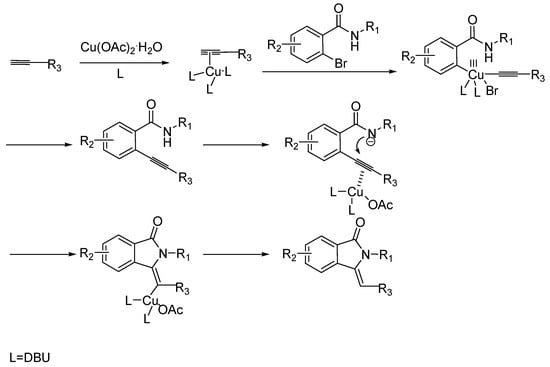
Scheme 5.
Possible Mechanism.
3. Experimental
3.1. General
Proton (1H-) and carbon (13C-) NMR spectra were recorded on a Varian AMX-300 spectrometer at 300 MHz and 100 MHz, respectively. Chemical shifts (δ) are reported in parts per million (ppm). Chloroform-d was used as an internal standard. Data are reported as follows: (s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet; dd = doublet of doublets; br = broad). Mass spectra were carried out in EI or ESI mode. High and low resolution mass spectra were recorded on a Finnigan MAT-95 and Finnigan LCQ-Dea-XP spectrometers. Infrared (IR) spectra were obtained on a Nicolet Magan 750 FT-IR spectrometer. Analytical TLC was performed on pre-coated silica gel 60F-254 plates. Microwave-assisted synthesis was carried out in an Initiator single-mode microwave synthesizer (Biotage, Uppsala, Sweden), equipped with an internal probe that monitors reaction temperature and pressure, and maintains the desired temperature by computer control. Reactions were conducted in 5 mL sealed vials.
3.2. General Procedure for the Preparation of (Z)-3-Methyleneisoindolin-1-ones
Compound 2{1} (0.75 mmol) was added to the mixture of Cu(OAc)2•H2O (30 mg, 0.15 mmol), DBU (293 μL, 2.00 mmol) and compound 1{1} (0.50 mmol) in EtOH (1.00 mL). The reaction vessel was sealed and the reaction mixture was stirred at 130 °C for 20 min under microwave irradiation. After cooling, the mixture was diluted with EtOAc. The organic layer separated and washed with 1 N HCl, brine, dried over anhydrous Na2SO4. After evaporation under vacuum, the residue was purified by flash column chromatography (petroleum ether/ethyl acetate = 20/1–10/1) to afford the desired product.
(Z)-2-Benzyl-3-benzylideneisoindolin-1-one (3{1}) [21]. 1H-NMR (CDCl3): δ 7.93 (d, J = 7.5 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.28–7.22 (m, 3H), 7.08–7.02 (m, 5H), 6.72 (s, 1H), 6.53 (d, J = 6.9 Hz, 2H), 4.94 (s, 2H); ESI-MS m/z 312 [M+H]+.
(Z)-3-Benzylideneisoindolin-1-one (3{2}) [21]. 1H-NMR (CDCl3): δ 8.17 (brs, 1H), 7.88 (d, J = 7.5 Hz, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.64 (t, J = 7.8 Hz, 1H), 7.52 (t, J = 7.2 Hz, 1H), 7.45–7.44 (m, 4H), 7.34–7.31 (m, 1H), 6.56 (s, 1H); ESI-MS m/z 222 [M+H]+.
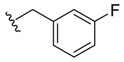
(Z)-2-(3-Fluorobenzyl)-3-benzylideneisoindolin-1-one (3{3}). 1H-NMR (CDCl3): δ 7.93 (d, J = 7.8 Hz, 1H), 7.75 (d, J = 7.5 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.29–7.22 (m, 3H), 7.06–6.97 (m, 3H), 6.81–6.77 (m, 1H), 6.74 (s, 1H), 6.33 (d, J = 7.5 Hz, 1H), 6.18 (d, J = 9.9 Hz, 1H), 4.91 (s, 2H); 13C-NMR (CDCl3) δ 168.8, 162.6 (d, J = 244.5 Hz), 139.4 (d, J = 7.1 Hz), 138.3, 134.4, 134.2, 132.3, 129.5, 129.4, 129.1, 127.9 (2C), 127.5, 123.6, 121.8, 119.5, 113.7, 113.5, 113.4, 113.1, 107.6, 44.4; IR (KBr) ν 3053, 1713, 1655, 1616, 1591, 1452, 1392, 1352, 1254, 1113, 955, 762, 702 cm−1; EI-MS m/z 329 (M+); HRMS (EI) Calcd for m/z C22H16NOF 329.1216, found 329.1210.
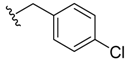
(Z)-2-(4-Chlorobenzyl)-3-benzylideneisoindolin-1-one (3{4}) [21]. 1H-NMR (CDCl3): δ 7.94 (d, J = 7.8 Hz, 1H), 7.75 (d, J = 7.5 Hz, 1H), 7.64 (t, J = 7.2 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.29–7.27 (m, 3H), 7.09–7.07 (m, 2H), 7.02 (d, J = 8.1 Hz, 2H), 6.74 (s, 1H), 6.45 (d, J = 8.1 Hz, 2H), 4.91 (s, 2H); ESI-MS m/z 346 [M+H]+.
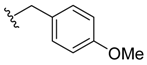
(Z)-2-(4-methoxybenzyl)-3-benzylideneisoindolin-1-one (3{5}) [21]. 1H-NMR (CDCl3): δ 7.91 (d, J = 7.5 Hz, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.51 (t, J = 7.5 Hz, 1H), 7.29–7.28 (m, 3H), 7.13–7.12 (m, 2H), 6.71 (s, 1H), 6.58 (d, J = 8.4 Hz, 2H), 6.44 (d, J = 8.1 Hz, 2H), 4.88 (s, 2H), 3.71 (s, 3H); ESI-MS m/z 342 [M+H]+.
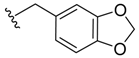
(Z)-2-(Benzo[d][1,3]dioxol-5-ylmethyl)-3-benzylideneisoindolin-1-one (3{6}). 1H-NMR (CDCl3): δ 7.91 (d, J = 7.2 Hz, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.52 (t, J = 7.2 Hz, 1H), 7.31–7.29 (m, 3H), 7.15–7.13 (m, 2H), 6.73 (s, 1H), 6.48 (d, J = 7.8 Hz, 1H), 6.06 (s, 1H), 5.94 (d, J = 7.8 Hz, 1H), 5.84 (s, 2H), 4.85 (s, 2H); 13C NMR (CDCl3) δ 168.9, 147.3, 146.3, 138.5, 134.6, 134.2, 132.1, 130.7, 129.7 (2C), 129.0, 128.0, 127.9 (2C), 127.4, 123.4, 119.8, 119.4, 107.7, 107.4, 107.1, 100.7, 44.4; IR (KBr) ν 3034, 1686, 1643, 1610, 1491, 1448, 1335, 1248, 945, 771, 700 cm−1; EI-MS m/z 355 (M+); HRMS (EI) Calcd for m/z C23H17NO3 355.1208, found 355.1216 .
(Z)-3-Benzylidene-2-phenylisoindolin-1-one (3{7}) [21]. 1H-NMR (CDCl3): δ 7.95 (d, J = 7.8 Hz, 1H), 7.86 (d, J = 8.4 Hz, 1H), 7.68 (t, J = 7.5 Hz, 1H), 7.55 (t, J = 7.8 Hz, 1H), 7.08 (m, 5H), 6.97–6.84 (m, 5H), 6.83 (s, 1H); ESI-MS m/z 298 [M+H]+.
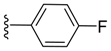
(Z)-3-Benzylidene-2-(4-fluorophenyl)isoindolin-1-one (3{8}). 1H-NMR (CDCl3): δ 7.95 (d, J = 7.2 Hz, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.69 (td, J = 7.8, 1.2 Hz, 1H), 7.54 (td, J = 7.5, 0.9 Hz, 1H), 7.06–6.94 (m, 5H), 6.88–6.85 (m, 3H), 6.78–6.75 (m, 2H); 13C NMR (CDCl3) δ 168.0, 161.1 (d, J = 245.1 Hz), 138.4, 134.4, 133.4, 132.5, 131.8, 129.3, 129.1 (2C), 128.8, 128.7, 127.6, 127.3 (2C), 126.8, 123.9, 119.4, 115.1, 114.9, 107.6; IR (KBr) ν 3435, 3066, 1711, 1645, 1508, 1387, 1219, 1124, 760, 692 cm−1; EI-MS m/z 315 (M+); HRMS (EI) Calcd for m/z C21H14NOF 315.1059, found 315.1054.
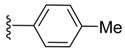
(Z)-3-Benzylidene-2-p-tolylisoindolin-1-one (3{9}) [25]. 1H-NMR (CDCl3): δ 7.94 (d, J = 7.2 Hz, 1H), 7.85 (d, J = 7.8 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 6.98–6.84 (m, 9H), 6.81 (s, 1H), 2.22 (s, 3H); ESI-MS m/z 312 [M+H]+.
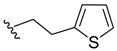
(Z)-3-Benzylidene-2-(2-(thiophen-2-yl)ethyl)isoindolin-1-one (3{10}). 1H-NMR (CDCl3): δ 7.86 (d, J = 7.2 Hz, 1H), 7.77 (d, J = 7.8 Hz, 1H), 7.62 (td, J = 7.5, 1.2 Hz, 1H), 7.51 (td, J = 7.2, 0.6 Hz, 1H), 7.44–7.38 (m, 5H), 7.00 (dd, J = 4.8, 1.2 Hz, 1H), 6.83 (s, 1H), 6.79–6.76 (m, 1H), 6.32 (d, J = 3.3 Hz, 1H), 3.94 (td, J = 8.1, 2.4 Hz, 2H), 2.74 (td, J = 8.1, 2.4 Hz, 2H); 13C-NMR (CDCl3) δ 168.5, 139.8, 138.3, 134.7, 134.5, 132.0, 129.5 (2C), 129.0, 128.4 (2C), 128.2, 127.8, 126.6, 125.2, 123.6, 123.3, 119.3, 106.6, 42.9, 28.3; IR (KBr) ν 3390, 2939, 1701, 1664, 1614, 1471, 1444, 1346, 1097, 997, 756, 694 cm−1; EI-MS m/z 331 (M+); HRMS (EI) Calcd for m/z C21H17NOS 331.1031, found 331.1035.
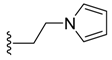
(Z)-2-(2-(1H-Pyrrol-1-yl)ethyl)-3-benzylideneisoindolin-1-one (3{11}) 1H-NMR (CDCl3): δ 7.86 (td, J = 7.5, 0.9 Hz, 1H), 7.77 (d, J = 7.2, 0.6 Hz, 1H), 7.63 (td, J = 7.5, 1.2 Hz, 1H), 7.52 (td, J = 7.5, 0.9 Hz, 1H), 7.49–7.37 (m, 5H), 6.82 (s, 1H), 6.07 (t, J = 2.1 Hz, 2H), 5.97 (t, J = 2.1 Hz, 2H), 4.01 (td, J = 7.5, 1.8 Hz, 2H), 3.68 (td, J = 7.8, 2.4 Hz, 2H); 13C-NMR (CDCl3) δ 168.4, 138.1, 134.5, 134.4, 132.2, 129.6 (2C), 129.2, 128.5 (2C), 128.0, 127.9, 123.3, 120.6 (2C), 119.3, 108.1 (2C), 106.4, 46.6, 42.2; IR (KBr) ν 2941, 1701, 1666, 1616, 1443, 1344, 1300, 1088, 1016, 712 cm−1; EI-MS m/z 314 (M+); HRMS (EI) Calcd for m/z C21H18N2O 314.1419, found 314.1415.
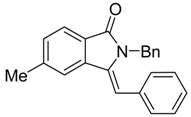
(Z)-2-Benzyl-3-benzylidene-5-methylisoindolin-1-one (3{12}) 1H-NMR (CDCl3): δ 7.81 (d, J = 7.8 Hz, 1H), 7.54 (s, 1H), 7.34 (d, J = 7.5 Hz, 1H), 7.28–7.21 (m, 3H), 7.08–7.00 (m, 5H), 6.68 (s, 1H), 6.51 (d, J = 8.4 Hz, 2H), 4.91 (s, 2H), 2.51 (s, 3H); 13C-NMR (CDCl3) δ 169.1, 142.8, 138.8, 136.9, 134.7, 134.4, 130.1, 129.6 (2C), 127.9 (2C), 127.8 (2C), 127.3, 126.6, 126.3 (2C), 125.7, 123.3, 119.8, 107.1, 44.8, 22.1; IR (KBr) ν 3032, 1695, 1649, 1624, 1491, 1444, 1344, 1151, 968, 696 cm−1; EI-MS m/z 325 (M+); HRMS (EI) Calcd for m/z C23H19NO 325.1467, found 325.1458.
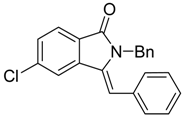
(Z)-2-Benzyl-3-benzylidene-5-chloroisoindolin-1-one (3{13}) [18]. 1H-NMR (CDCl3): δ 7.85 (d, J = 8.4 Hz, 1H), 7.71 (d, J = 0.9 Hz, 1H), 7.49 (dd, J = 8.1, 1.8 Hz, 1H), 7.29–7.25 (m, 3H), 7.06–7.04 (m, 5H), 6.69 (s, 1H), 6.50 (d, J = 8.1 Hz, 2H), 4.91 (s, 2H); ESI-MS m/z 346 [M+H]+.
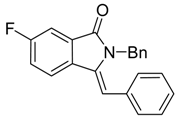
(Z)-2-Benzyl-3-benzylidene-6-fluoroisoindolin-1-one (3{14}). 1H-NMR (CDCl3): δ 7.71 (dd, J = 8.4, 4.2 Hz, 1H), 7.59 (dd, J = 7.5, 2.4 Hz, 1H), 7.36–7.22 (m, 4H), 7.10–7.02 (m, 5H), 6.67 (s, 1H), 6.54–6.51 (m, 2H), 4.93 (s, 2H); 13C-NMR (CDCl3) δ 167.8, 163.4 (d, J = 247.8 Hz), 136.5, 134.3, 133.5, 130.0, 129.9, 129.6 (2C), 128.0 (2C), 127.5, 126.8, 126.3 (2C), 121.3 (d, J = 8.6 Hz), 120.0, 119.7, 110.1, 109.9, 107.8, 45.0; IR (KBr) ν 3045, 1705, 1660, 1483, 1427, 1265, 1117, 702 cm−1; EI-MS m/z 329 (M+); HRMS (EI) Calcd for m/z C22H16NOF 329.1216, found 329.1213.
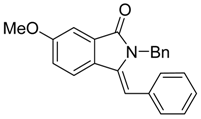
(Z)-2-Benzyl-3-benzylidene-6-methoxyisoindolin-1-one (3{15}) [18]. 1H-NMR (CDCl3): δ 7.63 (d, J = 8.1 Hz, 1H), 7.39 (d, J = 2.1 Hz, 1H), 7.34–7.23 (m, 3H), 7.18 (dd, J = 8.4, 2.7 Hz, 1H), 7.09–7.05 (m, 5H), 6.60 (s, 1H), 6.54–6.52 (m, 2H), 4.92 (s, 2H), 3.93 (s, 3H); ESI-MS m/z 342 [M+H]+.
(Z)-6-Benzyl-7-benzylidene-6,7-dihydropyrrolo[3,4-b]pyridin-5-one (3{16}). 1H-NMR (CDCl3): δ 8.80 (dd, J = 4.8, 1.8 Hz, 1H), 8.20 (dd, J = 7.5, 1.8 Hz, 1H), 7.45 (dd, J = 7.5, 4.5 Hz, 1H), 7.28–7.25 (m, 4H), 7.14–7.05 (m, 5H), 6.56 (dd, J = 7.8, 1.8 Hz, 2H), 4.99 (s, 2H); 13C-NMR (CDCl3) δ 166.7, 156.7, 153.4, 136.4, 134.1, 133.4, 131.5, 129.5 (2C), 128.1 (2C), 127.9 (2C), 127.7, 126.9, 126.4 (2C), 123.8, 121.6, 110.0, 44.7; IR (KBr) ν 3026, 1718, 1662, 1605, 1585, 1493, 1448, 1400, 1360, 1171, 976, 781, 700 cm−1; EI-MS m/z 312 (M+); HRMS (EI) Calcd for m/z C21H16N2O 312.1263, found 312.1259.
(Z)-3-(4-Fluorobenzylidene)-2-benzylisoindolin-1-one (3{17}) [26]. 1H-NMR (CDCl3): δ 7.93 (d, J = 7.2 Hz, 1H), 7.73 (d, J = 7.5 Hz, 1H), 7.63 (td, J = 6.3, 1.2 Hz, 1H), 7.56 (td, J = 6.3, 0.9 Hz, 1H), 7.09–6.88 (m, 7H), 6.64 (s, 1H), 6.55 (dd, J = 6.9, 1.8 Hz, 2H), 4.90 (s, 2H); ESI-MS m/z 330 [M+H]+.
(Z)-3-(4-Methxybenzylidene)-2-benzylisoindolin-1-one (3{18}). 1H-NMR (CDCl3): δ 7.93 (d, J = 7.2 Hz, 1H), 7.76–7.73 (m, 1H), 7.62 (td, J = 7.2, 1.2 Hz, 1H), 7.53 (td, J = 7.8, 1.5 Hz, 1H), 7.17 (t, J = 7.5 Hz, 1H), 7.11–7.06 (m, 4H), 6.92 (d, J = 7.5 Hz, 1H), 6.79 (s, 1H), 6.71 (s, 1H), 6.57 (m, 2H), 4.92 (s, 2H), 2.21 (s, 3H); 13C-NMR (CDCl3) δ 169.0, 138.5, 137.5, 136.9, 134.4, 134.2, 132.0, 130.3, 128.9, 128.1, 128.0, 127.9 (2C), 127.7, 126.6 (2C), 126.2 (2C), 123.4, 119.4, 107.7, 44.9, 21.2; IR (KBr) ν 2924, 1695, 1657, 1429, 1396, 1344, 980, 766, 698 cm−1; EI-MS m/z 325 (M+); HRMS (EI) Calcd for m/z C23H19NO 325.1467, found 325.1471.
(Z)-3-(4-methoxybenzylidene)-2-benzylisoindolin-1-one (3{19}) [27]. 1H-NMR (CDCl3): δ 7.92 (d, J = 7.6 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.53 (t, J = 7.6 Hz, 1H), 7.09–7.06 (m, 3H), 7.01 (d, J = 8.8 Hz, 2H), 6.79 (d, J = 8.4 Hz, 2H), 6.68 (s, 1H), 6.61–6.59 (m, 2H), 4.96 (s, 2H), 3.85 (s, 3H); EI-MS m/z 341 (M+).
(Z)-2-Benzyl-3-(cyclohexylmethylene)isoindolin-1-one (3{20}). 1H-NMR (CDCl3): δ 7.89 (d, J = 7.5 Hz, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.56 (t, J = 8.1 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.32–7.19 (m, 3H), 7.12 (d, J = 7.2 Hz, 2H), 5.40 (d, J = 10.8 Hz, 1H), 5.23 (s, 2H), 2.54–2.50 (m, 1H), 1.71–1.02 (m, 10H); 13C-NMR (CDCl3) δ 168.3, 138.4, 137.6, 131.7, 131.5, 128.6 (2C), 128.2, 127.7, 127.0 (2C), 125.8 (2C), 123.3, 119.0, 115.5, 44.9, 35.7, 33.7, 32.0, 25.7, 25.6; IR (KBr) ν 2929, 2848, 1701, 1660, 1437, 1402, 1362, 1126, 968, 762, 739, 696 cm−1; EI-MS m/z 317 (M+); HRMS (EI) Calcd for m/z C22H23NO 317.1780, found 317.1773.
4. Conclusions
In conclusion, we have developed a practical and convenient protocol for the preparation of (Z)-3-methyleneisoindolin-1-one libraries. Especially, only Z-isomers were obtained. This domino reaction is efficiently promoted by easily available Cu(OAc)2•H2O in the presence of DBU under microwave irradiation. Environmentally friendly EtOH was used as solvent. Various benzamide substituents, and ring substitutions including heteroaryl, aryl acetylenes and aliphatic alkynes could be applied to afford thre desired products in good to moderate yield with high stereoselectivity and high purity. Short reaction times and simple reaction conditions make this method suitable for the synthesis of (Z)-3-methyleneisoindolin-1-one libraries for biological and medicinal chemistry.
Acknowledgments
This work was financially supported by Natural Science Foundation of China grant (No. 81102395).
References
- Rys, V.; Couture, A.; Deniau, E.; Grandclaudon, P. First total synthesis of fumaridine. Tetrahedron 2003, 59, 6615–6619. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kiyota, Y.; Goto, G. Synthesis of Isoindolo[2,1-a]quinoline Derivatives and Their Effects on N2-Induced Hypoxia. Chem. Pharm. Bull. 1990, 38, 3024–3030. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Sasaki, M. An efficient method for the synthesis of enol ethers and enecarbamates. Total syntheses of isoindolobenzazepine alkaloids, lennoxamine and chilenine. Org. Biomol. Chem. 2007, 5, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Flitsch, W.; Peters, H. Wittig-reaktionen an imiden. Tetrahedron Lett. 1969, 10, 1161–1162. [Google Scholar] [CrossRef]
- Bousquet, T.; Fleury, J.F.; Daich, A.; Netchitailo, P. Oxidized isoquinolinoisoindolinone and benzazepinoisoindolinone alkaloids cores by convergent cyclisation processes: π-aromatic attack of thionium and oxonium species. Tetrahedron 2006, 62, 706–715. [Google Scholar] [CrossRef]
- Couture, A.; Deniau, E.; Grandclaudon, P.; Hoarau, C. A New Approach to Isoindolobenzazepines. A Simple Synthesis of Lennoxamine. Tetrahedron 2000, 56, 1491–1499. [Google Scholar] [CrossRef]
- Moreau, A.; Couture, A.; Deniau, E.; Grandclaudon, P. A New Route to Aristocularine Alkaloids: Total Synthesis of Aristoyagonine. J. Org. Chem. 2004, 69, 4527–4530. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Chang, L.J.; Wei, L.M.; Lin, C.F. A direct anionic cyclization of 2-alkynylbenzonitrile to 3-substituted-1(2H)-isoquinolones and 3-benzylideneisoindol-2-ones initiated by methoxide addition. Tetrahedron 1999, 55, 13193–13200. [Google Scholar] [CrossRef]
- Lu, W.D.; Lin, C.F.; Wang, C.J.; Wang, S.J.; Wu, M.J. Substituent effect on anionic cycloaromatization of 2-(2-substituted ethynyl)benzonitriles and related molecules. Tetrahedron 2002, 58, 7315–7319. [Google Scholar] [CrossRef]
- Reyes-Gonzalez, M.A.; Zamudio-Medina, A.; Ordonez, M. Practical and high stereoselective synthesis of 3-(arylmethylene)isoindolin-1-ones from 2-formylbenzoic acid. Tetrahedron Lett. 2012, 53, 5756–5758. [Google Scholar] [CrossRef]
- Hatoum, F.; Engler, J.; Zelmer, C.; Wißen, J.; Motti, C.A.; Lex, J.; Oelgemoller, M. Photodecarboxylative addition of carboxylates to phthalimides: A concise access to biologically active 3-(alkyl and aryl)methylene-1H-isoindolin-1-ones. Tetrahedron Lett. 2012, 53, 5573–5577. [Google Scholar] [CrossRef]
- Cao, H.; McNamee, L.; Alper, H. Syntheses of Substituted 3-Methyleneisoindolin-1-ones By a Palladium-Catalyzed Sonogashira Coupling−Carbonylation−Hydroamination Sequence in Phosphonium Salt-Based Ionic liquids. Org. Lett. 2008, 10, 5281–5284. [Google Scholar] [CrossRef] [PubMed]
- Couty, S.; Liegault, B.; Meyer, C.; Cossy, J. Synthesis of 3-(arylmethylene)isoindolin-1-ones from ynamides by Heck–Suzuki–Miyaura domino reactions. Application to the synthesis of lennoxamine. Tetrahedron 2006, 62, 3882–3895. [Google Scholar] [CrossRef]
- Couty, S.; Liegault, B.; Meyer, C.; Cossy, J. Heck–Suzuki–Miyaura Domino Reactions Involving Ynamides. An Efficient Access to 3-(Arylmethylene)isoindolinones. Org. Lett. 2004, 6, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Xu, B.A. Tandem Elimination−Cyclization−Suzuki Approach: Efficient One-Pot Synthesis of Functionalized (Z)-3-(Arylmethylene)isoindolin-1-ones. J. Org. Chem. 2008, 73, 7361–7364. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.G.; Khan, M.W. An expeditious synthesis of Z-3-alkylidene isoindolinones via combined palladium catalysed and friedel-crafts reactions. Tetrahedron Lett. 1997, 38, 6937–6940. [Google Scholar] [CrossRef]
- Kundu, N.G.; Khan, M.W.; Mukhopadhyay, R. Heteroannulation through combined palladium catalysed and Friedel-Crafts reactions strategy: Synthesis of 3-alkylidene isoindolin-1-ones. Tetrahedron 1999, 55, 12361–12376. [Google Scholar] [CrossRef]
- Hellal, M.; Cuny, G.D. Microwave assisted copper-free Sonogashira coupling/5-exo-digcycloisomerization domino reaction: Access to 3-(phenylmethylene)isoindolin-1-ones and related heterocycles. Tetrahedron Lett. 2011, 52, 5508–5511. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Ziccarelli, I.; Salerno, G. A new approach to isoindolinone derivatives by sequential palladium iodide-catalyzed oxidative aminocarbonylation–heterocyclization of 2-ethynylbenzamides. Tetrahedron Lett. 2012, 53, 6694–6696. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, K.H.; Kim, S.H.; Kim, J.N. Palladium-catalyzed oxidative arylation of trisubstituted olefin: an efficient synthesis of 3-(disubstituted)alkylidene-oxindoles. Tetrahedron Lett. 2013, 54, 170–175. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Zhang, X.J.; Jiang, Y.W.; Ma, D.W. Assembly of Substituted 3-Methyleneisoindolin-1-ones via a CuI/L-Proline-Catalyzed Domino Reaction Process of 2-Bromobenzamides and Terminal Alkynes. Org. Lett. 2009, 11, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xue, L.Y.; Wang, X.H.; Tu, M.S.; Liu, Y.P.; Tu, S.J. Microwave-assisted multicomponent reaction of aryl amidines: Regiospecific synthesis of new polysubstituted thiopyrano-, and pyrano[4,3-d]pyrimidines. Tetrahedron Lett. 2012, 53, 1261–1264. [Google Scholar] [CrossRef]
- Xie, Y.X.; Deng, C.L.; Pi, S.F.; Li, J.H.; Yin, D.L. Cu(OAc)2/Pyrimidines-Catalyzed Cross-coupling Reactions of Aryl Iodides and Activated Aryl Bromides with Alkynes under Aerobic, Solvent-free and Palladium-free Conditions. Chin. J. Chem. 2006, 24, 1290–1294. [Google Scholar] [CrossRef]
- Khan, M.W.; Kundu, N.G. A Highly Regio and Stereoselective Synthesis of (Z)-3-Aryl(alkyl)idene Isoindolin-1-ones via Palladium Catalyzed Annulation of Terminal Alkynes. Synlett 1997, 12, 1435–1437. [Google Scholar] [CrossRef]
- Cao, H.; McNamee, L.; Howard, A. Syntheses of Substituted 3-Methyleneisoindolin-1-ones By a Palladium-Catalyzed Sonogashira Coupling–Carbonylation–Hydroamination Sequence in Phosphonium Salt-Based Ionic liquids. Org. Lett. 2008, 10, 5281–5284. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.G.; Khan, M.W. Palladium-Catalysed Heteroannulation with Terminal Alkynes: A Highly Regio- and Stereoselective Synthesis of (Z)-3-Aryl(alkyl)idene Isoindolin-1-ones. Tetrahedron 2000, 56, 4777–4792. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).