Abstract
Graphite oxide and graphene oxides have been used as solid catalysts for the synthesis of 5,5-dialkyldipyrromethanes and calix[4]pyrroles in organic and aqueous solutions at room temperature.
1. Introduction
Graphene is a two dimensional sheet of sp2 hybridized carbon with remarkable thermal, mechanical and electronic properties [1,2,3,4]. Top down is an important method for the preparation of graphene by oxidation of graphite [5], as compared to other methods [6,7]. Graphite oxides containing OH, epoxy and carboxyl groups have been prepared by oxidation of graphite via minor modification of known methods [8,9] and their structures have been characterized by different spectroscopic methods [10,11,12]. The presence of polar groups is responsible for the acidic solutions formed when graphite and graphene oxides are suspended in aqueous media [13,14]. In organic synthesis polycarbon acids [15,16] and polyacids on carbon nanostructures [17,18,19,20] are considered more robust in aqueous as well as organic solvents than other solid acids [20,21,22,23] such as ion exchange resins [24], heteropolyacids [25,26,27] and layer transition metal oxides [28,29].
One of the most important methods developed for the mass production of graphene is exfoliation of graphite oxide in aqueous solution to single layers of graphene oxide. This exfoliation can be performed in aqueous solution alone or in presence of surfactants [30], polymers [31,32], ionic liquids [33] and polar solvents [34,35]. Aqueous suspensions of graphene oxide have been used in the oxidation of alcohols and cis-stilbene and the hydration of various alkynes to the corresponding aldehydes, acids and ketones under mild conditions [36,37]. The electrical conductivity of the graphene oxide differs from that of pristine graphene, hence various attempts have been made to deoxygenate graphene oxide to the maximum extent to regain the aromaticity and electrical conductivity. The hydroxyl and the epoxy groups and some carboxylic groups can be removed by reduction of graphite oxide by hydrazine, ascorbic acid and other reducing agents [38,39,40].
Porphyrinogens and more stable calix[4]pyrroles are important tetrapyrrolic macrocycles used in biosynthesis of porphyrinoids [41], supramolecular chemistry [42], anion receptor [43,44] and material chemistry [45,46,47]. They are synthesized by condensation of pyrroles with dialkylketones in the presence of aqueous acids [48,49], Lewis acids [50,51] and solid acids including zeolites [52], molecular sieves [53,54] and Amberlyst-15 [55] under different reaction conditions. Although various solid acids have been used in the synthesis of different calix[4]pyrroles, to the best of our knowledge graphite oxide and graphene oxides have not been used for this purpose. Hence, we report the synthesis of selected calix[4]pyrroles in aqueous suspensions of graphite oxide and graphene oxides under different reaction conditions (Scheme 1).
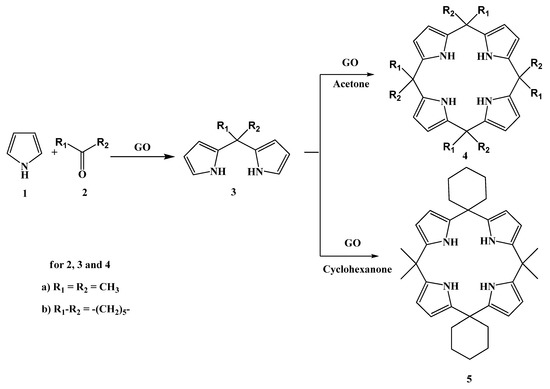
Scheme 1.
Synthesis of dipyrromethane and calix[4]pyrrole.
2. Results and Discussion
The reaction of acetone (2a) with pyrrole (1) in a suspension of graphite oxide (GO) in dichloromethane at room temperature gave dipyrromethane (3a) as the major product. The structure of 3a was confirmed by spectroscopic data, mixed melting point [56] and comparison of HPLC retention times with an authentic sample (Table 1). The reaction of 1 with cyclohexanone (2b) gave the corresponding dipyrromethane 3b in 70% yield. An analogous condensation of pyrrole and 2b in dichloromethane in the presence of the solid acid zeolite HY gave dipyrromethane 3b in 63% yield, along with other products, whereas the same reaction in the presence of HZSM in dichloromethane gave dipyrromethane 3b and calix[4]pyrrole 4b in 53 and 11% yield, respectively [52]. The reaction of 1 with 2a in the presence of Al-MCM-41 zeolite gave 3a and 4a in 12 and 70% yield, respectively [52]. The reaction of the above in the presence of AmberlystTM-15 gave 4a and N-confused calix[4]pyrrole in 83 and 14% yield, respectively [55]. The high yield of 4a in acetone may be explained by initial formation of 3a which on subsequent reaction with acetone leads to calix[4]pyrrole. This was also confirmed by reaction of 3a with 2b with the formation of 5 in 17% yield. (Table 1) [52]. The reaction of 1 and 2a was performed in other polar solvents and the results are reported in Table 1. A moderate yield of calix[4]pyrrole 4 was obtained when the reaction was performed in methanol or acetonitrile (Table 1). The graphite oxide is stable in organic solvents and it may be used several times without much loss of catalytic activity (Table 1, entry 14).

Table 1.
Graphite oxide catalyzed condensation of pyrrole and acetone in different solvents a.
The formation of calix[4]pyrrole 4a in excess acetone may be explained by initial formation of 3a which on subsequent reaction with acetone leads to 4a. The reaction of 3a with cyclohexanone lead to cailx[4]pyrrole 5 (Scheme 1).
The reaction of 1 and 2a in the presence of graphite oxide suspended in aqueous solution gave 3a as major product in 92% yield (Table 2). The reaction of pyrrole and ketones regardless of the ratio of starting compounds, forms the dialkyldipyrromethanes in the presence of weak acid in aqueous solutions [48]. The increase in the ratio of acetone decreases the yield of 3a and a minor amount of 4a was observed (Table 2).

Table 2.
Reaction of pyrrole and acetone in presence of graphite oxide in aqueous solution a.
The reaction of 1 and 2a in the presence of graphite oxide and SDS gave dipyrromethane (48%), calix[4]pyrrole (35%) and N-confused calix[4]pyrrole in 10% yield at room temperature in 1.5 to 3 h (Table 3).

Table 3.
Reaction of pyrrole and acetone in the presence of graphite oxide and surfactant and salts in aqueous solution a.
The addition of SDS to graphite oxide in aqueous solution forms the corresponding acid which catalyzes the formation of dipyrromethane in low and calix[4]pyrrole in high yields. The graphite oxides have been dispersed in SDS and poly(sodium 4-styrenesulphonate) in aqueous solution [32]. The reactions of 1 with 2a in the presence other surfactants and salts are given in Table 3. The reaction of 1 and 2a in the presence of reduced graphene oxide [57,58] in aqueous solution gave 3a as major product and 4a as minor product (Table 4).

Table 4.
Reaction of pyrrole and acetone in presence of reduced graphene oxide a.
The reduced graphene oxide agglomerizes in aqueous solution, but in the presence of organic solvents [59] and ionic liquids [60] it may be dispersed in aqueous solution. Suitable chemical modifications of reduced graphene oxides have been used to solubilize the graphene oxides in aqueous [60] and organic solvents [61]. The above results indicate that graphite and graphene oxide may be used as solid acids in various organic transformations.
3. Experimental
3.1. General
The infrared spectra (IR) were recorded on a Perkin-Elmer FT-1710 spectrophotometer. 1H-NMR spectra were recorded in CDCl3 on a Bruker Avance 400 MHz spectrophotometer with TMS as internal standard. UV-Vis spectra were recorded on Perkin-Elmer Lambda 35 spectrophotometer. Raman spectra were recorded on inVia Renishaw Raman spectrophotometer using a green (514 nm) laser. Powder XRD were recorded on a Bruker Discover 8 X-ray diffractometer. HPLC analysis was performed on a Waters 2998 using a Waters PAH C18 HPLC column (4.6 × 250 mm) and methanol as the eluent. Starting materials such as pyrrole (1) and acetone (2) were obtained from Acros USA and distilled immediately prior to use. The experimental operations were performed under ambient conditions. Neutral alumina was used for all the chromatographic purifications. Graphite powder was obtained from Alfa Aesar, USA.
3.2. Preparation of Authentic Samples
5,5-Dimethyldipyrromethane (3a) was prepared by following the literature procedure starting from acetone and pyrrole in ionic liquid [56] and aqueous solution [48]; m.p. 56 °C (lit [56] 55–57 °C);1H- NMR: 1.61 (s, 6H, –CH3), 6.08 (s, 2H, β-pyrrole), 6.1 (d, 2H, β-pyrrole), 6.5 (d, 2H, α-pyrrole), 7.64 (s, br, 2H, NH-pyrrole); HPLC retention time = 3.1 min.
Octamethylcalix[4]pyrrole (4a) was prepared by following a literature procedure [55] starting from acetone and pyrrole; m.p. 294 °C (lit [55] 296 °C ); 1H-NMR: 7.01 (4H, br s, NH), 5.89 (8H, d, J = 2.5 Hz, β-pyrrole), 1.50 (24 H, s); HPLC retention time = 3.8 min.
3.3. Preparation of Catalysts
3.3.1. Graphite Oxide
KMnO4 (9 g) was added in portions to a cooled (0 °C) solution of conc. H2SO4 (69 mL) containing graphite (3 g) and NaNO3 (1.5 g). The mixture was stirred at room temperature for 5 days. Distilled water (138 mL) was added slowly to the reaction mixture while the temperature was kept well below 98 °C for 3 h. The resultant bright-yellow suspension was diluted and a solution of H2O2 (6 mL, 30%) was added dropwise. The reaction mixture was centrifuged and washed to remove the remaining salts. The wet graphite oxide was dewatered by vacuum drying (50 °C). UV-Vis (λmax) (H2O) = 230 nm, (DMF) = 269 nm. FTIR (cm−1) = 3447 (OH), 1740 (C=O), 1636 (OH bending), 1091 (C-O). Raman spectra: 1350 (D band), 1584 (G band), D/G ratio: 0.85. XRD data: 10.5°.
3.3.2. Graphene Oxide
Aqueous colloids of single layer graphene oxide nanosheets were produced by exfoliation of graphite oxide dispersed in deionized water with ultrasonication [32].
3.3.3. Preparation of Reduced Graphene Oxide
Graphite oxide (75 mg) was dispersed in water (75 mL) with sonication. Sodium borohydride (600 mg) was added to the GO dispersion after the pH being adjusted to 9–10 with 5 wt% sodium carbonate solution. The mixture was then kept at 80 °C for 1 h under constant stirring. During reduction, the dispersion turned from dark brown to black accompanied by outgassing. UV-Vis (λmax) (H2O) = 270 nm. FTIR (cm−1) = 3440 (O-H), 1740 (C=O), 1091 (C-O). Raman spectra: 1350 (D band), 1584 (G band), D/G ratio: >1.
3.4. The Reaction of Pyrrole and Acetone in Organic Solvents
Equimolar amounts of pyrrole (14.4 mmol) and acetone (14.4 mmol) were taken up in dichloromethane (20 mL). Graphite oxide (10% w/w) was added in portions to the reaction mixture, which was stirred at ambient temperature for the appropriate time (as indicated in Table 1). The reaction progress was monitored by thin layer chromatography (TLC) with petroleum ether-chloroform. After the completion of reaction, the catalyst was removed by filtration and washed thoroughly with CH2Cl2 to dissolve all the contents. The filtrate was concentrated to give the crude product, which was subjected to column chromatography over neutral alumina eluting with petroleum ether-chloroform to afford pure calix[4]pyrrole and further elution of column gave dipyrromethane. The reaction mixture was analysed by HPLC. The HPLC yields are given in Table 1.
3.5. The Reaction of Pyrrole and Acetone in Aqueous Solution
Equimolar amounts of pyrrole (14.4 mmol) and acetone (14.4 mmol) were taken up in water (20 mL). Graphite oxide (10% w/w) was added in portions to the above reaction mixture that was stirred at ambient temperature for the appropriate time (Table 2). The reaction progress was monitored by thin layer chromatography (TLC) with petroleum ether-chloroform. After completion of reaction, the catalyst was removed by extraction with CH2Cl2. The organic layer was separated dried over sodium sulfate, filtered and the filtrate was concentrated to give the crude product, which was subjected to column chromatography over neutral alumina eluting with petroleum ether-chloroform to afford pure calix[4]pyrrole and further elution of column gave dipyrromethane. The reaction mixture was analysed by HPLC. The HPLC yields are given in Table 2.
3.6. The Reaction of Pyrrole and Acetone in Aqueous Solution in Presence of Suphonate Salts
Pyrrole (14.4 mmol), acetone (14.4 mmol) and surfactant/salt (0.034 mmol) were taken up in water (20 mL). Graphite oxide (10% w/w) was added in portions to the reaction mixture, which was stirred at ambient temperature for the appropriate time (Table 3). The reaction progress was monitored by thin layer chromatography (TLC) with petroleum ether-chloroform. After the completion of reaction, the catalyst was removed by extraction with CH2Cl2. The organic layer was separated dried over sodium sulfate, filtered and the filtrate was concentrated to give the crude product, which was subjected to column chromatography over neutral alumina eluting with petroleum ether-chloroform to afford pure calix[4]pyrrole and further elution of column gave dipyrromethane.The reaction mixture wasanalysed by HPLC. The HPLC yields are given in Table 3.
3.7. The Reaction of Pyrrole and Acetone Catalyzed by Reduced Graphene Oxide under Different Conditions
Equimolar amounts of pyrrole (14.4 mmol) and acetone (14.4 mmol) were taken up in different solvents (20 mL). Graphene oxide (10% w/w) was added in portions to the above reaction mixture that was stirred at ambient temperature for the appropriate time (Table 4). The reaction progress was monitored by thin layer chromatography (TLC) with petroleum ether-chloroform. After completion of reaction, the catalyst was removed by filtration or extraction. Filtrate was concentrated to give the crude product, which was subjected to column chromatography over neutral alumina eluting with petroleum ether-chloroform to afford pure calix[4]pyrrole and further elution of column gave dipyrromethane.The reaction mixture was analysed by HPLC. The HPLC yields are given in Table 4.
4. Conclusions
Graphite oxide and reduced graphene oxides have been used as solid acid catalysts for the room temperature preparation of dipyrromethanes and calix[4]pyrroles in organic and aqueous solutions.
Acknowledgements
S.M.S. Chauhan acknowledges the Department of Science and Technology and University of Delhi for financial assistance. Sweta Mishra acknowledges the UGC for Jounior Research Fellowship.
References
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thinfilm, hybrids and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratianc, K.R.; Ringer, S.P.; Thordason, P.; Gooding, J.J.; Braet, F. Carbon naonmaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.K.; Choi, M.-C.; Kong, J.-Y.; Kim, G.Y.; Kim, M.J.; Kim, S.-H.; Mishra, S.; Singh, R.P.; Ha, C.-S. Synthesis and drug delivery behaviour of chitosan-functionalized graphene oxide hybrid nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene based nanoassemblies for energy conversion. J. Phys. Chem. Lett. 2011, 2, 242–251. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morojov, S.V.; Jiang, D.; Zhang, Y.; Dobonos, S.V.; Greigovieva, I.V.; Firslov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical vapor deposition repair of graphene oxide: A route to highly-conductive graphene monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sunitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Mermoux, M.; Chabre, Y.; Rousseau, A. FTIR and 13C NMR study of graphite oxide. Carbon 1991, 29, 469–474. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Mermoux, M.; Chabre, Y. Formation of graphite oxide. Synth. Metals 1989, 34, 157–162. [Google Scholar] [CrossRef]
- Szabo, T.; Tombacz, E.; Illes, E.; Dekany, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Seredych, M.; Bendosz, T. Combine role of water and surface chemistry in relative adsorption of ammonia on graphite oxides. Langmuir 2010, 26, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yamaguchi, D.; Suganuma, S.; Nakajima, K.; Kato, H.; Hayashi, S.; Hara, M. Adsorption-enhanced hydrolysis of β-1,4-glucan on graphene-based amorphous carbon bearing SO3H, COOH and OH groups. Langmuir 2009, 25, 5068–5075. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Huang, M.; Ma, H.L.; Zhang, Z.-Q.; Gao, J.M.; Zhu, Y.L.; Han, X.J.; Guo, X.Y. Preparation of a carbon based solid acid catalyst by sulfonationg activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhang, L.; Wang, H.; Lv, P.; Yu, H. Sulfonated carbon nanotubes as a strong protonic acid catalyst. Carbon 2005, 43, 2405–2408. [Google Scholar] [CrossRef]
- Yu, H.; Jin, Y.; Li, Z.; Peng, F.; Wang, H. Synthesis and characterization of sulfonated single-walled carbon nanotubes and their performance as solid acid catalyst. J. Solid State Chem. 2008, 181, 432–438. [Google Scholar] [CrossRef]
- Liu, K.; Li, C.; Zhang, X.; Hua, W.; Yang, D.; Hu, J.; Yue, Y.; Gao, Z. Poly(styrene sulfonic acid)- grafted carbon nanotube as a stable protonic acid catalyst. Synth. Commun. 2010, 12, 217–221. [Google Scholar] [CrossRef]
- Salzman, C.G.; Liewellyn, S.A.; Tobias, G.; Ward, M.A.H.; Huh, Y.; Green, M.L.H. The role of carboxylated carbonaceous fragments in the functionalization and spectroscopy of a single walled carbon nanomaterial. Adv. Mater. 2007, 19, 883–887. [Google Scholar] [CrossRef]
- Wilson, K.; Clark, J.H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2000, 72, 1313–1319. [Google Scholar] [CrossRef]
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; El-Hiti, G.A.; Jayne, A.J.; Butters, M. Acetylation of aromatic ethers using acetic anhydride over solid acid catalysts in a solvent-free system. Scope of the reaction for substituted ethers. Org. Biomol. Chem. 2003, 1, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Harmer, M.A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, G.P.; Autino, J.C. Recent applications of heteropolyacids and related compounds in heterocycles synthesis. Mini-Rev. Org. Chem. 2009, 6, 359–366. [Google Scholar] [CrossRef]
- Palkovits, R.; Tajvidi, K.; Ruppert, A.M.; Procelewska, J. Heteropoly acids as efficient acid catalysts in the one-step conversion of cellulose to sugar alcohols. Chem. Commun. 2011, 47, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Kantam, L.M.; Ravindra, A.; Reddi, C.R.V.; Sreedar, B.; Choudary, B.M. Layered double hydroxide supported diisopropylamide, synthesis, characterization and application in organic reactions. Adv. Synth. Catal. 2006, 348, 569–578. [Google Scholar] [CrossRef]
- Tagusagawa, C.; Takagaki, A.; Takanabe, K.; Ebitani, K.; Hayashi, S.; Domen, K. Layered and nanosheet tantalum molybdate as strong solid acid catalysts. J. Catal. 2010, 270, 206–212. [Google Scholar] [CrossRef]
- Lomeda, J.R.; Doyle, C.D.; Kosynkin, D.V.; Hwang, W.-F.; Tour, J.M. Diazonium functionalization of surfactant-wrapped chemically converted graphene sheets. J. Am. Chem. Soc. 2008, 130, 16201–16206. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gong, K.; Xiao, P.; Xiao, M. Preparation and characterization of poly(vinyl acetate)-intercalated graphite oxide nanocomposite. J. Mater. Chem. 2000, 10, 933–935. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersion of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, W.; Zhang, J.; Qiao, X.; Zhang, J.; He, J.; Liu, C.Y. Stable dispersions of reduced graphene oxide in ionic liquids. J. Mater. Chem. 2010, 20, 5401–5403. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J. Mater. Chem. 2007, 17, 3678–3680. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Jia, H.-P.; Bielawski, C.W. Graphene oxide: A convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Pyun, J. Graphene oxide as catalyst: Application of carbon materials beyond nanotechnology. Angew. Chem. Int. Ed. 2011, 50, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Kumari, P.; Sinha, N.; Chauhan, P.; Chauhan, S.M.S. Isolation, synthesis and biomimetic reactions of metalloporphyrins in ionic liquids. Curr. Org. Synth. 2011, 8, 393–437. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Chauhan, S.M.S. Meso-functional calix[4]pyrrole: A solution phase study of anion directed self-assembly. J. Inc. Phen. Macrocyc. Chem. 2011, 70, 249–255. [Google Scholar] [CrossRef]
- Lee, C.H. Versatilities of calix[4]pyrrole based anion receptors. Bull. Korean Chem. Soc. 2011, 32, 768–778. [Google Scholar] [CrossRef]
- Gale, P.A. From anion receptors to transporters. Acc. Chem. Res. 2011, 44, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Danil de Namor, A.F.; Khalife, R. Calix[4]pyrrole derivative: Recognition of fluoride and mercury ions and extracting properties of the receptor based new material. J. Phys. Chem. B 2008, 112, 15766–15774. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Tong, C.C.; Haynes, C.J.E.; Adeosun, O.; Gross, D.E.; Karnas, E.; Sedenberg, E.M.; Quesada, R.; Sessler, J.L. Octafluorocalix[4]pyrrole: A chloride/bicarbonate antiport agent. J. Am. Chem. Soc. 2010, 132, 3240–3241. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, P.; Simak, O.; Novakova, Z.; Wimmer, Z.; Drasar, P. Asymmetrically substituted calix[4]pyrrole with chiral substituents. Org. Biomol. Chem. 2011, 9, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Rohand, T.; Dolusic, E.; Ngo, T.H.; Maes, W.; Dahaen, W. Efficient synthesis of aryldipyrromethanes in water and their application in synthesis of corroles and dipyrromethanes. ARKIVOC 2007, x, 307–324. [Google Scholar]
- Shao, S.J.; Yu, X.D.; Cao, S.Q. Synthesis of calix[4]pyrroles: A class of new molecular receptor. Chinese Chem. Lett. 1999, 10, 193–194. [Google Scholar]
- Shao, S.; Wang, A.; Yang, M.; Jiang, S.; Xianda, Y. Synthesis of meso-aryl-substituted calix[4]pyrroles. Synth. Commun. 2001, 31, 1421–1426. [Google Scholar] [CrossRef]
- Gao, G.H.; Lu, L.; Gao, J.B.; Zhou, W.J.; Yang, J.G.; Yu, X.Y.; He, M.Y. One step synthesis of dipyrromethanes in presence of ionic liquid [Hmim]BF4. Chin. Chem. Lett. 2005, 16, 900–902. [Google Scholar]
- Kishan, M.R.; Srinivas, N.; Raghavan, K.V.; Kulkarni, S.J.; Sarma, J.A.R.P.; Vairamani, M. A novel, shape-selective, zeolite-catalyzed synthesis of calix(4)pyrroles. Chem. Commun. 2001, 2226–2227. [Google Scholar] [CrossRef]
- Kishan, M.R.; Rani, V.R.; Murty, M.R.V.S.; Sita Devi, P.; Kulkarni, S.J.; Raghavan, K.V. Synthesis of calixpyrroles and porphyrins over molecular sieve catalysts. J. Mol. Catal. A Chem. 2004, 223, 263–267. [Google Scholar] [CrossRef]
- Kishan, M.; Rani, V.R.; Kulkarni, S.J.; Raghavan, K.V. A new environmentally friendly method for the synthesis of calix(4)pyrroles over molecular sieve catalysts. J. Mol. Cat. A Chem. 2005, 237, 155–160. [Google Scholar] [CrossRef]
- Chauhan, S.M.S.; Garg, B.; Bisht, T. Syntheses of calix[4]pyrroles by amberlyst-15 catalyzed cyclocondensations of pyrrole with selected ketones. Molecules 2007, 12, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.M.S.; Bisht, T.; Garg, B. 1-Arylazo-5,5-dimethyl dipyrromethanes: Versatile chromogenic probes for anions. Sensor Actuat B Chem. 2009, 141, 116–123. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, G.; Liu, J.; Sun, X. Evaluation criteria for reduced graphene oxide. J. Phys. Chem. C 2011, 115, 11327–11335. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersion in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Majumdar, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A. Engineered graphene oxide materials for application in water purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Limited samples of compounds 3a and 4a are available from the authors. |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).