Abstract
Arylhydrazonomalononitriles 1a,b react with phenylhydrazine to yield amidrazones 2a,b that cyclize to give 2-aryl-5-phenylhydrazono-2,5-dihydro-[1,2,4]-triazine-6-carbonitriles 5a,b upon reaction with dimethylformamide dimethylacetal (DMFDMA). Refluxing 5a,b in glacial acetic acid resulted in the formation of the pyrazolo-1,2,4-triazines 6a,b. Compounds 6a,b were also formed upon treatment of 3-amino-4-phenylhydrazono-1-phenyl-2-pyrazolin-5-ones 7a,b with DMFDMA. Reacting these triazinyl arylhydrazononitriles 5a,b with hydroxylamine hydrochloride in ethanolic sodium acetate afforded amidrazones 8a,b that are readily cyclized in refluxing dimethylformamide into [1,2,4]triazino[1,2,3]triazines 10a,b.
Introduction
Arylhydrazonomalononitriles 1 are synthetically useful reagents that have been utilized in the past as precursors to 4-arylazo-3,5-pyrazolediamines as well as [1,2,3]triazoleamines and pyrazolo[1,5-a]pyrimidines [1]. While reaction of 1 with hydrazine hydrate is established to yield pyrazolediamines that have been patented as a hair dyes, similar treatment with hydroxylamine hydrochloride has resulted, in our hands, in isolation of amidoximes that were utilized as precursors to other heterocycles [2,3]. It occurred to us that there might be value in trying to isolate amidrazones from the reactions of 1a,b with substituted hyrazines [4,5,6,7]. We report here the results of our reinvestigation of the behavior of compounds 1a,b toward phenylhydrazine. 1,2,3-Triazine derivatives are an important class of heterocyclic compounds useful in organic synthesis and as pharmaceuticals (e.g., as antimalarials) [8,9,10]. The work enabled development of an easy route to pyrazolo[3,4-e][1,2,4]triazines and [1,2,4]triazino[5,6-d][1,2,3]triazines (a new ring system) (Scheme 1) [11].
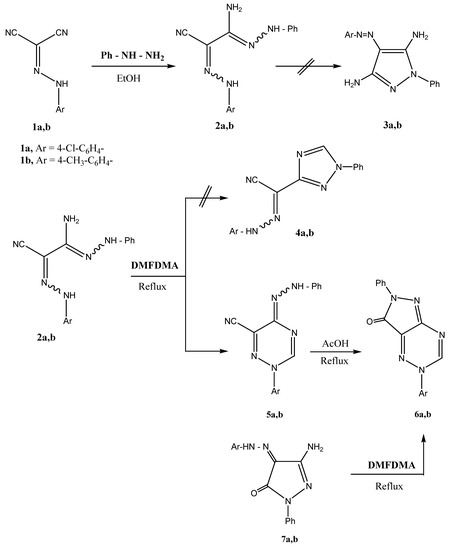
Scheme 1.
Proposed mechanism for the formation of 2-aryl-6-phenyl-2H-pyrazolo[3,4-e][1,2,4]triazin-7(6H)-ones 6a,b.
Results and Discussion
Reacting 2-arylhydrazonomalononitriles 1a,b with phenylhydrazine at room temperature in ethanol afforded 3-amino-2-arylhydrazono-3-phenylhydrazonopropanenitriles 2a,b. This is in contrast to the reported formation of 3a,b upon extended refluxing of 1a,b and phenylhydrazine in ethanol (Scheme 1) [3]. Although 2a,b may also exist in other tautomeric forms, only 2a,b were produced, as indicated from the 1H-NMR spectra that revealed D2O exchangeable amino signals at δ 6.09, 4.03 ppm and two hydrazone NH signals at δ 8.87, 9.67 and δ 11.10, 12.48 ppm, respectively. Compounds 2a,b, so formed, reacted readily and smoothly with dimethylformamide dimethylacetal (DMFDMA) to yield the products of condensation with elimination of two molecules of methanol and one molecule of dimethylamine. These can thus be formulated as the 2-aryl-5-phenylhydrazono-1,2,4-triazines 5a,b or the isomeric 1,2,4-triazoles 4a,b. Structures 5a,b could be established for these products based on the 1H-NMR spectra which revealed characteristic C-H signals at δ 9.67 and 8.76 ppm, respectively. If the products were 4a,b these signals should have appeared at higher field (δ ~ 8 ppm) [12]. Moreover the reaction product 5a,b cyclized upon refluxing in acetic acid to yield 6a,b. Heterocyclic ring systems similar to 6a,b were prepared by reacting 7 with acetic anhydride to give the methyl derivatives of 6 [13]. If the reaction products were 4a,b they should be recovered unreacted under these conditions. Compounds 6a,b could also be obtained via reaction of 7a,b with (DMFDMA) (cf. Scheme 1).
Compounds 5a,b reacted with hydroxylamine hydrochloride in ethanolic sodium acetate to yield 1,2,4-triazine-6-carboximidamides 8a,b. Refluxing the latter in dimethylformamide (DMF) resulted in cyclization via water elimination to yield the [1,2,4]triazino[5,6-d][1,2,3]triazines 10a,b (Scheme 2).
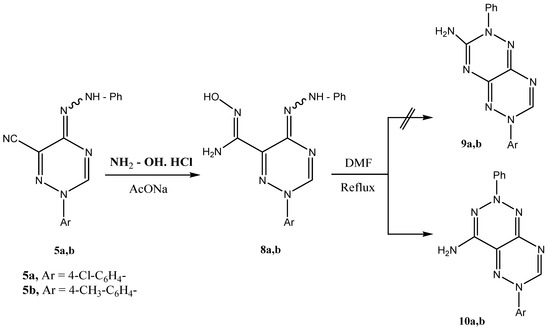
Scheme 2.
Proposed mechanism for the formation of 6-aryl-2-phenyl-2,6-dihydro-[1,2,4]triazino[5,6-d][1,2,3]triazin-4-amines 10a,b.
Possible Tiemann rearrangement of 8a,b prior to cyclization as reported earlier [14] for similar systems could be discounted as irradiation of the NH2 signal at δ 6.12 ppm did not enhance the integration of the aromatic multiplet at δ 7.44–8.02 ppm, as would be the case if the products were 9a,b. HMBC 15N-NMR indicated the amino protons at δ 6.14 ppm to have a 4J cross peak with the sp2 nitrogen at δ 280 ppm. A Tiemann rearrangement product should show such a cross peak with the sp3 nitrogen at δ 230 ppm (cf. Scheme 2).
Experimental
General procedures
Melting points were recorded on Gallenkamp apparatus and are uncorrected. Infrared spectra (KBr) were determined on a Perkin-Elmer 2000 FT-IR system. NMR measurements were determined on a Bruker DPX spectrometer, at 600 MHz for 1H-NMR and 125 MHz for 13C-NMR, in DMSO-d6 as solvent and using TMS as internal standard. Mass spectra were measured on MS 30 and MS 9 (AEI) spectrometers, with EI 70 eV. Elemental analyses were performed by using of LECO CHNS-932 Elemental Analyzer. Copies of the original data can be provided upon request.
Synthesis of arylhydrazonomalononitriles 1a,b
These compounds were prepared following the literature procedure [6]. A cold solution of aryldiazonium salt (10 mmol) was prepared by adding a sodium nitrite solution (1.4 g dissolved in 10 mL water) to a pre-cooled solution of arylamine hydrochloride (10 mmol of arylamine in 6 mL of 6 M HCl) with continuous stirring. The resulting solution of aryldiazonium salts were then added carefully to a cold ethanolic solution (50 mL) of malononitrile (0.66 g, 10 mmol) and sodium acetate trihydrate (2.8 g, 20 mmol). The mixture was stirred at room temperature for 1 h and the solid product formed was collected by filtration, washed with water and recrystallized from ethanol.
2-p-Chlorophenylhydrazonomalononitrile (1a). Yellow solid (83%), m.p. 186–188 °C (as reported in literature [15]); IR: υ = 3260 (NH), 2188.4 (CN), 2198 (CN) cm−1; 1H-NMR: δ = 7.26 (d, 2H, J = 8 Hz), 7.93 (d, 2H, J = 8 Hz), 13.08 (s, 1H, NH); 13C-NMR: δ = 117.8 (2 CN), 121.1, 123.6, 130.4, 139.8 (aromatic carbons), 159.2 (C(CN)2); MS, m/z (%), 204.0 (M+, 100), 126 (62), 111.0 (77); Anal. Calcd. for C9H5ClN4: C, 52.83; H, 2.46; Cl, 17.33; N, 27.38. Found: C, 52.80; H, 2.41; Cl, 17.28; N, 26.32.
2-p-Tolylhydrazonomalononitrile (1b). Yellow solid (74%), m.p. 169–170 °C (as reported in literature [16]); IR: υ = 3240 (NH), 2185.1 (CN), 2187 (CN) cm−1; 1H-NMR: δ = 2.6 (s, 3H, CH3), 7.02 (d, 2H, J = 8 Hz), 7.74 (d, 2H, J = 8 Hz), 12.6 (s, 1H, NH); 13C-NMR: δ = 23.6 (CH3), 117.4 (2 CN), 119.5, 125.3, 128.0, 135.9 (aromatic carbons), 157.6 (C(CN)2); MS, m/z (%), 184.1 (M+, 100), 91.1 (66); Anal. Calcd. for C10H8N4: C, 65.21; H, 4.38; N, 30.42. Found: C, 65.18; H, 4.32; N, 30.37.
Reaction of 1a,b with phenylhydrazine
A mixture of arylhydrazonomalononitrile 1a,b (10 mmol) and phenylhydrazine (1.08 g, 10 mmol) was dissolved in ethanol (50 mL) and the reaction progress was followed by TLC till completion after 24 h. The reaction mixture was cooled and the solid product, so formed, was then collected by filtration and recrystallized from ethanol.
3-Amino-2-p-chlorophenylhydrazono-3-phenylhydrazonopropanenitrile (2a). Yellow solid (71%), m.p. 133–135 °C; IR: υ = 3244 (br., NH2 and NH groups), 2223.5 (CN), 1632, 1624 (two C=N) cm−1; 1H-NMR: δ = 6.09 (s, 2H, NH2), 7.12 (d, 2H, J = 8 Hz), 7.64 (d, 2H, J = 8 Hz), 7.68 (m, 5H, phenyl), 8.87 (s, 1H, NH), 11.10 (s, 1H, NH); 13C-NMR: δ = 117.1 (CN), 113.9, 118.7, 120.6, 124.0, 129.3, 130.6, 138.7, 142.4 (aromatic carbons), 155.3 (C-CN), 156.8 (C-NH2); MS, m/z (%), 312.1 (M+, 100), 77 (55); Anal. Calcd. for C15H13ClN6: C, 57.60; H, 4.19; Cl, 11.34; N, 26.87. Found: C, 57.58; H, 4.14; Cl, 11.29; N, 26.80.
3-Amino-2-p-tolylhydrazono-3-phenylhydrazonopropanenitrile (2b). Yellow solid (68%), m.p. 148–150 °C; IR: υ = 3330 (br., NH2 and NH groups), 2181 (CN) 1630, 1620 (two C=N) cm−1; 1H-NMR: δ = 2.29 (s, 3H, CH3), 4.03 (s, 2H, NH2), 7.04 (d, 2H, J = 8 Hz), 7.46 (d, 2H, J = 8 Hz), 7.42 (m, 5H, phenyl), 9.67 (s, 1H, NH), 12.48 (s, 1H, NH); 13C-NMR: δ = 17.2 (CH3), 117.6 (CN), 116.1, 119.3, 119.8, 122.4, 124.2, 128.6, 131.1, 141.2 (aromatic carbons), 153.5 (C-CN), 154.8 (C-NH2); MS, m/z (%), 292.1 (M+, 100), 190.1 (52); Anal. Calcd. for C16H16N6: C, 65.74; H, 5.52; N, 28.75. Found: C, 65.47; H, 5.48; Cl, N, 28.70.
Reaction of 2a,b with DMFDMA
A mixture of 2a,b (10 mmol) and DMFDMA (1.2 g, 10 mmol) was dissolved in xylene (50 mL) and the reaction mixture was refluxed for 8 h, then concentrated under reduced pressure to the half of its original volume, cooled and the solid product, so formed, was then filtered and recrystallized from ethanol.
2-p-Chlorophenyl-5-phenylhydrazono-2,5-dihydro-1,2,4-triazine-6-carbonitrile (5a). Yellow solid (65%); m.p. 151–152 °C; IR: υ = 3350 (br., NH group), 2190.3 (CN) cm−1; 1H-NMR: δ = 7.02 (d, 2H, aryl o-protons), 7.52 (m, 7H, aromatic H), 9.67 (s, 1H, triazine H), 12.48 (s, 1H, NH); 13C-NMR: δ = 117.3 (CN), 114.8, 119.4, 121.3, 126.5, 127.9, 129.3, 138.4, 141.1 (aromatic carbons), 153.2 (C-CN), 154.7 (C-5, triazine ring), 158.6 (C-3, triazine ring); MS, m/z (%), 322.1 (M+, 100), 77.1 (32); Anal. Calcd. for C16H11ClN6: C, 59.54; H, 3.44; Cl, 10.98; N, 26.04. Found: C, 59.48; H, 3.40; Cl, 10.93; N, 25.97.
5-Phenylhydrazono-2-p-tolyl-2,5-dihydro-1,2,4-triazine-6-carbonitrile (5b). Yellow solid (62%); m.p. 224–225 °C; IR: υ = 3345 (br., NH group), 2187 (CN) cm−1; 1H-NMR: δ = 1.63 (s, 3H, CH3), 6.85 (d, 2H, aryl o-protons), 7.43 (m, 7H, aromatic H), 8.76 (s, 1H, triazine H), 11.03 (s, 1H, NH); 13C-NMR: δ = 13.8 (CH3), 116.9 (CN), 116.4, 120.2, 121.8, 124.2, 128.2, 129.8, 137.1, 140.8 (aromatic carbons), 153.7 (C-CN), 155.4 (C-5, triazine ring), 157.5 (C-3, triazine ring); MS, m/z (%), 302.1 (M+, 100), 77.1 (83); Anal. Calcd. for C17H14N6 C, 67.54; H, 4.67; N, 27.80. Found: C, 67.51; H, 4.64; N, 27.73.
Cyclization of phenylhydrazono-1,2,4-triazines 5a,b upon refluxing in AcOH
A mixture of 5a,b (10 mmol) was dissolved in glacial acetic acid (30 mL) and the reaction was refluxed for 6 h while the reaction was followed to completion by TLC. The reaction mixture was neutralized with Na2CO3 solution whereby a solid product was formed. The solid was collected by filtration and recrystallized from ethanol.
2-p-Chlorophenyl-6-phenyl-2,6-dihydro-pyrazolo[3,4-e][1,2,4]triazin-7-one (6a). Yellow solid (65%); m.p. 166–167 °C; IR: υ = 1689 (C=O) cm−1; 1H-NMR: δ = 6.62 (d, 2H, aryl o-protons), 7.34–7.98 (m, 7H, aromatic H), 7.67 (s, 1H, triazine H); 13C-NMR: δ = 114.7, 116.8, 119.1, 122.5, 128.7, 129.8, 134.1, 138.0, 139.6, 142.4 (aromatic carbons), 154.7 (C=O), 162.6 (C-3, triazine ring); MS, m/z (%), 323.1 (M+, 100), 225.1 (38), 77.1 (29); Anal. Calcd. for C16H10ClN5O: C, 59.36; H, 3.11; Cl, 10.95; N, 21.63. Found: C, 59.31; H, 3.04; Cl, 10.88; N, 21.56.
6-Phenyl-2-p-tolyl-2,6-dihydro-pyrazolo[3,4-e][1,2,4]triazin-7-one (6b). Yellow solid (67%); m.p. 194–196 °C; IR: υ = 1681 (C=O) cm−1; 1H-NMR: δ = 1.98 (s, 3H, CH3), 6.81- 7.56 (m, 10H, aromatic protons); 13C-NMR: δ = 16.2 (CH3), 115.7, 119.0, 120.8, 122.7, 124.6, 128.9, 130.0, 138.2, 140.9, 142.4 (aromatic carbons), 154.6 (C=O), 158.3 (C-3, triazine ring); MS, m/z (%), 303.1 (M+, 100), 211.1 (46); Anal. Calcd. for C17H13N5O C, 67.32; H, 4.32; N, 23.09. Found: C, 67.31; H, 4.27; N, 23.02.
Alternate route to 6a,b by refluxing pyrazolones 7a,b with DMFDMA (chemical evidence)
A mixture of 7a,b (10 mmol) and DMFDMA (1.2 g, 10 mmol) was dissolved in dry xylene (50 mL) and the solution was refluxed for 8 h. The reaction mixture was triturated as usual and the solid product, so formed, was then filtered and recrystallized from ethanol. Elemental analysis and NMR spectra matched those of 6a,b.
Reaction of phenylhydrazono-1,2,4-triazines 5a,b with hydroxylamine
A mixture of phenylhydrazono-1,2,4-triazine 5a,b (10 mmol), hydroxylamine hydrochloride (0.69 g, 10 mmol) and sodium acetate anhydrous (3 g, 25 mmol) in ethanol (25 mL) was heated under reflux for 5 h. The reaction mixture was poured to water while a solid product was formed. The solid product, so formed, was then collected by filtration and recrystallized from ethanol to give 8a,b.
2-p-Chlorophenyl-N′-hydroxy-5-phenylhydrazono-2,5-dihydro-1,2,4-triazine-6-carboximidamide (8a). Yellow solid (60%); m.p. 218 °C (with decomposition); IR: υ = 3330 (br., OH and NH groups) cm−1; 1H- NMR: δ = 6.15 (s, 2H, NH2), 6.75-7.34 (m, 9H, aromatic H), 7.52 (s, 1H, triazine H), 9.37 (s, 1H, OH group), 11.65 (s, 1H, NH group); 13C-NMR: δ = 114.7, 119.2, 122.8, 128.2, 129.1, 132.9, 138.1, 139.6 (aromatic carbons), 155.2 (C-6, triazine ring), 156.3 (C-5, triazine ring), 156.9 (C-3, triazine ring), 158.6 (C=NOH); MS, m/z (%), 355.1 (M+, 100), 171.1 (87), 77.1 (75); Anal. Calcd. for C16H14ClN7O: C, 54.01; H, 3.97; Cl, 9.96; N, 27.56. Found: C, 53.95; H, 3.91; Cl, 9.89; N, 27.49.
N′-Hydroxy-5-phenylhydrazono-2-p-tolyl-2,5-dihydro-1,2,4-triazine-6-carboximidamide (8b). Yellow solid (55%); m.p. 215 °C; IR: υ = 3320 (br., OH and NH groups) cm−1; 1H-NMR: δ = 2.24 (s, 3H, CH3), 5.57 (s, 2H, NH2), 6.52 (d, 2H, o-aryl protons), 7.06-7.94 (m, 7H, aromatic H), 7.64 (s, 1H, triazine H), 10.31 (s, 1H, OH group), 13.03 (s, 1H, NH group); 13C-NMR: δ = 18.1 (CH3), 115.2, 118.5, 122.8, 124.2, 126.3, 132.7, 134.2, 135.4 (aromatic carbons), 156.9 (C-6, triazine ring), 158.1 (C-5, triazine ring), 159.2 (C-3, triazine ring), 161.8 (C=NOH); MS, m/z (%), 335.1 (M+, 100), 105.1 (30); Anal. Calcd. for C17H17N7O: C, 60.88; H, 5.11; N, 29.24. Found: C, 60.84; H, 5.07; N, 29.18.
Cyclization of 1,2,4-triazine-6-carboximidamides 8a,b upon refluxing with DMF
A solution of 8a,b (10 mmol) in DMF (30 mL) was refluxed for 4 h. The reaction mixture was poured on HCl/ice mixture. The solid product, so formed, was then filtered and recrystallized from ethanol to give 10a,b.
6-p-Chlorophenyl-2-phenyl-2,6-dihydro-[1,2,4]triazino[5,6-d][1,2,3]triazin-4-amine (10a). Yellow solid (66%); m.p. 221–223 °C; IR: υ = 3350 (br., NH2 group) cm−1; 1H-NMR: δ = 6.14 (s, 2H, NH2), 7.44-7.8.04 (m, 9H, aromatic H), 9.51 (s, 1H, triazine H); 13C-NMR: δ = 115.4, 116.8, 119.0, 120.6, 123.8, 128.9, 139.0, 141.8 (aromatic carbons), 144.7 (C-6, 1,2,4-triazine ring), 146.4 (C-5, 1,2,4-triazine ring), 154.8 (C-NH2, 1,2,3-triazine), 157.2 (C-3, 1,2,4-triazine ring); MS, m/z (%), 337.1 (M+, 100), 171.0 (36), 77.1 (12); Anal. Calcd. for C16H12ClN7: C, 56.89; H, 3.58; Cl, 10.50; N, 29.03. Found: C, 56.84; H, 3.52; Cl, 10.45; N, 28.95.
2-Phenyl-6-p-tolyl-2,6-dihydro-[1,2,4]triazino[5,6-d][1,2,3]triazin-4-amine (10b). Yellow solid (70%); m.p. 181–183 °C; IR: υ = 3340 (br., NH2 groups) cm−1; 1H-NMR: δ = 2.36 (s, 3H, CH3), 6.13 (s, 2H, NH2), 6.68 (d, 2H, o-aryl protons), 6.87-7.62 (m, 7H, aromatic H), 9.34 (s, 1H, 1,2,4-triazine H); 13C-NMR: δ = 17.8 (CH3), 114.6, 115.6, 123.2, 125.0, 128.3, 128.8, 136.9, 137.2 (aromatic carbons), 147.2 (C-6, 1,2,4-triazine), 148.8 (C-5, 1,2,4-triazine), 150.1 (C-NH2, 1,2,3-triazine), 157.5 (C-3, 1,2,4-triazine); MS, m/z (%), 317.1 (M+, 100), 77.1 (37); Anal. Calcd. for C17H15N7: C, 64.34; H, 4.76; N, 30.90. Found: C, 64.28; H, 4.72; N, 30.81.
Conclusions
Arylhydrazonomalononitriles proved to be versatile readily obtainable starting materials for the synthesis of pyrazolo[3,4-e][1,2,4]triazines and 1,2,4-triazino[5,6-d][1,2,3]triazines derivatives (the latter being a new ring system).
Acknowledgements
Support of this work received from the University of Kuwait through research grant (SC04/06) and the facilities of Analab/SAF by research grant (GC01/01), (GC01/03) and (GS03/01) are gratefully acknowledged. Partial financial support from the College of Graduate Studies at Kuwait University for Doa’a M. Al-Dorri is highly appreciated.
References and Notes
- Riyadh, S.M.; Al-Matar, H.M.; Elnagdi, M.H. Studies with 2-Arylhydrazononitriles: Further Investigations on Reactivity of 2-Arylhydrazononitriles towards Hydroxylamine. J. Heterocycl. Chem. 2008, 45, 975–979. [Google Scholar] [CrossRef]
- Vidal, L.; (to L’Oreal). Hair Bleach composition and hair dye composition. U.S. Patent 6,916,432, 2005. [Google Scholar]
- Al-Mousawi, S.M.; El-Apasery, M.A.; Al-Kanderi, N.H. Microwave-assisted organic synthesis: The Gabriel approach as a route to new pyrazolylhydrazonoazoles. ARKIVOC 2008, 16, 268–278. [Google Scholar]
- Elnagdi, M.H.; Abdoula, S.O. Reactions with arylhydrazones of some α-cycloketones. J. Prakt. Chem. 1973, 315, 1009. [Google Scholar] [CrossRef]
- Elnagdi, M.H. Reactions with β-cycanoethylhydrazine 1: “A route for the preparation of pyrazolo[1,5-c]pyrimidines and pyrrolo[1,2-b]pyrazoles. Tetrahedron 1974, 30, 2791. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Elmoghayer, M.R.H.; Kandeel, E.M.; Ibrahim, M.K.A. Reactions with heterocyclic diazonium salts: Synthesis of some new pyrazolo[1,5-c]triazines, and 1,2,4-triazolo[1,5-c]triazines. J. Heterocyclic Chem. 1977, 14, 221. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Al-Matar, H.M.; Elnagdi, M.H. Studies with 2-arylhydrazono nitriles: Further investigations on reactivity of 2-arylhydrazono nitriles towards hydroxylamine. J. Heterocyclic Chem. 2008, 45, 975. [Google Scholar] [CrossRef]
- Stevens, M.F.G. The Medicinal Chemistry of 1,2,3-Triazines. Prog. Med. Chem. 1976, 13, 205. [Google Scholar] [PubMed]
- Curd, F.H.S.; Landquist, J.K.; Rose, F.L. Synthetic antimalarials. Part XII. Some 1:3:5-triazine derivatives. J Chem. Soc. 1947, 154–160. [Google Scholar] [CrossRef]
- Migawa, M.T.; Drach, J.C.; Townsend, L.B. Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(β-D-ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(β-D-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-β-D-ribofuranosyl)-1,5-dihydro-1,4,5, 6,7,8- hexaazaacenaphthylene as analogues of triciribine. J. Med. Chem. 2005, 48, 3840–3851. [Google Scholar] [PubMed]
- Bel’skaya, N.P.; Demina, M.A.; Sapognikova, S.G.; Fan, Z.; Zhang, H.; Dehaen, W.; Bakulev, V.A. Synthesis and oxidative cyclization of 2-arylhydrazono-2-cyanoacetamidines to 5-amino-2-aryl-2H-[1,2,3]triazole-4-carbonitrile. ARKIVOC 2008, xvi, 9. [Google Scholar]
- Pretsch, E.; Buhlmann, P.; Affolter, C. Structure Determination of Organic Compounds: Tables of Spectral Data, 3rd ed.; Springer-Velag: Berlin, Germany, 2000; p. 421. [Google Scholar]
- Shaban, M.A.; Abdou, N.A.; El-Meligie, S.; Taher, A. Novel pyrazolone derivatives: Synthesis and evaluation for analgesic activity. Alex. J. Pharm. Sci. 1990, 4, 93–95. [Google Scholar]
- Al-Matar, H.M.; Riyadh, S.R.; Elnagdi, M.H. 2-Arylhydrazononitriles in heterocyclic synthesis: a novel route to 1,3-diaryl-1,2,4-triazol-5-amines via a Tiemann rearrangement of arylhydrazonoamidoximes. ARKIVOC 2007, xiii, 53–62. [Google Scholar]
- Lythgoe, B.; Todd, A.R.; Topham, A. Synthesis of purine nucleosides. V. Coupling of pyrimidine derivatives with diazonium salts. Method for the preparation of 5-aminopyrimidines. J. Chem. Soc. 1944, 315. [Google Scholar] [CrossRef]
- Kulaeva, L.N.; Grabenko, A.D.; Pel’kis, P.S. Studies on 1,3-dithiolium derivatives. II. Oxidation of arylhydrazones of dithiomesoxalic acid diarylamides to 1,2-dithiolium derivatives. Inst. Org. Khim. Kiev. USSR 1978, 7, 909. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1a,b, 2a,b, 5a,b, 6a,b, 8a,b, 10a,b are available from the authors. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).