Abstract
An efficient synthesis of novel 1-aryl-3-(indole-3-yl)-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-ones from indoles and 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one using a Brønsted acid ionic liquid [Sbmim][HSO4] as catalyst is described. Satisfactory results with excellent yields and short reaction time were obtained in the experiments. The catalyst could be recovered conveniently and reused efficiently.
Introduction
In recent years, β-indolylketones have received much attention because they represent an important substructure in both biologically active compounds and natural products []. A simple and direct approach for their synthesis involves the conjugate addition of indole and α,β-unsaturated ketones in the presence of either protic or Lewis acids. During the last decade, several improved methods have been reported for the preparation of these compounds using various catalysts such as FAP [], I2 [], Zn-HAP [], CAN [], InBr3 [], PTSA [], Bi(NO3)3 [], HfCl4 and ScCl3 [], PVSA [], pyrrolidine and HClO4 [], GaI3 [], Zr(OTf)4 [], Bi(OTf)3 [] and so on. However, several of these reported procedures suffer from the drawbacks such as strong acidic conditions, long reaction times, complex handling and low yields of products. Hence, new efficient and green procedures are still in strong demand. Recently, ionic liquids (ILs) have been widely used as environmentally benign reaction media in organic synthesis owing to their unique properties of nonvolatility, nonflammability, and recyclability [,]. In particular, the synthesis of task-specific ILs with special functions according to the requirements of a specific reaction has become an attractive field [,]. Recently, Yang et al. [] reported the use of the Brønsted acid ionic liquid [Sbmim][HSO4] as catalyst for the hydrolysis of soybean isoflavone glycosides. In this process [Sbmim][HSO4] has good catalytic activities which are similar to those of sulfuric acid, giving a conversion of glycitin of more than 90%.
Due to their unique biological properties, 1,2,3-triazole derivatives have attracted much attention []. In this work, we studied the possibility to synthesize 1-aryl-3-(indol-3-yl)-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-ones using 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one as substrates instead of ordinary α,β-unsaturated ketones and employing the Brønsted acid ionic liquid [Sbmim][HSO4] as catalyst (Scheme 1). Herein, an efficient and practical method for the synthesis of target compounds is described and none of them has been reported yet in the literature.
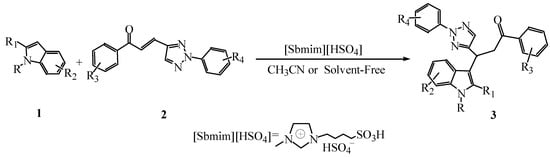
Scheme 1.
The synthesis of 1-aryl-3-(indol-3-yl)-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-ones catalyzed by the ionic liquid [Sbmim][HSO4].
Results and Discussion
Initially, to evaluate the effect of the catalyst [Sbmim][HSO4] under different reaction conditions, the reaction of indole and 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one was selected as a model reaction. The results were presented in Table 1. It was clear that the best solvent was acetonitrile and the best molar ratio of IL/substrate is 10% (Table 1, entry 3). The influence of the reaction time on the yield was also investigated as shown in Table 1, entries 3, 8–12. It was found that a higher yield occurred when the reaction time was 3 h (Table 1, entry 11), although, the yield did not change significantly when the reaction time was increased from 1 h to 5 h. For the purpose of saving energy, we chose 1 h as the reaction time. Hence, the best conditions employed a 0.1:1:1 mole ratio of [Sbmim][HSO4], indole and 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one at 80 °C for 1 h using acetonitrile as solvent.
The recycling performance of TSIL [Sbmim][HSO4] was also investigated in the reaction of indole and 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one. After the separation of product, the filtrate containing catalyst was distilled under vacuum to remove water and the resulting catalyst was reused directly for the next run.

Table 1.
Effect of the catalyst[Sbmim][HSO4] in the reaction of indole and 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one under different conditions. a
| Entry | Solvent | [Sbmim][HSO4] (mol %) | Time (h) | Yield (%)b |
|---|---|---|---|---|
| 1 | Ethyl acetate | 10 | 4 | 70 |
| 2 | Methanol | 10 | 4 | 87 |
| 3 | Acetonitrile | 10 | 4 | 95 |
| 4 | Acetonitrile | none | 4 | 0 |
| 5 | Acetonitrile | 2.5 | 4 | 79 |
| 6 | Acetonitrile | 5 | 4 | 91 |
| 7 | Acetonitrile | 15 | 4 | 94 |
| 8 | Acetonitrile | 10 | 0.5 | 79 |
| 9c | Acetonitrile | 10 | 1 | 95, 92, 90 |
| 10 | Acetonitrile | 10 | 2 | 98 |
| 11 | Acetonitrile | 10 | 3 | 99 |
| 12 | Acetonitrile | 10 | 5 | 97 |
a Reaction conditions: indole (2 mmol), 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (2 mmol) and catalyst in solvent (10 mL), 80 °C; b Isolated yield; c catalyst was recycled three times.
As shown in Table 1, Brønsted acidic ionic liquid [Sbmim][HSO4] can be recycled at least three times without any significant decrease in catalytic activity, the yields ranged from 95% to 90% (entry 9c). This indicated that the ionic liquid [Sbmim][HSO4] was an efficient and recyclable catalyst for the reaction. In order to check the generality of the procedure, a variety of substituted indoles were reacted with 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one. In general, the reaction proceeded easily under the optimum conditions described above and the adducts were isolated in excellent yields (Table 2).

Table 2.
[Sbmim][HSO4]-catalyzed synthesis of 1-aryl-3-(indole-3-yl)-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one.
| Entry | R | R1 | R2 | R3 | R4 | Mp (°C)a | Yields (%)b | |
|---|---|---|---|---|---|---|---|---|
| Ac | Bc | |||||||
| 3a | H | H | H | H | H | 148–150 | 93 | 89 |
| 3b | H | H | H | 4-CH3 | H | 157–159 | 98 | 97 |
| 3c | H | H | H | 4-OCH3 | H | 188–191 | 97 | 92 |
| 3d | H | H | H | 4-Cl | H | 140–143 | 95 | 96 |
| 3e | H | H | H | 4-Br | H | 143–146 | 96 | 93 |
| 3f | H | H | 5-Br | 2,4-Cl2 | H | 144–147 | 93 | 87 |
| 3g | H | CH3 | H | H | H | 163–165 | 97 | 95 |
| 3h | H | CH3 | H | H | 4-Br | 165–168 | 89 | 86 |
| 3i | H | CH3 | H | 2-Cl | H | 142–144 | 97 | 94 |
| 3j | CH3 | H | H | 4-OCH3 | H | 136–138 | 95 | 97 |
a Melting points were uncorrected; b Isolated yield; c Method A: [Sbmim][HSO4] (0.02 mmol), 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one (2 mmol), indoles (2 mmol), acetonitrile (10 mL), 80 °C, 1 h; Method B: [Sbmim][HSO4] (0.02 mmol), 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one (2 mmol), indoles (2 mmol), solvent-free, 90 °C, 1 h.
The results obtained indicated that the electron donating or withdrawing groups at the indole ring did not seem to affect the reaction significantly in terms of yields. As environmental consciousness in chemical research and industry has increased, the challenge for a sustainable environment has called for clean procedures that can avoid the use of organic solvents. Hence, we also examined the reaction of indoles and 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one under solvent-free condition, and the products were obtained in excellent yields (Table 2).
A proposed reaction mechanism for the conjugate addition of indole to 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one is presented in Scheme 2. The catalyst [Sbmim][HSO4] coordinates with the oxygen atom of 1-phenyl-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (2) to give intermediate 4. The electron rich β-position of indole ring then attacks the electron deficient conjugated carbon-carbon double bond of 4 to afford 5, followed by a hydrogen transfer to yield 6. Finally 6 rearranged to give target compound 3a and [Sbmim][HSO4] catalyzes the next cycle.

Scheme 2.
The proposed mechanism of synthesizing β-indolyketones catalyzed by ionic liquid [Sbmim][HSO4].
Conclusions
In summary, we have reported an efficient and simple method for synthesis of a series of novel 1-aryl-3-(indol-3-yl)-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one using 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one instead of ordinary α,β-unsaturated ketones as substrate and employing Brønstedacidic ionic liquid [Sbmim][HSO4] as catalyst. The corresponding adducts were synthesized in short reaction times successfully and isolated in excellent yields. It is an important supplement to the existing methods for the synthesis of β-indolylketones.
Experimental
General
All compounds were characterized by IR, 1H-NMR spectra and elemental analysis. The IR spectra were obtained as potassium bromide pellets with a FTS-40 spectrometer (BIO-RAD, U.S.A). The 1H-NMR spectra were obtained on a Varian Inova-400 spectrometer using CDCl3 or DMSO-d6 as solvent (as indicated under each entry below) and TMS as an internal standard, chemical shifts are given in ppm. Elemental analyses (C, H, N) were performed on a Perkin-Elmer Analyzer 2400. Melting points were determined using a Büchi B-540 instrument. All melting points are uncorrected. The Brønsted acid ionic liquid [Sbmim][HSO4] was synthesized according to a previous literature method []. 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one (2) was synthesized according to a previous literature report [].
General procedure for the synthesis of 1-aryl-3-(indol-3-yl)-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-ones 3a-3j
A mixture of indole (2 mmol), 1-aryl-3-(2-aryl-1,2,3-triazol-4-yl)propan-1-one (2 mmol) and [sbmim][HSO4] (0.2 mmol) was heated at 80 °C in acetonitrile (10 mL) or 90 °C under solvent-free conditions for 1 h with stirring (Scheme 1). The completion of the reaction was monitored by TLC. After cooling, the reaction mixture was poured onto crushed ice (30 g). The resulting precipitate was filtered under suction, and then recrystallized from ethanol to afford the pure product. The results are summarized in Table 2. Data of the compounds are shown below:
1-Phenyl-3-(indol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3a): Red powder. 1H-NMR (DMSO-d6) δ: 3.87 (dd, J = 6.0, 6.0 Hz, 1H, CH), 4.18 (dd, J = 8.4, 8.4 Hz, 1H, CH), 5.15 (t, 1H, J = 7.2 Hz, CH), 6.96–8.06 (m, 16H, ArH), 10.97 (s, 1H, NH); IR: ν 3342, 1680, 1594, 1457, 1357, 748 cm–1; Anal. Calcd. for C25H20N4O: C, 76.51; H, 5.14; N, 14.28. Found: C, 76.36; H, 5.18; N, 14.22.
1-(4-Methylphenyl)-3-(indol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3b): Light yellow powder. 1H-NMR (DMSO-d6) δ: 2.37 (s, 3H, CH3), 3.82 (dd, J = 6.0, 6.4 Hz, 1H, CH), 4.12 (dd, J = 8.4, 8.4 Hz, 1H, CH), 5.13 (t, 1H, J = 7.2 Hz, CH), 6.96–8.01 (m, 15H, ArH), 10.96 (s, 1H, NH); IR: ν 3359, 1674, 1595, 1456, 1337, 736 cm–1; Anal. Calcd. for C26H22N4O: C, 76.83; H, 5.46; N, 13.78. Found: C, 76.71; H, 5.51; N, 13.72.
1-(4-Methoxylphenyl)-3-(indol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3c): Light yellow powder. 1H-NMR (DMSO-d6) δ: 3.78 (dd, J = 6.4, 6.4 Hz, 1H, CH), 3.84 (s, 3H, OCH3) 4.08 (dd, J = 8.4, 8.8 Hz, 1H, CH), 5.12 (t, 1H, J = 7.2 Hz, CH), 6.95–8.04 (m, 15H, ArH), 10.95 (s, 1H, NH); IR: ν 3348, 2901, 1668, 1595, 1457, 1338, 735 cm–1; Anal. Calcd. for C26H22N4O2: C, 73.92; H, 5.25; N, 13.26. Found: C, 73.81 ; H, 5.32; N, 13.39.
1-(4-Chlorophenyl)-3-(indol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3d): Light yellow powder. 1H-NMR (DMSO-d6) δ: 3.85 (dd, J = 6.0, 6.0 Hz, 1H, CH), 4.17 (dd, J = 8.8, 8.8 Hz, 1H, CH), 5.12 (t, 1H, J = 7.2 Hz, CH), 6.96–8.17 (m, 15H, ArH), 10.97 (s, 1H, NH); IR: ν 3395, 1678, 1586, 1490, 1338, 737 cm–1; Anal. Calcd. for C25H19N4OCl: C, 70.34; H, 4.49; N, 13.12. Found: C, 70.48 ; H, 4.55; N, 13.01.
1-(4-Bromophenyl)-3-(indol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3e): Red powder. 1H-NMR (DMSO-d6) δ: 3.84 (dd, J = 6.0, 6.0 Hz, 1H, CH), 4.16 (dd, J = 8.4, 8.4 Hz, 1H, CH), 5.12 (t, 1H, J = 7.2 Hz, CH), 6.96–8.10 (m, 15H, ArH), 10.96 (s, 1H, NH); IR: ν 3389, 1677, 1583, 1491, 1338, 737 cm–1; Anal. Calcd. for C25H19N4OBr: C, 63.70; H, 4.06; N, 11.89. Found: C, 63.81; H, 4.11; N, 11.75.
1-(2,4-Dichlorophenyl)-3-(5-bromoindol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3f): Light yellow powder. 1H-NMR (DMSO-d6) δ: 3.77 (dd, J = 6.0, 6.4 Hz, 1H, CH), 4.04 (dd, J = 8.4, 8.8 Hz, 1H, CH), 5.03 (t, 1H, J = 7.6 Hz, CH), 7.16–8.06 ( m, 13H, ArH), 11.20 (s, 1H, NH); IR: ν 3323, 1689, 1581, 1461, 1340, 817, 753 cm–1; Anal. Calcd. for C25H17N4OCl2Br: C, 55.58; H, 3.17; N, 10.37. Found: C, 55.68; H, 3.10; N, 10.25.
1-Phenyl-3-(2-methylindol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3g): Red powder. 1H-NMR (400 MHz, DMSO-d6) δ: 2.44 (s, 3H, CH3), 3.81 (dd, J = 6.8, 6.8 Hz, 1H, CH), 4.25 (dd, J = 7.2, 7.6 Hz, 1H, CH), 5.09 (t, 1H, J = 7.6 Hz, CH), 6.85–8.01 (m, 15H, ArH), 10.84 (s, 1H, NH); IR (KBr): ν 3368, 2971, 1679, 1595, 1460, 1336, 752 cm–1; Anal. Calcd. for C26H22N4O: C, 76.83; H, 5.46; N, 13.78. Found: C, 76.91; H, 5.41; N, 13.89.
1-Phenyl-3-(2-methylindol-3-yl)-3-(2-(4-bromophenyl)-1,2,3-triazol-4-yl)propan-1-one (3h): Red powder. 1H-NMR (DMSO-d6) δ: 2.45 (s, 3H, CH3), 3.82 (dd, J = 6.8, 7.2 Hz, 1H, CH), 4.23 (dd, J = 7.2, 7.6 Hz, 1H, CH), 5.07 (t, 1H, J = 7.2 Hz, CH), 6.84–7.99 (m, 14H, ArH), 10.84 (s, 1H, NH); IR: ν 3378, 2898, 1675, 1593, 1489, 739 cm–1; Anal. Calcd. for C26H21N4OBr: C, 64.34; H, 4.36; N, 11.54. Found: C, 64.45; H, 4.30; N, 11.72.
1-(2-Chlorophenyl)-3-(2-methylindol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3i): Red powder. 1H-NMR (DMSO-d6) δ: 2.39 (s, 3H, CH3), 3.70 (dd, J = 6.8, 6.8 Hz, 1H, CH), 4.18 (dd, J = 8.0, 8.4 Hz, 1H, CH), 5.02 (t, 1H, J = 7.6 Hz, CH), 6.83–7.94 (m, 14H, ArH), 10.88 (s, 1H, NH); IR: ν 3380, 1692, 1588, 1460, 1354, 746 cm–1; Anal. Calcd. for C26H21N4OCl: C, 70.82; H, 4.80; N, 12.71. Found: C, 70.65; H, 4.83; N, 12.87.
1-(4-Methoxylphenyl)-3-(1-methylindol-3-yl)-3-(2-phenyl-1,2,3-triazol-4-yl)propan-1-one (3j): Light yellow powder. 1H NMR (DMSO-d6) δ: 3.72 (s, 3H, CH3) 3.76 (dd, J = 6.0, 6.0 Hz, 1H, CH), 3.84 (s, 3H, OCH3), 4.09 (dd, J = 8.4, 8.8 Hz, 1H, CH), 5.11 (t, 1H, J = 7.2 Hz, CH), 6.99–8.03 (m, 15H, ArH); IR: ν 3348, 2909, 1682, 1596, 1464, 731 cm–1; Anal. Calcd. for C27H24N4O2: C, 74.19; H, 5.54; N, 12.84. Found: C, 74.35; H, 5.59; N, 12.99.
Acknowledgements
We gratefully acknowledge support from the National Natural Science Foundation of China (No. 20862016, 20662009).
- Sample Availability: Available from the authors
References
- Zhan, Z.P.; Yang, R.F.; Lang, K. Samarium triiodide-catalyzed conjugate addition of indoles with electron-deficient olefins. Tetrahedron Lett. 2005, 46, 3859–3862. [Google Scholar] [CrossRef]
- Tahir, R.; Banert, K.; Sebti, S. Friedel–Crafts-type conjugate addition of indoles using fluorapatite doped zinc bromide as efficient solid catalyst. Appl. Catal. A Gen. 2006, 315, 147–149. [Google Scholar] [CrossRef]
- Banik, B.K.; Fernandez, M.; Alvarez, C. Iodine-catalyzed highly efficient Michael reaction of indoles under solvent-free condition. Tetrahedron Lett. 2005, 46, 2479–2482. [Google Scholar]
- Tahir, R.; Banert, K.; Solhy, A.; Sebti, S. Zinc bromide supported on hydroxyapatite as a new and efficient solid catalyst for Michael addition of indoles to electron-deficient olefins. J. Mol. Catal. A Chem. 2006, 246, 39–42. [Google Scholar] [CrossRef]
- Ko, S.K.; Lin, C.C.; Tu, Z.J.; Wang, Y.F.; Wang, C.C.; Yao, C.F. CAN and iodine-catalyzed reaction of indole or 1-methylindole with α,β-unsaturated ketone or aldehyde. Tetrahedron Lett. 2006, 47, 487–492. [Google Scholar]
- Bandini, M.; Cozzi, P.G.; Giacomini, M.; Melchiorre, P.; Selva, S. Sequential one-pot InBr3-catalyzed 1,4- then 1,2-nucleophilic addition to enones. J. Org. Chem. 2002, 67, 3700–3704. [Google Scholar] [CrossRef]
- Ji, S.J.; Wang, S.Y. An expeditious synthesis of β-indolylketones catalyzed by p-toluenesulfonic acid (PTSA) using ultrasonic irradiation. Ultrason. Sonochem. 2005, 12, 339–343. [Google Scholar] [CrossRef]
- Srivastava, N.; Banik, B.K. Bismuth nitrate-catalyzed versatile Michael reactions. J. Org. Chem. 2003, 68, 2109–2114. [Google Scholar] [CrossRef]
- Kawatsura, M.; Aburatani, S.; Uenish, J. Catalytic conjugate addition of heterocyclic compounds to α,β-unsaturated carbonyl compounds by hafnium salts and scandium salts. Tetrahedron 2007, 63, 4172–4177. [Google Scholar] [CrossRef]
- Ekbote, S.S.; Panda, A.G.; Bhor, M.D.; Bhanage, B.M. Polyvinylsulfonic acid as a novel Brønsted acid catalyst for Michael addition ofindoles to α,β-unsaturated ketones. Catal. Commun. 2009, 10, 1569–1573. [Google Scholar] [CrossRef]
- Li, D.P.; Guo, Y.C.; Ding, Y.; Xiao, W.J. Organocatalytic C3-selective Friedel–Crafts alkylations of indoles with α,β-unsaturated ketones. Chem. Commun. 2006, 799–801. [Google Scholar]
- Huang, Z.H.; Zou, J.P.; Jiang, W.Q. Gallium(III) triiodide catalyzed conjugate addition of indoles with α,β-unsaturated ketones. Tetrahedron Lett. 2006, 47, 7965–7968. [Google Scholar] [CrossRef]
- Shi, M.; Cui, S.C.; Li, Q.J. Zirconium triflate-catalyzed reactions of indole, 1-methylindole, and pyrrole with α,β-unsaturated ketone. Tetrahedron 2004, 60, 6679–6684. [Google Scholar] [CrossRef]
- Reddy, A.V.; Ravinder, K.; Goud, T.V.; Krishnaiah, P.; Raju, T.V.; Venkateswarlu, Y. Bismuth triflate catalyzed conjugate addition of indoles to α,β-enones. Tetrahedron Lett. 2003, 44, 6257–6260. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Pârvulescu ,V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef]
- Ranu, B.C.; Banerjee, S. Ionic liquid as catalyst and reaction medium. The dramatic influence of a task-specific ionic liquid, [bmIm]OH, in Michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles. Org. Lett. 2005, 7, 3049–3052. [Google Scholar] [CrossRef]
- Qiao, K.; Hagiwara, H.; Yokoyama, C. Acidic ionic liquid modified silica gel as novel solid catalysts for esterification and nitration reactions. J. Mol. Catal. A Chem. 2006, 246, 65–69. [Google Scholar] [CrossRef]
- Yang, Q.W.; Wei, Z.J.; Xing, H.B.; Ren, Q.L. Brønsted acidic ionic liquids as novel catalysts for the hydrolyzation of soybean isoflavone glycosides. Catal. Commun. 2008, 9, 1307–1311. [Google Scholar] [CrossRef]
- Hao, L.; Hui, X.P.; Zhang, Z.Y.; Guan, Z.W.; He, Y.L.; Yu, H.J. Studies on semisynthesis and antibacterial activity of 3-heterocyclicthiomethyl cephalosporins. Chem. J. Chin. Univ. 1999, 20, 1564–1569. [Google Scholar]
- Li, F.; Xie, Z.F.; Liu, F.M. Syntheses and fluorescent property of 5-(2-Phenyl-1,2,3-triazoly)-3-aryl pyrazoline derivatives. Chem. J. Chin. Univ. 2006, 6, 1058–1061. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).