Abstract
This study describes the synthesis of two new families of dendrimers based on the esterification of N-alkylated 3-amine-1-propanol with two different cores, adipic acid (1st and 2nd generations) and ethylenediamine (generation 1.5), both with carboxylic acid end groups, offering a wide variety of further modifications at the periphery. According to the cytotoxic evaluation of the dendrimers and their possible degradation products within cell lines, these materials could be considered as innocuous. In preliminary studies, the synthesized dendrimers proved to be potential enhancers of solubility of highly hydrophobic drugs, like methotrexate, widely used in chemotherapy.
1. Introduction
After at least three decades of study, dendrimers remain as one of the most exciting and promising molecular architectures for a great variety of applications in important fields such as catalysis [1], alternative sources of energy [2,3], and biomedical applications for both medical diagnostics and therapeutic applications, among others [4,5,6,7,8]. In the area of drug delivery, there is a constant search of new vehicles for many drugs with serious solubility and cytotoxicity problems that make it difficult for them to reach their main targets. Recently, progress has been made in the new field of nanomedicine, where nanoscale engineered materials, such as PAMAM (polyamidoamine) dendrimers, are used in several medical applications, as well as dendritic scaffolds in biology [9,10].
In this regard, the unique architecture and functionality of dendrimers at this scale make them exceptional carrier molecule candidates for use in medical applications. Thus, the present study describes the synthesis of two new families of poly(ester-amine) dendrimers, designed to be considered as flexible non-cytotoxic nanocarriers of poorly water-soluble drugs, like methotrexate (MTX, Figure 1), widely used in chemotherapy [11].
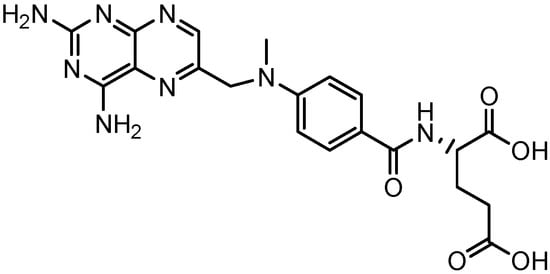
Figure 1.
Chemical structure of methotrexate (MTX).
To evaluate the cytotoxicity of the dendrimers and their possible degradation products, assays on different cell lines were performed. In addition, these new materials were also tested as enhancers of MTX solubility in water. Preliminary theoretical simulations were carried out to visualize the hydrophobic/hydrophilic balance once MTX has interacted with the dendrimers.
2. Results and Discussion
Taking into account the key features that materials with bio-applications should have, we designed and synthesized dendritic compounds addressing three important elements: i) high flexibility to enforce the encapsulation events and to improve solubility; ii) biodegradability by the presence of ester groups, since they are cleaved in the body and, at the same time, induce formation of hydrogen bonds; and iii) non-cytotoxicity by selecting carboxylic acids as end groups (pKa ~5), which should be practically deprotonated at the blood’s pH (7.4), leading to the formation of anionic entities that prevent adhesion to the walls of blood vessels by electrostatic repulsions [12]. Dendrimers with anionic components have shown to be less toxic and less hemolytic than their cationic counterparts [13,14,15,16]. To avoid acidic solutions, the carboxylic end groups can undergo an acid-base reaction with NaOH to produce the corresponding carboxylates.
The synthesis of compound 3 [17] (Scheme 1), from 3-aminopropan-1-ol (1) and tert-butyl acrylate (2) enabled us to obtain a 1st generation dendron bearing a single reactive point, a OH group, ready to be coupled via an esterification reaction to -COOH end groups of proposed cores (see Scheme 2 and Scheme 3). The reaction was allowed to proceed for 24 h, then the solvent and excess reactants were evaporated to give the product, quantitatively. The structure was corroborated by common spectroscopic techniques.
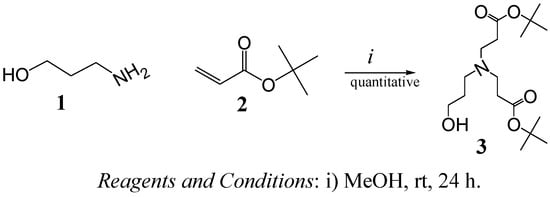
Scheme 1.
Synthesis of dendron 3.
In a convergent fashion, dendron 3 was coupled to two different cores with terminal carboxylic acids, one is adipic acid 4 (Scheme 2) and the other is compound 11 (Scheme 3) obtained from the alkylation of ethylenediamine and their subsequent hydrolysis.
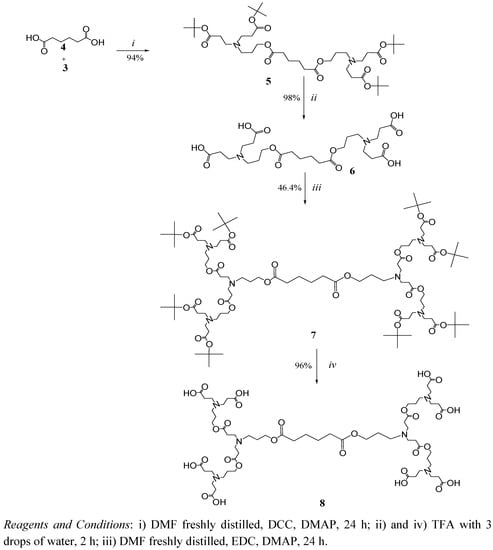
Scheme 2.
Synthesis of dendrimers with an adipic acid core
From Scheme 2, adipic acid and dendron 3 (1:6 mole ratio) were coupled in the presence of DCC/DMAP (dicyclohexylcarbodiimide/dimethylaminopyridine) to obtain compound 5 in high yield (96%) as an amber oil. The DMF solvent must be completely dried and freshly distilled; otherwise the yield drops to 30–35%. This new compound was characterized by 1H NMR, where the ratio of signals corresponding to the tert-butyl groups [36 H, 1.42 ppm (s)] was compared with those of the adipic core (8H) distributed in two groups of signals in 1.64 and 1.76 ppm. In the 13C-NMR spectrum, only those carbons related to ester groups in 171 and 173 ppm, corresponding to tert-butyl, and internal esters were observed, respectively. Finally, through FAB+ a m/z value of 773, corresponding to compound 5, was obtained.
The hydrolysis of compound 5 with trifluoroacetic acid (TFA) takes place in a selective manner to generate compound 6 (G1 dendrimer with carboxylic acid terminals) in 98% yield. The structure was corroborated by 1H-NMR, where the signal in 1.42 ppm, associated with the tert-butyl groups, had disappeared.
The DCC/DMAP coupling system was used to couple dendron 3 to compound 6 in order to obtain compound 7 (Scheme 2), like the coupling of adipic acid to dendron 3. Even though the DCC/DMAP coupling system usually gives excellent yields (86%, in this case), a serious inconvenience arose due to the presence of dicyclohexylurea (DCU), formed as by-product of the reaction with DCC, as the former was strongly encapsulated by the dendrimer (compound 7), which was confirmed by 1H-NMR (signals in 3.21 ppm, related to DCU).
Failed attempts to revert the encapsulation were made by filtration, centrifugation, and dialysis with flexible and rigid membranes; therefore, a change in the coupling system was required. EDC (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide) was chosen instead of DCC, since its urea is much more polar and more easily removed than the DCU by column chromatography; although the yield dropped to 46.4%, as shown in Scheme 2. In spite of the latter inconvenience, the strong encapsulation of highly insoluble DCU provides preliminary evidence of the ability of these compounds to enhance the solubility of hydrophobic compounds.
Thus, compound 7 was also obtained with the EDC/DMAP coupling system after chromatographic purification with ethyl acetate/NH4OH (1% v/v) as eluent. The structure was confirmed by 1H-NMR, where the ratio of signals in 1.44 and 1.65 ppm corresponds to the new relation of tert-butyl protons and the more protected methylene protons present in the structure of the dendron. Through FAB+, a m/z of 1,801 was obtained.
Compound 7 was hydrolyzed in TFA to obtain dendrimer 8 with 96% yield. This compound is highly hygroscopic and completely soluble in water, a desirable characteristic in materials with potential application as drug carriers.
On account of their chemical structure, these compounds exhibit two characteristic FT-IR bands. One, broad and weak around 3,500-2,200 cm-1 attributed to COOH end groups and another band around 1,650 cm-1 attributed to the carbonyl of the –COO-···+NHR3 salt. The formation of this kind of salts can produce duplicity in the NMR signals; therefore, the addition of aqueous HCl (drops) can be required. The hydrolyzed compound 3 shows no more duplicity of signals, hence all NMR spectra of hydrolyzed compounds were recorded after addition of a few drops of HCl (see Supplementary Materials).
A second family of dendrimers was synthesized starting from the alkylation of ethylenediamine with tert-butyl acrylate (Scheme 3) to obtain compound 10 as a white solid in quantitative yield. The structure of this compound was verified by 1H-NMR with signals at 1.44 ppm (tert-butyl groups) and a singlet at 2.53 ppm corresponding to the symmetric ethylenediamine core.
As before, the carboxylic acid end groups were obtained after the hydrolysis of compound 10 with TFA to obtain compound 11 with 99% yield as a poorly water-soluble solid. Compound 11 is less flexible and has less polar groups in comparison with compound 6, which exhibits the same number of terminal groups.
The coupling of compound 11 and dendron 3 allowed us to obtain compound 12. Its 1H-NMR reveals a singlet at 1.43 ppm corresponding to tert-butyl groups and a m/z of 1601 was found by FAB+. Compound 12 was hydrolyzed to obtain dendrimer 13 (G1.5) in 97% yield as a highly hygroscopic foamy solid. The FT-IR bands at 3,600 to 2,200 cm-1 and the disappearance of the singlet at 1.43 ppm in the NMR confirmed the hydrolysis.
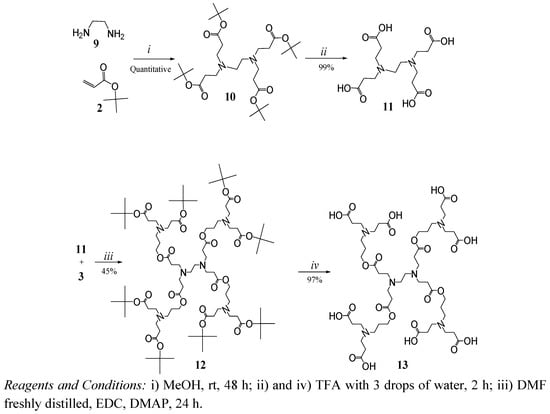
Scheme 3.
Synthesis of dendrimers with ethylenediamine core.
3. Biological Section
Since non-cytotoxicity is a necessary feature for any biomaterial, the harmlessness of dendrimers 8 and 13 and their possible degradation products (hydrolyzed compound 3, and compounds 6 and 11) was evaluated by assays on human lymphocytic cells (MT2) [18]. The results of the assays (performed in triplicate), as well as the standard error, are shown in Table 1, where a sample of polyglycidol (synthesized in our lab, see Experimental) was included as reference parameter of innocuity [20]. Although the main interest for the biological study was the verification of non-cytotoxycity of the new materials in normal cell lines, additional assays on human cancer cell lines were carried out for forthcoming studies.

Table 1.
Activity (%) of human cancer cell lines and human lymphocytic MT2 cells.a
| Compound | MT2 | U251 | PC-3 | K-563 | HCT-15 | MCF7 | SKLU-1 |
|---|---|---|---|---|---|---|---|
| 3 (hydrolyzed) | +3.4±2.0 | 10.6±1.2 | +6.6±5.0 | 6.5±1.2 | 7.8±1.4 | 24.0±1.2 | 17.9±1.3 |
| 4 | +3.2±1.5 | +13.7±2.9 | +3.7±0.8 | +12.0±1.3 | +6.5±1.0 | +11.1±6.0 | +5.1±0.5 |
| 6 | +3.0±1.5 | 1.6±1.0 | +5.2±0.2 | 15.3±5.8 | +3.5±3.3 | +21.4±5.4 | +2.3±0.8 |
| 8 | +6.1±1.0 | 9.8±0.4 | 12.6±3.6 | 7.7±0.0 | +5.9±2.0 | +10.2±2.2 | 2.4±0.4 |
| 11 | 6.7±2.2 | 19.7±5.0 | +1.7±1.0 | 11.8±2.4 | 9.9±3.3 | +9.8±4.2 | +5.4±1.3 |
| 13 | 1.3±0.6 | 11.2±0.5 | +9.6±2.1 | 1.3±0.3 | +4.4±4.0 | +6.6±1.2 | 2.1±0.1 |
| Polyglycidolb | +5.8±0.6 | 1.6±0.1 | +5.4±1.8 | 6.4±0.08 | 4.8±1.5 | 5.3±0.2 | 5.5±1.5 |
a: Cellular activity measured in presence of different compounds at 50 µM ± standard error. MT2: Human lymphocytes; Human cancer cell lines: U251 (human glyoblastoma); PC-3 (human prostatic adenocarcinoma); K562 (human chronic myelogenous leukemia cells); HCT-15 (human colorectal adenocarcinoma); MCF-7 (human mammary adenocarcinoma); SKLU-1 (human lung adenocarcinoma); b: MW = 4591; polydispersity = 1.8.
In Table 1 the + sign denotes cell growth compared with the control, which means that the compounds are non cytotoxic; and the values without + sign correspond to cell growth inhibition (%). From these results it can be observed that these new dendrimers, as well as their possible metabolites, exhibit cytotoxycity towards some cancer cell lines; however, they are harmless towards normal cells. The cytotoxycity observed for compound 11 and dendrimer 13 in normal cells is considered negligible.
Even though polyglycidol (reference compound) showed cytotoxycity in some cancer cell lines, it was also non cytotoxic towards normal cells (lymphocytes).
The performance of dendrimers 8 and 13 eventually might propitiate a synergistic effect [21] when the drug is included, since these materials already exhibit certain level of cytotoxycity toward some cancer cell lines. Concerning the possible metabolites 3 (hydrolyzed) and 11 (hydrosoluble molecules eliminable from the body), their differential effect in human glyoblastoma (U251) and human mammary adenocarcinoma (MCF-7) might be related to the selectivity of these cells, in terms of lipophilicity [22].
4. Dendrimers as Enhancers of Solubility: Preliminary Studies with MTX
MTX is an anticancer drug extensively used against breast and cervical cancer, although it is essentially insoluble in water at neutral pH [23]; therefore, it is an ideal candidate to be solubilized by dendrimers, ideally forming complexes. In order to avoid possible repulsive interactions owing to the dissociation of acid groups present in both, dendrimers and MTX, the dendrimer-drug complexes were formed in absence of water, initially grinding in a mortar the corresponding dendrimer with NaOH (equimolar amounts) to form the salt, followed by the addition of MTX to form the complex, as a paste, which was dissolved in deionized water up to 5 mL in a volumetric flask (see Experimental section). Water soluble complexes were obtained after sonication, centrifugation, and filtration of the precipitated MTX. The complexes remained stable at neutral pH during several days of storage in darkness.
Due to the very poor solubility of MTX in deionized water, our attempts to construct a calibration curve by UV-Vis at different concentrations failed, hence concentration values of MTX in the presence of dendrimers were unobtainable; however, from the measured absorbance values of dendrimer-MTX complex solutions in water in an appropriate dilution, and considering the Lambert-Beer law, it was possible to establish a ratio of concentrations to clearly show that both dendrimers, 8 and 13, significantly improve MTX solubility in water. To determine such a ratio of concentrations, it was necessary first to measure at least one absorbance value corresponding to MTX in water, which was achieved by means of the formation of a saturated solution of MTX in deionized water, and making the required dilutions to apply the Lambert-Beer law.
After a dilution of a saturated solution (0.3 mL up to 5 mL) of MTX, an absorbance (A302) of 0.457 at 302 nm was obtained. Applying Equations 1 and 2 and by algebraic treatment yields the concentration of MTX in water as a function of the constant (k):

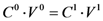

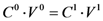
In a similar way, and making the appropriate dilutions (0.2 mL up to 5 mL), the measured absorbance values in water at 302 nm for the complexes formed between dendrimer 8 and 13 with MTX were 1.261 and 0.827 respectively.
Based on equations 1 and 2, and the maximum concentartion of MTX in water, the ratio of concentrations (the concentration of MTX in the presence of dendrimer ( 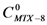 ) by the concentration of MTX in water alone (
) by the concentration of MTX in water alone ( 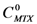 )), can be expressed as follows:
)), can be expressed as follows:

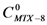 ) by the concentration of MTX in water alone (
) by the concentration of MTX in water alone ( 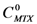 )), can be expressed as follows:
)), can be expressed as follows:

Thus, the presence of dendrimer 8 increases 4.14-times the concentration of MTX in water, while dendrimer 13 increases it 2.71-times. Figure 2 shows the UV-VIS spectra of MTX alone in water and dendrimer-MTX complexes. From Figure 2 a slight shift and best definition of maximum of MTX at 372 nm can be observed in the presence of dendrimers presumably due to dendrimer-MTX interactions via π-π * transitions.
With the experimental information described before it is possible to estimate a stoichiometry of interaction between dendrimers and MTX. Considering that the same initial concentration of each dendrimer (CD=1.7 mM) was used to form the complexes dendrimer-MTX, and comparing their performance to interact with MTX, we assumed a 1:1 stoichiometry for the dendrimer 8-MTX complex since the largest increment of MTX concentration in water is achieved with dendrimer 8.
Under this hypothesis, a 5:3 stoichiometry for dendrimer 13-MTX complex was estimated, taking into account the obtained concentration ratios by algebraic manipulation (see Experimental).
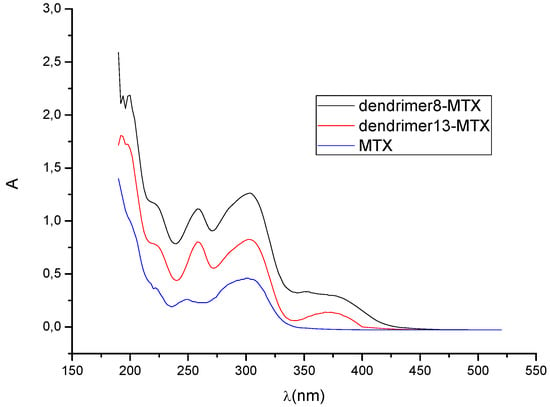
Figure 2.
UV-VIS spectra of dendrimer8-MTX and dendrimer13-MTX complexes and MTX in deionized water. The relative concentrations with regard to MTX in water are 2.71 to 13-MTX complex and 4.14 to 8-MTX complex.
Considering the estimated stoichiometries, possible conformations of the dendrimer-MTX complexes were explored by conformational search calculations in aqueous medium (continuum dielectric constant = 81). The Monte Carlo multiple minimum method (MCMM) was used [24,25], where random changes were made in torsion angles during the search.
Thus, considering, on one hand, carboxylate end groups (scenario at pH ~7), and, on the other hand, the experimentally observed complexation stoichiometry, ~1:1 and 5:3 complexes between dendrimer 8 and MTX and dendrimer 13 and MTX were constructed, respectively. From Figure 3a it can be suggested that the interaction dendrimer-drug is viable. Due to the flexible adipic core, the dendrimer exhibits a relaxed structure that surrounds the MTX and interacts with it via hydrogen bonds. In order to obtain additional information about the ability of dendrimers for isolating the hydrophobic MTX from polar environments (aqueous medium), surfaces that display hydrophobic and hydrophilic regions (in orange and blue respectively) were generated by an active-site mapping procedure that follows the Goodford algorithm [26].
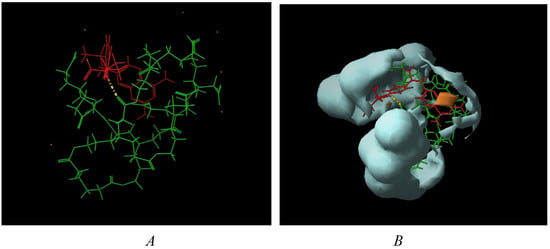
Figure 3.
(a) dendrimer 8-MTX; (b) Hydrophilic/hydrophobic balance.
In case of complex 8-MTX (Figure 3b), the drug is visibly shielded by the dendrimer, with the anionic end groups exposed to the aqueous medium, forming a hydrophilic bag with the drug inside.
In case of the less flexible dendrimer 13, the proposed complexation stoichiometry of 5:3 was minimized to obtain the conformation shown in Figure 4a, where the group of dendrimeric molecules surrounds the MTX, and several hydrogen bonds are formed.
According to the hydrophobic/hydrophilic balance, a homogeneous protection of MTX in an isolated bag formed by the dendrimeric cluster is observed (figure 4b). Thus, even when the experimental observation is the most reliable evidence of the interaction between the dendrimers and MTX, these preliminary calculations offer an illustrative rationalization of the experimental results.
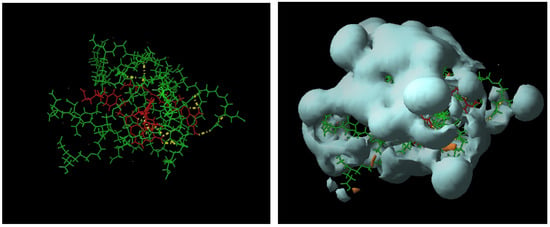
Figure 4.
(a) dendrimer 13-MTX (5:3); (b) Hydrophilic/hydrophobic balance.
5. Conclusions
The synthesis of two new families of ester-amine dendrimers constructed by esterification between 3-amine-1-propanol, previously N-alkylated, and two different cores was carried out. All dendrimers are monodisperse, highly soluble in water and both dendrimers and their metabolites, are innocuous to human cells. Studies on solubilization of MTX place these materials as potential solubility enhancers of hydrophobic molecules. According to the dendrimer: MTX ratio, estimated by UV-VIS spectra, the stoichiometry of the complexes depends on the molecular flexibility, directly related to the flexibility of the cores. Finally, the preliminary calculations to explore the conformations of the complexes dendrimer-MTX showed an effective isolation of MTX, where the hydrophilic surface generated by the dendrimers can be related with the increase of solubility observed for the MTX in aqueous media.
6. Experimental
6.1. General
Unless stated otherwise, chemicals were obtained from commercial sources and used without further purification. DMF was dried over CaH2 (5% w/v) overnight, filtered and freshly distilled at reduced pressure prior to its use. Uncorrected melting points were determined on an electrothermal 9100 Fisher and NMR spectra were recorded on a Bruker Avance 400 instrument. FT-IR spectra were measured on a Nicolet 6700 spectrometer. MS (FAB) mass spectra were measured with a JEOL AX-505 spectrometer and by ESI with a Bruker Daltonic DataAnalysis 3.2. Molecules 8, 13, MTX, and the complexes were equilibrated by conformational search in aqueous media with a continuum dielectric constant of 81 (Force Field OPLS2001) [27]), using the Monte Carlo statistical method [28] included in the Macromodel software. The algorithm of multiple minima (MCMM) [24,25] without limits on the number of variable torsions allowed in the search was used. Hydrophobic and hydrophilic surfaces were calculated using Maestro [29] with a box of 6 Å.
6.1.1. General Procedure for the Synthesis of tert-Butyl Ester Terminated Dendrimer
To an ice-cold suspension of carboxylic acid compound, a mixture of dendron (compound 3) and DMAP in dry DMF (in 12 mL), a solution of EDC (1-ethyl-3-[(3-dimethylamino)propyl]-carbodiimide) or DCC (dicyclohexilcarbodiimide) in DMF were added dropwise. The reaction mixture was stirred at 0°C for 1 h and then at room temperature, monitored by thin layer chromatography (TLC) until no more changes were visible (for these products 24 h were enough). The solvent was removed under reduced pressure to give an amber oil, which was purified by column chromatography on silica gel using ethyl acetate/ammonium hydroxide (1% v/v) as eluent.
(tert-Butyl 3-[(2-tert-butoxycarbonyl-ethyl)-(3-hydroxy-propyl)-amino]-propionate) (3). In a round-bottom flask purged with nitrogen and maintained in darkness, 3-aminopropan-1-ol (1, 5 g, 66.57 mmol) was diluted in methanol (30 mL) and then tert-butyl acrylate (2, 22.183 g, 173.08 mmol) was added. The mixture was stirred at room temperature for 24 h, the excess of tert-butyl acrylate and solvent were removed at vacuum to give a colorless oil in quantitative yield. FT-IR (cm-1): 3,413 (OH), 2,976, 2,939 (C-H aliph.), 1,723 (C=O); 1H-NMR (CDCl3) δ: 1.39 (s, 18H, -C(CH3)3), 1.64 (q, 2H, -CCH2C-), 2.35 (t, 4H, -CCH2COO-), 2.57 (t, 2H, -C(CH2)N-), 2.69 (t, 4H, -NCH2C-) ppm; 13C-NMR δ: (CDCl3, 77), 27.9 (-C(CH3)3), 28.2 (-C(CH2)2C-), 33.1 (-CCH2COO-), 49.2 (-NCH2C-), 52.7 (-C(CH2)2N-), 62.8 (HOCH2C-), 80.4(-COOC(CH3)3) and 171.6(-CCOOC-) ppm.
(4-Cascade:adipic acid [2-1,8]:(1-azabutylidyne):tert-butylpropyl ester) (5). To an ice-cold suspension of adipic acid (4, 1g, 6.84 mmol) compound 3 (13.6 g, 41.05 mmol) and DMAP (0.167 g, 1.37 mmol) dissolved in dry DMF (12 mL) and DCC (3.38 g, 16.41 mmol) in DMF (5 mL) were added in portions. When the reaction was finished the mixture was purified, as described above to give 5 (4.97 g, 94%) as an amber oil. FT-IR (cm-1): 2,973, 2,933 (C-H aliph.), 1,722 (C=O); 1H-NMR (acetone d6) δ: 1.42 (s, 36H, -C(CH3)3), 1.64 (m, 4H, -C(CH2)2C-), 1.76 (q, 4H, -CCH2C-), 2.34 (t, 12H, -CCH2COO-), 2.50 (t, 4H, -CCH2N-), 2.70 (t, 8H, -NCH2C-), 4.08 (t, 4H, -COOCH2C-) ppm; 13C-NMR δ: (acetone d6), 25 (-C(CH2)2C-), 27 (-CCH2COO-), 28 (-C(CH3)3), 34 (-(CH2)3CH2COO-), 34 (-CCH2COO-), 49.8 (-NCH2C-), 50 (-CCH2N-), 63 (-COOCH2C-), 79 (-COOC(CH3)3), 171 (-COO(CH3)3) and 173 (-CCOOC-) ppm; MS m/z (FAB+) 773.
(8-cascade:adipic acid [2-1,8]:(1-azabutylidyne):(6-oxo-5-oxa-1-azaoctylidyne):tert-butylpropyl ester) (7). To an ice-cold suspension of 6 (2.4 g, 4.39 mmol) compound 3 (11.65 g, 35.16 mmol) and DMAP (0.726 g, 5.86 mmol) in dry DMF (12 mL), and EDC (8.18 g, 52.69 mmol) in DMF (5 mL) were added in portions. When the reaction was finished, the mixture was purified as described above to give 7 (3.67 g, 46.4%) as an amber oil. FT-IR (cm-1): 2,973, 2,933 (C-H aliph.), 1,722 (C=O); 1H-NMR (MeOD) δ: 1.44 (s, 72H, -C(CH3)3), 1.65 (m, 4H, -C(CH2)2C-), 1.76 (q, 12H, -CCH2C-), 2.33 (t, 28H, -CCH2COO-), 2.50 (t, 12H-CCH2N-), 2.70 (t, 24H, -NCH2C-), 4.08 (t, 12H, -COOCH2C-). 13C (δ (ppm)): (MeOD): 24.3 (-C(CH2)2C-), 25.4 (-CCH2C-), 26.6 (-CCH2C-), 27.5 (-C(CH3)3), 32.2 (-CCH2COO-), 33.4 (-(CH2)3CH2COO-), 33.7 (-CCH2COO-), 49.6 (-NCH2C-, CCH2N-), 62.2 (-COOCH2C-), 79.3 (-COOC(CH3)3), 171.3 (-COO(CH3)3) 172.4 (-CCOOC-) ppm; MS: m/z (FAB+) 1,801 and 1,803 by ESI.
(4-Cascade:ethylenediamine[4-N,N,N´,N´]: tert-butyl propyl ester) (10). In a round-bottom flask purged with nitrogen and maintained in darkness, ethylenediamine (2 g, 33.27 mmol) was diluted in methanol (30 mL), and then tert-butyl acrylate (22.183 g, 173.08 mmol) was added. The mixture was stirred at room temperature for 24 h, the excess of tert-butyl acrylate and solvent were removed at vacuum to give a white solid in quantitative yield (19 g). p.f. 42 ºC. FT-IR (cm-1): 2,940, 2,818 (C-H aliph.); 1,715 (C=O); 1H-NMR (MeOD) δ: 1.47 (s, 36H, -C(CH3)3); 2.36 (t, 8H, -CCH2COO-); 2.53 (s, 4H, -N(CH2)2N-); 2.73 (t, 8H, -NCH2C-) ppm; 13C-NMR δ: (MeOD); 27 (-C(CH3)3); 33 (‑CCH2COO-); 55 (-N(CH2)2N-); 80 (-COOC(CH3)3); 172 (-COO(CH3)3) ppm.
(4-Cascade:ethylenediamine[4-N,N,N´,N´]:(6-oxo-5-oxa-1-azaoctylidyne):tert-butylpropyl ester) (12). To an ice-cold suspension of 11 (1.5 g, 4.3 mmol), compound 3 (11.51 g, 34.72 mmol) and DMAP (0.726 g, 5.68 mmol) in dry DMF (12 mL) and EDC (8.02 g, 51.66 mmol) in DMF (5 mL) were added in portions. When the reaction was finished, the mixture was purified as described above, to give 12 (3.1 g, 45%) as an amber oil. FT-IR (cm-1): 2,975, 2,818 (C-H aliph.) 1,723 (C=O); 1H-NMR (MeOD) δ: 1.43 (s, 72H, -C(CH3)3); 1.74 (q, 8H, -CCH2C-); 2.36 (t, 16H, -CCH2COO-); 2.48 (t, 8H, ‑CCH2COO-); 2.52 (t, 8H, -NCH2C-); 2.56 (s, 4H, -N(CH2)2N-); 2.72 (t, 16H, -NCH2C-); 2.80 (t, 8H, -CCH2N-); 4.10 (t, 8H, -COOCH2C-) ppm; 13C-NMR (MeOD) δ: 25 (-CCH2C-); 27 (-C(CH3)3); 33 (-CCH2COO-); 35 (-CCH2COO-); 48 (-NCH2C-); 49 (- NCH2C-); 50 (-CCH2N-); 55 (-N(CH2)2N-); 62 (-COOCH2C-); 80 (-COOC(CH3)3); 172 (-COO(CH3)3); 173 (-CCOOC-)ppm; MS m/z (FAB+) 1,601 and 1,603 by ESI.
6.1.2. General Procedure for the Hydrolysis of tert-Butyl Esters
tert-Butyl ester-terminated dendrimer (1 g) was dissolved in trifluoroacetic acid (TFA, 2 mL) with 3 drops of water. The hydrolysis was monitored by TLC. The TFA was evaporated under reduced pressure to give a viscous brown light oil, which was dissolved in methanol (10 mL) and evaporated under reduced pressure; this step was repeated from 3 to 5 times until no more TFA was present. As an alternative, the viscous oil can be triturated several times in hexane-dichloromethane (hex-DCM, 50/50) until no more TFA is present. Finally, the product was dried under high vacuum.
(4-Cascade:adipic acid [2-1,8]:(1-azabutylidyne):propionic acid) (6). Compound 5 (2 g, 2.58 mmol) was dissolved in TFA (4 mL). Once the general procedure was applied, the product was dried under high vacuum to give 6 (1.4 g, 98%) as a white, foamy and very hygroscopic product. FT-IR (cm-1): 2,220 to 3,600 (COOH broad and weak), 2,953 (C-H aliph), 1,738 and 1,662 (C=O); 1H-NMR (MeOH+HCl) δ: 1.66 (m, 4H, -C(CH2)2C-), 2.20 (q, 4H, -CCH2C-), 2.42 (t, 4H, -(CH2)3CH2COO -), 2.88 (t, 8H, -CCH2COO-), 3.48 (t, 8H, -NCH2C-), 3.74 (t, 4H-CCH2N-), 4.21 (t, 4H, -COOCH2C-) ppm; 13C-NMR δ: (MeOD): 23.0 (-C(CH2)2C-), 28.0 (-CCH2C-), 28.4 (-CCH2COO-), 33.0 (-(CH2)3CH2COO -), 48.5 (-NCH2C-), 49.5 (-CCH2N-), 61 (-COOCH2C-), 172 (-CCOOC-) and 171 (-CCOOH) ppm; MS m/z (ESI) 550.9.
(8-Cascade:adipic acid [2-1,8]:(1-azabutylidyne):(6-oxo-5-oxa-1-azaoctylidyne):propionic acid) (8). Compound 7 (1.5 g, 0.83 mmol) was dissolved in TFA (3 mL). Once the general procedure was applied, the product was dried under high vacuum to give 8 (1.08 g, 96%) as a white, foamy, very hygroscopic product. FT-IR (cm-1): 2,220 to 3,600 (COOH broad and weak), 2,943 (C-H aliph), 1,720 and 1,662 (C=O); 1H-NMR (MeOD) δ: 1.65 (q, 4H, -C(CH2)2C-), 1.96 (q, 12H, -CCH2C-), 2.25 (t, 4H, -OCCH2(CH2)2CH2CO-), 2.86-2.96 (m, 24H, -NCH2CH2COO-), 3.28 (m, 4H,-CCH2N-), 3.41-3.47 (2t, 24H, -NCH2C-), 3.64 (t, 8, -CCH2Nf-), 4.20 (t, 12H, -COOCH2C-)ppm; 13C-NMR δ: 25.2 (‑C(CH2)2C-), 26.7 (-OCCH2(CH2)2CH2CO-), 29.2, 34.4(-CCH2COO-), 50.8 (-NCH2C-,-CCH2N-), 60.6 (-COOCH2C-), 172.5, 175.7 (-CCOOC-) and 172.5 (-COOH) ppm; MS m/z (ESI) 1354.
(4-Cascade:ethylenediamine[4-N,N,N´,N´]: propionic acid) (11). Compound 10 (1 g, 1.89 mmol) was dissolved in TFA (2 mL). Once the general procedure was applied, the product obtained was triturated in dichlorometane-hexane (50/50) several times until compound 11 (0.66 g, 99%), a white solid, was precipitated. FT-IR (cm-1): 2,256 to 3,120 (COOH broad and weak); 2,992-3,011 (C-H aliph), 1,709, 1,651 (C=O); 1H-NMR (D2O) δ: 2.85 (t, 8H, -CCH2COOH), 3.46 (t, 8H, -NCH2C-), 3.72 (s, 4H, -N(CH2)2N-) ppm; 13C-NMR (D2O) δ: 28.3(-CCH2COO-), 47.8(-N(CH2)2N-), 50.3 (-NCH2C-) and 174.0 (-CCOOH) ppm.
(4-Cascade:ethylenediamine[4-N,N,N´,N´]:(6-oxo-5-oxa-1-azaoctylidyne):propionic acid) (13). Compound 12 (1 g, 0.62 mmol) was dissolved in TFA (2 mL). Once the general procedure was applied, the product was dried under high vacuum to give compound 13 (0.70 g, 97%) as a slightly brown, foamy, very hygroscopic product. FT-IR (cm-1): 2,220 to 3,600 (COOH broad and weak), 2,981 (C-H aliph), 1,724, 1,660 (C=O); 1H-NMR (MeOD + HCl) δ: 2.92 (q, 8H, -CCH2C-), 2.88 (t, 16H, -CCH2COO-), 2.69 (t, 8H, -CCH2COO-), 3.28 (s, 4H, -N(CH2)2N-), 3.43 (t, 8H, -NCH2C-), 3.64 (t, 16H, -NCH2C-), 3.84 (t, 8H, -CCH2N-), 4.21 (t, 8H, -COOCH2C-) ppm; 13C-NMR δ: (MeOD+HCl): 26.9 (-CCH2C-), 29.6 (-CCH2COO-), 50.7 (-CCH2N-, (-NCH2C- ), 54.2 (-N(CH2)2N-), 60.5 (-COOCH2C-), 171.9 (-CCOOC-) and 172.5 (-COOH) ppm; MS m/z (ESI) 1154.
6.1.3. Polyglycidol (Reference Compound for Biological Assays).
In a round bottom flask under N2 atmosphere, a solution of potassium methoxide (CH3OK) was prepared with potassium (0.8 g, 11.3 mmol) in previously distilled methanol (15 mL), and the solution was heated at 60 °C during 10 min. Afterwards, a solution of glycidol monomer (9.5 mL, 0.1427 mol) in methanol (5 mL) was added dropwise during 3 h. The mixture was heated at 85 °C for 24 h. Drops of 10% HCl were added to the cold solution until a pH = 7 was reached; next, the solution was filtered and evaporated under vacuum to give a yellow pale oil product. Mw = 4591, PDI = 1.8, FT-IR (cm-1) 3,261 (OH), 2,877 (CH2,CH), 1,059 and 1,127 (C-O); 1H-NMR (D2O) δ: 3.08-3.68 (m, CH, CH2), 2.8–2.85 (m, CH,CH2 oxirane ring); 13C-NMR (D2O) δ: 58.7-62.9 (CH2OH), 79.4-78.1 (C-HOR), 71.7–73.7(CH2OR) and 71.0-68.8 (CHOH) ppm.
6.2. Cell Lines Culture and Culture Medium
The dendrimers were screened in vitro (in triplicate) against human cancer cell lines: HCT-15 (human colorectal adenocarcinoma), MCF-7 (human mammary adenocarcinoma), K562 (human chronic myelogenous leukemia), U251 (human glyoblastoma), PC-3 (human prostatic adenocarcinoma), SKLU-1 (human lung adenocarcinoma), cell lines were supplied by the National Cancer Institute (USA). Besides human lymphocytes MT2 cell lines, human tumor cytotoxicity was also determined by using the protein-binding dye sulforhodamine B (SRB) in microculture assays to measure cell growth, as described in the protocols established by the NCI [30]. The cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 10,000 units/mL penicillin G sodium, 10,000 μg/mL streptomycin sulfate, 25 μg/mL amphotericin B (Gibco), and 1% non-essential amino acids (Gibco). They were maintained at 37 °C in a humidified atmosphere with 5% CO2. The viability of the cells used in the experiments exceeded 95%, as determined with trypan blue.
6.3. Cytotoxicity Assay
Cytotoxicity after treatment of the cancer cell lines and normal cells with the test compounds was determined using the protein-binding dye sulforhodamine B (SRB) in microculture assays to measure cell growth, as described in Monks et al., 1991). The cells were removed from the tissue culture flasks by treatment with trypsin, and diluted with fresh media. From these cell suspensions, 100 μL, containing 5,000—10,000 cells per well, were pipetted into 96-well microtiter plates (Costar) and the material was incubated at 37 °C for 24 h in a 5% CO2 atmosphere. Subsequently, a solution of the dendrimers obtained by diluting the stocks (100 μL) was added to each well. The cultures were exposed for 48 h to the compound at a concentration of 50 µM. After the incubation period, cells were fixed to the plastic substratum by the addition of 50 μL of cold 50% aqueous trichloroacetic acid. The plates were incubated at 4 °C for 1 h, washed with tap H2O, and air-dried. The trichloroacetic-acid-fixed cells were stained by the addition of 0.4% SRB. Free SRB solution was then removed by washing with 1% aqueous acetic-acid. The plates were then air-dried, and the bound dye was solubilized by adding 10 mM unbuffered Tris base (100 μL). The plates were placed on a shaker for 5 min, and the absorption was determined at 515 nm using an ELISA plates reader (Bio-Tex Instruments).
6.4. Formation of Dendrimer-MTX Complexes
A general procedure for complex formation involved, firstly, the salt formation of the corresponding dendrimer with NaOH (equimolar amounts), grinding in an agate mortar without solvent, followed by the addition of five equivalents of MTX to obtain a dendrimer-salt/MTX paste. This paste was dissolved in deionized water until reaching a 5 mL volume in a volumetric flask to give a water-soluble complex. The complex solution was sonicated for 20 min. and allowed to equilibrate in darkness overnight. Afterwards, the solution was centrifuged in a Cole Parmer 17250 centrifuge for 10 min. at 3400 rpm. The supernatant was filtered through an ANOTOP 25 syringe filter with a 400 nm pore size.
To determine the solubility of MTX in water, 5 mg of MTX was placed in a 5 mL volumetric flask, the solution was stirred for 30 min., sonicated for 20 min. and allowed to equilibrate in darkness overnight. Then, the solution was centrifuged for 10 min. at 3400 rpm. The supernatant was filtered through an ANOTOP 25 syringe filter with a 400 nm pore size. A dilution of 0.3 mL up to 5 mL was made to obtain a reading using the Lambert-Beer law. All data were recorded in triplicate in a UV-VIS spectrometer. The absorbance of MTX in deionized water was 0.457.
6.4.1. Dendrimer 8-MTX Complex
Compound 8 (11.7 mg, 0.866 × 10-5 mol) was ground with NaOH (2.7 mg, 6.93 × 10-5 mol) in a mortar, then MTX (25.5 mg, 5.61 × 10-5 mol) was added and ground for 20 min. This paste was dissolved in deionized water in a volumetric flask up to 5 mL. The solution was sonicated, centrifuged, and filtered according to the general procedure. In order to get a UV-VIS spectrum, a dilution of 0.2 mL up to 5 mL was made and the absorbances at 302 was recorded in triplicate. The absorbance was 1.261.
6.4.2. Dendrimer 13-MTX Complex
Compound 13 (14 mg, 0.866 × 10-5 mol) was ground with NaOH (2.7 mg, 6.93 × 10-5 mol) in an agate mortar; immediately MTX (25.5 mg, 5.61 × 10-5 mol) was added and ground for 20 min. This paste was dissolved in deionized water in a volumetric flask up to 5 mL. The solution was sonicated, centrifuged, and filtered according to the general procedure. In order to get a UV-VIS spectrum, a dilution of 0.2 mL up to 5 mL was made and the absorbances at 302 was recorded in triplicate. The absorbance was 0.827.
6.4.3. Calculation of Complex Stoichiometry
Starting from 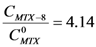 ; isolating CMTX-8 and dividing by CD, we have:
; isolating CMTX-8 and dividing by CD, we have: 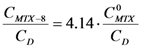 . If
. If 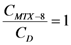 and CD = 1.7 mM,
and CD = 1.7 mM, 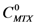 will be:
will be: 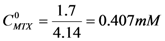 . With this result and evoking the concentration ratio for dendrimer 13, we have:
. With this result and evoking the concentration ratio for dendrimer 13, we have:
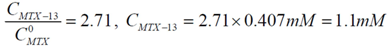
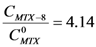 ; isolating CMTX-8 and dividing by CD, we have:
; isolating CMTX-8 and dividing by CD, we have: 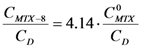 . If
. If 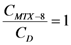 and CD = 1.7 mM,
and CD = 1.7 mM, 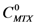 will be:
will be: 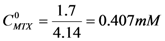 . With this result and evoking the concentration ratio for dendrimer 13, we have:
. With this result and evoking the concentration ratio for dendrimer 13, we have:
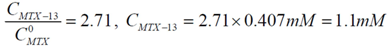
Thus, an estimated stoichiometry of 5:3 for dendrimer13-MTX complex can be obtained, multiplying and dividing by 3 to get a integer numbers, the follow expression:
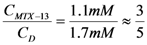
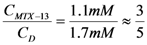
Supplementary Materials
Supplementary materials can be seen at http://www.mdpi.com/1420-3049/15/11/8082/s1.
Acknowledgments
This research was carried out with the support of Grants 81924 from CONACyT, IN101109 from PAPIIT and 4316 from ICyTDF. The authors kindly thank Gerardo Cedillo Valverde and Carmen Márquez for their technical assistance.
References
- Astruc, D.; Chardac, F. Dendritic catalysts and dendrimers in catalysis. Chem. Rev. 2001, 101, 2991–3031. [Google Scholar] [CrossRef]
- Segura, J.L.; Giacalone, F.; Gómez, R.; Martín, N.; Guldi, D.M.; Luo, C.; Swartz, A.; Riedel, I.; Chirvase, D.; Parisi, J.; Dyakonov, V.; Sariciftci, N.S.; Padinger, F. Design, synthesis and photovoltaic properties of [60]fullerene based molecular materials. Mater. Sci. Eng. C 2005, 25, 835–842. [Google Scholar] [CrossRef]
- Ramos, E.; Guadarrama, P.; Terán, G.; Fomine, S. Push–pull hyperbranched molecules. A theoretical study. J. Phys. Org. Chem. 2009, 22, 9–16. [Google Scholar]
- Stiriba, S.E.; Frey, H.; Haag, R. Dendritic polymers in biomedical applications: from potential to clinical use in diagnostics and therapy. Angew. Chem. Int. Ed. 2002, 41, 1329–1334. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nature Biotech. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Halkes, S.B.A.; Vrasidas, I.; Rooijer, G.R.; Van den Berg, A.J.J.; Liskamp, R.M.J.; Pieters, R.J. Synthesis and biological activity of polygalloyl-dendrimers as stable tannic acid mimics. Bioorg. Med. Chem. Lett. 2002, 12, 1567–1570. [Google Scholar] [CrossRef]
- Aulenta, F.; Hayes, W.; Rannard, S. Dendrimers: a new class of nanoscopic containers and delivery devices. Eur. Polym. J. 2003, 39, 1741–1771. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hal, M.; Kallos, G.; Martin, S. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. (Tokyo) 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Bertino, J.R.; Cronstein, B.N. Methotrexate (Milestones in Drug Therapy); Birkhäuser Verlag AG: Basel, Switzerland, 2000. [Google Scholar]
- Fernandez, L.; Calderón, M.; Martinelli, M.; Strumia, M.; Cerecetto, H.; González, M.; Silber, J.J.; Santo, M.J. Evaluation of a new dendrimeric structure as prospective drugs carrier for intravenous administration of antichagasic active compounds. Phys. Org. Chem. 2008, 21, 1079–1085. [Google Scholar] [CrossRef]
- Morgan, M.T.; Carnahan, M.A.; Immoos, C.E.; Ribeiro, A.A.; Finkelstein, S.; Lee, S.J.; Grinstaff, M.W. Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 2003, 125, 15485–15489. [Google Scholar]
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.; Szoka Jr, F.C. Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjugate Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weenerc, J.W.; Meijerc, E.W.; Paulusd, W.; Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125i-labelled polyamidoamine dendrimers in vivo. J. Control. Rel. 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Fuchs, S.; Kapp, T.; Otto, H.; Schoneberg, T.; Franke, P.; Gust, R.; Schluter, A.D. A set of surface-modified dendrimers with potential application as drug delivery vehicles: Synthesis, in vitro cytotoxicity, and intracellular localization. Chem. Eur. J. 2004, 10, 1167–1192. [Google Scholar] [CrossRef]
- Krishna, T.R.; Jain, S.; Tatu, U.S.; Jayaraman, N. Synthesis of water soluble and non-toxic poly(ether imine) dendrimers. Tetrahedron 2005, 61, 4281–4288. [Google Scholar] [CrossRef]
- Griffin, C.; Sharda, N.; Sood, D.; Nair, J.; McNulty, J.; Pandey, S. Selective cytotoxicity of pancratistatin-related natural Amaryllidaceae alkaloids: Evaluation of the activity of two new compounds. Canc. Cell Int. 2007, 7, 10. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Hester, S.R.; Levin, E.; Devine, D.V.; Brooks, D.E. In vitro Biological Evaluation of High Molecular Weight Hyperbranched Polyglycerols. Biomaterials 2007, 28, 4581–4590. [Google Scholar]
- Kainthan, R.K.; Brooks, D.E. In vivo biological evaluation of high molecular weight hyperbranched polyglycerols. Biomaterials 2007, 28, 4779–4787. [Google Scholar] [CrossRef]
- Barkin, R.L. Acetaminophen, aspirin, or ibuprofen in combination analgesic products. Am. J. Ther. 2001, 8, 433–442. [Google Scholar] [CrossRef]
- Saha, P.; Kim, K.J.; Yamahara, H.; Crandall, E.D.; Lee, V.H.L. Influence of lipophilicity on beta-blocker permeation across rat alveolar epithelial cell monolayers. J. Control. Release 1994, 32, 191–200. [Google Scholar] [CrossRef]
- Alvarez-Figueroa, M.J.; Delgado-Charro, M.B.; Blanco-Méndez, J. Passive and iontophoretic transdermal penetration of methotrexate. Int. J. Pharm. 2001, 212, 101–107. [Google Scholar] [CrossRef]
- Chang, G.; Guida, W.C.; Still, W.C. An internal coordinate monte carlo method for searching conformational space. J. Am. Chem. Soc. 1989, 111, 4379–4386. [Google Scholar] [CrossRef]
- Saunders, M.; Houk, K.N.; Wu, Y.D.; Still, W.C.; Lipton, M.; Chang, G.; Guida, W.C. Conformations of cycloheptadecane. a comparison of methods for conformational searching. J. Am. Chem. Soc. 1990, 112, 1419–1427. [Google Scholar]
- Goodford, P. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 1985, 28, 849–857. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the opls all-atom force field on conformational energetics and properties of organic liquid. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Kolossvary, I.; Guida, W.C. Low Mode Search. An efficient, automated computational method for conformational analisys: Application to cyclic and acyclic alkanes and cyclic peptides. J. Am. Chem. Soc. 1996, 118, 5011–5019. [Google Scholar] [CrossRef]
- Maestro, version 6.5.007, Schrödinger Inc.: New York, NY, USA, 2004.
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paul, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; Gray-Goodrich, M.; Campbell, H.; Mayo, J.; Boyd, M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. JNCI 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).