Abstract
A series of novel 1-[(2,6-dichloro-4-trifluoromethyl)phenyl]-3-aryl-1H-pyrazole-4-carbaldehydes 6a-i were synthesized using the Vilsmeier-Haack reagent. The structures of all the title compounds have been confirmed by elemental analysis, 1H-NMR and 13C-NMR and in addition, the structure of intermediate 5b was investigated by X-ray crystallography.
1. Introduction
The application of the Vilsmeier-Haack (VH) reagent (POCl3/DMF) for formylation of a variety of both aromatic and heteroaromatic substrates is well documented [1]. Besides this, the reagent has also been extensively used for effecting various chemical transformations with other classes of compounds. Many of these reactions have led to novel and convenient routes for the synthesis of various heterocyclic compounds [2]. A notable example that finds significant application in heterocyclic chemistry is the synthesis of 4-formylpyrazoles from the double formylation of hydrazones with the VH reagent [3,4]. These observations, coupled with the recent developments in the simple synthesis of pyrazole derivatives [5], especially 4-functionalized 1,3-diphenylpyrazoles as antibacterial [6], anti-inflammatory [7], antiparasitic [8], and antidiabetic drugs [9], prompted us to undertake the synthesis of pyrazole-4-carbaldehyde derivatives using the VH reagent.
It has been known for some time that fluorine atoms can lead to unexpected biological activity results due to the special properties of the fluorine atom, such as the high electronegativity of fluorine and the high carbon-fluorine bond energy [10]. As a consequence, trifluoromethyl-containing molecules have been found considerable utilization in the agrochemical and pharmaceutical industries [10,11,12], for example, pyrazole derivatives bearing trifluoromethyl groups, such as fipronil and analogs [13,14] are widely used phenylpyrazole insecticides applied in granular or bait form for residential and commercial control of turf grass pests, and celecoxib [15], widely prescribed to treat acute or chronic inflammation by providing symptomatic pain relief, are examples of such compounds. In this paper, we show a simple and efficient synthetic method using the VH reagent [4,16,17] that affords a series of novel 1-[(2,6-dichloro-4-trifluoromethyl)phenyl]-3-aryl-1H-pyrazole-4-carbaldehydes.
2. Results and Discussion
Phenylhydrazine 3 was synthesized from 2,6-dichloro-4-trifluoromethylphenylamine (1) through diazotization, reduction and hydration (Scheme 1). The reducing reagent plays an important role in the reaction. When the intermediate 2 was reduced by SnCl2, an almost quantitative yield of product was obtained. Use of Na2S2O3 or Zn as reducing reagents led to lower yields.
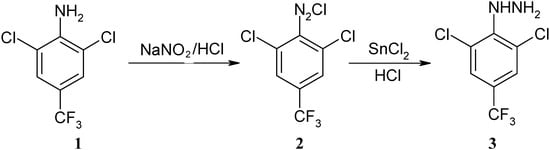
Scheme 1.
Synthesis of phenylhydrazine 3.
Phenylhydrazones 5 were next synthesized in almost quantitative yields by the reaction between the phenylhydrazine 3 and 3 or 4-substituted aryl methyl ketones, regardless of whether the ketones contained electron-withdrawing or electron-donating groups (Scheme 2).
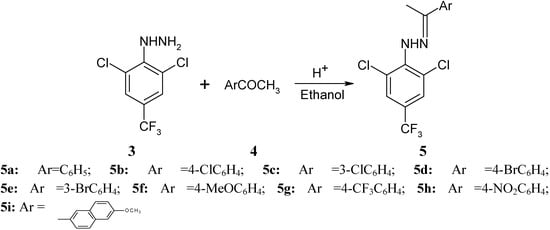
Scheme 2.
Synthesis of phenylhydrazones 5.
X-ray diffraction
To verify the structural assignment compound 5b was selected as an example for an X-ray diffraction study (Figure 1 and Table 1). The purified product 5b was dissolved in 50 % ethanol/acetone (1:1 v/v) and kept at room temperature for 5 days and single crystals of 5b were thus formed. CCDC 685556 contains the supplementary crystallographic data for this comound [18].
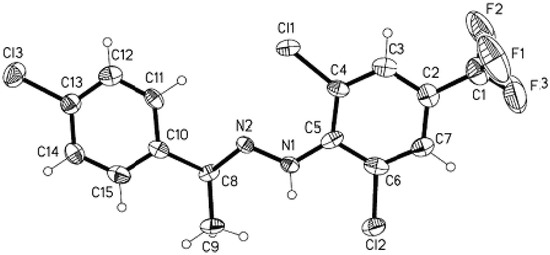
Figure 1.
ORTEP drawing of the compound 5b showing the atom numbering scheme.

Table 1.
Crystal data and summary of data collection and structure refinement.
| Compound | C15 H10 Cl3 F3 N2 |
|---|---|
| Color | Colorless |
| Formula weight | 381.60 |
| Crystal system | Monoclinic |
| Temperature,° | 25(298K) |
| Cell constants | |
| a (Å) | 16.8770(7) |
| b (Å) | 8.0054(8) |
| c (Å) | 24.119(2) |
| α (˚) | 90 |
| β (˚) | 99.654(2) |
| γ (˚) | 90 |
| Volume (Å3) | 3212.5(4) |
| Formula units | 8 |
| Calculated density (g/cm-3) | 1.578 |
| F(000) | 1536 |
| Absorption coefficient, mμ-3 | 0.599 |
| Limiting indices | -20<=h<=16, -7<=k<=9, -27<=l<=28 |
| Reflections collected / unique | 8110 / 2841 [R(int) = 0.0402] |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9370 and 0.8314 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 2841 / 0 / 208 |
| Goodness-of-fit on F2 | 1.015 |
| Final R indices | R1 = 0.1641, wR2 = 0.5152 |
| Largest diff. peak and hole (e Å-3) | 0.869 and -0.919 |
The Vilsmeier cyclization of hydrazones could provide an efficient route for the preparation of 1H -pyrazole-4-carbaldehydes. The reaction was carried out at 80–90 °C for 4 h using 3 equiv. of the VH reagent, affording a series of novel 1-[(2,6-dichloro-4-trifluoromethyl)phenyl]-3-aryl-1H-pyrazole-4-carbaldehydes in good yields (Scheme 3). The results are shown in Table 2. 1H-Pyrazole-4-carbaldehydes 6 cannot be obtained if the DMF used is not anhydrous.
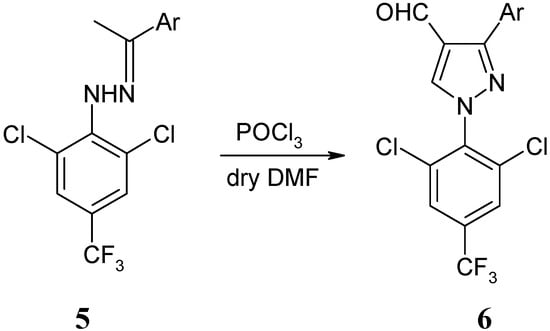
Scheme 3.
Synthesis of 1H-pyrazole-4-carbaldehydes 6.

Table 2.
Synthesis of 1H–pyrazole-4-carbaldehydes.
| Entry | Ar | Products(6) | Yield(%) a |
| 1 | C6H5 | 6a | 89 |
| 2 | 4-ClC6H4 | 6b | 88 |
| 3 | 3-ClC6H4 | 6c | 83 |
| 4 | 4-BrC6H4 | 6d | 86 |
| 5 | 3-BrC6H4 | 6e | 82 |
| 6 | 4-MeOC6H4 | 6f | 83 |
| 7 | 4-CF3C6H4 | 6g | 86 |
| 8 | 4-NO2C6H4 | 6h | 85 |
| 9 | 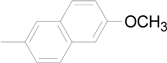 | 6i | 81 |
a Isolated yields with regard to the quantity of hydrazones 5
3. Experimental
3.1. General
All melting points were determined on an XT-4A apparatus and are uncorrected. TLC was performed using precoated silica gel GF254 (0.25mm), column chromatography was performed using silica gel (200–300 mesh). The 1H- and 13C-NMR spectra were measured at 25 °C at 300 and 75 MHz, respectively, on a Bruker Advance 300 spectrometer, using TMS as internal standard. J-values are given in Hz. The IR spectra were taken on a Bruker Vector 55 spectrometer. Elemental analyses were carried out with an EA 1112 elemental analyzer. All the reagents used were AR grade.
3.2. Synthesis of (2,6-Dichloro-4-trifluoromethyl)phenylhydrazine (3)
2,6-Dichloro-4-trifluoromethylphenylamine (1, 1.73 g, 7.5 mmol) was dissolved in concentrated HCl (10 mL) and water (10 mL) and cooled to 0 °C, sodium nitrite (0.62 g, 9.0 mmol) was added and the yellow solution was stirred at 0 °C for 2 h to get a solution of diazotizated compound 2. SnCl2 (2.85 g, 15 mmol) was dissolved in concentrated HCl (10 mL) and cooled to 7 °C, then the diazotizated solution of 2 was added slowly dropwise. After 1 h of reaction, the precipitate was filtered, washed with water and air-dried. The precipitate was neutralized by 25% NaOH in water to pH 8, the light yellow sediment was collected and air-dried to afford a brown solid 3 (1.53 g, 83%), m.p. 65–67 °C. 1H-NMR (CDCl3) δ: 7.52 (s, 2H, Ar-H), 5.85 (s, 1H, NH), 3.86 (br, 2H, NH2); 13C- NMR (CDCl3) δ: 137.1, 132.5 (q, J = 33.8 Hz), 130.3, 123.8, 122.3 (q, J = 271.8 Hz); IR (KBr) ν: 3517, 3418, 1622, 1583, 1492, 1329, 1281, 1144, 883 cm-1.
3.3. General procedure for synthesis of N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-(1-phenyl-ethylidene) hydrazines 5a-i
To a solution of compound 3 (12 mmol) in absolute C2H5OH (30 mL) was added substituted acetophenone 4 (10 mmol). Two drops of concentrated HCl were added to a stirred solution, and the mixture was refluxed for 1 h. The solid obtained on cooling was filtered, dried and recrystallized from C2H5OH to give compounds 5a-i.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-(1-phenylethylidene)hydrazine (5a): White solid, m.p. 90–91 °C; 1H-NMR (CDCl3) δ: 7.82 (d, J = 3.6 Hz, 2H, N’-Ar-H), 7.67 (s, 1H, NH), 7.58 (s, 2H, N-Ar-H), 7.34–7.42 (m, 3H, N’-Ar-H), 2.35 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 147.2, 140.8, 136.1, 132.5 (q, J = 33.8 Hz), 127.3, 126.8, 126.0, 125.8, 123.8, 122.3 (q, J = 271.8 Hz), 11.7; IR (KBr) ν: 3350, 3072, 2927, 1603, 1538, 1492, 1390, 1351, 1311, 1114, 1009, 887 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(4-chlorophenyl)ethylidene]hydrazine (5b): White solid, m.p. 118–120 °C; 1H-NMR (CDCl3) δ: 7.68–7.76 (m, 3H, N’-Ar-H and NH), 7.58 (s, 2H, N-Ar-H), 7.34–7.37 (m, 2H, N’-Ar-H), 2.33 (s, 3H, CH3); 13C NMR (CDCl3) δ: 146.1, 140.7, 136.3, 134.5 (q, J = 33.5 Hz), 128.3, 126.7, 126.2, 126.0, 123.9, 121.8 (q, J = 271.8 Hz), 11.8; IR (KBr) ν: 3360, 3070, 2924, 1605, 1532, 1490, 1392, 1352, 1303, 1114, 1004, 884 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(3-chlorophenyl)ethylidene]hydrazine (5c): White solid, m.p. 118–120 °C; 1H-NMR (CDCl3) δ: 7.83 (s, 1H, N’-Ar-H), 7.71–7.76 (m, 2H, N’-Ar-H and NH), 7.58 (s, 2H, N-Ar-H), 7.36–7.40 (m, 2H, N’-Ar-H), 2.32 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 146.1, 140.7, 136.3, 133.5 (q, J = 33.3 Hz), 129.2, 128.3, 127.5, 126.7, 126.1, 125.6, 123.9, 122.1 (q, J = 271.8 Hz), 12.1; IR (KBr) ν: 3358, 3073, 2926, 1603, 1536, 1489, 1390, 1352, 1313, 1108, 1011, 886 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(4-bromophenyl)-ethylidene]hydrazine (5d): White solid, m.p. 114–115 °C; 1H-NMR (CDCl3) δ: 7.65–7.69 (m, 3H, N’-Ar-H and NH), 7.58 (s, 2H, N-Ar-H), 7.48–7.52 (m, 2H, N’-Ar-H), 2.33 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 146.5, 141.7, 134.3, 132.5 (q, J = 33.5 Hz), 127.6, 127.1, 126.7, 125.6, 123.9, 121.9 (q, J = 271.8 Hz), 11.7; IR (KBr) ν: 3362, 3074, 2928, 1606, 1540, 1495, 1393, 1354, 1308, 1106, 1012, 887 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(3-bromophenyl)ethylidene]hydrazine (5e): White solid, m.p. 114–115 °C; 1H-NMR (CDCl3) δ: 7.94 (s, 1H, N’-Ar-H), 7.70–7.75 (m, 2H, N’-Ar-H and NH), 7.58 (s, 2H, N-Ar-H), 7.46–7.49 (m, 2H, N’-Ar-H), 2.32 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 147.1, 141.9, 139.3, 132.3 (q, J = 33.5 Hz), 128.3, 127.6, 127.1, 126.7, 126.0, 124.6, 123.9, 121.9 (q, J = 271.8 Hz), 11.9; IR (KBr) ν: 3360, 3072, 2924, 1600, 1538, 1492, 1390, 1356, 1307, 1115, 1019, 889 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(4-methoxyphenyl)ethylidene]-hydrazine (5f): White solid, m.p. 89–90 °C; 1H-NMR (CDCl3) δ: 7.81 (s, 1H, N’-Ar-H), 7.79 (s, 1H, N’-Ar-H), 7.67 (s, 1H, NH), 7.58 (m, 2H, N-Ar-H), 6.73–6.89 (m, 2H, N’-Ar-H), 3.83 (s, 3H, OCH3), 2.32 (s, 3H, CH3). 13C-NMR (CDCl3) δ: 158.1, 147.5, 136.2, 133.6 (q, J = 33.6 Hz), 127.8, 126.2, 125.6, 124.7, 121.9 (q, J = 270.2 Hz), 114.2, 55.1, 11.7; IR (KBr) ν: 3355, 3072, 2925, 1603, 1530, 1489, 1391, 1351, 1307, 1112, 1016, 887 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(4-trifluoromethylphenyl)-ethylidene]hydrazine (5g): White solid, m.p. 165–166 °C; 1H-NMR (CDCl3) δ: 7.91 (d, J = 8.4 Hz, 2H, N’-Ar-H), 7.78 (s, 1H, NH), 7.59–7.65 (s, 4H, N’ and N-Ar-H), 2.36 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 145.5, 144.7, 133.5 (q, J = 32.6 Hz), 130.4 (q, J = 32.1 Hz), 126.3, 125.8, 125.2, 124.6, 124.4, 123.2 (q, J = 270.2 Hz), 121.8 (q, J = 272.6 Hz), 11.9; IR (KBr) ν: 3351, 3070, 2926, 1601, 1535, 1490, 1389, 1350, 1313, 1116, 1021, 889 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(4-nitrophenyl)ethylidene]hydrazine (5h): Yellowish solid, m.p. 146–147 °C; 1H-NMR (CDCl3) δ: 8.24 (d, J = 6.0 Hz, 2H, N’-Ar-H), 7.88–7.97 (m, 3H, N’-Ar-H and NH), 7.60 (s, 2H, N-Ar-H), 2.38 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 147.5, 144.3, 143.9, 140.4, 132.8 (q, J = 33.6 Hz), 126.5, 125.1, 124.7, 123.6, 120.9 (q, J = 272.6 Hz), 11.8; IR (KBr) ν: 3320, 3073, 2926, 1583, 1513, 1393, 1339, 1307, 1117, 890 cm-1.
N-(2,6-Dichloro-4-trifluoromethyl)phenyl-N’-[1-(6-methoxy-naphthalen-2-yl)-ethylidene]hydrazine (5i): White solid, m.p. 191–193 °C; 1H-NMR (CDCl3) δ: 8.11 (d, J = 6.2 Hz, 1H, N’-Ar-H), 8.00 (s, 1H, N’-Ar-H), 7.71–7.78 (m, 3H, N’-Ar-H and NH), 7.58 (s, 2H, N-Ar-H), 7.14-7.17 (m, 2H, N’-Ar-H), 3.84 (s, 3H, OCH3), 2.43 (s, 3H, CH3); 13C-NMR (CDCl3) δ: 157.9, 147.5, 134.6, 133.2 (q, J = 32.5 Hz), 129.6, 128.2, 126.7, 126.2, 126.1, 125.3, 124.8, 123.8, 123.7, 121.7 (q, J = 271.6 Hz), 118.7, 105.5, 55.0, 11.7; IR (KBr) ν: 3353, 3069, 2938, 1602, 1528, 1487, 1390, 1346, 1305, 1127, 1026, 889 cm-1.
3.4. General procedure for synthesis of 1-[(2,6-dichloro-4-trifluoromethyl)phenyl]-3-aryl-1H-pyrazole-4- carbaldehydes 6a-i
POCl3 (0.5 g, 3.0 mmol) was added dropwise to an ice-cold stirred solution of hydrazone 5 (1.0 mmol) in dry DMF (4 mL), the reaction mixture was allowed to attain room temperature and then heated at 80 °C for 4 h. The resulting mixture was poured onto crushed ice, neutralized with dilute sodium hydroxide and left standing overnight. The pale yellow precipitate obtained was purified by flash column chromatography with ethyl acetate–petroleum ether mixture to yield the products 6a-i.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-phenyl-1H-pyrazole-4-carbaldehyde (6a): White solid, m.p. 147–149 °C; 1H-NMR (CDCl3) δ: 10.11 (s, 1H, CHO), 8.22 (s, 1H, pyrazole H), 7.79–7.83 (m, 4H, 1 and 3-Ar-H), 7.27–7.54 (m, 3H, 3-Ar-H); 13C-NMR (CDCl3) δ: 182.4, 153.8, 137.8, 135.0, 133.9 (q, J = 33.8 Hz), 129.0, 126.1, 125.8, 125.3, 124.6, 123.7, 122.6, 121.9 (q, J = 271.8 Hz); IR (KBr) ν: 3107, 3062, 2843, 1695, 1602, 1531, 1403, 1310, 1131, 850, 719 cm-1; Anal. Calcd. for C17H9Cl2F3N2O: C, 53.01; H, 2.36; N, 7.27. Found: C, 53.30; H, 2.45; N, 7.33%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(4-chlorophenyl)-1H-pyrazole-4-carbaldehyde (6b): White solid, m.p. 108–110 °C; 1H-NMR (CDCl3) δ: 10.08 (s, 1H, CHO), 8.22 (s, 1H, pyrazole H), 7.79–7.83 (m, 4H, 1 and 3-Ar-H), 7.45–7.48 (m, 2H, 3-Ar-H); 13C-NMR (CDCl3) δ: 183.6, 152.8, 138.8, 136.0, 133.8 (q, J = 33.8 Hz), 129.1, 128.5, 126.1, 125.8, 125.3, 123.7, 122.6, 121.9 (q, J = 271.8 Hz); IR (KBr) ν: 3107, 3060, 2845, 1695, 1600, 1529, 1401, 1314, 1130, 858, 719 cm-1; Anal. Calcd. for C17H8Cl3F3N2O: C, 48.66; H, 1.92; N, 6.68. Found: C, 48.77; H, 2.03; N, 6.60%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(3-chlorophenyl)-1H-pyrazole-4-carbaldehyde (6c): White solid, m.p. 105–106 °C; 1H-NMR (CDCl3) δ: 10.09 (s, 1H, CHO), 8.21 (s, 1H, pyrazole H), 7.83–7.91 (m, 3H, 1 and 3-Ar-H), 7.31–7.62 (m, 3H, 3-Ar-H); 13C-NMR (CDCl3) δ: 183.6, 152.8, 137.8, 136.0, 159.6, 133.8 (q, J = 33.8 Hz), 129.1, 128.5, 128.1, 126.1, 125.8, 125.2, 123.6, 122.7, 121.8 (q, J = 271.8 Hz); IR (KBr) ν: 3106, 3062, 2840, 1696, 1602, 1530, 1403, 1310, 1134, 856, 719 cm-1; Anal. Calcd. for C17H8Cl3F3N2O: C, 48.66; H, 1.92; N, 6.68. Found: C, 48.81; H, 1.90; N, 6.80%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(4-bromophenyl)-1H-pyrazole-4-carbaldehyde (6d): White solid, m.p. 116–118 °C; 1H-NMR (CDCl3) δ: 10.08 (s, 1H, CHO), 8.22 (s, 1H, pyrazole H), 7.74–7.79 (m, 4H, 1 and 3-Ar-H), 7.61–7.64 (m, 2H, 3-Ar-H); 13C-NMR (CDCl3) δ: 183.6, 152.8, 138.8, 136.0, 134.6, 133.8 (q, J = 33.8 Hz), 129.1, 128.5, 125.8, 125.3, 123.5, 122.6, 121.9 (q, J = 271.8 Hz); IR (KBr) ν: 3108, 3063, 2841, 1697, 1604, 1532, 1407, 1315, 1131, 858, 719 cm-1; Anal. Calcd. for C17H8BrCl2F3N2O: C, 44.00; H, 1.74; N, 6.04. Found: C, 43.87; H, 1.79; N, 5.96%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(3-bromophenyl)-1H-pyrazole-4-carbaldehyde (6e): White solid, m.p. 110–112 °C; 1H-NMR (CDCl3) δ: 10.09 (s, 1H, CHO), 8.22 (s, 1H, pyrazole H), 7.79–7.83 (m, 4H, 1 and 3-Ar-H), 7.59–7.62 (m, 1H, 3-Ar-H), 7.35–7.38 (m, 1H, 3-Ar-H); 13C-NMR (CDCl3) δ: 182.9, 153.8, 139.0, 136.7, 134.2, 133.8 (q, J = 33.8 Hz), 129.1, 128.5, 126.1, 125.9, 125.3, 123.7, 123.3, 122.6, 121.8 (q, J = 271.8 Hz); IR (KBr) ν: 3106, 3062, 2840, 1695, 1602, 1530, 1401, 1313, 1132, 856, 719 cm-1; Anal. Calcd. for C17H8BrCl2F3N2O: C, 44.00; H, 1.74; N, 6.04. Found: C, 43.70; H, 1.83; N, 6.36%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(4-methoxyphenyl)-1H-pyrazole-4-carbaldehyde (6f): White solid, m.p. 75–77 °C; 1H-NMR (CDCl3) δ: 10.08 (s, 1H, CHO), 8.20 (s, 1H, pyrazole H), 7.76–7.79 (m, 4H, 1 and 3-Ar-H), 7.00–7.03 (m, 2H, 3-Ar-H), 3.87 (s, 3H, OCH3); 13C-NMR (CDCl3) δ: 183.2, 160.4, 154.5, 136.1, 134.9, 133.8 (q, J = 33.8 Hz), 130.0, 128.2, 125.8, 125.7, 122.8, 121.9 (q, J = 271.6 Hz), 113.9, 55.0; IR (KBr) ν: 3110, 3060, 2963, 2850, 1695, 1600, 1530, 1403, 1312, 1130, 858, 719 cm-1; Anal. Calcd. for C18H11Cl2F3N2O2: C, 52.07; H, 2.67; N, 6.75. Found: C, 52.10; H, 2.65; N, 6.78%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(4-trifluoromethylphenyl)-1H-pyrazole-4-carbaldehyde (6g): White solid, m.p. 88–90 °C; 1H-NMR (CDCl3) δ: 10.11(s, 1H, CHO), 8.25 (s, 1H, pyrazole H), 8.01–8.03 (m, 2H, 3-Ar-H), 7.74–7.83 (m, 4H, 1 and 3-Ar-H); 13C-NMR (CDCl3) δ: 183.2, 151.8, 148.3, 139.1, 137.8, 136.9, 135.0, 133.9 (q, J = 33.8 Hz), 129.8 (q, J = 32.6 Hz), 129.0, 126.1, 123.7 (q, J = 270.1 Hz), 122.6, 121.9 (q, J = 271.8 Hz); IR (KBr) ν: 3110, 3060, 2842, 1695, 1600, 1528, 1400, 1313, 1130, 858, 719 cm-1; Anal. Calcd. for C18H8Cl2F6N2O: C, 47.71; H, 1.78; N, 6.18. Found: C, 47.84; H, 1.85; N, 6.23%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(4-nitrophenyl)-1H-pyrazole-4-carbaldehyde (6h): Yellowish solid, m.p. 206–208 °C; 1H-NMR (CDCl3) δ: 10.12 (s, 1H, CHO), 8.33–8.36 (m, 2H, 3-Ar-H), 8.27(s, 1H, pyrazole H), 8.13–8.15 (m, 2H, 3-Ar-H), 7.82 (s, 2H, 1-Ar-H); 13C-NMR (CDCl3) δ: 183.2, 151.8, 148.3, 139.1, 137.8, 136.9, 135.0, 133.8 (q, J = 33.8 Hz), 129.6, 126.1, 123.7, 122.6, 121.9 (q, J = 271.6 Hz); IR (KBr) ν: 3106, 3062, 2843, 1695, 1602, 1530, 1403, 1310, 1132, 858, 719 cm-1; Anal. Calcd. for C17H8Cl2F3N3O3: C, 47.47; H, 1.87; N, 9.77. Found: C, 47.56; H, 1.93; N, 9.62%.
1-[(2,6-Dichloro-4-trifluoromethyl)phenyl]-3-(6-methoxy-naphthalen-2-yl)-1H-pyrazole-4-carb-aldehyde (6i): White solid, m.p. 144–146 °C; 1H-NMR (CDCl3) δ: 10.19(s, 1H, CHO), 8.25 (s, 1H, pyrazole H), 7.80–7.92 (m, 5H, 1 and 3-Ar-H), 7.19–7.23 (m, 3H, 3-Ar-H), 3.94 (s, 3H, OCH3); 13C- NMR (CDCl3) δ: 183.2, 158.2, 155.0, 136.4, 134.9, 134.7, 133.8 (q, J = 33.8 Hz), 129.7, 128.3, 128.2, 127.0, 126.4, 125.8, 125.4, 123.5, 122.1, 121.9 (q, J = 271.6 Hz), 119.2, 105.4, 55.0; IR (KBr) ν: 3108, 3062, 2963, 2840, 1695, 1600, 1528, 1400, 1313, 1130, 858, 719 cm-1; Anal. Calcd. for C22H13Cl2F3N2O2: C, 56.79; H, 2.82; N, 6.02. Found: C, 56.65; H, 2.93; N, 6.15%.
4. Conclusions
In conclusion, The Vilsmeier cyclization of hydrazones provided an efficient route for the preparation of 1H-pyrazole-4-carbaldehydes, and we have successfully developed a general method for the synthesis in good yields of a series of 1-[(2,6-dichloro-4-trifluoromethyl)phenyl]-3-aryl-1H-pyrazole-4-carbaldehydes 6a-i using the Vilsmeier-Haack reagent. The structures of all the title compounds have been confirmed by elemental analysis, 1H-NMR and 13C-NMR and in addition, the structure of intermediate 5b was confirmed by X-ray crystallography.
Acknowledgements
We are grateful for financial support from Taizhou University (Project No. 2010PY21) and the Nature Science Foundation of China (Project No. 20905057).
- Sample Availability: Samples of the compounds 6a-i are available from the authors.
References and Notes
- Jones, G.; Stanforth, S.P. The Vilsmeier reaction of fully conjugated carbocycles and heterocycles. Org. React. 2001, 49, 1–315. [Google Scholar]
- Majo, V.J.; Perumal, P.T. Intramolecular cyclization of azides by iminium species. a novel method for the construction of nitrogen heterocycles under Vilsmeier conditions. J. Org. Chem. 1998, 63, 7136–7142. [Google Scholar] [CrossRef]
- Kira, M.A.; Abdel-Rahman, M.O.; Gadalla, K.Z. The Vilsmeier reaction-III, cyclization of hydrazones to pyrazoles. Tetrahedron Lett. 1969, 10, 109–110. [Google Scholar] [CrossRef]
- Selvi, S.; Perumal, P.T. Facile synthesis of [1]benzopyrano[4,3-c]pyrazoles, 1-aryl-3-(2-formamidophenyl)pyrazoles and 1-aryl-3-phenyl-4-alkylpyrazoles using Vilsmeier reagent. Indian J. Chem. 2002, 41B, 1887–1893. [Google Scholar]
- Elguero, J. Comprehensive Heterocyclic Chemistry; Katrizky, A., Ed.; Pregamon Press: Oxford, UK, 1984; p. 277. [Google Scholar]
- El-Emary, T.I.; Bakhite, E.A. Synthesis and biological screening of new 1,3-diphenylpyrazoles with different heterocyclic moieties at position-4. Pharmazie 1999, 54, 106–111. [Google Scholar]
- Bratenko, M.K.; Vovk, M.V.; Sydorchuk, I.J. Synthesis and antibacterial and antifungal activity of hydrazones and azines of 1-phenyl-3-R-4-formylpyrazole. Farm. Zh. 1999, 68–71. [Google Scholar]
- Rathelot, P.; Azas, N.; EL-Kashef, H.; Delmas, F.; Giorgio, C.D.; David, P.T.; Maldonado, V.P. 1,3-Diphenylpyrazoles: Synthesis andantiparasitic activities of azomethine derivatives. Eur. J. Med. Chem. 2002, 37, 671–679. [Google Scholar]
- Cottineau, B.; Toto, P.; Marot, C.; Pipaud, A.; Chenault, J. Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Biorg. Med. Chem. Lett. 2002, 12, 2105–2108. [Google Scholar] [CrossRef]
- Welch, J.T. Advances in the preparation of biologically active organofluorine compounds. Tetrahedron 1987, 43, 3123–3197. [Google Scholar] [CrossRef]
- Bertrand, F.; Pevere, V.; Quiclet-Sire, B.; Zard, S.Z. A xanthate transfer radical process for the introduction of the trifluoromethyl group. Org. Lett. 2001, 3, 1069–1071. [Google Scholar] [CrossRef]
- Song, J.J.; Tan, Z.; Reeves, J.T.; Gallou, F.; Yee, N.K.; Senanayake, C.H. N-heterocyclic carbene catalyzed trifluoromethylation of carbonyl compounds. Org. Lett. 2005, 7, 2193–2195. [Google Scholar]
- Caboni, P.; Sammelson, R.E.; Casida, J.E. Phenylpyrazole insecticide photochemistry, metabolism, and GABAergic action: Ethiprole compared with fipronil. J. Agric. Food Chem. 2003, 51, 7055–7061. [Google Scholar]
- Sammelson, R.E.; Casida, J.E. Synthesis of a tritium-tabeled, fipronil-based, highly potent, photoaffinity probe for the GABA receptor. J. Org. Chem. 2003, 68, 8075–8079. [Google Scholar] [CrossRef]
- Ranatunge, R.R.; Earl, R.A.; Garvey, D.S.; Janero, D.R.; Gordon Letts, L.; Martino, A.M.; Murty, M.G.; Richardson, S.K.; Schwalb, D.J.; Young, D.V.; Zemtseva, I.S. 3-(2-Methoxytetrahydrofuran-2-yl)pyrazoles: A novel class of potent, selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 6049–6052. [Google Scholar]
- Sridhar, R.; Perumal, P.T.; Etti, S.; Shanmugam, G.; Ponnuswamy, M.N.; Prabavathyc, V.R.; Mathivanan, N. Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg. Med. Chem. Lett. 2004, 14, 6035–6040. [Google Scholar] [CrossRef]
- Prakash, O.; Pannu, K.; Naithani, R.; Kaur, H. One-pot synthesis of oxime derivatives of 1,3-diphenylpyrazole-4-carboxaldehydes from acetophenone phenylhydrazones using Vilsmeier-Haack reagent. Synth. Commun. 2006, 36, 3479–3485. [Google Scholar] [CrossRef]
- CCDC 685556 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK; E-Mail: deposit@ccdc.cam.ac.uk.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).