Abstract
Substituted [1,4]thiazepino[2,3-h]quinolinecarboxylic acid 3 is prepared by PPA-catalyzed thermal lactamization of the respective 8-amino-7-[(2-carboxyethyl)thio]-1,4-dihydroquinoline-3-carboxylic acid 9. The latter synthon is obtained by reduction of the 8-nitro-1,4-dihydroquinoline precursor 8 which, in turn, is made accessible via interaction of 3-mercaptopropionic acid with 7-chloro-1-cyclopropyl-6-fluoro-8-nitro-1,4-dihydroquinoline-3-carboxylic acid 7 in the presence of triethylamine. A benzo-homolog of 3, namely tetrahydroquino[7,8-b]benzothiazepine-3-carboxylic acid 6, is analogously prepared via the raction of 2-mercaptobenzoic acid with 7, followed by reduction of the resulting 7-[(2-carboxyphenyl)thio]-8-nitro product 10 into the corresponding 8-amino derivative 11, and subsequent lactamization. The structures assigned to 3, 6 and 8-11 are based on microanalytical and spectral (IR, MS, NMR) data.
Introduction
Synthetic fluoroquinolones (e.g. ciprofloxacin (1a) [2]) represent a successful achievement towards the design and development of potent antiinfectious drugs [2,3], while some related derivatives, such as 1b [4], exhibit antitumor activity[4,5,6]. On the other hand, 2,3-dihyro-1,5-benzothiazpin-4(5H)-one (2a) and several derivatives thereof, e.g diltiazem [7] (2b, Figure 1), are of considerable interest both synthetically [8] and pharmacologically [9,10,11,12,13]. Depending on the nature of substituents at C-2, C-3 and N-5 of the parent skeleton (ring C, Figure 1), such derivatives exhibit coronary vasodilator [9], calcium antagonist [10], antidepressant [11], anticonvulsant [12] or antimicrobial [13] activities.
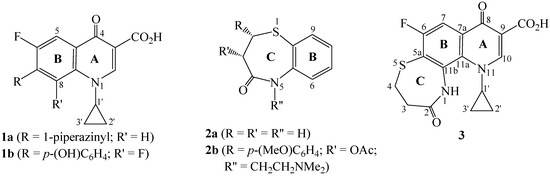
Figure 1.
Structures of 4-oxoquinolines 1a, 1b, dihydro-1,5-benzothiazepin-4(5H)-ones 2a, 2b and 2,8-dioxo-hexahydro[1,4]thiazepino[2,3-h]quinoline 3.
Several dibenzo[b,f][1,4]thiazepin-11(10H)-ones (e.g. 4, Figure 2) were also prepared [14,15,16,17], some of which were reported to exhibit activity against the HIV virus [15] or useful agents for the prevention and treatment of AIDS [16], while others act as leukotriene antagonists [17]. Compounds of type 4 have been readily transformed into 11-piperazinyldibenzo[b,f][1,4]thiazepines, exemplified by 11-{4-[2-(2-Hydroxyethoxy)-ethyl]piperazinyl}dibenzo[b,f][1,4]thiazepine, commonly known as quetiapine [18] (5, Figure 2).
The latter compound and its congeners are useful agents for treating anxiety [19] and substance-related disorders [20], act as calcium channel antagonists [21], neuroleptic and antipsychotic agents [22], and display antidopaminergic activity [23].
Herein, we wish to report on the synthesis of new heterocyclic ring systems incorporating a 4-oxopyridine entity condensed either to 1,5-benzothiazepinone (compound 3, Figure 1), or to dibenzo [b,f][1,4]thiazepinone (compound 6, Figure 2) as depicted in Scheme 1 and Scheme 2, respectively. The tricyclic system 3 encompasses the structural features both of fluoroquinolone (rings A, B) and 1,5-benzothiazepinone (rings B, C), while the tetracyclic assembly 6 incorporates fluoroquinolone (rings A, B) and dibenzo[1,4]thiazepinone (rings B, C, D) chemotypes. Such new hybrid heterocyclics might display interesting bioproperties.
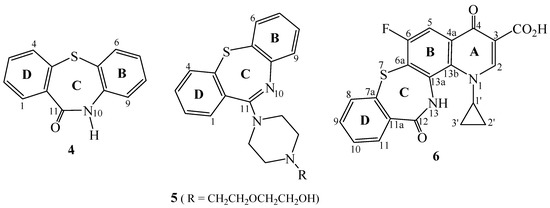
Figure 2.
Structures of dibenzo[b,f][1,4]thiazepine-11(10H)-ones 4, quetiapine 5 and tetrahydroquino[7,8-b][1,4]benzothiazepine 6.
Results and Discussion
Direct interaction between 7-chloro-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (7) [24,25] and 3-mercaptopropionic acid, in aqueous acetone containing triethylamine, produced the corresponding 7-[(2-carboxyethyl)thio]-8-nitro-1,4-dihydroquinoline derivative (8) (Scheme 1).
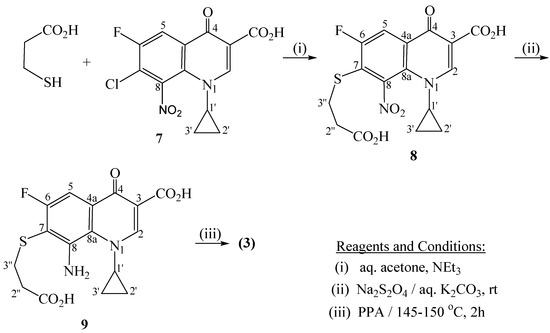
Scheme 1.
Synthesis of hexahydro[1,4]thiazepino[2,3-h]quinoline-9-carboxylic acid 3.
Herein, 3-mercaptopropionic acid acts as 'sulfur' nucleophile that displaces the C(7)- chlorine atom in the substrate (7). This reaction follows a nucleophilc aromatic substitution 'SN-Ar' (addition –elimination) path, and is facilitated by the presence of the electron withdrawing C(6)-fluoro, C(4)-keto, and C(8)-nitro groups. Reduction of the latter 8-nitro compound 8 with sodium dithionite in aqueous potassium carbonate gives the respective 8-amino derivative 9. In a separate step, compound 9 underwent lactamization upon heating with polyphosphoric acid (PPA) to afford a tricyclic system, namely 2,8-dioxohexahydro[1,4]thiazepino[2,3-h]quinoline-3-carboxylic acid (3). Likewise, the reaction of 2-mercaptobenzoic acid with 7 provided the corresponding 7-[(2-carboxyphenyl)thio]-8-nitro-1,4-dihydroquinoline derivative 10 which was then reduced to the respective 7-amino-1,4-dihydroquinoline-3-carboxylic acid 11 (Scheme 2). Subsequent lactamization of 11, using PPA, afforded the target tetracyclic product, namely 4,12-dioxotetrahydroquino[7,8-b]benzothiazepine-3-carboxylic acid (6). The elemental analyses and spectral (IR, MS, NMR) data of 3, 6 and 8-11, given in the Experimental part, are in conformity with the suggested structures. Thus, their MS spectra display the correct molecular ion peaks for which the measured HRMS data are in good agreement with the values calculated for the molecular formulae. Assignments of the 1H- and 13C- signals to the different respective protons and carbons are based on DEPT and 2D (COSY, HMQC, HMBC) experiments which showed correlations consistent with these assignments.
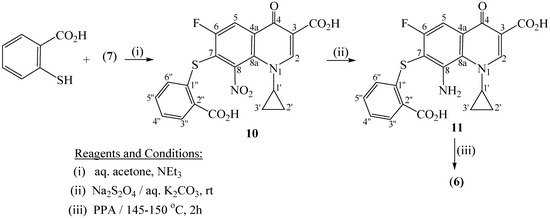
Scheme 2.
Synthesis of tetrahydroquino[7,8-b]benzothiazepine-3-carboxylic acid 6.
For compound 3, distinct ''three-bond'' (1H, 13C)–correlations are observed between H-10 and each of CO2H, C-8, C-11a and C-1', as well as between H-7 and each of C-8, C-11a and C-5a, and between H-1' and each of C-10 and C-11a. Corresponding long-range correlations are also observed for compounds 6 and 8-11 between H-2, H-5, H-1' and their neighbor carbons. The skeletal carbons of benzo-fused entity (ring B) in 3, 6 and 8-11 are recognizable by their doublet signals originating from scalar (through bond) coupling with the neighboring fluorine atom. Also, the C-3'' methylene carbon in 8 appears as a doublet due to through-space (dipolar) coupling with the nearby fluorine atom.
Experimental
General
Ethyl 3-(N,N-dimethylamino)acrylate, 2,4-dichloro-5-fluoro-3-nitrobenzoic acid and cyclopropyl-amine were purchased from Acros. 3-Mercaptopropionic acid and 2-mercaptobenzoic acid were purchased from Aldrich. Melting points were determined on a Gallenkamp capillary melting point apparatus and are uncorrected. 1H- (300 MHz), 13C-NMR (75 MHz) and DEPT spectra, and 2D (H-H COSY, HMQC, HMBC) experiments were measured on a Bruker DPX-300 instrument with Me4Si as internal reference and DMSO-d6 as solvent. High resolution mass spectra (HRMS) were measured in positive ion mode by Electrospray (ESI) on Bruker APEX-Qe 94 instrument. The samples were dissolved in acetonitrile, diluted in spray solution (methanol/water 1:1 v/v + 0.1% formic acid) and infused using a syringe pump with a flow rate of 2 µL/min. External calibration was conducted using the arginine cluster in a mass range m/z 175-871. Electron-impact mass spectra (EIMS) were obtained using a Varian MAT-212 spectrometer at 70 eV and at ion source temperature of 200 oC. IR spectra were recorded as KBr discs on a Nicolet Impact-400 FT-IR spectrophotometer. Elemental analyses were preformed at the Microanalytical Laboratory of the Hashemite University, Zarqa, Jordan.
7-Chloro-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid(7)
This compound [mp 256-257oC (decomp); Lit. [25] 261oC (decomp)] was prepared according to literature methods [24] by acid-catalyzed hydrolysis of the corresponding ethyl ester, in turn prepared from 2,4-dichloro-5-fluoro-3-nitrobenzoic acid, ethyl 3-(N,N-dimethylamino)acrylate and cyclopropyl- amine by following the stepwise synthetic procedures as reported for the corresponding methyl ester analog [mp 175-176 oC (decomp); Lit. [24] 174-176 oC (decomp)] [25,26].
7-[(2-Carboxyethyl)thio]-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8)
3-Mercaptopropionic acid (0.18 g, 1.7 mmol) was added to a stirred solution of 7 (0.5 g, 1.5 mmol) in aqueous acetone (45 mL, 1:2 v / v) and Et3N (6 mL) at room temperature (rt) and kept in the dark for 7 h. Thereafter, the reaction mixture was washed with CHCl3 (2 x 10 mL), the aqueous layer was separated and acidified with 3N HCl. The resulting yellow precipitate was collected and recrystallized from CHCl3. Yield 0.54 g (91%); mp 205-207oC; IR (cm-1) 3500, 3430, 3080, 2914, 2742, 1720, 1679, 1564, 1532, 1481, 1430, 1347, 1329, 1259; 1H-NMR δ 1.00/1.10 (2 m, 4H, 2H-2'/2H-3'), 2.45 (t, J = 6.7 Hz, 2H, H-2''), 3.20 (t, J = 6.7 Hz, 2H, H-3''), 3.70 (m, 1H, H-1'), 8.29 (d, 3JH-F = 9 Hz, 1H, H-5), 8.78 (s, 1H, H-2), 13.05 (br s, 2H, 2CO2H); 13C-NMR δ 11.1 (C-2'/C-3'), 30.5 (d, JC-F = 7.3 Hz, C-3''), 34.9 (C-2''), 39.6 (C-1'), 109.2 (C-3), 114.4 (d, 2JC-F = 25.6 Hz, C-5), 126.9 (d, 2JC-F = 24.7 Hz, C-7), 129.0 (d, 3JC-F = 7.9 Hz, C-4a), 131.3 (d, 4JC-F = 2.6 Hz, C-8a), 144.9 (d, 3JC-F = 1.5 Hz, C-8), 153.3 (C-2), 158.5 (d, 1JC-F = 248 Hz, C-6), 164.9 [C(3)CO2H], 172.7 [C(2'')CO2H], 175.6 (d, 4JC-F = 2.3 Hz, C-4). EIMS m/z (%): 396 (M+, 6), 378 (9), 352 (64), 334 (56), 307 (29), 269 (43), 263 (68), 233 (52), 200 (47), 191 (71), 172 (35), 108 (15), 55 (100); HRMS (EI): Calcd. for C16H13FN2O7S 396.04271; found 396.03918; Anal. calcd. for C16H13FN2O7S (396.35):C, 48.49; H, 3.31; N, 7.07; S, 8.09. Found: C, 48.26; H, 3.24; N, 7.16; S, 7.93.
8-Amino-7-[(2-carboxyethyl)thio]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (9)
A solution of sodium dithionite (0.87 g, 5 mmol) in water (5 mL) was added dropwise at rt to a stirred solution of 8 (0.4 g, 1.0 mmol) in water (20 mL) containing K2CO3 (0.96 g, 7 mmol). The reaction mixture was stirred at rt for an additional 8 h and then extracted with CHCl3 (2 X 10 mL). The aqueous layer was then acidified with 6N HCl, whereby the title compound was obtained as a pale yellow precipitate which was collected and recrystallized from CHCl3/MeOH. Yield 0.3 g (81%); mp 216-218oC; IR (cm-1) 3498, 3343, 3080, 3919, 1730, 3919, 1730, 1595, 1582, 1530, 1492, 1447, 1337, 1254, 1170; 1H-NMR δ 1.04, 1.16 (2 m, 4H, 2H-2' / 2H-3'), 2.44 (t, J = 7 Hz, 2H, 2H-2''), 2.93 (t, J = 7 Hz, 2H, 2H-3''), 4.53 (m, 1H, H-1'), 6.51 (br s, 2H, NH2), 7.20 (d, 3JH-F = 8.4 Hz, 1H, H-5), 8.72 (s, 1H, H-2), 14.70 (br s, 2H, 2CO2H); 13C-NMR δ 10.6 (C-2'/C-3'), 29.4 (C-3''), 34.6 (C-2''), 39.9 (C-1'), 97.0 (d, 2JC-F = 27.1 Hz, C-5), 107.2 (C-3), 109.9 (d, 2JC-F = 23.4 Hz, C-7), 126.9 (d, 4JC-F = 1 Hz, C-8a), 129.2 (d, 3JC-F = 9.8 Hz, C-4a), 145.2 (d, 3JC-F = 3.3 Hz, C-8), 151.5 (C-2), 161.4 (d, 1JC-F = 240 Hz, C-6), 166.0 [C(3)CO2H), 173.1 [ C(2″)-CO2H], 177.2 (d, 4JC-F = 2 Hz, C-4); HRMS(ESI): calcd for C16H16FN2O5S+ [M+H]+ : 367.07640, found: 367.07568; Anal. calcd. for C16H15FN2O5S (366.36):C, 52.45; H, 4.13; N, 7.65; S, 8.75. Found: C, 52.31; H, 4.08; N, 7.55; S, 8.48.
11-Cyclopropyl-6-fluoro-2,8-dioxo-1,2,3,4,8,11-hexahydro[1,4]thiazepino[2,3-h]quinoline-9-carboxylic acid (3)
Compound 9 (0.2 g, 0.55 mmol) was suspended in polyphosphoric acid (PPA, 7 g) and heated at 145-150 °C for 2h. The reaction mixture was then poured into water (30 mL) and stirred for 20 min. The resulting white precipitate was collected and recrystalised from CHCl3/MeOH. Yield 0.13g (68%); mp 307-309 oC; IR (cm-1) 3427, 3286, 3080, 2932, 1717, 1595, 1530, 1498, 1460, 1408, 1389, 1312, 1254, 1228, 1183; 1H-NMR δ 1.03 (m, 4H, 2H-2' / H-3'), 2.74 (t, J = 7 Hz, 2H, 2H-4), 3.53 (t, J = 7 Hz, 2H, 2H-3), 4.29 (m, 1H, H-1'), 7.94 (d, 3JH-F = 8.1 Hz, 1H, H-7), 8.79 (s, 1H, H-10), 10.02 (br s, 1H, N(1)H), 13.42 (br s, 1H, CO2H); 13C-NMR δ 9.5 ( C-2'/C-3'), 33.2 (C-4), 34.6 (C-3), 41.0 (C-1'), 108.2 (C-9), 108.6 (d, 3JC-F = 26.1 Hz, C-7), 126.6 (d, 2JC-F = 20.7 Hz, C-5a), 128.6 (d, 3JC-F = 8.6 Hz, C-7a), 133.7 (d, 3JC-F = 2.2 Hz, C-11a), 135.9 (d, 3JC-F = 1.6 Hz, C-11b), 152.5 (C-10), 159.6 (d, 1JC-F = 244 Hz, C-6), 165.5 (C(9)-CO2H), 172.0 (C-2), 176.8 (d, 4JC-F = 3 Hz, C-8); EIMS m/z (%): 348(M+, 27), 320(5), 276(15), 247(15), 233(12), 220(20), 193(8), 118(6), 152(4), 135(14), 108(9), 55(100); HRMS (ESI): calcd for C16H14FN2O4S+ [M+H]+: 349.06583. Anal. calcd. for C16H13FN2O4S (348.35):C, 55.17; H, 3.76; N, 8.04; S, 9.20. Found: C, 55.86; H, 3.74; N, 7.91; S, 9.27.
7-[(2-Carboxyphenyl)thio]-1-cyclopropyl -6-fluoro-8-nitro-4-oxo-1,4-dihydroquinolin-3-carboxylic acid (10)
Prepared from 2-mercaptobenzoic acid (0.26 g, 1.7 mmol) and 7 (0.5 g, 1.5 mmol) using the procedure and experimental conditions described above in the preparation of 8. The title compound was obtained as a white precipitate which was collected and recrystallized from chloroform/petroleum ether. Yield 0.62 g (93 %); mp 279-281oC. IR (cm-1) 3395, 3067, 2971, 2932, 2669, 2605, 1692, 1605, 1542, 1351, 1459, 1419, 1339, 1328, 1288, 1115; 1H-NMR δ 1.02, 1.18 (2 m, 4H, 2H-2' / 2H-3'), 3.74 (m, 1H, H-1'), 6.80 (dd, J = 7.9, 1.0 Hz, 1H, H-6''), 7.30 (ddd, J = 7.3, 7.5, 1 Hz, 1H, H-4''), 7.38 (ddd, J = 7.9, 7.3, 1.6 Hz, 1H, H-5''), 7.97 (dd, J = 7.5, 1.6 Hz,1H, H-3''), 8.33 (d, 3JH-F = 8 Hz, 1H, H-5), 8.82 (s, 1H, H-2), 13.75 (br s, 2H, 2CO2H); 13C-NMR δ 11.2 (C-2'/C-3'), 39.6 (C-1'), 109.4 (C-3), 115.0 (d, 2JC-F = 25.6 Hz, C-5), 124.3 (d, 2JC-F = 25.7 Hz, C-7), 126.9 (C-4''), 127.4 (C-6''), 128.5 (C-2''), 130.9 (d, 3JC-F = 7.8 Hz, C-4a), 131.6 (d, 3JC-F = 2.6 Hz, H-8a), 131.7 (C-3''), 133.7 (C-5''), 137.6 (C-1''), 146.3 (C-8), 153.3 (C-2), 155.1 (d, 1JC-F = 262 Hz, C-6), 165.0 [C(3)-CO2H], 168.1 [C(2″)-CO2H], 175.6 (d, 4JC-F = 2.2 Hz, C-4); HRMS (ESI): calcd. for C20H14FN2O7S+[M+H]+ : 445.05058 , found: 445.04989; Anal. calcd. for C20H13FN2O7S (444.39):C, 54.05; H, 2.95; N, 6.30; S, 7.22. Found: C, 54.23; H, 3.04; N, 6.18; S, 7.01.
8-Amino-7-[(2-carboxyphenyl)thio]-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (11)
A solution of sodium dithionite (0.52 g, 3.0 mmol) in water (5 mL) was added dropwise to a stirred solution of 10 (0.4 g, 0.9 mmol) in water (10 mL) containing dissolved K2CO3 (0.56 g. 4.0 mmol) at rt. Thereafter, the reaction mixture was stirred at rt for 5 h and then washed with CHCl3 (2 X 8 mL). The aqueous layer was neutralized with 6N HCl, the precipitated product was collected, dried, and recrystallized from CHCl3. Yield = 0.29 g (77%); mp 312-313 oC; IR (cm-1) 3491, 3379, 3086, 2771, 2610, 1704, 1678, 1582, 1524, 1453, 1350, 1235, 1138; 1H-NMR δ 1.09 (m, 4H, 2H-2'/2H-3'), 4.51 (m, 1H, H-1'), 6.49 (br s, 2H, NH2), 6.66 (d, J = 7.9 Hz, 1H, H-6''), 7.22 (dd, J = 6.8, 7.2 Hz, 1H, H-4''), 7.29 (d, 3JH-F = 8.3 Hz, 1H, H-5), 7.34 (dd, J = 7.9, 7.2 Hz, 1H, H-5''), 7.97 (d, J = 6.8 Hz, 1H, H-3''), 8.76 (s, 1H, H-2), 13.40 [br s, 1H, C(2'')-CO2H], 14.80 [br s, 1H, C(3)-CO2H]; 13C-NMR δ 10.6 (C-2'/C-3'), 39.8 ( C-1'), 97.2 (d, 2JC-F = 27.1 Hz, C-5), 107.5 (C-3), 107.6 (d, 2JC-F = 23.2 Hz, C-7), 125.2 (C-6''), 125.6 (C-4''), 127.3 (C-8a), 128.7 (C-2''), 130.3 (d, 3JC-F = 10.3 Hz, C-4a), 132.0 (C-3''), 133.2 (C-5''), 138.3 (C-1''), 146.0 (d, 3JC-F = 3 Hz, C-8), 151.7 (C-2), 161.4 (d, 1JC-F = 242 Hz, C-6), 166.0 [C(3)-CO2H], 168.1 [C(2'')-CO2H], 177.2 (d, 4JC-F = 3 Hz, C-4); EIMS m/z (%): 414 (M+, 8), 396 (10), 370 (64), 352 (82), 323 (100), 295 (31), 217 (46), 189 (59), 154 (19), 136 (50), 108 (11); HRMS (EI): calcd. for C20H15FN2O5S: 414.06854; found: 414.07027; Anal. calcd. for C20H15FN2O5S (414.41): C, 57.97; H, 3.65; N, 6.76; S, 7.74. Found: C, 57.68; H, 3.54; N, 6.72; S, 7.88.
1-cyclopropyl-6-fluoro-4,12-dioxo-1,4,12,13-tetrahydroquino[7,8-b]benzothiazepine-3-carboxylic acid (6)
Prepared from compound 11 (0.2 g, 0.48 mmol) by heating in PPA (7 g) at 145-150 °C for 2h. Work-up of the reaction mixture, as described for the preparation of 3, produced a brown precipitate which was collected, washed with cold EtOH (1 mL) and dried. Yield 0.14 g (74 %) mp 317-319 °C (decomp); IR (cm-1) 3433, 3247, 3086, 2929, 1713, 1674, 1614, 1349, 1490, 1466, 1425, 1383, 1330, 1259, 1235, 1193; 1H-NMR δ 0.36 , 1.06 (2m, 2H) and 0.91, 1.30 (2m, 2H) (2H-2' / 2H-3'), 4.37 (m, 1H, H-1'), 7.53 (m, 2H, H-9 + H-10), 7.62 (dd, J = 7.1, 1.4 Hz, 1H, H-8), 7.81 (dd, J = 7.3, 1.5 Hz, 1H, H-11), 7.90 (d, 3JH-F = 8.0 Hz, 1H, H-5), 8.80 (s, 1H, H-2), 10.99 (br s, 1H, N(1)-H), 13.30 (br s, 1H, CO2H); 13C-NMR δ 7.6, 11.9 ( C-2' /C-3'), 40.9 (C-1'), 108.3 (d, 2JC-F = 26 Hz, C-5), 108.5 (C-3), 128.7 (d, 3JC-F = 8.2 Hz, C-4a), 130.7 (C-9), 132.0 (C-11), 132.4 (d, 2JC-F = 22.4 Hz, C-6a), 132.6 (d, 4JC-F = 1.7 Hz, C-13a), 132.9 (C-10), 133.0 (C-8), 134.2 (d, 4JC-F = 1.9 Hz, C-13b), 135.3 (C-11a), 138.3 (C-7a), 152.6 (C-2), 157.3 (d, 1JC-F = 245 Hz, C-6), 165.5 (CO2H), 168.4 (C-12), 176.8 (d, 4JC-F = 2.9 Hz, C-4); EIMS m/z (%): 396 (M+, 43), 378 (5), 352 (100),337 (6), 323 (19), 319 (46), 295 (16), 269 (10), 241 (9), 214 (11), 196 (6), 168 (5), 157 (4), 107 (11); HRMS (ESI): calcd. for C20H14FN2O4S+ [M+H]+: 397.06583, found:397.06535; Anal. calcd. for C20H13FN2O4S (396.36): C, 60.60; H, 3.31; N, 7.07; S, 8.09. Found: C, 60.48; H, 3.23; N, 7.02; S, 7.87.
Acknowledgements
We wish to thank the Deanship of Scientific Research-The University of Jordan, Amman-Jordan for financial support.
References and Notes
- Part III: Abu Shuheil, M. Y.; Hassuneh, M. R.; Al-Hiari, Y. M.; Qaisi, A. M.; El-Abadelah, M. Heterocycles [h]-Fused onto 4-Oxoquinoline-3-carboxylic acid. Facile Synthesis and Antitumor Activity of Model Heterocycles [a]-Fused onto Pyrido[2,3-f] quinoxaline-3-carboxylic Acids. 2007, 71. in press. [Google Scholar] .
- Wise, R.; Andrews, J. M.; Edwards, L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob. Agents Chemother 1983, 23, 559–564. [Google Scholar] Felmingham, D.; O'Hare, M. D.; Robbins, M. J.; Wall, R. A.; Williams, A. H.; Cremer, A. W.; Ridgeway, G. L.; Gruneberg, R. N. Comparative in vitro studies with 4-quinolone antimicrobials. Drugs under experimental and clinical research. Drugs Exp. Clin. Res. 1985, 11, 317–329. [Google Scholar] Maurer, F.; Grohe, K. 2,4-Dichloro-5-fluorobenzoic acid. Ger. Offen. 1986, (Chem. Abstr. 1986, 105, 97158e). 3,435, 392. [Google Scholar] Petersen, U.; Bartel, S.; Bremm, K. D.; Himmler, T.; Krebs, A.; Schenke, T. The synthesis and biological properties of 6-fluoroquinolonecarboxylic acids. Bull. Soc. Chim. Belg. 1996, 105, 683–699. [Google Scholar]
- See for example: Okada, T.; Ezumi, K.; Yamakawa, M.; Sato, H.; Tsuji, T.; Tsushima, T.; Motokawa, K.; Komatsu, Y. Quantitative structure-activity relationships of antibacterial agents, 7-heterocyclic amine substituted 1-cyclopropyl-6,8-difluoro-4-oxoquinoline-3-carboxylic acids. Chem. Pharm. Bul. Jpn. 1993, 41, 126–131. [Google Scholar] ; (b)Grohe, K. in Quinolone Antibacterials. Springer-Verlag: Berlin, Heidelberg, 1998; 13–62. [Google Scholar] ; Li, Q.; Mitscher, L. A.; Shen, L. L. The 2-pyridone antibacterial agents: Bacterial topoisomerase inhibitors. Med. Res. Rev. 2000, 20, 231–293. [Google Scholar] ; Zhanel, G. G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A. S.; Embil, J.; Smith, H.; Hoban, D. A critical review of the fluoroquinolones: Focus on respiratory tract infections. J. Drugs 2002, 62, 13–59. [Google Scholar] ; Da Silva, A. D.; De Almeida, M. V.; De Souza, M. V. N.; Couri, M. R. C. Biological activity and synthetic metodologies for the preparation of fluoroquinolones, a class of potent antibacterial agents. Curr. Med. Chem. 2003, 10, 21–39. [Google Scholar] ; (f)Daneshtalab, M. Topics in Heterocyclic Chemistry. Volume 2, Heterocyclic Antitumor Antibiotics, Springer-Verlag: Berlin / Heidelberg, 2005; 153–173. [Google Scholar] ; Mistcher, L. A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar] .
- Elsea, H. S.; McGuirk, P. R.; Gootz, T. D.; Moynihan, M.; Osheroff, N. Drug features that contribute to the activity of quinolones against mammalian topoisomerase II and cultured cells: correlation between enhancement of enzyme-mediated DNA cleavage in vitro and cytotoxic potential. Antimicrob. Agents Chemother. 1993, 37, 2179–2186. [Google Scholar] Spitzner, J. R.; Chung, I. K.; Gootz, T. D.; McGuirk, P. R.; Muller, M. T. Analysis of eukaryotic topoisomerase II cleavage sites in the presence of the quinolone CP-115,953 reveals drug-dependent and -independent recognition elements. Mol. Pharmacol. 1995, 48, 238–249. [Google Scholar] Riesbeck, K.; Forsgren, A. CP-115,953 Stimulates cytokine production by lymphocytes. Antimicrob. Agents Chemother 1995, 39, 476–483. [Google Scholar] Bromberg, K. D.; Burgin, A. B.; Osheroff, N. Quinolone action against human topoisomerase II : Stimulation of enzyme-mediated double-stranded DNA cleavage. Biochemistry 2003, 42, 3393–3398. [Google Scholar]
- Yoshinari, T.; Mano, E.; Arakawa, H.; Kurama, M.; Iguchi, T.; Nakagawa, S.; Tanaka, N.; Okura, A. Stereo (C7)-dependent topoisomerase II inhibition and tumor growth suppression by a new quinolone, BO-2367. Jpn. J. Cancer Res. 1993, 84, 800–806. [Google Scholar] Okura, A.; Yoshinari, T.; Arakawa, H.; Nakagawa, S.; Mano, E.; Ushijima, R. Preparation of fluoropyridonecarboxylic acid derivatives as antitumor and antibacterial agents. PCT Int. Appl WO 9212146, 1992. [Chem. Abstr. 1993, 118, 147465]. [Google Scholar]
- Arakawa, H.; Mano, E.; Hakoda, N.; Yoshinari, T.; Nakagawa, S.; Okura, A. Potent antitumor activity of quinolone compounds with an unsaturated aminoazabicyclo group at the C-7 position of the quinolone ring. Anti-cancer Drug Des. 1996, 11, 221–229. [Google Scholar] Elsea, S. H.; Westergaard, M.; Burden, D. A.; Lomenick, J.-P.; Osheroff, N. Quinolones share a common interaction domain on topoisomerase II with other DNA. Biochemistry 1997, 36, 2919–2924. [Google Scholar] Kamat, A. M.; DeHaven, J. I.; Lamm, D. L. Quinolone antibiotics: A potential adjunct to intravesical chemotherapy for bladder cancer. Urology 1999, 54, 56–61. [Google Scholar] Nishijima, C.; Kawada, K.; Ohara, K.; Shinomiya, T.; Fukuda, S.; Nakamura, A.; Sawai, T.; Ikekita, M. Novel effects of quinolones to induce apoptosis in a hepatocellular carcinoma cell line, HepG2. Res. Comm. Biochem. Cell Mol. Biol. 2002, 6, 21–38. [Google Scholar] Huang, D.; Okada, K.; Komori, C.; Itoi, E.; Suzuki, T. Enhanced antitumor activity of ultrasonic irradiation in the presence of new quinolone antibiotics in vitro. Cancer Sci. 2004, 95, 845–849. [Google Scholar]
- This drug, namely (+)-cis-3-acetoxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxy-phenyl)-1,5-benzothiazepin-4(5H)-one, was developed and introduced in Japan as a cardio-vascular agent for the treatment of angina pectoris. The drug dilates peripheral arteries and arterioles; it also increases myocardial oxygen supply by relieving coronary artery spasm and reduces myocardial oxygen demand by decreasing heart rate and reducing overload.
- Krapcho, J.; Spitzmiller, E. R.; Turk, C. F. Substituted 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones and 3,4-dihydro-2-phenyl-(2H)-1,6-benzothiazocin-5(6H)-ones. J. Med. Chem. 1963, 6, 544–546. [Google Scholar] Zagorevskii, V. A.; Dudykina, N. V. Ring expansion in reduction of oximes. Zhur. Obshch. Khim. 1963, 33, 322–323. [Google Scholar] Levai, A.; Pazicha, G. Oxazepines and thiazepines. New procedures for the preparation of 2,3-dihydro-1,5-benzothiazepine-4(5H)-ones substituted in position 2. Synth. Commun. 1985, 15, 623–632. [Google Scholar] Ambrogi, V.; Grandolini, G. A convenient one pot synthesis of 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones. Synthesis 1987, 724–726. [Google Scholar] Mais, F. J.; Fiege, H. Preparation of 2,3-dihydro-1,5-benzothiazepin-4(5H)-ones as cocatalysts for Friedel-Craft’s chlorination of alkylbenzenes. Ger. Offen. DE 3, 800,386, 1989. [Google Scholar] Sharma, A. K.; Singh, G.; Yadav, A. K.; Prakash, L. Improved method for the synthesis of new 1,5-benzothiazepine derivatives as analogues of anticancer drugs. Molecules 1989. [Google Scholar]
- Glaser, R.; Sklarz, B. Stereochemistry and conformation in solution of diltiazem hydrochloride, a 1,5-benzothiazepine coronary vasodilator. J. Chem. Soc., Perkin Trans. 2 1989. [Google Scholar] Shibata, S. S.; Satake, N.; Hester, R. K.; Mochizuki, S.; Kosakai, K.; Ueyama, N.; Wakabayashi, S. Characterization of the vasoinhibitory actions of KT2-230, a new benzothiazepine vasodilator derivative on isolated rabbit vascular smooth muscles: An alpha-adrenergic- and serotonergic-receptor antagonist. Gen. Pharmacol. Vasc. Syst. 1991, 22, 443–448. [Google Scholar]
- Pitt, B. Diversity of calcium antagonists. Clin. Ther. 1997, 19, 3–17. [Google Scholar] Smith, D. H. G.; Neutel, J. M.; Weber, M. Comparisons of the effects of different long-acting delivery systems on the pharmacokinetics and pharmacodynamics of diltiazem. Am. J. Hypertens. 1999, 12, 1030–1037. [Google Scholar] Hagiwara, M.; Adachi-Akahane, S.; Nagao, T. High-affinity binding of [3H]DTZ323 to the diltiazem-binding site of L-type Ca2+ channels. Eur. J. Pharmacol. 2003, 466, 63–71. [Google Scholar] Hart, J.; Wilkinson, M. F.; Kelly, M. E. M.; Barnes, S. Inhibitory action of diltiazem on voltage-gated calcium channels in cone photoreceptors. Exp. Eye Res. 2003, 76, 597–604. [Google Scholar]
- Krapcho, J.; Turk, C. F.; Piala, J. Syntheses and pharmacological activity of compounds related to the antidepressant,5-(2-dimethylaminoethyl)-2,3-dihydro-2-phenyl-1,5-benzothiazepin-4(5H)-one (thiaze-sim). III. J. Med. Chem. 1968, 11, 361–364. [Google Scholar] [CrossRef]
- Sarro, G. D.; Chimirri, A.; Sarro, A. D.; Gitto, R.; Grasso, S.; Zappala, M. 5H-[1,2,4]Oxadiazolo[5,4-d][1,5]benzothiazepines as anticonvulsant agents in DBA/2 mice. Eur. J. Med. Chem. 1995, 30, 925–929. [Google Scholar]
- Singh, G.; Kumar, N.; Yadav, A. K.; Mishra, A. K. Syntheses of some new 1,5-benzothiazepine derivatives and their ribofuranosides as antimicrobial agents. Heteroat. Chem. 2002, 13, 620–625. [Google Scholar] [CrossRef]
- Kwak, B.-sung; Chung, K.-nam; Koh, K.-ho; Hwang, H.-jun. Method of preparing 10H-dibenzo[b,f]]1,4]thiazepin-11-one. PCT Int. Appl. WO 047722, 2004. [Google Scholar]
- Nicol, R. H.; Slater, M. J.; Hodgson, S. T. Preparation of dibenzothiazepinethiones as antiviral agents. PCT Int. Appl. WO 19607, 1992. [Google Scholar] Nicol, R. H.; Slater, M. J.; Hodgson, S. T. Preparation of 10,11-dihydrobenzo[b,f][1,4]thiazepin-11-ones as virucides. PCT Int. Appl. WO 19277, 1992. [Google Scholar]
- Hargrave, K. D.; Schmidt, G.; Engel, W.; Schromm, K. Preparation of dibenz[b,f][1,4]oxazepin (and thiazepin)-11(10H)-ones and-thiones for prevention and treatment of AIDS. Can. Pat. Appl. 2024040, 1991. [Google Scholar]
- Belanger, P. C.; Rokach, J.; Scheigetz, J. Preparation of 10,11-dihydrodibenzo[b,f][1,4]thiazepines as leukotriene antagonists. U.S. Pat. 4728735, 1988. [Google Scholar]
- Holkar, A. G.; Pise, A. Processes for the one-pot preparation of thiazepines and their pharmaceutical compositions. C. PCT Int. Appl. WO 020011, 2007. [Google Scholar] Tarur, V. R.; Sathe, D. G.; Naidu, A. V.; Aher, U. P.; Patil, S. S. A Process for the preparation of quetiapine hemifumarate. PCT Int. Appl. WO 004234, 2007. [Google Scholar] Buenger, G. S.; Alexander, A. Preparation of 11-{4-[2-(2-hydroxyethoxy)ethyl]piperazinyl}dibenzo[b,f][1,4]thiazepine (quetiapine) and its fumarate salt via chlorination of dibenzo[b,f][l,4]thiazepine-11(10H)one with phosphorus oxychloride. PCT Int. Appl. WO 135544, 2006. [Google Scholar] Bosch, L. J.; Burgarolas, M. M. C.; Chamorro, G. I. Process for preparing quetiapine and quetiapine fumarate by treatment of dibenzo[b,f][1,4]thiazepin-11(10H)-one with phosphorus oxychloride and then with 2-(2-piperazin-1-ylethoxy)ethanol. PCT Int. Appl. WO 117700, 2006. [Google Scholar] Harada, K.; Nishino, S.; Yoshii, K. Preparation of dibenzothiazepines and their intermediates. Jpn. Kokai Tokkyo Koho. JP 11199574, 1999. [Google Scholar]
- Davis, P. C.; Goldstein, J. M.; Grimm, S. W.; Winter, H. R.; Suckow, R. F. Method using 11-piperazin-1-yldibenzo[b,f][1,4]thiazepine for treating schizophrenia and other disorders. U.S. Pat. Appl. 217366, 2006. [Google Scholar]
- Davis, P. C.; Goldstein, J. M.; Grimm, S. W.; Suckow, R. F.; Winter, H. R. Use of 11-piperazin-1-yldibenzo[b,f][1,4]thiazepine or a pharmaceutically acceptable salt thereof to treat substance-related and other disorders, and oral pharmaceutical compositions. PCT Int. Appl. WO 073360, 2006. [Google Scholar]
- Li, R.; Farmer, P. S.; Wang, J.; Boyd, R. J.; Quilliam, M. A.; Walter, J. A.; Howlett, S. E. Molecular geometries of dibenzothiazepinone and dibenzoxazepinone calcium antagonist. Drug Design and Discovery 1995. [Google Scholar] Li, R.; Farmer, P. S.; Quillam, M. A.; Howlett, S. E. Dibenzothiazepinones as potential calcium channel antagonists. Drug Design and Discovery. 1993. [Google Scholar]
- Warawa, E. J.; Migler, B. M. Preparation of 11-{4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl} dibenzo[b,f][1,4]thiazepine as a neuroleptic and antipsychotic. Eur. Pat. Appl. EP 240228, 1987. [Google Scholar]
- Barker, A. C.; Copeland, R. J. Process for the preparation of a piperazinodibenzothiazepine with antidopaminergic activity. Eur. Pat. Appl. 1988. [Google Scholar]
- Sanchez, J. P.; Domagala, J. M.; Hagen, S. E.; Heifetz, C. L.; Hutt, M. P.; Nichols, J. B.; Trehan, A. K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J. Med. Chem. 1988, 31, 983–991. [Google Scholar]
- Grohe, K.; Heitzer, H. Cycloaracylation of enamines. I. Synthesis of 4-quinolone-3-carboxylic acids. Liebigs Ann. Chem. 1987, 29–37. [Google Scholar] Petersen, U.; Grohe, K.; Schenke, T.; Hagemann, H.; Zeiler, H. J.; Metzger, K. G. Preparation of 7-(azabicycloalkyl)-3-quinolinecarboxylates and -3-naphthyridinecarboxylates as bactericides and feed additives. Ger. Offen. 3 601 567, 1987. [Google Scholar]
- Pulla, R. M.; Venkaiah, C. N. An improved process for the preparation of quinolone derivatives, e.g. ciprofloxacin. PCT Int. Appl. WO 085 692, 2001. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.