Abstract
Three kinds of molecular complexes based on tetrathiafulvalene (TTF) and dialkylviologens were prepared and their crystal structures elucidated. While TTF-dimethylviologen complex forms a mixed stack arrangement of donors and acceptors in its crystal structure, TTF donors aggregate with long alkyl groups by CH/π and/or van der Waals interactions in a couple of TTF-heptylviologen complexes.
Introduction
Considerable attention has recently been paid in the field of supramolecular and materials chemistry to the incorporation of electroactive components into supramolecular assemblies. Among them, the supramolecular and macrocyclic assemblies derived from redox-controllable TTF 1 and dialkyl-4,4’-bipyridinium dications (dialkylviologens) such as 2, have evoked attention, not only as catenanes and rotaxanes, but also as molecular switches and potential molecular electronics devices [1]. The assembly of such kinds of molecular complexes relies strongly upon non-covalent charge-transfer (CT) interactions between donors and acceptors [2].
Since a dialkylviolgen 3 usually comprises two counteranions [3], it can form a unique molecular complex consisting of three molecular components when appropriate donors and/or acceptors are used. For example, Tamaoki et al. recently reported the formation of cocrystals consisting of dimethyl- viologen (methylviologen), anthraquinone disulfonate and hydroquinone with unique crystal structures mediated by electrostatic and CT interactions [4].
We have also directed our interests in preparing novel molecular or supramolecular complexes consisting of three (or more) molecular components in order to afford their assembling motifs and possible functionalities derived there from. In this paper, we wish to report on the preparation of three kinds of molecular crystals based on TTF and dialkylviologens with different alkyl chains and their crystal structures with different assembly motifs depending on the differences between alkyl chains and the ratios of components.
Results and Discussion
Preparation of Molecular Complexes 4-6
The preparation of molecular complexes is outlined in Scheme 1. When an equimolecular amount of TTF 1 was treated with dimethylviologen (paraquat, 2, R=CH3, X=Cl) in methanol at ambient temperature, the original brownish solution turned to green, indicating the appearance of a CT band in the visible region.
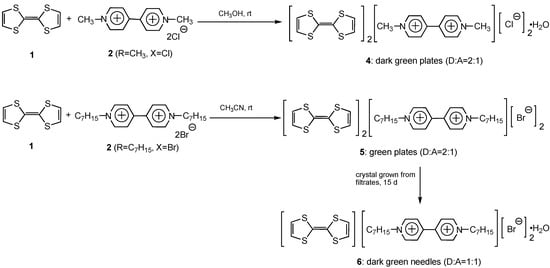
Scheme 1.
The greenish solid obtained after evaporating the solvent was then recrystallized from methanol to give dark green plates of complex 4. The donor-to-acceptor ratio of the resulting complex was found by X-ray analysis to be 2:1, with dichloride and a water molecule in the complex. Similar treatment of TTF 1 with diheptylviologen 2 (R=C7H15, X=Br) in acetonitrile solution gave green plates of complex 5 with same donor-to-acceptor ratio (but without a water molecule). When the filtrates of complex 5 were left standing for 15 d, different greenish crystals were obtained and the X-ray analysis data of these indicated the formation of another complex 6 with a 1:1 donor-to-acceptor ratio (vide infra).
Crystal Structures of Molecular Complexes 4-6
Single crystals of complexes 4-6 could be obtained by recrystallization from appropriate solvents and their X-ray diffraction data are summarized in Table 1.

Table 1.
Summary of crystal data for 4, 5 and 6.
| Parameter | 4 | 5 | 6 |
|---|---|---|---|
| Formula | C24H24N2OS8Cl2 | C36H46N2S8Br2 | C30H44N2OS4Br2 |
| Formula weight | 683.85 | 923.06 | 736.74 |
| Crystal system | orthorhombic | triclinic | triclinic |
| Space group | P2121211 | P-1 | P-1 |
| a/Å | 12.138(3) | 7.4789(14) | 9.038(2) |
| b/Å | 22.227(3) | 12.780(3) | 9.1628(15) |
| c/Å | 10.840(3) | 22.099(4) | 25.728(14) |
| α/degrees | 90 | 88.308(8) | 87.26(2) |
| β/degrees | 90 | 80.2890(15) | 80.254(14) |
| γ/degrees | 90 | 89.997(2) | 67.229(6) |
| V/Å3 | 2925(1) | 2081.0(7) | 1935.8(12) |
| Z | 4 | 3 | 3 |
| D (calc)/gcm-3 | 1.553 | 1.567 | 1.896 |
| No. of measured reflections | 3792 | 18475 | 6648 |
| No. of independent reflections | 3792 | 8034 | 3707 |
| No. of used reflections in refinement F>2σ | 26891 | 5986 | 2107 |
| No. of parameters refined | 334 | 479 | 397 |
| R | 0.035 | 0.101 | 0.095 |
| RW | 0.037 | 0.088 | 0.080 |
1 F>3σ
The crystal structure of complex 4 is shown in Figure 1 (left). Thus, the donors and anions form a similar mixed-stack arrangement in alternating DAD-DAD fashion with that observed in TTF•paraquat•2PF6 complex [2(b)]. The bond lengths of the central double bonds of TTF molecules are estimated to be 1.334–1.335 Å, indicating almost neutral states of the molecules without occurrence of electron transfer to acceptors. The interplanar distance for the TTF molecules is about 3.67 Å (Figure 1, right), which is close to the value observed for neutral TTF (3.62 Å) and longer than those observed for the stacked TTF units in its complex with TCNQ (3.47 Å) [5] and TTF•paraquat•2PF6 complex (3.54 Å) [2(b)], even though similar herringbone motifs are observed in the packing structures for these complexes. On the other hand, the interplanar distances of TTF and viologen molecules are about 3.41–3.46 Å, which are slightly shorter than those between the TTF molecules (3.67 Å). No close contacts appear to exist between viologens and chlorides, but the chloride anions, in turn, exist in the void of TTF and viologen molecules together with water molecules. On the whole, π–π intermolecular interaction is thought to play a significant role in the formation of complex 4. As expected, the room temperature conductivity of a single crystal of the complex was less than 10–6 S cm–1 and no intrinsic magnetic susceptibility was observed in the solid state, in spite of the observation of color change in the reaction between TTF and the viologen in the solution. This may be due to an insufficient charge-transfer between the donor/acceptor couple and/or the lost of paramagnetic spins in the solid state by singlet formation.
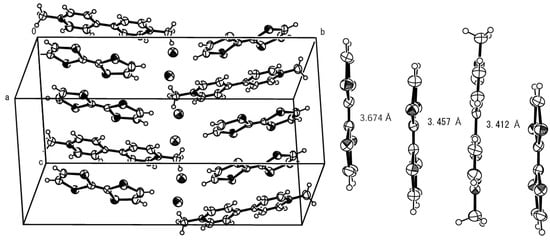
Figure 1.
Left: crystal structure of complex 4. Water molecules are removed for clarity. Right: side view of DDA and D arrangement with indication of interplanar distances.
The crystal structure of the second complex 5, with a donor-to-acceptor ratio of 2:1, is shown in Figure 2 (upper). As seen from the figure, quite different donor/acceptor molecule packing features are found in this complex, when compared to complex 4. Thus, no mixed-stack arrangement of DA molecules is observed but the bipyridiniums are stacked along the b-axis with relatively close contacts between bromide anions, while the TTF molecules are sandwiched between heptyl chains. The bipyridinium moiety is almost planar, with a dihedral angle between two pyridiniums of 4.36º, and the bond lengths of the central double bonds of TTF molecules are estimated to be 1.33 Å, indicating almost neutral states of the molecules. There exists two kinds of short S-S contacts of 3.66 Å and 3.58 Å between the next neighbor TTF molecules as shown in Figure 2 (lower), forming side-by-side and one-dimensional (1-D) tape-like structures also along the b-axis. Close contact is found between a carbon atom of the outer double bond in a TTF molecule and the hydrogen atom attached to the β–carbon of a heptyl group (3.07 Å), and in this context, CH/π [6] and/or van der Waals interactions between TTF molecules and alkyl groups appears to play a significant roll in the formation of complex 5.
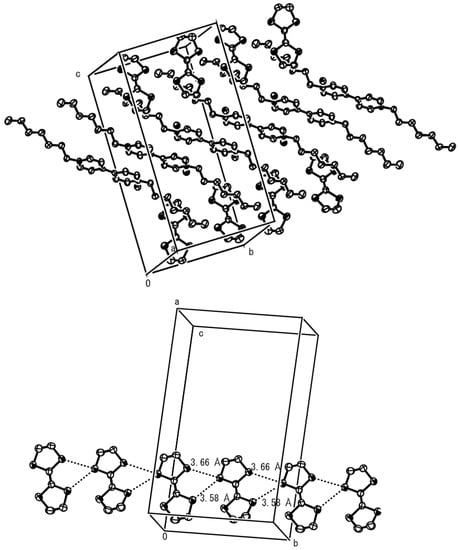
Figure 2.
Upper: crystal structure of complex 5. Lower: side-by-side arrangement of TTF molecules with indication of two kinds of short S-S contacts.
The crystal structure of complex 6, with a 1:1 donor-to-acceptor ratio, is illustrated in Figure 3 (upper). Again, no mixed-stack arrangement of DA molecules is observed in the complex and TTF molecules are arranged close to the heptyl chains. The bipyridinium moiety is non-planar in this case, with a dihedral angle between two pyridiniums of 28.23º, forming columnar structures. Though the bond lengths of the central double bonds of TTF molecules are somewhat long, about 1.38 Å, they are assumed to remain almost neutral because of their proximity to alkyl chains. There exists no S-S contact between neighboring TTF molecules, but nonetheless they locate in slipped positions with regards to each other, thereby forming a 1-D tape-like structure (Figure 3, lower).
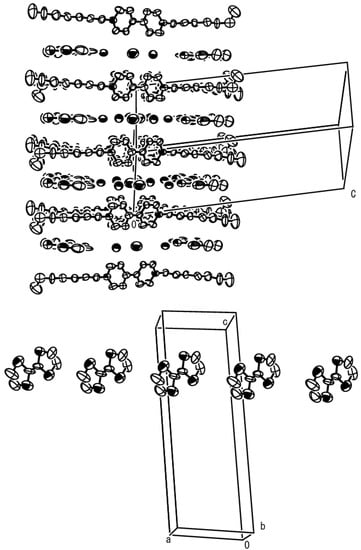
Figure 3.
Upper: crystal structure of complex 6. Lower: 1-D arrangement of TTF molecules.
Also in this case, a couple of close contacts are found between the carbon atoms of the outer double bond in a TTF molecule and the hydrogen atom attached to the β–carbon of a heptyl group (3.22 Å and 3.30 Å) and therefore, the formation of complex 6 is also regarded to be largely influenced by CH/π and/or van der Waals interactions between TTF molecules and alkyl groups.
Reflecting the structural features of complexes 5 and 6, the room temperature conductivity of a single crystal of each complex was less than 10–6 S cm–1 and at the same time, no intrinsic magnetic susceptibility was observed.
Thus, although the reasons are still not clear as to why these three complexes have different donor-to-acceptor ratios and different crystal structures, the fact that the molecular complexes like 5 or 6 are formed by CH/π and/or van der Waals interactions rather than CT interaction may give some clues toward understanding the preparation of supramolecular materials by crystal engineering [7].
Conclusions
Three kinds of molecular complexes 4-6 based on TTF and dialkylviologens were prepared and their crystals structures were clarified. A mixed stack arrangement of donors and acceptors was observed in the crystal structure of TTF-dimethylviologen complex 4 in alternating DAD-DAD fashion. Conversely, TTF donors were found to aggregate with long alkyl groups by CH/π and/or van der Waals interactions in a couple of TTF-diheptylviologen complexes 5 and 6, in spite of the differences between their donor-to-acceptor ratios and TTF arrangements.
Experimental
General
TTF, dimethylviologen (1,1’-dimethyl-4,4’-bipyridinium dichloride) and diheptylviologen (1,1’-di-n-heptyl-4,4’-bipyridinium dibromide) are commercially available as high grade reagents (Tokyo Kasei Kogyo Co.) and were used without further purification. Melting points were measured on a Yamato MP-21 apparatus and were uncorrected. UV-Vis spectra were taken on a JASCO Ubest-35 spectrometer. X-ray diffraction data were recorded using a Quantum CCD area detector on a Rigaku AFC-7R diffractometer at room temperature. CCDC deposition numbers 642858 – 642860 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Preparation of molecular complex 4
To a solution of TTF (50 mg, 0.24 mmol) in methanol (5 mL) was added dimethylviologen dichloride (63 mg, 0.24 mmol). After several minutes, the resulting solution was concentrated in vacuo to give a greenish solid, which was filtered and recrystallized from methanol for 1 week in a refrigerator to yield the complex 4 as dark green plates (38 mg, 60 %). M.p. > 300 ºC.
Preparation of molecular complex 5 and 6
A solution of TTF (50 mg, 0.24 mmol) in acetonitrile (10 mL) was mixed with diheptylviologen dibromide (126 mg, 0.24 mmol) in acetonitrile (10 mL). After several minutes the solvent was removed under reduced pressure to give a greenish solid, which was filtered and recrystallized from acetonitrile for 1 d to yield green plates of complex 5 (67 mg, 55 %). M.p. > 300 ºC. After separating these crystals and storing the filtrates over a period of two weeks, another complex 6 was obtained as dark green needles (25 mg, 20 %). M.p. > 300 ºC.
Acknowledgments
This work was partially supported by a grant-in-aid from Hyogo Science and Technology Association, which is gratefully acknowledged. B. R. is also grateful to a financial support from Kawanishi Memorial Shinmeiwa Foundation for her studies.
References and Notes
- Raymo, F. M.; Stoddart, J. F. Molecular Switches; Feringa, B. L., Ed.; Wiley-VCH: Weinheim, 2001; Chapter 7. [Google Scholar] Collier, C. P.; Mattersteig, G.; Wong, E. W.; Luo, Y.; Beverly, K.; Sampaio, J; Raymo, F. M.; Stoddart, J. F.; Heath, J. R. Science 2000, 289, 1172–1175. Becher, J.; Jeppensen, J. O. TTF Chemistry; Yamada, J., Sugimoto, T., Eds.; Kodansha–Springer: Berlin, Tokyo, 2004; Chapter 15. [Google Scholar]
- Simonsen, K. B.; Zong, K. Z.; Rogers, R. D.; Cava, M. P.; Becher, J. J. Org. Chem. 1997, 62, 679–686. Cooke, G.; de Cremiers, H. A.; Duclairoir, F. M. A.; Gray, M.; Vaqueiro, P.; Powell, A. V.; Rosair, G.; Rotello, V. M. Tetrahedron Lett. 2001, 42, 5089–5091.
- Monk, P. M. S. (Ed.) The Viologens: Physicochemical Properties, Synthesis and Applications of the Salts of 4,4’–Bipyridine; John Wiley & Son: Chichester, 1999.
- Kidowaki, M.; Tamaoki, N. Chem. Commun. 2003, 290–291.
- Kistenmacher, T. J.; Phillips, T. E.; Cowan, D. O. Acta. Cryst. B 1974, 30, 763–768. [CrossRef]
- Nishio, M.; Hirota, M.; Umezawa, Y. (Eds.) The Ch-Pi Interaction: Evidence, Nature, and Consequences (Methods in Stereochemical Analysis); Wiley-VCH: Weinheim, 1998.
- Desiraju, G. R. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311. Tiekink, E. R. T.; Vittal, J. (Eds.) Frontiers in Crystal Engineering; Wiley: Chichester, 2006.
- Sample Availability: Samples of compounds 4, 5 and 6 are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.