Abstract
L-Proline catalyzed additions of 13 different thiols to 11 different α-enone Michael acceptors in [bmim] PF6 are reported. Reasonable to high yields of the reaction products were isolated in most cases.
Introduction
Addition reactions of thiols to unsaturated ketones have been studied quite frequently, in particular the stereoselective version where chiral metal complexes are often used as catalysts [1,2]. Asymmetric reactions catalyzed by small molecule organic catalysts have become very attractive in recent years [3,4,5,6,7,8]. L-Proline, quinine and ephedrine, as well as 2-(S)-phenylaminomethyl-4-(S)-hydroxypyrrolidine and 2-(S)-(diphenylhydroxymethyl)piperidine and its 1-Boc derivatives have all previously been used as the catalysts for additions of thiols to unsaturated ketones, but they gave only moderate e.e. values [9,10,11,12,13,14].
Ionic liquids have emerged in recent years as “green” solvents for many organic reactions, including transition metal-catalyzed reactions [15,16,17,18,19,20,21,22,23], but they have been less frequently used as the media for organocatalyst catalyzed reactions. We [24] and Loh et al. [25] have simultaneously described the excellent results observed for L-proline catalyzed aldol reactions in ionic liquids. We have also reported [26] L-proline catalyzed additions of carbonyl compounds to β-nitrostyrenes. These reactions took place with high yields and with high e.e. using just 5 mol % of L-proline. Rasalkar very recently described [27] L-proline catalysed Michael additions of ketones to nitrostyrene. He tested several ionic liquids for this purpose and the best was found to be 1-methoxyethyl-3-methyl-imidazolium methanesulfonate ([MOEMIM]OMs), although to achieve good yields it was necessary to prolong the reaction times for up to 60 hours and use up to 40 mol % of the catalyst to achieve 75 % e.e. Hagiwara has recently described [28] organocatalyst catalysed additions of aliphatic aldehydes to methyl vinyl ketone in the ionic liquid [bmim]PF6. 2-(S)-(1-Morpholinomethyl)piperidine was found to be the best catalyst, but the yields of the product were only average and the e.e. values were just 11 –51 %. Yadav et al. have disclosed [29] the results of Michael additions of thiols to α,β-unsaturated ketones in [bmim]BF4/H2O and [bmim]PF6/H2O (2:1) mixtures. Similarly Ranu has reported [30] Michael addition of thiols and thiophosphate to α,β-unsaturated carbonyl compounds in [pmim]Br as the reaction medium as well as the catalyst. Salunkhe [31] has used ionic liquids as the medium for the quinidinium bromide catalyzed addition of dimethyl malonate to chalcone. The main aim of our current work was to examine the range of L-proline catalysed Michael additions of different thiphenols and thiols to several Michael acceptors.
Results and Discussion
The Michael addition of thiophenol to chalcone catalyzed by 5 mol % of L-proline went smoothly when 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim]PF6) was used as the solvent and nearly quantitative yields of the addition product were isolated after extraction of the ionic liquid with diethyl ether and quick chromatography on a short silica gel column. The possibility of re-use of the ionic liquid/catalyst system was also checked. The yields of the product were practically the same over four experiments (95%−90%), but dropped to 82% in the fifth experiment and to 74% at the sixth experiment. Our next aim was to explore the limits of Michael additions of thiols to substrates with activated double bonds (Scheme 1). The substrate structures are shown in Figure 1, those of the thiols in Figure 2 and those of the products in Figure 3. Results are summarized in Table 1.
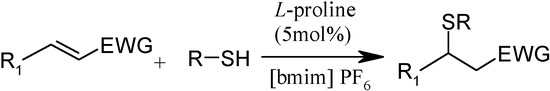
Scheme 1.
The results proved that addition of different thiols to chalcone is possible and high yields of the products were isolated. Exceptions were 2k, 2l and 2m, which did not afford any Michael addition products, possibly because all of them exist in their thione form. This was substantiated by the fact that 2h gave 27% of the product. High yields of the products were also achieved in additions to 1g and 1i, while low yields were observed in the addition to 1h, due to the steric hindrance. We were pleased to find that additions with 1e and 1f took place, albeit with low yields, because Ranu had previously claimed that additions of thiols to 1e are not possible [14]. Surprisingly, no product was isolated in the attempted additions of thiophenol to 2j, 2k and 2l, which is in accord with findings of Ranu [30]. A possible explanation for this could be the competitive reversible addition of thiophenol to the functional group.
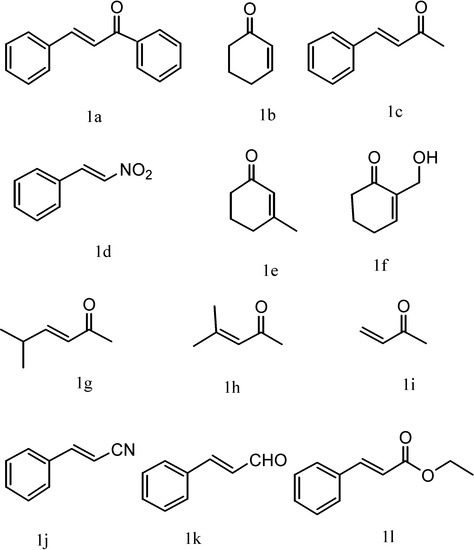
Figure 1.
Structures of substrates.
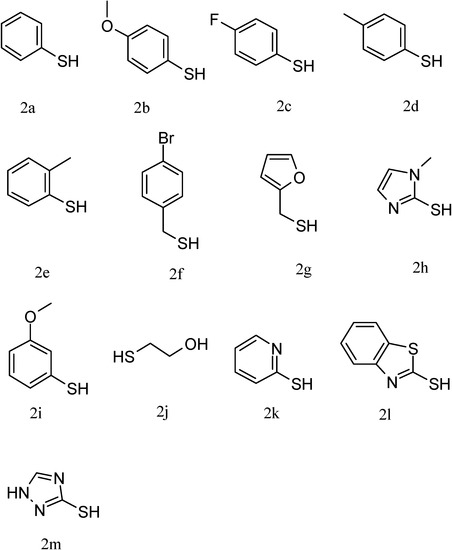
Figure 2.
Structures of thiols.
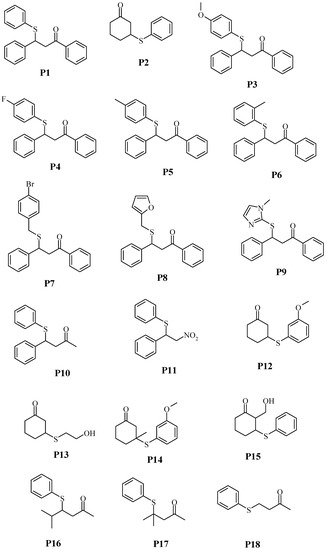
Figure 3.
Structures of products.
We were sorry to find that practically no stereoselectivity was observed for the L-proline catalysed additions of different thiols to neither chalcone or cyclohexene-1-one, although this is in accord with the results of Agami [11].

Table 1.
Michael additions of thiols to substrates containing activated double bonds.
| Entry | Substrate | Reagent | Time (min) | Yield (%) | Product |
|---|---|---|---|---|---|
| 1 | 1a | 2a | 10 | 99 | P1 |
| 2 | 1a | 2b | 5 | 89.8 | P3 |
| 3 | 1a | 2c | 20 | 86 | P4 |
| 4 | 1a | 2d | 10 | 91.5 | P5 |
| 5 | 1a | 2e | 65 | 74 | P6 |
| 6 | 1a | 2f | 30 | 74 | P7 |
| 7 | 1a | 2g | 40 | 83 | P8 |
| 8 a | 1a | 2h | 480 | 27 | P9 |
| 9 | 1b | 2a | 30 | 98 | P2 |
| 10 | 1b | 2i | 30 | 94 | P12 |
| 11 | 1b | 2j | 30 | 96 | P13 |
| 12 | 1c | 2a | 120 | 93 | P10 |
| 13 | 1d | 2a | 30 | 89 | P11 |
| 14 | 1e | 2i | 60 | 19 | P14 |
| 15 b | 1f | 2a | 60 | 18 | P15 |
| 16 | 1g | 2a | 30 | 75 | P16 |
| 17 | 1h | 2a | 30 | 30 | P17 |
| 18 | 1i | 2a | 30 | 95 | P18 |
a Reaction was performed at 90 °C. No product was detected at r.t./24 h. b The yield was determined from the 1H-NMR spectrum.
Conclusions
Our examination proved that ionic liquid is a solvent of choice for Michael additions of different thiols to a range of Michael acceptors catalyzed by L-proline. Products are isolated in reasonable to high yields by simple extraction into organic solvent.
Acknowledgements
We gratefully acknowledge the financial support of Slovak Granting Agency VEGA (Grant No 1/0072/03).
Experimental
General
NMR spectra were measured on a Varian Gemini 2000 spectrometer operating at 300 MHz for 1H and 75 MHz for 13C, respectively. Deuteriochloroform was used as solvent and tetramethylsilane was used as an internal standard.
General experimental procedure
L-Proline (5.75 mg, 5 mol%) and the chosen acceptor (1 mmol) was added to degassed [bmim]PF6 ionic liquid (1 mL) and the mixture was stirred for 10 min at r.t.. Thiol (1.1 mmol) was then added and the reaction mixture was stirred vigorously for the chosen time and at the chosen temperature (see Tables). The product was extracted with several portions of diethyl ether and the extract was chromatographed on a SiO2 column using 9 : 1 hexane/ethyl acetate in all cases. Products were isolated as pure materials and their structure was proven by 1H-NMR and 13C-NMR spectra and, in the case of new compounds, also by microanalysis.
Characterization of the products
1,3-Diphenyl-3-(phenylsulfanyl)propan-1-one (P1) 1H-NMR: 7.89 (d, 2H, J = 6.9Hz), 7.54-7.34 (m, 6H), 7.28-7.21 (m, 7H), 4.95 (dt, 1H, J = 8.1Hz, J = 6Hz), 3.61 (dd, 2H, J = 8.1Hz, J = 6Hz), which is in accord with the literature values [32].
3-(Phenylsulfanyl)cyclohexan-1-one (P2) 1H-NMR: 7.44-7.28 (m, 5H), 3.43 (m, 1H), 2.27 (2d, 1H, J = 4.8Hz), 2.42 (m, 3H-cycl.), 2.17 (m, 2H-cycl.), 1.17 (m, 2H-cycl.), which is in accord with [33].
3-(4-Methoxyphenylsulfanyl)-1,3-diphenylpropan-1-one (P3) 1H-NMR: 7.87 (d, 2H), 7.66-7.22 (m, 10H), 6.75 (d, 2H), 4.78 (t, 1H), 3.77 (s, 3H), 3.59 (m, 2H), which is in accord with [34].
3-(4-Fluorophenylsulfanyl)-1,3-32diphenylpropan-1-one (P4) 1H-NMR: 7.89 (d, 2H), 7.66-7.24 (m, 10H), 6.90 (t, 2H), 4.84 (t, 1H), 3.60 (m, 2H); 13C-NMR: 197.0, 164.5, 161.3, 145.0, 141.3, 136.9, 136.2, 136.1, 133.5, 132.9, 130.7, 129.2, 128.9, 128.6, 128.2, 127.9, 127.6, 116.2, 115.9, 49.3, 44.5.
1,3-Diphenyl-3-(p-tolylsulfanyl)propan-1-one (P5) 1H-NMR: 8.04 (d, 2H), 7.66-7.20 (m, 12H), 4.88 (dt, 1H, J = 6.3Hz, J = 1.8Hz), 3.60 (m, 2H), 2.29 (s, 3H), which is in accord with [35]
1,3-Diphenyl-3-(o-tolylsulfanyl)propan-1-one (P6) 1H-NMR: 7.87 (d, 2H), 7.55-7.12 (m, 12H), 4.89 (dt, 1H, J = 6Hz, J = 1.8Hz), 3.63 (m, 2H), 2.33 (s, 3H); 13C-NMR: 196.7, 151.5, 142.3, 141.6, 136.9, 133.4, 128.8, 128.7, 128.3, 128.3, 127.6, 110.6, 107.8, 45.3, 44.3, 28.1; Microanalysis: for C22H20OS (M.W. = 332.22) calc.: C, 79.48%; H, 6.06%; S, 9.64%; found: C, 79.51%; H, 6.11%; S, 9.49%.
3-(4-Bromobenzylsulfanyl)-1,3-diphenylpropan-1-one (P7) 1H-NMR: 7.84 (d, 2H), 7.54-7.05 (m, 12H), 4.42 (t, 1H), 3.48 (m, 4H); 13C-NMR: 196.8, 141.7, 137.2, 136.8, 133.4, 131.7, 130.8, 128.8, 128.2, 128.2, 127.6, 121.0, 45.4, 44.4, 35.4; Microanalysis: calc. for C22H19BrOS (M.W. = 411.36) C, 64.24%; H, 4.66%; Br, 19.42%; O, 3.89%; S 7.79%; found: C, 64.41%; H, 4.63%; Br, 19.18%; S, 7.71%.
3-(Furan-2-ylmethylsulfanyl)-1,3-diphenylpropan-1-one (P8) 1H-NMR: 7.86 (d, 2H), 7.54-7.22 (m, 9H), 6.29 (dt, 1H, J = 1.8Hz, J = 1.2Hz), 6.11 (d, 1H, J = 3.3Hz), 4.57 (dd, 1H, J = 6.3Hz, J = 1.8Hz), 3.54 (m, 2H); 13C-NMR: 197.4, 141.7, 140.9, 137.2, 133.9, 133.6, 133.5, 130.7, 129.0, 128.8, 128.5, 128.1, 128.0, 127.8, 126.8, 47.9, 45.1, 21.0; Microanalysis: calc. for C20H18O2S (M.W. = 322.43): C, 74.50%; H, 5.63%; S, 9.94%; found: C, 75.28%; H, 5.82%; S, 10.49%.
3-(1-Methyl-1H-imidazol-2-ylsulfanyl)-1,3-diphenylpropan-1-one (P9) 1H-NMR: 8.00 (d, 2H), 7.58-7.21 (m, 8H), 6.65 (d, 1H, J = 3.3Hz), 6.57 (d, 1H, J = 2.1Hz), 4.07 (d, 1H, J = 6Hz), 4.03 (d, 1H, J = 6Hz), 3.84 (dd, 1H, J = 9Hz, J = 8.7Hz), 3.62 (s, 3H); 13C-NMR: 196.4, 137.9, 136.7, 133.8, 129.3, 129.1, 128.7, 128.7, 128.2, 118.3, 115.4, 57.4, 42.0, 35.4; Microanalysis: calc. for C19H18N2OS (M.W. = 322.43): C, 70.78%; H, 5.63%; N, 8.69%; S, 9.94%; found: C, 70.62%; H, 5.52%; N, 8.56%; S, 9.69%.
4-Phenyl-4-Phenylsulfanylbutan-2-one (P10) 1H-NMR: 7.30-7.19 (m. 10H), 4.71 (t, 1H), 3.04 (m, 2H), 2.06 (s, 3H), which is in accord with [36].
(2-Nitro-1-phenylethyl)phenyl sulfide (P11) 1H-NMR: 7.41-7.26 (m, 10H), 4.91-4.76 (m, 2H), 4.71 (dd, 1H, J = 6.6Hz, J = 4.5Hz), which is in accord with [37].
3-(3-Methoxyphenylsulfanyl)cyclohexanone (P12) 1H-NMR: 7.23 (m, 2H), 7.01 (d, 1H, J = 18.5Hz), 6.96 (d, 1H, J = 7.8Hz), 6.83 (dd, 1H, J = 2.7Hz, J = 2.1Hz), 3.81 (s, 3H), 3.46 (m, 1H), 2.73 (dd, 1H, J = 4.8Hz, J = 3Hz), 2.41-2.22 (m, 3H), 2.17 (m, 2H), 1.75 (m, 2H), which is in accord with [10].
3-(2-Hydroxyethylsulfanyl)cyclohexanone (P13) 1H-NMR: 3.74 (m, 2H), 3.12 (m, 1H), 2.78 (m, 2H), 2.71 (m, 1H), 2.44-2.32 (m, 3H), 2.19-2.05 (m, 3H), 1.78-1.69 (m, 2H); 13C-NMR: 208.7, 61.1, 48.5, 42.9, 41.1, 33.9, 32.0, 24.3 Microanalysis: calc. for C8H14O2S (M.W. = 174.26): C, 55.14%; H, 8.10%; S, 8.40%; found: C, 55.19%; H, 8.14%; S, 8.49%.
3-(3-Methoxyphenylsulfanyl)-3-methylcyclohexanone (P14) 1H-NMR: 7.27 (t, 1H); 7.09 (m, 2H); 7.95 (dd, 1H, J = 2.4Hz, J = 0.9Hz); 3.83 (s, 3H); 2.53 (d, 1H); 2.35-2.15 (m, 4H); 1.95-1.80 (m, 3H); 1.30 (s, 3H); 13C-NMR: 209.6, 160.4, 123.3, 120.0, 115.7, 113.5, 112.9, 55.8, 53.6, 52.3, 40.8, 37.2, 28.9, 22.5; Microanalysis: calc. for C14H18O2S (M.W. = 250.36): C, 67.17%; H, 7.25%; S, 12.81%; found: C, 67.10%; H, 7.21%; S, 12.90%.
2-Hydroxymethyl-3-phenylsulfanylcyclohexanone (P15) 1H-NMR: 7.50-7.46 (m, 2H), 7.34-7.31 (m, 3H), 4.23-4.20 (dd, 1H, J = 6Hz, J = 2.1Hz), 4.09-3.99 (dd, 1H, J = 6.1Hz, J = 2.9Hz), 3.25 (dt, 1H, J = 4.2Hz), 2.56 (bs, 1H), 2.49 (m, 2H), 2.30, (m, 2H), 2.09 (m, 1H), 1.84 (m, 1H), 1.63 (m, 1H); 13C-NMR: 198.4, 138.9, 137.7, 137.2, 136.9, 136.7, 135.3, 62.4, 60.1, 56.8, 48.8, 32.8, 25.8; Microanalysis: calc. for C13H16O2S (M.W. = 236.34): C, 66.07%; H, 6.82%; S, 13.57%; found: C, 66.16%; H, 6.87%; S, 13.65%.
5-Methyl-4-phenylsulfanylhexan-2-one (P16) 1H-NMR: 7.43 (2d, 2H), 7.30-7.20 (m, 3H), 3.61 (m, 1H), 2.69 (d, 2H, J = 6.6Hz), 2.14 (s, 3H), 1.97 (m, 1H), 1.01 (d, 3H), 0.96 (d, 3H); 13C-NMR: 206.8, 135.7, 131.6, 128.9, 126.7, 50.7, 45.9, 31.9, 30.7, 19.5, 18.9; Micronalysis: calc. for C13H18OS (M.W. = 222.35): C, 70.22%; H, 8.16%; S, 14.42%; found: C,70.18%; H, 8.10%; S, 14.39%.
4-Methyl-4-phenylsulfanyl-pentan-2-one (P17) 1H-NMR: 7.38 (m, 2H), 7.20 (m, 3H), 2.52 (s, 2H), 1.98 (s, 3H), 1.22 (s, 6H), which is in accord with [38].
4-Phenylsulfanyl-butan-2-one (P18) 1H-NMR: 7.38-7.28 (m, 4H), 7.24 (t, 1H), 3.17 (t, 2H), 2.77 (t, 2H), 2.17 (s, 3H), which is in accord with [39].
References
- Kondo, T.; Mitsudo, T. Chem. Rev. 2000, 56, 3205.
- Garg, S.K.; Kumar, R.; Chakraborti, A.S. Synlett 2005, 1370.
- List, B.; Lerner, R.A.; Barbas, C.F., III. J. Am. Chem. Soc. 2000, 122, 2395.
- List, B. Synlett 2001, 1675.
- List, B. Tetrahedron 2002, 58, 5573.
- Grőger, H.; Wilken, J. Angew. Chem.Int. Ed. 2001, 40, 529. [CrossRef]
- Dalko, P.I.; Moisan, L. Angew. Chem. Int. Ed. 2001, 40, 3727. [CrossRef]
- Berkessel, A.; Grőger, A. (Eds.) Asymmetric Organocatalysis; Wiley-VCH: Weinheim, 2005.
- Helder, R.; Arends, R.; Bolt, W.; Hiemstra, H.; Wynberg, H. Tetrahedron Lett. 1977, 2185.
- Hiemstra, H.; Wynberg, H. J. Am. Chem. Soc. 1981, 103, 417.
- Agami, C.; Platzer, N.; Puchot, C.; Sevestre, H. Tetrahedron Lett. 1987, 53, 1091.
- Mukaiyana, T.; Ikegawa, A.; Suzuki, K. Chem. Lett. 1981, 165.
- Suzuki, K.; Ikegawa, A.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 1982, 55, 3277. [CrossRef]
- Kobayashi, S.; Ogawa, C.; Kawamura, M.; Sugiura, M. Synlett 1981, 983.
- Holbrey, J.D.; Seddon, K.R. Clean Prod. Processes 1999, 1, 223.
- Olivier-Bourbigou, H.; Magma, L. J. Mol. Catal. A. Chem. 2002, 182–183, 419.
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Chem. Rev. 2002, 102, 3667.
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Catal. Today 2002, 74, 157.
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, 2003.
- Sheldon, R. Chem. Commun. 2001, 2399.
- Song, C.E. Chem. Commun. 2004, 1033.
- Wilkes, J.S. J. Mol. Catal. A. Chem. 2004, 214, 11–17. [CrossRef]
- Welton, T. Coord. Chem. Rev. 2004, 248, 2459–2477.
- Kotrusz, P.; Kmentová, I.; Gotov, B.; Toma, Š. Chem. Commun. 2002, 2510. [CrossRef]
- Loh, J.S.; Feng, l.C.; Yang, H.Y.; Yang, J.Y. Tetrahedron Lett. 2002, 43, 8741.
- Kotrusz, P.; Toma, S.; Schmalz, H.G.; Adler, A. Eur. J. Org. Chem. 2004, 1577.
- Rasalkar, M.S.; Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M. J. Mol. Catal. A. Chem. 2005, 235, 267–270. [CrossRef]
- Hagiwara, H.; Okabe, T.; Hoshi, T.; Suzuki, T. J. Mol. Catal. A: Chem. 2004, 214, 167–174.
- Yadav, S.; Reddy, B.V.S.; Baishya, G. J. Org. Chem. 2003, 68, 7098.
- Ranu, B.C.; Dey, S.S. Tetrahedron 2004, 60, 4183.
- Dere, R.T.; Pal, R.R.; Patil, P.S.; Salunkhe, M.M. Tetrahedron Lett. 2003, 44, 5351.
- Narasaka, K.; Arai, N.; Okauchi, T. Bull. Chem. Soc. Jpn. 1993, 66, 2995. [CrossRef]
- da Silva, F.M.; Gomes, A.K.; Jones, J. Can. J. Chem. 1999, 77, 624.
- Skarzewski, J.; Zielinska-Blajet, M.; Turowska-Tyrk, I. Tetrahedron Asymmetry 2001, 12, 1923.
- Omote, M. Nippon Kagaku Kaishi 1972, 780, [Chem. Abstr.; 1972, 77, 33637f].
- Boldwell, J.R.; Patwardhan, B.H.; Dittmer, D.C. J. Org. Chem. 1984, 49, 4192.
- Cann, S. J. Chem. Soc. Perkin Trans. 2 1974, 817. [CrossRef]
- MacNicol, D.D.; McKendrick, J.J. J. Chem. Soc. Perkin Trans. 1 1974, 2493. [CrossRef]
- Wabnitz, T.C.; Spencer, J.B. Org. Lett. 2003, 5, 2141.
- Sample availability: Samples of the reaction products can be obtained from the author (P.K.)
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.