Abstract
The coupling reaction of benzoic acid and nicotinic acid hydrazides with N-protected L-amino acids including valine, leucine, phenylalanine, glutamic acid and tyrosine is reported. The target compounds, N-Boc-amino acid-(N`-benzoyl)- and N-Boc-amino acid-(N`-nicotinoyl) hydrazides 5a-5e and 6a-6e were prepared in very high yields and purity using N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl- methylene]-N-methyl-methanaminium hexafluorophosphate N-oxide (HATU) as coupling reagent. The antimicrobial activity of the Cu and Cd complexes of the designed compounds was tested. The products were deprotected affording the corresponding amino acid-(N`-benzoyl) hydrazide hydrochloride salts (7a-7e) and amino acid-(N`- nicotinoyl) hydrazide hydrochloride salts (8a-8e). These compounds and their Cu and Cd complexes were also tested for their antimicrobial activity. Several compounds showed comparable activity to that of ampicillin against S. aureus and E. coli.
Introduction:
Amino acids have proven to play a significant role in the synthesis of novel drug candidates with the use of non-proteinogenic and unnatural amino acids [1,2,3,4,5,6,7,8]. Recently a series of novel diflunisal hydrazide-hydrazone derivatives have demonstrated significant antimicrobial activity [9]. These synthesized compounds and additional hydrazide derivatives were screened for their wide range of biological activities, antimycobacterial activity against Mycobacterium tuberculosis, antimicrobial activities against various bacteria (Escherichia coli and Bacillus subtilis), fungi, and yeast species [10,11,12,13]. In addition, nicotinamide and nicotinic acid have been in use for 65 years due to their unusual antimicrobial spectrum. Many drugs possess modified pharmacological and toxicological properties when administered in form of metallic complexes [14,15,16]. In view of this it was interesting to synthesize several new compounds to evaluate the effect of amino acids on the bioactivity of both benzoic acid hydrazide and nicotinic acid hydrazide. It was also of interest to study the effect of metals such as Cu and Cd on the antibacterial activity of these compounds, thus both hydrazide ligands and complexes were tested for their antibacterial and antifungal activity.
Results and Discussion
Chemistry
For the synthesis of the desired compounds, the sequence of reactions shown in Scheme 1 was followed. The reaction of benzoic acid hydrazide (3) with different N-Boc-L-amino acids in dimethylformamide in the presence of triethylamine (Et3N) as a base and N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methyl-ene]-N-methylmethanaminium hexafluorophsphate N-oxide (HATU) as coupling reagent at 0°C gave the desired compounds 5a-5e. The structures of compounds 5a-5e were established by means of IR, 1H-NMR, 13C-NMR, and elemental analyses. The infrared spectra of these compounds showed the absorption peaks of the urethane and the amide carbonyls in the 1715-1727 cm-1 and 1663-1693 cm-1 ranges, respectively, and the absorption peak of the N-H in the 3226-3336 cm-1 range. The 1H-NMR showed the presence of three exchangeable signals for the NH and the 13C-NMR detected the presence of one urethane carbonyl and two amide carbonyls in the 153-173 ppm range. Similarly, nicotinic acid hydrazide (4) was allowed to react under the same conditions with the same N-Boc-L-amino acids to give the desired compounds 6a-6e. The structures of compounds 6a-6e were also established by means of IR, 1H-NMR, 13C-NMR, and elemental analyses. Compounds 5 and 6 were subjected to N-deprotection by passing HCl gas into a solution of these compounds in methylene chloride/ether. The latter series upon deprotection of Boc group afforded the new series of compounds 7 and 8 (Table 1, Table 2).
Compounds 5, 6, 7 and 8 were allowed to undergo complexation with Cu and Cd. All complexes were obtained by reacting one equivalent of the ligands 5, 6, 7 or 8 once with 2 equivalents of Cu(NO3)2 and once with 2 equivalents of Cd(CH3COO)2 in methanol.
The Cu and Cd complexes were later decomposed and their Cu and Cd contents were analyzed by atomic absorption to determine the ratio of complex formation of Cu and Cd to ligand. The atomic absorption analysis showed the formation of complexes Cu:L and Cd:L in the ratio (1:1).
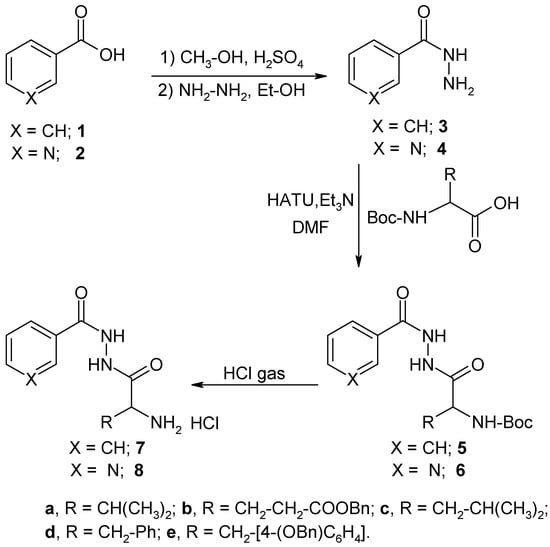
Scheme 1.

Table 1.
Yields, Color, Mps., and Elemental Analyses of Compounds 7 and 8.
| Cmpd | Yield (%) | Color | mp (°C) | Elemental Analysis (Found) | ||
|---|---|---|---|---|---|---|
| 7a | 75 | White | 244 | 53.04(52.89) | 6.68 (6.83) | 15.46(15.62) |
| 7b | 84 | White | 155 | 58.24(58.39) | 5.66 (5.89) | 10.72(10.96) |
| 7c | 93 | White | 190 | 54.64(54.91) | 7.05 (7.32) | 14.70(14.95) |
| 7d | 83 | White | 238 | 60.09(59.84) | 5.67 (5.92) | 13.14(13.32) |
| 7e | 89 | White | 233 | 64.86(64.58) | 5.68 (5.89) | 9.87 (9.59) |
| 8a* | 77 | White | 290 | --------------- | --------------- | --------------- |
| 8b* | 81 | White | 267 | --------------- | --------------- | --------------- |
| 8c* | 78 | White | 253 | --------------- | --------------- | --------------- |
| 8d* | 89 | White | 215 | --------------- | --------------- | --------------- |
| 8e* | 96 | White | 197 | --------------- | --------------- | --------------- |
* = Elemental analysis was unreliable due to their hygroscopic nature.

Table 2.
1H-NMR Data of Compounds 7 and 8.
| Compound | 1H-NMR (DMSO-d6): (δ) ppm |
|---|---|
| 7a | 0.89, 0.97 (2d, 6H, 2 CH3), 1.92 (m, 1H, CH), 4.89 (d, 1H, CH), 7.40-7.88 (m, 5H, aromatic), 8.38 (br.s, 2H, 2 NH), 10.64 (br.s, 2H, 2 NH). |
| 7b | 1.99 (m, 2H, CH2), 2.63, 2.75 (2m, 2H, CH2-CO), 4.55 (m, 1H, CH), 5.14 m, 2H, CH2-O), 7.21-7.90 (m, 10H, aromatic), 8.36 (br.s, 2H, 2 NH), 10.66 (br.s, 2H, 2 NH). |
| 7c | 0.91 (dd, 6H, 2 CH3), 1.63 (m, 2H, CH2), 1.81 (m, 1H, CH), 3.83 (m, 1H, CH), 7.46-7.87 (m, 5H, aromatic), 8.42 (br.s, 2H, 2 NH), 10.61 (br.s, 2H, 2 NH). |
| 7d | 3.07 (2m, 2H, CH2), 4.16 (m, 1H, CH), 7.22-7.89 (m, 10H, aromatic), 8.34 (br.s, 2H, 2 NH), 10.65 (br.s, 2H, 2 NH). |
| 7e | 3.08 (m, 2H, CH2), 4.29 (m, 1H, CH), 5.11 (br.s, 2H, CH2), 7.24-7.96 (m, 14H, aromatic), 8.45 (br.s, 2H, 2 NH), 10.59 (br.s, 2H, 2 NH). |
| 8a | δ 0.87, 0.96 (2d, 6H, 2 CH3), 1.96 (m, 1H, CH), 4.01 (m, 1H, CH), 7.79 (d, 2H, aromatic), 8.40 (br.s, 2H, 2 NH), 8.78 (d, 2H, aromatic), 10.62 (br.s, 2H, 2 NH). |
| 8b | 2.03 (m, 2H, CH2), 2.35 (m, 2H, CH2-CO), 4.09 (m, 1H, CH), 5.14 m, 2H, CH2-O), 7.33-7.82 (m, 7H, aromatic), 8.44 (br.s, 2H, 2 NH), 8.77 (d, 2H, aromatic), 10.66 ( br.s, 2H, 2 NH). |
| 8c | δ 0.92 (d, 6H, 2 CH3), 1.59 (m, 2H, CH2), 1.79 (m, 1H, CH), 4.07 (m, 1H, CH), 7.79 (d, 2H, aromatic), 8.39 (br.s, 2H, 2 NH), 8.75 (d, 2H, aromatic), 10.71 (br.s, 2H, 2 NH). |
| 8d | 3.09 (m, 2H, CH2), 4.33 (m, 1H, CH), 7.25-7.39 (m, 5H, aromatic), 7.85 (d, 2H, aromatic), 8.43 (br.s, 2H, 2 NH), 8.79 (d, 2H, aromatic), 10.79 (br.s, 2H, 2 NH). |
| 8e | 3.04 (m, 2H, CH2), 4.27 (m, 1H, CH), 5.14 (br.s, 2H, CH2), 7.24-7.47 (m, 9H, aromatic), 7.82 (d, 2H, aromatic), 8.45 (br.s, 2H, 2 NH), 8.79 (d, 2H, aromatic), 10.62 (m, 2H, 2 NH); |
Biological Test Results
The synthesized compounds 5, 6, 7 and 8 and their Cu and Cd complexes have been evaluated for their antimicrobial activity. The microdilution susceptibility test in Müller-Hinton Broth (Oxoid) and Sabouraud Liquid Medium (Oxoid) were used for the determination of antibacterial and antifungal activity [17]. The minimal inhibitory concentration (MIC) values listed in Table 3 show that all the test compounds have lower antifungal activity than clotrimazole (Canesten®, Bayer).

Table 3.
Minimal inhibitory concentration (MIC) of test compounds in μg/mL.
| Test compound | E. coli | S. aureus | C. albicans | Test compound | E. coli | S. aureus | C. albicans |
|---|---|---|---|---|---|---|---|
| ampicillin | 25 | 12.5 | --------- | (Cu:L),(1:1) of 6e | 100 | 12.5 | 200 |
| clotrimazole | ------- | -------- | 12.5 | (Cd:L),(1:1) of 5a | 100 | >200 | >200 |
| 5a | >200 | >200 | >200 | (Cd:L),(1:1) of 5b | >200 | 25 | >200 |
| 5b | >200 | >200 | >200 | (Cd:L),(1:1) of 5c | >200 | >200 | >200 |
| 5c | 100 | 100 | >200 | (Cd:L),(1:1) of 5d | >200 | 25 | >200 |
| 5d | 50 | >200 | >200 | (Cd:L),(1:1) of 5e | >200 | >200 | >200 |
| 5e | >200 | >200 | >200 | (Cd:L),(1:1) of 6a | >200 | >200 | >200 |
| 6a | >200 | >200 | >200 | (Cd:L),(1:1) of 6b | >200 | 100 | >200 |
| 6b | 100 | >200 | >200 | (Cd:L),(1:1) of 6c | 100 | >200 | >200 |
| 6c | 50 | >200 | >200 | (Cd:L),(1:1) of 6d | >200 | >200 | >200 |
| 6d | 100 | >200 | >200 | (Cd:L),(1:1) of 6e | 100 | >200 | >200 |
| 6e | 50 | >200 | >200 | (Cu:L),(1:1) of 7a | >200 | >200 | >200 |
| 7a | 100 | 100 | >200 | (Cu:L),(1:1) of 7b | >200 | >200 | >200 |
| 7b | 25 | >200 | >200 | (Cu:L),(1:1) of 7c | >200 | 100 | >200 |
| 7c | 100 | 50 | >200 | (Cu:L),(1:1) of 7d | >200 | 50 | >200 |
| 7d | 100 | >200 | >200 | (Cu:L),(1:1) of 7e | >200 | 50 | >200 |
| 7e | 200 | 50 | >200 | (Cu:L),(1:1) of 8a | >200 | >200 | >200 |
| 8a | 25 | >200 | >200 | (Cu:L),(1:1) of 8b | >200 | >200 | >200 |
| 8b | 100 | 100 | >200 | (Cu:L),(1:1) of 8c | >200 | >200 | >200 |
| 8c | 50 | >200 | >200 | (Cu:L),(1:1) of 8d | 100 | >200 | >200 |
| 8d | 100 | 100 | >200 | (Cu:L),(1:1) of 8e | >200 | 100 | >200 |
| 8e | 50 | 100 | >200 | (Cd:L),(1:1) of 7a | 100 | >200 | >200 |
| (Cu:L),(1:1) of 5a | 200 | 100 | >200 | (Cd:L),(1:1) of 7b | >200 | >200 | >200 |
| (Cu:L),(1:1) of 5b | >200 | >200 | 100 | (Cd:L),(1:1) of 7c | 100 | 12.5 | >200 |
| (Cu:L),(1:1) of 5c | 100 | 50 | >200 | (Cd:L),(1:1) of 7d | 50 | >200 | >200 |
| (Cu:L),(1:1) of 5d | >200 | 50 | >200 | (Cd:L),(1:1) of 7e | >200 | >200 | >200 |
| (Cu:L),(1:1) of 5e | 200 | 25 | >200 | (Cd:L),(1:1) of 8a | >200 | >200 | >200 |
| (Cu:L),(1:1) of 6a | >200 | 100 | >200 | (Cd:L),(1:1) of 8b | 100 | >200 | >200 |
| (Cu:L),(1:1) of 6b | >200 | >200 | >200 | (Cd:L),(1:1) of 8c | >200 | >200 | >200 |
| (Cu:L),(1:1) of 6c | >200 | >200 | >200 | (Cd:L),(1:1) of 8d | 100 | >200 | >200 |
| (Cu:L),(1:1) of 6d | 200 | 100 | >200 | (Cd:L),(1:1) of 8e | >200 | >200 | >200 |
The test compounds are more active against S. aureus and E. coli. Compounds (Cu:L),(1:1) of 6e and (Cd:L),(1:1) of 7c have antimicrobial activity against S. aureus comparable to that of ampicillin, while the activity of compounds (Cu:L),(1:1) of 5e, (Cd:L),(1:1) of 5b and (Cd:L),(1:1) of 5d is about 50% of that of ampicillin. Compounds 7b and 8a have antimicrobial activity against E. coli comparable to that of ampicillin, while the activity of compounds 5d, 6c, 6e, 8c, 8e, (Cd:L),(1:1) of 7d is about 50% of that of ampicillin.
Conclusions
The present work describes the synthesis of amino acid derivatives and its metal complexes which showed interesting results comparable to ampicillin. The uncomplexed compounds showed higher antimicrobial activity against E. coli compared to their respective complexes. While the complexes showed higher antimicrobial activity against S. aureus compared to their respective ligands. These results prompted us to further pursue in SAR as our future plane.
Experimental
General
Melting points were determined on a Mel-Temp apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded on a Bruker 300 MHz spectrometer with chemical shift values reported in δ units (ppm) relative to an internal standard (tetramethylsilane). Infrared data were obtained on a Perkin-Elmer 1600 series Fourier transform instrument as KBr pellets. Amino acids are abbreviated and designated following the rules of the IUPAC-IUB Commission of Biochemical Nomenclature (J. Biol. Chem., 1972, 247, 977). The abbreviations: HATU (N-[(dimethylamino)-1H-1,2,3-triazolo[4,5-b]pyridin-1-ylmethylene]-N-methyl-methanaminium hexafluorophosphate N-oxide); Boc (t-butyloxy-carbonyl); Et3N (triethylamine) and DMF (N,N-dimethylformamide) were used throughout the manuscript. Follow up of the reactions and checking the homogeneity of the compounds were made by TLC on silica gel-protected aluminum sheets (Type 60 F254, Merck) and the spots were detected by exposure to UV-lamp at λ 254 nm for few seconds. Elemental analyses were performed in the Chemistry Department, Faculty of Science, Cairo, University.
Chemistry
General Procedure for the Reaction of N-Boc-L-Amino Acids with Benzoic Acid Hydrazide.
A mixture of N-Boc-L-amino acid (1 mmol), HATU (0.38 g, 1 mmol) and Et3N (0.28 mL, 2 mmol) was stirred at 0°C for 3 minutes in DMF (2 mL). Then benzoic acid hydrazide (3, 0.122 g, 1 mmol) was added. The reaction mixture was stirred at 0°C for 1 hour and left overnight at room temperature. The reaction mixture was diluted with ethyl acetate (80 mL) and the mixture was washed successively with 5% aqueous citric acid solution (2°10 mL), saturated sodium bicarbonate solution (2°10 mL) and saturated sodium chloride solution (2°10 mL). The organic layer was dried over anhydrous sodium sulphate, filtered and the solvent was removed in vacuo. The crude product was crystallized from methylene chloride-hexane. In the case of N-Boc L-(O-benzyl)-tyrosine (5e) and N-Boc-L-phenylalanie (5d) the reaction mixture was poured into ice water, filtered, washed with 5% aqueous citric acid solution, saturated sodium bicarbonate solution and finally with water, dried and recrystallized from methylene chloride-hexane.
Boc-valyl(N`-benzoyl)-hydrazide (5a): White crystals, 0.32 g (95.4 % yield); mp 156-157°C; IR: 3308, 3226 (NH), 1715 (C=O, urethane), 1689 (C=O, amide), 1668 (C=O, amide) cm-1; 1H-NMR (CDCl3): δ 0.87, 0.95 (2d, 6H, 2 CH3), 1.35 (s, 9H, 3 CH3), 1.88 (m, 1H, CH), 4.93 (d, 1H, CH), 7.20-7.77 (m, 6H, 1 NH, aromatic), 9.17, 9.34 (2 br.s, 2H, 2 NH); 13C-NMR (CDCl3): δ 19.61, 19.70, 26.40, 28.62, 50.49, 80.69, 127.40, 129.00, 129.71, 131.79, 132.70, 136.58, 156.00, 164.62, 169.01; Anal. Calcd for C17H25N3O4: C, 60.88; H, 7.51; N, 12.53. Found: C, 60.72; H, 7.68; N, 12.81.
Boc-(o-benzyl)-glutamyl(N`-benzoyl)-hydrazide (5b): White powder, 0.38 g (80 % yield); mp 126°C; IR: 3336, 3253 (NH), 1753 (C=O, ester), 1727 (C=O, urethane), 1686 (C=O, amide), 1663 (C=O, amide) cm-1; 1H-NMR (CDCl3): δ 1.37 (s, 9H, 3 CH3), 1.90, 2.05 (2m, 2H, CH2), 2.60, 2.71 (2m, 2H, CH2-CO), 4.50 (m, 1H, CH), 5.15 m, 2H, CH2-O), 7.20-7.81 (m, 11H, 1 NH, aromatic), 9.17, 9.34 (2 br.s, 2H, 2 NH); 13C-NMR (CDCl3): δ 24.96, 28.16, 28.67, 49.70, 66.94, 80.72, 127.79, 128.65, 128.94, 128.95, 129.02, 131.87, 132.69, 136.10, 136.16, 153.64, 165.20, 169.82, 173.20; Anal. Calcd for C24H29N3O6: C, 63.28; H, 6.42; N, 9.22. Found: C, 63.02; H, 6.21; N, 8.96.
Boc-leucyl(N`-benzoyl)-hydrazide (5c): White crystals, 0.26 g (74 % yield); mp 147°C; IR: 3318, 3234 (NH), 1726 (C=O, urethane), 1689 (C=O, amide), 1672 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 0.91 (d, 6H, 2 CH3), 1.38 (s, 9H, 3 CH3), 1.51 (m, 2H, CH2), 1.72 (m, 1H, CH), 4.12 (m, 1H, CH), 6.85 (d, 1H, 1 NH), 7.2-7.77 (m, 5H, aromatic), 10.10, 10.40 (2 br.s, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 21.88, 23.00, 23.29, 24.47, 28.54, 51.64, 79.20, 126.00, 127.50, 131.65, 153.65, 156.11, 171.15; Anal. Calcd for C18H27N3O4: C, 61.87; H, 7.79; N, 12.03. Found: C, 62.05; H, 7.92; N, 12.26.
Boc-phenylalanyl(N`-benzoyl)-hydrazide (5d): White powder, 0.31 g (81 % yield); mp 168°C; IR: 3314, 3233 (NH), 1725 (C=O, urethane), 1692 (C=O, amide), 1673 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 1.30 (s, 9H, 3 CH3), 2.82, 3.08 (2m, 2H, CH2), 4.30 (m, 1H, CH), 7.04 (d, 1H, 1 NH), 7.18-7.92 (m, 10H, aromatic), 10.18, 10.29 (2 br.s, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 28.50, 37.68, 54.85, 126.58, 127.80, 128.40, 128.81, 129.59, 132.18, 153.62, 164.9, 173.10; Anal. Calcd for C21H25N3O4: C, 65.78; H, 6.57; N, 10.96. Found: C, 65.98; H, 6.78; N, 10.71.
Boc-(o-benzyl)-tyrosyl(N`-benzoyl)-hydrazide (5e): White crystals, 0.47 g (96 % yield); mp 177°C; IR: 3322, 3245 (NH), 1726 (C=O, urethane), 1693 (C=O, amide), 1673 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, 3 CH3), 2.85, 3.05 (2m, 2H, CH2), 4.25 (m, 1H, CH), 5.05 (br.s, 2H, CH2), 6.92-7.93 (m, 15H, aromatic, 1NH), 10.15, 10.46 (2s, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 26.52, 32.01, 53.48, 68.50, 77.52, 112.81, 125.90, 126.02, 126.81, 128.69, 130.21, 135.6, 153.62, 155.04, 163.81, 170.00; Anal. Calcd for C28H31N3O5: C, 68.69; H, 6.38; N, 8.58. Found: C, 68.51; H, 6.15; N, 8.73.
General Procedure for the Reaction of N-Boc-L-Amino Acids with Nicotinic Acid Hydrazide.
A mixture of N-Boc-L-amino acid (1 mmol), HATU (0.38 g, 1 mmol) and Et3N (0.15 mL, 1 mmol) was stirred at 0°C for 3 minutes in DMF (2 mL). Then nicotinic acid hydrazide (4, 0.123 g, 1 mmol) was added. The reaction mixture was stirred at 0°C for 1 hour and left overnight at room temperature. The reaction mixture was diluted with methylene chloride (80 mL) and the mixture was washed with 5% aqueous citric acid solution (2°10 mL), saturated sodium bicarbonate solution (2°10 mL) and saturated sodium chloride solution (2°10 mL). The organic layer was dried over anhydrous sodium sulphate, filtered and the solvent was removed in vacuo. The crude product was crystallized from methylene chloride-hexane.
Boc-valyl(N`-nicotinoyl)-hydrazide (6a): White crystals, 0.28 g (83.3 % yield); mp 209°C; IR: 3344, 3242 (NH), 1726 (C=O, urethane), 1708 (C=O, amide), 1661 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 0.86, 0.94 (2d, 6H, 2 CH3), 1.39 (s, 9H, 3 CH3), 1.96 (m, 1H, CH), 3.93 (m, 1H, CH), 6.81 (d, 1H, 1 NH), 7.77 (d, 2H, aromatic), 8.75 (d, 2H, aromatic), 10.12, 10.68 (2 br.s, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 18.7, 19.5, 28.6, 30.7, 58.7, 78.4, 121.7, 139.8, 150.7, 155.7, 164.1, 171.1; Anal. Calcd for C16H24N4O4: C, 57.13; H, 7.19; N, 16.66. Found: C, 57.34; H, 7.35; N, 16.41.
Boc-(o-benzyl)-glutamyl(N`-nicotinoyl)-hydrazide (6b): White powder, 0.42 g (92 % yield); mp 120°C; IR: 3334, 3243 (NH), 1740 (C=O, ester), 1727 (C=O, urethane), 1681 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 1.38 (s, 9H, 3 CH3), 1.84, 2.04 (2m, 2H, CH2), 2.32 (m, 2H, CH2-CO), 4.07 (m, 1H, CH), 5.14 m, 2H, CH2-O), 7.02 (m, 1H, 1 NH), 7.34 (br.s, 5H, aromatic), 7.77 (d, 2H, aromatic), 8.75 (d, 2H, aromatic), 10.13, 10.66 (2 br.s, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 26.6, 28.3, 30.0, 53.6, 66.2, 78.7, 121.6, 128.1, 128.4, 128.5, 128.7, 136.3, 139.7, 150.8, 156.0, 164.3, 171.1, 172.6, 175.8; Anal. Calcd for C23H28N4O6: C, 60.52; H, 6.18; N, 12.27. Found: C, 60.37; H, 6.32; N, 12.45.
Boc-leucyl(N`- nicotinoyl)-hydrazide (6c): White crystals, 0.25 g (71.3 % yield); mp 210°C; IR: 3301, 3222 (NH), 1710 (C=O, urethane), 1662 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 0.91 (d, 6H, 2 CH3), 1.38 (s, 9H, 3 CH3), 1.53 (m, 2H, CH2), 1.72 (m, 1H, CH), 4.13 (m, 1H, CH), 6.85 (d, 1H, 1 NH), 7.77 (d, 2H, aromatic), 8.71 (d, 2H, aromatic), 10.10, 10.70 (2 br.s, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 21.88, 23.00, 23.29, 24.47, 28.54, 51.64, 78.75, 121.64, 139.77, 150.48, 155.55, 163.97, 172.38; Anal. Calcd for C17H26N4O4: C, 58.27; H, 7.48; N, 15.99. Found: C, 58.02; H, 7.21; N, 16.26.
Boc-phenylalanyl(N`-nicotinoyl)-hydrazide (6d): White powder, 0.29 g (75.4 %) yield, mp 145°C; IR: 3309, 3234 (NH), 1726 (C=O, urethane), 1695 (C=O, amide), 1677 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 1.29 (s, 9H, 3 CH3), 2.82, 3.07 (2m, 2H, CH2), 4.31 (m, 1H, CH), 7.05-7.37 (m, 6H, aromatic, 1 NH), 7.81 (d, 2H, aromatic), 8.76 (d, 2H, aromatic), 10.33, 10.82 (2 br.s, 2H, 2 NH)); 13C-NMR (DMSO-d6): δ 28.12, 28.48, 37.82, 54.87, 78.4, 121.68, 126.62, 128.42, 129.57, 138.30, 139.70, 150.77, 155.67, 164.18, 171.70; Anal. Calcd for C20H24N4O4: C, 62.49; H, 6.29; N, 14.57. Found: C, 62.65; H, 6.50; N, 14.83.
Boc-(o-benzyl)-tyrosyl(N`-nicotinoyl)-hydrazide (6e): White crystals, 0.38 g (77.5 %) yield, mp 141°C; IR: 3311, 3237 (NH), 1727 (C=O, urethane), 1695 (C=O, amide), 1677 (C=O, amide) cm-1; 1H-NMR (DMSO-d6): δ 1.33 (s, 9H, 3 CH3), 2.83, 3.05 (2m, 2H, CH2), 4.26 (m, 1H, CH), 5.10 (br.s, 2H, CH2), 6.96-7.47 (m, 10H, aromatic, 1NH), 7.83 (d, 2H, aromatic), 8.78 (d, 2H, aromatic),10.36, 10.63 (2m, 2H, 2 NH); 13C-NMR (DMSO-d6): δ 28.20, 28.54, 37.03, 55.19, 69.50, 78.38, 114.77, 121.70, 127.99, 128.77, 130.63, 137.60, 140.06, 150.72, 155.68, 157.32, 164.08, 171.64; Anal. Calcd for C27H30N4O5: C, 66.11; H, 6.16; N, 11.42. Found: C, 65.82; H, 5.95; N, 11.59.
General Procedure for the Preparation of the Hydrochloride Salts 7 and 8.
5 or 6 (1 mmol) were dissolved in a mixture of methylene chloride (4 mL) and ether (4 mL). HCl gas was passed through the solution for 2 hours. A white precipitate that formed was filtered and washed with anhydrous ether. The crude product was recrystallized from methyl alcohol-anhydrous ether to give white crystals. The amino acid-(N`- nicotinoyl) hydrazide hydrochloride salts 8a-8e were obtained as hygroscopic salts.
General Procedure for the Preparation of the Cu Complexes.
A solution of Cu(NO3)2 (0.48 gm, 2 mmol) in methyl alcohol (2 mL) was added to a solution of 5, 6, 7 or 8 (1 mmol) in methyl alcohol (4 mL). A change was observed in the color of the solution. The Cu complexes were obtained as green crystals. The Cu complexes were later decomposed and their Cu content was analyzed by atomic absorption to determine the ratio of complex formation of Cu to ligand. The atomic absorption analysis showed the formation of complexes Cu:L in the ratio (1:1).
General Procedure for the Preparation of the Cd Complexes
A solution of Cd(CH3COO)2 (0.46 gm, 2 mmol) in methyl alcohol (2 mL) was added to a solution of 5, 6, 7 or 8 (1 mmol) in methyl alcohol (4 mL). The Cd complexes were obtained as white crystals. The Cd complexes were later decomposed and their Cd content was analyzed by atomic absorption to determine the ratio of complex formation of Cd to ligand. The atomic absorption analysis showed the formation of complexes Cd:L in the ratio (1:1).
In vitro antimicrobial activity
The microdilution susceptibility test in Müller-Hinton Broth (Oxoid) and Sabouraud Liquid Medium (Oxoid) were used for the determination of antibacterial and antifungal activity [17,18]. The utilized test organisms were: Escherichia coli (E. coli) ATCC 25922 as an example of Gram-negative bacteria, Staphylococcus aureus (S. aureus) ATCC 19433 as an example of Gram-positive bacteria and Candida albicans (C. albicans) as yeast-like fungi. Ampicillin trihydrate and clotrimazole were used as standard antibacterial and antifungal agents, respectively. Solutions of the test compounds, ampicillin trihydrate and clotrimazole were prepared in DMSO to a concentration of 1600 μg/mL. Twofold dilutions of the compounds were prepared (800, 400, … 6.25 μg/mL). Microorganism suspensions at 106 CFU/mL (Colony Forming Unit/mL) concentrations were inoculated to the corresponding wells. Plates were incubated at 36°C for 24 h to 48 h. The incubation chamber was kept sufficiently humid. At the end of the incubation period, the minimal inhibitory concentrations (MIC) were determined.
Acknowledgements
Author is very grateful to Dr. Adnan A. Bekhit, Department of Pharmaceutical Chemistry, and Dr. Elsayed Aboulmagd, Department of Microbiology, Faculty of Pharmacy, University of Alexandria, Egypt; for performing the antimicrobial evaluation.
References
- Barrett, D.; Tanaka, A.; Harada, K.; Ohki, H.; Watabe, E.; Maki, K.; Ikeda, F. Synthesis and biological activity of novel macrocyclic antifungals: acylated conjugates of the ornithine moiety of the lipopeptidolactone FR901469. Bioorg. Med. Chem. Lett. 2001, 11, 479–482. [Google Scholar] [CrossRef]
- Kovalainen, J. T.; Christians, J. A. M.; Kotisaari, S.; Laitinen, J. T.; Mannisto, P.T. Synthesis and in vitro pharmacology of a series of new chiral histamine H3-receptor ligands:2-(R and S)-amino-3-(1H-imidazol-4(5)-yl)propyl ether derivatives. J. Med. Chem. 1999, 42, 1193–202. [Google Scholar]
- El-Faham, A.; El Massry, A. M.; Amer, A.; Gohar, Y. M. A versatile synthetic route to chiral quinoxaline derivatives from amino acids precursors. Lett. Pept. Sci. 2002, 9, 49–54. [Google Scholar]
- Polyak, F.; Lubell, W. D. Rigid dipeptide mimics: Synthesis of enantiopure 5- and 7-benzyl, and 5,7-dibenzyl indolizidinone amino acids via enolization and alkylation of δ-oxo α,ω-di-[N-(9-(9-phenylfluorenyl))amino]azelate esters. J. Org. Chem 1998, 63, 5937–5949. [Google Scholar] Roy, S.; Lombart, H.-G.; Lubell, W. D.; Hancock, R. E. W.; Farmer, S. W. Exploring relationships between mimic configuration, peptide conformation and biological activity in indolizidin-2-one amino acid analogs of gramicidin S. J. Peptide Res. 2002, 60, 198–214. [Google Scholar]
- Okada, Y.; Tsukatani, M.; Taguchi, H.; Yokoi, T.; Bryant, S. D.; Lazarus, L. H. Amino acids and peptides. Design and synthesis of opioidmimetics containing pyrazinone ring and examination of their opioid receptor binding activity. Chem. Pharm. Bull. 1998, 46, 1374–82. [Google Scholar]
- Sun, G.; Uretsky, N. J.; Wallace, L. J.; Shams, G.; Weinstein, D. M.; Miller, D. D. Synthesis of chiral 1-(2`-amino-2`-carboxyethyl)-1,4-dihydro-6,7-quinoxaline-2,3-diones: α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor agonists and antagonists. J. Med. Chem. 1996, 39, 4430–38. [Google Scholar] [CrossRef]
- Marsham, P. R.; Wardleworth, J. M.; Boyle, F. T.; Hennequin, L. F.; Kimbell, R.; Brown, M.; Jackman, A. L. Design and synthesis of potent nonpolyglutamatable quinozoline antifolate thymidylate synthase inhibitors. Med. Chem. 1999, 42, 3809–20. [Google Scholar]
- Xia, Y.; Yang, Z.-Y.; Xia, P.; Bastow, K. F.; Nakanishi, Y.; Lee, K.-H. Antitumor agents. Part 202: Novel 2′-amino chalcones: design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2000, 10, 699–701. [Google Scholar]
- Kucukguzel, S. G.; Mazi, A.; Sahin, F.; Ozturk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar]
- Hassan, H. M.; Shedid, S. A. M. Synthesis and antimicrobial activity of some new Mannich bases, N-(2-hydroxy-1-naphthyl) amino acid, methyl ester and hydrazide derivatives. J. Serb. Chem. Soc. 1998, 63, 125–130. [Google Scholar]
- Dogan, H. N.; Rollas, S.; Erdeniz, H. Synthesis, structure elucidation and antimicrobial activity of some 3-hydroxy-2-naphthoic acid hydrazide derivatives. Farmaco 1998, 53, 462–7. [Google Scholar] [CrossRef]
- Cesur, Z.; Büyüktimkin, S.; Büyüktimkin, N.; Derbentli, Ţ. Synthesis and antimicrobial evaluation of some arylhydrazones of 4-[(2-methylimidazo[1,2-a]pyridine-3-yl)azo]benzoic acid hydrazine. Arch. Pharm. (Weinheim) 1990, 323, 141–4. [Google Scholar]
- Cocco, M. T.; Congiu, C.; Onnis, V.; Pusceddu, M. C.; Schivo, M. L.; De Logu, A. Synthesis and antimycobacterial activity of some isonicotinoylhydrazones. Eur. J. Med. Chem. 1999, 34, 1071–6. [Google Scholar] [CrossRef]
- Uĝur, A.; Mercimek, B.; Özler, M. A.; Şahin, N. Antimicrobial effects of bis(Δ2-2-imidazolinyl)-5,5`-dioxime and its mono- and tri-nuclear complexes. Trans. Met. Chem. 2000, 25, 421–25. [Google Scholar]
- Chohan, Z. H.; Farooq, M. A.; Scozzafava, A.; Supuran, C. T. Antibacterial Schiff bases of oxalyl-hydrazine/ diamide incorporating pyrrolyl and salicylyl moieties and their zinc(II) complexes. J. Enz. Inhib. Med. Chem. 2002, 17, 1–7. [Google Scholar] [CrossRef]
- Wu, G.; Wang, G.; Fu, X.; Zhu, L. Synthesis, crystal structure, stacking effect and antimicrobial studies of novel quaternary copper(II) complex with quinolone. Molecules 2003, 8, 287–296. [Google Scholar]
- Henderson B. In Textbook of Immunopharmacology, 3rd ed; Dale, M. M.; Foreman, J. C.; Fan, T-P. D. (Eds.) Blackwell Scientific Publications: London, 1994; pp. 16, 193.
- Murray, P. R.; Baron, E. J.; Pfaller, M. A.; F. C., Tenover; Yolken, R. H. Manual of clinical microbiology. In Antimicrobial Agents and Susceptibility Testing; Woods, G. L., Washington, J. A., Eds.; American Society for Microbiology: Washington, DC, 1995. [Google Scholar]
- Sample availability: Available from the author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.