Prospecting the Effects on Abalone (H. discus) Growth under Low-Salinity Stress after Feeding Citrus Peel (CP) and Ecklonia cava disuse (ECD) as Feed Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Breeding Management
2.2. Production of Feed for Abalones
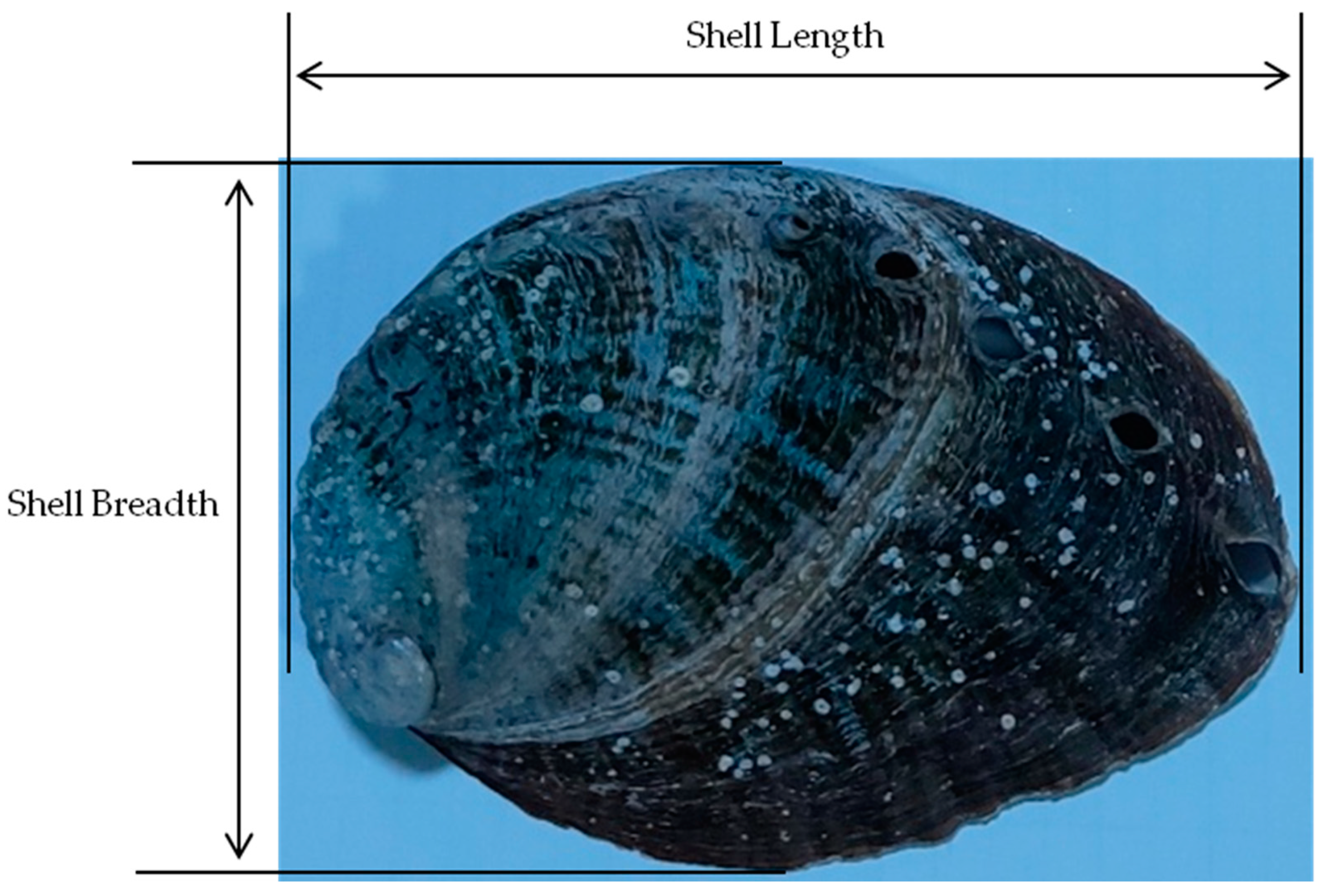
2.3. Survival Rate and Growth Rate Measurement Experiment According to Feed
2.4. Physiological Changes According to Rapid Low-Salinity Stress
2.5. Statistics
3. Results and Discussion
3.1. Changes in Survival Rate According to Feed
3.2. Changes in Growth Depending on Feed
3.3. Changes in Survival Rates Induced by Low-Salinity Stress
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, B.H.; Park, M.W.; Kim, T.I.; Son, M.H.; Lee, S.I. The effect of growth and survival rate on feeding rate of abalone, Haliotis discus hannai, rearing in net cage culture or indoor tank culture. Korean J. Malacol. 2014, 30, 227–234. [Google Scholar] [CrossRef][Green Version]
- Lee, S.M.; Yun, S.J.; Hur, S.B. Evaluation of dietary protein sources for abalone (Haliotis discus hannai). J. Aquacult. 1998, 11, 19–29. [Google Scholar]
- Kim, B.H.; Lee, S.M.; Go, C.S.; Kim, J.W.; Mueong, J.I. Optimum stocking density of juvenile abalone (Haliotis discus hannai) fed the formulated diet or macroalgae (Undaria). Korean J. Fish. Aquat. Sci. 1998, 31, 869–874. [Google Scholar]
- Lee, S.M.; Yun, S.Y.; Min, K.S.; Yoo, S.K. Evaluation of dietary carbohydrate sources for juvenile abalone (Haliotis discus hannai). J. Aquacult. 1998, 11, 133–140. [Google Scholar]
- Lee, S.M.; Lim, Y.S.; Moon, Y.B.; Yoo, S.K.; Rho, S. Effect of supplemental macroalgae and spirulina in the diets on growth performance in juvenile abalone (Haliotis discus hannai). J. Aquacult. 1998, 11, 31–38. [Google Scholar]
- Viana, M.T.; Lopez, L.M.; Salas, A. Diet development for juvenile abalone (Haliotis fulgens). Evaluation of two artificial diets and macroalgae. Aquaculture 1993, 117, 149–156. [Google Scholar] [CrossRef]
- Mai, K.; Mercer, J.P.; Donlon, J. Comparative studies on the nutrition of two species of abalone (Haliotis tuberculata) L. and (Haliotis discus hannai) Ino. III. Responses of abalone to various levels of dietary lipid. Aquaculture 1995, 134, 65–80. [Google Scholar] [CrossRef]
- Uki, N.; Kemuyama, A.; Watanabe, T. Development of semipurified test diets for abalone. Bull. Jpn. Soc. Sci. Fish. 1985, 51, 1825–1833. [Google Scholar] [CrossRef]
- Uki, N.; Kemuyama, A.; Watanabe, T. Nutrient evaluation of several sources in diets for abalone (Haliotis discus hannai). Bull. Jpn. Soc. Sci. Fish. 1985, 51, 1835–1839. [Google Scholar] [CrossRef]
- Uki, N.; Kemuyama, A.; Watanabe, T. Optimum protein level in diets for abalone. Bull. Jpn. Soc. Sci. Fish. 1986, 52, 1005–1012. [Google Scholar] [CrossRef][Green Version]
- Uki, N.; Sugiura, M.; Watanabe, T. Requirement of essential fatty acids in the abalone (Haliotis discus hannai). Bull. Jpn. Soc. Sci. Fish. 1986, 52, 1013–1023. [Google Scholar] [CrossRef]
- Mouly, P.P.M.; Arzouyan, C.G.; Gaydou, E.M.; Rstienne, J.M. Differentiation of citrus juices by factorial discriminant analysis using liquid chromatography of flavanone glycosides. J. Agric. Food Chem. 1994, 42, 70–79. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Martin, S.F.; Youtsey, C.O. Quantitative survey of narirutin, naringin, hesperidin and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Yun, A.Y.; Lee, K.W.; Kim, P.Y.; Jeong, H.S.; Kim, H.S.; Cho, S.H.; Kim, T.H. Substitution effect of Undaria pinnatifida with citrus(Citrus unshiu, Marcovitch) peel by-product in feed on growth, body composition and air exposure stressor of juvenile abalone (Haliotis discus, Reeve 1846). Aquac. Nutr. 2020, 26, 466–476. [Google Scholar] [CrossRef]
- Yasantha, A.; Jeon, Y.J. Screening for angiotensin 1-converting enzyme inhibitory activity of Ecklonia cava. J. Food Sci. Nutr. 2005, 10, 134–139. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, K.W.; Song, C.B.; Jeon, Y.J. Cytotoxin activities of green and brown seaweeds collected from jeju island against four tumor cell lines. J. Food Sci. Nutr. 2006, 11, 17–24. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.N.; Cha, S.H.; Ahn, G.N.; Jeon, Y.J. Comparison of antioxidant activities of enzymatic and methanolic extracts from Ecklonia cava stem and leave. J. Korean Soc. Food Sci. Nutr. 2006, 35, 1139–1145. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in citrus peel by products. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef]
- Latorre, D.; Pudu, P.; Valenti, P.; Gessani, S. Reciprocal interactions between lactoferrin and bactericidal endotoxins and their role in the regulation of the immune response. Toxins 2010, 2, 54–68. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef]
- Jwa, M.S.; Kang, K.P.; Choe, M.K.; Yeo, I.K. Effects of low salinity stresses on the physiological of disc abalone (Haliotis discus discus). J. Fish Pathol. 2009, 22, 293–303. [Google Scholar]
- Senevirathne, M.; Jeon, Y.J.; Ha, J.H.; Kim, S.H. Effective drying of citrus by-product by high speed drying: A novel drying technique and their antioxidant activity. J. Food Eng. 2009, 92, 157–163. [Google Scholar] [CrossRef]
- Johnson, J.K.; Rocheleau, T.A.; Hillyer, J.F.; Chen, C.C.; Li, J.; Christensen, B.M. A potential role for phenylalanine hydroxylase in mosquito immune responses. Insect Biochem. Mol. Biol. 2003, 33, 345–354. [Google Scholar] [CrossRef]





| Experimental Diets (%) | ||||
|---|---|---|---|---|
| Ingredients | Control | CP | ECD | ECD + CP |
| CP (Citrus Peel) | 6 | 3 | ||
| ECD (Ecklonia cava disuse) | 6 | 3 | ||
| Fish meal | 15.5 | 15.5 | 15.5 | 15.5 |
| Dextrin | 25 | 25 | 25 | 25 |
| Wheat flour | 6.5 | 0.5 | 0.5 | 0.5 |
| Casein | 20 | 20 | 20 | 20 |
| Sodium alginate | 23 | 23 | 23 | 23 |
| Mineral | 4 | 4 | 4 | 4 |
| Vitamin | 2 | 2 | 2 | 2 |
| Choline | 0.5 | 0.5 | 0.5 | 0.5 |
| Soybean oil | 1.5 | 1.5 | 1.5 | 1.5 |
| Fish oil | 2 | 2 | 2 | 2 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Proximate analysis (%) | ||||
| Crude protein | 28.8 | 28.8 | 28.5 | 28.6 |
| Crude lipid | 1.47 | 2.22 | 1.77 | 1.99 |
| Crude carbohydrate | 4.97 | 5.72 | 5.27 | 5.49 |
| Diets | Week | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 8 | 12 | |
| Control | 100 | 100 | 100 | 94 | 94 | 94 | 92 |
| CP | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ECD | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CP + ECD | 100 | 100 | 100 | 100 | 100 | 98 | 98 |
| Diets | Time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 24 | 48 | |
| Control | 100 | 100 | 100 | 100 | 100 | 74.1 |
| CP | 100 | 100 | 100 | 100 | 100 | 88.9 |
| ECD | 100 | 100 | 100 | 100 | 100 | 96.3 |
| CP + ECD | 100 | 100 | 100 | 100 | 100 | 70.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jwa, M.-S.; Hong, C.-Y. Prospecting the Effects on Abalone (H. discus) Growth under Low-Salinity Stress after Feeding Citrus Peel (CP) and Ecklonia cava disuse (ECD) as Feed Additives. J. Mar. Sci. Eng. 2021, 9, 707. https://doi.org/10.3390/jmse9070707
Jwa M-S, Hong C-Y. Prospecting the Effects on Abalone (H. discus) Growth under Low-Salinity Stress after Feeding Citrus Peel (CP) and Ecklonia cava disuse (ECD) as Feed Additives. Journal of Marine Science and Engineering. 2021; 9(7):707. https://doi.org/10.3390/jmse9070707
Chicago/Turabian StyleJwa, Min-Seok, and Chang-Yu Hong. 2021. "Prospecting the Effects on Abalone (H. discus) Growth under Low-Salinity Stress after Feeding Citrus Peel (CP) and Ecklonia cava disuse (ECD) as Feed Additives" Journal of Marine Science and Engineering 9, no. 7: 707. https://doi.org/10.3390/jmse9070707
APA StyleJwa, M.-S., & Hong, C.-Y. (2021). Prospecting the Effects on Abalone (H. discus) Growth under Low-Salinity Stress after Feeding Citrus Peel (CP) and Ecklonia cava disuse (ECD) as Feed Additives. Journal of Marine Science and Engineering, 9(7), 707. https://doi.org/10.3390/jmse9070707






