Abstract
Carnot’s reversible cycle net value was determined using the previously derived values for the transformations there occurring. A negative net value is obtained as a result, in contradiction with current thermodynamics, Clausius analysis based position that the net value for such a cycle is zero. The entropy function is introduced and the new criterion’s for spontaneity, reversibility and equilibrium are advanced.
Introduction
As it is known, currently accepted second law thermodynamics is based on Clausius work. However, when the former is applied to the analysis of a simple reversible cyclical process, the values obtained for the transformations therein occurring do not agree with those Clausius original analysis derived for the same transformations. This disagreement, which was exposed in part I, prompted, as discussed in part II, the development of a new method for the determination of the said values. The values thus obtained are in this work used for the determination of the net value of the reversible cyclical processes known as Carnot’s engine and refrigerator. The term net value refers of course to the total or combined value of all the transformations in those processes occurring.
Analysis
One cycle in the operation of what is known as Carnot’s reversible engine can be represented in terms of the transformations by it produced, as shown in Figure 1. According to it, Carnot’s reversible engine value, represented as V[Carnot’s E]rev , can be written as follows:
V[Carnot’s E]rev = V[Qc(Th)→Qc(Tc)]rev + V[Q(Th)→w]rev
The above expression, on substitution of the values for the transformations in it appearing, as expressed in eqs. 20 and 23 of part 2, reduces to:
and the net value for Carnot’s reversible engine instead of being zero as Clausius analysis demands, is actually negative.
Now, if in eq. 1 instead of using the real value of transformation [Qc(Th)→Qc(Tc)]rev we had used − Qc/Th + Qc/Tc we would have obtained a zero net value for Carnot’s reversible engine. Assigning this value to the said transformation was precisely Clausius position. However, as it has already been shown in part 2, the value for the said transformation is not − Qc/Th + Qc/Tc but zero, and consequently the value for Carnot’s reversible engine is not zero but −Q/Th.
A similar analysis to the one just performed on Carnot’s reversible engine can be worked out for Carnot’s reversible refrigerator by simply reversing the operations shown in Figure 1 as follows:
equation which, on substitution of the values for the transformations there shown, transforms into:
V[Carnot’s R]rev = V[Qc(Tc)→Qc(Th)]rev + V[w→Q(Th)]rev
The fact that the effects of one cycle in the operation of Carnot’s reversible engine are precisely reverted by one cycle in the operation of a Carnot’s reversible refrigerator working between the same two heat reservoirs, i.e. each and every body affected by the occurrence of one cycle in the operation of Carnot’s reversible engine being restored to its precise original condition by Carnot’s reversible refrigerator is clearly reflected in the following equation which combines the results expressed in eqs. 2 and 4:
In the light of the above result we will define a reversible circular process as that one in which each and everyone of the bodies associated to the transformations therein occurring returns to its original condition, without any change in other bodies remaining. It should here be realized that a significant difference exists between what has been defined as a reversible circular process and what is known as a reversible cyclical process, which in all propriety should be referred to as a semicircular process. In the latter, of all the bodies involved in the process only the working substance or variable body returns to its original condition; while both of the thermal reservoirs as well as the mechanical reservoir on the other hand, remain, at the end of one cycle, in a condition different from the one they originally had. By coupling however a reversible cyclical process with its inverse, a reversible circular process is produced.
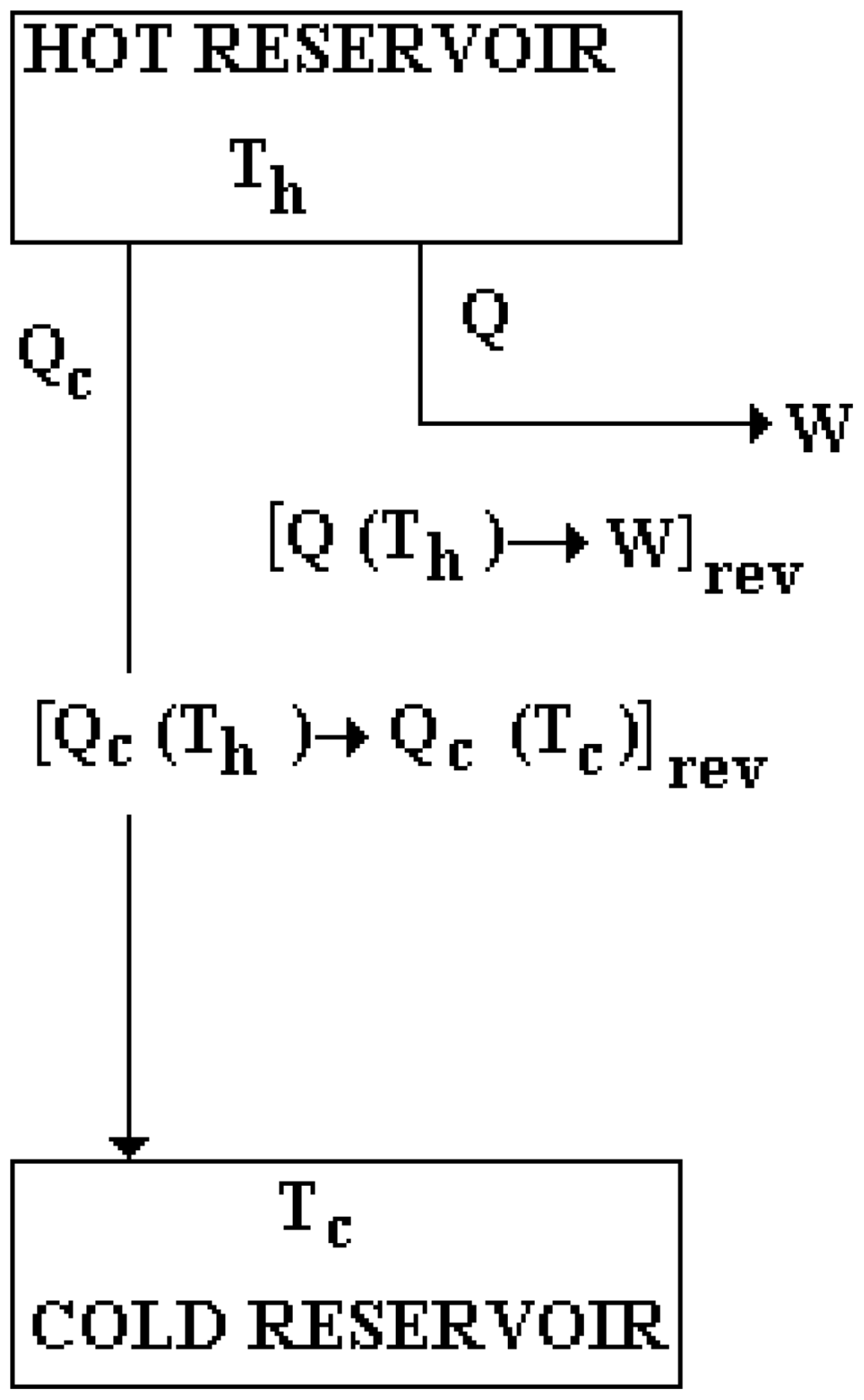
Figure 1.
Carnot’s reversible engine operation is separated into its two constitutive transformations: [Qc(Th)→Qc(Tc)]rev and [Q(Th)→w]rev .
From now on, the summations and integrals appertaining cyclical (cyc) and circular (circ) processes will be symbolized, respectively, as follows:
Thus, the mathematical expression for the value of the reversible circular process under consideration, at the light of the result expressed in eq. 5 and of the value equations by it subsumed such as eqs. 1 and 3, can in general be written as follows:
equation that tells us that the sum of the values of all the transformations involved in the reversible circular process is equal to zero.
In differential form eq. 6 can be written as follows:
Introduction now of the term dV to designate the algebraic sum shown in eq. 7, i.e.
, leads to:
in which the differential to be integrated is that constituted by all the elements of value of all the transformations involved in the reversible circular process.
∮dV = 0
Equations 6, 7 and 8 are, as should be obvious, valid for any reversible circular process because no change can remain after each and every one of the bodies involved in the reversible circular process have returned to their precise original condition. This is precisely what equations 6 to 8 express.
In what follows, we will transit from the “value of transformations” concept to the entropy function. The starting point of this endeavor will be the development of the mathematical expression for the combined value of all the transformations in a simple cyclical process, reversible or not.
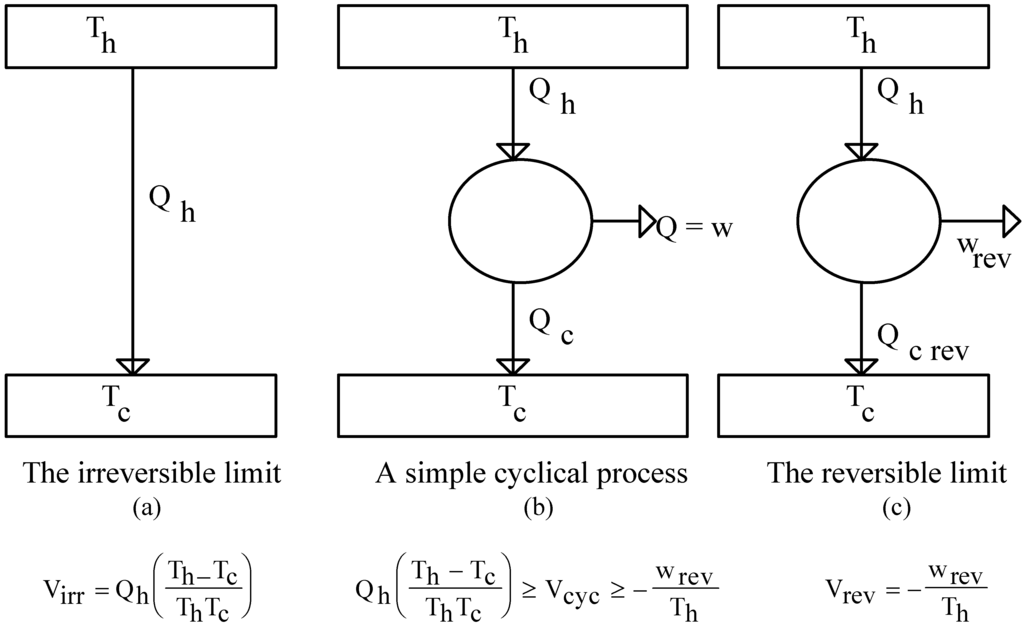
Figure 2.
A simple cyclical process (b), and its limiting irreversible (a), and reversible operations (c).
Let us consider the simple cyclical process depicted in Figure 2(b). In it Qh , Qc and w stand, respectively, for the amount of heat given off by the hot reservoir at Th, taken in by the cold reservoir at Tc, and transformed into work. The limits to the operation of this process are represented in Figure 2(a) and (c). In the reversible limit all of the heat given off by the hot reservoir is absorbed by the cyclical process, producing from it the maximum amount possible of work (wrev), discarding consequently to the cold reservoir the minimum possible amount of heat (Qc, rev). In the irreversible limit on the other hand, all of the heat given off by the hot reservoir finds its irreversible way to the cold reservoir, consequently, no work is at all produced. In the reversible limit by simply performing the inverse cycle, all of this process universe can be restored to its initial condition without leaving changes in other bodies. In the irreversible limit on the other hand, no part of the universe is susceptible of being restored to its original condition without changes in other bodies remaining. Any of the simple cyclical processes by them bounded will thus be partially reversible in the sense that in any of them only a portion of their universe is susceptible of being restored to its original condition, this portion being that involved in the production of whatever amount of work the said process is able to output. This can be understood if it is realized that by feeding this work to a reversible refrigerator it is possible to restore to its initial condition that portion of the universe involved in the production of the said work, without the occurrence of any other permanent change. Since the whole of the process universe is susceptible of being restored when w = wrev , while non is when w = 0, it follows that the above referred portion, i.e. the reversible fraction of the original universe, will be given by w/wrev. This quotient will be subsequently referred to as the reversibility degree and symbolized as ϕ. Obviously, the limit values for ϕ are 0 and 1, respectively, for the irreversible and reversible limits above described. The value of a simple cyclical process, Vcyc, will thus be bounded by the value of the irreversible limit that it acquires when its ϕ = 0, i.e. Vcyc = Virr = Qh(Th− Tc /ThTc), and the value of the reversible limit that it acquires when its ϕ = 1, i.e. Vcyc = Vrev = −wrev / Th . For any other ϕ, i.e. for 0 < w < wrev , Vcyc will be determined by the value contributions of the reversible and irreversible limits according to the relative weight each of those extremes have in the actual process. The reversible contribution to Vcyc , determined by the amount of heat transformed into work, will thus be given by −w / Th , which can also be written as (−w / wrev)(wrev / Th) , or as ϕVrev. The irreversible contribution, on the other hand, is determined by the heat being irreversibly transferred from Th to Tc . This amount of heat will be quantified as follows: That an amount of work w is produced, with w < wrev , means that of all the heat given off by the hot reservoir only the portion (Qh / wrev)w is being utilized for the production of w. The rest, Qh− (Qh / wrev)w or (1 − ϕ)Qh is consequently that being irreversibly transferred, and to this amount corresponds a value of (1 − ϕ)Qh(Th−Tc / Th Tc), which can also be written as (1 −ϕ)Virr. The value expression for our simple cyclical process can thus, at the light of the results just given, be written as:
or as
expression which upon simplification takes its final form as:
and from it the value for any simple cyclical process, reversible or not, can be obtained, fact that conveys to this equation the character of the general expression of value for simple cyclical processes
Vcyc = ϕVrev + (1 − ϕ)Virr
With eq.11 in addition to eq. 2 we have two equations describing the same property, namely, the total value of a reversible cyclical process. By combining them algebraically we find out that for these processes the following relationship also applies:
expression which, as should be noticed, happens to be the mathematical representation of Carnot’s theorem and consequently what Clausius flawed analysis incorrectly concluded to be the total value expression for simple reversible cyclical processes.
In restricting our discussion now to reversible processes, equation 11 will be extended to include reversible cyclical processes of any kind of complexity by taking into account the fact that any such process can be reduced to a certain number of simple reversible cyclical processes [1]. Based on this consideration eq. 11 can be written as follows:
in which both of the summations are taken over all the k heat reservoirs taking part in the reversible cyclical process, being the first one the algebraic sum of all the amounts of heat taken in or given off by the heat reservoirs, divided by the absolute temperature at which the heat exchange takes place, while the second stand for the algebraic sum of all the amounts of heat transformed into work or vice versa, divided by the absolute temperature at which the said transformation takes place.
Carnot’s theorem, given by eq.12, and the result expressed in eq. 5 allow us to express the first term of the equation of value represented in eq. 13, and the extension to circular processes of the second term, as follows:
and on reason of the fact that a reversible circular process is the result of the coupling of two reversible cyclical processes for each of which eq. 14 holds, it then follows that for such a process the following equation also holds:
Equations 15 and 16, representing the combined value of all the transformations in a reversible circular process, combine themselves to define Vcirc in accordance with eq. 6, i.e.
In differential form the counterparts to eqs 14, 15 and 16 can be written as follows:
The equation of value for reversible cyclical processes expressed in eq. 13 and its extension to reversible circular processes expressed in eq. 17 will be now, based on the results just obtained, written as follows:
In continuing now with our intended transition from the value of transformations to the entropy function, let us hear from Clausius [2]:
If the integral ∫dQ/T, corresponding to any given succession of variations of a body, be always equal to zero provided the body returns finally to its original condition, whatever the intervening conditions may be, then it follows that the expression under the integral sign, viz. dQ/T, must be the perfect differential of a quantity which depends only on the present condition of the body, and is altogether independent of the way in which it has been brought into that condition. If we denote this quantity by S, we may put dQ/T=dS.
As known, the name chosen by Clausius to designate this quantity S, was "entropy" [3].
In applying now the above Clausius statement to eqs. 18, 19 and 23 it can be seen that each of these three equations involve a perfect differential. This, because the integral of those differentials is always equal to zero when the body or bodies whose value behavior they describe return to their original condition.
In further detail and regarding eq. 23 it can be said that once the body composed itself by each and every one of the bodies taking any part in the process returns to its original condition, the integral of dVcirc vanishes. In realizing that the above referred collection of bodies constitute what is known as the universe of the process, and if following Clausius the symbol dSu , for universe entropy change, is used to designate this perfect differential, it can then be said that the entropy of the universe of a process returns to its original value once each and every one of the bodies in it involved return to their original condition.
With regard to eq. 18 it should be remembered that the term dQk / Tk refers to the heat given off or taken in by the k heat reservoir of temperature Tk . In realizing now that in a reversible cyclical process the heat given off by a thermal reservoir is heat taken in by the variable body and vice versa, taking these exchanges place with both bodies at essentially the same temperature, it is then possible to think of eq. 18 as the sum of all the quotients between the elements of heat taken in or given off by the variable body, and the absolute temperature at which those exchanges take place. Being this so it can clearly be seen from eq. 18, thus interpreted, that the integral of the perfect differential dQk / Tk vanishes every time the variable body returns to its original condition. Following Clausius, this perfect differential will be symbolized as dSc and designated as Clausius entropy. On these terms eq. 18 can either be interpreted by saying that no change in Clausius entropy remains in the variable body when it finally returns to its original condition, or equivalently , attending the original meaning of eq. 18 , by saying that the sum of the Clausius entropy changes sustained by the heat reservoirs, once a cycle has been completed, is equal to zero.
Being dSc the expression substituting dQk / Tk it follows that the change in Clausius entropy for a given body will be given by the quotient of the reversible heat by it exchanged and the absolute temperature at which the exchange takes place.
Referring now to eq. 19 it should be remembered that in it dQ stands for the heat produced from or transformed into work through transformations [Q(Tk)→w]rev or[w→Q(Tk)]rev , being Tk the absolute temperature at which the transformation takes place. The integral of this differential, as seen in eq. 19, vanishes every time a circle is completed i.e. at the end of the coupling of [Q(Tk)→w]rev with its inverse [w→Q(Tk)]rev or vice versa. Now, in being the mechanical body the gauge, the measuring rod of these transformations it follows that its evolution will thus correspond to the evolution of those transformations in such a way that the completion of a circle can be judged by the return of the said mechanical body to its original condition. Being this so, the value behavior of the said transformations can thus be ascribed to the behavior of the mechanical body. The fact that in the mechanical body representing the value of the said transformations no contradiction arises with its quality as a purely mechanical body which sustains no internal change of state in changing its position can be understood once it is realized that here its change in position is nothing more than the external, tangible manifestation of the occurrence of the thermodynamic changes expressed by transformations [Q(Tk)→w]rev or [w→Q(Tk)]rev . It is only in this sense that we refer to the mechanical body as being that representing the value behavior described by the perfect differential dQ/Tk . This expression will be henceforth referred to as the thermomechanical entropy and symbolized as dStm . Thus, as long as the change in position of the mechanical body be the expression of the effects of the referred transformations we will have that every time the said mechanical body returns to its original condition dStm will vanish.
Based on the discussion just given, which has brought upon the identification of value and entropy and with it the accomplishment of the intended transition above referred, and on eqs. 21 and 23, the following eq for dSu in terms of dSc and dStm can be written:
dSu = dSc + dStm
As seen, the correction of Clausius analysis flaw crystallizes in the term dStm . Its addition to dSc, as in eq. 24, produces the general expression for the entropy change of the universe of any process. This is the central equation of what, for the reason to become apparent later on, will be henceforth referred to as the negentropic formulation of the second law of thermodynamics, which will encompass all the new knowledge brought upon by the said correction. Some of this knowledge will arise from the following considerations.
In extending his analysis to irreversible processes Clausius was able to prove that the entropy of the universe of any such process could only increase or in the limit, for reversible processes, remain the same. Any decrease in such a quantity being likened to the spontaneous passage of heat from a colder to a hotter body. Clausius universe however, was not such. In not realizing, due to his already discussed analysis flaw, that in cyclical processes the reversible interconversions between heat and work bring forth an entropy effect separate and distinct from those associated to the exchanges of heat between the variable body and the heat reservoirs, he had made dStm = 0. His result was not then a general statement about dSu. No such judgment is possible without the concourse of dStm. What he had proved was thus that dSu≥0 when dStm = 0, or in other words, that in any circumstance dSc≥0.
Implicit in eq. 24 we will find the method for writing the entropy change for the universe of any process, it consisting in adding, if applicable, the thermomechanical entropy term to the expression written for the universe of the process following Clausius formulation. The sign of dStm on the other hand, will depend on whether there is a net production or consumption of work through transformations [Q(Tk)→w]rev and [w→Q(Tk)]rev , being negative in the former case and positive in the latter, while if no work is involved or if the effects of these two transformations are balanced, dStm will be equal to zero. As a consequence, dSu can be either positive, negative or zero depending on the sign and relative weight of dStm with respect to dSc.
Now, in terms of entropy, the value equations shown in eqs. 2, 4 and 5 can be written as follows:
ΔSu[Carnot’s circle]rev = 0
The fact that the entropy change for the universe of a reversible process is not necessarily zero can be seen from eqs. 25 and 26. It can be negative as shown in eq. 25 for Carnot’s reversible engine. It can be positive, as shown in eq. 26 for Carnot’s reversible refrigerator, or zero, as eq. 27 shows for Carnot’s reversible circle. That the entropy change for the universe of a process can be negative, a fact denied by Clausius work, is the reason behind the name chosen --negentropic-- for the formulation herein presented.
Now, if the gist of the second law, as Planck stated, consisted in such a law asserting that [4] ..."There exists in nature a quantity which changes always in the same sense in all natural processes", it only follows that such a quantity is dSc. From what has been before discussed it can be seen that nor the universe entropy change nor the thermomechanical entropy comply with the above noted characteristic of such a quantity. In being dSc>0 for any natural, irreversible process, it follows that:
where ξ stands for the degree of advancement of any natural process.
Equation 28 represents the negentropic formulation criterion for spontaneous change. This criterion differs from that furnished by Clausius analysis in that in his formulation is the universe entropy change the quantity signaling the direction of change. The fact however that this quantity could be positive, negative or zero for a reversible process rules it out as a criterion of spontaneity because even if it changed in the same direction for natural, irreversible processes, which as will be seen it doesn't, the possibility of the process considered being reversible would always be present, and couldn't be ruled out but with the concourse of yet another criterion.
Now, if as eq. 28 shows, a natural process occurs when there are states accessible to it for which
it then follows that it will cease to occur when no more states complying with the above written condition are accessible to it. The process will thus reach its equilibrium condition when Sc has reached its maximum value. This condition can be expressed as follow
and
(Sc)ξ+dξ > (Sc)ξ
The simultaneous fulfillment of the conditions expressed by eqs. 30 and 31 is the negentropic formulation criterion for locating the true equilibrium state.
Another change brought upon by the negentropic formulation refers to the reversibility criterion. As known, for the currently accepted, Clausius based formulation of the second law, a process A→B is reversible if , where the integral is taken over all the bodies that may be in any way affected by the process considered. The results obtained in eqs 3.25 and 3.26 show however that this is not the case. A reversible process can indeed occur with a non zero change in its universe entropy.
The true criterion of reversibility is embedded in eq. 8 in the form of the equation:
which can be expressed in words by saying that reversible is any process that can be completely reversed. For every process that can in no way be completely reversed it follows that:
and any such process is irreversible.
The Umbral Efficiency for Carnot’s Engine
As shown in Figure 3.2, the universe entropy change for a simple cyclical processes transit from a positive to a negative value as we move from the entropic, zero efficient operation represented by the irreversible limit to the efficient, negentropic, reversible limit. Between these limits there exists an operation with an efficiency η‘, 0 < η‘< ηrev such that ΔSu = 0. This operation can be identified by putting eq. 11 equal to zero and solving for the efficiency, Q /Qh. When this is done it is found that it is given by the following expression:
Thus, all the operations of Carnot’s engine for which the efficiency η is such that 0≤η<η' are entropic, while those where η'<η≤ηrev , are negentropic. The particular operation for which η= η' occurs with ΔSu = 0. In recognizing that this efficiency separates the entropic and negentropic operations is that it will be referred to as the umbral efficiency. Among other things, these results show that, as previously found for reversible processes, the entropy change for the universe of irreversible processes can also be either positive, negative or zero.
Final Considerations
The knowledge up to this point generated allow us to advance the considerations that follow, with which we will conclude this first incursion into the realm of the negentropic formulation.
(1) As can be seen from eq. 24, Clausius formulation of the second law is subsumed in the negentropic formulation as that special case for which dStm = 0.
(2) From the results herein obtained and also referring to eq. 24 it can be concluded that the equivalency between the law of increasing entropy and the second law of thermodynamics will not hold in all those cases in which ΔS[Q(T)→w] → ΔSC , because in these cases the universe entropy change will either be zero or negative. Being this so it follows that the expression the entropy of the universe tends to a maximum is not an equivalent statement of the second law of thermodynamics, whose more general statement continues to be : heat cannot, of itself, pass from a colder to a hotter body.
(3) The fact that the universe entropy change for reversible as well as irreversible processes can be either positive, negative or zero, rules out ΔSu as the criterion signaling the direction of change. This role is assumed by Clausius entropy which always change in the same direction, ΔSc>0, for natural, irreversible processes. This way, ΔSc plays in the negentropic formulation the same role played by itself, under the guise of ΔSu, in Clausius formulation.
(4) The knowledge generated in the umbral analysis of Carnot’s engine above performed could be the starting point for attempting a coherent thermodynamic description of the so-called self organizing phenomena which, in this perspective, would transit from chaos to order when their universes transit from entropic to negentropic, with the onset of organization taking place at that umbral point at which ΔSu = 0.
(5) The identification and further correction of Clausius flaw leads to a new vision, a new paradigm of natural phenomena: “the negentropic formulation of the second law of thermodynamics”, whose more salient features have been here presented. A plethora of questions, however, remains to be explored and eventually answered.
References and Notes
- Clausius, R. The Mechanical Theory of Heat; MacMillan: London, 1879; p. 87. [Google Scholar]
- Clausius, R. The Mechanical Theory of Heat; MacMillan: London, 1879; p. 90. [Google Scholar]
- Clausius, R. The Mechanical Theory of Heat; MacMillan: London, 1879; p. 107. [Google Scholar]
- Planck, M. Treatise on Thermodynamics; Dover: New York, 1990; p. 106. [Google Scholar]
© 1999 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.