Abstract
Recent advances in pharmaceutical technology, aimed at overcoming poor drug permeation across the intestinal–epithelial membrane and the challenges posed by the acidic gastrointestinal environment, have led to the development of orally administered somatostatin receptor ligands (SRLs). This development represents a promising step forward in the management of acromegaly, offering an alternative to the limitations associated with injectable SRLs. Several key clinical findings have emerged in the past two years, notably including the results from the extension phase of the MPOWERED trial, which evaluated oral octreotide capsules (OOCs), and the placebo-controlled PATHFNDR-1 trial using paltusotine. This prompted us to conduct a systematic review of the literature focusing on the efficacy of oral SRLs in controlling acromegaly, based on biochemical response. Of the 136 reports identified through our search on Medline and ClinicalTrials.gov, twelve were included, encompassing data from five interventional trials. Both OOCs and paltusotine demonstrated the ability to maintain biochemical control in patients previously controlled with injectable SRLs. While long-term maintenance was confirmed for OOCs, no data are yet available for paltusotine. Several gaps remain, such as the need for head-to-head comparisons between OOCs and paltusotine, and clinical trials in patients who have not received prior injectable SRL treatment.
1. Introduction
Acromegaly is characterized by an excessive production of growth hormone (GH) by the anterior pituitary gland. The prevalence of acromegaly is estimated at 5.9 cases per 100,000 individuals, with an incidence rate of approximately 0.38 cases per 100,000 person-years []. Medical therapy is recommended for patients with persistent disease despite surgical resection of the adenoma as well as for patients in whom surgery is not appropriate []. The long-acting formulations of the somatostatin receptor ligands (SRLs) octreotide and lanreotide are currently recommended as first-line medical therapy, while long-acting pasireotide could be considered as a second-line treatment []. SRLs bind to somatostatin receptors and mimic their physiological effects, leading to a reduction in GH secretion and subsequently insulin growth factor (IGF)-1 secretion by the liver.
Octreotide, lanreotide, and pasireotide are SRLs of a peptide nature, initially developed in injectable formulations due to their susceptibility to enzymatic degradation and low epithelial permeability in the intestine. They are administered through monthly intramuscular or subcutaneous injections of long-acting formulations. Injectable SRLs (iSRLs) exhibit numerous disadvantages such as injection site pain, nodules, bruising, inflammation, and scarring [,,]. A real-world survey showed that 70% of patients reported experiencing pain at the injection site lasting up to a week, with other common adverse effects including nodules (38%), swelling (28%), bruising (16%), scar tissue formation (8%), and inflammation (7%) []. In addition, nurses have only a small amount of confidence that the octreotide syringe will not be clogged and will be somewhat easy to use during injection [].
The development of oral formulations of SRLs has raised hope in mitigating these adverse effects and increasing compliance and quality of life []. Significant advances in oral formulations of SRLs have been made in the last decade. In particular, oral octreotide capsules (OOCs) have been developed through the enteric coating of capsules with a pH-dependent methacrylic acid copolymer preventing chemical and enzymatic degradation, but above all through the development of absorption enhancers to overcome low epithelial permeability []. In particular, the Transient Permeation Enhancer® technology (TPE®, Chiasma™, Jerusalem, Israel) has been used for the development of OOCs []. TPE® consists of an oily suspension of the peptide drug, sodium caprylate (C8), glyceryl monocaprylate and tricaprylate, and polyvinylpyrrolidone. C8 plays the role of a permeation enhancer by causing a transient opening of the intestinal epithelial tight junctions, thus creating a paracellular pathway for peptides with a molecular weight lower than 10 kDa (octreotide: 1.14 kDa) [].
The pharmacokinetic parameters, such as peak plasma concentration and area under the curve, are similar after oral administration of 20 mg octreotide or subcutaneous administration of 100 µg octreotide in human subjects []. The first results of phase III trials for OOCs were published in 2015 through the open-label CH-ACM-01 trial [], followed by the results of the randomized controlled treatment OPTIMAL trial in 2020 []. Subsequently, Mycapssa® (OOC) received regulatory approval from the US Food and Drug Administration (FDA) in June 2020 for the long-term maintenance and treatment of patients with acromegaly who have responded to and tolerated treatment with injectable octreotide or lanreotide. The European Medicines Agency delivered a marketing authorization for Mycapssa® in December 2022.
In addition to OOC, paltusotine, a novel oral SRL, has been recently developed by Crinetics Pharmaceuticals™ []. Paltusotine is original among oral SRLs due to its nonpeptide nature (Figure 1) and its high specificity for the somatostatin receptor subtype 2, while octreotide has an affinity for both somatostatin receptor subtype 2 and subtype 5. The placebo-controlled phase II ACROBAT Evolve trial (NCT03792555) was designed to assess the safety and efficacy of paltusotine in patients that are responders to long-acting iSRLs, but the results were not published due to the positive results of the ACROBAT Edge trial (NCT03789656). This open-label, phase II, single-arm study demonstrated satisfactory maintenance of biochemical response, leading the FDA to grant orphan drug designation to paltusotine for the treatment of acromegaly. Very interestingly, the first results of the placebo-controlled phase III PATHFNDR-1 trial (NCT04837040) evaluating oral paltusotine in patients with acromegaly previously controlled with iSRLs were recently published in June 2024 [].
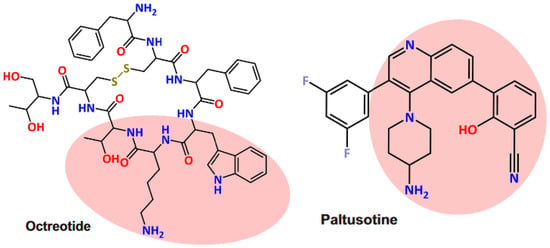
Figure 1.
Chemical structures of the somatostatin receptor ligands octreotide and paltusotine. Pink area highlights similar moieties. Reproduced from [] under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
This prompted us to conduct a systematic review of clinical trials of SRLs administrated via the oral route in patients with acromegaly by focusing on their efficacy based on the biochemical response, which is the strongest predictor of patient outcomes.
2. Materials and Methods
Our systematic review was presented according to the PRISMA 2020 statement [].
2.1. Eligibility Criteria
Table 1 shows the inclusion and exclusion criteria used to select the clinical trials for the review, according to the Population, Intervention, Comparison, Outcome, Study (PICOS) format.

Table 1.
Inclusion and exclusion criteria for the selection of studies.
2.2. Information Sources and Search Strategy
We conducted a search procedure using the PubMed/MEDLINE and ClinicalTrials.gov databases. The search strategy was based on the following words: “acromegaly”, “octreotide”, “lanreotide”, “pasireotide”, “paltusotine”, and “oral”. In addition, the references cited in the included studies were examined to select additional reports for screening. All reports published up to 15 June 2024 were included for further screening.
2.3. Selection and Data Collection Process
The two authors independently screened the titles and abstracts, excluding reports and studies based on the predefined inclusion and exclusion criteria. The remaining studies were integrally read to definitively judge on their inclusion. Disagreements on the eligibility of studies were resolved through discussion and consensus between the two authors. Data from each report were independently collected in Excel sheets by the two authors. No automation tool was used in the selection process or in data collection.
2.4. Data Items (Outcomes)
The primary outcome was the proportion of responders at the end of the treatment period, as determined by serum IGF-1 and GH levels.
2.5. Study Risk of Bias Assessment
The risk of bias in the included studies was independently evaluated by the two authors using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) and the ROBINS-I tool for uncontrolled trials []. Any discrepancies in judgment between the two authors were resolved through discussion.
2.6. Effect Measures
The effect measure of the primary outcome was the percentage of responders among enrolled participants.
2.7. Synthesis Methods
When the 95% confidence intervals of the proportion of responders were not available in the reports, they were determined using the modified Wald method (GraphPad Prism, version 9.5.0).
3. Results
3.1. Study Selection
The flowchart in Figure 2 illustrates the selection process for the studies included in our systematic review. Initially, 136 reports were identified through our search strategy. The majority (n = 87, 64.0%) were excluded based on the initial screening of their titles and abstracts due to non-adherence to our inclusion criteria. An additional fourteen records (10.3%) were excluded either due to non-retrieval or because the biochemical results were not yet available. The unpublished biochemical results were related to the following trials: ACROBAT Evolve (NCT03792555) [,], ACROBAT Advance (NCT04261712) [], and PATHFNDR-2 (NCT05192382) [].
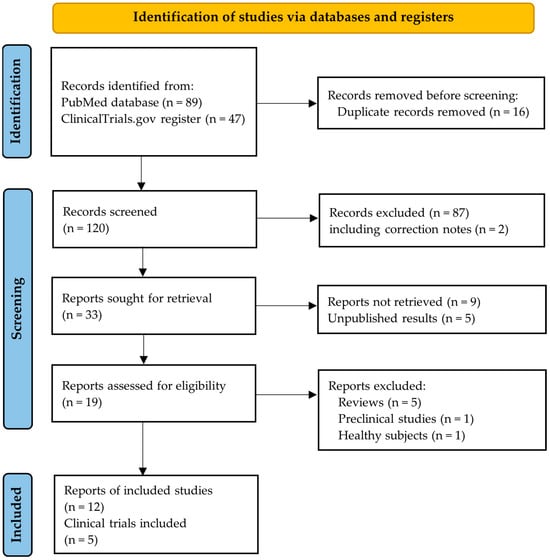
Figure 2.
PRISMA flowchart.
Of the nineteen studies assessed for eligibility, five were excluded since they were literature reviews, one which was a preclinical study on animals [], and another one involving a study population of healthy volunteers []. The interim results from the single-arm ACROBAT Advance study presented at the Endocrine Society’s annual meeting ENDO 2023 in June 2023 were not included in our review due to the lack of a peer-review process []. For the same reason, we did not include the results from the double-blind placebo-controlled PATHFNDR-2 study presented at ENDO 2024 in June 2024 [].
Ultimately, we included twelve reports from the following five clinical trials: CH-ACM-01 (NCT01412424), OPTIMAL (NCT03252353), MPOWERED (NCT02685709), ACROBAT Edge (NCT03789656), and PATHFNDR-1 (NCT04837040). Table 2 and Table 3 summarize the key characteristics of these five interventional trials. Three of these trials assessed the efficacy of OOCs (CH-ACM-01, OPTIMAL, and MPOWERED), while the remaining two evaluated oral paltusotine (ACROBAT Edge and PATHFNDR-1).

Table 2.
Characteristics of the selected studies.

Table 3.
Characteristics of participants within the studies included in the review.
All the selected trials were designed to include a core phase followed by a single-arm extension phase. The core phase consisted of open-label single arm trials (CH-ACM-01 and ACROBAT Edge), double-blind placebo-controlled trials (OPTIMAL and PATHFNDR-1), and a double-blind iSRL-controlled trial (MPOWERED). A total of 458 individuals with acromegaly, all of whom had achieved biochemical control on iSRLs, were enrolled in the core phases of these trials. Among them, 234 received OOCs, and 77 received paltusotine. The extension phases of these trials were structured as open-label single-arm studies, with biochemical results available for CH-ACM-01, OPTIMAL, and MPOWERED. The extension phases evaluating paltusotine are currently ongoing.
Biochemical measurements of serum IGF-I and GH levels were conducted using the iSYS analyzer with dedicated reagents (ImmunoDiagnostic Systems™, Boldon, UK) across all five clinical trials.
3.2. Risk of Bias
Table 4 and Table 5 present the assessment of the risk of bias in the randomized and uncontrolled interventional trials, respectively. Several potential biases were identified. In the CH-ACM-01 trial, at baseline in both the core and extension phases, 11% and 17% of participants, respectively, had IGF-1 levels exceeding 1.3 × ULN, despite having IGF-1 levels below this threshold at the time of inclusion. In the open-label MPOWERED trial, six participants were randomly assigned to the OOC group with IGF-I levels greater than 1.3 × ULN, whereas none in the iSRL group had IGF-1 levels above this threshold. Additionally, patients receiving the OOC had higher pre-inclusion doses of iSRLs. Moreover, the proportion of participants in the MPOWERED trial achieving IGF-1 levels below 1.0 times ULN at the end of the OOC treatment was not reported. In the PATHFNDR-1 trial, there were baseline imbalances between the paltusotine and placebo groups in terms of sex ratio, disease duration, and previous injectable octreotide dosage.

Table 4.
Assessment of the quality of the randomized interventional trials. Green indicates a low risk of bias, while orange signifies some concerns regarding the risk of bias.

Table 5.
Assessment of the quality of the uncontrolled interventional trials. Green indicates a low risk of bias, while orange signifies some concerns regarding the risk of bias.
3.3. Biochemical Response
Table 6 summarizes the rate of responders based on IGF-1 and GH levels in the five selected interventional trials. In the single-arm CH-ACM-01 trial, the rate of responders (i.e., IGF-1 < 1.3 × ULN) reduced from 91.4% at baseline to 64.2% after a 7-month treatment with OOC []. The reduction in the proportion of responders was only 6.3% during the extension phase.

Table 6.
Efficacy of oral SRLs based on biochemical response.
In the placebo-controlled OPTIMAL trial, all participants had IGF-1 levels lower than 1.3 × ULN at inclusion but this reduced to 71.4% after 9 months with OOC treatment. The OOC had higher efficacy than placebo in maintaining IGF-1 levels below 1.0 × ULN (+62.7%). The responder rate was 92.6% (78.7–100%) during the 11-month extension phase with OOC.
In the iSRL-controlled MPOWERED trial, the rate of participants with IGF-1 levels inferior to 1.3 × ULN remained stable after 8 months of OOC treatment. The efficacy was not inferior to that of iSRLs. It should be noted that only responders to the OOC during the run-in phase were enrolled in the randomized trial.
In the ACROBAT Edge trial, IGF-1 levels were expressed in concentrations rather than in percentage of responders. Nevertheless, the comparison between the levels at baseline and after paltusotine showed that monotherapy with paltusotine for 13 weeks maintained IGF-1 levels in patients previously on iSRL monotherapy.
Lastly, the core phase of the PATHFNDR-1 trial revealed that oral paltusotine for 8 months maintained IGF-1 levels below 1 × ULN in 83.3% of patients in comparison to 3.6% in the placebo group.
4. Discussion
The oral administration of SRLs, such as OOCs and oral paltusotine, represents an active area of research aiming to provide an efficient alternative to iSRLs in the management of acromegaly. It represents a genuine hope to improve the quality of life of patients with acromegaly. This development represents a promising step forward in the management of acromegaly, offering an alternative to the limitations associated with iSRLs. In particular, iSRLs exhibit disadvantages such as injection-site pain, nodules, swelling, bruising, inflammation, and scarring. Additionally, some patients may experience worsening symptoms of acromegaly and higher IGF-1 levels close to the date of their next injection [,]. However, a recent survey revealed that 65% of patients preferred subcutaneous injections administered once every fourth weeks using a pen at home, compared to oral capsules taken twice daily, suggesting that the required three-hour fasting period remains a significant burden []. The transition from injectable to oral SRLs should not compromise disease control. Our review indicates that the current data are reassuring in this regard.
The most studied formulation is OOCs, administered twice daily in a fasting state. The pharmacokinetic parameters in human subjects are similar after oral administration of 20 mg octreotide or subcutaneous administration of 100 µg octreotide, particularly regarding the peak plasma concentration and the area under the curve []. The mean of the apparent steady state elimination half-life ranged from 3.19 ± 1.07 h on 40 mg of oral octreotide, to 4.47 ± 2.02 h on 80 mg []. The first phase III trial, CH-ACM-01, demonstrated a biochemical response after seven months of treatment in approximately two-thirds of patients previously controlled by iSRLs. A similar magnitude of biochemical response was observed after nine months in the OPTIMAL trial, which also showed a clear superiority of the OOC compared to the placebo. Notably, the degree of baseline control on iSRLs was predictive of subsequent biochemical response to the OOC. In CH-ACM-01, the proportion of responders to the OOC reached 84.5% in patients with baseline IGF-1 levels below 1.0 × ULN, compared to 61.6% in the overall study population. The MPOWERED study was the first head-to-head comparison of an OOC with iSRLs in patients previously controlled with iSRLs. It demonstrated the non-inferiority of OOCs to iSRLs after an 8-month treatment period. In addition, it showed that the biochemical response remained satisfactory over three years of maintenance therapy.
In contrast to OOCs, paltusotine offers the advantage of once-daily administration. Paltusotine is associated with increased plasma concentrations to doses up to 40 mg, and is eliminated with a half-life of approximately 30 h []. Preclinical studies have demonstrated that paltusotine, an agonist of somatostatin receptor subtype 2, dose-dependently suppresses recombinant GH-releasing hormone-stimulated GH secretion in rats, and dose-dependently reduces IGF-1 levels []. In clinical trials conducted in humans, the ACROBAT Edge trial demonstrated that switching from iSRLs to oral paltusotine for thirteen weeks did not compromise biochemical control in patients previously well-managed on iSRL monotherapy. Recently published data from the PATHFNDR-1 trial further confirmed that paltusotine can maintain the biochemical response in over 80% of patients previously controlled with iSRLs, even after eight months of treatment.
Several gaps remain in the clinical validation of oral SRLs, including the need for direct head-to-head comparisons between OOCs and paltusotine. Furthermore, no peer-reviewed data are currently available on the efficacy of oral SRLs in patients who have not received prior treatment with injectable SRLs. However, the ongoing double-blind, placebo-controlled PATHFNDR-2 trial (NCT05192382) is assessing paltusotine in patients who were either treatment-naïve or previously treated but had discontinued medications for at least four months. Preliminary findings from PATHFNDR-2, presented at the ENDO 2024 meeting, reported that, after 24 weeks of treatment, 42.5% of patients in the paltusotine group achieved serum IGF-1 levels below 1.0 × ULN, compared to only 2.4% in the placebo group []. Finally, data on the long-term efficacy of paltusotine are still pending. The open-label phase II ACROBAT Advance trial (NCT04261712) is currently ongoing and is designed to evaluate the safety and efficacy of paltusotine over one year of treatment.
One limitation of our study is that the search strategy was restricted to the PubMed/MEDLINE and ClinicalTrials.gov databases. As a result, we may have missed some eligible studies. However, PubMed is one of the largest biomedical bibliographic databases, and ClinicalTrials.gov is a key reference database for interventional trials.
5. Conclusions
Recent advances in pharmaceutical technology have enabled the development of orally administered SRLs, providing a promising alternative to iSRLs in the management of acromegaly. OOCs have demonstrated efficacy in maintaining long-term biochemical control in patients previously stabilized on iSRLs. paltusotine has shown the ability to sustain biochemical control, but its long-term efficacy has yet to be conclusively established.
Author Contributions
Conceptualization, D.D.; methodology, D.D.; formal analysis, C.R. and D.D.; data curation, C.R. and D.D.; writing—original draft preparation, D.D.; writing—review and editing, C.R. and D.D.; supervision, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crisafulli, S.; Luxi, N.; Sultana, J.; Fontana, A.; Spagnolo, F.; Giuffrida, G.; Ferraù, F.; Gianfrilli, D.; Cozzolino, A.; Cristina De Martino, M.; et al. Global Epidemiology of Acromegaly: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2021, 185, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Bronstein, M.D.; Chanson, P.; Klibanski, A.; Casanueva, F.F.; Wass, J.A.H.; Strasburger, C.J.; Luger, A.; Clemmons, D.R.; Giustina, A. A Consensus Statement on Acromegaly Therapeutic Outcomes. Nat. Rev. Endocrinol. 2018, 14, 552–561. [Google Scholar] [CrossRef]
- Strasburger, C.J.; Karavitaki, N.; Störmann, S.; Trainer, P.J.; Kreitschmann-Andermahr, I.; Droste, M.; Korbonits, M.; Feldmann, B.; Zopf, K.; Sanderson, V.F.; et al. Patient-Reported Outcomes of Parenteral Somatostatin Analogue Injections in 195 Patients with Acromegaly. Eur. J. Endocrinol. 2016, 174, 355–362. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Kunz, P.L.; Webb, S.M.; Goldstein, G.; Khawaja, S.; McDonnell, M.; Boiziau, S.; Gueguen, D.; Houchard, A.; Ribeiro-Oliveira, A.; et al. PRESTO 2: An International Survey to Evaluate Patients’ Injection Experiences with the Latest Devices/Formulations of Long-Acting Somatostatin Analog Therapies for Neuroendocrine Tumors or Acromegaly. Adv. Ther. 2023, 40, 671–690. [Google Scholar] [CrossRef]
- Geer, E.B.; Sisco, J.; Adelman, D.T.; Ludlam, W.H.; Haviv, A.; Liu, S.; Mathias, S.D.; Gelbaum, D.; Shi, L. Patient Reported Outcome Data from Acromegaly Patients Treated with Injectable Somatostatin Receptor Ligands (SRLs) in Routine Clinical Practice. BMC Endocr. Disord. 2020, 20, 117. [Google Scholar] [CrossRef]
- Adelman, D.; Truong Thanh, X.-M.; Feuilly, M.; Houchard, A.; Cella, D. Evaluation of Nurse Preferences between the Lanreotide Autogel New Syringe and the Octreotide Long-Acting Release Syringe: An International Simulated-Use Study (PRESTO). Adv. Ther. 2020, 37, 1608–1619. [Google Scholar] [CrossRef]
- Chen, G.; Kang, W.; Li, W.; Chen, S.; Gao, Y. Oral Delivery of Protein and Peptide Drugs: From Non-Specific Formulation Approaches to Intestinal Cell Targeting Strategies. Theranostics 2022, 12, 1419–1439. [Google Scholar] [CrossRef]
- Kim, J.C.; Park, E.J.; Na, D.H. Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals. Pharmaceuticals 2022, 15, 1585. [Google Scholar] [CrossRef]
- Brayden, D.J.; Maher, S. Transient Permeation Enhancer® (TPE®) Technology for Oral Delivery of Octreotide: A Technological Evaluation. Expert Opin. Drug Deliv. 2021, 18, 1501–1512. [Google Scholar] [CrossRef]
- Tuvia, S.; Pelled, D.; Marom, K.; Salama, P.; Levin-Arama, M.; Karmeli, I.; Idelson, G.H.; Landau, I.; Mamluk, R. A Novel Suspension Formulation Enhances Intestinal Absorption of Macromolecules via Transient and Reversible Transport Mechanisms. Pharm. Res. 2014, 31, 2010–2021. [Google Scholar] [CrossRef]
- Tuvia, S.; Atsmon, J.; Teichman, S.L.; Katz, S.; Salama, P.; Pelled, D.; Landau, I.; Karmeli, I.; Bidlingmaier, M.; Strasburger, C.J.; et al. Oral Octreotide Absorption in Human Subjects: Comparable Pharmacokinetics to Parenteral Octreotide and Effective Growth Hormone Suppression. J. Clin. Endocrinol. Metab. 2012, 97, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Popovic, V.; Bidlingmaier, M.; Mercado, M.; van der Lely, A.J.; Biermasz, N.; Bolanowski, M.; Coculescu, M.; Schopohl, J.; Racz, K.; et al. Safety and Efficacy of Oral Octreotide in Acromegaly: Results of a Multicenter Phase III Trial. J. Clin. Endocrinol. Metab. 2015, 100, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Nachtigall, L.B.; Fleseriu, M.; Gordon, M.B.; Bolanowski, M.; Labadzhyan, A.; Ur, E.; Molitch, M.; Ludlam, W.H.; Patou, G.; et al. Maintenance of Acromegaly Control in Patients Switching From Injectable Somatostatin Receptor Ligands to Oral Octreotide. J. Clin. Endocrinol. Metab. 2020, 105, e3785–e3797. [Google Scholar] [CrossRef] [PubMed]
- McLaren, D.S.; Murray, R.D. Paltusotine, a Novel Oral Somatostatin Receptor Ligand in the Management of Acromegaly. J. Clin. Endocrinol. Metab. 2023, 108, e193–e194. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Casagrande, A.; Strasburger, C.J.; Bidlingmaier, M.; Snyder, P.J.; Guitelman, M.A.; Boguszewski, C.L.; Buchfelder, M.; Shimon, I.; Raverot, G.; et al. Acromegaly Disease Control Maintained after Switching from Injected Somatostatin Receptor Ligands to Oral Paltusotine. J. Clin. Endocrinol. Metab. 2024, dgae385. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, H.; Yu, J.; Hong, W.; Tian, X.; Qi, J.; Sun, S.; Zhao, C.; Wu, C.; Xu, Z.; et al. Prospect of Acromegaly Therapy: Molecular Mechanism of Clinical Drugs Octreotide and Paltusotine. Nat. Commun. 2023, 14, 962. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- A Study to Evaluate the Safety and Efficacy of Paltusotine for the Treatment of Acromegaly (ACROBAT Evolve). Available online: https://clinicaltrials.gov/study/NCT03792555?a=3 (accessed on 15 June 2024).
- Martin, S.; Bender, R.H.; Krasner, A.; Marmon, T.; Monahan, M.; Nelson, L. Development and Evaluation of the Acromegaly Symptom Diary. J. Patient Rep. Outcomes 2023, 7, 15. [Google Scholar] [CrossRef]
- A Study to Evaluate the Long-Term Safety and Efficacy of Paltusotine for the Treatment of Acromegaly (ACROBAT Advance). Available online: https://clinicaltrials.gov/study/NCT04261712 (accessed on 15 June 2024).
- A Study to Evaluate the Safety and Efficacy of Paltusotine for the Treatment of Acromegaly (PATHFNDR-2). Available online: https://clinicaltrials.gov/study/NCT05192382 (accessed on 15 June 2024).
- Zhao, J.; Wang, S.; Markison, S.; Kim, S.H.; Han, S.; Chen, M.; Kusnetzow, A.K.; Rico-Bautista, E.; Johns, M.; Luo, R.; et al. Discovery of Paltusotine (CRN00808), a Potent, Selective, and Orally Bioavailable Non-Peptide SST2 Agonist. ACS Med. Chem. Lett. 2023, 14, 66–74. [Google Scholar] [CrossRef]
- Madan, A.; Markison, S.; Betz, S.F.; Krasner, A.; Luo, R.; Jochelson, T.; Lickliter, J.; Struthers, R.S. Paltusotine, a Novel Oral Once-Daily Nonpeptide SST2 Receptor Agonist, Suppresses GH and IGF-1 in Healthy Volunteers. Pituitary 2022, 25, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Biller, B.M.K.; Casagrande, A.; Elenkova, A.; Boguszewski, C.L.; Jallad, R.; Hu, B. 12535 Efficacy and Safety of Once-Daily Oral Paltusotine in Medically Untreated Patients with Acromegaly: Results from the Phase 3, Randomized, Placebo-Controlled Pathfndr-2 Study. J. Endocr. Soc. 2024, 8, bvae163.1201. [Google Scholar] [CrossRef]
- Efficacy and Safety of Octreotide (MYCAPSSATM [Formerly OctreolinTM]) for Acromegaly. Available online: https://clinicaltrials.gov/study/NCT01412424 (accessed on 15 June 2024).
- Efficacy and Safety of Octreotide Capsules (MYCAPSSA) in Acromegaly. Available online: https://clinicaltrials.gov/study/NCT03252353 (accessed on 15 June 2024).
- Samson, S.L.; Nachtigall, L.B.; Fleseriu, M.; Jensterle, M.; Manning, P.J.; Elenkova, A.; Molitch, M.E.; Ludlam, W.H.; Patou, G.; Haviv, A.; et al. Durable Biochemical Response and Safety with Oral Octreotide Capsules in Acromegaly. Eur. J. Endocrinol. 2022, 187, 733–741. [Google Scholar] [CrossRef]
- Fleseriu, M.; Dreval, A.; Bondar, I.; Vagapova, G.; Macut, D.; Pokramovich, Y.G.; Molitch, M.E.; Leonova, N.; Raverot, G.; Grineva, E.; et al. Maintenance of Response to Oral Octreotide Compared with Injectable Somatostatin Receptor Ligands in Patients with Acromegaly: A Phase 3, Multicentre, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2022, 10, 102–111. [Google Scholar] [CrossRef]
- Comparison of Oral Octreotide Capsules to Injectable Somatostatin Analogs in Acromegaly. Available online: https://clinicaltrials.gov/study/NCT02685709 (accessed on 15 June 2024).
- Fleseriu, M.; Molitch, M.; Dreval, A.; Pokramovich, Y.; Bondar, I.; Poteshkin, Y.; Macut, D.; Obermayer-Pietsch, B.; Gilgun-Sherki, Y.; Haviv, A.; et al. MPOWERED Trial Open-Label Extension: Long-Term Efficacy and Safety Data for Oral Octreotide Capsules in Acromegaly. J. Clin. Endocrinol. Metab. 2023, 108, 3214–3222. [Google Scholar] [CrossRef]
- Gadelha, M.R.; Gordon, M.B.; Doknic, M.; Mezősi, E.; Tóth, M.; Randeva, H.; Marmon, T.; Jochelson, T.; Luo, R.; Monahan, M.; et al. ACROBAT Edge: Safety and Efficacy of Switching Injected SRLs to Oral Paltusotine in Patients With Acromegaly. J. Clin. Endocrinol. Metab. 2023, 108, e148–e159. [Google Scholar] [CrossRef]
- A Study to Evaluate the Safety and Efficacy of Paltusotine for the Treatment of Acromegaly (ACROBAT Edge). Available online: https://clinicaltrials.gov/study/NCT03789656 (accessed on 1 June 2024).
- A Study to Evaluate the Safety and Efficacy of Paltusotine for the Treatment of Acromegaly. Available online: https://clinicaltrials.gov/study/NCT04837040 (accessed on 15 June 2024).
- Maione, L.; Albrici, C.; Grunenwald, S.; Mouly, C.; Cimino, V.; Lecoq, A.-L.; Souberbielle, J.C.; Caron, P.; Chanson, P. IGF-I Variability Over Repeated Measures in Patients With Acromegaly Under Long-Acting Somatostatin Receptor Ligands. J. Clin. Endocrinol. Metab. 2022, 107, e3644–e3653. [Google Scholar] [CrossRef]
- Sisco, J.; Furumalm, M.; Yssing, C.; Okkels, A.; Zavisic, S. Preferences for Different Treatment Options among People Living with Acromegaly in the US. Curr. Med. Res. Opin. 2024, 40, 657–664. [Google Scholar] [CrossRef]
- Betz, S.F.; Markison, S.; Kusnetzow, A.K.; Athanacio, J.D.; Rico-Bautista, E.; Madan, A.; Johns, M.; Zhu, Y.F.; Schonbrunn, A.; Struthers, R.S. Suppression of Growth Hormone and Insulin-Like Growth Factor 1 in Rats After Oral Administration of CRN00808, a Small Molecule, Sst2 Selective Somatostatin Biased Agonist. Endocr. Rev. 2018, 39, i1–i1417. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).