Abstract
Bronchopulmonary dysplasia (BPD) is the most common complication after preterm birth. Pasteurized donor human milk (DHM) has increasingly become the standard of care for very preterm infants over the use of preterm formula (PF) if the mother’s own milk (MOM) is unavailable. Studies have reported beneficial effects of DHM on BPD. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) and observational studies on the effects of DHM on BPD and other respiratory outcomes. Eighteen studies met the inclusion criteria. Meta-analysis of RCTs could not demonstrate that supplementation of MOM with DHM reduced BPD when compared to PF (three studies, risk ratio (RR) 0.89, 95% confidence interval (CI) 0.60–1.32). However, meta-analysis of observational studies showed that DHM supplementation reduced BPD (8 studies, RR 0.78, 95% CI 0.67–0.90). An exclusive human milk diet reduced the risk of BPD, compared to a diet with PF and/or bovine milk-based fortifier (three studies, RR 0.80, 95% CI 0.68–0.95). Feeding raw MOM, compared to feeding pasteurized MOM, protected against BPD (two studies, RR 0.77, 95% CI 0.62–0.96). In conclusion, our data suggest that DHM protects against BPD in very preterm infants.
1. Introduction
The nutritional and immunological benefits of providing human milk to very preterm (gestational age (GA) < 32 weeks) or very low birth weight (VLBW, i.e., birth weight < 1500 g) infants have been increasingly recognized [,,,,]. Official bodies such as the World Health Organisation [], American Academy of Paediatrics [], or the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition [] recommend mother’s own milk (MOM) as the first choice in VLBW infant feeding and advocate for making strong efforts to promote lactation. When MOM is not available, pasteurized donor human milk (DHM) is the preferred choice [,]. When MOM and DHM are not available, preterm formula (PF) should be used [].
Although evidence exists regarding the protective effects of MOM in reducing the risk of necrotizing enterocolitis (NEC), late-onset sepsis (LOS), and retinopathy of prematurity (ROP) in VLBW infants, questions remain regarding whether DHM provides the same benefits [,,,,,,,]. DHM is usually donated by mothers of term infants and is pasteurized. Therefore, numerous MOM components which could contribute to protect against adverse outcomes of prematurity are reduced or absent in DHM [,,].
Bronchopulmonary dysplasia (BPD) is the most common complication of extreme preterm birth. Infants who develop BPD manifest aberrant or arrested pulmonary development and can experience lifelong alterations in cardiopulmonary function [,]. Optimal nutritional support is considered a cornerstone in the treatment/prevention of BPD [,]. Several studies have reported protective effects of DHM in the development of BPD [,]. However, a systematic review of the evidence in the literature has not been performed to date. Therefore, we aimed to conduct a systematic review and meta-analysis of the interventional and observational studies reporting data on the effects of pasteurized DHM on BPD as well as other indicators of pulmonary outcome in preterm infants.
2. Materials and Methods
A protocol was developed prospectively that detailed the specific objectives, criteria for study selection, the approach to assessing study quality, clinical outcomes, and statistical methodology. The study was carried out and reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) []. The PRISMA checklist for this report can be found in supplementary material (Table S1).
2.1. Data Sources and Search Strategies
A comprehensive literature search was conducted using PubMed/MEDLINE and EMBASE, from their inception to 1 December 2017. The search strategy for PubMed used the following terms, including Mesh terms: (breast milk OR infant feeding OR donor milk OR pasteurized human milk OR preterm formula) AND (preterm infant OR very low birth weight infant) AND (outcome OR bronchopulmonary dysplasia OR BPD). A similar strategy was used in the other databases. No language limit was applied. Translation was performed where necessary. Randomized controlled trials (RCTs) and observational studies were included in the review. Narrative reviews, systematic reviews, case reports, letters, editorials, and commentaries were excluded, but read to identify potential additional studies. Additional strategies to identify studies included use of “related articles” feature on PubMed, and use of “cited by” tool in Web of Science and Google Scholar.
2.2. Eligibility Criteria and Study Selection
Studies were included if they were RCTs, cohort studies or case-control studies, involving the use of DHM in very preterm (GA < 32 weeks) or VLBW (BW < 1500 g) infants, included a study and control group divided according to feeding policy, and reported results on BPD, days of mechanical ventilation (MV) or days on oxygen (O2). BPD was defined as oxygen dependency at 28 days (BPD28) or as oxygen dependency at 36 weeks post-menstrual age (BPD36). To identify relevant studies, two reviewers (E.V.-M., E.V.) independently screened the results of the searches and applied inclusion criteria using a structured form. Discrepancies were identified and resolved through discussion or in consultation with the other researchers.
2.3. Data Extraction and Assessment of Risk of Bias
Two investigators (E.V.-M., E.V.) extracted the data by using a data collection form designed for this review. The following information was collected: study type, number of patients, number and name of centres, study period, inclusion/exclusion criteria, patient characteristics (GA, BW), feeding intervention or observation (MOM, DHM, and/or PF, type of fortifier, duration of intervention/observation), and outcome (incidence of BPD, days on mechanical ventilation, days on oxygen). Two other investigators (M.P., G.C.) independently validated the accuracy of the extracted data.
Two reviewers (E.V.-M., E.V.) independently assessed risk of bias in each study, using two predetermined tools. Risk of bias in RCTs was assessed by using the Cochrane “Risk of Bias Assessment Tool” []. For each domain (random number generation, allocation concealment, blinding of intervention and outcome assessors, completeness of follow-up, selectivity of reporting, and other potential sources of bias) the risk was assessed as low, high, or unclear. Risk of bias in observational studies was assessed using the Newcastle-Ottawa scale for quality assessment of cohort and case-control studies []. This scale uses a rating system (range: 0–9) that gives points for selection (0–4), comparability (0–2), and outcome/exposure (0–3). Discrepancies during the data extraction and assessment of the risk of bias process were resolved by discussion and consensus among all reviewers.
2.4. Statistical Analysis
Studies were combined and analysed using comprehensive meta-analysis V 3.0 software (Biostat Inc., Englewood, NJ, USA). For dichotomous outcomes, the Mantel-Haenszel (MH) risk ratio (RR) with a 95% confidence interval (CI) was calculated from the data provided in the studies. For continuous outcomes, the mean difference (MD) with 95% CI was calculated. When studies reported continuous variables as median and range or interquartile range, we asked authors for mean and standard deviation (SD). If they did not provide the requested data, we estimated the mean and SD using the method of Wan et al. [].
Due to anticipated heterogeneity, summary statistics were calculated with a random-effects model. This model accounts for variability between studies as well as within studies. Subgroup analyses were conducted according to the mixed-effects model []. In this model, a random-effects model is used to combine studies within each subgroup and a fixed-effect model is used to combine subgroups and yield the overall effect. The study-to-study variance (tau-squared) is not assumed to be the same for all subgroups. This value is computed within subgroups and not pooled across subgroups. Statistical heterogeneity was assessed by Cochran’s Q statistic and by the I2 statistic, which is derived from Q and describes the proportion of total variation that is due to heterogeneity beyond chance []. We used the Egger’s regression test and funnel plots to assess publication bias. A probability value of less than 0.05 (0.10 for heterogeneity) was considered statistically significant.
3. Results
Based on the titles and abstracts of 1081 citations, we identified 139 potentially relevant studies (Figure 1), 18 of which met the inclusion criteria [,,,,,,,,,,,,,,,,,]. Seven studies [,,,,,,] were RCTs and 11 studies [,,,,,,,,,,] were observational. The main characteristics of the included RCTs are shown in Table 1, and the main characteristics of the included observational studies are shown in Table 2. Definitions of feeding type varied across studies. None of the studies had BPD as their primary outcome. To pool data, we classified feeding type comparisons into four categories: (1) DHM vs. PF; (2) MOM vs. DHM; (3) raw MOM vs. pasteurized MOM; and (4) raw MOM vs. frozen MOM.
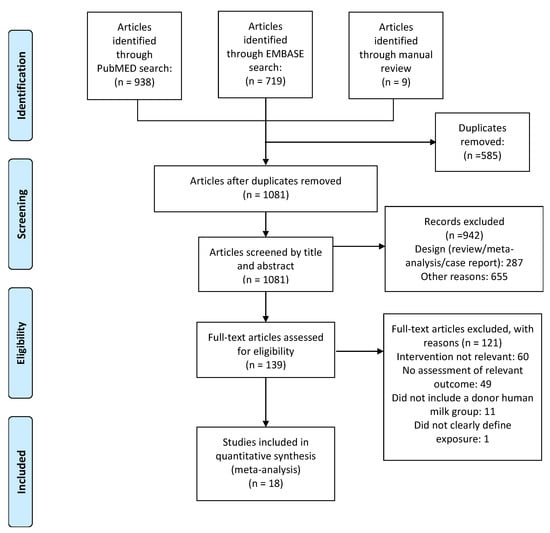
Figure 1.
Flow diagram of the literature search process.

Table 1.
Synoptic table of characteristics of included randomized controlled trials.

Table 2.
Synoptic table of characteristics of included observational studies.
3.1. Quality Assessment and Publication Bias
Six of the seven RCTs [,,,,,] included in the review scored low risk of bias for random sequence generation, allocation concealment, incomplete outcome data, selective reporting and other bias (Table A1). Four studies [,,,] scored high or unknown risk of bias on blinding (Table A1). The 12 observational studies included scored between 6 and 9 points on the Newcastle-Ottawa scale, out of a possible 9 points (Table A2). The median score of included studies was 7 points.
We found no evidence of publication bias in any of the analyses that we performed, either through visual inspection of funnel plots or Egger’s regression intercept. However, the limited amount of studies per outcome makes these tests inconclusive.
3.2. Randomized Controlled Trials: Donor Human Milk vs. Preterm Formula
Five RCTs [,,,,] randomized infants to receive DHM or PF when MOM was insufficiently available. Thus, they compared infants receiving MOM supplemented with DHM vs. infants receiving MOM supplemented with PF. Three of these studies [,,] reported on the rate of BPD36. As shown in Figure 2, meta-analysis could not detect a statistically significant effect of DHM on BPD36 (RR 0.89, 95% CI 0.60 to 1.32, p = 0.562). The RCT of Sullivan et al. used a DHM-based fortifier in the DHM group, and a bovine milk-based fortifier in the PF group. The studies of Schanler et al. [] and O’Connor et al. [] used a bovine milk-based fortifier in both groups. Exclusion of the study of Sullivan et al. [] for using a different type of fortifier did not significantly affect the results of the meta-analysis on BPD36 (RR 0.85, 95% CI 0.39 to 1.85, p = 0.676). One study [] reported on BPD28 and could not find any significant effect (RR 1.06, 95% CI 0.75 to 1.51, p = 0.724).
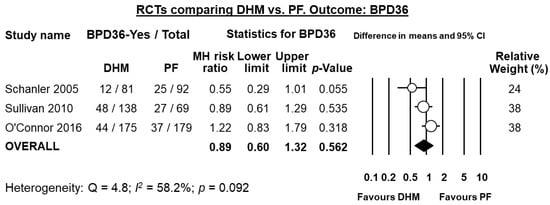
Figure 2.
Meta-analysis of randomized controlled trials assessing the effects of supplementation of MOM with DHM, compared with supplementation with PF, on risk of BPD36. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. DHM: donor human milk; PF: preterm formula; BPD36: bronchopulmonary dysplasia defined as oxygen dependency at 36 weeks post-menstrual age; MH: Mantel-Haenszel.
Three studies [,,] reported data on days on mechanical ventilation, and meta-analysis showed a significant reduction in this outcome for infants receiving DHM (MD −5.73 days, 95% CI −10.68 to −0.77, p = 0.023, Figure 3). Two studies [,] reported data on days on oxygen, and meta-analysis could not find a significant reduction in this outcome for infants receiving DHM (MD −9.11 days, 95% CI −24.82 to 6.60, p = 0.256).
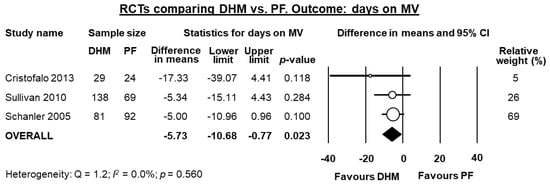
Figure 3.
Meta-analysis of randomized controlled trials assessing the effects of supplementation of MOM with DHM, compared with supplementation with PF, on mean days on MV. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. DHM: donor human milk; PF: preterm formula; MV: mechanical ventilation.
3.3. Observational Studies: Donor Human Milk vs. Preterm Formula
Eight observational studies compared infants receiving MOM supplemented with DHM to infants receiving MOM supplemented with PF [,,,,,,,] of which all reported data on BPD36. Three of these studies [,,] used a DHM-based fortifier in the DHM-group, and a bovine milk-based fortifier in the PF group. When these three studies were pooled, meta-analysis showed a significant protective effect of DHM on BPD36 (RR 0.80, 95% CI 0.68 to 0.95, p = 0.009, Figure 4). This protective effect of DHM on BPD36 was also observed when the five studies [,,,,] that did not use DHM-based fortifier were pooled (RR 0.71, 95% CI 0.53 to 0.95, p = 0.022, Figure 4) and when the 8 studies were combined in a mixed-effects meta-analysis (RR 0.78, 95% CI 0.67 to 0.89, p = 0.0005, Figure 4). The study of Ginovart et al. [] also reported data on BPD28 and found no significant difference in this outcome between the group receiving DHM and the group receiving PF (RR 1.18, 95% CI 0.68 to 2.05, p = 0.560).
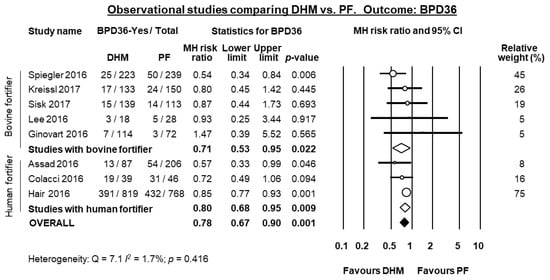
Figure 4.
Meta-analysis of observational studies assessing the effects of supplementation of MOM with DHM, compared with supplementation with PF, on risk of BPD36. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. DHM: donor human milk; PF: preterm formula; BPD36: bronchopulmonary dysplasia defined as oxygen depenency at 36 weeks post-menstrual age; MH: Mantel-Haenszel.
Four studies [,,,] reported data on mean days on mechanical ventilation, and meta-analysis showed a significant reduction in difference in means in the DHM-group (MD 2.14 days, 95% CI −4.08 to −0.21, p = 0.030, Figure 5). Three studies [,,] reported data mean on days on oxygen, and meta-analysis could not find a significant difference in mean days on oxygen in the DHM group (MD −2.78 days, 95% CI −6.32 to 0.76, p = 0.123, Figure 6).
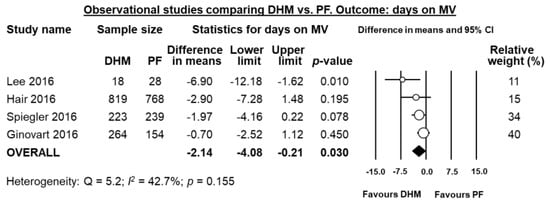
Figure 5.
Meta-analysis of observational studies assessing the effects of supplementation of MOM with DHM, compared with supplementation with PF, on mean days on mechanical ventilation. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. DHM: donor human milk; PF: preterm formula.
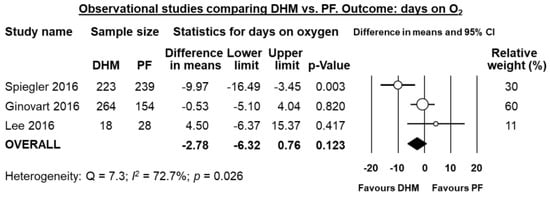
Figure 6.
Meta-analysis of observational studies assessing the effects of supplementation of MOM with DHM, compared with supplementation with PF, on mean days on oxygen. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. DHM: donor human milk; PF: preterm formula.
3.4. Mother’s Own Milk vs. Donor Human Milk
Five observational studies [,,,,] compared infants who received mainly MOM vs. infants who received mainly DHM. There were significant differences in group design. Giuliani et al. [] compared infants receiving >80% MOM (supplemented with <20% DHM) vs. infants receiving <20% MOM (supplemented with >80% DHM). Sisk et al. [] and Madore et al. [] compared infants receiving ≥50% MOM to infants receiving ≥50% DHM. The RCT of Schanler et al. [] randomized infants to receive either DHM or PF as supplementation when insufficient MOM was available, but also provided data on infants who only received MOM. We treated it as observational for this comparison, and compared infants who received exclusive MOM to infants who received MOM supplemented with DHM.
Four of the five studies reported on BPD36, and meta-analysis could not find a significant difference in BPD36 risk in the MOM-group compared to the DHM-group (RR 1.24, 95% CI 0.87 to 1.77, p = 0.231, Figure 7). The study of Giuliani et al. [] reported on BPD28 and did not find any significant effect of MOM on this outcome (RR 0.91, 95% CI 0.43 to 1.93, p = 0.804).
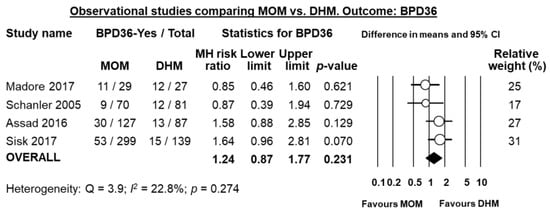
Figure 7.
Meta-analysis of observational studies assessing the effects of receiving mainly MOM vs. receiving mainly DHM, on risk of BPD36. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. MOM: mother’s own milk; DHM: donor human milk; BPD36: bronchopulmonary dysplasia defined as oxygen dependency at 36 weeks post-menstrual age; MH: Mantel-Haenszel.
3.5. Raw Mother’s Own Milk vs. Pasteurized Mother’s Own Milk
Two studies compared raw or fresh MOM to pasteurized MOM [,]. Cossey et al. [] carried out a RCT where infants were randomized to receive either raw or pasteurized MOM. Dicky et al. [] carried out a multi-centre observational trial comparing infants of centres where MOM was pasteurized and centres where it was not pasteurized. Individually, either study did not find a significant reduction in BPD36 risk in the raw MOM group when using unadjusted data (Figure 8), although Dicky et al. found a significant reduction on BPD36 risk in the raw MOM group when they adjusted their data for confounders (adjusted OR 0.40, 95% CI 0.27 to 0.67, p < 0.001). Since the study of Dicky et al. had a quasi-randomized design, we combined it with the RCT of Cossey et al. in a random effects meta-analysis. This analysis showed a significant reduction of BPD36 risk in the raw MOM group (RR 0.77, 95% CI 0.62 to 0.96, p = 0.018, Figure 8).
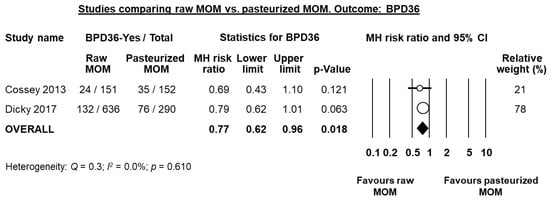
Figure 8.
Meta-analysis of studies assessing the effects of receiving raw MOM vs. receiving pasteurized MOM, on risk of BPD36. Circles (O) represent the effect sizes per study, and their size represents the relative weight of the study in the meta-analysis. Diamonds (◆) represent the pooled effect size. MOM: mother’s own milk; BPD36: bronchopulmonary dysplasia defined as oxygen dependency at 36 weeks post-menstrual age; MH: Mantel-Haenszel.
3.6. Raw Mother’s Own Milk vs. Frozen Mother’s Own Milk
One RCT [] studied the effect of freezing MOM vs. providing it fresh to infants. Infants were randomized to receive only freeze-thawed MOM (frozen at −20 °C for at least three days), or to receive fresh MOM or MOM refrigerated at 4 °C, supplemented with frozen MOM when no fresh MOM was available. The mothers of all included infants had intention to breastfeed. Supplementation in both groups was with pasteurized DHM when no MOM was available. The authors found an increase in BPD36 risk in the group receiving fresh MOM, although it was not significant (RR 1.47, 95% CI 0.98 to 2.21, p = 0.062).
4. Discussion
The present study is the first systematic analysis of evidence to date regarding the possible benefits of DHM on BPD. Meta-analysis of RCTs (three studies) could not demonstrate that supplementation of MOM with DHM had a significant effect on BPD36 risk, when compared to supplementation with PF. Meta-analysis of RCTs did find that supplementation with DHM significantly reduced the mean days on mechanical ventilation. Moreover, meta-analysis of observational studies (eight studies) showed a protective effect of DHM supplementation on BPD36 and on days on mechanical ventilation, but not on days on oxygen. Additionally, an exclusive human milk diet (i.e., MOM and/or DHM and DHM-derived fortifier) significantly reduced the risk of BPD36, when compared to a diet that included PF and/or bovine milk-based fortifier. We also found that feeding infants raw MOM, compared to feeding them pasteurized MOM, protected against BPD36. In conclusion, our data suggest that DHM could protect against BPD in very preterm/VLBW infants. However, the process of pasteurization appears to reduce the beneficial properties of human milk on BPD development.
Our study has several limitations. First, the number of included studies is small. Second, no RCT was primarily designed and powered to detect the effects of DHM on BPD. Third, most RCTs comparing the effects of DHM and PF have included infants receiving some or primarily MOM in both groups because of the inability to ethically assign feeding type []. The proportion of MOM and supplementation with either DHM or PF varied across studies. Fourth, there was substantial heterogeneity among the studies in population, timing of initiation, and duration of the intervention. Fifth, the definitions of days on mechanical ventilation and days on oxygen were heterogenous and frequently unclear, and the data itself were not always normally distributed. An additional limitation, inherent to any meta-analysis on BPD, is the heterogeneity of the definition of the condition [,,,]. Finally, many studies were observational, which potentially reduced the reliability of the results. Despite all these limitations, our meta-analysis shows an additional benefit of using DHM instead of PF in VLBW infants.
Out of 18 studies we included in our analysis, 12 were published in 2016 or 2017. The use of DHM in the NICU is a topic of much discussion, and the recency of most studies we included reflects this. As more centres start using DHM in clinical practice, and as more RCTs and observational studies are reported, the results of our meta-analyses could change or be confirmed further.
There are several hypothetical mechanisms by which human milk may exert a protective effect against BPD: (i) by improving the nutritional status and growth of the infants; (ii) by reducing postnatal inflammatory processes, such as NEC and LOS; (iii) by modulating the immune functions; and (iv) through the antioxidant properties of human milk. However, the term human milk feeding is frequently used to encompass both MOM and DHM, implying that the multiple beneficial outcomes attributed to MOM can be generalized to DHM [,]. This assumption may not be correct because of the important differences between DHM and MOM. As discussed below, part of these differences may be attributed to pasteurization, but there are additional factors that could play a role in the development of outcomes, such as BPD. DHM is typically donated by women who have delivered a term infant and this milk has different levels of nutrients and protective components, including cytokines, growth factors, and lactoferrin, than the milk provided by a mother for her own preterm infant [,]. Moreover, it has been suggested that the beneficial effects of MOM may be unique to the specific mother-infant dyad, thereby providing maximum protection to the mother’s own infant [,].
The concern about transmission of infectious agents through breast milk led to the obligatory requirement for pasteurization of DHM. Holder pasteurization (62.5 °C for 30 min) of DHM is performed in order to inactivate the microbial agents that may be present []. However, pasteurization also destroys or significantly decreases many of the protective elements in human milk, including, among others, lysozymes, secretory immunoglobulin A, growth factors, lactoferrin, antioxidants, and commensal bacteria [,,,,]. Although numerous studies have characterized the effects of Holder pasteurization on the biological properties of DHM (see [] for review), the impact of pasteurization on clinical outcomes has been scarcely investigated. Since DHM should always be pasteurized, it is ethically impossible to study the clinical impact of unpasteurized DHM. However, there are studies comparing pasteurized to unpasteurized MOM. The main purpose of this pasteurization of MOM is the prevention of cytomegalovirus (CMV) infection [].
We included two studies in our systematic review that reported the clinical outcomes of VLBW infants receiving unpasteurized or pasteurized MOM. The study of Cossey et al. [] was a single-centre RCT, whereas the study of Dicky et al. [] compared infants from 33 French NICUs that used pasteurized MOM to infants from 30 French NICUs that did not pasteurize MOM. Due to the quasi-randomized design of the study of Dicky et al., we combined it with the study of Cossey et al. in a meta-analysis, which showed that infants fed pasteurized MOM had a significantly higher risk of BPD than infants fed raw MOM (see Figure 8). Therefore, our data suggest that the possible protective effect of human milk on BPD decreases with pasteurization.
As mentioned above, Holder pasteurization not only inactivates all common pathogens in human milk but also affects the commensal microbiota []. The microbiota of breast milk has been associated with many of the benefits of raw MOM [], namely with respect to the prevention of NEC [,]. The beneficial effects on the prevention of NEC could not be repeated with pasteurized DHM [], which may be due to the loss of the microbiota [,]. The microbiota of breast milk can be “regrown” in pasteurized DHM with the inoculation of unprocessed MOM [] which awaits clinical testing. BPD has also been associated with changes in the microbiota of the airways [,] and may even be associated with the gut microbiota []. Unfortunately, we have no complete understanding about the origin, dynamics and biological function of the respiratory microbiome []. Nevertheless, the effect of MOM’s microbiota on the infant gut microbiota and possibly the infant respiratory microbiota may explain our finding on the loss of beneficial effects after pasteurization of human milk.
In some NICUs, MOM is routinely frozen to reduce the risk of CMV transmission []. In our systematic review we included one RCT [] which studied the effect of freezing MOM compared to providing fresh MOM. They found a close to significant increased risk of BPD in the group receiving fresh MOM (RR 1.47, 95% CI 0.98 to 2.21, p = 0.062). It is worth noting that this study was not powered to detect differences in BPD. Further studies are needed to elucidate the effect of MOM-freezing on neonatal outcomes.
Both MOM and DHM must be fortified to provide sufficient support for growth and development in the postnatal period of the VLBW infant [,,,]. At present, most NICUs use bovine milk-derived fortifiers. However, the recent availability of a DHM-derived fortifier has led to the introduction of the concept of “exclusive human milk diet” [,,,]. This diet consists of MOM, DHM if MOM is not adequately available, and fortification with DHM-derived fortifier. Our data suggest that this exclusive human milk diet may reduce the risk of developing BPD. However, this result should be interpreted with care. Most studies evaluating the use of an exclusive human milk diet [,,] do not isolate the individual effects of the DHM-derived fortifier, because the group receiving bovine products is a mixture of infants fed DHM plus bovine milk-derived fortifier and infants fed PF. Therefore, it is not possible to determine whether the lower risk of BPD was due to the benefit of DHM-derived fortifier or to the benefit of avoiding PF. Only the study of Assad et al. [] provided data on infants not exposed to PF and could not find a significant change in BPD risk when using DHM-derived fortifier, compared to bovine-derived fortifier.
Maintaining a sufficient (unpasteurized) MOM supply is challenging for mothers of VLBW infants, necessitating supplementation with either DHM or PF. Our results suggest the superiority of DHM over PF in reducing BPD, but we should not forget that MOM is more effective in the reduction of multiple morbidities and is less expensive to acquire than DHM. Therefore, NICU care providers should prioritize interventions to support initiation and maintenance of lactation and make efforts to identify and solve lactation problems in mothers of VLBW infants.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/10/2/238/s1, Table S1: PRISMA 2009 checklist.
Acknowledgments
We thank Nadja Haiden, Paula Sisk, Juliane Spiegler, and Sergio Verd for kindly providing additional data and clarification on their studies.
Author Contributions
E.V.-M. performed the search and selected studies for inclusion, collected data, performed the statistical analyses, contributed to the interpretation of the results, and drafted the initial manuscript. M.P. collected data, contributed to the statistical analysis and interpretation of the results, and reviewed and revised the manuscript. G.C. collected data, contributed to the statistical analysis and interpretation of the results, and revised the manuscript. F.M. supervised the data collection, contributed to the interpretation of results, and reviewed and revised the manuscript. B.W.K. contributed to the interpretation of the results and reviewed and revised the manuscript. E.V. conceptualized and designed the study, performed the search and selected studies for inclusion, supervised the data collection, contributed to the statistical analyses and interpretation of the results, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Assessment of risk of bias of included randomized controlled trials.
Table A1.
Assessment of risk of bias of included randomized controlled trials.
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Corpeleijn et al. 2016 [] | LR | LR | LR | LR | LR | LR | LR |
| Cossey et al. 2013 [] | LR | LR | HR | UR | LR | LR | LR |
| Cristofalo et al. 2013 [] | LR | LR | LR | LR | LR | LR | LR |
| O’Connor et al. 2016 [] | LR | LR | LR | LR | LR | LR | LR |
| Omarsdottir et al. 2015 [] | UR | UR | HR | UR | LR | LR | LR |
| Schanler et al. 2005 [] | LR | LR | HR | HR | LR | LR | LR |
| Sullivan et al. 2010 [] | LR | LR | HR | LR | LR | LR | LR |
LR: low risk of bias, HR: high risk of bias, UR: unknown risk of bias.

Table A2.
Assessment of methodological quality of included observational studies, using the Newcastle-Ottawa Scale.
Table A2.
Assessment of methodological quality of included observational studies, using the Newcastle-Ottawa Scale.
| Study Name | Study Design | Selection (0–4 Points) | Comparability (0–2) | Outcome (0–3) | Total (0–9) |
|---|---|---|---|---|---|
| Assad et al. 2015 [] | Retrospective cohort | 4 | 0 | 3 | 7 |
| Colacci et al. 2017 [] | Retrospective cohort | 4 | 0 | 3 | 7 |
| Dicky et al. 2017 [] | Retrospective cohort | 4 | 2 | 3 | 9 |
| Ginovart et al. 2016 [] | Retrospective cohort | 4 | 0 | 3 | 7 |
| Giuliani et al. 2012 [] | Retrospective case-control | 4 | 0 | 3 | 7 |
| Hair et al. 2016 [] | Retrospective cohort | 4 | 0 | 3 | 7 |
| Kreissl et al. 2017 [] | Prospective cohort | 4 | 0 | 3 | 7 |
| Lee et al. 2016 [] | Retrospective cohort | 4 | 0 | 2 | 6 |
| Madore et al. 2017 [] | Retrospective case-control | 4 | 2 | 3 | 9 |
| Sisk et al. 2017 [] | Retrospective cohort | 4 | 0 | 3 | 7 |
| Spiegler et al. 2016 [] | Prospective cohort | 4 | 2 | 3 | 9 |
References
- Hylander, M.A.; Strobino, D.M.; Dhanireddy, R. Human milk feedings and infection among very low birth weight infants. Pediatrics 1998, 102, e38. [Google Scholar] [CrossRef] [PubMed]
- Morales, Y.; Schanler, R.J. Human milk and clinical outcomes in vlbw infants: How compelling is the evidence of benefit? Semin. Perinatol. 2007, 31, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Giuliani, F.; Occhi, L.; Coscia, A.; Tonetto, P.; Marchino, F.; Fabris, C. Benefits of donor human milk for preterm infants: Current evidence. Early Hum. Dev. 2009, 85, S9–S10. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2014, CD002971. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low-and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; Hojsak, I. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.L.; Johnson, T.J.; Engstrom, J.L.; Fogg, L.F.; Jegier, B.J.; Bigger, H.R.; Meier, P.P. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J. Perinatol. 2013, 33, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Lau, C.; Hurst, N.M.; Smith, E.O.B. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Hylander, M.A.; Strobino, D.M.; Pezzullo, J.C.; Dhanireddy, R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J. Perinatol. 2001, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Sorita, A.; Carey, W.A.; Colby, C.E.; Murad, M.H.; Alahdab, F. Interventions to prevent retinopathy of prematurity: A meta-analysis. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shukla, V.V.; John, D.; Chen, C. Human milk feeding as a protective factor for retinopathy of prematurity: A meta-analysis. Pediatrics 2015, 136, e1576–e1586. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Parker, L.A.; Neu, J. Necrotizing enterocolitis and human milk feeding: A systematic review. Clin. Perinatol. 2017, 44, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Patel, A.; Esquerra-Zwiers, A. Donor human milk update: Evidence, mechanisms, and priorities for research and practice. J. Pediatr. 2017, 180, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Moukarzel, S.; Soberanes, L.; Dyer, R.A.; Albersheim, S.; Elango, R.; Innis, S.M. Relationships among different water-soluble choline compounds differ between human preterm and donor milk. Nutrients 2017, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Van Ormer, M.; Anderson-Berry, A. A comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Jain, L.; Schmidt, B.; Abman, S.; Bancalari, E.; Aschner, J.L. Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung diseases. Ann. Am. Thorac. Soc. 2014, 11, S146–S153. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.W.; Kallapur, S.; Newnham, J.; Jobe, A.H. Prenatal inflammation and lung development. Semin. Fetal Neonatal Med. 2009, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Wemhöner, A.; Ortner, D.; Tschirch, E.; Strasak, A.; Rüdiger, M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm. Med. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, J.; Preuß, M.; Gebauer, C.; Bendiks, M.; Herting, E.; Göpel, W.; Network, G.N. Does breastmilk influence the development of bronchopulmonary dysplasia? J. Pediatr. 2016, 169, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://s3.amazonaws.com/academia.edu.documents/37210401/0470699515.pdf?AWSAccessKeyId=AKIAIWOWYYGZ2Y53UL3A&Expires=1518487789&Signature=K9uRr67lWsWC8w5ktrWqgY652HU%3D&response-content-disposition=inline%3B%20filename%3D20_Qualitative_research_and_Cochrane_rev.pdf (accessed on 10 December 2017).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&as_vis=1&q=Newcastle-ottawa+quality+assessment+scale+cohort+studies&btnG= (accessed on 10 December 2017).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.; Rothstein, H.R. Subgroup analyses. In Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 149–186. [Google Scholar]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Chapter 16: Identifying and quantifying heterogeneity. In Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 123, pp. 2009–2124. [Google Scholar]
- Assad, M.; Elliott, M.; Abraham, J. Decreased cost and improved feeding tolerance in vlbw infants fed an exclusive human milk diet. J. Perinatol. 2016, 36, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Colacci, M.; Murthy, K.; DeRegnier, R.-A.O.; Khan, J.Y.; Robinson, D.T. Growth and development in extremely low birth weight infants after the introduction of exclusive human milk feedings. Am. J. Perinatol. 2017, 34, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; de Waard, M.; Christmann, V.; van Goudoever, J.B.; Jansen-van der Weide, M.C.; Kooi, E.M.; Koper, J.F.; Kouwenhoven, S.M.; Lafeber, H.N.; Mank, E. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: The early nutrition study randomized clinical trial. JAMA Pediatr. 2016, 170, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Cossey, V.; Vanhole, C.; Eerdekens, A.; Rayyan, M.; Fieuws, S.; Schuermans, A. Pasteurization of mother’s own milk for preterm infants does not reduce the incidence of late-onset sepsis. Neonatology 2013, 103, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Dicky, O.; Ehlinger, V.; Montjaux, N.; Gremmo-Féger, G.; Sizun, J.; Rozé, J.C.; Arnaud, C.; Casper, C. Policy of feeding very preterm infants with their mother’s own fresh expressed milk was associated with a reduced risk of bronchopulmonary dysplasia. Acta Paediatr. 2017, 106, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Ginovart, G.; Gich, I.; Verd, S. Human milk feeding protects very low-birth-weight infants from retinopathy of prematurity: A pre–post cohort analysis. J. Matern. Fetal Neonatal Med. 2016, 29, 3790–3795. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Prandi, G.; Coscia, A.; Cresi, F.; Di Nicola, P.; Raia, M.; Sabatino, G.; Occhi, L.; Bertino, E. Donor human milk versus mother’s own milk in preterm vlbwis: A case control study. J. Biol. Regul. Homeost. Agents 2012, 26, 19–24. [Google Scholar] [PubMed]
- Hair, A.B.; Peluso, A.M.; Hawthorne, K.M.; Perez, J.; Smith, D.P.; Khan, J.Y.; O’Donnell, A.; Powers, R.J.; Lee, M.L.; Abrams, S.A. Beyond necrotizing enterocolitis prevention: Improving outcomes with an exclusive human milk–based diet. Breastfeed. Med. 2016, 11, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, A.; Sauerzapf, E.; Repa, A.; Binder, C.; Thanhaeuser, M.; Jilma, B.; Ristl, R.; Berger, A.; Haiden, N. Starting enteral nutrition with preterm single donor milk instead of formula affects time to full enteral feeding in very low birth weight infants. Acta Paediatr. 2017, 106, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, J.H.; Kim, C.S.; Lee, S.L. Clinical findings according to feeding diets in very low birth weight infants: Human breast milk versus bovine milk-based formula. Neonat. Med. 2016, 23, 23–28. [Google Scholar] [CrossRef]
- Omarsdottir, S.; Casper, C.; Naver, L.; Legnevall, L.; Gustafsson, F.; Grillner, L.; Zweygberg-Wirgart, B.; Soderberg-Naucler, C.; Vanpee, M. Cytomegalovirus infection and neonatal outcome in extremely preterm infants after freezing of maternal milk. Pediatr. Infect. Dis. J. 2015, 34, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; Lambeth, T.M.; Rojas, M.A.; Lightbourne, T.; Barahona, M.; Anthony, E.; Auringer, S.T. Necrotizing enterocolitis and growth in preterm infants fed predominantly maternal milk, pasteurized donor milk, or preterm formula: A retrospective study. Am. J. Perinatol. 2017, 34, 676–683. [Google Scholar] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e561. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016, 316, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Madore, L.S.; Bora, S.; Erdei, C.; Jumani, T.; Dengos, A.R.; Sen, S. Effects of donor breastmilk feeding on growth and early neurodevelopmental outcomes in preterm infants: An observational study. Clin. Ther. 2017, 39, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1591. [Google Scholar] [CrossRef] [PubMed]
- Ginovart, G.; Gich, I.; Gutiérrez, A.; Verd, S. A fortified donor milk policy is associated with improved in-hospital head growth and weight gain in very low-birth-weight infants. Adv. Neonatal Care 2017, 17, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.; Lee, M.K.; Kagawa, M. Ethical challenges in infant feeding research. Nutrients 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H.; Bancalari, E.H. Controversies about the definition of bronchopulmonary dysplasia at 50 years. Acta Paediatr. 2017, 106, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.B.; Feng, R.; Schmidt, B.; Aschner, J.L.; Ballard, R.A.; Hamvas, A.; Reynolds, A.M.; Shaw, P.A.; Jobe, A.H. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann. Am. Thorac. Soc. 2015, 12, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Beam, K.S.; Aliaga, S.; Ahlfeld, S.K.; Cohen-Wolkowiez, M.; Smith, P.B.; Laughon, M.M. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J. Perinatol. 2014, 34, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.; Villamor, E. Probiotic supplementation in preterm infants does not affect the risk of bronchopulmonary dysplasia: A meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.; Schmidt, S.; King, T.; Israel-Ballard, K.; Amundson Mansen, K.; Coutsoudis, A. The effect of simulated flash-heat pasteurization on immune components of human milk. Nutrients 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Gormaz, M.; Torres-Cuevas, I.; Cernada, M.; Kuligowski, J.; Cubells, E.; Escobar, J.; Vento, M. Role of human milk in oxidative stress associated with prematurity. J. Pediatr. Biochem. 2013, 3, 169–177. [Google Scholar] [CrossRef]
- Hamprecht, K.; Goelz, R. Postnatal cytomegalovirus infection through human milk in preterm infants: Transmission, clinical presentation, and prevention. Clin. Perinatol. 2017, 44, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; Kouwenhoven, S.M.P.; Paap, M.C.; van Vliet, I.; Scheerder, I.; Muizer, Y.; Helder, O.K.; van Goudoever, J.B.; Vermeulen, M.J. Intake of own mother’s milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology 2012, 102, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Niño, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Vongbhavit, K.; Underwood, M.A. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin. Ther. 2016, 38, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Neu, J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Harrison, N.A.; Parker, L.A.; Padgett, K.A.; Lemas, D.J.; Marcial, G.E.; Li, N.; Carr, L.E.; Neu, J.; Lorca, G.L. Personalization of the microbiota of donor human milk with mother’s own milk. Front. Microbiol. 2017, 8, 1470. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Sato, M.; Go, H.; Ogasawara, K.; Kanai, Y.; Maeda, H.; Chishiki, M.; Shimizu, H.; Mashiyama, F.; Goto, A.; et al. The microbiome of the lower respiratory tract in premature infants with and without severe bronchopulmonary dysplasia. Am. J. Perinatol. 2017, 34, 80–87. [Google Scholar] [PubMed]
- Lohmann, P.; Luna, R.A.; Hollister, E.B.; Devaraj, S.; Mistretta, T.-A.; Welty, S.E.; Versalovic, J. The airway microbiome of intubated premature infants: Characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr. Res. 2014, 76, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, D.J.; Niemarkt, H.J.; Benninga, M.A.; Budding, A.E.; van Kaam, A.H.; Kramer, B.W.; Pantophlet, C.M.; van Weissenbruch, M.M.; de Boer, N.K.; de Meij, T.G. Development of severe bronchopulmonary dysplasia is associated with alterations in fecal volatile organic compounds. Pediatr. Res. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D.; Sontag, M.K.; Harris, J.K.; Miller, J.I.; Morrow, L.; Robertson, C.E.; Stephens, M.; Poindexter, B.B.; Abman, S.H.; Mourani, P.M. Airway microbial community turnover differs by bpd severity in ventilated preterm infants. PLoS ONE 2017, 12, e0170120. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Hanson, C.; Lyden, E.; Dugick, L.; Ruybal, L.; Anderson-Berry, A. Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients 2014, 6, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Lyden, E.; Weishaar, K.; Elliott, E.; Wu, R.; White, K.; Timm, H.; Anderson-Berry, A. Comparison of a powdered, acidified liquid, and non-acidified liquid human milk fortifier on clinical outcomes in premature infants. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Adamkin, D.H.; Radmacher, P.G. Fortification of human milk in very low birth weight infants (VLBW <1500 g birth weight). Clin. Perinatol. 2014, 41, 405–421. [Google Scholar] [PubMed]
- Radmacher, P.G.; Adamkin, D.H. Fortification of human milk for preterm infants. Semin. Fetal Neonatal Med. 2017, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).