Abstract
In this work, we carried out studies of the chemical composition of hexane, chloroform and ethanol extracts from two samples of the lichen Parmotrema hypoleucinum collected in Algeria. Each sample of the lichen P. hypoleucinum was collected on two different supports: Olea europaea and Quercus coccifera. Hexane extracts were prepared, in Soxhlet; each hexane extract was fractionated by its solubility in methanol; the products soluble in methanol were separated (cold): 1-Hexane, 2-Hexane; and the products insoluble in methanol (cold): 1-Cires, 2-Cires. A diazomethane esterified sample of 1-Hexane, 2-Hexane, 1-Cires and 2-Cires was analyzed by GC-MS, and the components were identified as methyl esters. In the 1-Hexane and 2-Hexane fractions, the methyl esters of the predominant fatty acids in the lichen were identified: palmitic acid, linoleic acid, oleic acid and stearic acid; a hydrocarbon was also identified: 13-methyl-17-norkaur-15-ene and several derivatives of orsellinic acid. In the 1-Cires and 2-Cires fractions, the previous fatty acids were no longer observed, and only the derivatives of orsellinic acid were found. The analysis of the 1-Hexane, 2-Hexane fractions by HPLC-MS/MS allows us to identify different chemical components, and the most characteristic products of the lichen were identified, such as Atranol, Chloroatranol, Atranorin and Chloroatranorin. In the fractions of 1-Cires and 2-Cires, the HPLC-MS/MS analysis reveals that they are very similar in their chemical components; the characteristic products of this lichen in this fraction are Atranorin and Chloroatranorin. In the extracts of chloroform, 1-Chloroform and 2-Chloroform, the analysis carried out by HPLC-MS/MS shows small differences in their chemical composition at the level of secondary products; among the products to be highlighted for this work, we have chloroatranorin, the stictic acid, norstictic acid and other derivatives. In the analysis of the most polar extracts carried out in ethanol: 1-Ethanol and 2-Ethanol, HPLC-MS/MS analysis shows very similar chemical compositions in these two extracts with small differences. In these extracts, the following acids were identified as characteristic compounds of this lichen: constictic acid, stictic acid, substictic acid and methylstictic acid. In the HPLC–MS/MS analysis of all these extracts, alectoronic acid was not found.
1. Introduction
Lichens live in symbiotic associations between fungi and algae and/or cyanobacteria, and in addition to these two symbiotic partners (photobiont and mycobiont) classically described, a third partner can also be integrated: epi and/or endophytic fungi as well as bacteriobiont or associated bacterial communities [] which are important constituents of many of them. The production of various unique extracellular secondary metabolites known as lichen substances is the result of this symbiosis. The specific condition in which lichens live is the reason for the production of many metabolites that provide good protection against negative physical and biological influences. [] The majority of lichens-forming fungi belong to Ascomycetes Lecanoromycetes []. Due to the vast genetic diversity and interactions with various environmental factors, lichens have unique profiles of primary and secondary metabolites (i.e., lichen substances) with interesting physiochemical properties [].
In this paper, we describe our studies on the chemical composition of the extracts of Parmotrema hypoleucinum, Lecanorales Order, family Parmeliaceae, which, with 2765 species all over the world, is the largest family [].
In Algeria, the Parmeliaceae family is very present compared to other families []; four Parmotrema have been identified so far: P. perlatum, P. reticulatum, P. robustrum and P. hypoleucinum. The last one is very common in the Mediterranean area []; it belongs to the Lecanorales Order and to the Parmeliaceae Family. It corresponds to a foliaceous lichen that can be up to 12 cm in diameter. In general, the lobes are very irregular, wide and raised, often forming tufts on small branches and can appear like curly lettuce. The upper side has a grayish appearance, and the lower side is largely white, which allows it to be easily distinguished from other similar species that have a dark back side. This species has black cilia and marginal Soralies.
Several activities of these lichen molecules, mainly those resulting from the polymalonate acetate pathway, are of interest for cosmetics: photoabsorbing, antioxidant and inducing melanogenesis. These properties have been studied for a still limited number of secondary metabolites (Mitrović, 2011) (Figure 1).
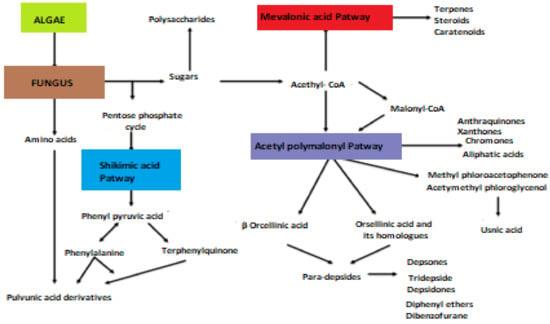
Figure 1.
Biosynthetic pathways of lichen secondary metabolites (Elix, 1996; Stocker wörgötter, 2008).
The natural products isolated from different lichens (such as Usnic acid, Lobaric acid, Atranorin, Protolichesterinic acid and Salazinic acid) have good antibiotic activities against Gram-positive bacteria and are also active against pathogenic dermatophyte fungi []. Other products found in lichens, such as anthraquinones derivatives, bianthrones and hypericin, have an inhibitory action on the activity of viral enzymes, such as the integrase of HIV-1 and HSV-1 [,] and also on enzymes such as lipooxygenases, histidine decarboxylase and tyrosinase; other derivatives inhibit the biosynthesis of Leukotriene B4 (LTB4) [,,].
Polyphenolic products isolated from lichens have limitations, low solubility and, above all, toxicity. Usnic acid is a polyphenolic compound very common in lichens that has good activity, among others, against microorganisms of the Mycobacterium genus. In the 1950s, Shibata and Miura [] made modifications and derivatizations of the functional groups of usnic acid to carry out the structure–activity correlation study to enhance the biological activity profile.
Usnic and polyporic acids have a good growth inhibition activity of L1210 leukemic cells; in subsequent research [,], several derivatives of these acids have been prepared to enhance antitumor activity, but none of these derivatives have exceeded the activities presented by usnic and polyporic acids.
Kumar and Muller have prepared a series of analogs of barbatic, diffractaic and obtusatic acids isolated from lichens to evaluate the effects of inhibition of the biosynthesis of LTB4 and as antiproliferative agents. Some of these derivatives show good potential as LTB4 biosynthesis inhibitors [,].
Lichens can also have xanthones, that shown enzyme modulation that are therapeutic targets, such as protein kinase C [], topoisomerase II [,], acetylcholinesterase [] and monoamine oxidases []; antiretrovirals [,], antimalarials [,], antihypertensives [], anti-inflammatory, cytotoxics [] and antitumors [,]. For this reason, the use of the base skeleton of xanthone is justified to prepare derivatives with bioactive potential.
Many depsidones isolated from lichens and higher plants have important activities, including the inhibition of enzymatic activity [], antimycobacterial, anti-inflammatory, analgesic, antitumor, cytotoxic and antiviral activity [,,]. In the work published in 2015 by James C Lendemer and collaborators [], they studied and delineated the Parmotrema species in eastern North America. Using morphological, chemical, reproductive and ecological characters, they define four species for this group: P. hypoleucinum, P. hypotropum, P. perforatum and P. subrigidum.
This group has found P. hypoleucinum and P. subrigidum to be momophyletic, the latter comprising two chemotypes that differ in the presence or absence of norstictic acid in addition to alectoronic acid.
Due to the pharmacological potential presented by compounds isolated in lichens, it was decided to study the chemical composition of the lichen Parmotrema hypoleucinum (J. Steiner) Hale, collected on two different supports in the area of Lac Tonga in Algeria.
2. Results and Discussion
Parmotrema hypoleucinum (J. Steiner) Hale is an epiphytic lichen collected in Algeria and studied in order to determine its metabolic composition and chemical fingerprint. P hypoleucinum (J. Steiner) Hale was collected in two different supports, the first one in Lac Tonga (Sector Brabtia) on Olea europaea and the second one in Lac Tonga (Sector Brabtia) on Quercus coccifera. The metabolic compositions of each lichen sample were studied by sequential extraction, first of all, with hexane in Soxhlet for low polarity products. The remaining vegetable mass was placed with chloroform at room temperature to obtain the chloroform extract for the products of intermediate polarity, and finally, the vegetable mass was extracted with ethanol at room temperature for the products with higher polarity.
The hexane extract of each sample was dissolved in hot methanol and allowed to cool slowly to obtain the products insoluble in cold methanol. In this way, the products insoluble in methanol were separated: 1-Cires and 2-Cires; remaining soluble: 1-Hexane and 2-Hexane. Initially, an aliquot of the samples 1-Hexane, 2-Hexane, 1-Cires and 2-Cires were esterified with diazomethane to esterify the acid groups of the existing compounds. These esterified samples were analyzed by GC-MS to identify compounds of lower polarity.
The different extracts obtained were:
- From P hypoleucinum (J. Steiner) Hale on Olea europaea
- 1-Hexane, 1-Cires, 1-C 1-Ethanol
- From Parmotrema hypoleucinum (J. Steiner) Hale on Quercus coccifera.
- 2-Hexane, 2-Cires, 2-Choroform, 2-Ethanol
Chemical analysis of the hexane extract soluble in MeOH esterified with diazometane, 1-Hexan and 2-Hexane of Parmotrema hypoleucinum collected from two different phorophytes by GC/MS
The 1-Hexane and 2-Hexane samples were esterified with diazomethane to esterify the existing acid groups to their methyl esters for GC-MS analysis, being the natural products of the acids indicated in Table 1 for 1-Hexane sample and Table 2 for 2-Hexane sample.

Table 1.
Sample Parmotrema hypoleucinum (in Olea europaea), Hexane extract part soluble in MeOH esterified whit diazomethane, 1-Hexane.

Table 2.
Sample Parmotrema hypoleucinum (in Quercus coccifera), Hexane extract part soluble in MeOH esterified whit diazometane, 2-Hexane.
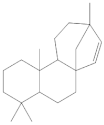
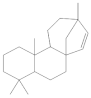
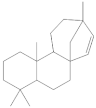
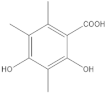
The 1-Hexane sample was analyzed by GC-MS, and eight products were identified, among them palmitic, linoleic, oleic and stearic acids and an unidentified compound. Figure 2 and Table 1. The esterified 2-Hexane sample was also analyzed by GC-MS, identifying six products, palmitic, linoleic, oleic and stearic acid, a phenolic compound 2,4-dihydroxy-3,5,6-trimethylbenzoic acid and 13-methyl-17-norkaur-15-ene. Figure 3 and Table 2.
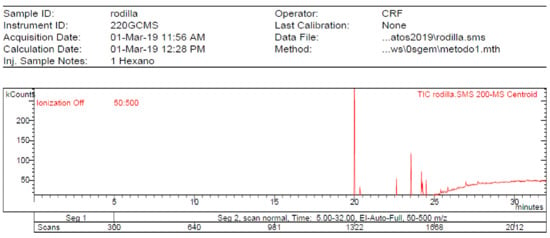
Figure 2.
Chromatogram of Hexane extract part soluble in MeOH esterified with diazomethane, 1-Hexane.
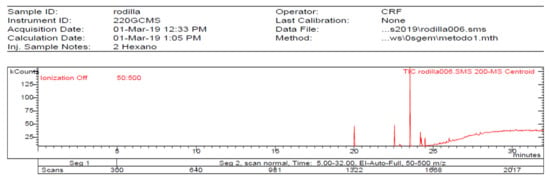
Figure 3.
Chromatogram of Hexane extract part soluble in MeOH esterified whit diazomethane, 2-Hexane.
The compound identified as Hibaene (13-methyl-17-norkaur-15-ene) is the product with a retention time of 23:53 in GC-MS; its mass spectrum shows the molecular ion at 272 and comes from the dehydration of alcohol (-)-ent-Kauran-16α-ol in the ionization source of the mass spectrometer. The natural product should be the alcohol (-)-ent-Kauran-16α-ol.
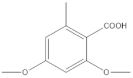
P hypoleucinum (J. Steiner) Hale, on Quercus coccifera, the 2-Hexane sample, it has a lower number of components, and all were identified in the 1-Hexane sample. The most important fatty acids in the extracts were identified as palmitic, linoleic, oleic and stearic acids. 2,4-Dihydroxy-3,5,6-trimethylbenzoic acid and (-)-ent-Kauran-16α-ol alcohol is also identified in the two samples, 1-Hexane and 2-Hexane. Only in the 1-Hexane sample 4-hydroxy-2-methoxy-3,5,6-trimethylbenzoic acid and 2,4-dihydroxy-3,6-dimethylbenzoic acid are also identified.
For the fractions that have been obtained by crystallization from the crude hexane extract by solubilization in hot methanol, 1-Cires and 2-Cires are also esterified with diazomethane for GC-MS analysis.
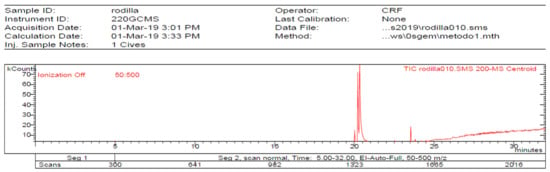
Figure 4.
Chromatogram of Hexane extract part insoluble in MeOH esterified whit diazomethane, 1-Cires.

Table 3.
Sample Parmotrema hypoleucinum (in Olea europaea), Hexane extract part insoluble in MeOH esterified whit diazomethane, 1-Cires.
In the 2-Cires sample, three products have been identified from the methanol-insoluble part of the hexane extract of P. hypoleucinium (Quercus coccifera). Figure 5 and Table 4.
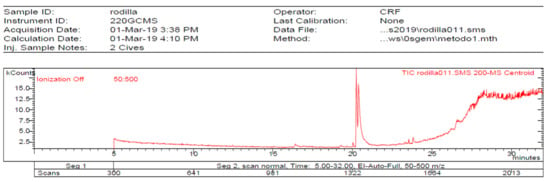
Figure 5.
Chromatogram of Hexane extract part insoluble in MeOH esterified whit diazometane, 2-Cires.

Table 4.
Sample Parmotrema hypoleucinum (in Quercus coccifera), Hexane extract part insoluble in MeOH esterified whit diazomethane, 2-Cires.
In the 1-Cires sample, there is a compound that could not be identified; this product does not appear in the 2-Cires sample. The kaurene-skeletal alcohol is found in 1-Cires and was not found in the 2-Cires sample. Of the other three compounds, two were identified in 1-Cires and 2-Cires: 2,4-dihydroxy-3,5,6-trimetylbenzoic acid and 2,4-dihydroxy-3,6-dimethylbenzoic acid.
In the 1-Cires sample, 4-hydroxy-2-methoxy-3,6-dimethylbenzoic acid is also identified, and this product does not appear in the 2-Cires sample; instead, the 2,4-dimethoxy-6-methylbenzoic acid appears in 2-Cires.
In the HPLC—MS/MS analysis of the 1-Hexane and 2-Hexane fractions of P. hypoleucinium, 95 products were detected in the 1-Hexane fraction, in which we proposed 78 structures (and 16 not identified). In total, 91 products were found in the 2-Hexane fraction, of which we proposed 78 structures (and 13 no identified ones).
Figure 6 and Figure 7 show the HPLC chromatograms that allowed this analysis and identification of the indicated compounds to be carried out; the complete result is shown in Table 5.
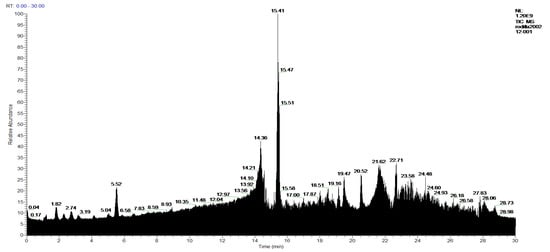
Figure 6.
Chromatogram of Hexane extract part soluble in MeOH, 1-Hexane.
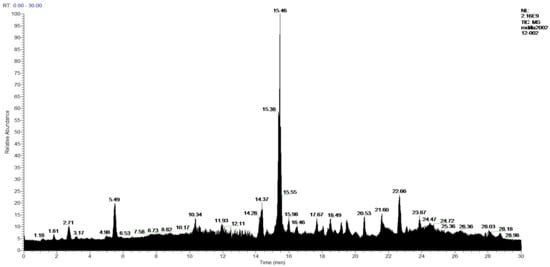
Figure 7.
Chromatogram of Hexane extract part soluble in MeOH esterified whit diazomethane, 2-Hexane.

Table 5.
Samples Parmotrema hypoleucinum (in Olea europea), Hexane extract part soluble in MeOH 1-Hexane and Parmotrema hypoleucinum (in Quercus coccifera), Hexane extract part soluble in MeOH, 2-Hexane.
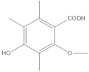
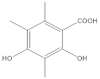
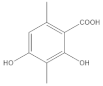
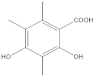
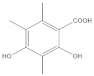
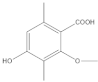
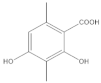
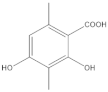
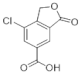
The most characteristic products of the 1-Hexane and 2-Hexane extracts of the lichens in the two samples are 3-methylorsellinic acid, atranol, 4-formylbenzoic acid, chloroatranol, p-coumaric acid, atranorin and chloroatranorin.
In the fatty acid profile, we find the following acids common to the two samples: 2-hydroxy-10-undecenoic, 2-undecenedioic, undecanedioic, trans-2-dodecenedioic, 9-hydroxy-10,12-pentadecadienoic, tridecanedioic, 9Z-octadecenedioic, 10-acyl-9-formyl-13-hydroxyoctadeca-6,11-dienoic, octadecanedioic, 2-oxopalmitic, nonadecanoic, arachidic and 12-triacontenedioic acids.
Among the acids that have a cyclohexanecarboxylic base, the following derivatives were also found, as described in the publication of the lichen Physcia mediterranea Nimis [], the 3,5-dimethoxycyclohexanecarboxylic acids, 6-(hydroxymethyl)-3,5-bis(methoxycarbonyl)-2,4-dimethylcyclohex-1-ene-1-carboxylic, 3,5,6-hydroxymethyl-2,4-dimethylcyclohex-1-ene-1-carboxylic, 5-formyl-3-hydroxymethyl-2,4,6-trimethylcyclohex-1-ene-1-carboxylic, 3,5-dihydroxy-2,4,6-trimethylcyclohex-1-ene-1-carboxylic, 5-formyl-3,6-dihydroxymethyl-2,4-dimethylcyclohex-1-ene-1-carboxylic, 2,4-dihydroxy-3,5,6-trimethylcyclohexane-1-carboxylic, 4-hydroxy-2,5-dimethylcyclohex-1-ene-1-carboxylic, 6-(1-oxopentyl)-cyclohex-1-ene-1-carboxylic acids.
Lactones were also identified: N-dodecanoyl-L-homoserine lactone and fukinanolide, other derivatives such as leoidin, lecideoidin, an alcohol such as 7,10,12-nonadecatrien-1-ol and a triterpenic acid identified as ursolic acid (found in the two extracts).
In the comparison of the characteristic polyphenolic compounds of the lichens in these two samples, 20 compounds were identified: 4-O-demethyldivaricatic, barbatic, 8-hydroxybarbatic, baeomycesic, allo-protolichesterinic, 4′-O-demethylsekikaic acids, 8-hydroxydiffractaic, glomelliferonic occurs in Xanthoparmelia subincerta [], muronic is detected in P. praesorediosum [], murolic, lichesterylic [], praesorediosic, 19-acetoxyprotolichesterinic, diploicin, leprolomin, methyl 3-formyl-2-hydroxy-4-((4-methoxy-2-methylbenzoyl)oxy)-6-methylbenzoate, superpicrolichenic and 3-formyl-2-hydroxy-4-((2-(14-hydroxypentadecyl)-4-methyl-5-oxo-2,5-dihydrofuran-3-carbonyl)oxy)-6-methylbenzoic acids.
In the 1-Hexane sample, the following compounds were not identified: 3,5-dimethylorselinic acid, 9,10-dihydroxy-8-oxo-12-octadecenoic acid, octadecanedioic acid and 18-hydroxylinoleic acid, which were found in the 2-Hexane sample. The following compounds were not identified in the 2-Hexane sample: barbatic acid, tetraoxodocosanoic acid and superpicrolichenic acid; these products were identified in the 1-Hexane sample too. In the 1-Hexane sample, there are 17 products that could not be identified and in the 2-Hexane 13.
In the analysis of the 1-Cires and 2-Cires fractions, obtained by precipitation of the initial hexane extract by HPLC-MS/MS, it has been possible to detect in 1-Cires 56 products, of which 11 products were not identified. In 2-Cires, 53 products were detected, and 13 products could not be identified. For the acids and diacids identified in 1-Cires there are 23 products, and in 2-Cires, we have 27 products. As benzoic acids or derivatives we have p-coumaric acid and 6,7-dihydroxycoumarin. Among the polyphenolic compounds and esters, 1-Cires and 2-Cires were identified: allo-protolichestrinic acid, atranorin, 7-chloro-3-oxo-1,3-dihydroisobenzofuran-5-carboxylic acid, chloroatranorin, 8-hydroxydiffractaic acid and 19-acetoxylichestrinic acid.
The chromatograms and the analysis of the 1-Cires and 2-Cires compounds are shown in Figure 8 and Figure 9 and Table 6.
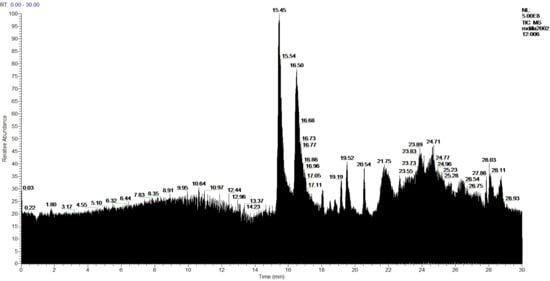
Figure 8.
Chromatogram of Hexane extract part insoluble in MeOH, 1-Cires.
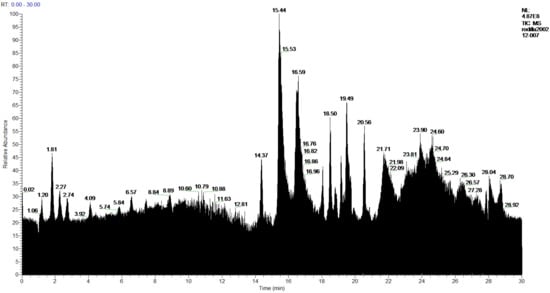
Figure 9.
Chromatogram of Hexane extract part insoluble in MeOH, 2-Cires.

Table 6.
Samples Parmotrema hypoleucinum (in Olea europea), Hexane extract part insoluble in MeOH, 1-Cires and Parmotrema hypoleucinum (in Quercus coccifera), Hexane extract part insoluble in MeOH, 2-Cires.
In the work carried out in 2016, in a general analysis of the chemical relationship in the group of Parmotrema perforatum (Parmeliaceae, Ascomycota), each sorediate species is descended from an apotheciate species with the same secondary chemicals []. The lichen Parmotrema hypoleucinum is derived from the ancestor Parmotrema perforatum represented by its secondary metabolites in stictic, constictic and norstictic acids. These acids have not been extracted in the Hexane extract, and they do not exist in the part insoluble in methanol, 1-Cires and 2-Cires nor in the part soluble in methanol: 1-Hexane and 2-Hexane.
The products identified in the 1-Chloroform and 2-Chloroform extracts are shown in the chromatograms in Figure 10 and Figure 11 and the results of the identification of the compounds in Table 7.
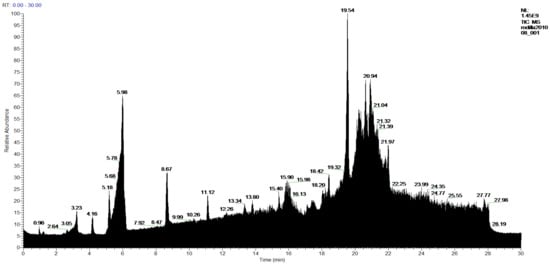
Figure 10.
Chromatogram of 1-Chloroform extract from P. hypoleucinum.
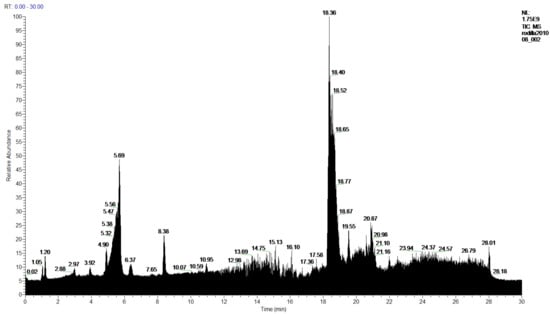
Figure 11.
Chromatogram of 2-Chloroform extract from P. hypoleucinum.

Table 7.
Samples Parmotrema hypoleucinum (in Olea europea), Chloroform extract, 1-Chloroform and Parmotrema hypoleucinum (in Quercus coccifera), Chloroform extract, 2-Chloroform.
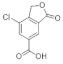
In the analysis of 1-Chloroform and 2-Chloroform extracts, the acids of the secondary metabolites that define the Sorediate species are identified for Parmotrema hypoleucinum; these acids are as follows. Constictic acid is also detected in Parmotrema tinctorum; Norstictic acid, according to mycologia 2015, this compound appeared to be present in variable concentrations throughout the thallus. Often the medulla of a lobe tested negative while the medulla adjacent to the apothecia tested positive or vice versa. Stictic acid and other derivatives identified as Substictic acid, there are depsidone were detected in Parmotrema tinctorum, P. grayanum, also in P. robustum and P. andinum [].
In sample 1-Chloroform, 60 products were detected, of which 52 products were identified, and 8 products were not identified, and in the sample 2-Chloroform, 59 compounds were detected, of which 52 products were identified and 7 unidentified.
As in the previous analyses, fatty acids, hydroxy acids, oxo acids, some xanthones and flavones have been found, in addition to menegazziaic, siphullelic, protocetraric, conphysodalic, cryptostictic, menegazziaic isomer, gyrophoric, lecanoric and muronic acids among others.
In the extracts of the more polar products made with ethanol, in the 1-Ethanol sample 57 products were found, of which 54 products were identified, and 3 compounds were not identified. Figure 12 and Table 8. In the sample 2-Ethanol analyzed, 54 compounds were detected, of which 52 products were identified, and 2 compounds were not identified. Figure 13 and Table 8.
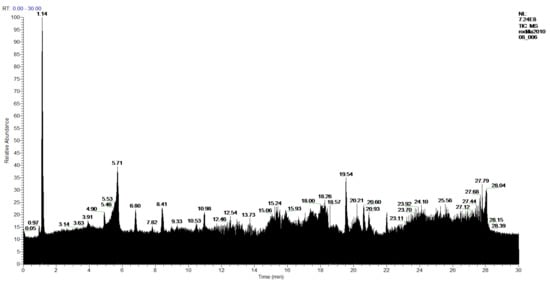
Figure 12.
Chromatogram of 1-Ethanol extract from P. hypoleucinum.

Table 8.
Samples Parmotrema hypoleucinum (in Olea europea), Ethanol extract, 1-Ethanol and Parmotrema hypoleucinum (in Quercus coccifera), Ethanol extract, 2-Ethanol.
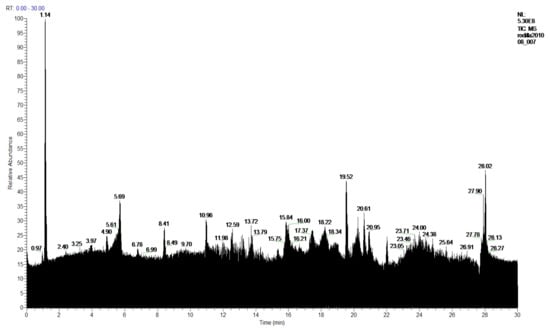
Figure 13.
Chromatogram of 2-Ethanol extract from P. hypoleucinum.
Among the compounds identified in the 1-Ethanol and 2-Ethanol samples were also found the acids that define this lichen Parmotrema hypoleucinum, the Constictic and Stictic acids and a derivative such as methylstictic acid.
The products analyzed by GC-MS were identified by their mass spectra and compared with the mass spectra of the NIST and Wiley databases.
3. Materials and Methods
3.1. Lichen Material
Parmotrema hypoleucinum is a foliose epiphytic lichen, which was collected on Quercus coccifera and on Olea europea at Lake Tonga (Sector Brabtia), at an altitude of 2.20 m above sea level, coordinate 36°51′38″ N; 08°28′46″ E in June 2017. The area of lake tonga is 2600 ha communicating with the sea through the artificial channel of the Messida.
This station is located in the national park of el kala (80,000 ha) Figure 14, classified as a biosphere reserve by UNESCO in 1990, located in the extreme northeast of Algeria.
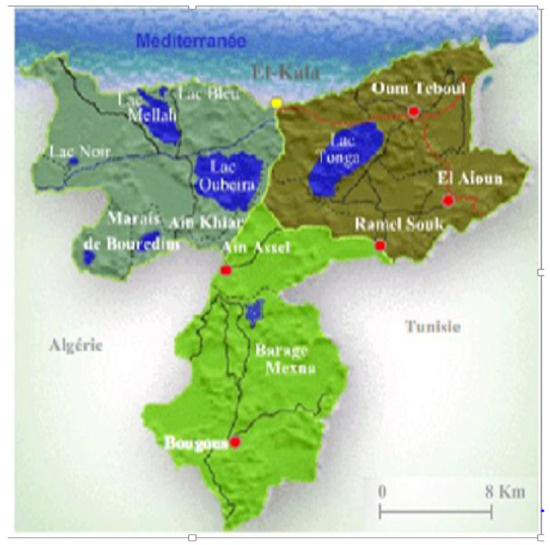
Figure 14.
Location of El Kala National Park (P.N.E.K., 2010).
Parmotrema hypoleucinum (J. Steiner) Hale was identified by Professor Monia Ali Ahmed lichenologist and research director of the Pathology of Ecosystems team at the University of Badji-Mokhtar, Annaba, Algeria. This sample has been deposited in Badji-Mokhtar University, Annaba, code AAM-2.
3.2. HPLC Orbitrap
3.2.1. Sample Preparation
Hexane extraction was carried out for each lichen sample; for Parmotrema hypoleucinium (J. Steiner), Hale was made from 40 g of powder material for the two samples. The extraction was carried out in Soxhlet apparatus with hot n-Hexane for 24 h; after this time, the solvent was evaporated to obtain the Parmotrema hypoleucinium (Olea europaea) n-Hexane extract 0.66 g, which represents (1.65%) and Parmotrema hypoleucinium (Quercus coccifera)) n-Hexane extract 0.27 g, which represent (0.68%). The n-Hexane extracts are then dissolved in hot methanol and allowed to cool to room temperature so that insoluble products crystallize. With this treatment, the methanol insoluble part (cires) and the cold methanol soluble part (Hexane) are obtained for each n-Hexane extract. For P hypoleucinium, the part insoluble in methanol produced 1-Cires of 0.379 g and 2-Cires of 0.058 g; the part soluble in methanol produced 1-Hexane of 0.281 g and 2-Hexane of 0.219 g.
The vegetable mass recovered and dried from the extractions with Hexane was placed to extract with chloroform at room temperature for 5 days. After this time, the chloroform extract was filtered, and the solvent was evaporated, obtaining the 1-Chloroform extract with a weight of 0.226 g, which represents (5.65%) and the 2-Chloroform extract with a weight of 0.196 g, which represents (4.90%).
After being extracted the vegetable mass with chloroform was placed with ethanol for 5 days to prepare the Ethanol extracts. Evaporation of the solvent gave the ethanol extracts: for 1-Ethanol, a mass of 2385 g was recovered, which represented 5.96%, and for the 2-Ethanol extract, it presented a mass of 2.417 g, which represented 6.04%.
3.2.2. Instruments
For the GCMS analysis, an Agilent MS220 mass spectrometer coupled to a 7890A GC was used.
HPLC analyses were carried out on an orbitrap Thermo q-Exactive mass spectrometer coupled to a Vanquish HPLC.
3.2.3. GCMS Parameters
The oven temperature was initially set to 50 °C, held for 5 min and then a ramp of 30 °C/min was applied up to 270 °C that was held for 5 additional mins. A VF-5 ms columns was used, with a length of 30 m, inner diameter 0.25 mm and layer width of 0.25 micron.
MS spectra were acquired in EI mode with a mass range from 50 uma to 600 uma.
3.2.4. LC Parameters
For the HPLC separation, a Kinetex XB-C18 (Phenomenex) with a particle size of 2.6 microns, 100 mm in length and a diameter of 2.1 mm was used as column. As solvent A, water with 0.1% of formic acid was used, and as solvent B, acetonitrile was chosen. The column flow war 0.200 mL/min. The following gradient was used (in Table 9):

Table 9.
Solvents used, % of solvent A and % of solvent B.
3.2.5. MS Parameters
For the ionization electrospray in negative mode was used, with the following parameters: Electrospray voltage −3.8kV, Sheath gas 30, Aux gas 10, drying gas temperature 310 ºC. Capillary temp. 320 and S-lens value of 55.0.
The acquisition was performed in a mass range from 100 to 1000 uma, and an auto MS2 program was used with a fragmentation voltage of 30.
4. Conclusions
Due to the biological importance of lichens isolated natural products, studies of the chemical composition of two samples of the lichen Parmotrema hypoleucinum, collected on two different supports: Olea europaea and Quercus coccifera, in Algeria, were carried out. For each sample, the extracts of hot n-Hexane, Chloroform at room temperature and Ethanol at room temperature were carried out.
The n-Hexane extract of each sample is dissolved in hot methanol and allowed to cool slowly so that the products insoluble in methanol precipitate. The parts soluble in methanol are obtained by filtration, the solvent is evaporated, and they are designated as 1-Hexane and 2-Hexane fractions for each sample, respectively. The product insoluble in methanol, washed and dried, are designated as the 1-Cires and 2-Cires fractions, respectively. An aliquot sample of the fractions: 1-Hexane, 2-Hexane, 1-Cires and 2-Cires, were esterified with diazomethane to produce the methyl esters of the existing acids.
Esterified samples of 1-Hexane and 2-Hexane were analyzed by GC-MS to identify components of lower polarity. In both samples, the methyl esters of 2,4-dihydroxy-3,5,6-trimethylbenzoic acids, palmitic acid, linoleic acid, oleic acid, stearic acid and the hydrocarbon 13-methyl-17-norkaur-15-ene (Probably the natural product will be (-)-ent-kauran-16α-ol). 4-Hydroxy-2-methoxy-3,5,6-trimethylbenzoic and 2,4-dihydroxy-3,6-dimethylbenzoic acid methyl esters and a product that does not it has been possible to identify mass 288; these products were not found in the esterified sample of 2-Hexane.
The 1-Cires and 2-Cires esterified samples were analyzed by GC-MS to separate and identify the components of lower polarity. In the analysis carried out, 2,4-dihydroxy-3,5,6-trimethylbenzoic and 2,4-dihydroxy-3,6-dimethylbenzoic acids were found as existing products in the two samples. In the 2-Cires esterified sample, 2,4-dimethoxy-6-methylbenzoic acid, which does not exist in the esterified 1-Cires fraction, was also identified in this analysis. In the esterified fraction 1-Cires, the analysis also identified 4-hydroxy-2-methoxy-3,6-dimethylbenzoic acid, the hydrocarbon 13-methyl-17-norkaur-15-ene, and a product that was not identified with a mass of 312, which were not found in the esterified sample of 2-Cires.
The analysis, identification and comparison of the components of the original fractions of 1-Hexane and 2-Hexane by HPLC-MS/MS indicate that they are very similar and show almost no differences, except for some very minor components; in these fractions of low polarity, the most characteristic components P hypoleucinum will be: Atranol, Chloroatranol, Atranorin and Chloroatranorin; a triterpenic acid identified as Ursolic acid has also been found in both samples.
The analysis of the original 1-Cires and 2-Cires fractions by HPLC-MS/MS shows that they are very similar, presenting some small differences in some minor components. The predominant components identified are the fatty acids indicated in the Tables in addition to the predominant product Atranorin and Chloroatranorin, which will be characteristic for this lichen.
In the analysis carried out by HPLC-MS/MS of the original extracts of 1-Chloroform and 2-Chloroform of the two samples of P hypoleucinum and in the comparison of their components, small differences were found in some secondary compounds. In this analysis, the following acids can be considered as characteristic products of the lichen: Constictic acid, Norstictic acid (this acid was only found in the 1-Chloroform extract), Stictic acid, Substictic acid and Chloroatranorin.
The HPLC-MS/MS analysis of the original extracts of 1-Ethanol and 2-Ethanol did not suggest great differences, and most of their components were identified; the following compounds were found as more representative of the lichen: Constictic acid, Stictic acid, Substictic acid; Methylstictic acid was only identified in the extract of 1-Ethanol.
In this work, a more complete study of the components of the different extracts made from P. hypoleucinum was carried out due to the importance of the biological activities. In the most important identified products, in general, their biological activities were referred to derivatives of orsellinic acid, atranol, chloroatranol, atranorin, chloroatranorin, stictic acid and norstictic acid.
For these components, their antioxidant activities, apoptotic effects, cytotoxic, antimicrobial and antitumor activity are already known.
Author Contributions
Conceptualization, J.M.R. and A.A.M.; methodology, J.M.R., A.A.M., L.S. and D.D.; software, C.R. and M.K.; validation, L.S., D.D. and J.M.R.; formal analysis, C.R., N.S. and M.K.; investigation, A.A.M., J.M.R., L.S., N.S. and M.K.; writing-original draft preparation, J.M.R., A.A.M., L.S. and M.K.; writing-review and editing, J.M.R., A.A.M., and D.D.; funding acquisition, DGRSDT. All authors have read and agreed to the published version of the manuscript.
Funding
DGRSDT: Badji Mokhtar University, Algeira. We thank the Ministry of Higher Education and Scientific Research (MESRS) and the General Directorate of Scientific Research and Technological Development (DGRSDT) of Algeria for the financial support of this study. The authors thank to FibEnTech-UBI, which is financed by National Funds from Fundação para a Ciência e a Tecnologia (FCT) and Project UIDB/00195/2020 (financing by the FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Nash, T.H. Lichen Biology; Cambridge University Press: Cambridge, UK, 2008; p. 498. [Google Scholar]
- Ranković, B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Tehler, A.; Wedin, M. Systematics of Lichenized Fungi. In Lichen Biology, 2nd ed.; Cambridge University Press: London, UK, 2008; Chapter 17; pp. 336–352. [Google Scholar]
- Nguyen, K.V.; Thi Do, N.T.; Chandna, A.; Pham, C.V.; Doan, P.M.; Nguyen, A.Q.; Nguyen, C.K.T.; Larsson, M.; Escalante, S.; Olowokure, B.; et al. Antibiotic use and resistance in emerging economies: A situation analysis for Viet Nam. BMC Public Health 2013, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Amrani, S.; Seaward, M.R.D.; Sipman, H.J.M.; Feuerer, T. Lichenological exploration of Algeria II: Checklist of lichenized, lichenicolous and allied fungi. Herzogia 2018, 31, 817–892. [Google Scholar] [CrossRef]
- Nash, T.H.; Ryan, B.D.; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Arizona State University Lichen Herbarium: Tempe, AZ, USA, 2002; Volume 1. [Google Scholar]
- Lawrey, J.D. The chemical ecology of lichen mycoparasites: A review. Can. J. Bot. 1995, 73, S1. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K.; Chung, G.A.C.; Skúlason, V.G.; Gissurarson, S.R.; Vilhelmstóttir, M. Antimycobacterial activity of lichen metabolites in vitro. Eur. J. Pharm. Sci. 1998, 6, 141. [Google Scholar] [CrossRef]
- Cohen, P.A.; Hudson, J.B.; Towers, G.H.N. Antiviral activities of antraquinones, bianthrones and hypericin derivatives from lichens. Experientia 1996, 52, 180. [Google Scholar] [CrossRef]
- Neamati, N.; Hong, H.; Mazumder, A.; Wang, S.; Sunder, S.; Nicklaus, M.C.; Milne, G.W.A.; Proska, B.; Pommier, Y. Depsides and depsidones as inhibitors of HIV-1 integrase: Discovery of novel inhibitors through 3D database searching. J. Med. Chem. 1997, 40, 942. [Google Scholar] [CrossRef]
- Kumar, S.K.C.; Müller, K. Depsides as non-redox inhibitors of leukotriene B4 biosynthesis and HaCaT cell growth. 1. Novel analogues of barbatic and diffractaic acid. Eur. J. Med. Chem. 1999, 35, 1035–1042. [Google Scholar] [CrossRef]
- Kumar, S.K.C.; Müller, K. Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J. Nat. Prod. 1999, 62, 817. [Google Scholar] [CrossRef]
- Kumar, S.K.C.; Müller, K. Depsides as non-redox inhibitors of leukotriene B4 biosynthesis and HaCaT cell growth. 2. Novel analogues of obtusatic acid. Eur. J. Med. Chem. 2000, 35, 405. [Google Scholar] [CrossRef]
- Shibata, S.; Miura, Y. Antibacterial effects of Lichen substances. 1. Comparative studies of various lichen substances. Japn. Med. J. 1948, 1, 518. [Google Scholar] [CrossRef]
- Cain, B.F. Potential anti-tumour agents. IV. Polyporic acid series. J. Chem. Soc. 1966, 1041–1045. [Google Scholar] [CrossRef]
- Takai, M.; Uehara, Y.; Beisler, J.A. Usnic acid derivatives as potential antineoplastic agents. J. Med. Chem. 1979, 22, 1380. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, L.; Fresco, P.; Pinto, E.; Saouza, E.; Pinto, M.; Gonçalves, J. Inhibition of protein kinase C by synthetic xanthone derivatives. Bioorg. Med. Chem. 2003, 11, 1215. [Google Scholar] [CrossRef]
- Kwok, Y.; Zeng, Q.; Hurley, L.H. Topoisomerase II-mediated site-directed alkylation of DNA by psorospermin and its use in mapping other topoisomerase II poison binding sites. Proc. Natl. Acad. Sci. USA 1998, 95, 13531. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Jung, J.; Lee, C.; Kwon, Y.; Na, Y. Synthesis of new xanthone analogues and their biological activity test-cytotoxicity, topoisomerase II inhibition, and DNA cross-linking study. Bioorg. Med. Chem. 2007, 17, 1163. [Google Scholar] [CrossRef]
- Piazzi, L.; Belluti, F.; Bisi, A.; Gobbi, S.; Rizzo, S.; Bartolini, M.; Andrisano, V.; Recanatini, M.; Rampa, A. Cholinesterase inhibitors: SAR and enzyme inhibitory activity of 3-[omega-(benzylmethylamino)alkoxy]xanthen-9-ones. Bioorg. Med. Chem. 2007, 15, 575. [Google Scholar] [CrossRef]
- Nuñez, M.B.; Maguna, F.P.; Okulik, N.B.; Castro, A.E. QSAR modeling of the MAO inhibitory activity of xanthones derivatives. Bioorg. Med. Chem. 2004, 14, 5611. [Google Scholar] [CrossRef]
- Ng, T.B.; Huang, B.; Fong, W.P.; Yeung, H.W. Anti-human immunodeficiency virus (anti-HIV) natural products with special emphasis on HIV reverse transcriptase inhibitors. Life Sci. 1997, 10, 933. [Google Scholar] [CrossRef]
- Groweiss, A.; Cardellina, I.J.H.; Boyd, M.R. HIV-Inhibitory Prenylated Santhones and Flavones from Maclura tinctoria. J. Nat. Prod. 2000, 63, 1537–1539. [Google Scholar] [CrossRef]
- Portela, C.; Afonso, C.M.M.; Pinto, M.M.M.; Ramos, M.J. Computational studies of new potential antimalarial compounds-Stereoelectronic complementarity with the receptor. J. Comput.-Aided Mol. Des. 2003, 17, 583. [Google Scholar] [CrossRef] [PubMed]
- Riscoe, M.; Kelly, J.X.; Winter, R. Xanthones as antimalarial agents: Discovery, mode of action, and optimization. Cur. Med. Chem. 2005, 12, 2539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kang, J.; Chen, I.; Teng, C.; Lin, C. Antihypertensive and vasorelaxing activities of synthetic xanthone derivatives. Bioorg. Med. Chem. 2002, 10, 567. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 2003, 66, 1124. [Google Scholar] [CrossRef]
- Gobbi, S.; Rampa, A.; Bisi, A.; Belluti, F.; Valenti, P.; Caputo, A.; Zampiron, A.; Carrara, M. Synthesis and antitumor activity of new derivatives of xanthen-9-one-4-acetic acid. J. Med. Chem. 2002, 45, 4931. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517. [Google Scholar] [CrossRef]
- Hamano, K.; Kinoshitaokami, M.; Hemmi, A.; Sato, A.; Hisamoto, M.; Matsuda, K.; Yoda, K.; Haruyama, H.; Hosoya, T.; Tanzawa, K. Folipastatin, a new depsidone compound from Aspergillus unguis as an inhibitor of phospholipase A2. Taxonomy, fermentation, isolation, structure determination and biological properties. J. Antibiot. 1992, 45, 1195. [Google Scholar] [CrossRef][Green Version]
- Permana, D.; Lajis, N.H.; Mackeen, M.M.; Ali, A.M.; Kitajima, M.; Takayama, H. Isolation and bioactivities of constituents of the roots of Garcinia atroviridis. J. Nat. Prod. 2001, 64, 976. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Mishina, Y.; Tomiyasu, H.; Litaudon, M.; Cosson, J.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Furukawa, H. Cancer chemopreventive agents. New depsidones from Garcinia plants. J. Nat. Prod. 2001, 64, 147. [Google Scholar] [CrossRef]
- Lendemer, J.C.; Allen, J.L.; Noel, N. The Parmotrema acid test: A look at species delineation in the P. perforatum group 40 y later. Mycologia 2015, 107, 1120–1129. [Google Scholar] [CrossRef]
- Kumar, K.; Siva, B.; Sarma, V.U.M.; Mohabe, S.; Reddy, A.M.; Boustie, J.; Tiwari, A.K.; Rao, N.R.; Babu, K.S. UPLC–MS/MS quantitative analysis and structural fragmentation study of five Parmotrema lichens from the Eastern Ghats. J. Pharm. Biomed. Anal. 2018, 156, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Kerboua, M.; Ahmed, M.A.; Samba, N.; Aitfella-Lahlou, R.; Silva, L.; Boyero, J.F.; Raposo, C.; Rodilla, J.M.L. Phytochemical Investigation of New Algerian Lichen Species: Physcia mediterranea Nimis. Molecules 2021, 26, 1121. [Google Scholar] [CrossRef] [PubMed]
- Elix, J.A. A Catalogue of Standardized Chromatographic Data and Biosynthetic Relationships for Lichen Substances, 3rd ed.; The Author: Canberra, Australia, 2014. [Google Scholar]
- Solberg, Y. Chemical Constituent of the Lichen Species Cetraria islandica. J. Hattori Bot. Lab. 1986, 60, 391. [Google Scholar]
- Widhelm, T.J.; Egan, R.S.; Bertoletti, F.R.; Asztalos, M.J.; Kraichak, E.; Leavitt, S.D.; Lumbsch, H.T. Picking holes in traditional species delimitations: An integrative taxonomic reassessment of the Parmotrema perforatum group (Parmeliaceae, Ascomycota). Bot. J. Linn. Soc. 2016, 182, 868–884. [Google Scholar] [CrossRef][Green Version]
- Torres-Benítez, A.; Rivera-Montalvo, M.; Sepúlveda, B.; Castro, O.N.; Nagles, E.; Mario, J.; Simirgiotis, M.J.; García-Beltrán, O.; Areche, C. Metabolomic Analysis of Two Parmotrema Lichens: P. robustum (Degel.) Hale and P. andinum (Mull. Arg.) Hale Using UHPLC-ESI-OT-MS-MS. Molecules 2017, 22, 1861. [Google Scholar] [CrossRef]
- Elix, J.A.; Wardlaw, J.H. 4-O-Methylconhypoprotocetraric acid, a new β-orcinol depsidone from the lichen Xanthoparmelia competita. Aust. J. Chem. 2000, 53, 1009–1010. [Google Scholar] [CrossRef]
- David, F.; Elix, J.A.; Samsudin, M.W. Two new aliphatic acids from the lichen Parmotrema praesorediosum. Aust. J. Chem. 1990, 43, 1297–1300. [Google Scholar] [CrossRef]
- Huynh, B.L.C.; Duong, T.H.; Do, T.M.L.; Pinnock, T.G.; Pratt, L.M.; Yamamoto, S.; Watarai, H.; Tanahashi, T.; Nguyen, K.P.P. New gamma-lactone carboxylic acids from the lichen Parmotrema praesorediosum (Nyl.) hale, parmeliaceae. Rec. Nat. Prod. 2016, 10, 332–340. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).