Phenolic Acids from Fructus Chebulae Immaturus Alleviate Intestinal Ischemia-Reperfusion Injury in Mice through the PPARα/NF-κB Pathway
Abstract
:1. Introduction
2. Results
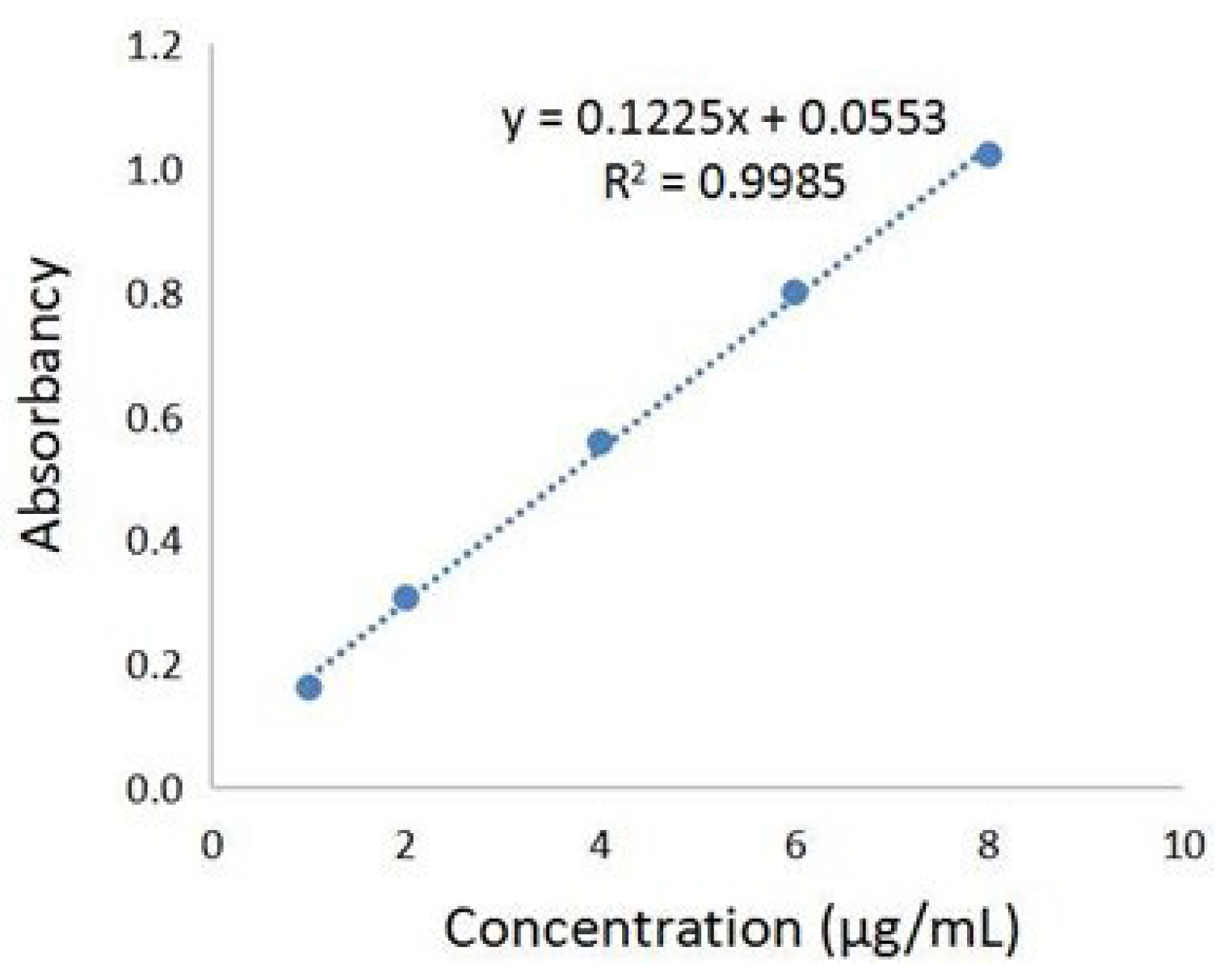
2.1. Determination of Total Phenolic Acids Content in Chebulae Fructus Immaturus Extract
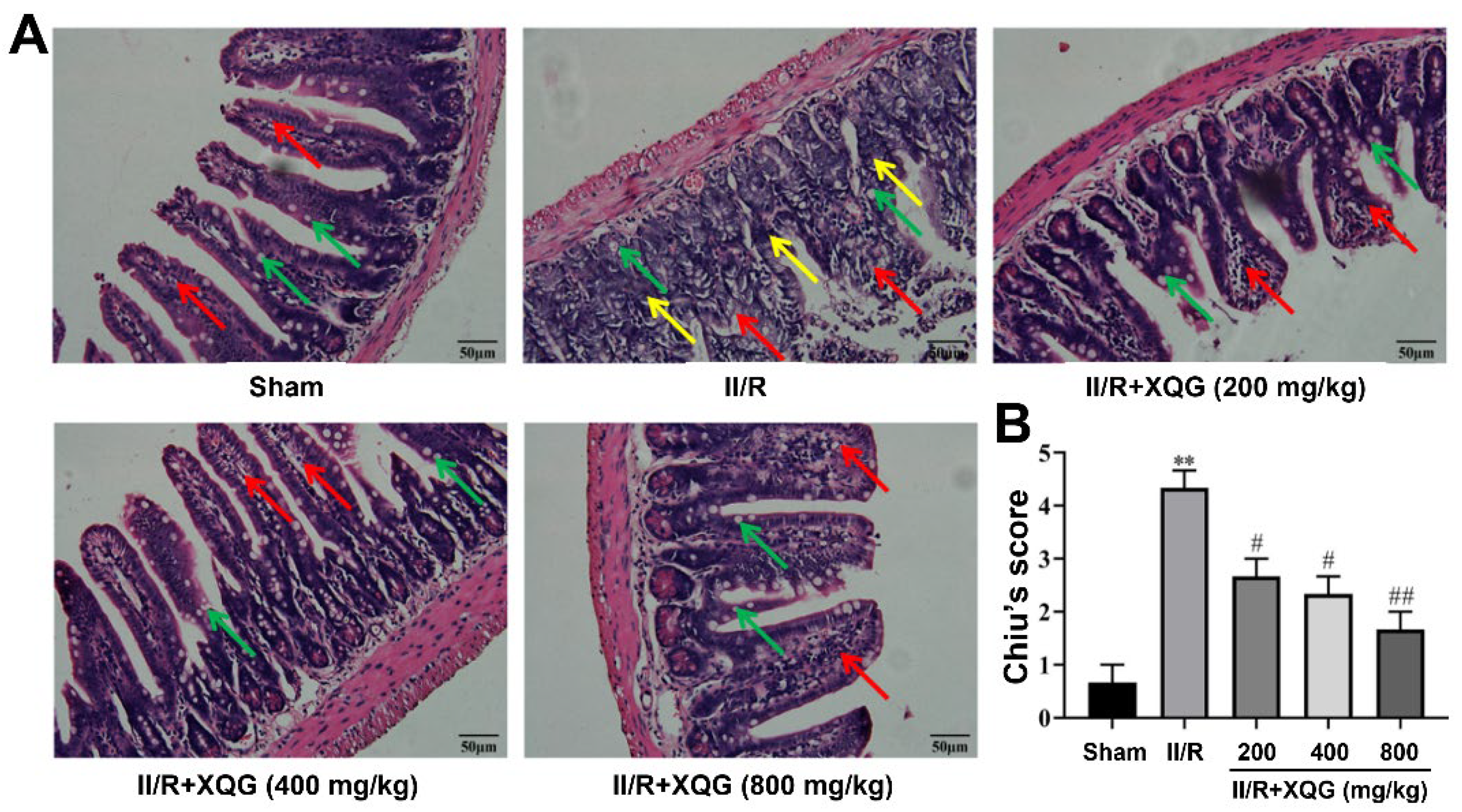
2.2. XQG Ameliorated II/R Induced Intestinal Morphological Damage
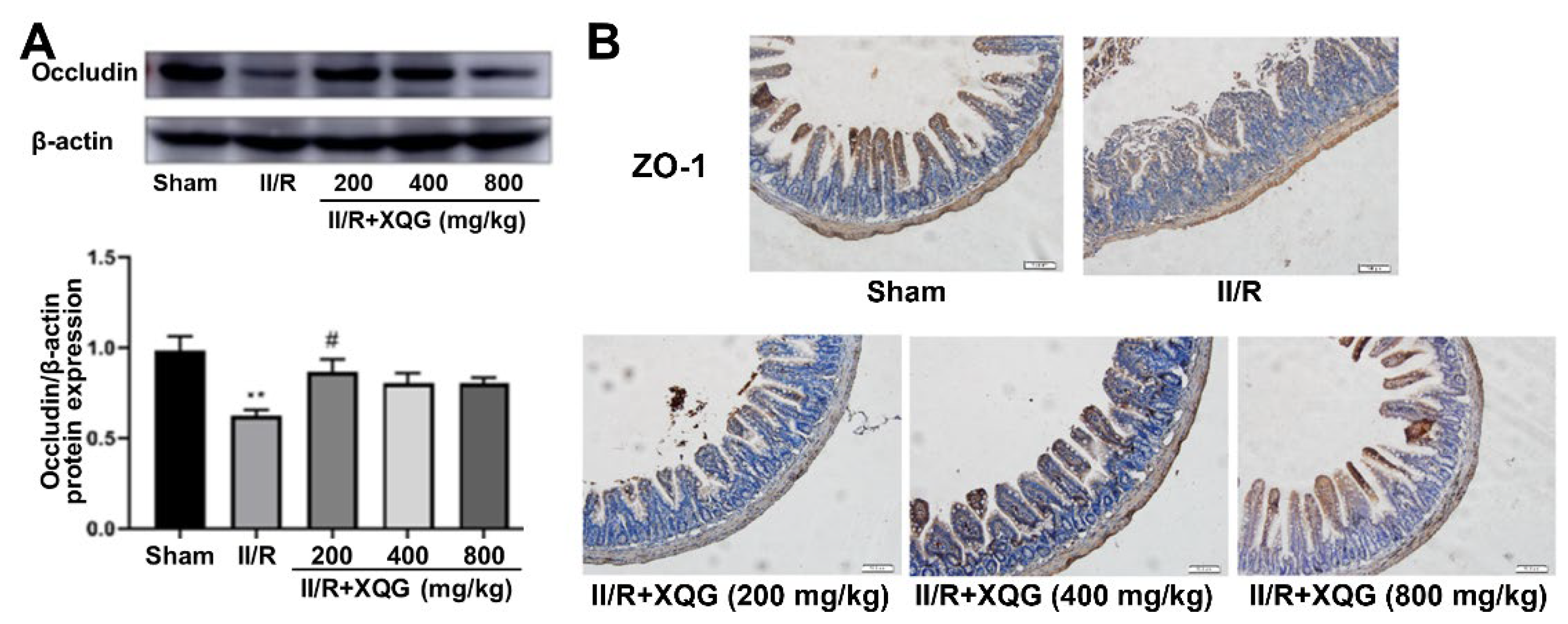
2.3. XQG Ameliorated II/R-Induced Intestinal Barrier Injury
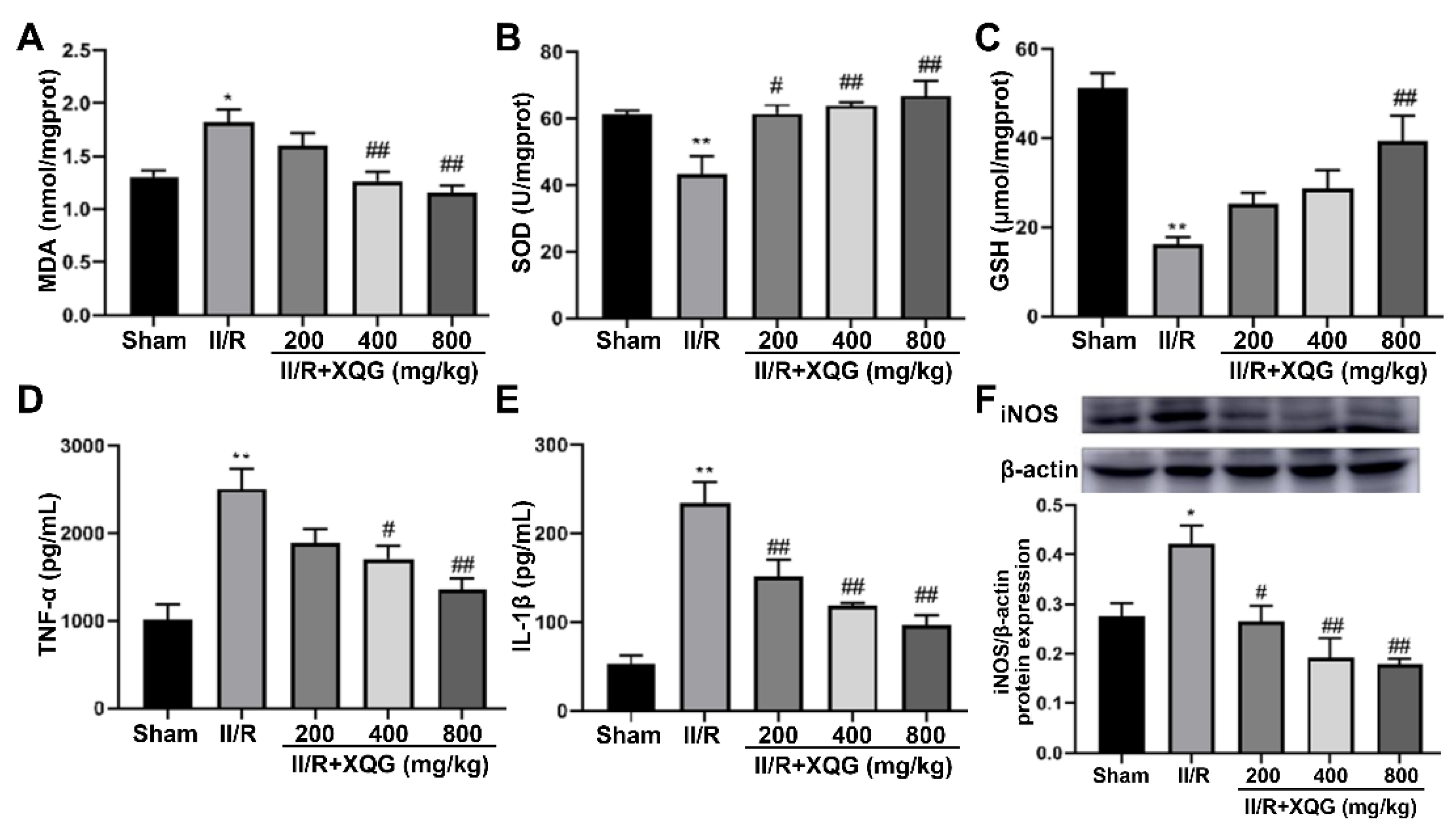
2.4. XQG Alleviated II/R-Induced Oxidative Stress and the Inflammatory Response
2.5. XQG Alleviated II/R-Induced Cell Apoptosis
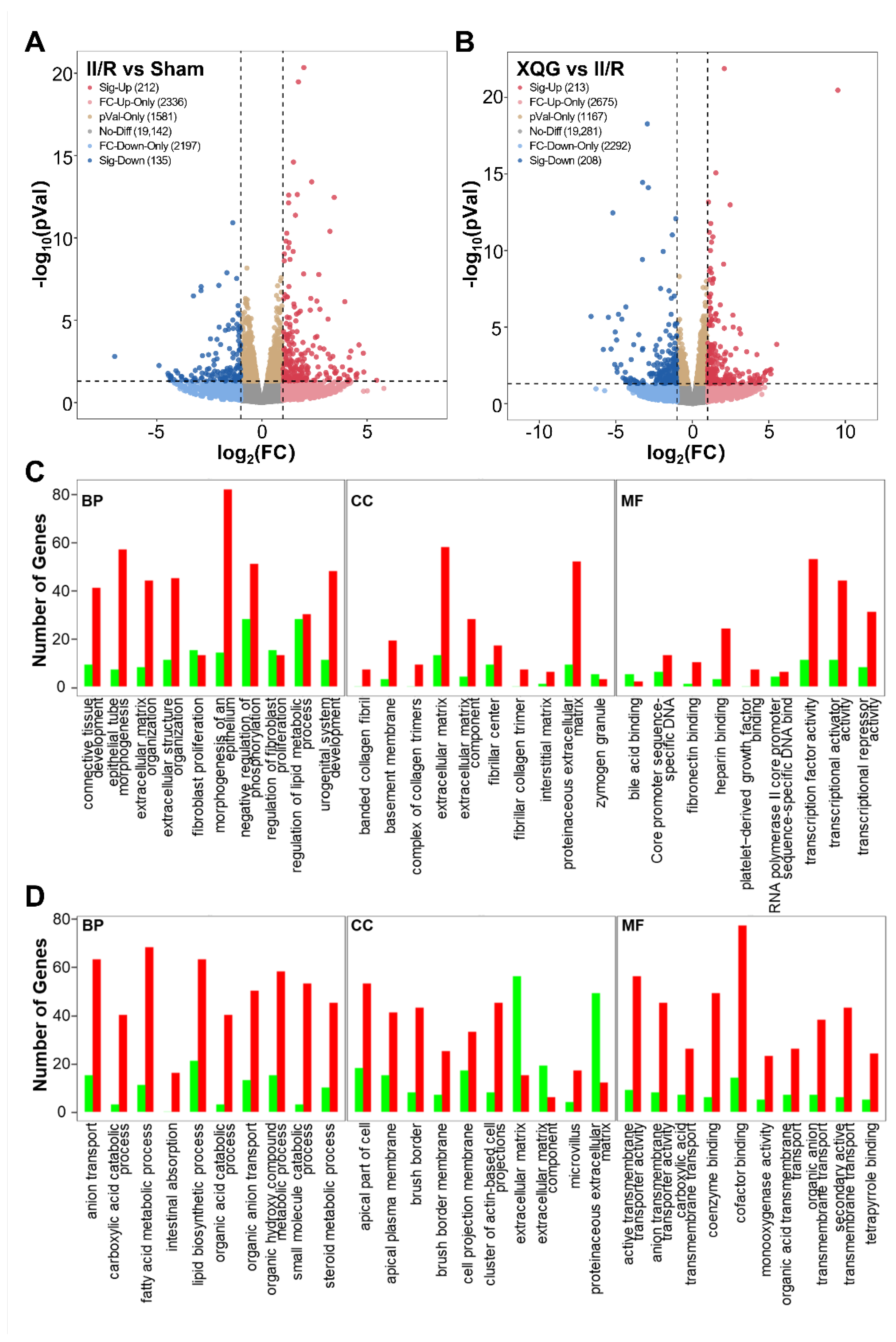
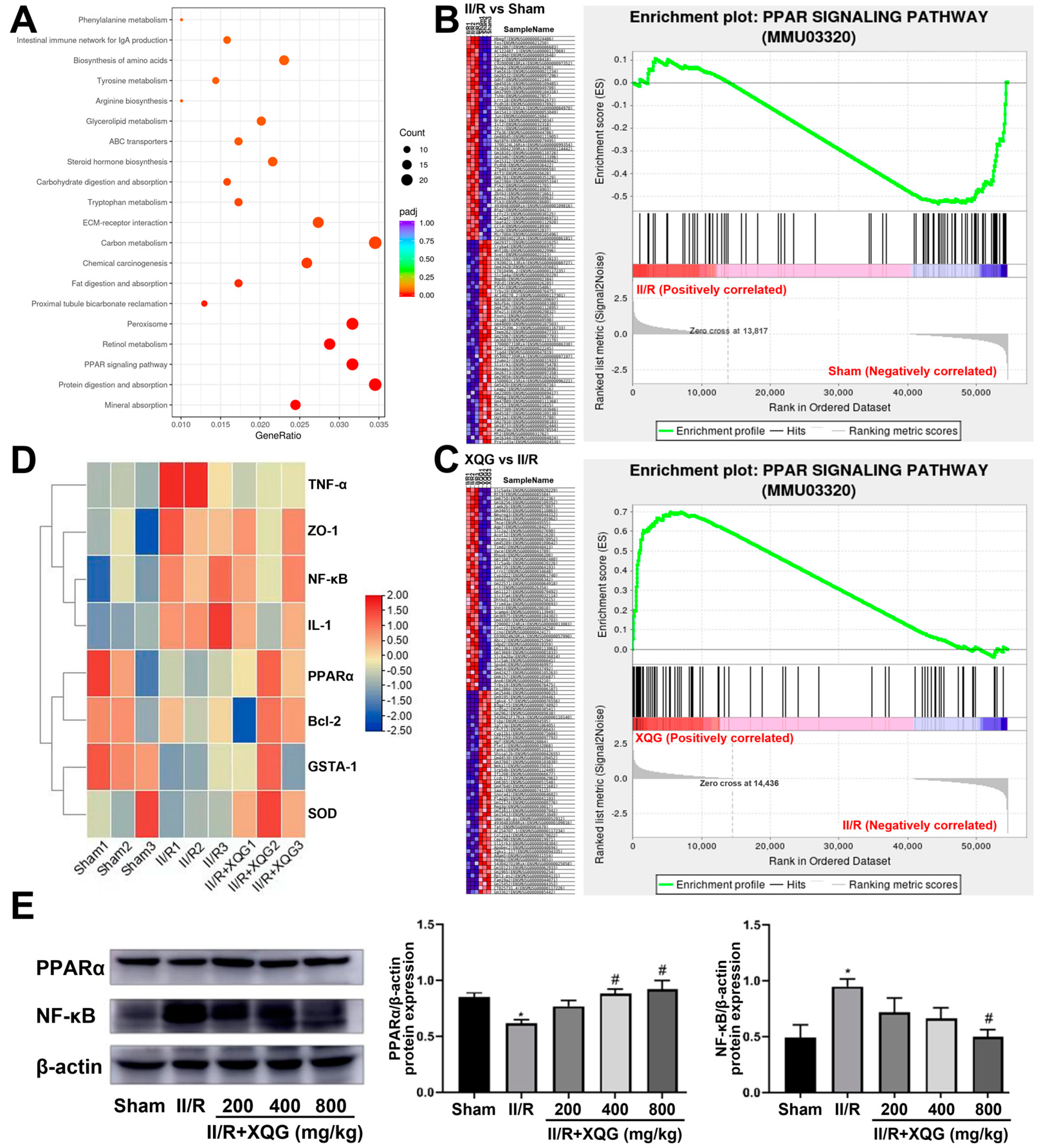
2.6. XQG Pretreatment Alters Gene Expression in II/R Mice
2.7. XQG Alleviated II/R-Induced Injury via the PPARα/NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Main Instruments and Reagents
4.2. Animals
4.3. Histopathological Examination
4.4. Biochemical Indicators Detection
4.5. Immunohistochemical Analysis
4.6. Transcriptome Sequencing
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Akbari, G. Emerging roles of microRNAs in intestinal ischemia/reperfusion-induced injury: A review. J. Physiol. Biochem. 2020, 76, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, H.; Wang, S.; Tang, G.; Zhai, C.; Shen, L. Ferroptosis: A Novel Therapeutic Target for Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2021, 9, 688605. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, Y.; Xiao, J.; Shang, J.; Tan, Q.; Ping, F.; Huang, W.; Wu, F.; Zhang, H.; Zhang, X. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020, 27, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, C.; Xiao, H.; Chen, M.; Yang, Z.; Liang, Z.; Wang, H.; Liu, Y.; Yang, Y.; Wang, Q. Ethyl pyruvate: A newly discovered compound against ischemia-reperfusion injury in multiple organs. Pharmacol. Res. 2021, 171, 105757. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Chai, D.; Peng, J.; Xia, Y.; Hu, R.; Jiang, H. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging 2020, 12, 12943–12959. [Google Scholar] [CrossRef]
- Wen, S.; Li, X.; Ling, Y.; Chen, S.; Deng, Q.; Yang, L.; Li, Y.; Shen, J.; Qiu, Y.; Zhan, Y.; et al. HMGB1-associated necroptosis and Kupffer cells M1 polarization underlies remote liver injury induced by intestinal ischemia/reperfusion in rats. FASEB J. 2020, 34, 4384–4402. [Google Scholar] [CrossRef]
- Li, L.; Shu, F.; Wang, X.Q.; Wang, F.; Cai, L.; Zhao, X.; Lv, H.G. Propofol alleviates intestinal ischemia/reperfusion injury in rats through p38 MAPK/NF-kappaB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1574–1581. [Google Scholar]
- de Holanda, G.S.; Dos Santos Valenca, S.; Carra, A.M.; Lichtenberger, R.C.L.; Franco, O.B.; Ribeiro, B.E.; Rosas, S.L.B.; Santana, P.T.; Castelo-Branco, M.T.L.; de Souza, H.S.P.; et al. Sulforaphane and Albumin Attenuate Experimental Intestinal Ischemia-Reperfusion Injury. J. Surg. Res. 2021, 262, 212–223. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Zou, X.; Shi, Y.; Liu, Q.; Huyan, T.; Su, J.; Wang, Q.; Zhang, F.; Li, X.; et al. FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-gamma coactivator-1alpha-mediated mitochondrial biogenesis. Br. J. Pharmacol. 2020, 177, 432–448. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhou, Y.; Chen, T.; Lei, J.C.; Jiang, X.J. AMPK/SIRT1 Pathway is Involved in Arctigenin-Mediated Protective Effects Against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2020, 11, 616813. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway. J. Mol. Cell Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, C. Pemafibrate Pretreatment Attenuates Apoptosis and Autophagy during Hepatic Ischemia-Reperfusion Injury by Modulating JAK2/STAT3beta/PPARalpha Pathway. PPAR Res. 2021, 2021, 6632137. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, L.; Cui, Y.; Qi, Z.; Huang, X.; Cai, L.; Zhang, T.; Yin, Y.; Lu, Z.; Xiang, J. Roles of PPARgamma/NF-kappaB signaling pathway in the pathogenesis of intrahepatic cholestasis of pregnancy. PLoS ONE 2014, 9, e87343. [Google Scholar]
- Zhang, J.; Cheng, P.; Dai, W.; Ji, J.; Wu, L.; Feng, J.; Wu, J.; Yu, Q.; Li, J.; Guo, C. Fenofibrate Ameliorates Hepatic Ischemia/Reperfusion Injury in Mice: Involvements of Apoptosis, Autophagy, and PPAR-alpha Activation. PPAR Res. 2021, 2021, 6658944. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Adhikari-Devkota, A.; Dirar, A.I.; Hassan, M.M.; Adhikari, A.; Belwal, T.; Devkota, H.P. Fruits of Terminalia chebula Retz.: A review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytother. Res. 2020, 34, 2518–2533. [Google Scholar] [CrossRef]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef]
- Li, K.; Lin, Y.; Li, B.; Pan, T.W.; Wang, F.; Yuan, R.Q.; Ji, J.J.; Diao, Y.P.; Wang, S.Y. Antibacterial constituents of Fructus Chebulae Immaturus and their mechanisms of action. BMC Complement. Med. Ther. 2016, 16, 183. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Zhang, J.S.; Liu, X.G.; Liu, J.; Li, K.; Pan, T.W.; Wang, S.Y.; Diao, Y.P. Protective effect of phenolic acids from Chebulae Fructus immaturus on carbon tetrachloride induced acute liver injury via suppressing oxidative stress, inflammation and apoptosis in mouse. Nat. Prod. Res. 2020, 34, 3249–3252. [Google Scholar] [CrossRef]
- Li, K.; Han, X.W.; Li, R.Z.; Xu, Z.R.; Pan, T.W.; Liu, J.; Li, B.; Wang, S.Y.; Diao, Y.P.; Liu, X.G. Composition, Antivirulence Activity, and Active Property Distribution of the Fruit of Terminalia chebula Retz. J. Food Sci. 2019, 84, 1721–1729. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Jia, Y.; Cui, R.; Wang, C.; Feng, Y.; Li, Z.; Tong, Y.; Qu, K.; Liu, C.; Zhang, J. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020, 32, 101534. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ji, X.; Liang, H.; Liu, Y.; Wang, B.; Sun, L.; Li, W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018, 9, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin alphaVbeta5 receptor. J. Cell. Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Turan, I.; Ozacmak, H.S.; Ozacmak, V.H.; Barut, F.; Arasli, M. Agmatine attenuates intestinal ischemia and reperfusion injury by reducing oxidative stress and inflammatory reaction in rats. Life Sci. 2017, 189, 23–28. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.T.; Ye, L.; Tang, J.J.; Zhang, Z.Z.; He, X.H. Dexmedetomidine Ameliorates Lung Injury Induced by Intestinal Ischemia/Reperfusion by Upregulating Cannabinoid Receptor 2, Followed by the Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 6120194. [Google Scholar] [CrossRef]
- Alexandrova, A.; Petrov, L.; Georgieva, A.; Kessiova, M.; Tzvetanova, E.; Kirkova, M.; Kukan, M. Effect of MG132 on proteasome activity and prooxidant/antioxidant status of rat liver subjected to ischemia/reperfusion injury. Hepatol. Res. 2008, 38, 393–401. [Google Scholar] [CrossRef]
- Wen, S.H.; Li, Y.; Li, C.; Xia, Z.Q.; Liu, W.F.; Zhang, X.Y.; Lei, W.L.; Huang, W.Q.; Liu, K.X. Ischemic postconditioning during reperfusion attenuates intestinal injury and mucosal cell apoptosis by inhibiting JAK/STAT signaling activation. Shock 2012, 38, 411–419. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Duval, C.; Fruchart, J.C.; Staels, B. PPAR alpha, fibrates, lipid metabolism and inflammation. Arch. Mal. Coeur. Vaiss. 2004, 97, 665–672. [Google Scholar]
- Cuzzocrea, S.; Mazzon, E.; Di Paola, R.; Peli, A.; Bonato, A.; Britti, D.; Genovese, T.; Muia, C.; Crisafulli, C.; Caputi, A.P. The role of the peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in the regulation of acute inflammation. J. Leukoc. Biol. 2006, 79, 999–1010. [Google Scholar] [CrossRef]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef]
- Staels, B.; Koenig, W.; Habib, A.; Merval, R.; Lebret, M.; Torra, I.P.; Delerive, P.; Fadel, A.; Chinetti, G.; Fruchart, J.C.; et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 1998, 393, 790–793. [Google Scholar] [CrossRef]
- Korbecki, J.; Bobinski, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhao, J.; Muhammad, I.; Zhang, Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS ONE 2018, 13, e0202368. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, B.; Liu, J.; Qiu, F.; Diao, Y.; Lei, Y.; Liu, J.; Zhang, W. Phenolic Acids from Fructus Chebulae Immaturus Alleviate Intestinal Ischemia-Reperfusion Injury in Mice through the PPARα/NF-κB Pathway. Molecules 2022, 27, 5227. https://doi.org/10.3390/molecules27165227
Liu J, Li B, Liu J, Qiu F, Diao Y, Lei Y, Liu J, Zhang W. Phenolic Acids from Fructus Chebulae Immaturus Alleviate Intestinal Ischemia-Reperfusion Injury in Mice through the PPARα/NF-κB Pathway. Molecules. 2022; 27(16):5227. https://doi.org/10.3390/molecules27165227
Chicago/Turabian StyleLiu, Junjie, Bin Li, Jing Liu, Feng Qiu, Yunpeng Diao, Yuxin Lei, Jianjun Liu, and Wei Zhang. 2022. "Phenolic Acids from Fructus Chebulae Immaturus Alleviate Intestinal Ischemia-Reperfusion Injury in Mice through the PPARα/NF-κB Pathway" Molecules 27, no. 16: 5227. https://doi.org/10.3390/molecules27165227
APA StyleLiu, J., Li, B., Liu, J., Qiu, F., Diao, Y., Lei, Y., Liu, J., & Zhang, W. (2022). Phenolic Acids from Fructus Chebulae Immaturus Alleviate Intestinal Ischemia-Reperfusion Injury in Mice through the PPARα/NF-κB Pathway. Molecules, 27(16), 5227. https://doi.org/10.3390/molecules27165227





