Abstract
Approximately 100–400 million people from more than 100 countries in the tropical and subtropical world are affected by dengue infections. Recent scientific breakthroughs have brought new insights into novel strategies for the production of dengue antivirals and vaccines. The search for specific dengue inhibitors is expanding, and the mechanisms for evaluating the efficacy of novel drugs are currently established, allowing for expedited translation into human trials. Furthermore, in the aftermath of the only FDA-approved vaccine, Dengvaxia, a safer and more effective dengue vaccine candidate is making its way through the clinical trials. Until an effective antiviral therapy and licensed vaccine are available, disease monitoring and vector population control will be the mainstays of dengue prevention. In this article, we highlighted recent advances made in the perspectives of efforts made recently, in dengue vaccine development and dengue antiviral drug.
1. Introduction
Dengue virus (DENV) one of the human arboviruses from the Flaviviridae family, places a significant impact amongst 125 tropical and subtropical regions. Approximately 390 million infections affect the global population annually []. Out of the 390 million cases, 500,000 to 1,000,000 infections are severe cases that lead to fatalities. Endemicity is observed in more than 100 countries, including Africa, the Eastern Mediterranean, the Americas, Southeast Asia, and the Western Pacific. The latter three are the most severely afflicted, with Asia accounting for 70% of the worldwide illness load []. Large scale dengue outbreaks occurred in several countries in the recent past, including the 2019 epidemics in Nepal [] the 2019 outbreaks in Dhaka, Bangladesh [] also including the unexpected massive outbreaks in Xishuangbanna (a border area of China), Myanmar, and Laos in 2019 []. According to a prediction model developed by Messina, J.P. et al., 2019, the number of people infected with dengue would grow by 2.25 billion between 2015 and 2080 []. In addition to these global trends, rising temperatures attributed to climate change have raised concerns that dengue will worsen in already endemic areas due to faster viral amplification, increased vector survival, reproduction, and biting rate, ultimately leading to longer transmission seasons and a greater number of human infections, with a greater number of severe infections expected. Temperature rises may worsen the problem by allowing for increased dissemination and transmission in low-risk or currently dengue-free areas of Asia, Europe, North America, and Australia. Hence, the World Health Organization (WHO) has recently announced that dengue infection is one of the top ten most significant threat to global health in 2019.
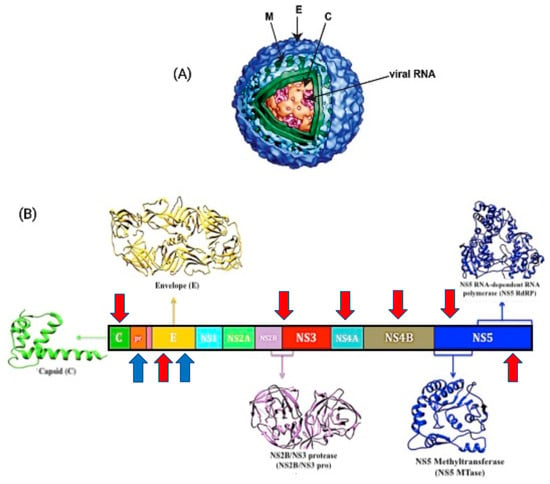
DENV is an enveloped virus with icosahedral symmetry and a genomic size of around 11 kb []. It has a positive single-stranded RNA genome that encodes for a single open reading frame and can be translated into three structural proteins, the core (C), premembrane/membrane (prM/M), and envelope (E), as well as seven non-structural (NS) proteins, namely NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5 []. Its structural glycoprotein E is in charge of cell identification and encouraging host entrance, which is accomplished by a fusion process between the viral envelope and the cell membrane, while the NS protein aids viral genome replication [].
DENV is spread to humans by female Aedes mosquitos in four different serotypes (DENV1–4). The four serotypes are further subdivided into phylogenetic groups, each with its unique genotype. The icosahedral viral genome, which expresses itself as the DENV1–4 serotypes with 65–70% sequence identity, is the taxonomically distinguishing component []. The genome sequence categorizes serotypes into different lineages with high genetic diversity []. The regional assimilation of viral serotypes and genotypes from local geographical proximity, as well as their extensive dispersion, can lead to regional population movement and trans-border economic activity []. Furthermore, viral genotypes may differ dependent on geographical distribution, epidemic potency, and other factors. To assist the tracing of DENV outbreak isolates and aiding the control of the infection, Yamashita, A. and colleagues presented a comprehensive database of DENV sequences containing both serotype and genotype data together with the epidemiological data (Figure 1) [].
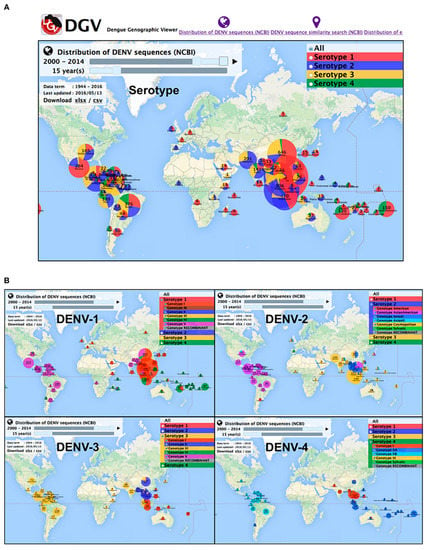
Figure 1.
Overview of DENV serotypes and genotype global distribution in 2000–2014. (A) DENV serotypes distribution (B) DEN genotypes distribution for each serotype. Source: Yamashita et al., 2016 [].
During the infection, the virus enters the body and infiltrates local macrophages, and multiplies. Locally infected cells then move from the site of infection to lymph nodes, where monocytes and macrophages are recruited and become infection targets. As a result, the infection multiplies and the virus spreads via the lymphatic system. During this primary viremia which manifests itself within 24 h, many mononuclear cells, including blood-derived monocytes, were activated. Bone marrow cells have also been found to be vulnerable to DENV infection. The viral load is quite high in severe cases, and many critical organs are impacted. Infected macrophages generate a variety of signaling proteins, including interferons, cytokines, chemokines, TNF, and other mediators, which are responsible for a variety of symptoms. Consequently, these mediators have an effect on the body’s hemostatic system. Fluid begins to leak from blood vessels, causing blood volume to drop and resulting in low blood pressure and inadequate supply of oxygen to critical organs such as the brain. Dengue also affects bone marrow, preventing it from producing enough platelets resulting in blood clotting deficiency and increasing the risk of bleeding (Figure 2).
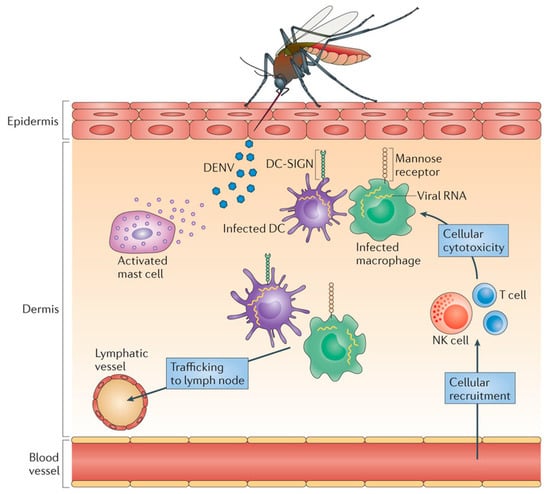
Figure 2.
Dengue pathogenesis. Released viral particles may infect adjacent cells (mostly monocytes or dendritic cells (DCs)) or trigger local immune cells. A local inflammatory response to DENV in the skin induces the recruitment of vasculature-derived leukocytes, including natural killer (NK) cells and T cells, which enhance the death of virus-infected cells at the injection site. DENV is then expected to spread to draining lymph nodes through lymphatic channels, causing systemic infection. These localized inflammatory reactions occur several days before any symptoms appear. Adapted with permission from ref. []. Copyright 2013, St. John, A. et al.
Dengue infection can be asymptomatic and symptomatic in some infected persons. The common clinical symptoms experienced by an infected person include headaches, fever, fatigue, urticaria, body aching, lymphitis, and leukocytopenia. Dengue hemorrhagic fever (DHF) develops in severe instances and is characterized by hemostasis irregularities and increased vascular permeability, the latter of which can lead to hypovolemic shock (dengue shock syndrome, DSS) []. In a person who has not previously been infected by any flavivirus, known as primary infection, the ratio of IgM and IgG is high. Whilst in secondary infection, the host is immunologically sensitized to dengue or other flavivirus infection and the ratio of both immunoglobulins is low.
Despite the lack of specific antiviral therapy and contentious approved vaccine by the US Food and Drug Administration (FDA), dengue maintains as a major worry in 129 endemic countries. Hence, dengue prevention continues to rely on disease surveillance and vector population control [,]. This review focuses on the current status and challenges of DENV antiviral and vaccines development, hence shedding some light on our direction in overcoming dengue disease.
2. Dengue Vaccine Development
In the development of dengue vaccines, a thorough understanding of immune responses to DENV aid in the formulation of an effective approach []. Live-attenuated vaccines, inactivated vaccines, recombinant subunit vaccines, and nucleic acid (DNA) vaccines are the primary forms of dengue vaccines currently under research (Figure 3).
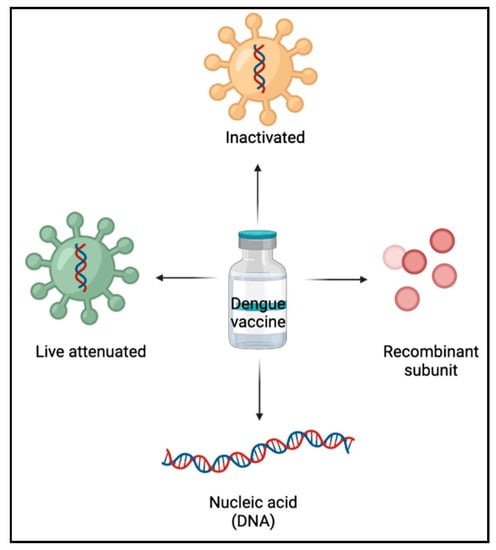
Figure 3.
Types of dengue vaccines.
These types of vaccines confer protection by increasing the immune responses to the E protein and non-structural protein 1 of the dengue virus (DENV) (NS1). The vaccine candidates that have progressed to the clinical trial stage are summarized in Table 1 below.

Table 1.
DENV vaccines currently under development.
2.1. Live-Attenuated Vaccines
Antigenic compounds synthesized from a living pathogen that has been engineered to be less virulent or avirulent are known as live-attenuated vaccines. Once administered, the viruses multiply locally, eliciting neutralizing antibodies and cell-mediated immune responses against the four dengue virus serotypes. These vaccines demonstrate the benefits of delivering protective antigens while offering long-term immune protection []. Using recombinant DNA technology, several live dengue attenuated vaccines have been developed.
Dengvaxia, also known as CYD-TDV, is the only approved tetravalent live-attenuated dengue vaccine candidate [,,]. Receiving FDA approval in 2015, this vaccine is now accessible in more than 20 countries. Its usage has been allowed with strict restrictions on the recipients’ age and serostatus []. It offers high effectiveness in preventing dengue disease caused by DENV serotypes 1–4 and is safe in people who have had a past dengue infection, i.e., those who are seropositive. To date, the FDA has approved the usage in individuals 9 through 16 years of age with laboratory-confirmed previous dengue infection and living in endemic areas []. However, for those who are seronegative, the vaccine increases the chance of having severe dengue when the person has a spontaneous dengue illness about 3 years following immunization. Vaccination in the naive subjects stimulates the development of neutralizing antibodies against all four DENV serotypes. Specific antibodies against one or a few serotypes dominate this response, whereas reactions against the other serotypes are mostly due to cross-reactive antibodies. Moreover, it produces serotype-specific and cross-reactive T cell responses against DENV structural antigens. Therefore, the seronegative individuals may thus constitute a subclinical attenuated ‘primary-like’ illness. Vaccination in this group also results in different immunological effects depending on the serotypes. When compared to cross-protection evoked by vaccination in seropositive individuals, minimal cross-protection in seronegative individuals can be observed, posing a greater risk of inducing antibody-dependent enhancement (ADE) [,,]. Consequently, WHO recommends that this vaccine is only given to seropositive individuals.
Tetravax (TV003/TV005), on the other hand, differs significantly from CYD-TDV in terms of the viral particle structure, infectivity, and immunogenicity [,]. To reduce DENV virulence, researchers utilized three untranslated regions (UTRs) deletions and structural gene prM/E chimerization. In comparison to TV003/TV005, CYD had a greater risk of viremia, lesser dengue virus type 2 resistance, and a reduced level of adaptive immune response [].
TAK-003, often known as DENVax, is a live-attenuated chimeric tetravalent dengue vaccine []. At present, it is still in phase III of clinical investigations. The backbone of this vaccine is a weakened DENV2 strain (PDK-53) that contains the prM/E parts of all serotypes. The vaccine was proven to be immunogenic and well-tolerated in multiple phase I and II clinical studies, independent of the participants’ age or serostatus. TAK-003 showed a DENV serotype-dependent protective effectiveness, similar to its predecessor Dengvaxia, but with higher levels of DENV2 neutralizing antibodies and lower DENV3 and DENV4 protection rates, consequently, its safety profile is not entirely known []. Although a previous clinical study indicated it triggers CD8+ T lymphocytes directed at NS1, NS3, and NS5 in patients that have never been infected with DENV [], this data were not included in later clinical studies.
In addition to live-attenuated dengue vaccines is TDEN F17/F19 [,]. In a phase II trial, this vaccine was proven to be a safe, well-tolerated, and immunogenic DENV vaccine candidate. One month following the second dosage, antibody responses to all four DENV types were recorded in more than half of the infants/toddlers and all of the children []. The vaccines used in these studies were lyophilized monovalent vaccines that were combined into a tetravalent vaccine at the point of administration.
Conclusively, based on the experiences obtained during the development prospective live-attenuated vaccines, the WHO have highlighted the guidelines on the quality, safety, and efficacy of the dengue tetravalent vaccines (live, attenuated). Furthermore, according to FDA, dengue live-attenuated vaccine, particularly Dengvaxia, elicits dengue-specific immune responses against the four dengue virus serotypes after injection. However, the exact mechanism of protection has yet to be discovered [].
2.2. Inactivated Vaccines
Inactivated vaccines are antigenic compounds made up of denatured substances from other microbes such as bacteria and viruses that can provide protection against the live pathogen []. This vaccine stimulates immunity by using antigens from the capsid (c), membrane (M), envelope (E), and non-structural 1 (NS1) protein, although composite vaccinations provide superior protection compared to single-type immunizations.
The tetravalent purified formalin-inactivated virus (TPIV), which contains four non-active dengue serotypes, is an example of an inactivated vaccine now in clinical trials []. TDENV PIV/AS03B is now being investigated in a clinical study with various dosing regimens [,]. In both flavivirus-naive and experienced groups of infected individuals, TDEV PIV was well tolerated and immunogenic. Furthermore, this type of vaccine is safer than live-attenuated vaccines since there is no risk of reactivation and immunological balance is better regulated.
2.3. Recombinant Subunit Vaccine
Following the failure and controversy surrounding Dengvaxia®, recombinant subunit vaccination options have regained some interest. In this type of immunization, antigenic proteins produced by prokaryotic or eukaryotic cells generate long-lasting protective/therapeutic immune responses []. By far the most prevalent DENV recombinant subunit candidates are the envelope E protein or shortened variants. These rely on the formation of neutralizing antibodies to prevent DENV from infecting its host cells.
Despite the ease with which recombinant dengue proteins may be expressed in E. coli, there are potential concerns of being exposed to endotoxin contaminants and protein misfolding []. V180, which is made up of a shortened version of the protein DEN-80, is the most promising subunit vaccine [,]. Recombinant subunit vaccinations are more likely than live-attenuated vaccinations to induce steady immune responses against all DENV serotypes, decreasing the likelihood of the ADE effect [].
2.4. DNA Vaccine
In the development of DNA vaccines, no less than a gene encoding specific antigens were incorporated into the plasmid. The E glycoprotein anchored to the prM protein mediates the first interaction between DENV and host cells, hence, it becomes the primary target for inducing neutralizing antibodies. Through in vivo injection of the expressed antigens, the vaccines induce both arms of the immune system which are the T cell responses and antibody production [].
D1ME100 is an example of a DNA vaccine that has been clinically tested on Aotus nancymaae monkeys and humans [,]. In the initial phase of immunization, the vaccine proved to be safe and tolerable. Notwithstanding, with only about half of those who had high-dose immunization producing neutralizing antibodies, this immunogenicity produced was found to be weak. Furthermore, in those who received low-dose immunization, no neutralizing antibody response was detected []. Another example is the tetravalent dengue vaccine (TVDV), which is made up of four plasmids carrying the prM and E encoding genes from each DENV serotype [,]. The robust DENV-specific IFN T-cell response generated in 79% of the highest vaccination dosage recipients [], however, is cause for concern. Despite its lack of immunogenicity, this vaccine has been shown to be stable, simple to make, inexpensive, and large-scale production. Plasmid modification with extremely efficient promoters, alternative delivery strategies, multiple doses, and co-immunization with adjuvants are recommended as solutions to the drawbacks of this form of vaccination.
2.5. An Ideal Vaccine against Dengue
Despite the fact that a dengue vaccine is now available, which is an important step forward, the long-lasting protective efficacy against each of the four dengue virus serotypes has yet to be confirmed. The characteristics of DENV, as well as the immunological protection and pathologic processes involved, including the transmission and epidemiology of dengue illness, have all hindered the development of a dengue vaccine. Vaccine development also has encountered conceptual problems since its inception, including the biology of the viruses that cause it, immunological protection and pathogenesis processes, and transmission and epidemiology.
Moreover, in endemic regions, the establishment of a protective antibody response against the infection is acquired through recurrent viral exposure. After being infected with one of the four DENV serotypes, the patient can be infected with any of the other serotypes. However, the infected individuals most likely do not manifest the clinical symptoms as a consequence of cross-protection.
The ideal dengue vaccine is abridged from the fact that it must elicit a multitypic response similar to that of people living in endemic areas, aided by persistent exposure and perhaps symptomatic or asymptomatic reinfection. An explicit elaboration on factors influencing vaccine responses, such as the pre-vaccination environment, as well as the significant challenges that face the development of an efficient/protective dengue vaccine, such as the presence of multiple serotypes, ADE, and cross-reactivity with other flaviviruses, have also been discussed to enhance the search of reliable dengue vaccine []. Hence, we concluded the key barriers to dengue vaccine development include a lack of a proper mechanistic investigation in pathogenesis and ADE.
4. Conclusions
In the past five years, several potential vaccine candidates and antiviral agents with anti-DENV activity have been identified. Some vaccine candidates are now undergoing clinical trials to test their safety and effectiveness in humans. Furthermore, since most clinical vaccine trials are conducted in naive people, it will be critical to expand these studies to discover if individuals who have already been infected with dengue can be efficiently and safely vaccinated.
On the other hand, the need to develop well-protective antiviral properties with moderate toxicity, low likelihood of viral resistance, and adequate stability to ensure absorption and dispersion has stymied antiviral research against dengue. Hence, continuous advancements in screening methodologies, X-ray modeling, and easily accessible databases provide a promising foundation for the discovery of novel therapies, but additional examination of existing medications is also required. Combination treatment appears to be the best antiviral method for addressing the current anti-DENV medication development roadblocks.
Author Contributions
N.S.L. designed the study; H.N. carried out the data collection, data analysis and interpretation; N.S.L. and H.N., drafted the article; R.V. edited the article. All authors read and approved the final article. Authors contributed equally for the preparation of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Research University Individual grant from Universiti Sains Malaysia (1001/CIPPM/8012305). This study also funded by Malaysian Ministry of Education through the Higher Institution Centre of Excellence (HICoE) Grant No: 311/CIPPM/4401005.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmed, A.M.; Mohammed, A.T.; Vu, T.T.; Khattab, M.; Doheim, M.F.; Mohamed, A.A.; Abdelhamed, M.M.; Shamandy, B.E.; Dawod, M.T.; Alesaei, W.A.; et al. Prevalence and burden of dengue infection in Europe: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2093. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue and Severe Dengue. Dengue Fact Sheet. Available online: http://www.searo.who.int/entity/vector_borne_tropical_diseases/data/data_factsheet/en/ (accessed on 11 January 2021).
- EDCD (Epidemiology and Disease Control Division). Updates on Dengue Fever. Available online: http://edcd.gov.np/resources/download/dengue-update-as-of-1st-ashoj-2076 (accessed on 22 October 2021).
- Hossain, M.S.; Siddiqee, M.H.; Siddiqi, U.R.; Raheem, E.; Akter, R.; Hu, W. Dengue in a crowded megacity: Lessons learnt from 2019 outbreak in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 2020, 14, e0008349. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, Y.; Shan, X.; Li, D.; Ma, D.; Li, T.; Long, S.; Wang, X.; Pan, Y.; Chen, J.; et al. Co-circulation of three dengue virus serotypes led to a severe dengue outbreak in Xishuangbanna, a border area of China, Myanmar, and Laos, in 2019. Int. J. Infect. Dis. 2021, 107, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- St. John, A.L.; Abraham, S.N.; Gubler, D.J. Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat. Rev. Microbiol. 2013, 11, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Manoharan, M. Dengue Virus. In Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–359. [Google Scholar]
- Nncube, N.B.; Ramharack, P.; Soliman, M.E.S. Using bioinformatics tools for the discovery of Dengue RNA-dependent RNA polymerase inhibitors. PeerJ 2018, 6, e5068. [Google Scholar] [CrossRef]
- Rico-Hesse, R. Microevolution and virulence of dengue viruses. Adv. Virus Res. 2003, 59, 315. [Google Scholar]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.S.; Ismail, H.I.H.M.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Yamashita, A.; Sakamoto, T.; Sekizuka, T.; Kato, K.; Takasaki, T.; Kuroda, M. DGV: Dengue Genographic Viewer. Front. Microbiol. 2016, 7, 875. [Google Scholar] [CrossRef]
- Tiga-Loza, D.C.; Martínez-Vega, R.A.; Undurraga, E.A.; Tschampl, C.A.; Shepard, D.S.; Ramos-Castañeda, J. Persistence of symptoms in dengue patients: A clinical cohort study. Trans. R. Soc. Trop. Med. Hyg. 2021, 114, 355–364. [Google Scholar] [CrossRef]
- Alkuriji, M.A.; Al-Fageeh, M.B.; Shaher, F.M.; Almutairi, B.F. Dengue Vector Control: A Review for Wolbachia-Based Strategies. Biosci. Biotechnol. Res. Asia 2020, 17, 507–515. [Google Scholar] [CrossRef]
- Selvarajoo, S.; Liew, J.W.K.; Tan, W.; Lim, X.Y.; Refai, W.F.; Zaki, R.A.; Sethi, N.; Wan Sulaiman, W.Y.; Lim, Y.A.L.; Vadivelu, J.; et al. Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: A cross-sectional study. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Wellekens, K.; Betrains, A.; De Munter, P.; Peetermans, W. Dengue: Current state one year before WHO 2010–2020 goals. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Whitehead, S.S.; Durbin, A.P.; Pierce, K.K.; Elwood, D.; McElvany, B.D.; Fraser, E.A.; Carmolli, M.P.; Tibery, C.M.; Hynes, N.A.; Jo, M.; et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl. Trop. Dis. 2017, 11, e0005584. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Durbin, A.P.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Diehl, S.A.; Elwood, D.; Jarvis, A.P.; et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J. Infect. Dis. 2015, 212, 702–710. [Google Scholar] [CrossRef]
- Sirivichayakul, C.; Barranco-Santana, E.A.; Esquilin-Rivera, I.; Oh, H.M.L.; Raanan, M.; Sariol, C.A.; Shek, L.P.; Simasathien, S.; Smith, M.K.; Velez, I.D.; et al. Safety and Immunogenicity of a Tetravalent Dengue Vaccine Candidate in Healthy Children and Adults in Dengue-Endemic Regions: A Randomized, Placebo-Controlled Phase 2 Study. J. Infect. Dis. 2016, 213, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Eckels, K.H.; Carletti, I.; De La Barrera, R.; Dessy, F.; Fernandez, S.; Putnak, R.; Toussaint, J.F.; Sun, W.; Bauer, K.; et al. A phase II, randomized, safety and immunogenicity study of a re-derived, live-attenuated dengue virus vaccine in healthy adults. Am. J. Trop. Med. Hyg. 2013, 88, 73–88. [Google Scholar] [CrossRef]
- Bauer, K.; Esquilin, I.O.; Cornier, A.S.; Thomas, S.J.; Del Rio, A.I.Q.; Bertran-Pasarell, J.; Ramirez, J.O.M.; Diaz, C.; Carlo, S.; Eckels, K.H.; et al. A phase II, randomized, safety and immunogenicity trial of a re-derived, live-attenuated dengue virus vaccine in healthy children and adults living in puerto rico. Am. J. Trop. Med. Hyg. 2015, 93, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.C.; Lin, L.; Martinez, L.J.; Ruck, R.C.; Eckels, K.H.; Collard, A.; De La Barrera, R.; Paolino, K.M.; Toussaint, J.F.; Lepine, E.; et al. Phase 1 randomized study of a tetravalent dengue purified inactivated vaccine in healthy adults in the United States. Am. J. Trop. Med. Hyg. 2017, 96, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Koren, M.; Lin, L.; Martinez, L.J.; Eckels, K.H.; Campos, M.; Jarman, R.G.; de la Barrera, R.; Lepine, E.; Febo, I.; et al. Safety and immunogenicity of different formulations of a tetravalent dengue purified inactivated vaccine in healthy adults from Puerto Rico: Final results after 3 years of follow-up from a randomized, placebo-controlled phase I study. Am. J. Trop. Med. Hyg. 2020, 102, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.A.G.; Clements, D.E.; Bett, A.J.; Sagar, S.L.; Ter Meulen, J.H. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 2011, 29, 7267–7275. [Google Scholar] [CrossRef]
- Manoff, S.B.; Sausser, M.; Falk Russell, A.; Martin, J.; Radley, D.; Hyatt, D.; Roberts, C.C.; Lickliter, J.; Krishnarajah, J.; Bett, A.; et al. Immunogenicity and safety of an investigational tetravalent recombinant subunit vaccine for dengue: Results of a Phase I randomized clinical trial in flavivirus-naïve adults. Hum. Vaccines Immunother. 2019, 15, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Beckett, C.G.; Tjaden, J.; Burgess, T.; Danko, J.R.; Tamminga, C.; Simmons, M.; Wu, S.J.; Sun, P.; Kochel, T.; Raviprakash, K.; et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011, 29, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Danko, J.R.; Kochel, T.; Teneza-Mora, N.; Luke, T.C.; Raviprakash, K.; Sun, P.; Simmons, M.; Moon, J.E.; De La Barrera, R.; Martinez, L.J.; et al. Safety and immunogenicity of a tetravalent dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am. J. Trop. Med. Hyg. 2018, 98, 849–856. [Google Scholar] [CrossRef]
- Kochel, T.J.; Raviprakash, K.; Hayes, C.G.; Watts, D.M.; Russell, K.L.; Gozalo, A.S.; Phillips, I.A.; Ewing, D.F.; Murphy, G.S.; Porter, K.R. A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine 2000, 18, 3166–3173. [Google Scholar] [CrossRef]
- Nivarthi, U.K.; Swanstrom, J.; Delacruz, M.J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Kirkpatrick, B.D.; Pierce, K.K.; Diehl, S.A.; Katzelnick, L.; et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Tully, D.; Griffiths, C.L. Dengvaxia: The world’s first vaccine for prevention of secondary dengue. Ther. Adv. Vaccines Immunother. 2021, 9, 251513552110158. [Google Scholar] [CrossRef]
- FDA. Dengvaxia FDA 2019. Available online: https://www.fda.gov/vaccines-blood-biologics/dengvaxia (accessed on 26 October 2021).
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2015, 14, 45–54. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Katzelnick, L.C.; Russell, P.K.; Markoff, L.; Aguiar, M.; Dans, L.R.; Dans, A.L. Ethics of a partially effective dengue vaccine: Lessons from the Philippines. Vaccine 2020, 38, 5572–5576. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; What makes this vaccine different from the Sanofi-Pasteur CYDTM vaccine? Expert Rev. Vaccines 2016, 15, 509–517. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Young, E.F.; Stoops, M.J.; Henein, S.R.; Adams, E.C.; Baric, R.S.; de Silva, A.M. Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (Tak-003). PLoS Negl. Trop. Dis. 2021, 15, e0009258. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Lim, M.Q. CD4+ and CD8+ T-cell immunity to Dengue—Lessons for the study of Zika virus. Immunology 2017, 150, 146–154. [Google Scholar] [CrossRef]
- Watanaveeradej, V.; Gibbons, R.V.; Simasathien, S.; Nisalak, A.; Jarman, R.G.; Kerdpanich, A.; Tournay, E.; De La Barrerra, R.; Dessy, F.; Toussaint, J.F.; et al. Safety and immunogenicity of a rederived, live-attenuated dengue virus vaccine in healthy adults living in Thailand: A randomized trial. Am. J. Trop. Med. Hyg. 2014, 91, 119–128. [Google Scholar] [CrossRef]
- Putnak, R.; Barvir, D.A.; Burrous, J.M.; Dubois, D.R.; D’Andrea, V.M.; Hoke, C.H.; Sadoff, J.C.; Eckels, K.H. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: Immunogenicity and protection in mice and rhesus monkeys. J. Infect. Dis. 1996, 174, 1176–1184. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Thomas, S.J.; Ruxrungtham, K. Dengue vaccine: Global development update. Asian Pac. J. Allergy Immunol. 2020, 38, 178–185. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Recombinant Protein–Based Dengue Vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Tripathi, N.K. Production and purification of recombinant proteins from Escherichia coli. ChemBioEng Rev. 2016, 3, 116–133. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Yamamura, A.M.Y.; Freire, M.S.; Trindade, G.F.; Bonaldo, M.; Galler, R.; Alves, A.M.B. DNA vaccines against dengue virus type 2 based on truncate envelope protein or its domain III. PLoS ONE 2011, 6, 20528. [Google Scholar] [CrossRef] [PubMed]
- Maves, R.C.; Oré, R.M.C.; Porter, K.R.; Kochel, T.J. Immunogenicity and protective efficacy of a psoralen-inactivated dengue-1 virus vaccine candidate in Aotus nancymaae monkeys. Vaccine 2011, 29, 2691–2696. [Google Scholar] [CrossRef]
- Izmirly, A.M.; Alturki, S.O.; Alturki, S.O.; Connors, J.; Haddad, E.K. Challenges in Dengue Vaccines Development: Pre-existing Infections and Cross-Reactivity. Front. Immunol. 2020, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.; Padmanabhan, R. Molecular targets for flavivirus drug discovery. Antivir. Res. 2009, 81, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Sawasdee, N.; Junking, M.; Srisawat, C.; Choowongkomon, K.; Yenchitsomanus, P. A Peptide Inhibitor Derived from the Conserved Ectodomain Region of DENV Membrane (M) Protein with Activity Against Dengue Virus Infection. Chem. Biol. Drug Des. 2015, 86, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, D.; Peng Chin, S.; Lim Chong, W.; Zain, S.M.; Abd Rahman, N.; Sanghiran Lee, V. Dynamics and binding interactions of peptide inhibitors of dengue virus entry. J. Biol. Phys. 2019, 45, 63–76. [Google Scholar] [CrossRef]
- Yang, C.C.; Hu, H.S.; Lin, H.M.; Wu, P.S.; Wu, R.H.; Tian, J.N.; Wu, S.H.; Tsou, L.K.; Song, J.S.; Chen, H.W.; et al. A novel flavivirus entry inhibitor, BP34610, discovered through high-throughput screening with dengue reporter viruses. Antivir. Res. 2019, 172, 104636. [Google Scholar] [CrossRef]
- Faustino, A.F.A.A.; Guerra, G.M.; Huber, R.G.; Hollmann, A.; Domingues, M.M.; Barbosa, G.M.; Enguita, F.J.; Bond, P.J.; Castanho, M.A.R.B.R.B.; Da Poian, A.T.; et al. Understanding Dengue Virus Capsid Protein Disordered N-Terminus and pep14-23-Based Inhibition. ACS Chem. Biol. 2015, 10, 517–526. [Google Scholar] [CrossRef]
- Smith, J.L.; Sheridan, K.; Parkins, C.J.; Frueh, L.; Jemison, A.L.; Strode, K.; Dow, G.; Nilsen, A.; Hirsch, A.J. Characterization and structure-activity relationship analysis of a class of antiviral compounds that directly bind dengue virus capsid protein and are incorporated into virions. Antivir. Res. 2018, 155, 12–19. [Google Scholar] [CrossRef]
- Bhakat, S.; Delang, L.; Kaptein, S.; Neyts, J.; Leyssen, P.; Jayaprakash, V. Reaching beyond HIV/HCV: Nelfinavir as a potential starting point for broad-spectrum protease inhibitors against dengue and chikungunya virus. RSC Adv. 2015, 5, 105. [Google Scholar] [CrossRef]
- Raut, R.; Beesetti, H.; Tyagi, P.; Khanna, I.; Jain, S.K.; Jeankumar, V.U.; Yogeeswari, P.; Sriram, D.; Swaminathan, S. A small molecule inhibitor of dengue virus type 2 protease inhibits the replication of all four dengue virus serotypes in cell culture. Virol. J. 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.W.; Mao, F.; Ye, Y.; Li, J.; Xu, C.L.; Luo, X.M.; Chen, J.; Shen, X. Policresulen, a novel NS2B/NS3 protease inhibitor, effectively inhibits the replication of DENV2 virus in BHK-21 cells. Acta Pharmacol. Sin. 2015, 36, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.F.; Nitsche, C.; Graf, D.; Bartenschlager, R.; Klein, C.D. Phenylalanine and Phenylglycine Analogues as Arginine Mimetics in Dengue Protease Inhibitors. J. Med. Chem 2015, 58, 7733. [Google Scholar] [CrossRef]
- Behnam, M.A.M.; Graf, D.; Bartenschlager, R.; Zlotos, D.P.; Klein, C.D. Discovery of Nanomolar Dengue and West Nile Virus Protease Inhibitors Containing a 4-Benzyloxyphenylglycine Residue. J. Med. Chem. 2015, 58, 9354–9370. [Google Scholar] [CrossRef]
- Li, L.; Basavannacharya, C.; Chan, K.W.K.; Shang, L.; Vasudevan, S.G.; Yin, Z. Structure-guided Discovery of a Novel Non-peptide Inhibitor of Dengue Virus NS2B–NS3 Protease. Chem. Biol. Drug Des. 2015, 86, 255–264. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Manzano, M.; Teramoto, T.; Pilankatta, R.; Padmanabhan, R. High-throughput screening for the identification of small-molecule inhibitors of the flaviviral protease. Antivir. Res. 2016, 134, 6–16. [Google Scholar] [CrossRef]
- Rothan, H.A.; Abdulrahman, A.Y.; Khazali, A.S.; Nor Rashid, N.; Chong, T.T.; Yusof, R. Carnosine exhibits significant antiviral activity against Dengue and Zika virus. J. Pept. Sci. 2019, 25, e3196. [Google Scholar] [CrossRef]
- Bhowmick, S.; Alissa, S.A.; Wabaidur, S.M.; Chikhale, R.V.; Islam, M.A. Structure-guided screening of chemical database to identify NS3-NS2B inhibitors for effective therapeutic application in dengue infection. J. Mol. Recognit. 2020, 33, e2838. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Khan, B.A.; Hameed, S.; Batool, F.; Saleem, H.N.; Mughal, E.U.; Saeed, M. Synthesis and evaluation of novel S-benzyl-and S-alkylphthalimide-oxadiazole-benzenesulfonamide hybrids as inhibitors of dengue virus protease. Bioorg. Chem. 2020, 96, 103567. [Google Scholar] [CrossRef]
- Ghosh, I.; Talukdar, P. Molecular docking and pharmacokinetics study for selected leaf phytochemicals from Carica papaya Linn. against dengue virus protein, NS2B/NS3 protease. World Sci. News 2019, 124, 264–278. [Google Scholar]
- Lim, W.Z.; Cheng, P.G.; Abdulrahman, A.Y.; Teoh, T.C. The identification of active compounds in Ganoderma lucidum var. antler extract inhibiting dengue virus serine protease and its computational studies. J. Biomol. Struct. Dyn. 2020, 38, 4273–4288. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Munir, B.; Naeem, S.; Yameen, M.; Iqbal, S.Z.; Ahmad, A.; Mustaan, M.A.; Noor, M.W.; Nadeem, M.A.; Ghaffar, A. Exploration of Carica papaya bioactive compounds as potential inhibitors of dengue NS2B, NS3 and NS5 protease. Pak. J. Pharm. Sci. 2020, 33, 355–360. [Google Scholar] [PubMed]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antivir. Res 2019, 162, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.N.; Hariono, M.; Salleh, H.M.; Chong, S.-L.; Yee, L.S.; Zahari, A.; Wahab, H.A.; Derbré, S.; Awang, K. Chemical Constituents From Endiandra kingiana (Lauraceae) as Potential Inhibitors for Dengue Type 2 NS2B/NS3 Serine Protease and its Molecular Docking. Nat. Prod. Commun. 2019, 14, 1934578X19861014. [Google Scholar] [CrossRef]
- Salleh, H.M.; Chong, S.L.; Othman, R.; Hazni, H.; Ahmad, K.; Mohd Yusof, M.Y.Z.; Fauzi, N.W.; Wahab, H.A.; Liew, S.Y.; Awang, K. Dengue protease inhibition activity of selected Malaysian medicinal herbs. Trop. Biomed. 2019, 36, 357–366. [Google Scholar]
- Yao, X.; Ling, Y.; Guo, S.; He, S.; Wang, J.; Zhang, Q.; Wu, W.; Zou, M.; Zhang, T.; Nandakumar, K.S. Inhibition of dengue viral infection by diasarone-I is associated with 2′O methyltransferase of NS5. Eur. J. Pharmacol. 2018, 821, 11–20. [Google Scholar] [CrossRef]
- Saleem, H.N.; Batool, F.; Mansoor, H.J.; Shahzad-ul-Hussan, S.; Saeed, M. Inhibition of Dengue Virus Protease by Eugeniin, Isobiflorin, and Biflorin Isolated from the Flower Buds of Syzygium aromaticum (Cloves). ACS Omega 2019, 4, 1525–1533. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, M.-H.; Lee, J.-Y.; Hwang, I.; Yoon, G.Y.; Kim, H.S.; Kwon, Y.-C.; Ahn, D.-G.; Kim, K.-D.; Kim, B.-T.; et al. Structure-Based Virtual Screening: Identification of a Novel NS2B-NS3 Protease Inhibitor with Potent Antiviral Activity against Zika and Dengue Viruses. Microorganisms 2021, 9, 545. [Google Scholar] [CrossRef]
- Nobori, H.; Toba, S.; Yoshida, R.; Hall, W.W.; Orba, Y.; Sawa, H.; Sato, A. Identification of Compound-B, a novel anti-dengue virus agent targeting the non-structural protein 4A. Antiviral Res. 2018, 155, 60–66. [Google Scholar] [CrossRef]
- Van Cleef, K.W.R.; Overheul, G.J.; Thomassen, M.C.; Marjakangas, J.M.; Van Rij, R.P. Escape mutations in NS4B render dengue virus insensitive to the antiviral activity of the paracetamol metabolite AM404. Antimicrob. Agents Chemother. 2016, 60, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Moquin, S.A.; Simon, O.; Karuna, R.; Lakshminarayana, S.B.; Yokokawa, F.; Wang, F.; Saravanan, C.; Zhang, J.; Day, C.W.; Chan, K.; et al. NITD-688, a pan-serotype inhibitor ofthe dengue virus NS4B protein, shows favorable pharmacokinetics and efficacy in preclinical animal models. Sci. Transl. Med. 2021, 13, eabb2181. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Tseng, C.K.; Wu, Y.H.; Kaushik-Basu, N.; Lin, C.K.; Chen, W.C.; Wu, H.N. Characterization of the activity of 2′-C-methylcytidine against dengue virus replication. Antivir. Res. 2015, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vernekar, S.K.V.; Qiu, L.; Zhang, J.; Kankanala, J.; Li, H.; Geraghty, R.J.; Wang, Z. 5′-silylated 3′-1,2,3-triazolyl thymidine analogues as inhibitors of West Nile Virus and Dengue virus. J. Med. Chem. 2015, 58, 4016–4028. [Google Scholar] [CrossRef]
- Bullard, K.M.; Gullberg, R.C.; Soltani, E.; Steel, J.J.; Geiss, B.J.; Keenan, S.M. Murine efficacy and pharmacokinetic evaluation of the flaviviral NS5 capping enzyme 2-thioxothiazolidin-4-one inhibitor BG-323. PLoS ONE 2015, 10, e0130083. [Google Scholar] [CrossRef][Green Version]
- Stahla-Beek, H.J.; April, D.G.; Saeedi, B.J.; Hannah, A.M.; Keenan, S.M.; Geiss, B.J. Identification of a Novel Antiviral Inhibitor of the Flavivirus Guanylyltransferase Enzyme. J. Virol. 2012, 86, 8730. [Google Scholar] [CrossRef] [PubMed]
- Brecher, M.; Chen, H.; Li, Z.; Banavali, N.K.; Jones, S.A.; Zhang, J.; Kramer, L.D.; Li, H. Identification and Characterization of Novel Broad-Spectrum Inhibitors of the Flavivirus Methyltransferase. ACS Infect. Dis. 2016, 1, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Hermida, L.; Bernardo, L.; Ramirez, R.; Guillen, G. Domain III of the envelope protein as a dengue vaccine target. Expert Rev. Vaccines 2010, 9, 137–147. [Google Scholar] [CrossRef]
- Yildiz, M.; Ghosh, S.; Bell, J.A.; Sherman, W.; Hardy, J.A. Allosteric inhibition of the NS2B-NS3 protease from dengue virus. ACS Chem. Biol. 2013, 8, 2744–2752. [Google Scholar] [CrossRef]
- Wu, H.; Bock, S.; Snitko, M.; Berger, T.; Weidner, T.; Holloway, S.; Kanitz, M.; Diederich, W.E.; Steuber, H.; Walter, C. Novel dengue virus NS2B/NS3 protease inhibitors. Antimicrob. Agents Chemother. 2015, 59, 1100–1109. [Google Scholar] [CrossRef]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- Zou, J.; Xie, X.; Wang, Q.-Y.; Dong, H.; Lee, M.Y.; Kang, C.; Yuan, Z.; Shi, P.-Y. Characterization of Dengue Virus NS4A and NS4B Protein Interaction. J. Virol. 2015, 89, 3455–3470. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Chan, W.L.; Ding, M.; Leong, S.Y.; Nilar, S.; Seah, P.G.; Liu, W.; Karuna, R.; Blasco, F.; Yip, A.; et al. Lead optimization of spiropyrazolopyridones: A new and potent class of dengue virus inhibitors. ACS Med. Chem. Lett. 2015, 6, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Y.; Dong, H.; Zou, B.; Karuna, R.; Wan, K.F.; Zou, J.; Susila, A.; Yip, A.; Shan, C.; Yeo, K.L.; et al. Discovery of Dengue Virus NS4B Inhibitors. J. Virol. 2015, 89, 8233–8244. [Google Scholar] [CrossRef] [PubMed]
- Tunghirun, C.; Narkthong, V.; Chaicumpa, W.; Chimnaronk, S. Interference of dengue replication by blocking the access of 3′ SL RNA to the viral RNA-dependent RNA polymerase. Antivir. Res. 2020, 182, 104921. [Google Scholar] [CrossRef]
- Manjula, S.; Kumaradhas, P. Evaluating the suitability of RNA intervention mechanism exerted by some flavonoid molecules against dengue virus MTase RNA capping site: A molecular docking, molecular dynamics simulation, and binding free energy study. J. Biomol. Struct. Dyn. 2020, 38, 3533–3543. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).